Abstract
Introduction
Voriconazole (VRZ) is the recommended standard treatment for life-threatening invasive aspergillosis. The plasma concentration of VRZ should be determined to optimise treatment results and reduce side effects. This study aimed to compare the correlation and concordance of ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) and enzyme-multiplied immunoassay technique (EMIT) to determine VRZ plasma concentration in clinical practice.
Methods
An isotopically labelled internal standard UPLC-MS/MS method was established, validated, and subsequently applied to determine VRZ concentration. The UPLC-MS/MS method was also compared with a commercial EMIT method regarding results correlation and concordance.
Results
The calibration curve of UPLC-MS/MS was linear from 0.1 to 10 mg/L, the inter- and intra-day relative standard deviations (RSDs), and the stability of quality control samples were less than 15 %, satisfying the Bioanalytical Method Validation Guidelines. A total of 122 plasma samples were collected and analyzed using both methods. UPLC-MS/MS and EMIT showed a high correlation (r = 0.9534), and Bland-Altman analysis indicated a mean absolute bias of 1.035 mg/L and an average bias of 27.56 % between UPLC-MS/MS and EMIT. The paired Wilcoxon test and Bland-Altman analysis revealed poor consistency between the two methods. Furthermore, we compared the effects of different methods in clinical applications. Two threshold values for treatment efficacy (1.0 mg/L) and safety (5.5 mg/L) were established, and considerable discordance was observed between the original EMIT and UPLC-MS/MS results at both thresholds (p < 0.05). Nevertheless, the adjusted EMIT results were not inconsistent with the UPLC-MS/MS results regarding the efficacy (p = 0.125) and safety (p = 1.0) thresholds.
Conclusions
The isotopically labelled internal standard UPLC-MS/MS method is established and well applied in the clinical setting. A strong correlation but discordance was found between UPLC-MS/MS and EMIT, indicating that switching from UPLC-MS/MS to EMIT was unsuitable. However, the adjusted EMIT results may serve as a reliable surrogate when UPLC-MS/MS results cannot be obtained when necessary.
Keywords: Voriconazole, UPLC-MS/MS, EMIT, Concordance, Therapeutic drug monitoring
1. Introduction
Invasive aspergillosis is a potentially life-threatening infection in patients with long-term or severe immune system dysfunction [1]. Voriconazole (VRZ), a second-generation triazole with broad-spectrum antifungal effects, is the recommended standard therapy for the primary treatment of invasive aspergillosis [2,3]. VRZ is metabolised in the liver by hepatic cytochrome P450 isoenzymes, predominantly CYP2C19, CYP3A4, and CYP2C9, with nonlinear pharmacokinetic properties [4]. Despite the use of a standardised dosing strategy, large inter- and intra-individual variability in VRZ plasma concentrations has been observed, with little correlation between the VRZ dose and the measured plasma concentration [5]. Multiple variables, such as age, sex, liver disease, drug-drug interactions, cytochrome P450 polymorphisms, and inflammation, may influence VRZ plasma concentrations [5,6]. Because VRZ plasma concentrations are correlated with efficacy and safety, current guidelines recommend performing VRZ therapeutic drug monitoring (TDM) regularly to improve treatment outcomes and avoid adverse effects, such as psychiatric and neurotoxicity, visual disturbances, prolonged QT interval, and hepatotoxicity [7]. VRZ has a narrow therapeutic range and is recommended between 1.0 and 5.5 mg/L in Europe [8,9], Japan [10], and Australia [11], and 0.5–5.0 mg/L in China [12]. Consequently, a precise and accurate method for determining VRZ is necessary.
Currently, analytical methods based on chromatography, such as high-performance liquid chromatography combined with a UV detector (HPLC-UV) [13] and ultra-performance liquid chromatography coupled with tandem mass spectrometry (UPLC-MS/MS) [14], have been used to determine VRZ concentrations. Among them, UPLC-MS/MS is the most sensitive and selective method, and by using a collisional activated dissociation, the molecular ions and specific fragment ions are selected for quantitative determination. UPLC-MS/MS has been the “gold standard” method, particularly when performed with stable isotopically labelled internal standard (SIL-IS). Although UPLC-MS/MS has excellent sensitivity and specificity, it is inappropriate for regular TDM because of the high equipment cost, technical complexity, extensive pre-treatment, and time-consuming procedures. Recently, an innovative commercial kit that utilises the enzyme-multiplied immunoassay (EMIT) method was developed and authorised for VRZ TDM in China. Compared with chromatography, EMIT has efficient pre-treatment procedures, full automation, and high-throughput fast determination with a short turnover.
Only a few studies have examined the concordance between HPLC and EMIT, and their findings have been contradictory [15,16]. To the best of our knowledge, no comparison between UPLC-MS/MS and EMIT has been performed. Therefore, this study aimed to develop and validate a more specific UPLC-MS/MS assay for VRZ determination based on SIL-IS. We also evaluated the concordance between the UPLC-MS/MS and EMIT measurements. Furthermore, we assessed the clinical relevance of any discrepancies in TDM data between LC-MS/MS and EMIT.
2. Materials and methods
2.1. Enzyme-multiplied immunoassay technique (EMIT)
The enzyme-multiplied immunoassay technique (EMIT) is a simple, commercial, and rapid method commonly used to measure various drugs. A known drug quantity is chemically labelled with an enzyme such as glucose-6-phosphate dehydrogenase, and antibodies specific to that drug bind to the drug-enzyme complex, reducing enzyme activity. A biological sample containing the same drug releases the enzyme-labelled drug from the antibody complex, thereby increasing the enzymatic activity. Enzyme activity correlates with the drug concentration in the specimen and is measured by absorbance changes resulting from the activity of the enzyme on a particular substrate.
2.1.1. Chemicals and reagents
The VRZ calibrator (Lot:20220701), quality control (Lot:20220801), and test kits for the EMIT assay (No.20220801) were purchased from Zhuhai Livzon Diagnostics Inc. (Guangdong, China).
2.1.2. Preparation of standard solution and quality control samples
The EMIT reagents were commercially supplied and verified by Zhuhai Livzon Diagnostics Inc. (Guangdong, China). Therefore, the preparation procedures were performed in accordance with the manufacturer's instructions. Briefly, a calibration curve was constructed using six working solutions at concentrations of 0, 0.6, 1.7, 4.1, 8.3, and 17.0 mg/L and determined via SIEMENS Viva-ProE® System. A four-point logarithmic curve was used to obtain the calibration curve. A three-level quality control sample with a theoretical mean concentration and range was used, and if the results fulfilled the range, the method was considered verified. The mean concentration ranged as follows: low-quality control sample: 1.3, 1.04–1.56 mg/L; medium-quality control sample: 5.6, 4.48–6.72 mg/L; high-quality control sample: 11.0, 8.80–13.20 mg/L.
2.1.3. Sample collection and preparation
A total of 122 whole blood samples with at least 2 mL volume of steady-state trough VRZ concentration were obtained from the Southwest Hospital of the Army Medical University from January 2023 to May 2023. Blood samples were centrifuged at 4000 rpm for 5 min within 2 h of collection. The resulting supernatant was analyzed using the SIEMENS Viva-ProE® System with EMIT technology. The residual supernatant samples were used for UPLC-MS/MS analysis.
2.2. Established and validated of UPLC-MS/MS analysis
2.2.1. Chemicals and reagents
A reference standard for VRZ (purity: 99 %; Lot No. S0801AS) was purchased from Dalian Meilun Biotechnology Co., Ltd. (Dalian, China). Voriconazole-d3 (VRZ-d3), an internal standard (IS, Purity: 99 %; Lot No. Y19S11w121334), was purchased from Shanghai Yuanye Biotechnology Co., Ltd. (Shanghai, China). High-performance liquid chromatography (HPLC)-grade methanol (MeOH) and acetonitrile (ACN) were obtained from Dikma Technologies, Inc. (Beijing, China). Formic acid (FA, HPLC grade) was purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). Ultrapure water (UPW) was generated using a Milli-Q water purification system (Millipore Corp., Bedford, MA, United States). Drug-free human plasma was obtained from a regional plasma bank (The Army Medical University, China).
2.2.2. Chromatographic parameters
The HPLC system consisted of a DGU-20A 5R degassing unit, SIL-30 AC autosampler, CTO-30A column oven, and two LC-30 AD liquid-phase pumps (Shimadzu Corporation, Japan). A BEH C18 column (2.1 × 50 mm, 1.7 μm, Water, United States) was used to enrich and separate VRZ and VRZ-d3. Gradient elution was performed using a mobile phase consisting of 0.1 % FA in UPW (phase A) and ACN (phase B) at a flow rate of 0.350 mL/min. A gradient program ran through as follows: 0–0.5 min, 10 % B; 0.5–0.6min, 10–90 % B; 0.6–2.2 min, 90 % B; 2.2–2.5 min, 90–10 % B; 2.5–4.0 min, 10 % B. The column and autosampler were kept at 40 °C and 4 °C, respectively. After each injection, the sample manager was subjected to a needle wash process with methanol/water (50:50, v/v).
2.2.3. Mass spectrometric parameters
A Sciex QTRAP 5500 mass spectrometer (AB Sciex Pte Ltd., Singapore) equipped with a Turbo V source was used for the sample characterisation. It was equipped with an electrospray ionisation (ESI) source. Quantification was performed in the positive ESI mode [ESI (+)], with multiple reaction monitoring (MRM) as the acquisition mode. The ion spray voltage and the source temperature (TEM) were set to 5500 V and 500 °C, respectively. The curtain gas (CUR), nebuliser gas (Gas1), and heater gas (Gas2) were set at 35, 40, and 40 pis, respectively. Each transition had a dwell time of 200 ms in the multiple reaction monitoring mode. The other important parameters are listed in Table 1. The UPLC-MS/MS system control and data analysis were performed using the Analyst MD software (Version 1.6.2, AB Sciex Pte. Ltd., Singapore).
Table 1.
Parameters for the precursor-to-product ion transitions DP, EP, CE, and CXP of voriconazole and voriconazole-d3.
| Analytes | Precursor Ion (m/z) | Product Ion (m/z) | DP (V) | EP (V) | CE (V) | CXP (V) |
|---|---|---|---|---|---|---|
| Voriconazole | 350.2 | 281.1 | 76.0 | 10.0 | 29.1 | 12.8 |
| Voriconazole-d3 | 353.2 | 284.0 | 89.2 | 11.2 | 23.7 | 13.0 |
CE, collision energy; CXP, collision cell exit potential; DP, declustering potential; EP, entrance potential.
2.2.4. Preparation of solutions, calibration standards and quality control samples
Stock solutions of VRZ and VRZ-d3 at concentrations of 1 mg/mL were obtained by precisely weighing appropriate amounts of VRZ and VRZ-d3, followed by their dissolution in MeOH. VRZ stock solutions were further diluted with MeOH:H2O (1:1, v/v) to prepare working solutions. All the stock solutions and working solutions were kept at a −70 °C refrigerator.
Calibration samples were obtained by adding the corresponding working solutions to blank human plasma. The standard curve samples (CSs) concentrations were 0.1, 0.2, 0.5, 1.0, 2.0, 5.0, and 10.0 mg/L. The concentrations of the plasma quality controls (QCs) were 0.1, 0.2, 1.0, and 8.0 mg/L for the lower limit of quantification (LLOQ), low QC (LQC), medium QC (MQC), high QC (HQC). The IS working solution of 20 ng/mL for plasma analysis was diluted with methanol from a 1 mg/mL stable isotopically SIL-IS stoke solution.
2.2.5. Sample selection and extraction procedure
The clinical samples were centrifuged, as determined by the EMIT method and collected for UPLC-MS/MS. All CSs, QCs, and clinical samples were extracted using a protein precipitation (PPT) method. For the plasma, the following procedure was used: 950 μL of MeOH containing IS (20 ng/mL of VRZ-d3) were added to 50 μL of plasma (CS, QC, blank matrix, and clinical samples) in a 1.5 mL centrifuge tube with the exception of the double blank plasma where methanol was added. The mixture was then vortexed for 2 min. The precipitate was removed by centrifugation at 13 000 rpm for 5 min at 4 °C. Then, 20 μL supernatant was added to 980 μL of UPW, shaken for 1 min and centrifuged at 13 000 rpm for 5 min at 4 °C. Finally, 2 μL of the supernatant solution was loaded onto the LC-MS/MS column for analysis.
2.2.6. Method validation
The present method was optimised and validated using cryopreserved plasma as a matrix, according to the Bioanalytical Method Validation Guidance of the U.S. Food and Drug Administration (FDA) in 2018 (U.S. Food and Drug Administration, 2018).
-
(1)
Selectivity
Selectivity was evaluated by comparing the instrument responses to LLOQs and blank human plasma samples obtained from six donors. If the analyte response in blank plasma was <20 % of the LLOQ and 5 % of the IS, then the selectivity of the method was considered acceptable.
-
(2)
Linearity and Lower Limit of Quantification
A calibration standard was prepared by adding a known concentration of the test substance to a blank matrix that covered the corresponding concentration range and formed a calibration curve. The ratio of the standard peak area to the IS peak area was plotted against the ratio of the standard concentration/IS to construct the calibration curves. Linearity was evaluated using calibration standards over three consecutive days, and a 1/x2 weighting factor was used for linear regression.
The LLOQ of the assay, defined as the lowest concentration on the standard curve at which the signal-to-noise ratio was much larger than 10, was quantified with an accuracy ranging from 80.0 to 120.0 % of the theoretical value and a precision of <20.0 %.
-
(3)
Accuracy and Precision
Intra-day accuracy and precision were assessed by measuring QC samples at four concentrations (LLOQ, LQC, MQC, and HQC) in six replicates. The inter-day accuracy and precision were established by repeating the intra-day validation procedure in three consecutive batches prepared on different days. The accuracy was calculated as the relative error (RE,%) and was acceptable when the RE was within ±20 % and ±15 % for theoretical values (LLOQ) and other QC levels, respectively. The precision was expressed as relative standard deviation (RSD, %). The precision was considered acceptable when the RSD was ±15 % (±20 % for the LLOQ).
-
(4)
Matrix Effect and Recovery
Matrix effects at both the LQC and HQC levels were determined using the IS normalized matrix factor (IMF) by dividing the peak area ratios of the analyte/IS with the plasma matrix from six different sources by those without the plasma matrix. Inter-subject variability expressed by the RSD should be ≤15 %.
The recoveries were determined by comparing the peak areas of the extracted QC samples with those of the analytes spiked into the blank sample extracts at the same concentration. The experiment was performed using three concentrations (LQC, MQC, and HQC) of VRZ, and each concentration was measured five times. IS recoveries were calculated using the same methodology.
-
(5)
Stability
To assess variable conditions during sample analysis, the stability was determined by analysing the LQC and HQC samples under different storage conditions. The storage conditions included short-term (room temperature (20–25 °C) for 26 h), auto-sampler (4 °C for 28 h), three freeze-thaw cycles (from −70 °C to room temperature), and long-term (−70 °C for 60 days). All analytes were considered stable when the calculated concentrations were within ±15 % of the theoretical values.
2.3. Statistical analysis
All data were statistically analyzed using GraphPad Prism Software (version 8.0.3, CA, USA) and IBM SPSS Statistics v22 (IBM, Armonk, NY). Results are presented as mean ± standard deviation (SD) or median (minimum to maximum). Linear regression analysis and Deming linear regression were used to evaluate the correlation between assays, Pearson correlation coefficient and Intraclass correlation coefficient (ICC) analysis were performed. To evaluate their concordance, the paired Wilcoxon test, Bland–Altman analysis of absolute bias and percentage differences were performed, Fisher's test was used to validate the relationship between UPLC-MS/MS and EMIT, whereas McNemar's test (with binomial distribution) was used to examine their discordance.
3. Results
3.1. The EMIT assay
A four-point logarithmic curve was used to obtain the formula for the calibration curve between 0 and 17.0 mg/L. And the formula was as follows: A = Ro+K*(1/(1 + exp[-(a+b*(lnC))])); where Ro = 2.70673 × 102,K = 1.95232 × 102,a = −8.1708,and b = 7.7770. The calculated concentrations of the three-level quality control samples based on the calibration curve were 1.20 mg/L, 5.45 mg/L, and 11.30 mg/L, respectively, all of which fulfilled the ranges and were considered verified.
3.2. The UPLC-MS/MS assay
3.2.1. The optimisation of experimental conditions for UPLC-MS/MS
Fig. 1 shows the product-ion spectra of VRZ and VRZ-d3. First, the scanning mode was optimised to achieve the highest sensitivity for VRZ and VRZ-d3. When detected in positive ion mode, the experiments revealed that these compounds had more significant and higher precursor ions at m/z 350.2 and m/z 353.2. Subsequently, the product ions were scanned by collision-induced dissociation (CID), and the main fragment ions were at m/z 281.1 and 127.0. The ion at m/z 281.1 exhibited a higher response than that at m/z 127.0. Consequently, 281.1 m/z was used as a product ion for the quantitative analysis of VRZ to improve the response of MS and the sensitivity of the analytical method. To improve sensitivity, an acid modifier (formic acid) was added to the mobile phase. Formic acid can provide H+, which greatly improves the VRZ and IS signals, thereby increasing the detection limit of VRZ.
Fig. 1.
Product ion spectra and proposed fragmentation patterns of voriconazole (A) and voriconazole-d3 (B).
In this study, analytes were extracted from human plasma using the MeOH-induced protein precipitation method, which is simple and efficient for high-throughput screening. Satisfactory analyte recoveries were obtained under these conditions. Although the PPT may introduce endogenous components that can cause matrix effects during LC-MS/MS analysis, this problem can be avoided by optimising the chromatographic conditions and selecting an SIL-IS. This analytical method used VRZ-d3 as an IS for the following reasons: VRZ-d3 has almost the same physical and chemical properties as VRZ, and its retention and ionisation behaviours are consistent, which avoids the matrix effect and increases the recovery rate and other conditions, leading to reliable detection results.
3.2.2. LC-MS/MS method validation
-
(1)
Selectivity
Fig. 2 illustrates the chromatograms of the blank plasma, LLOQ, and patient samples for each drug and IS. The retention times of VRZ and VRZ-d3 were 2.21 and 2.19 min, respectively. As shown in Fig. 2, no interference peaks were observed near the retention time of the analyte, indicating that no endogenous substances interfered with the quantitative analysis of VRZ and VRZ-d3.
-
(2)
Linearity and Lower Limit of Quantification
Fig. 2.
Typical MRM chromatograms of voriconazole [1] and voriconazole-d3 (IS, 2): (a) a double blank plasma (without analytes and IS); (b) LLOQ plasma sample spiked with 0.1 mg/L; (d) human plasma sample from patient administered VRZ. Abbreviations: MRM, multiple reaction monitoring; LLOQ, lower limit of quantification; VRZ, voriconazole.
Calibration curves for VRZ indicated excellent linearity over the concentration ranges of 0.1–10 mg/L. The representative linear regression equation for VRZ was Y = 0.00278X+0.532 (r = 0.9997). As shown in Table 2, the LLOQ was 2 mg/L, indicating good precision and accuracy.
-
(3)
Accuracy and Precision
Table 2.
Intra- and inter-day precision and accuracy for voriconazole.
| Intra-day (n = 6) |
Inter-day (n = 24) |
|||||
|---|---|---|---|---|---|---|
| Theoretical concentration (mg/L) | Determined concentration (mean ± SD ng/mL) | Precision (RSD,%) | Accuracy (RE,%) | Determined concentration (mean ± SD ng/mL) | Precision (RSD,%) | Accuracy (RE,%) |
| 0.10 | 0.099 ± 0.01 | 8.4 | −0.8 | 0.097 ± 0.01 | 11.8 | −3.2 |
| 0.20 | 0.20 ± 0.02 | 8.1 | −1.8 | 0.19 ± 0.02 | 7.9 | −2.8 |
| 1.00 | 1.00 ± 0.10 | 10.4 | 0.4 | 1.03 ± 0.08 | 7.4 | 2.5 |
| 8.00 | 7.48 ± 0.24 | 3.2 | −6.6 | 7.94 ± 0.55 | 6.9 | −0.7 |
RSD, relative standard deviations; SD, standard deviation.
Data relating to both inter- and intra-day accuracy and precision are shown in Table 2. The RE values for intra- and inter-day accuracies were between −6.6 % and 2.5 %. The RSD values for precision were ≤11.7 % for VRZ. Both the accuracy and precision were within the acceptance criteria. These results suggest that the proposed method exhibits satisfactory accuracy, precision, and reproducibility.
-
(4)
Matrix Effect and Recovery Rate Analyses
The data related to the matrix effect and recovery are presented in Table 3. In human plasma, the IMF were 100.6 % and 92.9 % for LQCS and HQCS. The RSDs of the of matrix effect were ≤5.1 % at both LQC and HQC levels. The data showed that the matrix effect was negligible. The recovery rates for LQC,MQC, and HQC were 100.5 %, 102.6 %, and 109.9 %. The RSDs of the recoveries of analyte at all QC levels were ≤6.8 %. These results suggest that the recovery rates of the substances were quite high and reproducible.
-
(5)
Stability
Table 3.
Recovery and matrix effect of voriconazole in human plasma.
| Analyte | Nominal concentration (mg/L) | Recovery (%) |
IS normalized matrix factor (%) |
||
|---|---|---|---|---|---|
| Mean | RSD | Mean | RSD | ||
| Voriconazole | 0.2 | 100.5 ± 2.8 | 2.7 | 100.6 ± 5.0 | 5.0 |
| 1.0 | 102.6 ± 7.0 | 6.8 | |||
| 8.0 | 109.9 ± 2.2 | 2.0 | 92.9 ± 4.7 | 5.1 | |
| Voriconazole-d3 | 0.02 | 111.6 ± 6.4 | 5.8 | ||
RSD, relative standard deviations; SD, standard deviation.
The stability was assessed under various conditions. The stability test results demonstrated that these analytes remained stable in plasma samples stored under the described conditions. The detailed data are listed in Table 4.
Table 4.
Stability of voriconazole in human plasma under different storage conditions.
| Storage conditions | Concentration (mg/L) |
RE (%) | RSD (%) | |
|---|---|---|---|---|
| Nominal | Measured | |||
| short temperature | 0.2 | 0.19 ± 0.02 | −4.8 | 9.9 |
| 8.0 | 8.00 ± 0.52 | 0.0 | 6.5 | |
| Auto-sampler | 0.2 | 0.19 ± 0.02 | −4.5 | 9.3 |
| 8.0 | 8.16 ± 0.61 | 1.9 | 7.4 | |
| Freeze-thaw | 0.2 | 0.20 ± 0.02 | 1.3 | 11.1 |
| 8.0 | 8.15 ± 0.50 | 1.9 | 6.2 | |
| long-term | 0.2 | 0.19 ± 0.01 | −6.7 | 6.7 |
| 8.0 | 8.14 ± 0.50 | 1.7 | 6.4 | |
RE, relative error; RSD, relative standard deviations; SD, standard deviation.
3.3. Correlation and concordance between UPLC–MS/MS and EMIT method
3.3.1. Total correlation and concordance analysis
A total of 122 plasma samples were determined by UPLC-MS/MS and EMIT methods. VRZ concentrations measured by UPLC-MS/MS ranged from 0.054 mg/L to 9.69 mg/L, with median concentration at 3.045 mg/L (1.45–4.71 mg/L), while VRZ concentrations measured by EMIT showed a larger range from 0.04 mg/L to 14.510 mg/L with median concentration at 4.065 mg/L (1.997–5.980 mg/L). The median concentrations of VRZ obtained using EMIT was 133.5 % of that obtained using UPLC-MS/MS.
To determine if there was any significant correlation between the two methods, two linear regression methods were employed: least squares linear regression and Deming linear regression. The y-axis in Fig. 3A displays the EMIT results, whereas the x-axis represents the UPLC-MS/MS results. The least squares linear regression curve is shown by the black solid line, with the 95 % CI area in pink, the regression equation is as follows: YEMIT = 1.28*UPLC-MS/MS +0.081. The Deming regression curve is represented by the red dotted line, the Deming linear regression equation is somewhat different, with a little higher slope as follows: YEMIT = 1.32*UPLC-MS/MS-0.052. The Pearson correlation coefficient (r = 0.976) and the Intraclass correlation coefficient analysis (r = 0.941) also showed a strong correlation.
Fig. 3.
The linear regression curve and Bland-Altman analysis for UPLC-MS/MS and EMIT. (A) The linear regression curve for UPLC-MS/MS and EMIT. The least squares linear regression curve is represented by the black solid line, with the 95 % CI area in pink. The Deming linear regression curve is represented by the red dotted line. (B) Bland-Altman analysis of the absolute bias between UPLC-MS/MS and EMIT and (C) percentage bias between UPLC-MS/MS and EMIT. Mean bias is shown as a blue dotted line, and ±95 % CI is represented as a red dotted line.
Regarding concordance, the paired Wilcoxon test analysis showed a significant difference between the two methods (p < 0.0001), indicating that the two methods were discordant. Furthermore, results from the Bland-Altman analysis also confirmed this discordance. According to the Bland-Altman analysis, the mean absolute bias between EMIT and UPLC-MS/MS was 1.033 mg/L (95 % CI: 0.77–42.840 mg/L) (Fig. 3B); the average bias in percentage between EMIT and UPLC-MS/MS was 27.56 % (95 % CI: 2.159–52.97 %) (Fig. 3C), indicating a mean positive bias for EMIT. Both the paired Wilcoxon test and Bland-Altman analysis revealed poor consistency between the two methods.
3.3.2. Correlation and concordance analysis in different concentration groups
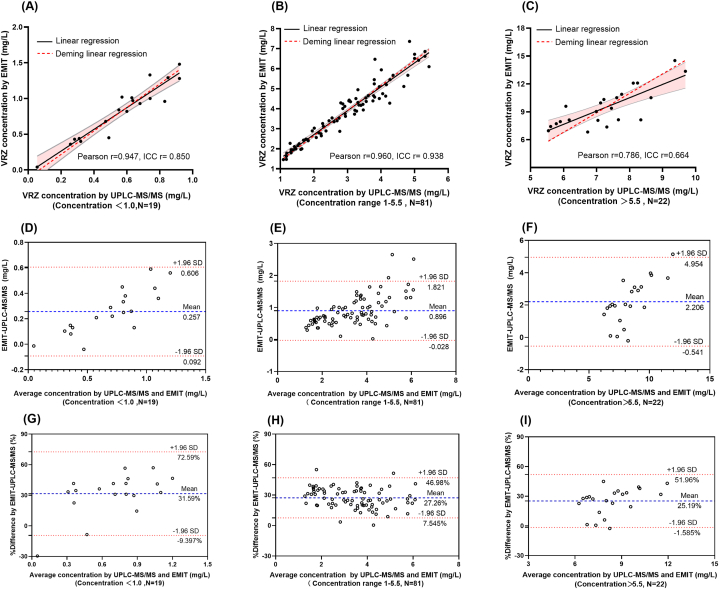
According to guidelines, the effective therapeutic range for VRZ is recommended to be between 1.0 and 5.5 mg/L. Therefore, adhering to these guidelines, the samples were classified into three groups: the sub-therapeutic group (concentrations below 1.0 mg/L), the therapeutic group (concentrations ranging from 1.0 to 5.5 mg/L), and the potential side-effect group (concentrations above 5.5 mg/L). Subsequently, the correlations and consistencies between these groups were evaluated. From a distributional standpoint, a predominant proportion of the samples (66.40 %, 81/122) manifested within the therapeutic group, whereas 15.57 % (19/122) of the samples were observed in the sub-therapeutic group, and 18.03 % (22/122) in the potential side effect group. The sub-therapeutic group exhibited moderate correlation (Pearson r = 0.897, ICC r = 0.850) (Fig. 4A); therapeutic group exhibited the strongest correlation and the narrowest 95 % CI (Pearson r = 0.960, ICC r = 0.938) (Fig. 4B), whereas the potential side-effect group exhibited the reverse (Pearson r = 0.786, ICC r = 0.664) (Fig. 4C).
Fig. 4.
The linear regression curve and Bland-Altman analysis for UPLC-MS/MS and EMIT in different concentration groups. (A) The linear regression curve for UPLC-MS/MS and EMIT in a sub-therapeutic, (B) therapeutic, and (C) potential side-effect groups. In A, B, and C, the least squares linear regression curve is represented by the black solid line, with the 95 % CI area in pink, and the Deming linear regression curve is represented by the red dotted line. (D) Bland-Altman analysis of the absolute bias between UPLC-MS/MS and EMIT in sub-therapeutic, (E) therapeutic, and (F) potential side-effect groups. (G) Bland-Altman analysis of the bias in percentage between UPLC-MS/MS and EMIT in sub-therapeutic, (H) therapeutic, and (I) potential side-effect groups. (D–I) The mean bias is shown as a blue dotted line, and ±95 % CI is represented as a red dotted line.
The Bland-Altman analysis pointed out that less than 5 % of samples were located outside the 95 % CI. Nevertheless, the mean absolute bias between EMIT and UPLC-MS/MS in sub-therapeutic group, therapeutic group and potential side-effect group were 0.257 mg/L (95 % CI: 0.092–0.606 mg/L) (Fig. 4D), 0.896 mg/L (95 % CI: 0.028–1.821 mg/L) (Fig. 4E) and 2.206 mg/L (95 % CI: 0.541–4.954 mg/L) (Fig. 4F), respectively; The average bias in percentage between EMIT and UPLC-MS/MS in those three groups were 31.59 % (95 % CI: 9.397–72.59 %) (Fig. 4G), 27.26 % (95 % CI: 7.545–46.98 %) (Fig. 4H) and 25.17 % (95 % CI: 1.585–51.96 %) (Fig. 4I), respectively, indicating concentration-dependent downward trend of bias in percentage. The paired Wilcoxon test revealed a statistically significant difference between the two methods across all three groups (p < 0.0001). Both the paired Wilcoxon test and Bland-Altman analysis revealed consistent results, with poor concordance between the two methods.
3.4. Comparative evaluation of EMIT method and UPLC-MS/MS method and implications for clinical therapy
Given that the EMIT method measured larger amounts than the UPLC-MS/MS method, we aimed to determine whether this bias affected the clinical therapy. To assess the treatment efficacy (1.0 mg/L) and safety (5.5 mg/L), two threshold values were established. Using UPLC-MS/MS as a reference method, a comparison was made between the original EMIT concentration and the adjusted EMIT concentration based on Deming linear regression. Fisher's test was used to evaluate the correlation between the two methods, and a significant difference indicated a significant correlation. On the other hand, the McNemar test was used to evaluate the discordance of the two methods, and a statistic significant suggests a lack of concordance between the two methods. Consequently, directly switching from UPLC-MS/MS to EMIT is not acceptable in clinical situations.
To address this issue, we adjusted the EMIT results using the Deming linear regression equation and assessed their correlation and concordance with UPLC-MS/MS. As shown in Table 5, the results demonstrate a significant correlation between the original EMIT concentration and the UPLC-MS/MS concentration (p < 0.0001), as well as between the adjusted EMIT concentration and UPLC-MS/MS concentration. However, a discordance was observed between the original EMIT concentration and the UPLC-MS/MS concentration at the efficacy (p = 0.016) and safety thresholds (p = 0.001). Nevertheless, the adjusted EMIT results exhibited no inconsistency with the UPLC-MS/MS results for either the efficacy threshold (p = 0.125) or safety threshold (p = 1.0). These findings imply that, although there is a discordance between the EMIT and UPLC-MS/MS results, the adjusted EMIT results can serve as a reliable surrogate when UPLC-MS/MS results cannot be obtained when necessary. However, a UPLC-MS/MS analysis is required for further verification.
Table 5.
Contingency tables used two alternative cut-off values to compare TDM-based indications by UPLC-MS/MS and EMIT or adjusted EMIT by Deming Linear regression.
| VRZ determined by EMIT with cut-off at 1 mg/L | Total | p-value | VRZ determined by EMIT and adjusted by DLR with threshold at 1 mg/L | Total | p value | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| VRZ determined by UPLC-MS/MS with a cut-off at 1 mg/L | <1 | ≥1 | <1 | ≥1 | ||||||
| <1 | 12 | 7 | 19 | p Fisher<0.0001 | <1 | 15 | 4 | 19 | p Fisher<0.0001 | |
| ≥1 | 0 | 103 | 103 | ≥1 | 0 | 103 | 103 | |||
| Total | 12 | 110 | 122 | p McNemar = 0.016a | 15 | 107 | 122 | p McNemar = 0.125 | ||
| VRZ determined by EMIT with cut-off at 5.5 mg/L | VRZ determined by EMIT and adjusted by DLR with threshold at 5.5 mg/L | |||||||||
| VRZ determined by UPLC-MS/MS with a cut-off at 5.5 mg/L | ≤5.5 | >5.5 | ≤5.5 | >5.5 | ||||||
| ≤5.5 | 89 | 11 | 100 | p Fisher<0.0001 | ≤5.5 | 99 | 1 | 100 | p Fisher<0.0001 | |
| >5.5 | 0 | 22 | 22 | >5.5 | 1 | 21 | 22 | |||
| Total | 89 | 33 | 122 | p McNemar=0.001a | 100 | 22 | 122 | p McNemar = 1.0 | ||
DLR, dynamic linear regression; EMIT, enzyme multiplied immunoassay technique; UPLC-MS/MS, ultra-performance liquid chromatography-tandem mass spectrometry; VRZ, voriconazole.
p Fisher significance implies a significant correlation. In contrast, p McNemar's test indicates a significant discordance.
4. Discussion
Implementing VRZ therapeutic drug monitoring is essential to ensure optimal therapeutic outcomes and minimise the risk of adverse effects associated with drug therapy [17,18]. In this study, we developed and validated a novel isotopically labelled internal standard UPLC-MS/MS quantitative method to determine VRZ plasma concentration, which can improve accuracy, reduce variability, and adjust sample preparation losses. However, the entire procedure is time-consuming, usually taking approximately 3 h to obtain the first drug concentration result. However, the subsequent analyses are significantly faster, with a turnaround time of approximately 2–3 min/sample [19]. However, owing to the irregular arrival of clinical specimens, UPLC-MS/MS is not suitable for routine clinical monitoring. Notably, UPLC-MS/MS instruments have both high sensitivity and considerable cost and demonstrate exceptional accuracy in their analytical capabilities. However, if a malfunction happens, the troubleshooting process may extend for 3–5 days, posing uncertainty for daily routine analysis. Consequently, an alternative determination method that is simple, rapid, and user-friendly is required. The EMIT method is a commercially available analytical technique that is widely used for routine therapeutic drug monitoring of immunosuppressants [19,20] and anti-seizure drugs [21]. The EMIT approach is simple to execute and requires only ordinary laboratory equipment and no sophisticated equipment. Only 30 min were required before the first drug concentration was obtained, making EMIT suitable for high-throughput analysis.
Previous studies have compared HPLC with EMIT methods for the quantitative analysis of VRZ. However, controversial results have emerged. One study demonstrated a strong correlation and consistency between HPLC and EMIT [22], whereas two other studies reported contradictory findings indicating a lack of concordance [16,23]. Other bioassay or immunoassay methods also showed controversial results compared to HPLC [15,24]. HPLC-UV is susceptible to interference from co-eluting compounds with similar wavelengths or matrix components that exhibit similar absorbance characteristics. The specificity of HPLC-UV is inferior to that of UPLC-MS/MS.
To the best of our knowledge, this is the first study to report the correlation and concordance between EMIT and UPLC-MS/MS methods using an isotope internal standard. Despite the assertion of “no cross-reactivity” in the EMIT assay, a comparison between the average concentrations obtained from EMIT and UPLC-MS/MS revealed a notable overestimation of 33.5 % by the EMIT method, consistent with previous studies which compared it with the HPLC method [16,23]. However, the bias in this study is more modest. Notably, the EMIT method consistently yielded higher values and greater variability than the UPLC-MS/MS method. One plausible explanation for this bias is the misidentification of metabolites such as voriconazole-N-oxide as VRZ [16]. Another contributing factor could be the influence of pathophysiological conditions. Many factors, such as age, race, genetics, and drug interactions, may influence the VRZ concentration [25]. In this study, we evaluated the differences between adults and the elderly. Although the elderly displayed more variability than adults, no statistically significant difference was identified (data not shown). Moreover, the EMIT assay instruction manual states that variables such as albumin and triglycerides do not affect the results when they are within a specified range. However, the possible influence of these indicators on the other methods remains uncertain.
This type of bias theoretically affects dose adjustments guided by TDM results. When switching from the UPLC-MS/MS to the EMIT method, the percentage of sub-therapeutic group samples decreased from 15.57 % to 9.84 %. In contrast, the safety group samples increased from 18.03 % to 27.05 %, indicating exaggerated treatment effects and overestimating the risk of side effects. Significant discordance was eliminated when we adjusted the EMIT concentration based on the Deming linear regression equation. These findings indicate that the results from EMIT and UPLC-MS/MS cannot be interchanged; however, if alternation is required, the adjusted EMIT concentration should be applied. Furthermore, when monitoring shifts, physicians should be informed, and an optimal option for dosage optimisation should be made to prevent drug-related adverse effects.
5. Conclusion
In summary, we established a more specific and accurate isotype internal standard UPLC-MS/MS method to determine VRZ in plasma. A strong correlation but discordance was found between UPLC-MS/MS and EMIT in determining VRZ concentrations. The higher values obtained from the EMIT may be due to cross-reactions between voriconazole-N-oxide and interference from other drugs. Therefore, it is advisable to use UPLC-MS/MS to monitor VRZ levels and avoid fluctuations. Direct switching from UPLC-MS/MS to EMIT is unacceptable in clinical situations. However, if this assay is not available, the adjusted EMIT results may be a surrogate when necessary.
Author contributions
Mingjie Yu: Conceived and designed the experiments; Performed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Jun Yang: Performed the experiments; Conceived and designed the experiments; contributed reagents, materials, analysis tools or data; analyzed and interpreted the data.
Lirong Xiong: Performed the experiments; Contributed reagents, materials, analysis tools or data.
Shipeng Zhan: Analyzed and interpreted the data; Lin Cheng: Analyzed and interpreted the data; Yongchuan Chen: Conceived and designed the experiments; Wrote the paper.
Fang Liu: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Ethical approval
This study was approved by the Ethics Committee of the Southwest Hospital of Army Medical University (No. KY2023084).
Funding
The authors gratefully acknowledge financial support from the Medical Research Project of Science and Health of Chongqing (2019ZDXM052), Chongqing Clinical Pharmacy Key Specialties Construction Project (425Z2M2), Social Science Program of Army Medical University (2019XRW10), and Talent Program of Army Medical University (XZ-2019-505-073).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Yongchuan Chen, Email: zwmcyc@163.com.
Fang Liu, Email: liufang0209@163.com.
References
- 1.Patterson T.F., Thompson G.R., 3rd, Denning D.W., Fishman J.A., Hadley S., Herbrecht R., et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the infectious diseases society of America. Clin. Infect. Dis. 2016;63(4):e1–e60. doi: 10.1093/cid/ciw326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ullmann A.J., Aguado J.M., Arikan-Akdagli S., Denning D.W., Groll A.H., Lagrou K., et al. Diagnosis and management of Aspergillus diseases: executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin. Microbiol. Infect. 2018;24(Suppl 1):e1–e38. doi: 10.1016/j.cmi.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Denning D.W., Cadranel J., Beigelman-Aubry C., Ader F., Chakrabarti A., Blot S., et al. Chronic pulmonary aspergillosis: rationale and clinical guidelines for diagnosis and management. Eur. Respir. J. 2016;47(1):45–68. doi: 10.1183/13993003.00583-2015. [DOI] [PubMed] [Google Scholar]
- 4.Tang D., Yan M., Song B.L., Zhao Y.C., Xiao Y.W., Wang F., et al. Population pharmacokinetics, safety and dosing optimization of voriconazole in patients with liver dysfunction: a prospective observational study. Br. J. Clin. Pharmacol. 2021;87(4):1890–1902. doi: 10.1111/bcp.14578. [DOI] [PubMed] [Google Scholar]
- 5.Jin H., Wang T., Falcione B.A., Olsen K.M., Chen K., Tang H., et al. Trough concentration of voriconazole and its relationship with efficacy and safety: a systematic review and meta-analysis. J. Antimicrob. Chemother. 2016;71(7):1772–1785. doi: 10.1093/jac/dkw045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pascual A., Calandra T., Bolay S., Buclin T., Bille J., Marchetti O. Voriconazole therapeutic drug monitoring in patients with invasive mycoses improves efficacy and safety outcomes. Clin. Infect. Dis. 2008;46(2):201–211. doi: 10.1086/524669. [DOI] [PubMed] [Google Scholar]
- 7.Maertens J.A., Rahav G., Lee D.G., Ponce-de-Leon A., Ramirez Sanchez I.C., Klimko N., et al. Posaconazole versus voriconazole for primary treatment of invasive aspergillosis: a phase 3, randomised, controlled, non-inferiority trial. Lancet. 2021;397(10273):499–509. doi: 10.1016/S0140-6736(21)00219-1. [DOI] [PubMed] [Google Scholar]
- 8.Ashbee H.R., Barnes R.A., Johnson E.M., Richardson M.D., Gorton R., Hope W.W. Therapeutic drug monitoring (TDM) of antifungal agents: guidelines from the British Society for Medical Mycology. J. Antimicrob. Chemother. 2014;69(5):1162–1176. doi: 10.1093/jac/dkt508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laverdiere M., Bow E.J., Rotstein C., Autmizguine J., Broady R., Garber G., et al. Therapeutic drug monitoring for triazoles: a needs assessment review and recommendations from a Canadian perspective. Can. J. Infect Dis. Med. Microbiol. 2014;25(6):327–343. doi: 10.1155/2014/340586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamada Y., Tokimatsu I., Mikamo H., Kimura M., Seki M., Takakura S., et al. Practice guidelines for therapeutic drug monitoring of voriconazole: a consensus review of the Japanese Society of Chemotherapy and the Japanese Society of Therapeutic Drug Monitoring. J. Infect. Chemother. 2013;19(3):381–392. doi: 10.1007/s10156-013-0607-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chau M.M., Kong D.C., van Hal S.J., Urbancic K., Trubiano J.A., Cassumbhoy M., et al. Consensus guidelines for optimising antifungal drug delivery and monitoring to avoid toxicity and improve outcomes in patients with haematological malignancy. Intern. Med. J. 2014;44(12b):1364–1388. doi: 10.1111/imj.12600. 2014. [DOI] [PubMed] [Google Scholar]
- 12.Chen K., Zhang X., Ke X., Du G., Yang K., Zhai S. Individualized medication of voriconazole: a practice guideline of the division of therapeutic drug monitoring, Chinese pharmacological society. Ther. Drug Monit. 2018;40(6):663–674. doi: 10.1097/FTD.0000000000000561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chawla P.K., Dherai A.J., Ashavaid T.F. Plasma voriconazole estimation by HPLC. Indian J. Clin. Biochem. 2016;31(2):209–214. doi: 10.1007/s12291-015-0507-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wen Z.P., Zhang H., Li Q., Hu C.J., Yang C.L., Fan X.H., et al. UPLC-MS/MS method for the determination of voriconazole plasma concentration from pediatric patients with hematologic tumors: an application toward personalized therapy. Biomed. Chromatogr. 2022;36(6) doi: 10.1002/bmc.5356. [DOI] [PubMed] [Google Scholar]
- 15.Pena-Lorenzo D., Rebollo N., Sanchez-Hernandez J.G., Zarzuelo-Castaneda A. Comparison of ultra-performance liquid chromatography and ARK immunoassay for therapeutic drug monitoring of voriconazole. Ann. Clin. Biochem. 2021;58(6):657–660. doi: 10.1177/00045632211041887. [DOI] [PubMed] [Google Scholar]
- 16.Li X., Li W., Li M., Zhang Z., Liu S., Chen Z. Correlation between enzyme multiplied immunoassay technique and high-performance liquid chromatography in the quantification of voriconazole in a paediatric population. Scand. J. Clin. Lab. Invest. 2021;81(2):121–126. doi: 10.1080/00365513.2020.1868048. [DOI] [PubMed] [Google Scholar]
- 17.Luong M.L., Al-Dabbagh M., Groll A.H., Racil Z., Nannya Y., Mitsani D., et al. Utility of voriconazole therapeutic drug monitoring: a meta-analysis. J. Antimicrob. Chemother. 2016;71(7):1786–1799. doi: 10.1093/jac/dkw099. [DOI] [PubMed] [Google Scholar]
- 18.Resztak M., Sobiak J., Czyrski A. Recent advances in therapeutic drug monitoring of voriconazole, mycophenolic acid, and vancomycin: a literature review of pediatric studies. Pharmaceutics. 2021;13(12) doi: 10.3390/pharmaceutics13121991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou H., Xiang H., Cai J., Wang Y., Zhang M., Han Y., et al. Comparison of a point-of-care testing with enzyme-multiplied immunoassay technique and liquid chromatography combined with tandem mass spectrometry methods for therapeutic drug monitoring of mycophenolic acid: a preliminary study. Ther. Drug Monit. 2021;43(5):630–636. doi: 10.1097/FTD.0000000000000861. [DOI] [PubMed] [Google Scholar]
- 20.Kaneko T., Fujioka T., Suzuki Y., Nagano T., Sato Y., Asakura S., et al. Comparison of whole-blood tacrolimus concentrations measured by different immunoassay systems. J. Clin. Lab. Anal. 2018;32(9) doi: 10.1002/jcla.22587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xia Y., Long J.Y., Shen M.Y., Dong N., Guo H.L., Hu Y.H., et al. Switching between LC-ESI-MS/MS and EMIT methods for routine TDM of valproic acid in pediatric patients with epilepsy: what clinicians and researchers need to know. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.750744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ding J., Lin X., Wen Y., Jiang G., Lyu P., Ouyang H. Comparative study between high performance liquid chromatography and enmyme-amplified immunoassay method for monitoring voriconazole concentration in human plasma. Chinese Journal of Clinical Pharmacy. 2020;29(4):266–271. [Google Scholar]
- 23.LI. J. Zhuo S., Chen S., Liu C., Xie R. Correlation analysis of voriconazole plasma concentrations detected by HPLC and EMIT. China Pharmaceuticals. 2022;31(22):56–59. [Google Scholar]
- 24.Steinmann J., Huelsewede J., Buer J., Rath P.M. Comparison and evaluation of a novel bioassay and high-performance liquid chromatography for the clinical measurement of serum voriconazole concentrations. Mycoses. 2011;54(5):e421–e428. doi: 10.1111/j.1439-0507.2010.01938.x. [DOI] [PubMed] [Google Scholar]
- 25.Cheng L., Xiang R., Liu F., Li Y., Chen H., Yao P., et al. Therapeutic drug monitoring and safety of voriconazole in elderly patients. Int. Immunopharm. 2020;78 doi: 10.1016/j.intimp.2019.106078. [DOI] [PubMed] [Google Scholar]