Abstract
Various diseases (e.g., hypertension and diabetes) are risk factors for the exacerbation of coronavirus 2019 (COVID-19). Patients with chronic obstructive pulmonary disease (COPD) and interstitial lung disease (ILD) tend to develop severe COVID-19. Patients with severe COVID-19 present with acute respiratory distress syndrome (ARDS), and many COVID-19-related ARDS survivors eventually develop fibrosis. However, the appropriate management of patients with COVID-19 and ILD and post-COVID-19 ILD remains unclear. Thus, a better understanding of the pathology that exacerbates COVID-19 in patients with ILD is needed. We report the autopsy results of a patient with COVID-19 and combined pulmonary fibrosis and emphysema, whose lung organization and fibrosis progressed after the acute phase of infection. Histopathological findings suggest that fatal pulmonary fibrosis persists after the negative conversion of SARS-CoV-2. Elucidating the cause of death by autopsy may help determine therapeutic strategies in patients with COVID-19 and ILD. Vaccination and early administration of anti-inflammatory drugs or antifibrotic agents may be crucial for preventing disease progression and fatal lung fibrosis. This report aims to clarify the histopathological features of COVID-19 in patients with ILD via autopsy and discuss treatment strategies.
Keywords: COVID-19, SARS-CoV-2, Autopsy, Interstitial lung disease, Acute respiratory distress syndrome
Highlights
-
•
We report an autopsy case of COVID-19 with COPD and ILD.
-
•
COPD and ILD are potential risk factors for severe COVID-19.
-
•
Many survivors of COVID-19-related ARDS eventually develop fibrosis.
-
•
Proper management of post-COVID-19 ILD has not yet been established.
-
•
Establishing appropriate treatments for severe COVID-19 with fibrosis is needed.
1. Introduction
Since the first reported case of viral pneumonia caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in Wuhan, China, coronavirus disease 2019 (COVID-19) has spread worldwide [1]. Various diseases, such as hypertension and diabetes, are risk factors for the exacerbation of COVID-19. Moreover, patients with chronic obstructive pulmonary disease (COPD) and interstitial lung disease (ILD) tend to develop severe COVID-19 [2,3]. The main histological findings in severe COVID-19 include alveolar exudates, acute respiratory distress syndrome (ARDS) with hyaline membrane, and acute organizing pneumonia [4]. Many survivors of acute COVID-19-related ARDS, especially those with a disease duration of >3 weeks, eventually develop fibrosis [5]. A cohort study revealed that ILD, predominantly organizing pneumonia, develops in approximately 5 % of patients after SARS-CoV-2 infection [6]. However, the appropriate management of patients with COVID-19 and ILD remains unclear. Performing autopsies may improve our understanding of the pathogenesis of COVID-19 and underlying diseases such as ILD and help establish therapeutic strategies for patients with these comorbidities [7]. We report the autopsy results of a patient with COVID-19 and pulmonary fibrosis and emphysema (CPFE), in whom respiratory failure persisted despite treatment with remdesivir, steroids, and anticoagulants. Vaccines and immunosuppressants, such as tocilizumab and baricitinib, were unavailable in Japan when this patient was infected with SARS-CoV-2. The autopsy revealed the organizing stage of diffuse alveolar damage (DAD) but negative SARS-CoV-2 staining, suggesting that SARS-CoV-2 infection may promote worsening lung fibrosis despite diminished SARS-Cov2 infection. This report aims to clarify the histopathological features of COVID-19 in patients with ILD based on the autopsy results and discuss treatment strategies.
2. Case presentation
In November 2020, a 67-year-old man was admitted to a local hospital with a fever and sore throat for 3 days. The patient had hypertension that was treated with an angiotensin II receptor blocker. The patient had no history of lung disease but had a history of heavy smoking (66 pack years). He had no history of travel or contact with an individual with COVID-19. Because no SARS-CoV-2 vaccine was available in Japan at that time, the patient was not vaccinated. At the local hospital, chest computed tomography (CT) revealed emphysematous changes and ground glass opacity in both lower lung lobes (Fig. 1A and B). The SARS-CoV-2 antigen test was positive. The patient was transferred to another hospital on the same day because he needed 2 L/min oxygen supplementation. The SARS-CoV-2 reverse transcription polymerase chain reaction (RT-PCR; Beckman Coulter, Inc.) was positive with a cycle threshold value of 28.6. Despite the administration of methylprednisolone (mPSL) 500 mg/day and ceftriaxone 2 g/day, his breathing condition deteriorated, and he was referred to our hospital on Day 6.
Fig. 1.
Antemortem chest radiograph and CT scan findings. (A, B) CT scan on Day 4 reveals emphysema in both the upper and middle lobes and ground glass opacities and fibrosis mainly in the bilateral lower lobes. (C) Chest radiograph on Day 6 shows hyperlucency in the right upper lung field and ground glass opacities in both lower lung fields. CT, computed tomography.
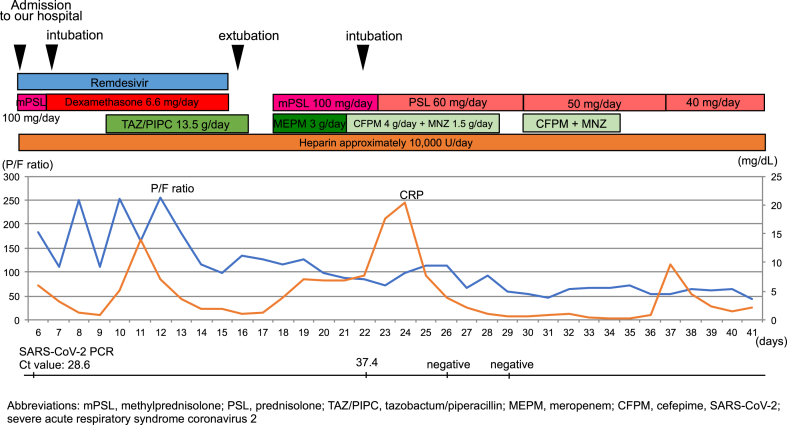
Fig. 2 summarizes the patient's clinical course after arrival at our hospital. On arrival, an examination revealed the following: temperature, 36.8 °C; blood pressure, 151/91 mmHg; pulse rate, 95/min; respiratory rate 28/min; and oxygen saturation, 95 % with 4 L/min oxygen supplementation. Chest auscultation revealed fine crackles in the left lower lung fields. A chest radiograph showed hyperlucency in the right upper field and ground glass opacities in both lower lung fields (Fig. 1C). His blood test revealed leukopenia, lymphopenia, and elevated levels of transaminases, ferritin, Krebs von den Lungen-6, C-reactive protein, and D-dimer. The arterial blood gas showed respiratory alkalosis (Table 1).
Fig. 2.
Clinical course of the patient.
Table 1.
Laboratory results obtained upon admission to our hospital.
| Hematology | Biochemistry | Arterial Blood Gas | |||
|---|---|---|---|---|---|
| WBC | 2800/μL | TP | 6.16 g/dL | (O2 4L/min, RR 28/min) | |
| Neut | 78.5 % | Alb | 3.02 g/dL | pH | 7.47 |
| Lym | 16.2 % | BUN | 16.1 mg/dL | pO2 | 73.3 Torr |
| Mo | 5.3 % | Cr | 0.74 mg/dL | pCO2 | 31.1 Torr |
| Eo | 0 % | T-bil | 0.8 mg/dL | HCO3− | 22.4 mmol/L |
| Baso | 0 % | AST | 40 U/L | Lac | 2.5 mmol/L |
| ALT | 40 U/L | ||||
| RBC | 486 × 106/μL | LDH | 355 U/L | ||
| Hb | 16.1 g/dL | ALP | 148 U/L | ||
| Ht | 44.3 % | Na | 135 mg/dL | ||
| Plt | 154 × 103/μL | K | 4.0 mg/dL | ||
| Cl | 101 mg/dL | ||||
| Coagulation | Serology | ||||
| PT | 12.0 sec | CRP | 5.90 mg/dL | ||
| APTT | 27.2 sec | Ferritin | 803.7 ng/mL | ||
| D-dimer | 1.19 μg/mL | KL-6 | 553 U/mL | ||
We administered remdesivir and dexamethasone for COVID-19 and heparin to prevent thromboembolism, and the patient was intubated the next day. On Day 11, the patient developed a fever, and the lung opacities were dense on the chest radiograph. After administering tazobactam/piperacillin for suspected ventilator-associated pneumonia (VAP), his condition improved. His sputum culture grew only indigenous oral bacteria. Remdesivir and dexamethasone were discontinued on Day 16, and he was extubated the next day. However, his breathing condition and the chest radiography findings worsened 2 days after extubation. Meropenem and mPSL were not effective, and he was reintubated on Day 22. SARS-CoV-2 RT-PCR (Rosche Diagnostics, Inc.) results on days 26 and 29 were negative, and no causative bacteria for VAP were detected in the sputum culture. We suspected organized pneumonia after COVID-19, but the response to 1 mg/kg/day of prednisolone was poor. The patient died of respiratory failure on Day 42 of the illness.
Autopsy imaging 2 hours after death showed worsened emphysema in the right upper lobe compared with the image at the time of disease onset and dense infiltration and traction bronchiectasis in the bilateral lower lobes (Fig. 3A and B). Dilatation of the right ventricle and hepatomegaly were also observed. The autopsy was conducted 4 hours after death (Fig. 4A–E). Marked emphysematous changes were observed in both lungs (left, 640 g; right, 670 g), mainly in the upper lobes. The cut surface was rough and hard, and organization with whitish spots was observed. Other gross findings included right cardiac dilatation, hepatomegaly, and splenomegaly. Table 2, Table 3 summarize the autopsy findings.
Fig. 3.
A chest computed tomography scan 2 hours after death shows (A) worsened emphysema in the right upper lobe and (B) dense infiltration and traction bronchiectasis in the bilateral lower lobes.
Fig. 4.
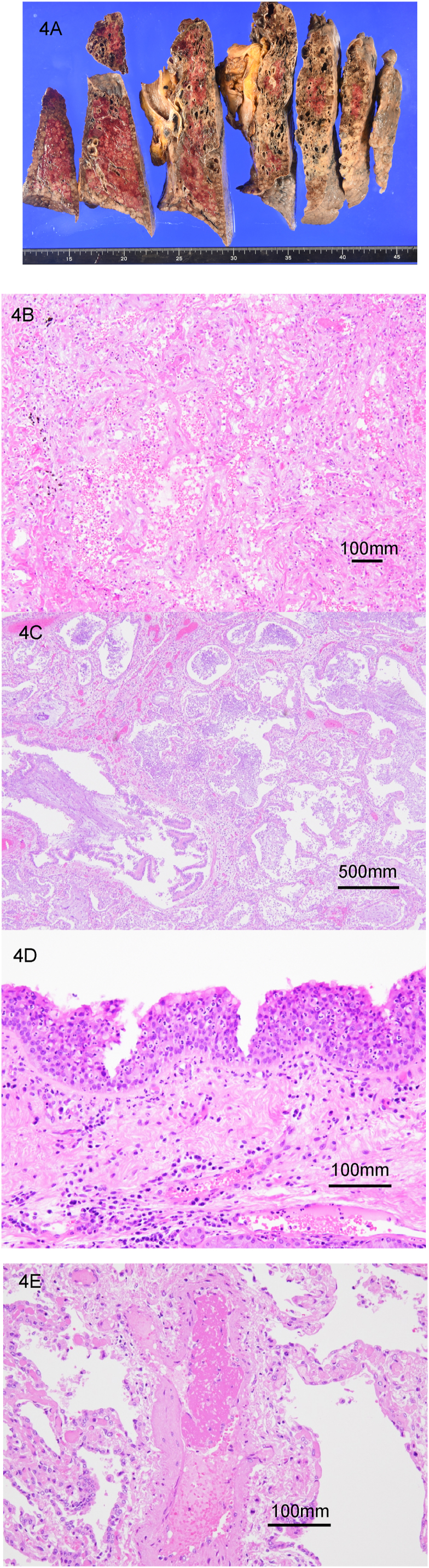
Pathologic findings of the lungs and trachea on autopsy in a Japanese man who died from COVID-19. (A) Gross findings show congested lungs and marked emphysematous changes mainly in the upper lung lobes. Microscopic lung findings show (B) organization in the left upper lobe, (C) accumulation of neutrophils in an organized air cavity in the right lower lobe, (D) neutrophilic infiltration in the tracheal mucosa, and (E) thrombus in a pulmonary vessel. (Hematoxylin and eosin stain; B, D, and E × 200; C × 40).
Table 2.
Macroscopic and microscopic findings in each organ.
| Pathological anatomical findings | |
|---|---|
| Major lesions | 1) SARS-CoV-2 infection (DAD, bronchopneumonia) |
| 2) CPFE | |
| 3) Prostate cancer (right lobe, latent cancer) | |
| Minor lesions | 1) Left ventricular hypertrophy |
| 2) Liver congestion | |
| 3) Splenomegaly | |
| 4) Bilateral nephrosclerosis | |
| 5) Atherosclerosis | |
| 6) Right jugular vein thrombosis | |
| 7) Colorectal adenoma (transverse colon, sigmoid colon) | |
| 8) Colon diverticulum | |
| 9) Postoperative right knee arthroplasty | |
Abbreviations: SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
DAD, diffuse alveolar damage; CPFE, combined pulmonary fibrosis and emphysema.
Table 3.
Summary of the pathological anatomical findings of our case.
| Organs | Weight | Gross findings | Microscopic findings | SARS-CoV-2 antigen |
|---|---|---|---|---|
| Lung | 640 g (left) | Left: Emphysema in the left upper lobe, interstitial change in the left lower lobe | DAD (organizing stage) | negative |
| 670 g (right) | Right: Bulla in the upper lobe | DAD (organizing stage), inflammatory cells in alveoli, neutrophil infiltration in the trachea | negative | |
| Kidney | 195 g (left), 200 g (right) | No remarkable change | Glomerular sclerosis, lymphocyte infiltration in the interstitium | ND |
| Heart | 395 g | Right cardiac dilatation | Concentric left ventricular hypertrophy | ND |
| Liver | 1455 g | Hepatomegaly | Acute liver congestion | ND |
| Spleen | 135 g | Splenomegaly | No remarkable change | ND |
| Adrenal glands | 8.4 g (left), 11.9g (right) | Adrenal atrophy | No remarkable change | ND |
Abbreviations: DAD, diffuse alveolar damage; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; ND, no data.
Microscopically, an enlarged air cavity with fibrosis, which was assumed to be CPFE, was observed. Hyaline membranes were detected in focal areas, and fibrosis was present in the alveolar spaces. These findings suggest an exudative and organizing phase of ARDS. Neutrophil accumulation was noted in the bronchial and tracheal mucosa. No bacterial or fungal infection was confirmed histologically. Thromboembolism was observed in only one small pulmonary artery and was unlikely to have caused the fatal deterioration of respiratory distress. The other histological findings included cardiac hypertrophy, mural thrombus in the right carotid vein that was probably secondary to central venous catheter insertion, and adenoma in the transverse and sigmoid colon. Prostate cancer was incidentally found but was probably not connected to his death. Infected cells were not detected by SARS-CoV-2 immunostaining (Gene Tex, Inc.) in the lungs, likely because several weeks had passed after a negative SARS-CoV-2 RT-PCR.
3. Discussion
The typical autopsy findings of COVID-19 include exudates in the alveoli, DAD, and acute organizing pneumonia [4]. In two COVID-19 autopsy case studies, DAD and lung congestion were observed in all cases [8,9]. An analysis of 407 COVID-19 autopsy cases [10] demonstrated that vascular changes associated with proliferative and exudative DAD with hyaline membrane deposition is a distinctive feature of SARS-CoV-2 infection. The main lung autopsy finding in our case was also DAD. The mortality rate of patients with COVID-19 who develop ARDS is high (range: 52.5%–93.0 %) [11]. Moreover, patients with COVID-19 and ILD are at a higher risk of developing severe disease or mortality due to decreased respiratory function [2]. SARS-CoV-2 infection can lead to acute exacerbation of ILD [12], with histopathological findings similar to or indistinguishable from the histopathology of ARDS [13]. However, the specific autopsy findings of patients with COVID-19 and ILD are unknown. Similarly, the specific autopsy findings of COVID-19 with ILD were not obvious in our case. Patients with COPD and COVID-19 have relatively severe symptoms and poor prognoses [14]. Although our patient was not diagnosed with ILD or emphysema before admission to our hospital, CPFE was presumed based on a history of heavy smoking, CT images, and autopsy findings. CPFE may have exacerbated his respiratory dysfunction. Regardless of the presence of ILD, organization and fibrotic changes in the lungs may persist after recovery from severe COVID-19 [6].
SARS-CoV-2 infection may promote self-perpetuating fibrosis in patients with ILD, resulting in a worse prognosis. In our case, SARS-CoV-2 immunostaining in the lungs was negative and a long period passed from the SARS-CoV-2 infection to death. Negative immunostaining in the lungs suggests that the pulmonary fibrosis is self-perpetuating, wherein viral replication is controlled by antiviral agents. Thus, fatal lung fibrosis may proceed even after the patient with ILD is negative for SARS-CoV-2.
Although genetic analysis of the virus was not performed in our case, the main genotype of SARS-CoV-2 prevalent in Japan at that time was B.1.1.214 [15]. Thus, the virus was likely of the same genotype in our case. Disease severity may differ by genotype [16], and molecular characterization should be encouraged to accumulate the necessary information.
The National Institutes of Health recommended dexamethasone and remdesivir for patients with COVID-19 who need oxygen supplementation and suggested a prophylactic dose of anticoagulation for hospitalized patients at the time our patient was hospitalized [17]. Accordingly, we administered these medications and an antimicrobial drug for bacterial pneumonia. However, the respiratory failure progressed even after a negative SARS-CoV-2 RT-PCR. The autopsy revealed the organizing phase of DAD, suggesting that a long period had passed since the SARS-CoV-2 infection. Neither fatal pulmonary embolism nor bacterial/fungal superinfection (a major complication of COVID-19) was found during the autopsy. Our patient needed a very high fraction of inspiratory oxygen (FiO2), even after a negative SARS-CoV-2 PCR result. Prolonged breathing of high FiO2 (0.9 ≤ FiO2) causes hyperoxic lung injury (HALI) [18]. Histopathological findings of HALI include exudative pulmonary edema and fibroproliferation of the lung and hyaline membranes, which also occur in ARDS. Our autopsy findings suggest that both SARS-CoV-2 infection and HALI caused irreversible fatal lung injury.
The effectiveness of anti-inflammatory drugs, such as baricitinib [19] or tocilizumab [20], was reported after our case. Antibody cocktail treatment for mild cases may prevent disease progression [21]. The condition of our patient had already progressed to a severe state when the antiviral drug was administered. Thromboembolism and bacterial coinfection were not detected due to the administration of anticoagulants and antibacterial agents. Acute exacerbations of ILD or organizing pneumonia after COVID-19 were suspected and additional corticosteroids were administered, but the response was poor. Appropriate management for severe cases with widespread pulmonary fibrosis and organizing pneumonia that persists in the subacute and chronic phases has not been established. Uemasu et al. demonstrated a good response to high-dose corticosteroid therapy, including steroid pulse, in three cases of post-COVID-19 ILD [22]. Myall KJ et al. reported that early treatment of post-COVID-19 ILD with corticosteroids was well tolerated and associated with rapid and significant improvement [6]. Another study indicated that the administration of tocilizumab improved the lung parenchyma interstitial lesions affected by COVID-19 [23]. Umemura et al. conducted an interventional study in patients with COVID-19 requiring mechanical ventilation and reported that the length of mechanical ventilation was significantly shorter in the nintedanib group compared with historical controls [24]. However, data on prolonged corticosteroid therapy, anti-inflammatory therapy, and antifibrotic agents in patients with post-COVID-19 ILD is scarce, and the safety and efficacy of these agents remain controversial. Lung transplantation can be a life-saving therapeutic option for some patients with COVID-19-related end-stage lung disease. Globally, lung transplants have been successfully used in a limited number of patients [25]; however, only a small number of donors are available and the wait period to receive a lung transplant after being registered on the lung transplantation list can be very long.
In our case, the autopsy results suggested prolonged lung fibrosis after the negative SARS-CoV-2 test may have caused the patient's death. Autopsies of patients with COVID-19 can improve our understanding of the pathogenesis and clinical condition of post-COVID-19 lung fibrosis [26]. An accumulation of autopsy findings is necessary to clarify the cause of death in COVID-19 patients with ILD and to improve treatment strategies for patients with COVID-19 and ILD.
4. Conclusion
We report the autopsy of a patient with fatal COVID-19 and ILD and emphysema who died despite receiving the recommended treatment. The autopsy revealed that ARDS and fibrosis caused by SARS-CoV-2 infection caused the death. Fatal pulmonary fibrosis may be induced by SARS-CoV-2 infection in patients with severe COVID-19, especially those with ILD. Understanding the pathological features through autopsy and establishing appropriate treatments for severe cases of extensive pulmonary fibrosis and organization is important. Case accumulation and clinical trials are needed to determine appropriate treatment, such as prolonged administration of corticosteroids, anti-inflammatory drugs, and antifibrotic agents.
Ethics statement
Written informed consent for the publication of this report was obtained from the patient's family by the author.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability
No data was used for the research described in the article.
CRediT authorship contribution statement
Risa Kudo: Conceptualization, Writing – original draft. Takeshi Kawaguchi: Conceptualization, Data curation, Visualization, Writing – review & editing. Masatoshi Kimura: Data curation, Resources. Yuuki Rikitake: Resources. Chihiro Iwao: Data curation, Resources. Mao Rikitake: Data curation, Resources. Kosho Iwao: Data curation, Resources. Ayako Aizawa: Data curation. Yumi Kariya: Data curation. Motohiro Matsuda: Data curation. Shunichi Miyauchi: Supervision. Ichiro Takajo: Supervision. Yuichiro Sato: Resources, Supervision. Yujiro Asada: Resources, Supervision. Taiga Miyazaki: Supervision, Writing – review & editing. Kunihiko Umekita: Conceptualization, Supervision, Validation, Visualization, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.World Health Organization Novel coronavirus situation report 2019-nCoV; 1. 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200121-sitrep-1-2019-ncov.pdf/
- 2.Lee H., Choi H., Yang B., et al. Interstitial lung disease increases susceptibility to and severity of COVID-19. Eur. Respir. J. 2021;58 doi: 10.1183/13993003.04125-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beltramo G., Cottenet J., Mariet A.S., et al. Chronic respiratory diseases are predictors of severe outcome in COVID-19 hospitalised patients: a nationwide study. Eur. Respir. J. 2021;58 doi: 10.1183/13993003.04474-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shanmugam C., Mohammed A.R., Ravuri S., Luthra V., Rajagopal N., Karre S. COVID-2019 - a comprehensive pathology insight. Pathol. Res. Pract. 2020;216 doi: 10.1016/j.prp.2020.153222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guler S.A., Ebner L., Aubry-Beigelman C., et al. Pulmonary function and radiological features 4 months after COVID-19: first results from the national prospective observational Swiss COVID-19 lung study. Eur. Respir. J. 2021;57 doi: 10.1183/13993003.03690-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Myall K.J., Mukherjee B., Castanheira A.M., et al. Persistent post-COVID-19 interstitial lung disease. an observational study of corticosteroid treatment. Ann Am Thorac Soc. 2021;18:799–806. doi: 10.1513/AnnalsATS.202008-1002OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sessa F., Salerno M., Pomara C. 2021. Autopsy Tool in Unknown Diseases: the Experience with Coronaviruses (SARS-CoV, MERS-CoV, SARS-CoV-2). Medicina (Kaunas) p. 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carsana L., Sonzogni A., Nasr A., et al. Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: a two-centre descriptive study. Lancet Infect. Dis. 2020;20:1135–1140. doi: 10.1016/S1473-3099(20)30434-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Menter T., Haslbauer J.D., Nienhold R., et al. Postmortem examination of COVID-19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings in lungs and other organs suggesting vascular dysfunction. Histopathology. 2020;77:198–209. doi: 10.1111/his.14134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.D'Errico S., Zanon M., Montanaro M., et al. More than pneumonia: distinctive features of SARS-Cov-2 infection. From autopsy findings to clinical implications: a systematic review. Microorganisms. Oct 23 2020:8. doi: 10.3390/microorganisms8111642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caramaschi S., Kapp M.E., Miller S.E., et al. Histopathological findings and clinicopathologic correlation in COVID-19: a systematic review. Mod. Pathol. 2021;34:1614–1633. doi: 10.1038/s41379-021-00814-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drake T.M., Docherty A.B., Harrison E.M., et al. Outcome of hospitalization for COVID-19 in patients with interstitial lung disease. an international multicenter study. Am. J. Respir. Crit. Care Med. 2020;202:1656–1665. doi: 10.1164/rccm.202007-2794OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oda K., Ishimoto H., Yamada S., et al. Autopsy analyses in acute exacerbation of idiopathic pulmonary fibrosis. Respir. Res. 2014;15:109. doi: 10.1186/s12931-014-0109-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gómez Antúnez M., Muiño Míguez A., Estrada B.A.D., et al. Clinical characteristics and prognosis of COPD patients hospitalized with SARS-CoV-2. Int. J. Chronic Obstr. Pulm. Dis. 2020;15:3433–3445. doi: 10.2147/COPD.S276692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de H., Nakata Y., Nagashima M., et al. Molecular epidemiological features of SARS-CoV-2 in Japan, 2020-1. Virus Evol. 2022;8(1):veac034. doi: 10.1093/ve/veac034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsuchiya K., Yamamoto N., Hosaka Y., et al. Molecular characterization of SARS-CoV-2 detected in Tokyo, Japan during five waves: identification of the amino acid substitutions associated with transmissibility and severity. Front. Microbiol. 2022;13 doi: 10.3389/fmicb.2022.912061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Instititutes of health Ther. Manag. Adults COVID. 2021;19 https://www.covid19treatmentguidelines.nih.gov/management/therapeutic-management [Google Scholar]
- 18.Kallet R.H., Matthay M.A. Hyperoxic acute lung injury. Respir. Care. 2013;58:123–141. doi: 10.4187/respcare.01963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalil A.C., Patterson T.F., Mehta A.K., et al. Baricitinib plus Remdesivir for hospitalized adults with Covid-19. N. Engl. J. Med. 2021;384:795–807. doi: 10.1056/NEJMoa2031994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.REMAP-CAP Investigators. Gordon A.C., Mouncey P.R., et al. Interleukin-6 receptor antagonists in critically ill patients with covid-19. N. Engl. J. Med. 2021;384:1491–1502. doi: 10.1056/NEJMoa2100433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Razonable R.R., Pawlowski C., O'Horo J.C., et al. Casirivimab-Imdevimab treatment is associated with reduced rates of hospitalization among high-risk patients with mild to moderate coronavirus disease-19. EClinicalmedicine. 2021;40 doi: 10.1016/j.eclinm.2021.101102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anghel A.M., Niculae C.M., Manea E.D., et al. The impact of tocilizumab on radiological changes assessed by quantitative chest CT in severe COVID-19 patients. J. Clin. Med. 2022:11. doi: 10.3390/jcm11051247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uemasu K., Yasuda Y., Hirayama Y., et al. Post-COVID-19 interstitial lung disease presenting with profound hypoxemia: report of three cases demonstrating a good response to high-dose corticosteroid therapy. J. Infect. Chemother. 2022;28:321–325. doi: 10.1016/j.jiac.2021.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Umemura Y., Mitsuyama Y., Minami K., et al. Efficacy and safety of nintedanib for pulmonary fibrosis in severe pneumonia induced by COVID-19: an interventional study. Int. J. Infect. Dis. 2021;108:454–460. doi: 10.1016/j.ijid.2021.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.King C.S., Mannem H., Kukreja J., et al. Lung transplantation for patients with COVID-19. Chest. 2022;161:169–178. doi: 10.1016/j.chest.2021.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aljerian K., BaHammam A.S. COVID-19: lessons in laboratory medicine, pathology, and autopsy. Ann. Thorac. Med. 2020;15:138–145. doi: 10.4103/atm.ATM_173_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.