Abstract
In this investigation woven cotton fabric was dyed with madder dye under different dyeing conditions such as in the presence of without mordant, single mordant and mixed mordant. The thermal behaviour of non-mordanted,single mordanted and mixed chemical mordanted with madder dyed cotton fabrics were investigated through thermogravimetric analysis. Further, the fundamental molecular arrangement of dyed cotton fabric was confirmed by the Fourier transformer–Infrared spectroscopy, and the electronic orientation of dye molecule, and after adsorption of cellulose structure is confirmed from Ultra–Violet spectroscopy. HOMO and LUMO calculations are evaluated from the gaussian software. The interaction and binding energies between inhibitor (dye molecule) and cellulose surface are evaluated from molecular dynamic simulation using BIOVIA material studio software.
Keywords: Dye, Madder, HOMO and LUMO, Cellulose, Adsorption andBinding energy
1. Introduction
Natural dyes are sustainable, bio-degradable, and eco-friendly nature, due to which the revival of natural dyeing technology has become so important [1]. Most synthetic dyes are toxic and carcinogenic, whereas the natural dyes are eco-friendly, have no adverse effects on humans' skin, and most natural dye extracts possess medicinal values [2,3].Natural plant-based dyes are possessing a lot of medical values such as antimicrobial, anti-inflammatory, wound healing, antioxidants, and so on., due to their chemical composition of anthraquinones, flavonoids, tannins, naphthoquinones etc., [4].Rubia tinctorum dye is one of the furthermostextensively known plants used as a natural dye. The use of rubiaceae species is littered all over historical literature, especiallyRubiatinctorumiscommonly known as madder. The Oldest ever finding of a textile dyed with madder was a cotton fabric the dates back to 3000 BCE and was discovered in Mohenjjo-Daro in the Indus Valley. The Madder supplies the dye compound within its roots, which contains anthraquinonoid molecules or its derivatives. Morethan, 13,000 different species of the Rubiacaecs family are currently known. The genus Rubia has nearly 80 different species containing various amounts of anthraquinone derivatives. Aglycone anthraquinone compounds are present in the Rubia genus and the analysis of several glycosidic anthraquinoneshasbeen reported. Further, thespecies include Rubia cordifolia (munjeet), Rubia peregrine L. (wild madder), and Galium Verum L. (ladies’ bedstraw). The Rubia tinctorum roots were covered with a rind, underneath which invention the anthraquinone dye moleculegiving a dark red colour. The extraction and analysis of Rubia tinctorumgive the dye molecules which areresponsible for the production of colour. In this process, 36 different anthraquinone molecules are discovered from Rubiatinctorumroot. The production of madder had become enormously important and profitablein the 16th Century at the Ottoman market which was the biggest in the world. In the 17th Century, this was massively sorted after the Turkish Red dye technique.
Previously reported, M. Sadeghi-Kiakhaniet al., madder dye was treated with wool and nylon which is exhibitedinthe kinetic and adsorption isotherm studies [5]. M. Yusuf et al. have reported henna and madder dye studies on the effect of tin (II) chloride mordant through woollen yarn [6].R.S. Blackburn et al., reported the extraction and analysis of madder dye in historical textiles. In his work describes the 68 anthraquinone colourant that has been discovered in (Rubia spp.) madder [7].A. Jahangiri et al., reported the Tannin-based Biomordants are treated with wool through madder dye which was tested in colorimetric, tensile assay and fastness respectively [8].T. Hussainet al. reported the mordants gives the various shade (colours) on the cotton fabric of range from red to violet shades [9].N. Jahan et al. reported the natural dye of madder which has compared with cotton and silk fabric. In this process, alum and copper sulphate was used as mordanted. This silk fabric was observed deeper than the cotton fabrics [10].
This present work explores the adsorption phenomenon of madder dye on the cellulose structure. This may be confirmed through theoretical data analysis of madder dye molecule (1,2 – Dihydroxy anthraquinone) absorbed onto cellulose unit. This research article has newly introduced the theoretical analysis of the adsorption process which is novel on the adsorption process.
2. Materials and methods
Madder dye was extracted from the dried root of the Rubia tinctorumplant. The loom-made fabric was scoured and bleached well. The specification of cotton fabric is as follows Ends per inch(EPI)-62,Picks per inch(PPI)-68, warp &weft count 60SNe, areal density 285 g/m2.Spectrophotometric measurements were performed on a UV–Vis PerkinElmer LAMBDA-35 spectrophotometer.Thermogravimetric analysis was carried out using STA 2500 Regulus (Netzsch, Germany) in the presence of nitrogen atmosphere at different heat rate.
In this investigation, cotton fabric was scoured and bleached with sodium hydroxide and Hydrogen peroxide respectively. Subsequently the dyed with Rubia tinctorum plant extract in five different pH ranges such as 3,5,7,9.11.and 13 to understand the impact of different pH. Further to this, thermodynamic studies were carried out with the temperature range of 100 °C,250 °C, and 250 °C to find out the impact of different thermal ranges and Activation energy (Ea), Free energy (ΔG), Enthalpy (ΔH) and Entropy (ΔS) against different temperature. Then the dyeing was carriedout with single mordant (Fe2SO4) and mixed mordant (Fe2SO4 + Alum) in five different concentration such as 3 %.4 %,5 %,6 %,and 7 %. Finally the TGA analysis was carried out for all the single mordanted and mixed mordanted dyed samples.
3. Experimental
The thermal decomposition of the samples was monitored using Thermogravimetric analyser at the temperature range between 30 and 500 °C under a nitrogen atmosphere ataheating rate of 10 °C per min by using an alumina TG crucible.The interaction between alizarin dye and the cellulose surface was investigated from molecular dynamic simulation using BIOVIA material studio software. The cellulose (100) was chosen as the surface for simulation. The crystalline structure of the cellulose was obtained from the literature [11]. The interaction between the alizarin dye and cellulose was carried out using the COMPASS force field in a simulation box (32.804 x 41.520 × 32.697 Å) with periodic boundary conditions. Allfour layers of cellulose surface were kept “frozen” at fixed positions and one alizarin dye molecule was allowed to freely interact with the surface. The interaction energyand binding energies between inhibitor and cellulose surface are calculated as follows.
WhereEtotalis the total energy of the metal surface and absorbed alizarin dye molecule, Esurface is the energy of the cellulose surface, and Einhibitor is the energy of the dye.
3.1. Adsorption process
The pre-treated cotton has been used as the absorbent of 100 ppm madder dye. The specimen size of the absorbent is 2 × 2 cm which was immersed for 1 h in the mechanical shaker. After the adsorption process, the absorbate was recorded in UV–Vis spectroscopy.
4. Result and discussion
4.1. FT-IR spectroscopy
The FT-IR spectra of madder (dye molecule) and cellulose (cotton) before and after adsorption is shown in Fig. 1A – F at different pH of 3, 5, 7, 9, and 11 respectively. The FT-IR spectra were recorded in the ATR mode through % of absorption and wavelength. Through this FT-IR analysis demonstrate the functional frequency assignment at different pH level (3, 5, 7, 9, 11 and 13). It was found that the intensity of the adsorption peak of the samples was lower at before adsorption state than after adsorption. This reveals that the dye molecules are closely absorbed on the cotton surface.
Fig. 1.
FT-IR spectra of before and after adsorption of madder and cotton at different pH A) pH 3, B) pH 5, C) pH 7, D) pH 9, E) pH 11, and F) pH13.
The ATR FT-IR peak values of before and after adsorption at different pH ranges are tabulated in Table 1. The ATR FT-IR absorption peaks reveal that the cellulose molecular orientation. The absorption peak in the range of 1103–1105 cm−1 represents the ring asymmetric stretching and the peaks range at 1156–1159 cm−1 suggested the C–O–C asymmetric stretching of the cellulose unit. The absorption peaks observed in the range 1315–1320 cm−1 reveals that the CH2 wagging vibration in cellulose structure. The peaks range at 1423–1427 cm−1 confirmed the CH2 symmetric bending vibration in cellulose structure. Absorption peaks range at 3271–3290, 3325, and 3335 cm−1, which confirmed that the O–H stretching vibration of cellulose structure [12,13].
Table: 1.
ATR FT-IR peak values of before and after adsorption at different pH.
| S. No | pH3 |
pH5 |
pH7 |
pH9 |
pH11 |
pH13 |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before adsorption | After adsorption | Before adsorption | After adsorption | Before adsorption | After adsorption | Before adsorption | After adsorption | Before adsorption | After adsorption | Before adsorption | After adsorption | |
| 1 | 1105 | 1104 | 1104 | 1104 | 1105 | 1105 | 1104 | 1104 | 1103 | 1104 | 1104 | 1105 |
| 2 | 1159 | 1156 | 1156 | 1156 | 1155 | 1156 | 1156 | 1156 | 1153 | 1156 | 1156 | 1156 |
| 3 | 1315 | 1318 | 1318 | 1317 | 1319 | 1317 | 1320 | 1318 | 1319 | 1318 | 1320 | 1317 |
| 4 | 1426 | 1427 | 1424 | 1426 | 1426 | 1426 | 1426 | 1426 | 1426 | 1427 | 1426 | 1423 |
| 5 | 1640 | 1650 | 1637 | 1637 | 1633 | 1631 | 1631 | 1641 | 1639 | 1634 | 1637 | 1643 |
| 6 | 2853 | 2852 | 2857 | 2857 | 2855 | 2857 | 2725 | 2740 | 2572 | 2571 | 2584 | 2891 |
| 7 | 2903 | 2901 | 2902 | 2904 | 2911 | 2896 | 2899 | 2894 | 2898 | 2895 | 2892 | 3058 |
| 8 | 3271 | 3290 | 3277 | 3293 | 3273 | 3283 | 3293 | 3285 | 3285 | 3286 | 3289 | 3289 |
| 9 | 3330 | 3333 | 3335 | 3330 | 3331 | 3330 | 3333 | 3325 | 3328 | 3326 | 3330 | 3330 |
4.2. UV–Visible spectroscopy
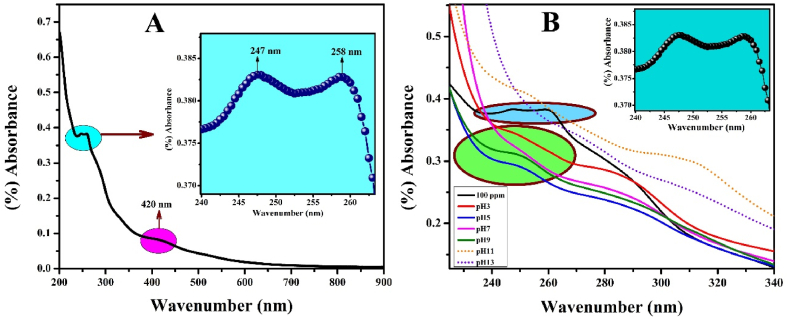
The UV–Visible spectra of madder dye are shown in Fig. 2A. The Peak values are obtained in the range of 247 nm and 258 nm demonstrates that the σ–σ* and π– π* electronic transition of madder dye molecular structure. The peak value of 420 nmrepresentsthe n–π* transition. Further, the UV–Visible spectra of madder dye at different pH are shown in Fig. 2B.
Fig. 2.
A) UV–Visible spectroscopy of madder dye and B) UV–Visible spectroscopy of madder dye at different pH.
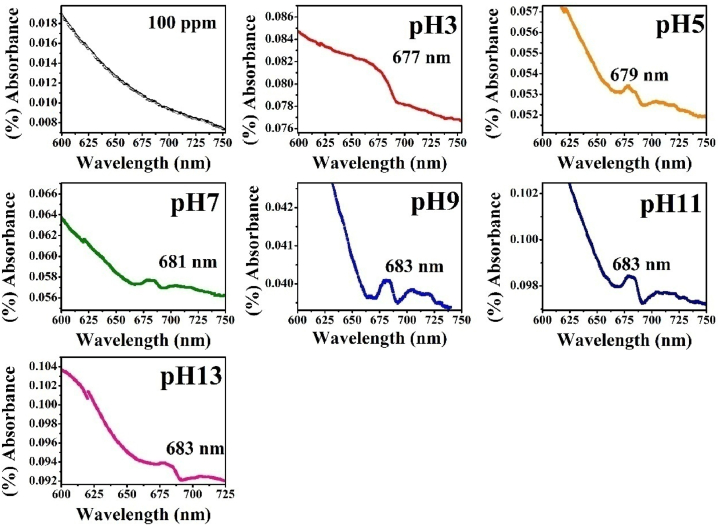
The wavelength of 600–750 nm UV–Visible spectra of madder dye at different pH conditions are shown in Fig. 3. The absorption of peak values of different pH conditions such as pH3, pH5, pH7, pH9, pH11, and pH13 are as follows 677, 679, 681, 683, 683, and 683 nm respectively. This confirms that the n–π* transition has shifted to a higher wavelength region known as red shift [14].
Fig. 3.
UV–Visible spectroscopy in the range of 600–750 nm of madder dye at different pH.
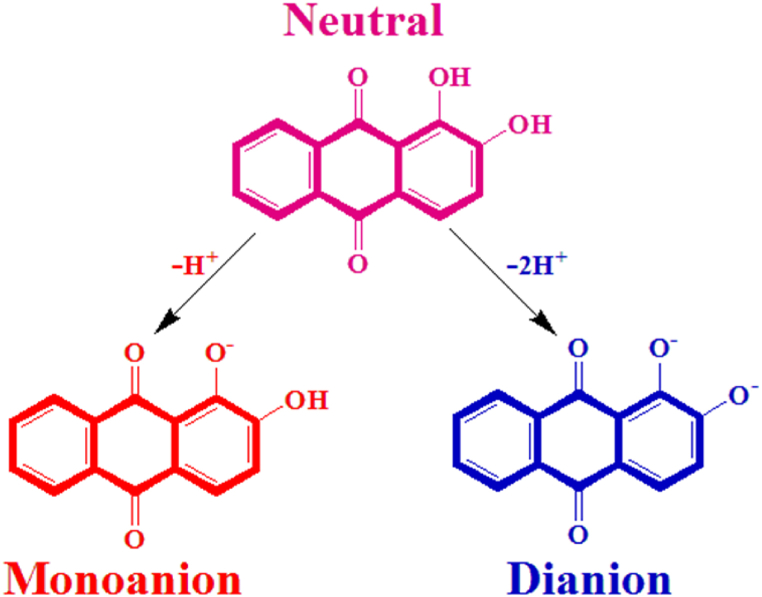
The madder dye produces monoanion during lower pH ranges such as pH3, pH5, and pH7, whereas dianion during higher pH9, pH11, and pH13 exhibit the dianion [14] and the structures are shown in Fig. 4. The dianion is effectively absorbed on the cotton surface than the monoanion. So, the higher pH is preferred to the adsorption phenomenon.
Fig. 4.
Formation of monoanion and dianion in the madder dye molecule.
4.3. Thermogravimetric analysis
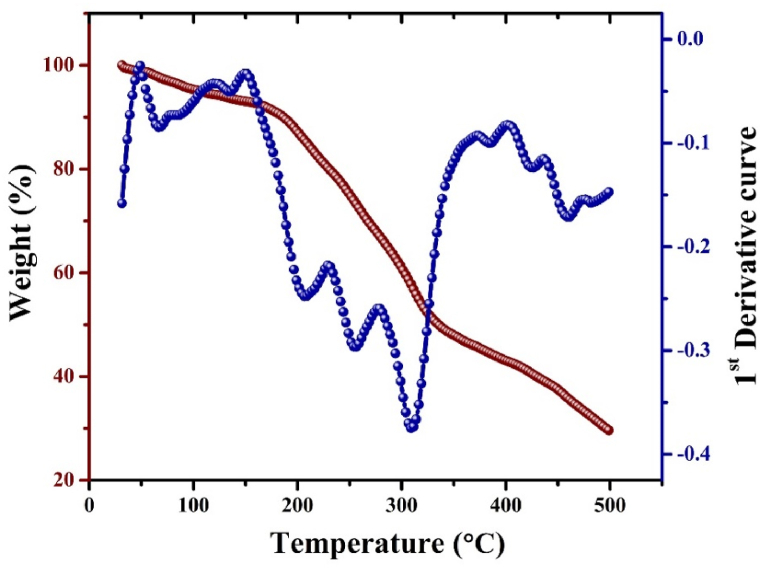
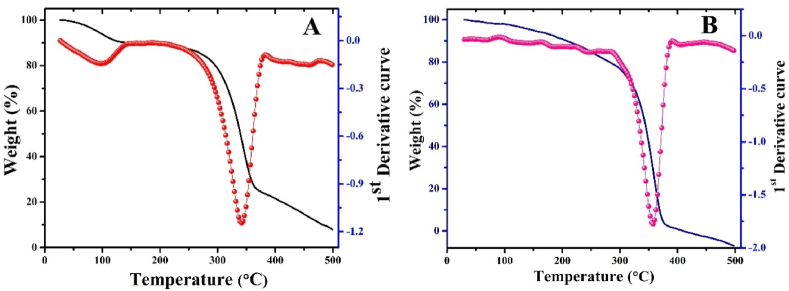
Thermogravimetric analysis used to determine the thermal stability of the madder dye and its thermogram is shown in Fig. 5. Three-stage degradation is observed in the madder dye. In the first stage, the temperature range between 30 and 100 °C, 4.6 % of water molecules are decomposed. Second stage degradation observed in the temperature range from 101 to 150 °C where 2.28 % of weight loss observed due to loss of hydroxy molecule. The third stage of degradation is observed in the temperature range between 150 and 500 °Cand the weight loss of 63.49 % was decomposed due to the organic moiety of madder dye [15].The thermogram of undyed cotton and dyed cotton are shown in Fig. 6A and B. Both thermograms reveal that the three-stage degradation. The first stage degradation of undyed and dyed cotton at a temperature between 30 and 100 °C. The weight loss of 6.19 % and 2.05 % due to decomposed water molecules or moisture presence in the cellulose structure [16]. The second stage of decomposition is observed in the temperature 101–250 °C. The weight loss of 6.54 % and 13.33 % are the primary thermal decomposition of cellulosic materials and depolymerization of hemicellulose [17].
Fig. 5.
Thermogram of madder dye.
Fig. 6.
Thermogram of A) undyed cotton and B) Dyed cotton.
The third stage of decomposition was observed in the temperature between 251 and 500 °C, the weight loss is observed of 70.42 % and overall weight loss of 92.14 % observed at a temperature from 30 to 500 °C, which explores the decomposition of drastic cleavage of the glycosidic linkage of cellulose structure and reduce the polymerization degree in leading to the formation of CO2, H2O, and variety of hydrocarbon derivatives [17,18]. But in the case of dyed cotton at a temperature range between 251 and 400 °C, the weight loss of 83.80 % is observed and the dyed cotton observed at a temperature between 30 and 400 °C where the weight loss of 99 % decomposed the glycosidic linkage of cellulose structure [17]. The thermogram of treated cotton is completely decomposed, which confirms that the removal of the impurities of cotton through pre-treatment.
4.4. Theoretical calculation
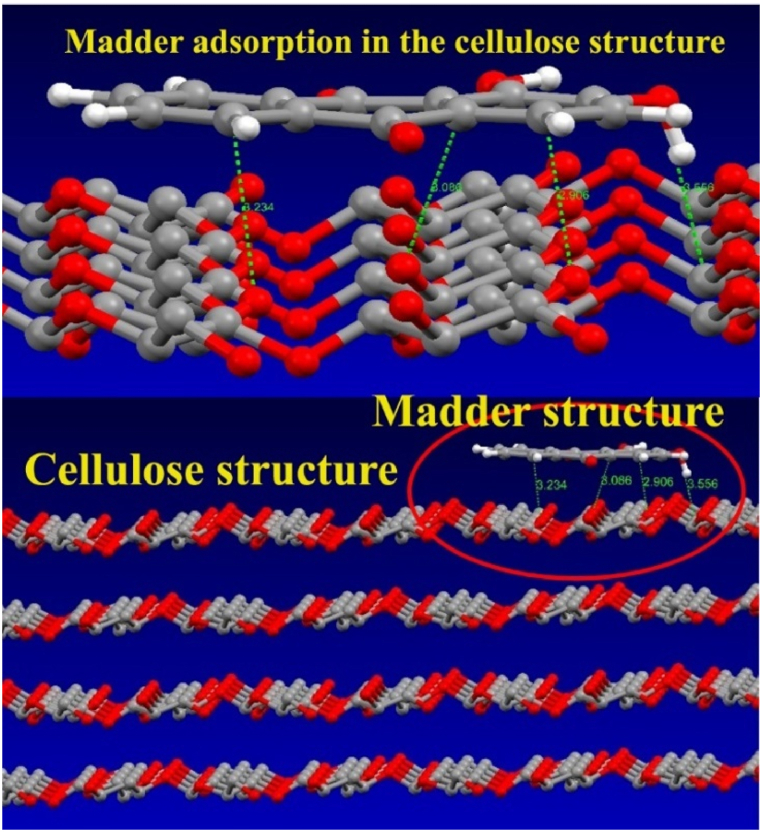
Molecular dynamics simulation is a favourable platform fora deeper understanding of the interaction between the dye molecule and cellulose surface. This simulation is carried out to explore the adsorption mechanism of these two systems. A molecular dynamics simulation study is used to investigate the most favourable configuration of the interacted madder dye molecule and cellulose surface. The side view of the investigated madder dye molecule and cellulose surface (100) is shown in Fig. 7.
Fig. 7.
Madder adsorption in cellulose structure.
The figure reveals that the dye molecule absorbs tightly on the cellulose surface with a parallel/flat orientation of each other. The parallel orientation of the dye molecule and cellulose surface covers the larger area of the surface. The binding energy of the adsorption system is 30.5035 kcal/mol. The higher value of the binding energy suggests the more stable adsorption of the system.
4.5. HOMO and LUMO structure of madder
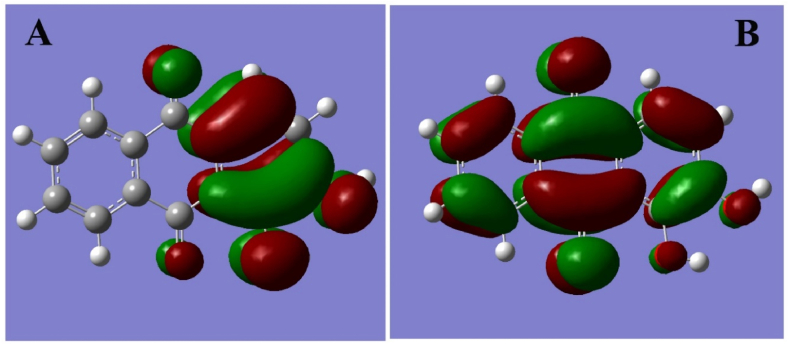
The HOMO and LUMO structures of madder are shown in Fig. 8 A and Fig. 8B respectively, and these values are calculated from the FOPT type through RB3LYPmethods.Thebasis set = 6-31G, charge = 0, the spin is a singlet. From this study, the total energy is observed of −838.9759 a.u, the RMS gradient norm of 0.00002141 a.u., the dipole moment is observed of 3.3601 Debye, the observed point group is C1. HOMO and LUMO values are −2.7807 and −6.4064 obtained respectively. The energy gap between HOMO and LUMO structures is 3.6256.
Fig. 8.
HOMO and LUMO structure of Madder.
4.6. Thermodynamical analysis of madder dye
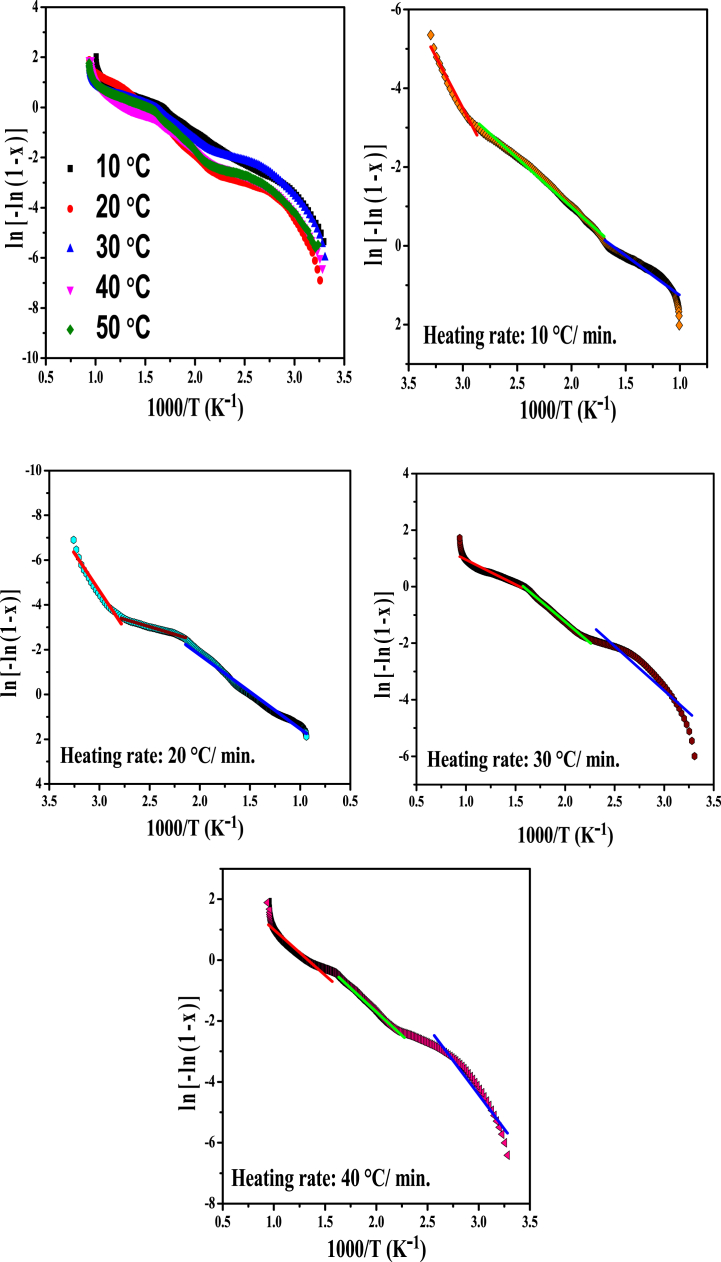
Madder dye was carried out thermally analyzed in different heating rate like 10°, 20°, 30°, 40° and 50° per minute are shown in Fig. 9 and degradation percentage are tabulated Table 2.
Fig. 9.
Thermogram of madder dye at different heating rate.
Table: 2.
Degrading behaviour of madder dye at different heat rate.
| S. No | Heating rate (°C) | Weight loss (%) |
Residue (%) | ||
|---|---|---|---|---|---|
| Temperature (°C) | |||||
| 30–100 °C | 100–400 °C | 400–800 °C | |||
| 1 | 10 | 6.51 | 67.37 | 26.04 (725 °C) | 0.08 |
| 2 | 20 | 3.48 | 56.57 | 31.37 | 8.58 |
| 3 | 30 | 7.53 | 55.49 | 28.33 | 8.65 |
| 4 | 40 | 4.45 | 48.15 | 46.95 (770 °C) | 0.45 |
| 5 | 50 | 3.75 | 49.66 | 28.42 | 18.17 |
When increasing heating rate 10°–50° the initial weight loss is observed of 6.51, 3.48, 7.53, 4.45 and 3.75 % at 30–100° due to loss of moisture in the madder dye. Further increasing the temperature 100–400 °C the weight loss observed of 67.37, 56.57, 55.49, 48.15 and 49.66 % which has been second degradation of –OH group of madder dye molecule.
Further increasing the temperature 400–800 °C the weight loss observed of 26.04, 31.37, 28.33, 46.95 and 28.42 % due to loss of quinone moiety of madder dye, which has been confirmed the madder dye was thermally more stable. The activation energy also calculated The Coats-Redfern method.
Fig. 10 shows the three step degradation behaviour observed of madder dye at different heating rate. The first step denotes the dehydration step or lose of moisture in the Madder dye. The second step describes the loss of –OH group and third step suggested the decomposition of madder dye molecule.
Fig. 10.
Plot of ln[-ln(1 - x)] vs 1000/T at different heating rate of madder dye.
Activation energy (Ea), Free energy (ΔG), Enthalpy (ΔH) and Entropy (ΔS) of madder dye are measured through thermal analysis at different heating rate at 100 °C are shown in Table 3. The different heating rate of madder dye obtained Gibbs free energy (ΔG) are shown in positive values in the range of 99.9267–108.2486 kJ mol−1which has been thermally not spontaneous process. The lowest value of ΔG observed of 99.92 kJ mol−1. The entropy negative values of (ΔS) are observed range of −0.1291 to −0.2484 kJ mol−1. The Enthalpy (ΔH) positive values are observed in the range of 10.8758–52. 9288 kJ mol−1. The lowest and highest values of ΔH are observed in the heating rate 50° and 20 °C per minute. Maximum and minimum Ea value is observed of 56.0500 and13.9770 kJ mol−1 at heating rate 20° and 50 °C per minute respectively.. .
Table: 4.
Thermodynamic parameters of madder dye at different heating rate at temperature 250 °C.
| S. No. | Heating rate | Stage | Temp. | Ea (kJ mol−1) | ΔS (kJ mol−1) | ΔH (kJ mol−1) | ΔG (kJ mol−1) |
|---|---|---|---|---|---|---|---|
| 1 | 10 °C/min. | 2 | 250 | 20.6469 | −0.2427 | 16.2985 | 143.2168 |
| 2 | 20 °C/min. | 2 | 250 | 11.1980 | −0.2777 | 6.8497 | 152.0953 |
| 3 | 30 °C/min. | 2 | 250 | 24.6638 | −0.2347 | 20.3154 | 143.0738 |
| 4 | 40 °C/min. | 2 | 250 | 25.8167 | −0.2356 | 21.4684 | 144.6755 |
| 5 | 50 °C/min. | 2 | 250 | 32.3961 | −0.2205 | 28.0477 | 143.3729 |
The Thermodynamic parameter at 450 °C are shown in Table 5. The positive ΔG value are observed in the range from 178.0351 to 197.5309 kJ mol−1 at heating rate 40° and 10 °C per minute, which is confirm the thermally non-spontaneous process. The negative ΔS value observed in the range from −0.2030 to −0.2583 kJ mol−1 at heating rate 40° and 10 °C per minute respectively. The positive ΔH values are obtained in the range between 10.81374 and 31.29486 kJ mol−1 at heating rate 10° and 40 °C per minute respectively. The maximum and minimum Ea value are observed of 37.306 and 16.825 kJ mol−1at heating rate 40° and 10 °C respectively.
Table: 5.
Thermodynamic parameters of madder dye at different heating rate at temperature 450 °C.
| S. No. | Heating rate | Stage | Temp. | Ea (kJ mol−1) | ΔS (kJ mol−1) | ΔH (kJ mol−1) | ΔG (kJ mol−1) |
|---|---|---|---|---|---|---|---|
| 1 | 10 °C/min. | 3 | 450 | 16.825 | −0.2583 | 10.81374 | 197.5309 |
| 2 | 20 °C/min. | 3 | 450 | 27.438 | −0.2410 | 21.42678 | 195.6777 |
| 3 | 30 °C/min. | 3 | 450 | 26.106 | −0.2336 | 20.09475 | 188.9728 |
| 4 | 40 °C/min. | 3 | 450 | 37.306 | −0.2030 | 31.29486 | 178.0351 |
| 5 | 50 °C/min. | 3 | 450 | 28.241 | −0.2312 | 22.23011 | 189.3764 |
Table: 3.
Thermodynamic parameters of madder dye at different heating rate at temperature (100 °C).
| S. No. | Heating rate | Stage | Temp. | Ea (kJ mol−1) | ΔS (kJ mol−1) | ΔH (kJ mol−1) | ΔG (kJ mol−1) |
|---|---|---|---|---|---|---|---|
| 1 | 10 °C/min. | 1 | 100 | 43.7170 | −0.1590 | 40.6158 | 99.9267 |
| 2 | 20 °C/min. | 1 | 100 | 56.0500 | −0.1291 | 52.9488 | 101.0891 |
| 3 | 30 °C/min. | 1 | 100 | 14.5698 | −0.2472 | 11.4686 | 103.6714 |
| 4 | 40 °C/min. | 1 | 100 | 24.9283 | −0.2317 | 21.8271 | 108.2486 |
| 5 | 50 °C/min. | 1 | 100 | 13.9770 | −0.2484 | 10.8758 | 103.5327 |
Thermodynamic parameters at 250 °C are shown in Table 4. The positive ΔG values are appeared in the range from 143.0738 to 152.0953 kJ mol−1 at heating rate 30° and 20° per minute, which confirms the thermally non-spontaneous process. The ΔS negative values are observed in the range from −0.2205 to −0.2777 kJ mol−1 at heating rate 50° and 20° per minute respectively. The positive ΔH values are obtained in the range from 6.8497 to 28.0477 kJ mol−1 at heating rate from 20° to 50 °C per minute. The maximum and minimum Ea values are obtained of 32.3961 and 11.1980 kJ mol−1 at the heating rate of 50° and 20 °C per minute respectively.
4.7. Thermogravimetric curve analysis of madder dyed fabric
Thermogravimetric analysis was carried out for all the dyed samples with or without mordanted to analyse correlation between the thermal residue and colour strength. For that TGA test was carried out and its results are shown in graphical and tabulated format.
4.7.1. Thermogravimetric studies of non-mordanted dyed sample
The thermogravimetric curve of scoured and bleached cotton at different madder dye(%) are shown in Fig. 11A. The First step degradation are carried out 30–100 °C due to loss of moisture present in the scoured and bleached cotton with from 1 % to 5 % madder dye. When increasing madder dye 1 %–5 % the first step degradation increases 1.28 %–47.20 %. Further, increasing weight loss from 100 to 250 °C the second step weight loss are obtained due to loss of –OH group present in the madder dye molecule and cellulose molecular structures are starting to decomposition.
Fig. 11.
Thermogravimetric curve of madder dye absorbed cotton A) without mordant, B) madder dye scoured and bleached cotton with different madder dye concentration (1 %–5 %) in 3 % Fe2SO4, C)madder dye scoured and bleached cotton with different madder dye concentration (1 %–5 %) in 4 % Fe2SO4, D) madder dye scoured and bleached cotton with different madder dye concentration (1 %–5 %) in 5 % Fe2SO4, E) madder dye scoured and bleached cotton with different madder dye concentration (1 %–5 %) in 6 % Fe2SO4 and E) madder dye scoured and bleached cotton with different madder dye concentration (1 %–5 %) in 7 % Fe2SO4.
At this stage the maximum degradation is observed from scoured and bleached cotton with 1 % madder dye (20.28 %). The minimum degradation is observed of 0.58 % which is scoured and bleached cotton with 5 % madder dye.
The third step degradation is observed from 250 to 500 °C due to loss of back bone of cellulose molecular structure and molecule of madder dye. In this stage the maximum degradation observed of 74.23 % from scoured and bleached cotton with 3 % madder dye. The maximum residue present in the scoured and bleached cotton with 1 % madder dye and the weight loss of 15.68 % is observed.
4.7.2. Thermogravimetric studies of madder dyed sample with 3 % single mordant (Fe2SO4)
The Thermogravimetric curve of madder dye scoured and bleached cotton with different madder dye concentration (1 %–5 %) and 3 % Fe2SO4 are shown in Fig. 11B.
The first step degradation observed at 30–100 °C due to loss of moisture. The minimum weight loss observed of 8.70 % in scoured and bleached cotton with combination of 2 % madder dye and 3 % Fe2SO4. The maximum weight loss observed of 15.57 % in scoured and bleached cotton with combination of 1 % madder dye and 3 % Fe2SO4. The second step degradation observed from 100 to 250 °C due to loss of –OH group present in the madder dye molecule and degradation start from cellulose molecular structure. The maximum weight loss (14.05 %) observed from scoured and bleached cotton with combination of 1 % Madder dye with 3 % single mordanted.
The third step degradation observed from 250 to 500 °C due to loss of bank bone of made dye molecule, cellulose molecular structure and Fe2SO4. The maximum weight loss observed (53.77 %) from scoured and bleached cotton with combination of 4 % madder dye with 3 % single mordant.
The minimum weight loss observed as 38.81 % for 3 % madder dye with 3 % single mordanted. The maximum residue observed for 3 % madder dyed with 3 % single mordanted of 50.76 % which is thermally more stable than the other madder dye concentration of 1 %, 2 %, 4 % and 5 % respectively.
4.7.3. Thermogravimetric studies of madder dyed sample with 4 % single mordant (Fe2SO4)
Scoured and bleached cotton with combination of madder dye (1 %–5 %) in 4 % of Fe2SO4 which thermogravimetric curves are shown in Fig. 11C. First step decomposition occurs between 30 and 100 °C due to loss of moisture. The maximum weight loss observed of 15.16 % in scoured and bleached cotton with combination 3 % madder dye and 4 % Fe2SO4. The minimum weight loss (7.80 %) is obtained in scoured and bleached cotton with combination of 5 % madder dye and 4 % Fe2SO4.The second weight loss is observed at 100–250 °C due to loss of madder dye molecule and decomposition starts from cellulose molecular structure in this stage 13.99 % weight loss observed in scoured and bleached cotton with the combination of 2 % of madder dye and 4 % of Fe2SO4. The third step degradation obtained temperature range between 250 and 500 °C due to loss of cellulose and madder dye molecular structure back bone. The maximum weight loss (72.33 %) observed from scoured and bleached cotton within the combination of 5 % madder dye and 4 % of Fe2SO4.
The minimum weight loss observed The maximum residue observed from scoured and bleached cotton with combination of 1 % madder dye and 4 % of Fe2SO4in this results shows the pristine combination is thermally more stable than the other combination.
4.7.4. Thermogravimetric studies of madder dyed sample with 5 % single mordant (Fe2SO4)
The thermogravimetric analysis of bleached cotton treated with different madder dye concentration (1 %–5 %) with combination of 5 % Fe2SO4. Thermograms are shown in Fig. 11D. The first degradation observed at temperature range between 30 and 100 °C at this range lowest degradation (4.19 %) is observed in scoured and bleached cotton with combination of 1 % Madder dye with 5 % of Fe2SO4. The highest degradation is observed (5.52 %) from scoured and bleached cotton with combination of 5 % madder dye with 5 % of Fe2SO4. Further, the second stage degradation starts from 100 to 250 °C due to loss of madder dye and cellulose molecular structure. The lowest degradation observed of 4.56 % in scoured and bleached cotton with combination of 1 % madder dye with 5 % of Fe2SO4. The maximum degradation found to be 15.76 % for cotton dyed through 5 % madder dye with 4 % single mordanted. The third stage decomposition starts from 250 to 500 °C due to complete back bone decomposition of structural skeletal of cellulose and madder dye molecule. Lowest decomposition obtained as 35.24 % from the cotton with combination of 3 % madder dye with 4 % single mordanted. Highest degradation value of 60.81 % observed from scoured and bleached cotton with 4 % madder dye and 5 % Fe2SO4. The maximum residue of 49.5 % was observed from cotton with combination of 1 % madder with 4 % single mordanted. This composition is thermally more stable than the other composition.
4.7.5. Thermogravimetric studies of madder dyed sample with 6 % single mordant (Fe2SO4)
Thermogravimetric analysis of bleached cotton with various madder dye concentration from 1 % to 5 % and 6 % of Fe2SO4. This thermograms are shown in Fig. 11E. The first stage decomposition detected at the temperature between 30 and 100 °C due to loss of moisture. The lowest weight loss observed of 5.09 % in the cotton with combination of 4 % madder dye with 6 % single mordanted. Maximum weight loss observed of 10.59 %, in cotton fabric with combination of 2 % madder dye with 6 % single mordanted. The second weight loss observed at the range between 100 and 250 °C due to decomposition starts cellulose and madder dye molecule. The minimum weight loss observed of 5.09 % for cotton in 3 % madder dye with 6 % single mordanted. The maximum weight loss in this thermal range was obtained of 10.59 % in scoured and bleached cotton in 2 % madder dye with 6 % single mordanted.
Third stage decomposition starts at the temperature between 250 and 500 °C due to complete decomposition of cellulose and madder dye backbone of the molecule. The minimum weight loss observed of 25.45 % in cotton with the combination of 4 % madder dye with 6 % single mordanted. The maximum weight loss observed of 79.55 % in cotton with chemical composition of 5 % madder dye with 6 % single mordanted. The maximum residue obtained of 58.36 % is present in the cotton with the composition of 4 % madder dye with 6 % single mordanted. This composition of absorbed cotton have thermally more stable than the other composition absorbed cotton.
4.7.6. Thermogravimetric studies of madder dyed sample with 7 % single mordant (Fe2SO4)
Thermogravimetric curve of bleached cotton with different madder dye concentration from 1 % to 5 % in the presence of 7 % Fe2SO4 are shown in Fig. 11F. The first degradation observed at 30–100 °C due to loss of moisture present in the absorbed material. The lowermost values are observed of 8.51 % in scoured and bleached cotton with the combination of 2 % madder dye with 7 % single mordanted. The highest weight loss observed in at this temperature range of 18.01 % for cotton with the combination of 3 % madder dye with 7 % single mordanted. The second stage degradation obtained at temperature range between 100 and 250 °C. At this temperature the lower weight loss observed of 5.51 % for cotton dyed through 5 % of madder dye with 7 % of single mordanted. The highest weight loss observed of 15.28 % for cotton dyed through 5 % of madder dye with 7 % of single mordanted.Third stage degradation observed at temperature range between 250 and 500 °C due to complete decomposition of cellulose and madder dye backbone of the molecule. The lowest decomposition observed of 8.51 % for cotton dyed through composition of 2 % madder dye with 7 % single mordanted. The maximum decomposition obtained of 18.01 % in cotton with combination of 3 % madder dye with 7 % of single mordanted due to loss of moisture present in the absorbed cotton fabric.
The second stage decomposition detected at the temperature between 100 and 250 °C, at this temperature the lowest weight loss observed at 5.51 % in cotton dyed through the combination of 5 % of madder dye with 7 % single mordanted. The maximum weight loss observed of 15.28 % in scoured and bleached cotton in 2 % of madder dye with 7 % single mordanted. Third stage degradation found to be at temperature range from 250 to 500 °C due to complete decomposition of madder dye and cellulose backbone molecular structure. At this temperature the minimum weight loss observed of 37.80 % in scoured and bleached cotton with chemical composition of 2 % of madder dye with 7 % of single mordanted.
The maximum weight loss observed of 43.06 % in scoured and bleached cotton with combination of 5 % of madder dye with 7 % of single mordanted.The maximum residue observed of 39.07 % in scoured and bleached cotton with combination 5 % of madder dye with 7 % of single mordanted. This combination have thermally more stable than the other absorbed cotton.
4.8. Thermogravimetric analysis of mixed mordanted cotton samples
4.8.1. Thermogravimetric studies of madder dyed sample without any mordant. Alum)
Thermogravimetric analysis of Mordant cotton with different madder dye concentration 1–5 % without any mordant concentration. The Thermograms are shown in Fig. 12A.This mordant cotton without mordant concentration also observed in three stage degradation. The first stage degradation is found to be at temperature range between 30 and 100 °C due to loss of moisture present in the mordant cotton. At this temperature the lowest weight loss obtained of 5.87 % in mordant cotton and 5 % madder dye. The highest weight loss of 13.74 %, which is detected from dyed cotton.
Fig. 12.
Thermogravimetric curve of Mordant A) cotton with 1–5 % of madder dye without Fe2SO4, B) cotton with 1–5 % of madder dye with 3 % of Fe2SO4/Alum, C)cotton with 1–5 % of madder dye with 4 % of Fe 2SO4/Alum, D) cotton with 1–5 % of madder dye with 3 % of Fe2SO4/Alum, E) cotton with 1–5 % of madder dye with 6 % of Fe2SO4/Alum and F) cotton with 1–5 % of madder dye with 7 % of Fe2SO4/Alum.
The second stage of degradation observed at the temperature range between 100 and 250 °C. This decomposition starts from cellulose and madder dye. At this temperature range lowest weight loss observed of 3.25 % in dyed cotton with 5 % of madder dye. The highest weight loss observed of 9.55 % in dyed cotton with 2 % of madder dye composition. Third stage of weight loss observed from at the temperature range between 250 and 500 °C. At this temperature weight loss which confirms the complete decomposition of backbone of the cellulose and madder dye molecular structure. The maximum residue observed from dyed cotton with 5 % of madder dye combination and the remaining residue weight obtained of 6.11 % which is confirms the madder dye was thermally more stable.
4.8.2. Thermogravimetric studies of madder dyed sample with 3 % mixed mordant (Fe2SO4 + pot. Alum)
Thermogravimetric analysis of various madder dye concentration from 1 % to 5 % with mordant used in the presence of 3 % Fe2SO4/Alum. The weight loss thermogram are shown in Fig. 12B. The first stage degradation obtained at temperature range between 30 and 100 °C due to loss of moisture present in the material. The minimum weight loss observed at this temperature range is 11.14 % in mordant cotton with combination of 5 % madder dye and 3 % of 3 % Fe2SO4/Alum solution. The maximum weight loss observed of 14.74 % in mordant cotton with the combination of 1 % madder dye and 3 % of 3 % Fe2SO4/Alum solution. The second stage degradation observed at temperature from 100 to 250 °C. At this temperature weight loss observed due to loss of cellulose and madder dye starts to decomposition in this range, the lowest weight loss observed from 7.63 % in mordant cotton with the composition of 3 % madder dye and 3 % of mixed mordanted solution. Similarly, the maximum weight loss observed from mordant cotton with the chemical composition of 4 % madder dye and 3 % of Fe2SO4 solution.
Third stage of degradation shown at temperature range between 250 and 500 °C due to complete decomposition of cellulose and madder dye backbone of the molecule. The minimum weight loss observed of 45.37 % in mordant cotton with the combination of 1 % madder dye and 3 % of Fe2SO4 solution. The maximum weight loss observed of 54.97 % in mordant cotton with the combination of 5 % madder dye and 3 % of Fe2SO4 solution.
The mordant cotton with the combination of 4 % madder dye and 3 % of Fe2SO4 solution takes places a complete decomposition done at the temperature of 320 °C. The maximum residue observed of 27.77 % from mordant cotton with the combination of 1 % madder dye and 3 % of Fe2SO4solution. This composition is thermally more stable than the other composition.
4.8.3. Thermogravimetric studies of madder dyed sample with 4 % mixed mordant (Fe2SO4 + pot. Alum)
The thermogravimetric analysis mordant cotton with the combination of various madder dye concentration from 1 % to 5 % with 4 % of 3 % Fe2SO4/Alum solution. Thermogram and weight loss values of pristine are shown in Fig. 12C. Three stage degradation observed from this combination of mordant cotton. First stage observed at temperature between 30 and 100 °C due to loss of moisture present in the various concentration of madder dye absorbed mordant cotton with combination of 4 % 3 % Fe2SO4/Alum solution. The minimum weight loss observed of 5.68 % in mordant cotton with the combination of 3 % madder dye with 4 % of mixed mordanted solution. The maximum weight loss observed of 20.06 % from 2 % of madder dye concentration with 4 % mixed mordanted solution. Second stage of decomposition observed at temperature range between 100 and 250 °C due to cellulose and madder dye molecule starting to decomposed. At this temperature the lowest weight loss observed from mordant cotton with the combination of 1 % madder dye with 4 % mixed mordanted solution which decomposed value found to be 14 %.
Third stage degradation found to be at temperature range between 250 and 500 °C due to complete decomposition of madder dye and cellulose molecular backbone. The minimum decomposition observed of 37.74 % from mordant cotton with combination of 1 % madder dye with 4 % mixed mordanted solution. The maximum weight loss observed of 63.47 % in the mordant cotton with the combination of 3 % madder dye with 4 % of mixed mordanted material which have completely decomposed at the temperature of 450 °C. The maximum residue (30.08 %) present in the mordant cotton with the combination of 1 % madder dye with 4 % mixed mordanted solution. This combination of material thermally more stable than the other combination.
4.8.4. Thermogravimetric studies of madder dyed sample with 5 % mixed mordant (Fe2SO4 + pot. Alum)
From the thermogram reveals that the three stage degradation appear which shown in Fig. 12D. The first stage degradation appear at temperature range between 30 and 100 °C due to loss of moisture present in the material. At this temperature range the lowest weight loss observed of 9.65 % in mordant cotton with the combination of 3 % madder dye and 5 % of 3 % Fe2SO4/Alum solution. The maximum weight loss observed of 14.51 % with the combination of 4 % madder dye with 5 % of mixed mordanted solution. The second stage of degradation obtained at temperature range between 100 and 250 °C due to loss of cellulose and madder dye molecule starts too decomposed. The lowest weight loss observed of 8.63 % in mordant cotton with the combination of 4 % madder dye with 5 % of mixed mordanted solution. The maximum weight loss found to be 25.11 % in mordant cotton with the combination of 3 % madder dye with 5 % of mixed mordanted solution.
Third stage decomposition observed at temperature between 250 and 500 °C due to complete decomposition of cellulose and madder dye molecule. The minimum weight loss observed of 31.79 % in mordant cotton with the combination of 5 % madder with 5 % of mixed mordanted solution. The maximum weight loss found to be 64.33 % in mordant cotton with 3 % madder with 5 % of mixed mordanted solution. This combination of madder dye with 5 % of mixed mordanted cotton has complete decomposition at 450 °C and the maximum residue has observed of 42.45 %. This composition is more stable than the other combination of absorbed mordant cotton.
4.8.5. Thermogravimetric studies of madder dyed sample with 6 % mixed mordant (Fe2SO4 + pot. Alum)
The thermogravimetric analysis of mordant cotton with various concentration of madder dye (1 %–5 %). Three stage degradation has appears and this thermogram are shown in Fig. 12E. First stage degradation observed at temperature range between 30 and 100 °C due to loss of moisture present in the materials. The lowest weight loss observed of 7.63 % in mordant cotton with the combination of 5 % madder dye and 6 % of Fe2SO4/Alum solution. Similarly, the maximum weight loss (17.51 %) found to be mordant cotton with the combination of 2 % madder dye with 6 % of mixed mordanted solution. Second stage degradation appear at temperature range between 100 and 250 °C due to decomposition of cellulose and madder dye molecule. The lowest weight loss (7.63 %) observed from mordant cotton with the combination of 5 % madder dye with 6 % of mixed mordanted solution. The maximum weight loss obtained of 17.59 % in mordant cotton with the chemical composition of 2 % madder dye with 6 % of mixed mordanted solution. Third stage degradation observed at temperature range between 250 and 500 °C due to complete decomposition of cellules and madder dye molecules. The lowest weight loss of 22.34 % observed of mordant cotton with the combination of 5 % madder dye with 6 % of mixed mordanted solution. The highest weight loss observed of 40.19 % in mordant cotton with the chemical composition of 1 % madder dye with 6 % of mixed mordanted solution with the residue of 39.20 %. The maximum residue of 39.20 % obtained from mordant cotton with the chemical composition of 2 % madder dye with 6 % of mixed mordanted. This composition is thermally more stable than the other combination.
4.8.6. Thermogravimetric studies of madder dyed sample with 7 % mixed mordant (Fe2SO4 + pot. Alum)
Thermogravimetric analysis of mordant cotton with the combination of various madder dye concentration 1–5 % and Fe2SO4 solution, in this combination three stage degradation has been observed. This thermogram are shown in Fig. 12F. First stage of degradation observed at temperature range between 30 and 100 °C due to loss of moisture present in the molecule. The lowest weight loss of 9.41 % has observed from mordant cotton with the combination of 5 % of madder dye and 7 % of Fe2SO4/Alum solution. Highest weight loss of 13.17 % has obtained from mordant cotton with the combination of 1 % madder dye with 7 % of mixed mordanted solution. The second stage of degradation are observed at temperature range between 100 and 250 °C due to decomposition starting of cellulose and madder dye molecule. At this temperature range the lowest weight loss of 12.29 % has observed from mordant cotton with the combination of 5 % of madder dye 7 % of mixed mordanted solution.
The highest weight loss of 17.67 % has observed of mordant cotton with the combination of 2 % of madder dye 7 % of mixed mordanted solution.Third stage degradation has been observed from 250 to 500 °C due to complete decomposition of madder dye and cellulose molecule. The lowest decomposition weight of 22.61 % which exhibits the mordant cotton with the combination of 2 % madder dye with 7 % of mixed mordanted solution.
5. Conclusion
Natural impurities and colouring matters from the woven cotton fabric, such as hemicelluloses, pectin, wax, and colouring matters, which have been removed through the scouring and bleaching process. The dyeing mechanism of madder dye on cotton in different pH using FTIR spectroscopy and UV–Vis spectroscopy have been studied. The fabric in different concentration between 1 % and 5 % without mordanting condition have been dyed. The fabric in different concentrations between the ranges of 1 %–5 % using a single mordant (ferrous sulphate mordant) concentration has been dyed successfully.The fabric in different concentrations between the ranges of 1 %–5 % using mixed mordant (ferrous sulphate mordant and Alum) concentration has been dyed successfully.The colour strength of the dyed samples due to the impact of the different mordanting condition have been analyzed successfully.Correlation analysis between the colour strength with residue % in TGA analysis has been analyzed.Correlation analysis between the Antimicrobial activities with residue % in TGA analysis has been analyzed.Finally, the molecular interaction between the dye and the fibre has been studied theoretically. The values discussed in the theoretical study may be used for future studies with physically dyed samples for checking the trend pattern obtained theoretically. Suggested future studies will help to formulate optimum and economically sustainable natural dye process especially using madder dyes for obtaining fast colours.
The adsorption of madder dye in the basic medium is due to the formation of dianion which is provided through UV–Visible spectroscopy. ATR FT-IR spectra confirmed the molecular orientation of the cellulose structure before and after adsorption. The absorption peak values are slightly shifted which confirms the madder dye is absorbed on the cellulose structure. This may be further confirmed through theoretical calculation through molecular dynamic simulation using BIOVIA material studio software. The HOMO and LUMO values are–2.78 and −6.40 observed respectively.
Data availability statement
Data included in the article.
CRediT authorship contribution statement
B. SenthilKumar: Project administration, Conceptualization. Sundar M: Writing – original draft, Investigation, Formal analysis. Ramasubbu P: Resources. . Dominic J: Software, Investigation. Gowri Shannkari B: Resources. Chitra Devi S: Writing – review & editing.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:Dr.B.Senthil kumar reports equipment, drugs, or supplies was provided by The central instrumentation facility of Gandhigram rural institute.Dr.B.Senthil kumar.
Contributor Information
B. SenthilKumar, Email: senthil.b1980@gmail.com.
Sundar M, Email: msundarmtech@gmail.com.
Ramasubbu P, Email: racprabha@yahoo.com.
Dominic J, Email: dominiccharleschemist85@gmail.com.
Gowri Shannkari B, Email: gowrishannakaribalaji16@gmail.com.
Chitra Devi S, Email: chitradevi1986@gmail.com.
References
- 1.Boominathan S., Magesh G., Balakrishnan S. Antimicrobial and Mosquito Repellent finish on cotton fabric using Coleus Aromaticus Leaf extract. J. Nat. Fibers. 2021:1–11. doi: 10.1080/15440478.2020.1870620. [DOI] [Google Scholar]
- 2.Sanches R.A. Organic cotton, lyocell and SPF: a comparative study. Int. J. Cloth. Sci. Technol. Sep. 2015;27(5):692–704. doi: 10.1108/IJCST-07-2014-0090. [DOI] [Google Scholar]
- 3.Boominathan S., Karthi V., Balakrishanan S. Optimization of process parameters on color strength and antimicrobial activities of cotton fabric dyed with Rubia cordifolia extract. J. Nat. Fibers. 2020 doi: 10.1080/15440478.2020.1818347. [DOI] [Google Scholar]
- 4.Senthil Kumar B., Patchiyappan K.M., Senthil Kumar C.B. Process optimization of antimicrobial treatment on tencel knit fabrics using chitosan biopolymer. J. Test. Eval. 2022;49(5):3637–3645. doi: 10.1520/JTE20200382. [DOI] [Google Scholar]
- 5.Sadeghi-Kiakhani M. Eco-friendly dyeing of wool and nylon using madder as a natural dye: kinetic and adsorption isotherm studies. Int. J. Environ. Sci. Technol. 2015;12:2363–2370. doi: 10.1007/s13762-015-0770-9. [DOI] [Google Scholar]
- 6.Yusuf M., Shahid M., Ibrahim M., Ahmad S., Ali M., Mohammad F. Dyeing studies with henna and madder: a research on effect of tin (II) chloride mordant. J. Saudi Chem. Soc. 2015;19:64–72. [Google Scholar]
- 7.Blackburn R.S. 2017. Natural Dyes in Madder (Rubia Spp) and Their Extraction and Analysis in Historical Textiles Coloration Technology; pp. 1–13. [DOI] [Google Scholar]
- 8.Jahangiri A., et al. Natural dyeing of wool by madder (Rubia tinctorum L.) root extract using Tannin-based Biomordants: colorimetric, fastness and tensile assay. Fibers Polym. 2018;19(10):2139–2148. doi: 10.1007/s12221-018-8069-3. [DOI] [Google Scholar]
- 9.Hussain T., Inayat F. Colour gamut of pad-steam dyed cotton fabric from natural extracts of Rubia tinctorum and Rubia cordifolia. J. Text. Eng. Fash. Technol. 2019;5(3):148–152. doi: 10.15406/jteft.2019.05.00195. [DOI] [Google Scholar]
- 10.Jahan N., Datta E. A comparative study on dyeing of cotton and silk fabric using madder as a natural dye. IOSR J. Polym. Text. Eng. 2015;2(2):5–11. doi: 10.9790/019X-0220511. [DOI] [Google Scholar]
- 11.Nishiyama Y., Sugiyama J., Chanzy H., Langan P. Crystal structure and Hydrogen Bonding system in cellulose Iα from Synchrotron X-ray and Neutron fiber Diffraction. J. Am. Chem. Soc. 2003;125(47):14300–14306. doi: 10.1021/ja037055w. [DOI] [PubMed] [Google Scholar]
- 12.Nelson M.L., O'Connor R.T. Relation of Certain Infrared Bands to cellulose Crystallinity and crystal Lattice type . Part II . A New Infrared Ratio for Estimation of Crystallinity in celluloses I and II. J. Appl. Polym. Sci. 1964;8:1325–1341. [Google Scholar]
- 13.Yang Y.P., Zhang Y., Lang Y.X., Yu M.H. Structural ATR-IR analysis of cellulose fibers prepared from a NaOH complex aqueous solution. IOP Conf. Ser. Mater. Sci. Eng. 2017;213(1) doi: 10.1088/1757-899X/213/1/012039. [DOI] [Google Scholar]
- 14.Mohandoss S., Stalin T. Photochemical and computational studies of inclusion complexes between β-cyclodextrin and 1,2-dihydroxyanthraquinones. Photochem. Photobiol. Sci. 2017;16(4):476–488. doi: 10.1039/c6pp00285d. [DOI] [PubMed] [Google Scholar]
- 15.Marzec A., Szadkowski B., Rogowski J., Maniukiewicz W., Szynkowska M.I., Zaborski M. Characteristics of hybrid pigments made from alizarin dye on a mixed oxide host. Materials. 2019;12 doi: 10.3390/ma12030360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Števulova N., Hospodárova V., Eštoková A. Study of thermal analysis of Selected cellulose fibres. Geosci. Eng. 2016;62(3):18–21. doi: 10.1515/gse-2016-0020. [DOI] [Google Scholar]
- 17.Poletto M., Zattera A.J., Forte M.M.C., Santana R.M.C. Thermal decomposition of wood: influence of wood components and cellulose crystallite size. Bioresour. Technol. 2012;109:148–153. doi: 10.1016/j.biortech.2011.11.122. [DOI] [PubMed] [Google Scholar]
- 18.Matheus Poletto V., Pistor I., Zattera A.J. Intech; 2013. Structural Characteristics and Thermal Properties of Native Cellulose; pp. 45–68. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in the article.