Abstract
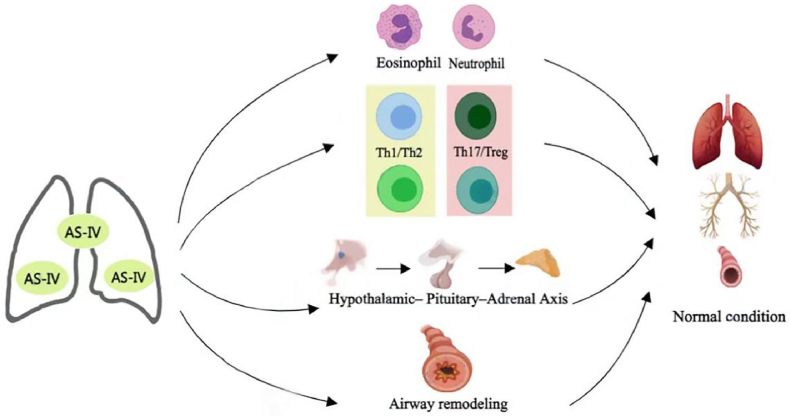
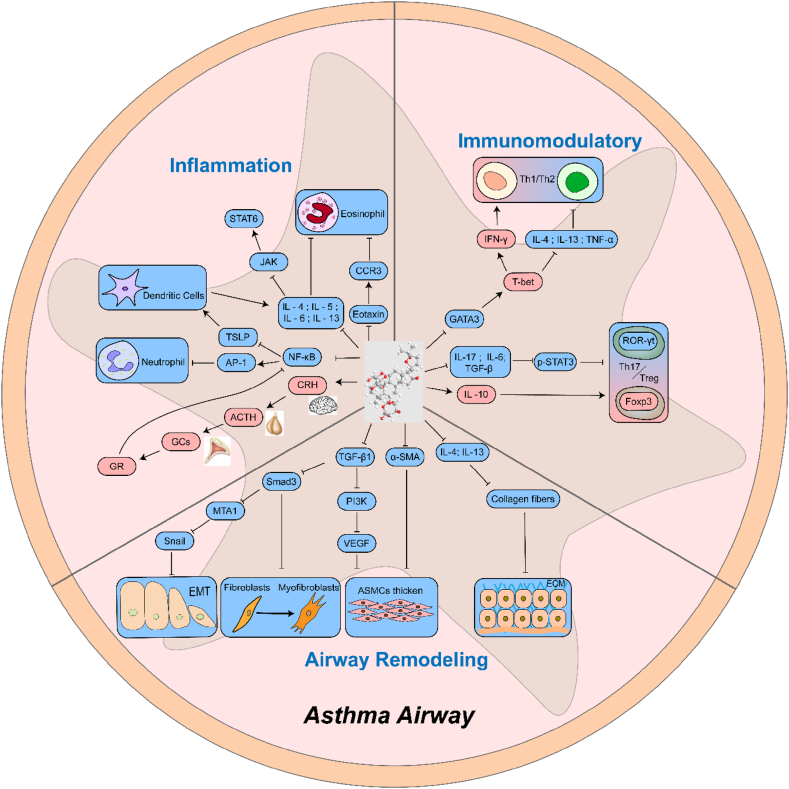
Asthma is a common chronic respiratory disease, and its treatment is a core problem and challenge in clinical practice. Glucocorticoids (GCs) are the first-line therapy for the treatment of asthma. Local and systemic adverse reactions caused by GCs create obstacles to the treatment of asthma. Therefore, the research target is to find a new, safe, and effective therapeutic medicine at present. Natural products are an important source for treating asthma with low cost and low toxicity. Astragaloside IV (AS-IV) is an active ingredient of traditional Chinese medicine Astragalus mongholicus Bunge. Previous studies have indicated that AS-IV plays a therapeutic role in the treatment of asthma by inhibiting airway inflammation and remodeling the airway, and by regulating immunity and neuroendocrine function (Fig. 1) . It has a variety of biological characteristics such as multi-target intervention, high safety, and good curative effect. This article reviews the specific mechanism of AS-IV for the treatment of asthma to provide references for subsequent research.
1. Introduction
Asthma is a highly heterogeneous disease characterized by cough, chest tightness, dyspnea, and wheezing [1]. As chronic respiratory inflammation is dominated by eosinophil infiltration, the basic features of this condition include reversible airflow limitation, airway hyperresponsiveness, and airway remodeling, which are important pathological features [2]. Asthma can be classified as atopic or non-atopic T helper 2 (Th2)–type immune response associated with eosinophilia or a non-Th2 response associated with neutrophilia [3]. As a major global health problem affecting all age groups [4], there are currently about 334 million asthma patients worldwide, and the prevalence is increasing every year [5]. The prevalence of adult asthma in Asia ranges from 0.7 % to 11.9 % (with an average of no more than 5 %), and the average prevalence of asthma has also been increasing in recent years [6]. A survey from February 2010 to August 2012 in eight provinces/cities in seven regions of mainland China indicated that the prevalence rate of asthma among people older than 14 years was 1.24 %, and newly diagnosed asthma patients accounted for 26 % [7]. In 2015, there were approximately 45.7 million asthma patients older than 20 years [8]. Approximately 1000 people worldwide die from asthma every day [9]. A study indicated that only 20.1 % of 8000 asthma patients aged 18–50 years in 11 European countries had achieved asthma control in 2012, and 44.0 % of them had received oral glucocorticoid (GC) therapy [10]. In mainland China, only 28.7 % of 3069 asthma patients achieved asthma control in 2008 [11]. From November 2012 to June 2013, 55.1 % of the patients did not achieve asthma control in the survey of 4125 asthma patients older than 17 years in 34 provinces and cities in mainland China [12]. According to the classification of asthma control level defined by global initiative for asthma, a survey indicated that the overall asthma control rate was only 28.5 % of 3875 asthma patients in outpatient clinics in 30 provinces, cities, and urban areas in China in 2017 [13]. Globally, 40 % of asthma patients have not achieved asthma control [14]. Currently, common treatment methods for asthma include GCs, bronchodilators, leukotriene modulators, and biologically targeted drugs [4]. GCs are the most effective drugs for controlling asthmatic airway inflammation [15]. Long-term and/or systemic use of GCs may cause adverse effects such as osteoporosis, skin atrophy, diabetes, glaucoma, hypertension, and growth retardation in children [16]. Of the patients, 5%–10 % still do not respond well to high-dose inhaled corticosteroids and systemic GCs [17]. The treatment of asthma is encountering challenges, so it is of great significance to find new, safe, and effective treatment drugs.
Traditional Chinese medicine has a history of treating asthma for thousands of years, as recorded in Treatise on Febrile and Other Diseases by Zhang Zhongjing during the Eastern Han Dynasty. Natural products are considered relatively safe compared to chemical products [18]. Due to its low cost and relative safety, the utilization of natural products in clinical research and drug development for treating various diseases has been recommended [19]. For example, recent studies have unveiled that natural products could treat diabetes and improve efficacy when combined with a variety of herbal extracts [20]. Researchers had come up with the idea of combining various herbal extracts to attempt to develop new drugs to treat diabetes [21]. Polyherbal dip tea was developed through this idea, which could treat diabetes by significantly inhibiting alpha-amylase [22]. In addition, ointment prepared from infusion extract of polyherbal tea bag formulation could significantly improve wound healing activity in diabetic and nondiabetic rats [23]. Modern medicine has found that all types of traditional Chinese medicine and its extracts have significant therapeutic effects on asthma [24]. In recent years, animal research on astragaloside IV (AS-IV) in the prevention and treatment of chronic asthma has made progress [25]. The mechanism of AS-IV in the treatment of asthma is elucidated from the aspects of inhibiting airway inflammation and remodeling, and regulating immunity and neuroendocrine function. This article aims to further study the mechanism of AS-IV in the treatment of asthma and the development of safer, more effective, and more affordable drugs. This review is as follows.
2. Distribution and description of plants
AS-IV is a monomer extracted from Astragalus mongholicus Bunge, one of the largest genera of flowering plants in the Leguminosae family. A. mongholicus Bunge is widely distributed in temperate and arid regions. It is an annual or perennial herb, subshrub, or shrub; is usually glabrous, with sharp or hairy stipule and bract margins; is often with minute subsessile glands; and grows in rocky soil [26]. A. mongholicus Bunge was first recorded in Shennong's Classic of the Materia Medica more than 2000 years ago [27,28]. Currently, it is widely used in the treatment of respiratory diseases [29].
3. Physicochemical properties of astragaloside IV
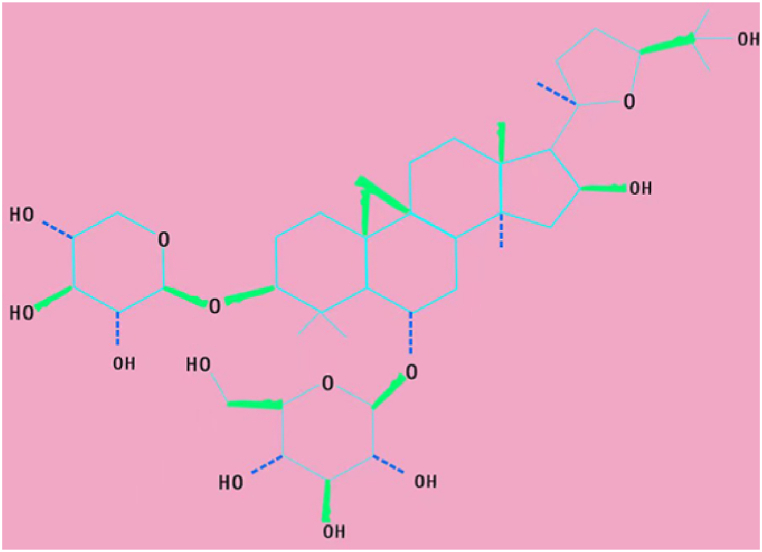
AS-IV (Fig. 2), 3-O-β-d-xylopyranosyl-6-O-β-d-glucopyranosyl-cycloastragenol (C41H68O14), is a lanolin alcohol–type tetracyclic triterpenoid saponin with a relative molecular weight of 784.97. It is a white or light-yellow powder; easily soluble in methanol, ethanol, and acetone; and insoluble in low-polarity organic solvents such as ethyl acetate and chloroform [30]. The limitations of thermal stability and water solubility of AS-IV can be overcome by extraction and separation methods such as ultrafiltration, high-speed centrifugation, reflux, ultrasonic extraction, water extraction, and alcohol precipitation [31]. Cycloastragenol is the active form of AS-IV; it can activate telomerase and lengthen telomeres and has anti-inflammation, anti-oxidation, antivirus, anti-fibrotic, anti–ischemia–hypoxia injury, anti–lipid accumulation, and immunomodulation properties [32].
Fig. 1.
Mechanism of AS-IV in the treatment of asthma. AS-IV plays a therapeutic role in the treatment of asthma by inhibiting airway inflammation and remodeling the airway, and regulating immunity and neuroendocrine function. (Fig. 1 was created with BioRender.com)
Fig. 2.
Chemical structure of AS-IV.
4. Pharmacokinetics of astragaloside IV
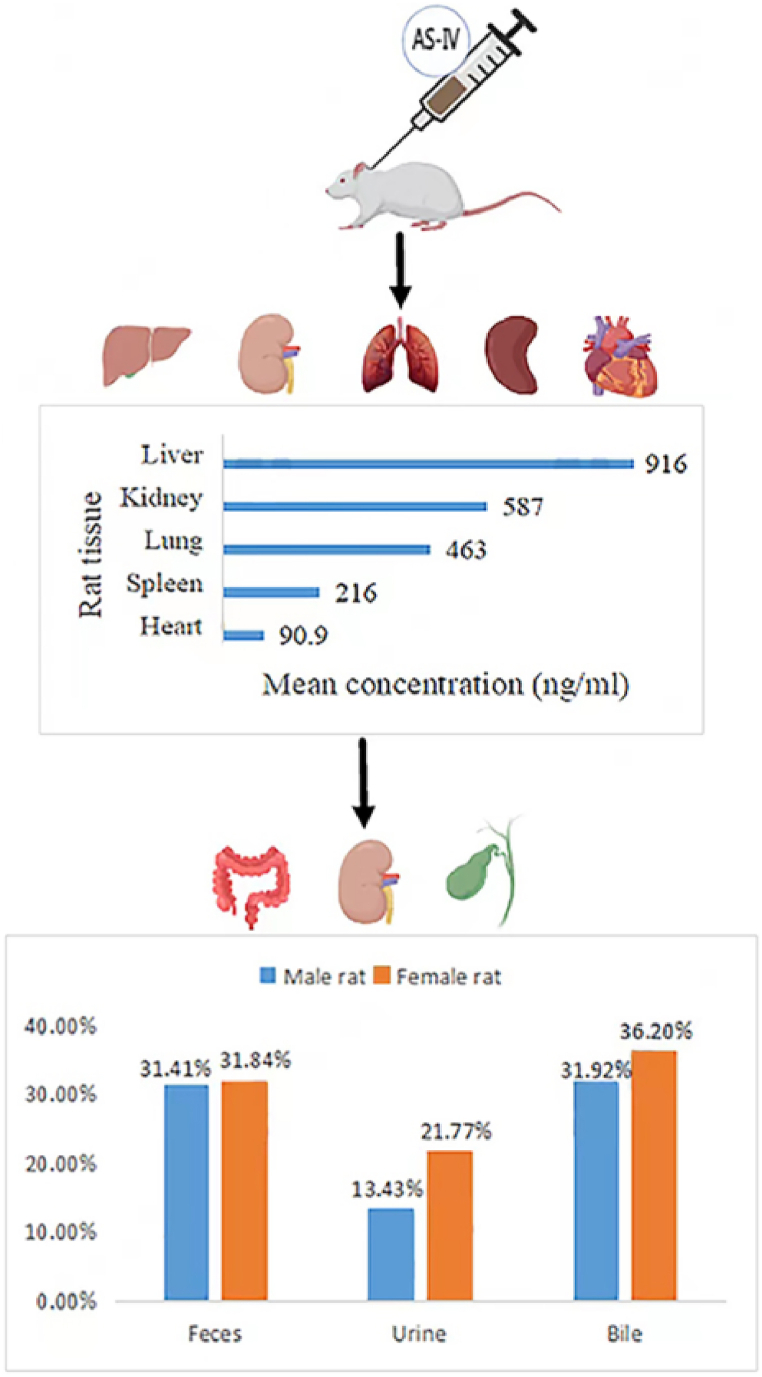
AS-IV has low absorption and bioavailability, the absolute bioavailability of oral administration AS-IV is 3.66 % in rats and only 7.4 % in beagle dogs [33]. This may be related to poor intestinal permeability, high molecular weight, low lipophilicity and its paracellular transport [34]. After oral administration of 10 mg kg−1 astragaloside, the elimination half-life (t1/2) of AS-IV in Beagle dogs was 229.71 min, and the area under curve (AUC) was 204.05 μg h ml−1 [33]. Meanwhile, AS-IV was widely distributed in the heart, liver, spleen, lung, kidney and other organs of rats after intravenous injection [35]. The pharmacokinetics of intravenous injection of AS-IV in rats is presented in Fig. 3. The plasma protein binding rate of AS-IV was about 90 % within a concentration range of 250–1000 ng ml−1 [33]. After intravenous administration of 4 mg kg−1 of AS-IV to rats, the concentrations in the liver, kidney, lung, spleen, and heart tissues were 916 ± 506, 587 ± 301, 463 ± 494, 216 ± 114, and 90.9 ± 45.7 ng ml−1 (mean ± standard deviation, n = 6), respectively [36]. In beagle dogs, after intravenous injection of 0.5, 1 and 2 mg kg−1 of AS-IV, the plasma t1/2 was 177.18, 196.58 and 241.59 min, respectively, and the AUC were 126.24, 276.28 and 724.51 μg h ml−1, respectively [33]. About 50 % of AS-IV is metabolized in the body [37]. It is slowly cleared primarily by the liver and excreted in feces, bile, and urine. The 24-h fecal, urinary, and biliary excretion rates were 31.41 %, 13.43 %, and 31.92 % in male rats and 31.84 %, 21.77 %, and 36.20 % in female rats, respectively [35]. There was a study of intravenous astragaloside in healthy patients, the mean maximum plasma concentration (Cmax) values of AS-IV were 2.12, 3.59, 3.71 and 5.17 g ml−1 after single doses of 200, 300, 400 and 500 ml, respectively. The corresponding mean values of AUC were 4.38, 9.75, 13.59 and 18.22 μg h ml−1, respectively, and the mean values of elimination t1/2 were 2.14, 2.59, 2.62 and 2.69 h, respectively. Cumulative urinary excretion of AS-IV was 3.91 % within 24 h after adminis-tration of 500 ml [38]. A study on rats and dogs injected with Astragalus extract (mainly composed of Astragalus polysaccharides and Astragalus saponins) for 3 months found no obvious side effects [39]. In addition, no significant adverse effects such as hepatotoxicity and nephrotoxicity were observed in rats after oral administration of AS-IV (10 mg kg−1·day−1) for 14 weeks [40]. AS-IV did not accumulate in human plasma after seven consecutive days of intravenous astragaloside injection and was safe and well tolerated after single doses of 200–600 mL and multiple doses of 500 mL over 7 days [38]. These studies prove that AS-IV is a safe drug. Table 1 shows the pharmacokinetics of intravenous astragaloside.
Fig. 3.
The pharmacokinetics process of AS-IV in rats. After intravenous administration of 4 mg/kg of AS-IV to rats, the concentrations in the liver, kidney, lung, spleen, and heart tissues were 916, 587, 463, 216, and 90.9 ng/mL, respectively [36]. It was slowly cleared primarily by the liver and excreted in feces, bile, and urine. The 24-h fecal, urinary, and biliary excretion rates were 31.41 %, 13.43 %, and 31.92 % in male rats and 31.84 %, 21.77 %, and 36.20 % in female rats, respectively [35]. (Fig. 3 was created with BioRender.com)
Table 1.
Intravenous astragaloside pharmacokinetics.
| Parameters | Dose | AUC0 →∞ | t1/2 | Cmax | MRT | CL |
|---|---|---|---|---|---|---|
| Male Rats [35] | 0.75 mg kg | 289 μg min·ml−1 | 98.1min | 3.79 μg ml−1 | 122min | 0.005 l kg·min−1 |
| 1.5 mg kg | 402 μg min·ml−1 | 34.0min | 6.98 μg ml−1 | 95.2min | 0.007 l kg·min−1 | |
| 3 mg kg | 520 μg min·ml−1 | 67.2min | 7.79 μg ml−1 | 98.7min | 0.006 l kg·min−1 | |
| Female Rats [35] | 0.75 mg kg | 153 μg min·ml−1 | 66.9 min | 5.18 μg ml−1 | 47.4min | 0.005 l kg·min−1 |
| 1.5 mg kg | 298 μg min·ml−1 | 71.8min | 4.80 μg ml−1 | 90.1min | 0.003 l kg·min−1 | |
| 3 mg kg | 551 μg min·ml−1 | 131.6min | 7.24 μg ml−1 | 149min | 0.003 l kg·min−1 | |
| Beagle dogs [33] | 0.5 mg kg | 126.24 μg min·ml−1 | 177.18min | – | 222.61min | 0.004 l kg·min−1 |
| 1 mg kg | 276.28 μg min·ml−1 | 196.58min | – | 232.48min | 0.004 l kg·min−1 | |
| 2 mg kg | 724.51 μg min·ml−1 | 241.59min | – | 241.80min | 0.003 l kg·min−1 | |
| Volunteers [38] | 200 ml | 4.38 μg h·ml−1 | 2.14h | 2.12 μg ml−1 | – | – |
| 300 ml | 9.75 ± 1.31 μg h·ml−1 | 2.59 ± 0.25h | 3.59 ± 0.46 μg ml−1 | – | – | |
| 400 ml | 13.59 ± 1.90 μg h·ml−1 | 2.62 ± 0.26h | 3.71 ± 0.32 μg ml−1 | – | – | |
| 500 ml | 18.22 ± 5.60 μg h·ml−1 | 2.69 ± 0.42h | 5.17 ± 1.27 μg ml−1 | – | – |
5. Mechanism of astragaloside IV in the treatment of asthma
Airway inflammation plays an important role in asthma. A variety of inflammatory cells and immune cells are involved in the occurrence and development of asthma (Fig. 4).
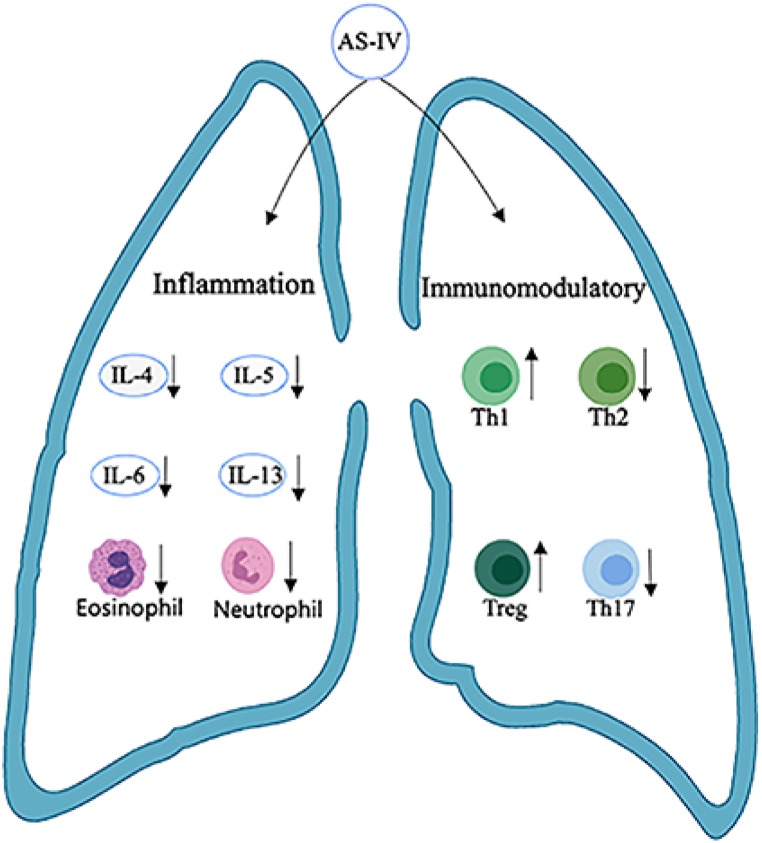
Fig. 4.
Mechanism of AS-IV inhibiting airway inflammation and regulating immunity. AS-IV inhibits airway inflammation by reducing IL-4, IL-5, IL-6, IL-13, eosinophils, and neutrophils. The immunoregulatory mechanism of AS-IV is realized by improving the imbalance of Th1/Th2 cells and Th17 cells/Tregs. (Fig. 4 was created with BioRender.com)
5.1. Mechanism of astragaloside IV in inhibiting airway inflammation
5.1.1. Astragaloside IV can reduce the production of inflammatory cytokine
Asthma is a chronic airway inflammatory disease, and cytokines such as interleukin-4 (IL-4), interleukin-5 (IL-5), and interleukin-13 (IL-13), which are secreted by Th2 cells, are involved in the occurrence of asthma [41]. The janus kinase Janus Kinase/signal transducer and activator of transcription (JAK/STAT) signaling pathway, which consists of the tyrosine kinase JAK family and the transcription factor STAT family, is an important cytokine signaling pathway that plays an essential role in T-cell proliferation and differentiation [42]. IL-4, IL-5, and IL-13 promote T-cell proliferation and differentiation by activating STAT6 and JAK2 signaling pathways [43]. The anti-inflammatory effect of AS-IV functions by downregulating the expression of p-JAK2/p-STAT6 protein and inhibiting the activation of the JAK/STAT6 pathway by significantly inhibiting the expression of IL-4, IL-5, interleukin-6 (IL-6), and IL-13 [44]. Thymic stromal lymphopoietin (TSLP) is an inflammatory cytokine that plays a key role in the development of Th2 airway inflammation in asthma [45]. TSLP can activate dendritic cells to promote the initiation and development of Th2 inflammation and activate mast cells to secrete Th2 cytokines such as IL-4, IL-5, and IL-13. However, Th2-related cytokines promote the secretion of TSLP by airway epithelial cells, which further exacerbates airway inflammation [46]. The nuclear factor-kappa B (NF-κB) pathway plays an essential role in the regulation of TSLP expression [47]. Studies have indicated that AS-IV exerts an anti-inflammatory effect by inhibiting the NF-κB pathway and reducing TSLP expression [48,49].
5.1.2. Astragaloside IV can reduce eosinophil infiltration
Eosinophils are considered key cells and therapeutic targets in asthma [50]. Approximately 50 % of patients with asthma have chronic airway inflammation dominated by eosinophil infiltration [51]. Massive infiltration of eosinophils in the airway is closely related to the acute exacerbation and severity of asthma [52]. IL-5 is a key cytokine for eosinophil growth, activation, and survival and, together with IL-4 and IL-13, can enhance the inflammatory response in asthmatic airways [53]. Eotaxin is a member of the CC chemokine family characterized by two adjacent l-cysteines near the N-terminus [54]. CC chemokine receptor 3 (CCR3) is a receptor protein mainly expressed in eosinophils. Eotaxin has the strongest affinity with CCR3. Eotaxin/CCR3 mediates the infiltration of eosinophils in the airways of asthma patients [55]. Eotaxin mediates the migration of eosinophils from blood vessels to inflammatory lung tissues by interacting with CCR3 [56]. The blockade of CCR3 has been shown to result in a reduction of eosinophilic infiltration in animal models of asthma [57]. A study has shown that astragaloside can block CCR3 [58]. We guess that the reason AS-IV can prevent the activation of the eotaxin/CCR3 signaling pathway to reduce the infiltration of eosinophils in the airway of asthmatic mice is that it downregulates the expression of CCR3 protein and reduces cytokines such as IL-4, IL-5, and IL-13 and chemokine eotaxin [57,58].
5.1.3. Astragaloside IV can reduce neutrophil infiltration
Studies have indicated that approximately 50 % of patients with asthma have airway inflammation dominated by non-eosinophilic infiltration, mostly neutrophil infiltration [59]. Neutrophilic asthma accounts for only 5%–20 % of all asthma cases but utilizes more than 50%–80 % of asthma-related medical resources, with high hospitalization rate and mortality rate, critically affecting the lives of asthma patients and becoming a significant burden to society and the public health system [60,61]. It has been indicated that the transcription of inflammatory genes is regulated by the NF-κB signaling pathway, and the upregulation of the NF-κB pathway can cause neutrophil inflammation [62]. AS-IV can reduce neutrophil activation and infiltration by blocking NF-κB- and AP-1-mediated inflammatory signaling pathways, resulting in the inhibition of nuclear translocation of NF-κB, reduction of inflammatory gene expression, and secretion of proinflammatory mediators [63].
5.1.4. Other possible mechanisms by which astragaloside inhibits airway inflammation
The inflammatory mechanism of asthma is complex, and new studies have found that astragaloside may have other mechanisms to inhibit airway inflammation, such as Astragaloside IV ameliorates airway inflammation in an established murine model of asthma by Inhibiting the mTORC1 Signaling Pathway [64].
5.2. Immunomodulatory mechanism of astragaloside IV
5.2.1. Improve T helper 1/Th2 cell imbalance
The initiation and development of asthma is closely related to the involvement of multiple immune cells, with a critical role played by a diverse set of functional helper T cells differentiated from naive CD4+ T cells [65]. It is well-established that allergen-specific Th2 cells play central roles in developing allergic asthma [66]. Previous studies have indicated that asthma is caused by the enhanced differentiation of Th2 cell subsets and the inhibition of T helper 1 (Th1) cell differentiation under the stimulation of allergens [67]. GATA3 is the main transcription factor inducing Th2 cell differentiation, and T-bet mainly regulates Th1 cell differentiation [68]. AS-IV restored the imbalance of Th1/Th2 cells by inhibiting Th2 cell differentiation and enhancing Th1 cell differentiation because AS-IV downregulated the expression of GATA3 in the lung tissues of ovalbumin-induced asthmatic mice and decreased the levels of inflammatory Th2 cytokines such as IL-4, IL-13, and TNF-α in the serum of asthmatic mice, and it also upregulated the expression of T-bet [69,70].
5.2.2. Improve T helper 17 cell/regulatory T cell imbalance
Previous studies have indicated that the pathogenesis of asthma is associated with an immune-mediated imbalance of T helper 17 (Th17) cells and regulatory T cells (Tregs) [71]. Tregs are a new type of CD4+ T cells existing in both humans and mice and were first proposed by Sakaguchi et al. [72]. Th17 cells, which are helper T cells independent of Th1 and Th2 cells, are a novel CD4+ T-cell subset first described by Infante-Duarte in recent years [73]. Studies have indicated that the decrease of Tregs and the increase of Th17 cells are important pathogenesis of asthma [74]. IL-17 is a major effector secreted by Th17 cells promoting the recruitment of inflammatory cells to inflammatory regions and promoting an inflammatory response [75]. IL-6 and transforming growth factor beta (TGF-β) induce initial Th17 cell differentiation and exacerbate asthma by promoting STAT3 phosphorylation and activating its downstream transcriptional regulator RORγt [76,77]. Foxp3, a specific transcription factor of Tregs, plays an important role in regulating the development and function of Tregs [78]. Interleukin-10 is a cytokine with potent anti-inflammatory properties, which can inhibit the synthesis and release of proinflammatory cytokines and inhibit the synthesis of inflammatory cells [79]. The anti-inflammatory effect of AS-IV on restoring Th17 cell/Treg balance is achieved by inhibiting Th17 cell differentiation and promoting Treg differentiation because AS-IV can inhibit inflammatory factors such as IL-17, TGF-β, and IL-6 to reduce the expression of RORγt mRNA and increase the expression of IL-10 and Foxp3 mRNA [69,80].
5.3. Neuroendocrine regulation mechanism of astragaloside IV
5.3.1. Astragaloside IV could regulate the hypothalamic–pituitary–adrenal axis
GCs are the primary drugs for the treatment of asthma [15], and the secretion of GCs is controlled by the hypothalamic–pituitary–adrenal axis (HPAA) [81]. GCs can bind to glucocorticoid receptors (GRs) to inhibit or activate the transcription of genes in the nucleus. GRα is the main mediating receptor that can inhibit NF-κB, STAT family, and other inflammatory factors to exert a strong anti-inflammatory effect in vivo, whereas GRβ mainly plays a negative regulatory role [82,83]. A study have shown that asthmatics with HPAA dysfunction are more prone to asthma triggers and exacerbations [84]. AS-IV reduces airway inflammation in asthma patients by enhancing the function of the HPAA to restore neuroendocrine-immune dysfunction to normal. AS-IV can reduce the downstream inflammatory mediators such as IL-4 and IL-6 by promoting the activation of GCs, increasing the expression of GRα, and inhibiting the dephosphorylation of IκB and NF-κB [83,85].
5.4. Mechanism of astragaloside IV in inhibiting airway remodeling
5.4.1. Astragaloside IV inhibits extracellular matrix deposition
Abnormal deposition of extracellular matrix (ECM) proteins is a key factor in the development of tissue remodeling that results in asthma and impaired lung function in these diseases [86]. TGF-β is a multifunctional and pleiotropic growth factor, a superfamily that exhibits three mammalian isoforms (TGF-β1, TGF-β2, and TGF-β3) in humans, with TGF-β1 targeting the ECM. TGF-β1 can induce the formation of fibroblasts and enhance the production of ECM [87]. Previous studies have indicated that TGF-β levels are significantly increased in patients with airway remodeling asthma [88]. Smad proteins are transcription factors that regulate gene expression, and Smad3 signaling is required for allergen-induced airway remodeling and accumulation of airway myofibroblasts [89]. TGF-β1 promotes airway fibrosis and collagen deposition by regulating the cytokine Smad protein [90]. AS-IV reduces ECM deposition by blocking the TGF-β1/Smad3 signaling pathway and inhibiting collagen expression in fibroblasts and myofibroblast transformation [91].
5.4.2. Astragaloside IV inhibits airway epithelial-to-mesenchymal transition
Epithelial-to-mesenchymal transition (EMT), a key step in airway remodeling, is accompanied by a decrease in epithelial marker E-cadherin; an increase in mesenchymal markers such as N-cadherin, vimentin, Fnl, α-SMA; and the activation of the transcription factors Snail and Twist in fibroblast proliferation [92]. It can lead to decreased GC sensitivity and critically affect prognosis [93]. Studies have indicated that TGF-β1 promotes EMT in the asthmatic airway by regulating the Smad3 signaling pathway [94]. AS-IV reverses the formation of pulmonary EMT by regulating the TGF-β1/Smad3 signaling pathway, resulting in the inhibition of the expression of MTA1 and Snail [95].
5.4.3. Astragaloside IV inhibits the proliferation of airway smooth muscle cells
Thickening of airway smooth muscle (ASMC) is another important feature of airway remodeling in asthma. Excessive proliferation of airway smooth muscle cells promotes irreversible airflow obstruction and airway hyperresponsiveness, modifies the extracellular matrix, promotes the production of inflammatory factors, and finally accelerates the process of airway remodeling [95]. α-SMA is a characteristic marker of airway smooth muscle [68,96]. Vascular endothelial growth factor (VEGF) is involved in angiogenesis and increases airway wall thickness, which is closely related to airway remodeling in asthma [97]. Studies have indicated that the thickening of airway smooth muscle is characteristic of VEGF overexpression in asthmatic mouse models [98]. TGF-β1 can induce the proliferation of airway smooth muscle cells and, at the same time, promote the release of VEGF from airway smooth muscle cells through the PI3K pathway [99]. Studies have confirmed that AS-IV inhibits the proliferation of airway smooth muscle cells by downregulating the expression of TGF-β1, VEGF, and α-SMA in ovalbumin-induced asthmatic mice [100,101].
5.4.4. Astragaloside IV inhibits the proliferation of reticular basement membrane
Thickening of the basement membrane is an important feature of airway remodeling, which occurs mainly in the reticular layer, called the reticular basement membrane (RBM) [102]. This is called subepithelial fibrosis, which is caused by the increased production of type I, III, and IV collagen fibers by underlying connective tissue cells [103]. Subepithelial fibrosis is strongly associated with asthma severity and decreased lung function [104]. IL-4 and IL-13 are considered key mediators of tissue fibrosis in asthma pathophysiology [105]. TGF-β1 is a pro-fibrotic cytokine that induces fibroblasts to proliferate and differentiate into myofibroblasts and promotes extracellular matrix synthesis [99]. TGF-β1 expression correlated with the degree of subepithelial fibrosis, and TGF-β1 levels were significantly elevated in severe asthma patients with significant eosinophilic airway inflammation [106]. Studies have indicated that AS-IV can reduce collagen deposition and subepithelial fibrosis by downregulating the expression of TGF-β1 and the levels of IL-4 and IL-13 in lung tissues [100,101].
In conclusion, AS-IV plays a therapeutic role in the treatment of asthma by inhibiting airway inflammation and remodeling, and regulating immunity and neuroendocrine function (Fig. 5).
Fig. 5.
Mechanism of AS-IV inhibiting airway inflammation and remodeling, and regulating immunity and neuroendocrine function. AS-IV inhibits and alleviates airway inflammation in asthma by preventing the activation of JAK/STAT6, NF-κB, and eotaxin/CCR3 signaling pathways and inhibiting the generation of inflammatory factors such as IL-4, IL-5, IL-6, and IL-13. AS-IV improves Th1/Th2 cell and Th17 cell/Treg imbalance by preventing the activation of GATA3 and STAT3 signaling pathways, respectively. AS-IV can reduce airway remodeling by downregulating the expression of TGF-β1 and α-SMA and reducing the production of inflammatory factors such as IL-4 and IL-13.
5.5. Other possible mechanisms of astragaloside IV therapy for asthma
The mechanism of AS-IV in the treatment of asthma has not been fully studied currently. In addition, new research has found that the antioxidant effects of AS-IV can reduce lung damage in asthmatic mice. For example, AS-IV can ameliorate the lesions caused by bronchitis in rats through activating the Nrf2/Keap1 pathway, which plays a protective role on anti-oxidative stress injury [107]. AS-IV alleviates PM2.5-caused lung toxicity by inhibiting inflammasome-mediated pyroptosis via NLRP3/caspase-1 axis inhibition in mice [108].
6. Conclusion and future perspectives
Asthma is a major global health problem affecting all age groups with increasing incidence. Traditional treatment methods have drug resistance and adverse reactions. Hence, there is an urgent need to find new treatment methods. Natural products are valuable sources of new medicines. AS-IV is a known Astragalus extract with outstanding properties. Previous studies have indicated that AS-IV has a significant effect on the treatment of asthma. AS-IV plays a therapeutic role in the treatment of asthma by inhibiting airway inflammation and remodeling, and regulating immunity and neuroendocrine function. This article reviews the effects and mechanism of AS-IV in the treatment of asthma to provide a reference for subsequent research.
However, some issues warrant further clarification in future research before the clinical usage of this natural compound. First, because of the multi-target nature of AS-IV, its mechanism for the treatment of asthma has not been fully elucidated and hence needs additional validation by more studies in the future. Through network pharmacology, research has demonstrated that traditional medicines indicated a synergistic effect through multiple targets and pathways [109]. Therefore, we propose to explore possible targets of AS-IV through network pharmacology and to search for drugs or natural products that can act synergistically with AS-IV. We hope to create a successful combination of asthma medications. Therefore, we propose to explore possible targets of AS-IV through network pharmacology and to search for drugs or natural products that can act synergistically with AS-IV. We hope to create a successful combination of asthma medications.
Second, most of the existing studies focus on the cellular level, and there is a dearth of corresponding clinical data to evaluate the efficacy and corresponding dose in humans, which should be investigated further.
Third, the pharmacokinetics of AS-IV in humans has not been fully elucidated. The metabolites of AS-IV in the body also need to be further examined. How metabolites interact with AS-IV requires further investigation for an in-depth understanding of their biological activities. Therefore, we recommend more in vivo studies to understand the metabolism of AS-IV.
Fourth, Low absorption and bioavailability of AS-IV limits clinical use. Intravenous injections may avoid this drawback. However, its paracellulartransport may alsobe the major direction to explore the absorption enhancement strategy of AS-IV, We have found that chitosan and sodium deoxycholate can be used as absorption enhancers based on its transport mechanism [34]. Meanwhile, various novel drug delivery systems such as nanoemulsions, nanostructured lipid carriers and self-microemulsifying drug delivery system are constantly being investigated, and the above novel drug carriers can improve the solubility and bioavailability of astragaloside to a certain extent [110]. In addition, exosomes are extracellular vesicles secreted by a variety of living cells and are natural nanocarriers. Almost all exosomes released by cells will eventually enter the blood circulatory system or be absorbed by other cells [111]. Exosomes are being extensively studied as novel drug carriers. Delivery of AS-IV through exosomes can effectively improve drug activity and bioavailability, and is expected to become a therapeutic method for future clinical research.
In summary, many studies have revealed that AS-IV can treat asthma, and more in vivo and in vitro studies are needed to further confirm the specific mechanism of AS-IV in the treatment of asthma. It is hoped that with more in-depth research, the therapeutic role of AS-IV in the treatment of asthma will be clinically and widely accepted.
Authors’ contributions
Qingsong Huang and Chuantao Zhang provided the article ideas. Fanyi Yuan searched the literature, wrote the first draft of the manuscript. Yang Yang draught mechanism diagrams. Li Liu processed some information on medicinal chemistry. Yi Zhu and Yilu Chai are responsible for processing the form, Pengcheng Zhou, Keling Chen and Wenjun Tang contributed to the manuscript revision. All authors read and approved the submitted version.
Data availability statement
No data was used for the research described in the article.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The is work is funded by The National Natural Science Foundation of China (82374291); 2022 “Tianfu Qingcheng Plan” Tianfu Science and Technology Leading Talents Project (Chuan Qingcheng No. 1090); The National TCM Clinical Excellent Talents Training Program (National TCM Renjiao Letter [2022] No. 1); “100 Talent Plan” Project of Hospital of Chengdu University of Traditional Chinese Medicine(Hospital office [2021] 42); Special subject of scientific research of Sichuan Administration of Traditional Chinese Medicine (2021MS093, 2021MS539, 2023MS608); Sichuan Philosophy and Social Science Planning Major Programs (SC22ZD010).
Contributor Information
Qingsong Huang, Email: qs_huang@126.com.
Chuantao Zhang, Email: zhangchuantao@cdutcm.edu.cn.
Abbreviations
- t1/2
Half-life
- AUC
Area under curve
- MRT
Mean retention time
- CL
Total clearance
- Cmax
Mean maximum plasma concentration
- GCs
Glucocorticoids
- AS-IV
Astragaloside IV
- Th2 cells
T helper 2 cells
- IL-4
Interleukin-4
- IL-5
Interleukin-5
- IL-13
Interleukin-13
- JAK
Janus Kinase
- STAT
Signal transducer and activator of transcription
- IL-6
Interleukin-6
- IL-10
Interleukin-10
- TSLP
Thymic stromal lymphopoietin
- NF-κB
Nuclear factor-kappa B
- CCR3
CC chemokine receptor 3
- Th1 cells
T helper 1 cells
- Th17 cells
T helper 17 cells
- Tregs
Regulatory T cells
- TGF-β
Transforming growth factor beta
- HPAA
Hypothalamic– pituitary–adrenal axis
- GRs
Glucocorticoid receptors
- ECM
Extracellular matrix
- EMT
Epithelial cell-to- mesenchymal transition
- ASMC
Airway smooth muscle
- VEGF
Vascular endothelial growth factor
- RBM
The reticular basement membrane
References
- 1.Reddel H.K., Bacharier L.B., Bateman E.D., et al. Global initiative for asthma strategy 2021. Executive summary and rationale for key changes. Arch. Bronconeumol. 2022;58(1):35–51. doi: 10.1016/j.arbres.2021.10.003. [DOI] [PubMed] [Google Scholar]
- 2.Komlósi Z.I., van de Veen W., Kovács N., et al. Cellular and molecular mechanisms of allergic asthma. Mol. Aspect. Med. 2022;85 doi: 10.1016/j.mam.2021.100995. [DOI] [PubMed] [Google Scholar]
- 3.Michaeloudes C., Abubakar-Waziri H., Lakhdar R., et al. Molecular mechanisms of oxidative stress in asthma. Mol. Aspect. Med. 2022;85 doi: 10.1016/j.mam.2021.101026. [DOI] [PubMed] [Google Scholar]
- 4.Reddel H.K., Bacharier L.B., Bateman E.D., et al. Global initiative for asthma strategy 2021: executive summary and rationale for key changes. J. Allergy Clin. Immunol. Pract. 2022;10(1s):S1–S18. doi: 10.1016/j.jaip.2021.10.001. [DOI] [PubMed] [Google Scholar]
- 5.Papi A., Brightling C., Pedersen S.E., et al. Asthma. Lancet. 2018;391(10122):783–800. doi: 10.1016/S0140-6736(17)33311-1. [DOI] [PubMed] [Google Scholar]
- 6.Song W.J., Kang M.G., Chang Y.S., et al. Epidemiology of adult asthma in Asia: toward a better understanding. Asia Pac Allergy. 2014;4(2):75–85. doi: 10.5415/apallergy.2014.4.2.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin J., Wang W., Chen P., et al. Prevalence and risk factors of asthma in mainland China: the CARE study. Respir. Med. 2018;137:48–54. doi: 10.1016/j.rmed.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 8.Huang K.W., Yang T., Xu J.Y., et al. Prevalence, risk factors, and management of asthma in China: a national cross-sectional study. Lancet. 2019;394(10196):407–418. doi: 10.1016/S0140-6736(19)31147-X. [DOI] [PubMed] [Google Scholar]
- 9.Ramakrishnan R.K., Al Heialy S., Hamid Q. Role of IL-17 in asthma pathogenesis and its implications for the clinic. Expert Rev Respir Med. 2019;13(11):1057–1068. doi: 10.1080/17476348.2019.1666002. [DOI] [PubMed] [Google Scholar]
- 10.Price D., Fletcher M., van der Molen T. Asthma control and management in 8,000 European patients: the REcognise Asthma and LInk to Symptoms and Experience (REALISE) survey. NPJ Prim Care Respir Med. 2014;24 doi: 10.1038/npjpcrm.2014.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Su N., Lin J., Chen P., et al. Evaluation of asthma control and patient's perception of asthma: findings and analysis of a nationwide questionnaire-based survey in China. J. Asthma. 2013;50(8):861–870. doi: 10.3109/02770903.2013.808346. [DOI] [PubMed] [Google Scholar]
- 12.Zhong N., Lin J., Zheng J., et al. Uncontrolled asthma and its risk factors in adult Chinese asthma patients. Ther. Adv. Respir. Dis. 2016;10(6):507–517. doi: 10.1177/1753465816663978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang J.T., Lin W.Q., Xin Z., et al. Survey results of the control level of outpatient bronchial asthma patients in 30 provinces and cities in my country. Chin. J. Tuberc. Respir. Dis. 2017;40(7):494–498. [Google Scholar]
- 14.Agusti A., Fabbri L., Lahousse L., et al. Single inhaler triple therapy (SITT) in asthma: systematic review and practice implications. Allergy. 2022;77(4):1105–1113. doi: 10.1111/all.15076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reddel H.K., Bateman E.D., Schatz M., et al. A practical guide to implementing SMART in asthma management. J. Allergy Clin. Immunol. Pract. 2022;10(1S):S31–S38. doi: 10.1016/j.jaip.2021.10.011. [DOI] [PubMed] [Google Scholar]
- 16.Amrani Y., Panettieri R.A., Ramos-Ramirez P., et al. Important lessons learned from studies on the pharmacology of glucocorticoids in human airway smooth muscle cells: too much of a good thing may be a problem. Pharmacol. Ther. 2020;213 doi: 10.1016/j.pharmthera.2020.107589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henderson I., Caiazzo E., McSharry C., et al. Why do some asthma patients respond poorly to glucocorticoid therapy? Pharmacol. Res. 2020;160 doi: 10.1016/j.phrs.2020.105189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hou Y., Wang X.B., Zhang Y., et al. Highland mate: edible and functional foods in traditional medicine for the prevention and treatment of hypoxia-related symptoms. Curr. Opin. Pharmacol. 2021;60:306–314. doi: 10.1016/j.coph.2021.07.018. [DOI] [PubMed] [Google Scholar]
- 19.Atanasov A.G., Zotchev S.B., Dirsch V.M., et al. Natural products in drug discovery: advances and opportunities. Nat. Rev. Drug Discov. 2021;20(5):200–216. doi: 10.1038/s41573-020-00114-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patwekar M., Patwekar F., Mezni A., et al. Assessment of antioxidative and alpha-amylase potential of polyherbal extract. Evid Based Complement Alternat Med. 2022;2022 doi: 10.1155/2022/7153526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quazi A., Patwekar M., Patwekar F., et al. In vitro alpha-amylase enzyme assay of hydroalcoholic polyherbal extract: proof of concept for the development of polyherbal teabag formulation for the treatment of diabetes. Evid Based Complement Alternat Med. 2022;2022 doi: 10.1155/2022/1577957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patwekar M., Quazi A., Faheem I., et al. In vitro inhibitory effect on alpha amylase enzyme by polyherbal dip tea in diabetes. Indo Global J. Pharmaceut. Sci. 2022;12:156–165. [Google Scholar]
- 23.Quazi A., Patwekar M., Patwekar F., et al. Evaluation of wound healing activity (excision wound model) of ointment prepared from infusion extract of polyherbal tea bag formulation in diabetes-induced rats. Evid Based Complement Alternat Med. 2022;2022 doi: 10.1155/2022/1372199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Y., Chen Y.J., Xiang C., et al. Discovery of potential asthma targets based on the clinical efficacy of Traditional Chinese Medicine formulas. J. Ethnopharmacol. 2020;252 doi: 10.1016/j.jep.2020.112635. [DOI] [PubMed] [Google Scholar]
- 25.Yuan X.J., Sun S.Q., Wang S.C., et al. Effects of astragaloside IV on IFN-gamma level and prolonged airway dysfunction in a murine model of chronic asthma. Planta Med. 2011;77(4):328–333. doi: 10.1055/s-0030-1250408. [DOI] [PubMed] [Google Scholar]
- 26.Abd Elrahim Abd Elkader H.T., Essawy A.E., Al-Shami A.S. Astragalus species: phytochemistry, biological actions and molecular mechanisms underlying their potential neuroprotective effects on neurological diseases. Phytochemistry. 2022;202 doi: 10.1016/j.phytochem.2022.113293. [DOI] [PubMed] [Google Scholar]
- 27.Pistelli L., Bertoli A., Lepori E., et al. Antimicrobial and antifungal activity of crude extracts and isolated saponins from Astragalus verrucosus. Fitoterapia. 2002;73(4):336–339. doi: 10.1016/s0367-326x(02)00087-4. [DOI] [PubMed] [Google Scholar]
- 28.Auyeung K.K., Han Q.B., Ko J.K. Astragalus membranaceus: a review of its protection against inflammation and gastrointestinal cancers. Am. J. Chin. Med. 2016;44(1):1–22. doi: 10.1142/S0192415X16500014. [DOI] [PubMed] [Google Scholar]
- 29.Li L., Hou X., Xu R., et al. Research review on the pharmacological effects of astragaloside IV. Fundam. Clin. Pharmacol. 2017;31(1):17–36. doi: 10.1111/fcp.12232. [DOI] [PubMed] [Google Scholar]
- 30.Chen T.Q., Yang P.Y., Jia Y.J. Molecular mechanisms of astragaloside‑ IV in cancer 562 therapy (Review) Int J Mol Med. 2021;47(3) doi: 10.3892/ijmm.2021.4846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ren S., Zhang H., Mu Y., et al. Pharmacological effects of Astragaloside IV: a literature review. J. Tradit. Chin. Med. 2013;33(3):413–416. doi: 10.1016/s0254-6272(13)60189-2. [DOI] [PubMed] [Google Scholar]
- 32.Yu Y., Zhou L., Yang Y., et al. Cycloastragenol: an exciting novel candidate for age-associated diseases. Exp. Ther. Med. 2018;16(3):2175–2182. doi: 10.3892/etm.2018.6501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Q., Zhu L.L., Chen G.G., et al. Pharmacokinetics of astragaloside iv in beagle dogs. Eur. J. Drug Metab. Pharmacokinet. 2007;32(2):75–79. doi: 10.1007/BF03190995. [DOI] [PubMed] [Google Scholar]
- 34.Huang C.R., Wang G.J., Wu X.L., et al. Absorption enhancement study of astragaloside IV based on its transport mechanism in caco-2 cells. Eur. J. Drug Metab. Pharmacokinet. 2006;31(1):5–10. doi: 10.1007/BF03190635. [DOI] [PubMed] [Google Scholar]
- 35.Zhang W.D., Zhang C., Liu R.H., et al. Preclinical pharmacokinetics and tissue distribution of a natural cardioprotective agent astragaloside IV in rats and dogs. Life Sci. 2006;79(8):808–815. doi: 10.1016/j.lfs.2006.02.032. [DOI] [PubMed] [Google Scholar]
- 36.Chang Y.X., Sun Y.G., Li J., et al. The experimental study of Astragalus membranaceus on meridian tropsim: the distribution study of astragaloside IV in rat tissues. J. Chromatogr., B: Anal. Technol. Biomed. Life Sci. 2012;911:71–75. doi: 10.1016/j.jchromb.2012.10.024. [DOI] [PubMed] [Google Scholar]
- 37.Du Y., Zhang Q., Chen G.G., et al. Pharmacokinetics of Astragaloside IV in rats by liquid chromatography coupled with tandem mass spectrometry. Eur. J. Drug Metab. Pharmacokinet. 2005;30(4):269–273. doi: 10.1007/BF03190631. [DOI] [PubMed] [Google Scholar]
- 38.Xu M., Yin J., Xie L., et al. Pharmacokinetics and tolerance of toal astragalosides after intravenous infusion of astragalosides injection in healthy Chinese volunteers. Phytomedicine. 2013;20(12):1105–1111. doi: 10.1016/j.phymed.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 39.Yu S.Y., Ouyang H.T., Yang J.Y., et al. Subchronic toxicity studies of Radix Astragali extract in rats and dogs. J. Ethnopharmacol. 2007;110(2):352–355. doi: 10.1016/j.jep.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 40.Gui D., Guo Y., Wang F., et al. Astragaloside IV, a novel antioxidant, prevents glucose-induced podocyte apoptosis in vitro and in vivo. PLoS One. 2012;7(6) doi: 10.1371/journal.pone.0039824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hou L., Zhu L., Zhang M., et al. Participation of antidiuretic hormone (ADH) in asthma exacerbations induced by psychological stress via PKA/PKC signal pathway in airway-related vagal preganglionic neurons (AVPNs) Cell. Physiol. Biochem. 2017;41(6):2230–2241. doi: 10.1159/000475638. [DOI] [PubMed] [Google Scholar]
- 42.Kiu H., Nicholson S.E. Biology and significance of the JAK/STAT signalling pathways. Growth Factors. 2012;30(2):88–106. doi: 10.3109/08977194.2012.660936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cai B., Cai J.P., Luo Y.L., et al. The specific roles of JAK/STAT signaling pathway in sepsis. Inflammation. 2015;38(4):1599–1608. doi: 10.1007/s10753-015-0135-z. [DOI] [PubMed] [Google Scholar]
- 44.Yang X., Wang F. The effect of astragaloside IV on JAK2-STAT6 signalling pathway in mouse model of ovalbumin-induced asthma. J. Anim. Physiol. Anim. Nutr. 2019;103(5):1578–1584. doi: 10.1111/jpn.13114. [DOI] [PubMed] [Google Scholar]
- 45.Zhou B., Comeau M.R., De Smedt T., et al. Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice. Nat. Immunol. 2005;6(10):1047–1053. doi: 10.1038/ni1247. [DOI] [PubMed] [Google Scholar]
- 46.Rochman Y., Leonard W.J. Thymic stromal lymphopoietin: a new cytokine in asthma. Curr. Opin. Pharmacol. 2008;8(3):249–254. doi: 10.1016/j.coph.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ryu W.I., Lee H., Kim J.H., et al. IL-33 induces Egr-1-dependent TSLP expression via the MAPK pathways in human keratinocytes. Exp. Dermatol. 2015;24(11):857–863. doi: 10.1111/exd.12788. [DOI] [PubMed] [Google Scholar]
- 48.Zhang W.J., Hufnagl P., Binder B.R., et al. Antiinflammatory activity of astragaloside IV is mediated by inhibition of NF-kappaB activation and adhesion molecule expression. Thromb Haemost. 2003;90(5):904–914. doi: 10.1160/TH03-03-0136. [DOI] [PubMed] [Google Scholar]
- 49.Bao K.F., Yu X., Wei X., et al. Astragaloside IV ameliorates allergic inflammation by inhibiting key initiating factors in the initial stage of sensitization. Sci. Rep. 2016;6 doi: 10.1038/srep38241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rose C.E., Jr., Lannigan J.A., Kim P., et al. Murine lung eosinophil activation and chemokine production in allergic airway inflammation. Cell. Mol. Immunol. 2010;7(5):361–374. doi: 10.1038/cmi.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McCann J.R., Mason S.N., Auten R.L., et al. Early-life intranasal colonization with nontypeable Haemophilus influenzae exacerbates juvenile airway disease in mice. Infect. Immun. 2016;84(7):2022–2030. doi: 10.1128/IAI.01539-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mallah N., Rodriguez-Segade S., Gonzalez-Barcala F.J., et al. Blood eosinophil count as predictor of asthma exacerbation. A meta-analysis. Pediatr. Allergy Immunol. 2021;32(3):465–478. doi: 10.1111/pai.13403. [DOI] [PubMed] [Google Scholar]
- 53.Dewson G., Cohen G.M., Wardlaw A.J. Interleukin-5 inhibits translocation of Bax to the mitochondria, cytochrome c release, and activation of caspases in human eosinophils. Blood. 2001;98(7):2239–2247. doi: 10.1182/blood.v98.7.2239. [DOI] [PubMed] [Google Scholar]
- 54.Conroy D.M., Williams T.J. Eotaxin and the attraction of eosinophils to the asthmatic lung. Respir. Res. 2001;2(3):150–156. doi: 10.1186/rr52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Palmqvist C., Wardlaw A.J., Bradding P. Chemokines and their receptors as potential targets for the treatment of asthma. Br. J. Pharmacol. 2007;151(6):725–736. doi: 10.1038/sj.bjp.0707263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pease J.E., Williams T.J. Eotaxin and asthma. Curr. Opin. Pharmacol. 2001;1(3):248–253. doi: 10.1016/s1471-4892(01)00044-3. [DOI] [PubMed] [Google Scholar]
- 57.Ok I.S., Kim S.H., Kim B.K., et al. Pinellia ternata, Citrus reticulata, and their combinational prescription inhibit eosinophil infiltration and airway hyperresponsiveness by suppressing CCR3+ and Th2 cytokines production in the ovalbumin-induced asthma model. Mediators Inflamm. 2009;2009 doi: 10.1155/2009/413270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gu X., Jiang D., Wang Y., et al. Effects of astragaloside IV on eosinophil activation induced by house dust mite allergen. Mol. Med. Rep. 2012;6(1):115–120. doi: 10.3892/mmr.2012.869. [DOI] [PubMed] [Google Scholar]
- 59.Zhang J., Zhu Z., Zuo X., et al. The role of NTHi colonization and infection in the pathogenesis of neutrophilic asthma. Respir. Res. 2020;21(1):170. doi: 10.1186/s12931-020-01438-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Peters M.C., Kerr S., Dunican E.M., et al. Refractory airway type 2 inflammation in a large subgroup of asthmatic patients treated with inhaled corticosteroids. J. Allergy Clin. Immunol. 2019;143(1):104–113. doi: 10.1016/j.jaci.2017.12.1009. e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hansbro P.M., Kim R.Y., Starkey M.R., et al. Mechanisms and treatments for severe, steroid-resistant allergic airway disease and asthma. Immunol. Rev. 2017;278(1):41–62. doi: 10.1111/imr.12543. [DOI] [PubMed] [Google Scholar]
- 62.Wang D., Paz-Priel I., Friedman A.D. NF-kappa B p50 regulates C/EBP alpha expression and inflammatory cytokine-induced neutrophil production. J. Immunol. 2009;182(9):5757–5762. doi: 10.4049/jimmunol.0803861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang W.J., Frei B. Astragaloside IV inhibits NF- κ B activation and inflammatory gene expression in LPS-treated mice. Mediators Inflamm. 2015;2015 doi: 10.1155/2015/274314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jin H., Wang L., Li B., Cai C., Ye J., Xia J., Ma S., Jin H., Wang L., Li B., Cai C., Ye J., Xia J., Ma S. Astragaloside IV ameliorates airway inflammation in an established murine model of asthma by inhibiting the mTORC1 signaling pathway. Evid Based Complement Alternat Med. 2017;2017 doi: 10.1155/2017/4037086. Epub 2017 Oct 25. PMID: 29234390; PMCID: PMC5676443. Evid Based Complement Alternat Med. 2017;2017:4037086. doi: 10.1155/2017/4037086. Epub 2017 Oct 25. PMID: 29234390; PMCID: PMC5676443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Loxham M., Davies D.E., Blume C. Epithelial function and dysfunction in asthma. Clin. Exp. Allergy. 2014;44(11):1299–1313. doi: 10.1111/cea.12309. [DOI] [PubMed] [Google Scholar]
- 66.León B., Ballesteros-Tato A. Modulating Th2 cell immunity for the treatment of asthma. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.637948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Holgate S.T. Innate and adaptive immune responses in asthma. Nat Med. 2012;18(5):673–683. doi: 10.1038/nm.2731. [DOI] [PubMed] [Google Scholar]
- 68.Pang L., Yu P., Liu X., et al. Fine particulate matter induces airway inflammation by disturbing the balance between Th1/Th2 and regulation of GATA3 and Runx3 expression in BALB/c mice. Mol. Med. Rep. 2021;23(5):378. doi: 10.3892/mmr.2021.12017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Huang X., Tang L., Wang F., et al. Astragaloside IV attenuates allergic inflammation by regulation Th1/Th2 cytokine and enhancement CD4(+)CD25(+)Foxp3 T cells in ovalbumin-induced asthma. Immunobiology. 2014;219(7):565–571. doi: 10.1016/j.imbio.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 70.Qiu Y.Y., Zhu J.X., Bian T., et al. Protective effects of astragaloside IV against ovalbumin-induced lung inflammation are regulated/mediated by T-bet/GATA-3. Pharmacology. 2014;94(1–2):51–59. doi: 10.1159/000362843. [DOI] [PubMed] [Google Scholar]
- 71.Cosmi L., Liotta F., Maggi E., et al. Th17 cells: new players in asthma pathogenesis. Allergy. 2011;66(8):989–998. doi: 10.1111/j.1398-9995.2011.02576.x. [DOI] [PubMed] [Google Scholar]
- 72.Sakaguchi S., Sakaguchi N., Asano M., et al. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 1995;155(3):1151–1164. [PubMed] [Google Scholar]
- 73.Infante-Duarte C., Horton H.F., Byrne M.C., et al. Microbial lipopeptides induce the production of IL-17 in Th cells. J. Immunol. 2000;165(11):6107–6115. doi: 10.4049/jimmunol.165.11.6107. [DOI] [PubMed] [Google Scholar]
- 74.Zhou Y., Zhao H., Wang T., et al. Anti-inflammatory and anti-asthmatic effects of TMDCT decoction in eosinophilic asthma through Treg/Th17 balance. Front. Pharmacol. 2022;13 doi: 10.3389/fphar.2022.819728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Monteleone I., Sarra M., Pallone F., et al. Th17-related cytokines in inflammatory bowel diseases: friends or foes? Curr. Mol. Med. 2012;12(5):592–597. doi: 10.2174/156652412800620066. [DOI] [PubMed] [Google Scholar]
- 76.Rincon M., Irvin C.G. Role of IL-6 in asthma and other inflammatory pulmonary diseases. Int. J. Biol. Sci. 2012;8(9):1281–1290. doi: 10.7150/ijbs.4874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tanaka S., Suto A., Iwamoto T., et al. Sox5 and c-Maf cooperatively induce Th17 cell differentiation via RORγt induction as downstream targets of Stat3. J. Exp. Med. 2014;211(9):1857–1874. doi: 10.1084/jem.20130791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xu L., Li J., Zhang Y., et al. Regulatory effect of baicalin on the imbalance of Th17/Treg responses in mice with allergic asthma. J. Ethnopharmacol. 2017;208:199–206. doi: 10.1016/j.jep.2017.07.013. [DOI] [PubMed] [Google Scholar]
- 79.Tang J.F., Guan S.H., Wang Z.G. [Roles of interleukin-10 differentiated dendritic cell of allergic asthma patients in T-lymphocyte proliferation in vitro] Zhonghua Yixue Zazhi. 2012;92(40):2851–2854. [PubMed] [Google Scholar]
- 80.Xu S.Y., Hu X.D., Yang Z.L., et al. Effects of astragaloside Ⅳ on inflammatory response and percentage of peripheral blood Th17 cells in mice with ulcerative colitis. China J. Chin. Mater. Med. 2022;47(2):469–475. doi: 10.19540/j.cnki.cjcmm.20210827.401. [DOI] [PubMed] [Google Scholar]
- 81.Caramori G., Nucera F., Mumby S., et al. Corticosteroid resistance in asthma: cellular and molecular mechanisms. Mol. Aspect. Med. 2022;85 doi: 10.1016/j.mam.2021.100969. [DOI] [PubMed] [Google Scholar]
- 82.Kim S.H., Kim D.H., Lavender P., et al. Repression of TNF-alpha-induced IL-8 expression by the glucocorticoid receptor-beta involves inhibition of histone H4 acetylation. Exp. Mol. Med. 2009;41(5):297–306. doi: 10.3858/emm.2009.41.5.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liu H.S., Shi H.L., Huang F., et al. Astragaloside IV inhibits microglia activation via glucocorticoid receptor mediated signaling pathway. Sci. Rep. 2016;6 doi: 10.1038/srep19137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chida Y., Sudo N., Sonoda J., et al. Early-life psychological stress exacerbates adult mouse asthma via the hypothalamus-pituitary-adrenal axis. Am. J. Respir. Crit. Care Med. 2007;175(4):316–322. doi: 10.1164/rccm.200607-898OC. [DOI] [PubMed] [Google Scholar]
- 85.Fudong Zhao J.D., Xie Jinyu, et al. Effects of Chinese herbs for replenishing shen and strengthening qi on some indexes of neuro-endocrino-immune network in asthmatic rats. Chinese Journal of Integrated Traditional and Wester. 2007;27(8):715–719. [PubMed] [Google Scholar]
- 86.Liu G., Philp A.M., Corte T., et al. Therapeutic targets in lung tissue remodelling and fibrosis. Pharmacol. Ther. 2021;225(8) doi: 10.1016/j.pharmthera.2021.107839. [DOI] [PubMed] [Google Scholar]
- 87.Hyytiäinen M., Penttinen C., Keski-Oja J. Latent TGF-beta binding proteins: extracellular matrix association and roles in TGF-beta activation. Crit. Rev. Clin. Lab Sci. 2004;41(3):233–264. doi: 10.1080/10408360490460933. [DOI] [PubMed] [Google Scholar]
- 88.Al-Alawi M., Hassan T., Chotirmall S.H. Transforming growth factor β and severe asthma: a perfect storm. Respir. Med. 2014;108(10):1409–1423. doi: 10.1016/j.rmed.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 89.Le A.V., Cho J.Y., Miller M., et al. Inhibition of allergen-induced airway remodeling in Smad 3-deficient mice. J. Immunol. 2007;178(11):7310–7316. doi: 10.4049/jimmunol.178.11.7310. [DOI] [PubMed] [Google Scholar]
- 90.Yao Z., Fu Y. Glycyrrhizic acid restrains airway inflammation and remodeling in asthma via the TGF-β1/Smad signaling pathway. Exp. Ther. Med. 2021;21(5):461. doi: 10.3892/etm.2021.9892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li N., Feng F., Wu K., et al. Inhibitory effects of astragaloside IV on silica-induced pulmonary fibrosis via inactivating TGF-β1/Smad3 signaling. Biomed. Pharmacother. 2019;119 doi: 10.1016/j.biopha.2019.109387. [DOI] [PubMed] [Google Scholar]
- 92.Rout-Pitt N., Farrow N., Parsons D., et al. Epithelial mesenchymal transition (EMT): a universal process in lung diseases with implications for cystic fibrosis pathophysiology. Respir. Res. 2018;19(1):136. doi: 10.1186/s12931-018-0834-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sun Z., Ji N., Ma Q., et al. Epithelial-mesenchymal transition in asthma airway remodeling is regulated by the IL-33/cd146 Axis. Front. Immunol. 2020;11:1598. doi: 10.3389/fimmu.2020.01598. no. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fan Q., Jian Y. MiR-203a-3p regulates TGF-β1-induced epithelial-mesenchymal transition (EMT) in asthma by regulating Smad3 pathway through SIX1. Biosci. Rep. 2020;40(2) doi: 10.1042/BSR20192645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Qian W., Cai X., Qian Q., et al. Metastasis-associated protein 1 promotes epithelial-mesenchymal transition in idiopathic pulmonary fibrosis by up-regulating Snail expression. J. Cell Mol. Med. 2020;24(11):5998–6007. doi: 10.1111/jcmm.15062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang B., Tang L., Shi S., et al. Qufeng xuanbi formula ameliorates airway remodeling in asthmatic mice by suppressing airway smooth muscle cell proliferation through MEK/ERK signaling pathway. Evid Based Complement Alternat Med. 2022;2022 doi: 10.1155/2022/1525110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lee K.S., Park S.J., Kim S.R., et al. Inhibition of VEGF blocks TGF-beta1 production through a PI3K/Akt signalling pathway. Eur. Respir. J. 2008;31(3):523–531. doi: 10.1183/09031936.00125007. [DOI] [PubMed] [Google Scholar]
- 98.Hu L., Li L., Zhang H., et al. Inhibition of airway remodeling and inflammatory response by Icariin in asthma. BMC Complement Altern Med. 2019;19(1):316. doi: 10.1186/s12906-019-2743-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Makinde T., Murphy R.F., Agrawal D.K. The regulatory role of TGF-beta in airway remodeling in asthma. Immunol. Cell Biol. 2007;85(5):348–356. doi: 10.1038/sj.icb.7100044. [DOI] [PubMed] [Google Scholar]
- 100.Du Q., Chen Z., Zhou L.F., et al. Inhibitory effects of astragaloside IV on ovalbumin-induced chronic experimental asthma. Can. J. Physiol. Pharmacol. 2008;86(7):449–457. doi: 10.1139/y08-053. [DOI] [PubMed] [Google Scholar]
- 101.Du Q., Gu X.Y., Feng G.Z., et al. [Effects of astragaloside IV on the expressions of transforming growth factor-β1 and thymic stromal lymphopoietin in a murine model of asthma] Zhonghua Yixue Zazhi. 2011;91(44):3139–3142. [PubMed] [Google Scholar]
- 102.Trejo Bittar H.E., Yousem S.A., Wenzel S.E. Pathobiology of severe asthma. Annu. Rev. Pathol. 2015;10:511–545. doi: 10.1146/annurev-pathol-012414-040343. no. [DOI] [PubMed] [Google Scholar]
- 103.Payne D.N., Rogers A.V., Adelroth E., et al. Early thickening of the reticular basement membrane in children with difficult asthma. Am. J. Respir. Crit. Care Med. 2003;167(1):78–82. doi: 10.1164/rccm.200205-414OC. [DOI] [PubMed] [Google Scholar]
- 104.Roche W.R., Beasley R., Williams J.H., et al. Subepithelial fibrosis in the bronchi of asthmatics. Lancet. 1989;1(8637):520–524. doi: 10.1016/s0140-6736(89)90067-6. [DOI] [PubMed] [Google Scholar]
- 105.Zhou L.F., Zhang M.S., Hu A.H., et al. Selective blockade of NF-kappaB by novel mutated IkappaBalpha suppresses CD3/CD28-induced activation of memory CD4+ T cells in asthma. Allergy. 2008;63(5):509–517. doi: 10.1111/j.1398-9995.2007.01580.x. [DOI] [PubMed] [Google Scholar]
- 106.Vignola A.M., Chanez P., Chiappara G., et al. Transforming growth factor-beta expression in mucosal biopsies in asthma and chronic bronchitis. Am. J. Respir. Crit. Care Med. 1997;156(2 Pt 1):591–599. doi: 10.1164/ajrccm.156.2.9609066. [DOI] [PubMed] [Google Scholar]
- 107.Zhang Z., Cheng X., Ge D., et al. Protective effects of astragaloside IV combined with budesonide in bronchitis in rats by regulation of nrf2/keap1 pathway. Med Sci Monit. 2018;24:8481–8488. doi: 10.12659/MSM.911150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Huang D., Shi S., Wang Y., et al. Astragaloside IV alleviates PM2.5-caused lung toxicity by inhibiting inflammasome-mediated pyroptosis via NLRP3/caspase-1 axis inhibition in mice. Biomed. Pharmacother. 2022;150 doi: 10.1016/j.biopha.2022.112978. [DOI] [PubMed] [Google Scholar]
- 109.Yuan H.D., Ma Q.Q., Cui H.Y., et al. How can synergism of traditional medicines benefit from network pharmacology? Molecules. 2017;22(7):1135. doi: 10.3390/molecules22071135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Luo K.P., Jiang Y.J., Xu M.H., Yang W.B., Yang L. Research progress on astragaloside IV-loaded novel drug delivery systems. Chin. Tradit. Herb. Drugs. 2023;54(18):6118–6127. [Google Scholar]
- 111.He J., Ren W.H., Wang W., et al. Exosomal targeting and its potential clinical application. Drug Delivery and Translational Research. 2022;12(10):2385–2402. doi: 10.1007/s13346-021-01087-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.