Abstract
This is a slow‐fast atrioventricular nodal reentrant tachycardia (AVNRT) case wherein the fractionation map‐guided cryoablation of the slow pathway (SP) successfully terminated the tachycardia. In this case, the Advisor™ HD Grid catheter and fractionation map in the EnSite™ X EP system with relatively high‐sensitive settings were useful for detecting the target SP area. Direct AVNRT termination by cryomapping at the fractionated potential area might be a quick and safe ablation strategy, which may provide a new workflow for SP ablation.
Keywords: atrioventricular nodal reentrant tachycardia, cryoablation, fractionation map, slow pathway ablation, three‐dimensional mapping system
Anatomical slow pathway (SP) ablation is a widely accepted strategy for treating typical slow‐fast atrioventricular nodal reentrant tachycardia (AVNRT), but the target SP potential lacks objectivity, which often requires multiple radiofrequency applications. We previously reported that a fractionated potential area highlighted by the LUMIPOINT™ module (RHYTHMIA HDx™, Boston Scientific) in the triangle of Koch (ToK) matched the SP location, and the ablation strategy targeting this area reduced redundant radiofrequency applications. 1 Based on that data, we present a slow‐fast AVNRT case wherein the fractionation map‐guided (EnSite™ X EP system, Abbott Laboratories) cryoablation of the SP successfully terminated the AVNRT.
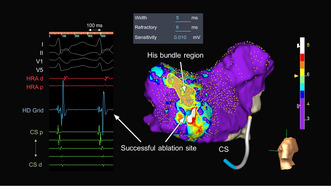
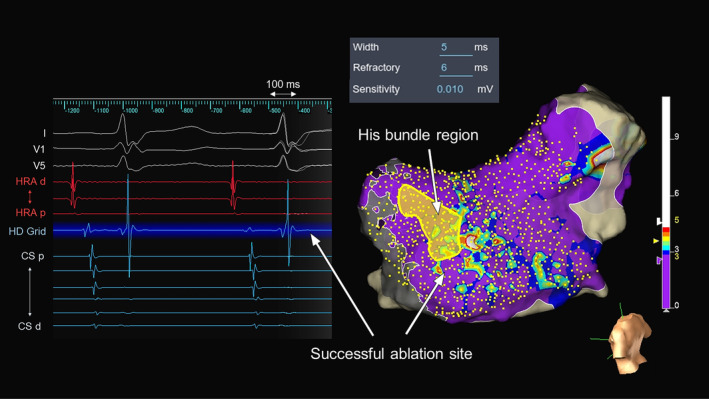
A 56‐year‐old man without overt structural heart disease was referred to our institution for catheter ablation of an adenosine‐sensitive narrow QRS tachycardia (171 beats/min). During sinus rhythm, the atrio‐His (AH) and His‐ventricular (HV) intervals were 70 and 50 ms, respectively. A decrease in the coupling interval of the atrial extrastimulus induced a tachycardia with a right bundle branch block morphology and AH jump during an isoproterenol administration. The tachycardia exhibited a cycle length (CL) of 290 ms, HV interval of 50 ms, and ventriculoarterial interval of −10 ms with the earliest atrial activation in the His‐bundle region (Figure 1A). A scanned single premature ventricular extrastimulus during His‐bundle refractoriness failed to reset the tachycardia. A V‐A‐V response and uncorrected/corrected post‐pacing interval—tachycardia CLs of 216 ms (>115 ms) and 214 ms, respectively, suggested a diagnosis of slow‐fast AVNRT. An ultra‐high‐density three‐dimensional (3D) map in the ToK was created with an EnSite™ X EP system and a high‐density multipolar grid catheter (Advisor™ HD Grid, Sensor Enabled™, Abbott) during slow‐fast AVNRT with the bipolar recording settings filtered between 30 and 300 Hz (Figure 1B). The fractionation map was displayed with fractionated signal settings of the width (5 ms), refractory time (6 ms), and roving sensitivity (0.01 mV). The fractionated potential area with a fractionation threshold of ≥8, highlighted in white, was seen in the mid‐septal region (Figure 2), and cryomapping at −30°C with a 6 mm tip cryoablation catheter (Freezor Xtra, Medtronic) successfully terminated the tachycardia within 14 s in that area (Figure 3). Therefore, we delivered two cryothermal applications at −70°C for 240 s twice at that site, which successfully rendered the AVNRT noninducible.
FIGURE 1.
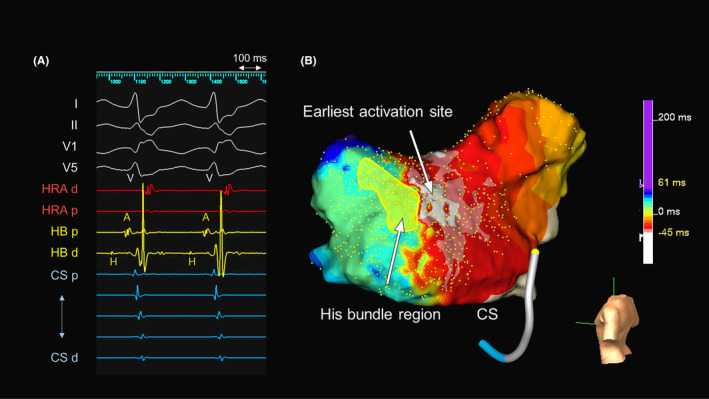
(A) Intracardiac electrograms and (B) an activation map during slow‐fast atrioventricular reentrant tachycardia (AVNRT). A, atrial; CS, coronary sinus; d, distal; H, His bundle; HB, His bundle; HRA, high right atrium; p, proximal; V, ventricle.
FIGURE 2.
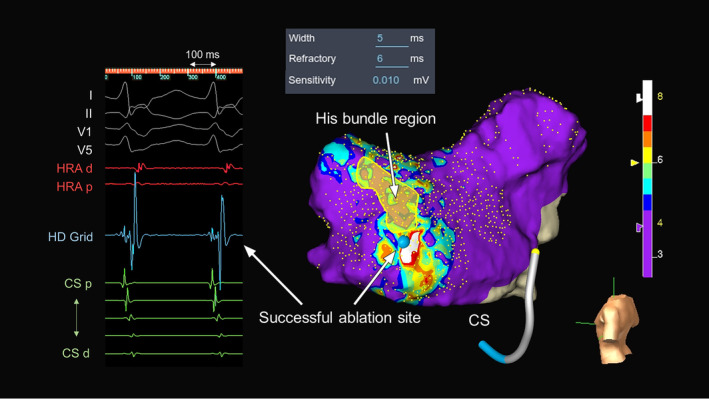
Fractionation map created during tachycardia in the triangle of Koch with the local intracardiac electrograms in the white‐colored area where the most fractionated signals are detected in the map. The color bar colors the higher fractionated potential area (fractionation threshold ≥8) as white and the lower fractionated potential area (fractionation threshold <4) as purple. Fractionation thresholds between 4 and 8 to 4 are colored blue, light blue, green, yellow, orange, and red. CS, coronary sinus; d, distal; HRA, high right atrium; p, proximal.
FIGURE 3.
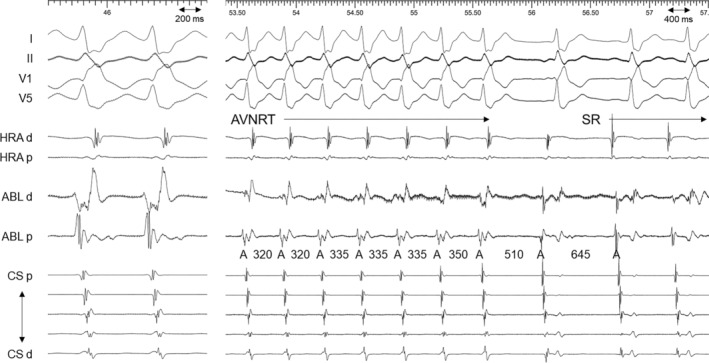
Intracardiac electrograms from a cryoablation catheter just before (left panel) and during the cryothermal application (right panel). The cryothermal energy was delivered in the white‐colored area in the mid‐septal region. A, atrial; ABL, ablation catheter; AVNRT, atrioventricular reentrant tachycardia; CS, coronary sinus; d, distal; HRA, high right atrium; p, proximal; SR, sinus rhythm.
We previously reported that fractionated potential areas with fractionated peaks of ≥8.4 in the LUMIPOINT™ module during slow‐fast AVNRT corresponded to 90% of the successful SP ablation sites, 1 which suggested a potential target of an SP ablation. Previous studies reported that the rightward inferior extension of the SP itself contains a relatively large amount of connexin 43, suggesting relatively fast conduction. 2 Given that, and as in our previous publication, the fractionated potentials may be responsible for the atrial insertion of the SP or transition cells. This setting was originally based on the RHYTHMIA HDx™ Mapping System, but other 3D mapping systems also provide the same features to detect fractionated potentials. In this case, the Advisor™ HD Grid catheter and fractionation map in the EnSite™ X EP system with relatively high‐sensitive settings of the width (5 ms), refractory time (6 ms), and roving sensitivity (0.01 mV) were also useful for detecting the target SP area. 3 The EnSite™ X EP system has improved the suppression of the noise level and acquired 18 532 points during this procedure, which allowed the creation of a reliable fractionation map. A fractionation Map during sinus rhythm was also created, but the successful ablation site did not correspond to the fractionated area (Figure 4). Therefore, it appeared that the fractionated potentials were exclusively acquired during the tachycardia. However, this setting of the fractionation map may need to be adjusted depending on the case. Determining the optimal settings for the fractionation map for the SP would be warranted in further studies. Furthermore, electrophysiologists need to ensure that the Advisor™ HD Grid catheter makes contact with the right atrial tissue in the ToK area in order to record sharp fractionated potentials, but the omnipolar technology of the Advisor™ HD Grid catheter may provide additional information to delineate the SP location showing the propagation direction or conduction speed. 4 Direct AVNRT termination by cryomapping at the fractionated potential area might be a quick and safe ablation strategy to avoid the risk of atrioventricular block, 5 which may provide a new workflow for the SP ablation.
FIGURE 4.
Fractionation map created during sinus rhythm in the triangle of Koch and the local intracardiac electrograms at the successful ablation site. The color bar colors the higher fractionated potential area (fractionation threshold ≥5) as white and the lower fractionated potential area (fractionation threshold <3) as purple. Fractionation thresholds between 3 and 5 to 3 are colored blue, light blue, green, yellow, orange, and red. CS, coronary sinus; d, distal; HRA, high right atrium; p, proximal.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest for this article.
ETHICS APPROVAL STATEMENT
Not applicable.
PATIENT CONSENT STATEMENT
The patient has provided consent for publication.
CLINICAL TRIAL REGISTRATION
Not applicable.
ACKNOWLEDGEMENTS
We appreciate the help in proofreading our manuscript by Mr. John Martin.
Wakamatsu Y, Nagashima K, Watanabe R, Hirata S, Okumura Y. Termination of slow‐fast atrioventricular reentrant tachycardia by a single cryoablation of the slow pathway guided by a fractionation map. J Arrhythmia. 2023;39:969–972. 10.1002/joa3.12932
REFERENCES
- 1. Wakamatsu Y, Nagashima K, Kaneko Y, Mori H, Tsutsui K, Maegaki M, et al. Novel ablation strategy targeting the slow pathway visualized by ultrahigh‐resolution mapping in typical slow‐fast atrioventricular nodal reentrant tachycardia. Circ Arrhythm Electrophysiol. 2023;16(3):e011497. [DOI] [PubMed] [Google Scholar]
- 2. Mazgalev TN, Ho SY, Anderson RH. Anatomic‐electrophysiological correlations concerning the pathways for atrioventricular conduction. Circulation. 2001;103(22):2660–2667. [DOI] [PubMed] [Google Scholar]
- 3. Launer H, Clark T, Dewland T, Henrikson CA, Nazer B. An automated fractionation mapping algorithm for mapping of scar‐based ventricular tachycardia. Pacing Clin Electrophysiol. 2019;42(8):1133–1140. [DOI] [PubMed] [Google Scholar]
- 4. Massé S, Magtibay K, Jackson N, Asta J, Kusha M, Zhang B, et al. Resolving myocardial activation with novel Omnipolar electrograms. Circ Arrhythm Electrophysiol. 2016;9(7):e004107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Okishige K, Okumura K, Tsurugi T, Yotsukura A, Nanbu T, Sugiura H, et al. Japan ablation registry: cryoablation in atrioventricular nodal reentrant tachycardia ("JARCANRET study"): results from large multicenter retrospective investigation. J Interv Card Electrophysiol. 2020;58(3):289–297. [DOI] [PubMed] [Google Scholar]


