Abstract
Background
Traditional risk scores for recurrent atrial fibrillation (AF) following catheter ablation utilize readily available clinical and echocardiographic variables and yet have limited discriminatory capacity. Use of data from cardiac imaging and deep learning may help improve accuracy and prediction of recurrent AF after ablation.
Methods
We evaluated patients with symptomatic, drug‐refractory AF undergoing catheter ablation. All patients underwent pre‐ablation cardiac computed tomography (cCT). LAVi was computed using a deep‐learning algorithm. In a two‐step analysis, random survival forest (RSF) was used to generate prognostic models with variables of highest importance, followed by Cox proportional hazard regression analysis of the selected variables. Events of interest included early and late recurrence.
Results
Among 653 patients undergoing AF ablation, the most important factors associated with late recurrence by RSF analysis at 24 (+/−18) months follow‐up included LAVi and early recurrence. In total, 5 covariates were identified as independent predictors of late recurrence: LAVi (HR per mL/m2 1.01 [1.01–1.02]; p < .001), early recurrence (HR 2.42 [1.90–3.09]; p < .001), statin use (HR 1.38 [1.09–1.75]; p = .007), beta‐blocker use (HR 1.29 [1.01–1.65]; p = .043), and adjunctive cavotricuspid isthmus ablation [HR 0.74 (0.57–0.96); p = .02]. Survival analysis demonstrated that patients with both LAVi >66.7 mL/m2 and early recurrence had the highest risk of late recurrence risk compared with those with LAVi <66.7 mL/m2 and no early recurrence (HR 4.52 [3.36–6.08], p < .001).
Conclusions
Machine learning‐derived, full volumetric LAVi from cCT is the most important pre‐procedural risk factor for late AF recurrence following catheter ablation. The combination of increased LAVi and early recurrence confers more than a four‐fold increased risk of late recurrence.
Keywords: atrial fibrillation, cardiac computed tomography, catheter ablation, left atrium, machine learning
We found that machine learning‐derived, full volumetric LAVi from cardiac computed tomography is the most important pre‐procedural risk factor for late AF recurrence following catheter ablation. The combination of increased LAVi and early recurrence conferred more than a four‐fold increased risk of late recurrence in our retrospective study of 653 patients undergoing catheter ablation for symptomatic, drug refractory atrial fibrillation.
1. INTRODUCTION
The prevalence of atrial fibrillation (AF) is increasing worldwide. AF is associated with increased risks of thromboembolic events, new‐onset heart failure, and all‐cause mortality. 1 , 2 Catheter ablation of AF has been shown to be superior to drug therapy for the prevention of recurrent AF and has been shown to improve outcomes in select patients. Despite technological advances, AF recurrence rates after catheter ablation remain suboptimal, occurring in 30%–50% of patients within 1 year. 3
AF recurrence – also termed “late recurrence” – is defined as AF, atrial flutter, or atrial tachyarrhythmia detected after a 3‐month blanking period following catheter ablation. 4 Recurrence within this 3‐month blank period is termed “early recurrence.” While early recurrence may be transient and due to inflammation, it has also been associated with late recurrence. 5 , 6 , 7 , 8 Though prediction of recurrent AF has traditionally focused on preoperative patient characteristics, there has been renewed interest in early recurrence as a predictor of late recurrence. 9
Risk factors for late recurrence and predictive models developed with regression techniques have been studied extensively. 10 While these models have included readily available clinical information and discrete elements from imaging reports, they have been limited by modest to poor discriminatory capacity and they do not take advantage of actual imaging data like CT geometries. 10 Despite the known importance of left atrial volume (LAV) in predicting AF recurrence, 11 full volumetric left atrial assessment has not been widely used in prognostic models due to the time‐consuming nature of manual segmentation.
Deep learning – a subset of machine learning – can be used to automate the otherwise time‐consuming process of cCT‐derived LAVi computation and generate a full volumetric assessment of the left atrium. Deep learning‐derived LAVi is particularly relevant to late AF recurrence, as patients routinely undergo cCT as part of the necessary pre‐ablation evaluation. The objective of this study was to use and evaluate machine learning techniques to define the risk of recurrent AF using a combination of clinical, procedural, and imaging variables.
2. METHODS
2.1. Patient population
A total of 990 consecutive patients underwent pulmonary vein isolation for symptomatic, antiarrhythmic drug‐refractory AF between January 2014 and June 2019 at the MedStar Heart and Vascular Institute in Washington, DC, USA. Among these patients, 337 were excluded either because they were lost to follow‐up before the end of the three‐month blanking period (112) or because they did not have preoperative cCT (225), resulting in 653 patients available for analysis. This study was approved by Georgetown‐Medstar Institutional Review Board with a waiver of informed consent.
2.2. Clinical variables
Demographic information including age and sex was collected from a review of the electronic medical record. Clinical variables including height, weight, and medications were gathered from a preoperative clinic visit. Body mass index and body surface area (BSA) were calculated using established formulas. Comorbidities were obtained including coronary artery disease, heart failure, peripheral artery disease, hypertension, hyperlipidemia, diabetes mellitus, obstructive sleep apnea, and stroke / transient ischemic attack history. CHA2DS2‐VASc at the time of ablation was calculated using these data. 12 AF was classified as paroxysmal or non‐paroxysmal (persistent or long‐standing persistent) at the time of ablation. The number of prior cardioversions and catheter ablations for AF or atypical atrial flutter were recorded.
2.3. Cardiac computed tomography protocol and LAVi computation
Preoperative cCT was acquired using a high‐resolution 256‐slice scanner (Brilliance iCT, Royal Philips, Amsterdam, Netherlands) with 0.625 mm detector collimation, matrix 512 × 512, and rotation time 330 ms. Images were acquired using prospectively ECG‐triggered cCT at 40% of the cardiac cycle, scanning the chest from carina to diaphragm during the angiodynamic administration of contrast medium. The contrast protocol included administration of 60 mL iohexol (Omnipaque, GE Healthcare) intravenously at a rate of 5 mL/s.
LAV was generated using a deep learning framework described in detail elsewhere. 13 In brief, the framework begins by using a ResNet50 classification model to select images within the series that include the left atrium and then uses a UNet image segmentation model to select the left atrium. Derived from a dataset of 85,477 cCT images from 337 patients, the LAV computation framework achieved accuracies of 98% in the image classification task and 88.5% mean dice score in the image segmentation task with a resulting coefficient of determination value of 0.968 in the LAV estimation task, relative to manually segmented controls. LAV was then indexed to BSA and calculated as left atrial volume index (LAVi) using the formula LAV/BSA and reported in mL/m2. Data S1 contains additional details and a pictorial representation of this method.
2.4. Ablation procedural protocol
Femoral venous access was obtained and transseptal puncture was performed under intracardiac echocardiographic and/or fluoroscopic guidance. Ablation was performed with either an open‐irrigated radiofrequency ablation catheter or cryoballoon ablation catheter. 3D electroanatomic mapping and voltage mapping were performed using high‐fidelity mapping catheters to confirm electrical isolation of all pulmonary veins. If the pulmonary veins were not electrically quiescent, endocardial radiofrequency energy was delivered to complete the ablation. Patients with a history of typical atrial flutter or inducible cavotricuspid isthmus (CTI)‐dependent atrial flutter intraoperatively underwent adjunctive CTI radiofrequency ablation. A subset of patients in the cohort underwent hybrid ablation (catheter + surgical ablation during the same procedure). The epicardial portion of the hybrid ablation was carried out with pericardial access via subxiphoid approach. In our institution, most hybrid ablation patients also underwent clipping of the left atrial appendage.
2.5. Post‐ablation follow‐up
Patients were continued on anticoagulation postoperatively. The decision of whether to continue antiarrhythmic medications was made on a case‐by‐case basis at the discretion of the treating physician. Late recurrence was defined according to the 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement that describes clinical AF recurrence as a documented episode of AF, atrial flutter, or atrial tachyarrhythmia lasting >30 s on ECG or Holter monitor after a 3‐month blanking period. 4 Early recurrence was defined as a documented episode of AF, atrial flutter, or atrial tachyarrhythmia within the 3‐month blanking period. Routine ECG and 24‐h Holter monitors were performed at follow‐up visits of 1 month, 3 months, and 12 months after ablation, with additional visits and use of Holter monitor and/or long‐term continuous monitoring based on symptoms. This method is the standard of care at our institution with a goal of capturing clinical – or symptomatic – recurrence in patients who underwent catheter ablation for symptomatic atrial fibrillation. 14 Patients lost to follow‐up before 3 months were excluded.
2.6. Statistical analysis
Continuous variables are expressed as mean ± standard deviation and compared using two‐sided Student's t‐test. Categorical numbers are expressed as absolute numbers and percentages and compared using Pearson's Chi‐square test for independence. We used a two‐step machine learning process to select the most important covariates for the model. First, we used random survival forest (RSF) to impute the missing covariates and provide variable importance ranking, which eliminated variables with little predictive power without assuming any statistical models. Large importance values indicate variables with predictive ability, whereas zero or negative values identify non‐predictive variables to be filtered out during regression analysis. Following RSF, Cox proportional hazard regression was performed on variables of highest importance, resulting in independent predictors of late recurrence. Model discrimination was performed using the concordance index (C‐index). Survival tree analysis was performed to elucidate patient subgroups at the highest risk of late recurrence. Cut‐off values were calculated using the survival tree method “Multivariable Survival Trees.” This is a modified classification and regression trees (CART) procedure with three steps: (1) growing a large initial tree, (2) pruning it back to obtain a sequence of subtrees, and (3) selecting the optimal tree size. Finally, Kaplan–Meier curve analysis and log‐rank testing were performed to determine differences in rates of recurrence between selected groups. Statistical significance was defined as two‐sided p‐values <.05. Statistical analyses were performed using R (The R Project, https://www.r‐project.org/).
3. RESULTS
3.1. Patient demographics, comorbidities, and imaging variables
Among 653 patients in the study cohort, the mean age was 63.8 ± 10.1 years, and 34% (n = 219) of patients were female. The mean follow‐up period was 24 months (+/− 18 months). The majority of patients had paroxysmal AF (62%, n = 404) as compared to non‐paroxysmal AF (38%, n = 249). The energy source used to achieve PVI was cryoballoon for 75% (n = 489) and radiofrequency for 25% (n = 170). A subset of patients underwent adjunctive cavotricuspid isthmus (CTI) ablation for atrial flutter (34%; n = 222). A minority of patients underwent hybrid ablation (10%, n = 68). Patients with or without late recurrence had similar prevalence of most comorbidities as shown in Table 1. Mean CHA2DS2‐VASc was higher in the late recurrence group (3.2 ± 1.9 vs. 2.7 ± 1.8, p < .001). Heart failure (35% vs. 24%, p = .003) and beta blocker use (67% vs. 57%, p = 0.012) were more prevalent in the late recurrence group. The mean LAV for the cohort was 139.7 ± 46.5 cm3, and mean LAVi was 66.0 ± 21.1 cm3/m2. Patients with late recurrence had higher LAV (151.4 ± 49 vs. 129.8 ± 42 cm3, p < .001) and LAVi (72.1 ± 21.9 vs. 60.9 ± 18.9 cm3/m2, p < .001). Patients with adjunctive CTI ablation for atrial flutter less frequently experienced late recurrence (28% vs. 39%, p = .004), and those undergoing hybrid ablation were more likely to experience late recurrence (14% vs. 7%, p = .007). Patients with late recurrence were also more likely to experience early recurrence than those without (40% vs. 17%, p < .001).
TABLE 1.
Baseline characteristics.
| Total (n = 653) | |
|---|---|
| Demographics and Comorbidities | |
| Age, years | 63.8 (10.1) |
| Female gender | 219 (34) |
| BMI, kg/m2 | 30.6 (6.6) |
| Heart failure | 191 (29) |
| Coronary Artery disease | 207 (32) |
| Peripheral artery disease | 51 (8) |
| Hypertension | 496 (76) |
| Hyperlipidemia | 395 (60) |
| Diabetes mellitus | 151 (23) |
| Obstructive sleep apnea | 244 (37) |
| Stroke/TIA history | 99 (15) |
| CHA2DS2‐VASc | 2.9 (1.9) |
| Paroxysmal AF | 404 (62) |
| Prior cardioversion(s) | 227 (35) |
| Prior ablation(s) | 171 (26) |
| Medications | |
| Aspirin | 160 (25) |
| Anticoagulation | 551 (84) |
| Beta blocker | 402 (62) |
| Calcium channel blocker | 137 (21) |
| Antiarrhythmic | 357 (55) |
| Statin | 298 (46) |
| Procedural variables | |
| PVI Energy Source: Cryoballoon | 489 (75) |
| Adjunctive CTI Ablation for Atrial Flutter | 222 (34) |
| Hybrid Ablation | 68 (10) |
| Imaging phenotypes | |
| LAV, cm3 | 139.9 (46.7) |
| LAVi, cm3/m2 | 66.2 (21.1) |
| Early recurrence | 182 (28) |
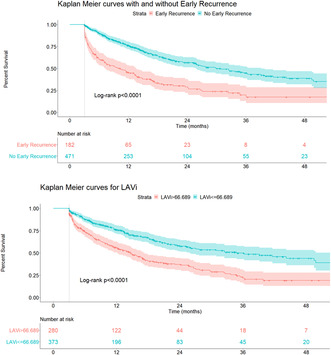
As depicted with Kaplan Meier analysis, patients with LAVi >66.7 cm3/m2 had a higher risk for late recurrence (HR 2.10 [1.67–2.64], p < .001 by log‐rank test) (Figure 1), as did patients with early recurrence (HR 2.62 [2.05–3.34], p < .001 by log rank rest) (Figure 1). Patients with both LAVi >66.7 cm3/m2 and early recurrence had the highest late recurrence risk as compared to patients with LAVi <66.7 cm3/m2 and no early recurrence (HR 4.52 [3.36–6.08], p < .001).
FIGURE 1.
Kaplan–Meier curves for LAVi and early recurrence. The cut‐off of LAVi was obtained from survival tree analysis. Patients with LAVi >66.7 cm3/m2 were at higher risk for late AF recurrence (HR 2.10 [1.67–2.64], log‐rank p < .001) as were patients with early recurrence (HR 2.62 [2.05–3.34], log‐rank p < .001).
3.2. Recurrence risk prediction
The RSF was used to derive variable importance for recurrence prediction for all 26 clinical, procedural, and imaging covariates. The most important variables for predicting late recurrence included (in decreasing importance): early recurrence, LAVi, statin use, beta blocker use, female sex, heart failure, atrial fibrillation type (paroxysmal vs. non‐paroxysmal), adjunctive CTI ablation for atrial flutter, cryoballoon PVI energy source, CHADS‐VASc, body mass index, peripheral artery disease, and stroke/TIA history (Figure 2). Of these 13 variables, five covariates were identified as independent predictors of late recurrence in the Cox proportional hazards multivariable regression: LAVi (HR 1.01 [1.01–1.02]; p < 0.001), early recurrence (HR 2.42 [1.90–3.09]; p < 0.001), statin use (HR 1.38 [1.09–1.75]; p = 0.007), beta blocker use (HR 1.29 [1.01–1.65]; p = 0.043), and adjunctive CTI ablation for atrial flutter (HR 0.74 [0.57–0.96]; p = .022). The resulting C‐index was 0.70 (Table 2).
FIGURE 2.
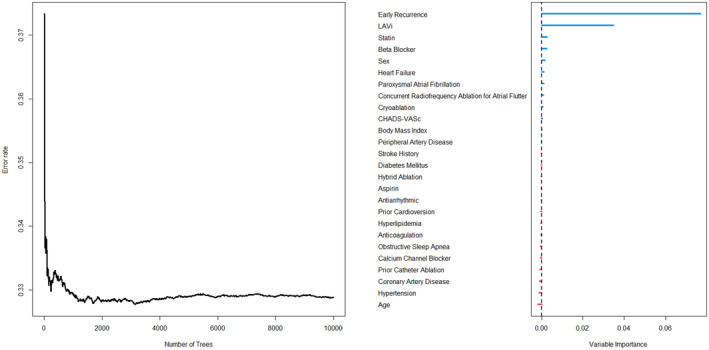
Variable importance from random survival forest (RSF). In this machine learning method for variable selection, large importance values indicate variables with predictive ability, whereas zero or negative values identify non‐predictive variables to be filtered out during regression analysis. The most important variables in the model were early recurrence and LAVi. Predictors of highest importance in RSF were selected for Cox proportional hazards regression analysis.
TABLE 2.
Multivariable Cox proportional hazards regression.
| Model concordance: 0.70 | ||
|---|---|---|
| Hazard ratio (95% CI) | p‐value | |
| Body Mass Index | 0.98 (0.96–1.00) | 0.086 |
| Peripheral Artery Disease | 0.68 (0.44–1.05) | 0.0789 |
| Beta Blocker | 1.29 (1.01–1.65) | 0.043 |
| Statin | 1.41 (1.11–1.78) | 0.0043 |
| Concurrent | ||
| Radiofrequency Ablation for Atrial Flutter | 0.74 (0.57–0.96) | 0.0218 |
| Early Recurrence | 2.53 (1.98–3.23) | <0.0001 |
| LAVi | 1.02 (1.01–1.02) | <0.0001 |
Abbreviation: LAVi, left atrial volume index.
The bolded values in Table 2 represent those that met statistical significance as indicated by P value below 0.05.
4. DISCUSSION
Our study leveraged deep learning techniques to perform a full volumetric left atrial assessment and used machine learning statistical methods to identify variables of highest importance to late recurrence prediction. The main findings of the study are that (1) adjunctive CTI ablation for atrial flutter was predictive of freedom from recurrence regardless of pulmonary vein isolation energy source, (2) statin and beta blocker use was independently predictive of recurrence, (3) deep‐learning‐derived LAVi from cCT is the most important preoperative predictor of late recurrence for patients undergoing catheter ablation, and (4) early recurrence further improves the prognostic model as compared to preoperative variables alone.
4.1. Clinical variables and risk prediction
In our study, clinical variables including heart failure, stroke/TIA history, CHA2DS2‐VASc, AF type, and prior cardioversion(s) were associated with late recurrence. These associations can be found in numerous other observational studies. In fact, in a review of 13 prognostic models, there were 25 different clinical variables associated with late recurrence but only three variables (age, type of AF, and left atrial size) were statistically significant predictors in more than half of the studies. 10 , 15 After variable selection with RSF and Cox proportional hazard regression, the statistically significant predictors in this study included LAVi, early recurrence, adjunctive CTI ablation for atrial flutter, statin use, and beta blocker use.
Adjunctive CTI ablation for known or inducible atrial flutter had a protective effect against late AF recurrence. Although atrial flutter following pulmonary vein isolation is a relatively rare phenomenon, it could reasonably account for some of the 11% absolute difference in late recurrence observed between patients undergoing adjunctive atrial flutter ablation and those who did not. 16 Additionally, preoperative beta blocker and statin use were found to be independently predictive of late recurrence, although variable importance of these two medications was significantly lower than early recurrence and LAVi (Figure 2). Beta blocker and statin use could be markers of cardiovascular disease severity as a risk factor for recurrence, as suggested by larger left atrial volumes in patients taking these medications in our study (data not shown). Data on medication doses and post‐ablation use were not available for analysis, limiting the generalizability of these findings.
4.2. Significance of left atrial volume index
Left atrial dilatation has been well described in the pathogenesis and perpetuation of AF. 17 Left atrial wall irregularities and user‐dependence limit precision and accuracy of echocardiographically derived measures of left atrial enlargement relative to cCT‐derived LAVi. 18 In the context of atrial fibrillation, cCT‐derived LAVi is superior to echocardiographically derived LA diameter and LAVi for predicting late AF recurrence. 19 Despite this, prior prognostic models have most commonly used readily available, echocardiographically derived measures. 10 Studies that utilize cCT‐derived LAVi for post‐ablation prognostication arrive at these measurements through manual or semi‐automated segmentation, 20 , 21 , 22 , 23 a time‐intensive process that potentially limits its traction for routine clinical use despite widespread availability of pre‐ablation cCT. Our study leveraged deep learning to overcome these barriers and compute LAVi from cCT with both speed and accuracy. RSF analysis revealed that LAVi is more important than any other pre‐procedural clinical factor for recurrence prediction. Other machine‐learning‐derived morphologic features of the left atrium, pulmonary veins, and left atrial myocardium have shown early promise for improving prognostic models' discriminative ability but require further replication and validation. 24 , 25 These findings support the continued pursuit of machine‐learning techniques as a component of risk stratification.
4.3. Significance of early recurrence
Most recurrence prognostication focuses on variables that are available preoperatively; however, there has been renewed interest in early recurrence as a predictor of late recurrence. 9 , 26 The association has been observed similarly in patients undergoing either radiofrequency ablation or cryoablation. 27 These findings challenge the notion that recurrence during the 3‐month blanking period is a transient and clinically insignificant result of periprocedural inflammation. 6 , 28 Our study showed a similar rate of early recurrence (28%) to that reported in the literature and a similarly strong association with late recurrence. In fact, early recurrence was the only parameter of greater importance than LAVi in RSF analysis. While patients with either early recurrence or large LAVi had approximately a two‐fold increased risk of late recurrence, patients with both LAVi >66.7 cm3/m2 and early recurrence had more than four times the risk of late recurrence as compared to patients with LAVi <66.7 cm3/m2 and no early recurrence. This finding is consistent with a recent publication by Kim et al. which showed that left atrial diameter, early recurrence, and non‐paroxysmal AF type are the most significant predictors for late recurrence. 9 Although early recurrence cannot influence the decision of whether or not to pursue an ablation like traditional preoperative factors, it may nonetheless be an important prognostic indicator.
4.4. Study strengths and limitations
Strengths of this study include the use of deep learning for cCT‐derived LAVi and use of machine learning statistical methods that eliminate variables with little predictive power without assuming statistical models. Although the sample size is modest, this study is the largest, to our knowledge, that utilizes deep‐learning‐derived, full‐volumetric LAVi from cCT for recurrence risk prediction. Limitations include the retrospective study design with patients from a single healthcare system, use of ICD‐9/10 billing to code for comorbidities, and the inaccessibility of complete echocardiographic data. Another limitation is the use of routine electrocardiogram and 24‐h Holter monitoring to determine early and late recurrence. Although this AF detection strategy is consistent with the 2017 expert consensus statement for the definition of clinical AF recurrence, the lack of continuous monitoring may limit detection of subclinical recurrence. Finally, we acknowledge the potential for selection bias associated with >10% of patients excluded from the study for loss to follow‐up.
5. CONCLUSIONS
Machine learning‐derived, full volumetric LAVi from cCT is the most important pre‐procedural variable for predicting late AF recurrence following catheter ablation. The combination of increased LAVi and early recurrence confers more than a four‐fold increased risk of late AF recurrence. Thus, machine learning methods can help risk stratify patients undergoing AF catheter ablation.
FUNDING INFORMATION
MA and SEP acknowledge support from the CAP‐AI program (led by Capital Enterprise in partnership with Barts Health NHS Trust and Digital Catapult and funded by the European Regional Development Fund and Barts Charity) and Health Data Research UK (HDR UK – an initiative funded by UK Research and Innovation, Department of Health and Social Care (England) and the devolved administrations, and leading medical research charities; www.hdruk.ac.uk). SEP acknowledges support from the National Institute for Health Research (NIHR) Biomedical Research Centre at Barts, from the SmartHeart EPSRC program grant (www.nihr.ac.uk; EP/P001009/1) and the London Medical Imaging and AI Center for Value‐Based Healthcare. SEP has received funding from the European Union's Horizon 2020 research and innovation program under grant agreement No. 825903 (euCanSHare project). JPP is supported by R01AG074185 from the National Institutes of Aging. He also receives grants for clinical research from Abbott, the American Heart Association, the Association for the Advancement of Medical Instrumentation, Bayer, Boston Scientific, iRhythm and Philips and serves as a consultant to Abbott, Abbvie, ARCA biopharma, Bayer, Boston Scientific, Bristol Myers Squibb (Myokardia), Element Science, Itamar Medical, LivaNova, Medtronic, Milestone, ElectroPhysiology Frontiers, ReCor, Sanofi, Philips, and Up‐to‐Date.
CONFLICT OF INTEREST STATEMENT
Authors declare no conflict of interests for this article.
ETHICS STATEMENT
This study was approved by Georgetown‐Medstar Institutional Review Board with waiver of informed consent.
Supporting information
Data S1:
ACKNOWLEDGEMENTS
The authors thank the MedStar Heart and Vascular Institute and Georgetown University Medical Center for their support of this project. The authors thank medical students Andrew Wilbur, Kirsten Linnartz, and Jacqueline Anders for their assistance with data abstraction.
Brahier MS, Zou F, Abdulkareem M, Kochi S, Migliarese F, Thomaides A, et al. Using machine learning to enhance prediction of atrial fibrillation recurrence after catheter ablation. J Arrhythmia. 2023;39:868–875. 10.1002/joa3.12927
Steffen E. Petersen and Jose D. Vargas contributed equally to this work.
REFERENCES
- 1. Andrade J, Khairy P, Dobrev D, Nattel S. The clinical profile and pathophysiology of atrial fibrillation: relationships among clinical features, epidemiology, and mechanisms. Circ Res. 2014;114(9):1453–1468. 10.1161/CIRCRESAHA.114.303211 [DOI] [PubMed] [Google Scholar]
- 2. Wolf PA, Dawber TR, Thomas HE Jr, Kannel WB. Epidemiologic assessment of chronic atrial fibrillation and risk of stroke: the Framingham study. Neurology. 1978;28(10):973–977. 10.1212/wnl.28.10.973 [DOI] [PubMed] [Google Scholar]
- 3. Deng H, Bai Y, Shantsila A, Fauchier L, Potpara TS, Lip GYH. Clinical scores for outcomes of rhythm control or arrhythmia progression in patients with atrial fibrillation: a systematic review. Clin Res Cardiol. 2017;106(10):813–823. 10.1007/s00392-017-1123-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga L, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation: executive summary. Europace. 2018;20(1):157–208. 10.1093/europace/eux275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Andrade JG, Khairy P, Verma A, Guerra PG, Dubuc M, Rivard L, et al. Early recurrence of atrial tachyarrhythmias following radiofrequency catheter ablation of atrial fibrillation. Pacing Clin Electrophysiol. 2012;35(1):106–116. 10.1111/j.1540-8159.2011.03256.x [DOI] [PubMed] [Google Scholar]
- 6. Jiang H, Lu Z, Lei H, Zhao D, Yang B, Huang C. Predictors of early recurrence and delayed cure after segmental pulmonary vein isolation for paroxysmal atrial fibrillation without structural heart disease. J Interv Card Electrophysiol. 2006;15(3):157–163. 10.1007/s10840-006-9003-y [DOI] [PubMed] [Google Scholar]
- 7. Oral H, Knight BP, Ozaydin M, Tada H, Chugh A, Hassan S, et al. Clinical significance of early recurrences of atrial fibrillation after pulmonary vein isolation. J Am Coll Cardiol. 2002;40(1):100–104. 10.1016/s0735-1097(02)01939-3 [DOI] [PubMed] [Google Scholar]
- 8. Lee SH, Tai CT, Hsieh MH, Tsai CF, Lin YK, Tsao HM, et al. Predictors of early and late recurrence of atrial fibrillation after catheter ablation of paroxysmal atrial fibrillation. J Interv Card Electrophysiol. 2004;10(3):221–226. 10.1023/B:JICE.0000026915.02503.92 [DOI] [PubMed] [Google Scholar]
- 9. Kim YG, Boo KY, Choi JI, Choi YY, Choi HY, Roh SY, et al. Early recurrence is reliable predictor of late recurrence after radiofrequency catheter ablation of atrial fibrillation. JACC Clin Electrophysiol. 2021;7(3):343–351. 10.1016/j.jacep.2020.09.029 [DOI] [PubMed] [Google Scholar]
- 10. Dretzke J, Chuchu N, Agarwal R, Herd C, Chua W, Fabritz L, et al. Predicting recurrent atrial fibrillation after catheter ablation: a systematic review of prognostic models. Europace. 2020;22(5):748–760. 10.1093/europace/euaa041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Njoku A, Kannabhiran M, Arora R, Reddy P, Gopinathannair R, Lakkireddy D, et al. Left atrial volume predicts atrial fibrillation recurrence after radiofrequency ablation: a meta‐analysis. Europace. 2018;20(1):33–42. 10.1093/europace/eux013 [DOI] [PubMed] [Google Scholar]
- 12. Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor‐based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137(2):263–272. 10.1378/chest.09-1584 [DOI] [PubMed] [Google Scholar]
- 13. Abdulkareem M, Brahier MS, Zou F, Taylor A, Thomaides A, Bergquist PJ, et al. Generalizable framework for atrial volume estimation for cardiac CT images using deep learning with quality control assessment. Front Cardiovasc Med. 2022;9:822269. 10.3389/fcvm.2022.822269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Calhoun P, Su X, Nunn M, Fan J. Constructing multivariate survival trees: the MST package for R. J Stat Softw. 2018;83(12):1–21. 10.18637/jss.v083.i12 [DOI] [Google Scholar]
- 15. Garvanski I, Simova I, Angelkov L, Matveev M. Predictors of recurrence of AF in patients after radiofrequency ablation. Eur Cardiol. 2019;14(3):165–168. 10.15420/ecr.2019.30.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Baman JR, Kaplan RM, Diaz CL, Peigh G, Bavishi AA, Trivedi A, et al. Characterization of atrial flutter after pulmonary vein isolation by cryoballoon ablation. J Interv Card Electrophysiol. 2020;57(2):233–240. 10.1007/s10840-019-00560-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nattel S, Burstein B, Dobrev D. Atrial remodeling and atrial fibrillation: mechanisms and implications. Circ Arrhythm Electrophysiol. 2008;1(1):62–73. 10.1161/CIRCEP.107.754564 [DOI] [PubMed] [Google Scholar]
- 18. Arsanjani R, Flint N, Beigel R, Khachatryan T, Shalev A, Shturman A, et al. Comparison of accuracy of left atrial area and volume by two‐dimensional trans‐thoracic echocardiography versus computed tomography. Am J Cardiol. 2019;123(7):1180–1184. 10.1016/j.amjcard.2018.12.047 [DOI] [PubMed] [Google Scholar]
- 19. Sohns C, Sohns JM, Vollmann D, Lüthje L, Bergau L, Dorenkamp M, et al. Left atrial volumetry from routine diagnostic work up prior to pulmonary vein ablation is a good predictor of freedom from atrial fibrillation. Eur Heart J Cardiovasc Imaging. 2013;14(7):684–691. 10.1093/ehjci/jet017 [DOI] [PubMed] [Google Scholar]
- 20. Abecasis J, Dourado R, Ferreira A, Saraiva C, Cavaco D, Santos KR, et al. Left atrial volume calculated by multi‐detector computed tomography may predict successful pulmonary vein isolation in catheter ablation of atrial fibrillation. Europace. 2009;11(10):1289–1294. 10.1093/europace/eup198 [DOI] [PubMed] [Google Scholar]
- 21. Costa FM, Ferreira AM, Oliveira S, Santos PG, Durazzo A, Carmo P, et al. Left atrial volume is more important than the type of atrial fibrillation in predicting the long‐term success of catheter ablation. Int J Cardiol. 2015;184:56–61. 10.1016/j.ijcard.2015.01.060 [DOI] [PubMed] [Google Scholar]
- 22. D'Ambrosio G, Romano S, Alothman O, Frommhold M, Borisov G, El Garhy M, et al. Computed tomography‐derived left atrial volume index, sex, and age to predict the presence and the extent of left atrial low‐voltage zones in patients with atrial fibrillation: the ZAQ score. J Cardiovasc Electrophysiol. 2020;31(4):895–902. 10.1111/jce.14391 [DOI] [PubMed] [Google Scholar]
- 23. Maier J, Blessberger H, Nahler A, Hrncic D, Fellner A, Reiter C, et al. Cardiac computed tomography‐derived left atrial volume index as a predictor of Long‐term success of Cryo‐ablation in patients with atrial fibrillation. Am J Cardiol. 2021;1(140):69–77. 10.1016/j.amjcard.2020.10.061 [DOI] [PubMed] [Google Scholar]
- 24. Firouznia M, Feeny AK, LaBarbera MA, McHale M, Cantlay C, Kalfas N, et al. Machine learning‐derived fractal features of shape and texture of the left atrium and pulmonary veins from cardiac computed tomography scans are associated with risk of recurrence of atrial fibrillation Postablation. Circ Arrhythm Electrophysiol. 2021;14(3):e009265. 10.1161/CIRCEP.120.009265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Atta‐Fosu T, LaBarbera M, Ghose S, Schoenhagen P, Saliba W, Tchou PJ, et al. A new machine learning approach for predicting likelihood of recurrence following ablation for atrial fibrillation from CT. BMC Med Imaging. 2021;21(1):45. 10.1186/s12880-021-00578-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hodges G, Bang CN, Torp‐Pedersen C, Hansen ML, Schjerning AM, Hansen J, et al. Significance of early recurrence of atrial fibrillation after catheter ablation: a nationwide Danish cohort study. J Interv Card Electrophysiol. 2021;60(2):271–278. 10.1007/s10840-020-00741-x [DOI] [PubMed] [Google Scholar]
- 27. Vaishnav AS, Levine E, Coleman KM, Beldner SJ, Chinitz JS, Bhasin K, et al. Early recurrence of atrial fibrillation after pulmonary vein isolation: a comparative analysis between cryogenic and contact force radiofrequency ablation. J Interv Card Electrophysiol. 2020;57(1):67–75. 10.1007/s10840-019-00639-3 [DOI] [PubMed] [Google Scholar]
- 28. Li XP, Dong JZ, Liu XP, Long DY, Yu RH, Tian Y, et al. Predictive value of early recurrence and delayed cure after catheter ablation for patients with chronic atrial fibrillation. Circ J. 2008;72(7):1125–1129. 10.1253/circj.72.1125 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1:


