Abstract
Background
Acute respiratory infections (ARIs) are common in children and can range in severity from mild self-limiting illnesses to more severe conditions such as pneumonia and respiratory failure. Data on the epidemiology of viral and bacterial pathogens causing ARIs in children are scarce in this region. This study aimed to investigate the epidemiology and clinical manifestations of pathogens in children aged ≤5 years presenting with severe acute respiratory infection (SARI) in Bangkok, Thailand. The impact of rapid multiplex PCR-based testing on clinical management is also explored.
Methods
This cross-sectional study enrolled consecutive children aged ≤5 years presenting with SARI at a tertiary care centre in Bangkok, Thailand, between 2019 and 2020. Nasopharyngeal swabs were collected once at admission, and viral and bacterial pathogens were tested using the QIAstat-Dx respiratory panel.
Results
A total of 169 children were enrolled in this study. At least one pathogenic virus was detected in 91.7 % of participants. Based on the final diagnoses made upon discharge, 30.2 % had upper respiratory tract infection, whereas 66.3 % had lower respiratory tract infection. Pneumonia was the most common diagnosis (59.2 %). The most common pathogen identified was rhino/enterovirus (45.2 %), followed by respiratory syncytial virus (31.6 %) and parainfluenza virus (14.2 %). Co-infection was found in 15.4 % and was not associated with increased disease severity.
Conclusions
This study provides additional insights into the pathogen profiles, clinical diagnosis, and co-infection combinations of ARIs in hospitalized children. This information is useful for diagnosis and treatment of ARIs, as well as implementation of appropriate infection control measures and guidance for future vaccine policy development.
Keywords: Epidemiology, Clinical manifestations, viral, Bacterial, Respiratory pathogens, Children, Severe acute respiratory infections
Highlights
-
•
Rapid multiplex PCR-based testing allows rapid identification of pathogens causing acute respiratory infection in children.
-
•
At least one pathogenic virus can be detected in 91.7 % of the children.
-
•
Co-infection was not associated with increased disease severity.
1. Introduction
Acute respiratory infections (ARIs) are common problems in children and associated with considerable morbidity and mortality among children aged five years and younger [1]. In children, ARIs are often caused by viruses including influenza virus, respiratory syncytial virus (RSV), parainfluenza virus (PIV), human metapneumovirus (HMPV), human adenovirus (ADV), human rhinovirus (HRV), human bocavirus (BoV) and seasonal coronavirus (CoV) [[2], [3], [4], [5]]. However, to a lesser degree, some bacteria, including Streptococcus pneumoniae, Mycoplasma pneumoniae and Bordetella pertussis, can cause respiratory infections in children [[6], [7], [8], [9]]. Viral and bacterial respiratory infections cannot be completely distinguished by their clinical presentation. Thus, further laboratory investigations play an important role in identifying etiologic pathogens and allowing physicians to understand the clinical manifestations and epidemiology of the circulating pathogens.
The diagnosis of infectious diseases has been greatly improved by molecular-based multiplex assays, which allow the detection of multiple pathogens simultaneously in a single assay [10]. Recent technologies allow a short turnaround time of 1–2 h and require minimal technical expertise to obtain accurate results [11]. These tools allow the rapid diagnosis of infectious diseases at the point of care. Recent studies in adults have found the use of rapid molecular-based point-of-care tests for respiratory tract infection resulted in reduced hospital stay duration, improved influenza detection and antiviral use compared to standard clinical care [12,13]. Another study by Almannaei et al. found that antimicrobial prescriptions were more appropriate for influenza A viruses and bacterial organisms (Mycoplasma pneumoniae, Chlamydia pneumoniae and Bordetella pertussis) after implementing rapid molecular-based testing [14]. In Thailand, rapid multiplex PCR-based testing is not routinely used due to its relatively high cost of approximately 100 US dollars per test. In comparison, conventional single polymerase chain reaction (PCR) testing costs 40–50 US dollars, while antigen testing costs 10–15 US dollars per antigen.
Previous studies have investigated whether respiratory viral co-infection impacted the clinical course or disease severity compared to single infection [15,16]. Aberle et al. reported that among infants with lower respiratory tract infection, those co-infected with RSV had a decreased interferon-gamma response in peripheral blood mononuclear cells and an increase in severity of the disease compared to those co-infected with other viruses [16]. Another study by Tabatabai et al. found that co-infection was more frequent in young children, and co-infection with RSV and HRV was associated with more severe respiratory symptoms [17].
In Thailand, data on the prevalence of viral or bacterial pathogens causing ARIs often relies on the use of antigen testing or single conventional or real-time PCR [3,4,6]. Although affordable, these methods have a restricted range of pathogens which they can identify. At the time this study was conducted, use of rapid multiplex PCR-based point-of-care testing was limited to private hospitals. This study aimed to explore the epidemiology of circulating respiratory pathogens in children ≤5 years of age who were hospitalized with severe acute respiratory tract infection (SARI) as defined by the World Health Organization (WHO); an acute respiratory infection with documented or history of fever (measured temperature ≥38 °C), cough onset within the last 10 days, and requiring hospitalization, using the multiplex PCR-based testing [18]. The findings of this study will provide further insights into the epidemiology, clinical diagnosis, and the prevalence of co-infections in cases with ARIs. The secondary objective of this study is to explore the possible impact of multiplex PCR-based point-of-care testing on clinical management.
2. Materials and methods
2.1. Study design
Children aged ≤5 years who presented to King Chulalongkorn Memorial Hospital, Bangkok, Thailand between September 2019 and December 2020 (pre-pandemic and early COVID-19 pandemic) with clinical characteristics of SARI according to WHO definition were assessed for eligibility. Exclusion criteria were immunocompromised or immunosuppressed children, children with chronic lung disease, genetic disorders, or congenital abnormalities, and hospitalization >7 days due to possible nosocomial infections. After obtaining informed written consent from parents or guardians, researchers obtained nasopharyngeal swabs and placed them in specimen collection tubes. Nasopharyngeal swab samples were collected only once at admission, for each case. Demographic and clinical data were collected at enrolment. Patients were followed from the date of admission until discharge from the hospital.
This study was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization-Good Clinical Practice Guidelines (ICH-GCP). The Institutional Review Board of the Faculty of Medicine, Chulalongkorn University (IRB 494/62) approved this study.
2.2. Laboratory testing
Nasopharyngeal swab samples were tested for 18 viral and 3 bacterial targets using the QIAstat-Dx respiratory panel (QIAGEN, Les Ulis, France) which utilizes multiplex PCR testing. The molecular targets included adenovirus, bocavirus, coronavirus 229E, coronavirus HKU1, coronavirus NL63, coronavirus OC43, human metapneumovirus A + B, influenza A, influenza A subtype H1, influenza A subtype H1N1pdm2009, influenza A subtype H3, influenza B, parainfluenza virus 1, parainfluenza virus 2, parainfluenza virus 3, parainfluenza virus 4, respiratory syncytial virus A + B, rhinovirus/enterovirus, Mycoplasma pneumoniae, Legionella pneumophilla and Bordetella pertussis [19]. Each QIAstat Respiratory panel cartridge contains a full process Internal Control (IC), which is a MS2 bacteriophage in dried format which is automatically rehydrated upon sample loading. The IC verifies all steps of the analysis process during testing, including sample resuspension/homogenization, lysis, nucleic acid purification, reverse transcription, and PCR. The test cartridge has two distinct loading ports and can be inoculated directly with a dry swab or with transport medium. In this study, nasopharyngeal swab samples were placed in the 500 μL viral transport medium and subsequently homogenized through vigorous inversion of the vial. A total of 300 μL of suspension was transferred to the cartridge using the transfer pipette provided. The inoculated cartridge was placed on the instrument. Amplification curves and cycle threshold (Ct) values can be viewed for detected pathogens. The test is considered valid if it was completed normally with valid IC test result. Between September 2019 and September 2020, the QIAstat-Dx respiratory panel used in this study did not include detection of SARS-CoV-2. We began using the SARS-CoV-2-incoporated version of the QIAstat-Dx respiratory panel after it was imported to Thailand in October 2020.
Testing was carried out in the laboratory of the Centre of Excellence in Clinical Virology, Department of Pediatrics, Faculty of Medicine, Chulalongkorn University in Bangkok, Thailand.
2.3. Virus genotyping of viruses
Samples tested positive for RSV and/or rhino/enterovirus were genotyped using the leftover suspension that had not been transferred to the cartridge. Viral RNA was extracted from nasopharyngeal swabs using the magLEAD 12gC automated extraction system (Precision System Science, Chiba, Japan). Complementary DNA (cDNA) was synthesized using the ImProm II reverse transcription system (Promega, Madison, WI, USA) according to the manufacturer's instructions. Semi-nested PCR was used to amplify the partial G and F genes, as previously described [20].
For rhinovirus genotyping, cDNA was subjected using semi-nested PCR to amplify the VP4/VP2 region as previously described [21]. Purified PCR products were subjected to Sanger sequencing.
2.4. Phylogenetic analysis
The nucleotide sequences were deposited in the GenBank database under the accession numbers OR038392-OR038430. To identify the RSV genotypes in this study, the partial G gene sequences of RSV (nucleotide positions 634–963 with respect to the prototypic reference strain A2 (accession number M74568) and the reference strain B (accession number AF013254) were used. Sequence alignment was performed using the Clustal X programme. The phylogenetic tree was reconstructed using the neighbor‐joining method and bootstrap values of 1000 replicates in the MEGA X software [22]. HRV nucleotide sequences (390 bp in length) were compared with reference prototypes from the GenBank database. The neighbor‐joining approach with bootstrap values of 1000 replicates was used to reconstruct phylogenetic trees in MEGA X.
2.5. Statistical analysis
Descriptive data was reported with counts and percentages, mean and standard deviations or median and interquartile range (IQR), as appropriate. Logistic regression was performed to study associations between demographic and clinical parameters such as age, sex, diagnosis, length of hospital stay, duration of oxygen use and antibiotic treatment in single versus multiple viral infections. Statistical analysis was performed using Stata 17 (College Station, TX, USA). A p-value <0.05 was considered statistically significant.
3. Results
3.1. Demographic and clinical characteristics of the patient
Between September 2019 and December 2020, 185 hospitalized children aged ≤5 years presenting SARI were assessed for eligibility. Sixteen children were excluded due to congenital diseases, immunosuppressed status, or refusal of the parents to participate in the study. In total, 169 samples had been collected; 86/169 (50.9 %) were collected in 2019 and the remaining 49.1 % were collected in 2020. The majority of study participants were male (62.1 %) and 144/169 (85.2 %) had no underlying comorbidities. The median age was 18.9 months (range:16 days to 57 months). Among the 25 children with comorbidities, the most prevalent conditions were reactive airway disease (44 %), followed by allergic rhinitis (20 %), premature birth (8 %), and glucose-6-phosphate dehydrogenase deficiency (8 %). The characteristics of the study participants are listed in Table 1. In addition to fever and cough, children also presented with other symptoms such as nausea, vomiting, diarrhea, seizures, rash, conjunctivitis, ear discharge, and epistaxis.
Table 1.
Demographic and clinical characteristics of the patients.
| Characteristics | N (%) or median (IQR) from 169 samples | |
|---|---|---|
| Age | ||
| 0–24 months | 113 (66.9 %) | |
| 25–60 months | 56 (33.1 %) | |
| Median age (months) (IQR) [range] | 18.9 (7–30) [0.5–57] | |
| Sex | ||
| Male | 105 (62.1 %) | |
| Female | 64 (37.9 %) | |
| Year of sample collection | ||
| 2019 | 86 (50.9 %) | |
| 2020 | 83 (49.1 %) | |
| Median height (cm) | 79.3 (14.8) | |
| Median weight (kg) | 10.7 (4.4) | |
| Presence of comorbidities | ||
| Yes | 25 (14.8 %) | |
| -Reactive airway disease | 11 | |
| -Premature birth | 2 | |
| -Allergic rhinitis | 5 | |
| -Asthma | 1 | |
| -Cow's milk protein allergy | 1 | |
| -G6PD | 2 | |
| -Right upper lung bronchial stenosis | 1 | |
| -Laryngomalacia | 1 | |
| -Asymptomatic small VSD | 1 | |
| -Bilateral extrarenal pelvis | 1 | |
| -Renal cyst | 1 | |
| No | 144 (85.2 %) | |
| Presence of other symptoms | ||
| Vomiting | 72 (42.6 %) | |
| Nausea | 50 (29.6 %) | |
| Diarrhea | 37 (21.9 %) | |
| Seizure | 9 (5.3 %) | |
| Seizure with rash | 1 (0.6 %) | |
| Rash | 7 (4.1 %) | |
| Others (conjunctivitis, ear discharge, epistaxis) | 3 (1.8 %) | |
| Diagnosis | ||
| Upper respiratory tract infection | ||
| Acute nasopharyngitis (common cold) | 41 (24.3 %) | |
| Croup | 10 (5.9 %) | |
| Lower respiratory tract infection | ||
| Bronchitis | 3 (1.8 %) | |
| Acute bronchiolitis | 9 (5.3 %) | |
| Pneumonia | 100 (59.2 %) | |
| Others (Chikungunya, UTI, meningitis, acute viral infection) | 6 (3.6 %) | |
| Numbers of pathogen detected | ||
| No pathogen detected | 14 (8.3 %) | |
| Single pathogen | 129 (76.3 %) | |
| Double pathogen | 25 (14.8 %) | |
| Triple pathogen | 1 (0.6 %) | |
| Median (IQR) length of stay (days) | 4 (2–5) | |
| ICU admission | ||
| Yes | 11 (6.5 %) | |
| No | 158 (93.5 %) | |
| Requirement of oxygen support | ||
| Yes | 100 (59.2 %) | |
| No | 68 (40.2 %) | |
| No information | 1 (0.6 %) | |
| Median (IQR) duration of oxygen use (days) | 2 (0–3) | |
| Requirement of mechanical ventilation | ||
| Yes | 7 (4.1 %) | |
| No | 162 (95.9 %) | |
| Use of antibacterial agents | ||
| Yes | 63 (37.3 %) | |
| No | 106 (62.7 %) | |
IQR; interquartile range, cm; centimeter, kg; kilogram, G6PD; glucose-6-phosphate dehydrogenase deficiency, VSD; ventricular septal defect, UTI; Urinary tract infection, ICU; intensive care unit.
The diagnosis was classified into upper and lower respiratory tract infections. A total of 30.2 % had upper respiratory tract infection, whereas 66.3 % had lower respiratory tract infection. Pneumonia (59.2 %) was the most common diagnosis. There were six cases in which the final diagnosis was not respiratory tract infection. The median (IQR) duration of the hospital stay was 3 (2–5) days. There were 6.5 % and 4.1 % of study participants admitted to the intensive care unit and requiring mechanical ventilation, respectively.
3.2. Distribution of respiratory pathogens
Of the 169 samples, 155 samples were confirmed to be positive for at least one viral pathogen (91.7 %). A single pathogen was detected in 76.3 %, while a double pathogen and a triple pathogen were detected in 14.8 % and 0.6 %, respectively. Rhino/enterovirus (RV/EV) was the most detected virus throughout the year (45.2 %) as shown in Fig. 1. The second and third most common viruses detected were RSV (31.6 %) and PIV (14.2 %), respectively. None of the cases tested positive for bacterial infection. The number of admissions and samples available decreased dramatically between March and September 2020 due to the lockdown policy in response to the COVID-19 pandemic. Following the relaxation of COVID-19 restrictions in Thailand in late September 2020, there was a gradual rise in number of admissions due to respiratory infection. Despite the utilization of the updated SARS-CoV-2-incorporated respiratory panel from October 2020 onwards, SARS-CoV-2 was not detected in this study.
Fig. 1.
Monthly distribution of respiratory pathogens between September 2019 (month 9) and December 2020 (month 12). The numbers of positive cases for each respiratory pathogen are represented as different colours: green, RV/EV; red, RSV; orange, PIV; pink, seasonal CoV; beige, ADV; violet, BoV; light blue, HMPV; brown, Influenza virus; grey, no pathogen. The number above each bar graph indicates the total samples collected from pediatric patients in each month.
RV/EV, rhino/enterovirus; RSV, respiratory syncytial virus; PIV, parainfluenza virus; ADV, adenovirus; BoV, bocavirus; CoV; coronavirus HMPV, human metapneumovirus. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Co-infections were found in 26 of 169 children (15.4 %). RE/EV and RSV were the most common co-infection in this study (Table S1). Other common coinfections were ADV + RV/EV, and BoV + RE/EV. Triple infection was detected in only one case, which was PIV type 1, PIV type 3 and RV/EV. Multiple infection was not associated with increased disease severity, as shown by the comparable clinical parameters between children with single infection and multiple infection, and the non-significant odds ratios presented in Table S2. However, females were more likely to have multiple infection than males (odds ratio = 2.56 (95%CI 1.06–6.28); p = 0.04). Additionally, this study found that dual RSV (RSV co-infected with another virus) infection was not associated with increased disease severity compared to single RSV infection as presented in Table S3.
3.3. Viral pathogens and clinical diagnosis
After categorizing pathogens based on the final clinical diagnoses made upon discharge, this study revealed the most prevalent pathogens causing the acute nasopharyngitis were RV/EV (24.4 %), followed by influenza A (22.0 %), and PIV (22.0 %), as demonstrated in Table 2. Cases of influenza A virus (n = 9) could be further subgrouped into H1N1pdm09 (n = 6), H3 (n = 1), Flu B (n = 1), and untypable (n = 1).
Table 2.
Frequencies of the three most common pathogens classified by clinical diagnoses made upon discharge.
| Frequencies of the top three pathogens | |
|---|---|
| Acute nasopharyngitis (n = 41) | Rhino/enterovirus (n = 10) |
| Influenza A (n = 9) | |
| Parainfluenza virus (n = 9) | |
| Croup (n = 10) | Parainfluenza virus (n = 5) |
| Coronavirus NL63 (n = 3) | |
| Rhino/enterovirus (n = 1) | |
| Bronchitis (n = 3) | Respiratory syncytial virus (n = 3) |
| Rhino/enterovirus (n = 2) | |
| Acute bronchiolitis (n = 9) | Rhino/enterovirus (n = 6) |
| Respiratory syncytial virus (n = 2) | |
| Pneumonia (n = 100) | Rhino/enterovirus (n = 48) |
| Respiratory syncytial virus (n = 38) | |
| Parainfluenza virus (n = 7) |
In contrast, PIV was the most common pathogen in croup. PIV was detected in 50 % of the cases, followed by seasonal CoV (30 %) and RV/EV (10 %). For lower respiratory tract infection, RSV was detected in all cases diagnosed with bronchitis, and 66.7 % of the bronchitis cases were co-infected with RV/EV. Acute bronchiolitis and pneumonia were mainly caused by RV/EV and RSV, and less likely PIV.
Two participants were treated with empirical antiviral agents (oseltamivir), which was later discontinued after the test results showed that both were infected with PIV type I. Thirty-seven percent of the children (63/169) were prescribed antibacterial agents due to clinical symptoms of superimposed secondary bacterial infection following viral infection. Although 37 % of antibacterial agent prescriptions in this study were attributed to suspected bacterial superimposed infections, only 2.9 % of cases (5 out of 169) were confirmed to have bacteria in their endotracheal secretion culture, including Haemophilus influenzae in four cases and Streptococcus pneumoniae in one case.
3.4. Molecular characterization of RSV and HRV
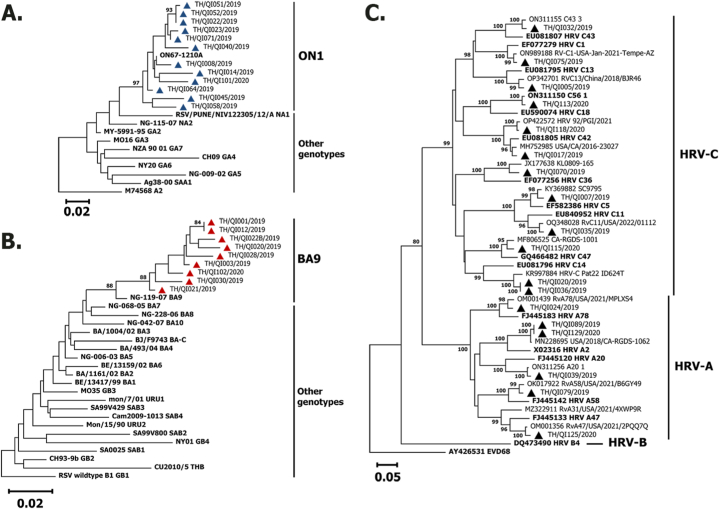
RSV-A (n = 12) detected among the children belonged to the ON1 clade, while RSV-B (n = 9) belonged to the BA9 clade (Fig. 2A–B). Among HRV strains (n = 18), the phylogenetic analysis of the VP4/VP2 viral protein coding regions indicated that the strains were clustered into HRV-A (n = 6) and HRV-C (n = 12) (Fig. 2C). HRV-B was not detected in the present study. The HRV-A strains belonged to subgroups A78, A2, A20, A58, and A47, while the HRV-C strains belonged to subgroup C43, C1, C13, C18, C42, C36, C5, C11, C47, and C14. Enterovirus D68 (AY426531) was served as an outgroup for the HRV phylogenetic tree.
Fig. 2.
Phylogenetic analysis of RSV subgroup A (A) and RSV subgroup B (B) based on the partial nucleotide sequences of the second hypervariable region of the G gene (330 bp) and phylogenetic analysis of the HRV VP4/VP2 region (C). The trees were constructed using the neighbor‐joining algorithm implemented in MEGA X. Bootstrap values > 80 % are displayed at the branch nodes. Samples of this study are shown by triangles. Other reference genotypes are shown in bold letters. Reference sequences were obtained from GenBank (GA1-GA7, SAA1, NA1-NA4 and ON1 for RSV-A and GB1-GB4, SAB1-SAB4, URU1, URU2, THB and BA1-BA10 for RSV-B). The scale bar represents the nucleotide substitution rate. Enterovirus D68 (EV‐D68) was served as an outgroup for the HRV phylogenetic tree.
4. Discussion
In this study, we explore the epidemiology of circulating respiratory pathogens in children ≤5 years of age who were hospitalized with SARI. A large percentage of the cases were caused by RV/EV, which was further subdivided into HRV-A and HRV-C, followed by RSV. The overwhelming majority of viral pathogens in pediatric patients with ARIs, with RSV and HRV being the most common causes of lower respiratory tract infection, is consistent with the findings reported by other studies, both before and after the COVID-19 pandemic [[23], [24], [25]]. In this study, RSV-A was detected more frequently than RSV-B. Further characterization of the RSV detected in this study showed that RSV-A belonged to the ON1 genotype, whereas RSV-B belonged to the BA9 genotype. The RSV-A genotype ON1 and the RSV-B genotype BA9 have been circulating in Thailand for more than a decade [26].
Even during the COVID-19 pandemic, HRV remained one of the most common respiratory pathogens in children under five years old [27,28]. Our previous study showed that HRV was detected in 27.5 % of the samples collected from Thai children ≤ 5 years of age presenting with influenza-like illness (ILI) between July and December 2020, of which 44 % were HRV-A, 7 % were HRV-B and 36 % were HRV-C [27]. In hospitalized children enrolled in this study, HRV was detected in 45.2 % of all pathogens identified. Unlike other studies where bacterial pathogens were detected in 2.1–8.8 % of pediatric cases [14,24], our study did not detect any bacterial pathogens. A previous study of atypical pneumonia in Thai children reported that Mycoplasma pneumoniae can be detected in children aged 2–5 years [9]. In contrast, other studies showed that Mycoplasma pneumoniae was found in children older than 4 years of age [8,25]. Bordetella pertussis can be detected in infants younger than 6 months whose mothers did not receive tetanus-diphtheria and pertussis (Tdap) vaccination during pregnancy, as infants have not yet completed the full primary immunization against pertussis [24,29]. Since this study had a very small number of infants younger than 6 months, it is not surprising that pertussis could not be detected in this group.
In our study, the viral-viral co-infection rate was found to be 15.4 % and mainly involved HRV and RSV. In the literature, the reported rate of viral–viral coinfection in respiratory tract infection ranges between 9.5 % and 46.9 % [28,[30], [31], [32]]. The clinical significance of multiple viral pathogens has been previously explored, but there is still conflicting evidence regarding the impact of viral co-infections on disease severity [33]. RSV alone or in combination with other viruses are reported to be more severe than other viruses in young children [16,34]. In addition, influenza-infected adults and children who were co-infected with non-rhinovirus had a higher severity than rhinovirus coinfection [35]. A study by Mazur et al. found that RSV co-infection with adenovirus and influenza virus was associated with more severe disease [15]. In contrast, other studies showed that there were no significant differences between patients with single viral infection and dual viral infection [25,36].
Overall, we recorded 37.3 % of antibacterial agent prescriptions in viral respiratory tract infection. Most of these antibacterial agents were prescribed on days 3–5 of the illness when clinically indicated for superimposed secondary bacterial infection. A recent systematic review showed that Streptococcus pneumoniae was the most common bacteria that were co-infected among hospitalized cases of influenza A(H1N1)2009pdm pneumonia [37], and the incidence of bacterial co-infection was as high as 23 %. In addition, sequential infection by RSV and bacterial pathogens has been documented since RSV induces adherence of Streptococcus pneumoniae, Pseudomonas aeruginosa, and Haemophilus influenzae to airway epithelial cells [38]. Given that rapid multiplex PCR-based testing in this study could not identify common bacterial pathogens that follow viral respiratory infections, antibacterial agents prescribed to pediatric patients in the present study were not discontinued even after the availability of the respiratory panel results. A previous study also reported an insignificant change in prescribing medication after the release respiratory panel testing results [14]. However, in specific instances where respiratory panel tests detected Mycoplasma pneumoniae, Bordetella pertussis, or Chlamydia pneumoniae, treatment would be adjusted to the most suitable antibiotic [14]. It is important to note that in the present study, Mycoplasma pneumoniae and Bordetella pertussis were not detected, so such treatment change did not apply. Recent studies found that the use of multiplex PCR test in pediatric patients was associated with a shorter antimicrobial day of therapy [23,39], but our study could not identify such association. Manatrey-Lancaster et al. found that the impact of respiratory panel testing on antimicrobial duration of therapy varies by clinical setting, and reductions were observed only among patients in the emergency department setting, as opposed to the hospitalized group [40]. Another study by Dinh et al. found no significant changes in hospitalization costs, hospital length of-stay, or rates of ventilator-associated pneumonia following the adoption of respiratory viral panel testing [41].
Regarding antiviral prescription, there were two patients who received empirical oseltamivir, which was later discontinued after the test results showed that they were infected with PIV type I. The benefit of rapid molecular-based panel testing on appropriate antiviral prescription has been previously reported [14], although data on the cost-effectiveness between rapid multiplex PCR-based testing versus single PCR for influenza is still lacking.
To the best of our knowledge, this study was the first study in Thailand that explored the circulating pathogens, clinical diagnosis, and co-infection combinations of ARIs in hospitalized children using the multiplex PCR point-of-care testing. Due to the lack of control group without multiplex PCR testing, data presented in this study were insufficient to determine the impact of multiplex PCR testing on clinical management such as diminishing antibiotic duration and usage, reducing duration of hospitalization and total inpatient hospitalization costs. Additionally, this study was conducted in a tertiary hospital in Bangkok and may not be generalizable to other areas in Thailand. The COVID-19 pandemic lockdown in 2020 resulted in the disruption of patient enrolment and sample collection of this study [42]. Furthermore, personal protective measures such as staying home, wearing masks, and washing hands have played a role in reducing cross-infection and the risk of viral respiratory tract infection resulting in the decreased number of outpatient and emergency department visits with respiratory tract infection in 2020. Due to the small sample size and the disruption in patient enrolment, the seasonality of viral pathogens could not be determined. Furthermore, a large proportion of RSV- and RV/EV-positive samples cannot be further amplified and sequenced for genotyping due to the limited sensitivity of the conventional PCR assay.
In conclusion, this study provides additional insights into the pathogen profiles, clinical diagnosis, and co-infection combinations of ARIs in hospitalized children. This information is useful for diagnosis and treatment of ARIs, as well as implementation of appropriate infection control measures and guidance for future vaccine policy development. More studies on the cost effectiveness and benefits of molecular-based point-of-care testing are required to better understand how to implement this test in children.
Ethics statement
This study was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization-Good Clinical Practice Guidelines (ICH-GCP). The Institutional Review Board of the Faculty of Medicine, Chulalongkorn University (IRB 494/62) approved this study.
Funding statement
This work is supported by the Research Grant of the Centre of Excellence in Clinical Virology, Department of Pediatrics, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand.
Data availability statement
Data will be made available on request.
CRediT authorship contribution statement
Nasamon Wanlapakorn: Writing – review & editing, Writing – original draft, Supervision, Methodology, Formal analysis, Conceptualization. Ilada Thongpan: Writing – original draft, Methodology, Conceptualization. Nasiri Sarawanangkoor: Writing – original draft, Formal analysis. Preeyaporn Vichaiwattana: Methodology. Chompoonut Auphimai: Project administration, Methodology. Donchida Srimuan: Project administration. Thaksaporn Thatsanathorn: Project administration. Lalida Kongkiattikul: Writing – review & editing, Project administration. Stephen J. Kerr: Writing – review & editing, Methodology, Formal analysis. Yong Poovorawan: Writing – review & editing, Supervision, Funding acquisition, Formal analysis, Conceptualization.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Yong Poovorawan reports financial support was provided by Chulalongkorn University. Yong Poovorawan reports equipment, drugs, or supplies was provided by QIAGEN GmbH.
Acknowledgements
We thank all participants for their contributions to the project. We thank the pediatric residents of King Chulalongkorn Memorial Hospital for data collection from hospitalized patients and Ms. Farsai Chiewbangyang and Ms. Nichanan Nettakul for their assistance with data cleaning and analysis.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e22300.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
References
- 1.Perin J., Mulick A., Yeung D., et al. Global, regional, and national causes of under-5 mortality in 2000-19: an updated systematic analysis with implications for the Sustainable Development Goals. Lancet Child Adolesc Health. 2022;6:106–115. doi: 10.1016/S2352-4642(21)00311-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu Y., Li W., Yang B., et al. Epidemiological and virological characteristics of respiratory tract infections in children during COVID-19 outbreak. BMC Pediatr. 2021;21:195. doi: 10.1186/s12887-021-02654-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chieochansin T., Samransamruajkit R., Chutinimitkul S., et al. Human bocavirus (HBoV) in Thailand: clinical manifestations in a hospitalized pediatric patient and molecular virus characterization. J. Infect. 2008;56:137–142. doi: 10.1016/j.jinf.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soonnarong R., Thongpan I., Payungporn S., et al. Molecular epidemiology and characterization of human coronavirus in Thailand, 2012-2013. SpringerPlus. 2016;5:1420. doi: 10.1186/s40064-016-3101-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pratheepamornkull T., Ratanakorn W., Samransamruajkit R., et al. Causative agents of severe community aquired viral pneumonia among children in Eastern Thailand. Southeast Asian J Trop Med Public Health. 2015;46:650–656. [PubMed] [Google Scholar]
- 6.Chinthanate S., Wanlapakorn N., Puenpa J., et al. Pertussis in Thai adult and pediatric patients presenting with prolonged acute cough. Southeast Asian J Trop Med Public Health. 2018;49:447–455. [Google Scholar]
- 7.Izu A., Nunes M.C., Solomon F., et al. All-cause and pathogen-specific lower respiratory tract infection hospital admissions in children younger than 5 years during the COVID-19 pandemic (2020-22) compared with the pre-pandemic period (2015-19) in South Africa: an observational study. Lancet Infect. Dis. 2023;S1473–3099(23) doi: 10.1016/S1473-3099(23)00200-1. 00200-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ling Y., Zhang T., Guo W., et al. Identify clinical factors related to Mycoplasma pneumoniae pneumonia with hypoxia in children. BMC Infect. Dis. 2020;20:534. doi: 10.1186/s12879-020-05270-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lochindarat S., Suwanjutha S., Prapphal N., et al. Mycoplasma pneumoniae and Chlamydophila pneumoniae in children with community-acquired pneumonia in Thailand. Int. J. Tubercul. Lung Dis. 2007;11:814–819. [PubMed] [Google Scholar]
- 10.Ouafi M., Dubos F., Engelman I., et al. Rapid syndromic testing for respiratory viral infections in children attending the emergency department during COVID-19 pandemic in Lille, France, 2021-2022. J. Clin. Virol. 2022;153 doi: 10.1016/j.jcv.2022.105221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parčina M., Schneider U.V., Visseaux B., et al. Multicenter evaluation of the QIAstat Respiratory Panel-A new rapid highly multiplexed PCR based assay for diagnosis of acute respiratory tract infections. PLoS One. 2020;15 doi: 10.1371/journal.pone.0230183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brendish N.J., Malachira A.K., Clark T.W. Molecular point-of-care testing for respiratory viruses versus routine clinical care in adults with acute respiratory illness presenting to secondary care: a pragmatic randomised controlled trial protocol (ResPOC) BMC Infect. Dis. 2017;17:128. doi: 10.1186/s12879-017-2219-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brendish N.J., Malachira A.K., Armstrong L., et al. Routine molecular point-of-care testing for respiratory viruses in adults presenting to hospital with acute respiratory illness (ResPOC): a pragmatic, open-label, randomised controlled trial. Lancet Respir. Med. 2017;5:401–411. doi: 10.1016/S2213-2600(17)30120-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Almannaei L., Alsaadoon E., AlbinAli S., et al. A retrospective study examining the clinical significance of testing respiratory panels in children who presented to a tertiary hospital in 2019. Access Microbiol. 2022;4 doi: 10.1099/acmi.0.000332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mazur N.I., Bont L., Cohen A.L., et al. Severity of respiratory syncytial virus lower respiratory tract infection with viral coinfection in HIV-Uninfected children. Clin. Infect. Dis. 2017;64:443–450. doi: 10.1093/cid/ciw756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aberle J.H., Aberle S.W., Pracher E., et al. Single versus dual respiratory virus infections in hospitalized infants: impact on clinical course of disease and interferon-gamma response. Pediatr. Infect. Dis. J. 2005;24:605–610. doi: 10.1097/01.inf.0000168741.59747.2d. [DOI] [PubMed] [Google Scholar]
- 17.Tabatabai J., Ihling C.M., Manuel B., et al. Viral etiology and clinical characteristics of acute respiratory tract infections in hospitalized children in southern Germany (2014-2018) Open Forum Infect. Dis. 2023;10:ofad110. doi: 10.1093/ofid/ofad110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization WHO surveillance case definitions for ILI and SARI Influenza surveillance and monitoring. https://www.who.int/teams/global-influenza-programme/surveillance-and-monitoring/case-definitions-for-ili-and-sari Available from:
- 19.Leber A.L., Lisby J.G., Hansen G., et al. Multicenter evaluation of the QIAstat-dx respiratory panel for detection of viruses and bacteria in nasopharyngeal swab specimens. J. Clin. Microbiol. 2020;58 doi: 10.1128/JCM.00155-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Auksornkitti V., Kamprasert N., Thongkomplew S., et al. Molecular characterization of human respiratory syncytial virus, 2010-2011: identification of genotype ON1 and a new subgroup B genotype in Thailand. Arch. Virol. 2014;159:499–507. doi: 10.1007/s00705-013-1773-9. [DOI] [PubMed] [Google Scholar]
- 21.Linsuwanon P., Payungporn S., Samransamruajkit R., et al. High prevalence of human rhinovirus C infection in Thai children with acute lower respiratory tract disease. J. Infect. 2009;59:115–121. doi: 10.1016/j.jinf.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumar S., Stecher G., Li M., et al. Mega X: molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018;35:1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tiew W.T., Chen Y.C., Hsiao H.L., et al. Impact of multiplex polymerase chain reaction syndromic panel on antibiotic use among hospitalized children with respiratory tract illness during COVID-19 pandemic. J. Microbiol. Immunol. Infect. 2023;S1684–1182(23) doi: 10.1016/j.jmii.2023.01.009. 00009-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lamrani Hanchi A., Guennouni M., Rachidi M., et al. Epidemiology of respiratory pathogens in children with severe acute respiratory infection and impact of the multiplex PCR film array respiratory panel: a 2-year study. Internet J. Microbiol. 2021;2021 doi: 10.1155/2021/2276261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nolan V.G., Arnold S.R., Bramley A.M., et al. Etiology and impact of coinfections in children hospitalized with community-acquired pneumonia. J. Infect. Dis. 2018;218:179–188. doi: 10.1093/infdis/jix641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thongpan I., Mauleekoonphairoj J., Vichiwattana P., et al. Respiratory syncytial virus genotypes NA1, ON1, and BA9 are prevalent in Thailand, 2012-2015. PeerJ. 2017;5 doi: 10.7717/peerj.3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thongpan I., Vichaiwattana P., Vongpunsawad S., et al. Upsurge of human rhinovirus infection followed by a delayed seasonal respiratory syncytial virus infection in Thai children during the coronavirus pandemic. Influenza Other Respir Viruses. 2021;15:711–720. doi: 10.1111/irv.12893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cason C., Zamagni G., Cozzi G., et al. Spread of respiratory pathogens during the COVID-19 pandemic among children in the northeast of Italy. Front. Microbiol. 2022;13 doi: 10.3389/fmicb.2022.804700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muloiwa R., Wolter N., Mupere E., et al. Pertussis in africa: findings and recommendations of the global pertussis initiative (GPI) Vaccine. 2018;36:2385–2393. doi: 10.1016/j.vaccine.2018.03.025. [DOI] [PubMed] [Google Scholar]
- 30.Akkoc G., Dogan C., Bayraktar S., et al. Evaluation of viral respiratory pathogens in children aged under five hospitalized with lower respiratory tract infections. North Clin Istanb. 2022;9:162–172. doi: 10.14744/nci.2021.69923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang H., Chen S., Zhang X., et al. Detection and clinical characteristics analysis of respiratory viruses in hospitalized children with acute respiratory tract infections by a GeXP-based multiplex-PCR assay. J. Clin. Lab. Anal. 2020;34 doi: 10.1002/jcla.23127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.El Kholy A.A., Mostafa N.A., Ali A.A., et al. The use of multiplex PCR for the diagnosis of viral severe acute respiratory infection in children: a high rate of co-detection during the winter season. Eur. J. Clin. Microbiol. Infect. Dis. 2016;35:1607–1613. doi: 10.1007/s10096-016-2698-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goka E.A., Vallely P.J., Mutton K.J., et al. Single and multiple respiratory virus infections and severity of respiratory disease: a systematic review. Pediatr Respir Rev. 2014;15:363–370. doi: 10.1016/j.prrv.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ueno F., Tamaki R., Saito M., et al. Age-specific incidence rates and risk factors for respiratory syncytial virus-associated lower respiratory tract illness in cohort children under 5 years old in the Philippines. Influenza Other Respir Viruses. 2019;13:339–353. doi: 10.1111/irv.12639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Esper F.P., Spahlinger T., Zhou L. Rate and influence of respiratory virus co-infection on pandemic (H1N1) influenza disease. J. Infect. 2011;63:260–266. doi: 10.1016/j.jinf.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rhedin S., Lindstrand A., Rotzén-Östlund M., et al. Clinical utility of PCR for common viruses in acute respiratory illness. Pediatrics. 2014;133:e538–e545. doi: 10.1542/peds.2013-3042. [DOI] [PubMed] [Google Scholar]
- 37.MacIntyre C.R., Chughtai A.A., Barnes M., et al. The role of pneumonia and secondary bacterial infection in fatal and serious outcomes of pandemic influenza a(H1N1)pdm09. BMC Infect. Dis. 2018;18(1):637. doi: 10.1186/s12879-018-3548-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hendaus M.A., Jomha F.A., Alhammadi A.H. Virus-induced secondary bacterial infection: a concise review. Ther Clin Risk Manag. 2015;11:1265–1271. doi: 10.2147/TCRM.S87789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Subramony A., Zachariah P., Krones A., et al. Impact of multiplex polymerase chain reaction testing for respiratory pathogens on healthcare resource utilization for pediatric inpatients. J. Pediatr. 2016;173:196–201.e2. doi: 10.1016/j.jpeds.2016.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Manatrey-Lancaster J.J., Bushman A.M., Caligiuri M.E., Rosa R. Impact of BioFire FilmArray respiratory panel results on antibiotic days of therapy in different clinical settings. Antimicrob Steward Healthc Epidemiol. 2021;1(1):e4. doi: 10.1017/ash.2021.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dinh J., Hinkle C.F., Law A.C., et al. Effect of respiratory viral panel adoption on antibiotic use in ventilated patients. Ann Am Thorac Soc. 2023 Sep 25 doi: 10.1513/AnnalsATS.202304-326OC. Epub ahead of print. PMID: 37748086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Suntronwong N., Thongpan I., Chuchaona W., et al. Impact of COVID-19 public health interventions on influenza incidence in Thailand. Pathog. Glob. Health. 2020;114:225–227. doi: 10.1080/20477724.2020.1777803. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.