Abstract
Study Objectives:
This study evaluated the effects of early time-restricted eating (eTRE) on shifting the timing of sleep among late sleepers. Primary outcomes included actigraphy- and sleep diary–derived sleep onset, midsleep phase, and wake time with total sleep time as a secondary outcome.
Methods:
Fifteen healthy adults with habitual late sleep timing were randomized to receive either eTRE or sleep and nutrition hygiene (control) via a single 30-minute synchronous video session. Participants completed an initial 1-week baseline phase followed by a 2-week intervention phase. Measures included continuous sleep monitoring and sleep and nutrition diaries.
Results:
Linear mixed-effects modeling demonstrated that eTRE significantly advanced sleep timing compared with controls. Self-reported sleep onset (56.1 [95% confidence interval: 20.5, 91.7] minutes), midpoint (19.5 [7.2, 31.9] minutes), and offset (42.2 [2.9, 81.5] minutes) each moved earlier in eTRE as compared with controls. Similarly, objectively determined sleep onset (66.5 [29.6, 103.4] minutes), midpoint (21.9 [9.1, 34.7] minutes), and offset (39.3 [1.3, 77.3] minutes) each moved earlier in eTRE as compared with controls. Total sleep time showed a nonsignificant increase in the eTRE group as compared with controls.
Conclusions:
Late sleepers who were instructed in a single session about eTRE significantly advanced their sleep timing, especially sleep onset. eTRE shows potential as a clinical strategy for advancing sleep timing in late sleepers.
Clinical Trial Registration: Registry: Chinese Clinical Trial Registry; Name: FAST Asleep: It’s All About Timing; URL: https://www.chictr.org.cn/showproj.html?proj=122504; Identifier: ChiCTR2100043691.
Citation:
Blum DJ, Hernandez B, Zeitzer JM. Early time-restricted eating advances sleep in late sleepers: a pilot randomized controlled trial. J Clin Sleep Med. 2023;19(12):2097–2106.
Keywords: early time-restricted eating, sleep, treatment
BRIEF SUMMARY
Current Knowledge/Study Rationale: Early time-restricted eating is a newer technique that is commonly used to adjust the behavior and timing of the food-entrainable oscillator. We wished to determine whether this technique could also aid in the adjustment of sleep timing.
Study Impact: We found that, with only a single, 30-minute intervention, we were able to move the timing of sleep to an earlier hour in late sleepers. While a small pilot study, our findings indicate the potential clinical effectiveness of this intervention in individuals with late sleep.
INTRODUCTION
The timing of sleep is influenced by several factors, including internal preference (“chronotype”), which is driven by the central circadian pacemaker in the suprachiasmatic nucleus, and social constraints (eg, work, school, family). Late bedtimes are often associated with poor health outcomes, including depression,1 suicidality,2 impaired cognitive performance,3 insulin resistance, obesity, and diabetes4,5 and may lead to short sleep duration due to fixed, early-morning responsibilities. Individuals who prefer a later bedtime may find it difficult to “try” to go to sleep earlier, as the suprachiasmatic nucleus provides a strong wake signal in the hours just before normal bedtime.6 Consistent use of morning light, and avoidance of evening light, can help some individuals go to sleep earlier by shifting the timing of the suprachiasmatic nucleus to an earlier hour.7 However, given the ubiquity of device use at night (ie, smartphones, tablets, etc8,9), absolute avoidance of light at night is problematic. One potential alternative mechanism to move sleep timing earlier is to engender changes in the food-entrainable oscillator.
The food-entrainable oscillator is a powerful secondary clock in mammals capable of synchronizing peripheral tissues, entraining body temperature, and helping to adjust sleep and wake independently of light–dark rhythms.10 Preliminary work has shown that leveraging the food-entrainable oscillator using early time-restricted eating (eTRE; eg, eating only between 8 am to 4 pm) may rapidly reset peripheral clocks and reduce jet lag symptoms, including disruptions to sleep, mood, and concentration.11 This method consists of 2 primary components: a fast of longer than 16 hours followed by a large meal in the targeted “morning.” Synchronizing peripheral clocks using time-restricted eating also appears to optimize metabolic health (eg, lowering hemoglobin A1c, reducing insulin response), independently of weight loss, but only when done early as time-restricted eating beginning at midday (eg, 12 pm to 8 pm) has produced mixed results.12,13 Based on these emerging data, strategic fasting (ie, eTRE) could be a potentially helpful countermeasure to circadian desynchrony and its negative sequelae.
Whereas prior eTRE research has predominantly focused on leveraging the food-entrainable oscillator to improve metabolic functioning, our primary goal was to use eTRE to improve sleep. Specifically, we sought to explore if eTRE could be used to advance the timing of sleep. As a secondary outcome we examined if eTRE increased the amount of sleep (total sleep time [TST]).
METHODS
Participants
From February 2021 to October 2021, community-based adults were recruited via local advertisements on the NYU Shanghai campus and referrals from the broader community. Participants were included in the study if they (1) self-reported late sleep timing (ie, difficulties falling asleep before 1 am) and (2) were fluent in English (ie, did not require translation services to engage in the study). Exclusion criteria included (1) currently active shift workers, (2) history of cardiometabolic health issues (eg, diabetes, cardiovascular disease), or (3) history of a diagnosed eating disorder.
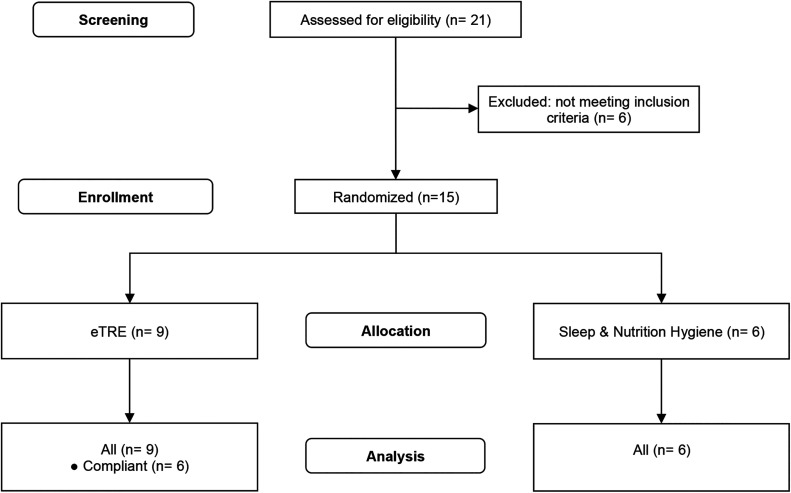
The online inquiry form was completed by 21 individuals (Figure 1), 6 of whom were not eligible for this study. The remaining 15 individuals completed screening, met criteria, and were consented to participate in the study during the screening interview. This study was approved by the Institutional Review Board at NYU Shanghai and was registered at http://www.ChiCTR.org.cn.
Figure 1. CONSORT diagram.
Flowchart of participants through the trial. Three individuals allocated to receive eTRE did not comply with the study protocol. CONSORT = Consolidated Standards of Reporting Trials, eTRE = early time-restricted eating.
Procedures
Study design
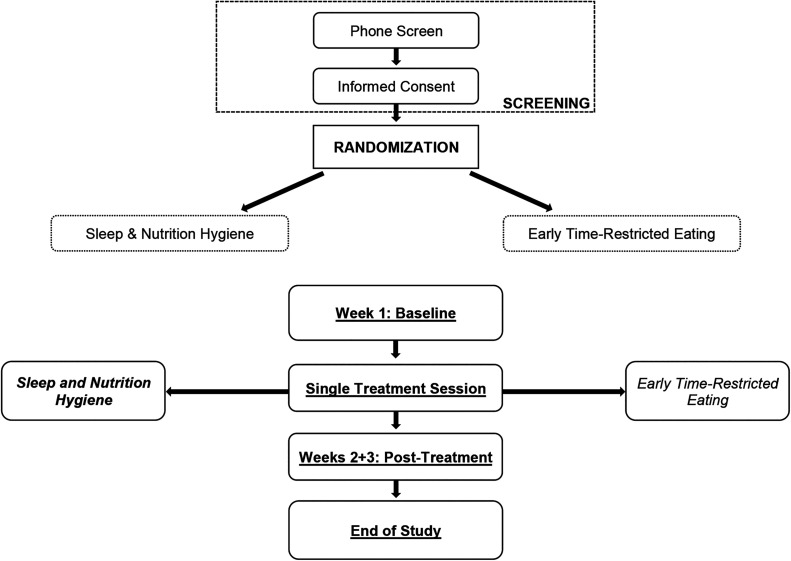
This study (Figure 2) was a randomized controlled trial design with 2 parallel groups comparing eTRE with a sleep and nutrition hygiene control treatment. Participants were randomized using block randomization (block size = 6).
Figure 2. Study design.
Flowchart of participants through the trial.
Screening
Individuals who were interested in participating initially completed a brief online questionnaire that included the basic inclusion/exclusion criteria and contact information. Individuals who met initial eligibility were then invited to conduct a phone interview with a sleep specialist/licensed psychologist to confirm eligibility using a brief clinical interview, cardiovascular health screening tool,14 and the Eating Disorder Examination questionnaire (EDE 6.015). Individuals with low risk on both questionnaires (ie, a negative history of 1 or more cardiometabolic health issues, 2 or more risk factors for cardiometabolic disease, or an EDE total < 1.27) and met inclusion/exclusion criteria following the clinical interview were randomized to either the active treatment or placebo condition.
Treatment overview
The treatment phase of the study included a baseline appointment and one 30-minute video session, the content of which varied between the 2 arms of the study (eTRE or sleep and nutrition hygiene). At the baseline appointment, participants completed web-based self-reported questionnaires administered via Qualtrics (Qualtrics, Provo, UT), reviewed instructions for wearing the Readiband V sleep and activity monitor (Fatigue Science, Inc, Vancouver, BC, Canada), were taught how to complete the sleep and nutrition diary, and scheduled their 30-minute intervention session. All participants wore a Readiband and tracked their sleep and nutrition (ie, the first and last intake of food or beverage excluding water) daily for a total of 3 weeks (1 week baseline, 2 weeks posttreatment). After 3 weeks of sleep and nutrition monitoring, participants completed the poststudy questionnaires and returned the Readibands.
Measures
Phone screen
Prospective participants were asked questions about their age, sleep timing, pregnancy, work schedule, history of and risk for cardiovascular health issues (AHA/ACSM [American Heart Association/American College of Sports Medicine] Health/Fitness Facility Preparticipation Screening Questionnaire), and history of and risk for an eating disorder (EDE 6.0). The AHA/ACSM Health/Fitness Facility Preparticipation Screening Questionnaire is a 32-item questionnaire that assesses the risk for cardiovascular health issues. It is split into 2 sections: (1) history of cardiometabolic diagnoses and symptoms and (2) current cardiovascular risk factors. Potential participants were excluded if they endorsed ≥ 1 item on section 1 and/or endorsed ≥ 2 items on section 2.
The EDE 6.0 is a valid and reliable 28-item questionnaire that assesses the risk for an eating disorder.16 The EDE 6.0 has high internal consistency (Cronbach’s α coefficient is 0.95) and high discriminative validity (area under the receiver operating characteristic curve [AUC] of 0.96). A cutoff score > 1.27 was used to determine risk for an eating disorder.16,17
Self-report measures
Participants completed self-report measures assessing sleep quality, daytime sleepiness, mood, and anxiety during the baseline week and at the end of the study.
The Pittsburg Sleep Quality Index (PSQI) is a widely used 19-item questionnaire that assesses sleep quality, sleep latency, sleep duration, sleep efficiency, sleep disturbances, sleep medication use, and daytime energy. The PSQI has good internal consistency (Cronbach’s α = 0.70–0.83), with higher scores (> 5) indicating poor sleep quality.18
The Epworth Sleepiness Scale (ESS) was used to assess excessive daytime sleepiness. It is one of the most commonly used self-report questionnaires and has shown to have good internal consistency (Cronbach’s α ranges from 0.73 to 0.86) and moderate concurrent validity with objective tests of sleepiness.19
Mood was assessed using the 9-item Patient Health Questionnaire (PHQ-9), a 9-item depression measure that assesses depressive symptoms over the past 2 weeks. The PHQ-9 reports excellent internal consistency (Cronbach’s α = 0.89).20
Anxiety was assessed using the 7-item Generalized Anxiety Disorder (GAD-7). The GAD-7 is a 7-item questionnaire that assesses anxious symptoms and reports excellent internal consistency (Cronbach’s α = 0.92).21
Sleep diary
Prospective sleep patterns were recorded using a daily sleep diary based on the Consensus Sleep Diary.22 The Consensus Sleep Diary is the gold-standard self-reported prospective measure of sleep and requires participants to answer the following questions upon awakening to start their day: bedtime, lights out, sleep-onset latency, number of awakenings, wake after sleep onset, wake time, rise time. Based on these values, additional aspects of sleep were calculated including sleep-onset time, midsleep phase (MSP), TST, time in bed, and sleep efficiency.
To assess for fasting duration, the sleep diary also included daily prompts for the first intake of food or beverage (excluding water) and the last intake of food or beverage (excluding water).
Wrist activity monitor
Readiband V5 wrist activity monitors were used to objectively measure movement, which can be used to impute sleep patterns. This device uses a proprietary algorithm to impute sleep and wake and performs comparably to research-grade actigraphy (ie, Philips Respironics Actiwatch 223). For this study, analyses of Readiband V5 data focused on sleep-onset time, MSP, wake time, and TST.
Treatment conditions
Early time-restricted eating
The eTRE intervention consisted of one 30-minute session conducted on synchronous video by a licensed clinical psychologist. This session included a novel strategy for advancing sleep timing in which participants were instructed to adhere to a water-only fast for ≥ 16 hours between last food intake and first intake of food anchored to the desired rise time (eg, fasting from 4 pm until 8 am) and sleep and nutrition hygiene (ie, 2-process model of sleep and wake, normal sleep architecture, sleep hygiene). Participants were instructed to adhere to this eTRE protocol for 2 weeks (14 days) after attending the virtual session.
Sleep and nutrition hygiene
Participants randomized to the control condition also received one 30-minute session conducted on synchronous video by a licensed clinical psychologist. The control group received information related to the 2-process model of sleep and wake, normal sleep architecture, sleep hygiene (eg, maintain regular sleep schedule, don’t exercise too close to bedtime), and nutrition hygiene (eg, don’t eat within 2 hours of bedtime, refrain from caffeine 6–8 hours prior to bedtime).
Data analysis
Linear mixed-effects modeling was used to calculate primary outcome measures using SAS version 9.4 PROC MIXED (SAS Institute, Cary, NC). Changes in actigraphy- and sleep diary–derived sleep-onset time, MSP, and wake time were analyzed as primary outcome measures. Secondary outcomes included changes in actigraphy- and sleep diary–derived TST as well as changes in self-reported sleep (ESS, PSQI) and mood (PHQ-9, GAD-7). Pre- to post-treatment changes in questionnaire data were analyzed using 2-sided unpaired t tests or Mann-Whitney U tests for nonnormal data. Significance level was set at .05; 2-sided P values are reported on all tests.
We powered our sample size based on a prior report examining the impact of time-restricted eating on sleep that reported odds ratios of 7.5 (flying eastward from the United States to South Korea) and 16.2 (flying westward back to the United States) regarding symptoms of jet lag.11 We conservatively, therefore, predicted a medium effect size of our intervention. As such, using G*Power 3.0 (Universität Kiel, Germany), we estimated that a sample size of 12 (6 eTRE, 6 control) was sufficient to detect a clinically significant improvement in sleep timing based on an effect size of 0.5, power of 0.8, and 2 measurements comparing actigraphy- and sleep log–derived MSP pre- and post-intervention.
RESULTS
Participant characteristics
A total of 15 participants (male = 4, female = 10, preferred not to disclose = 1) were randomized to receive eTRE (n = 9) or sleep and nutrition hygiene (n = 6). There were no statistically significant differences between the groups in terms of age, sex ratio, education, ethnicity, marital status, or body mass index (Table 1). The groups were also similar in their mood (GAD-7, PHQ-9) scores, with approximately half of the participants from each group endorsing mild symptoms of anxiety or depression (Table 1). Groups were similar in their summary sleep (ESS, PSQI; Table 1) and showed no statistically significant differences at baseline with regard to both sleep diary–derived and wrist activity monitor–derived sleep parameters (Table 2).
Table 1.
Baseline participant demographic characteristics for both eTRE and control groups.
| eTRE (n = 9) | Control (n = 6) | P * | |
|---|---|---|---|
| Age, mean (SD), y | 29.6 (9.9) | 22.0 (4.6) | .11 |
| Female, n (%) | 6 (67) | 4 (67) | 1.00 |
| Bachelor’s/graduate degree, n (%) | 5 (56) | 3 (50) | 1.00 |
| Ethnicity, n (%) | |||
| East Asian | 5 (56) | 5 (83) | .65 |
| South Asian | 1 (11) | 0 | |
| White | 2 (22) | 0 | |
| Latinx | 1 (11) | 0 | |
| Mixed | 0 | 1 (17) | |
| Single, n (%) | 7 (78) | 6 (100) | .49 |
| BMI, mean (SD) | 23.1 (5.1) | 18.9 (1.4) | .04 |
| (n = 8) | (n = 6) | ||
| ESS | 8.8 (3.8) | 7.2 (5.2) | .52 |
| ≥ 11, n (%) | 3 (37) | 2 (33) | 1.00 |
| PSQI | 8.5 (3.0) | 7.2 (4.1) | .49 |
| ≥ 5, n (%) | 8 (100) | 5 (83) | .43 |
| GAD-7 | 4.0 (2.9) | 4.3 (5.4) | .88 |
| ≥ 10, n (%) | 0 (0) | 1 (17) | .43 |
| PHQ-9 | 4.8 (4.0) | 4.2 (5.3) | .82 |
| ≥ 5, n (%) | 5 (63) | 2 (33) | .59 |
Difference between groups tested with unpaired t tests for age, BMI, ESS, PSQI, GAD-7, and PHQ-9, and with Fisher’s exact test 2-sided probability for Female, Bachelor’s/graduate degree, Ethnicity, and Single. BMI = body mass index, ESS = Epworth Sleepiness Scale, eTRE = early time-restricted eating, GAD-7 = 7-item Generalized Anxiety Disorder, PHQ-9 = 9-item Patient Health Questionnaire.
Table 2.
Sleep characteristics.
| Wrist Activity Monitor | Sleep Diary | |||||||
|---|---|---|---|---|---|---|---|---|
| eTRE | Control | eTRE | Control | |||||
| Baseline | Treatment | Baseline | Treatment | Baseline | Treatment | Baseline | Treatment | |
| TST (hours) | ||||||||
| Compliant only | 6.86 (0.77) | 7.12 (0.80) | 7.09 (0.61) | 7.55 (0.63) | ||||
| All participant | 6.85 (0.81) | 7.07 (0.74) | 6.43 (0.79) | 6.43 (1.04) | 7.13 (0.63) | 7.55 (0.63) | 6.41 (0.98) | 6.71 (1.51) |
| SON (time) | ||||||||
| Compliant only | 1:12 (71.9 min) | 0:24 (57.7 min) | 1:14 (43.7 min) | 0:16 (48.5 min) | ||||
| All participant | 1:02 (65.5 min) | 0:29 (52.3 min) | 1:47 (28.1 min) | 2:09 (32.3 min) | 1:02 (38.8 min) | 0:26 (44.8 min) | 2:03 (35.4 min) | 2:00 (52.6 min) |
| WT (time) | ||||||||
| Compliant only | 8:32 (51.9 min) | 8:04 (48.2 min) | 8:24 (36.2 min) | 7:56 (42.6 min) | ||||
| All participants | 8:24 (54.8 min) | 8:07 (47.3 min) | 8:39 (55.1 min) | 8:59 (64.3 min) | 8:19 (43.8 min) | 8:07 (45.7 min) | 8:36 (63.1 min) | 8:48 (64.4 min) |
| MSP (time) | ||||||||
| Compliant only | 4:52 (58.7 min) | 4:14 (46.7 min) | 4:40 (45.1 min) | 3:58 (47.0 min) | ||||
| All participants | 4:39 (54.7 min) | 4:18 (45.5 min) | 5:09 (39.3 min) | 5:34 (40.3 min) | 4:29 (44.4 min) | 4:07 (47.4 min) | 5:16 (47.4 min) | 5:21 (40.8 min) |
Baseline and treatment sleep characteristics as determined by objective (wrist activity monitor) and self-report (sleep diary) measures for both eTRE and control groups. Data are shown as mean (SD). eTRE = early time-restricted eating, MSP = midsleep phase, SON = sleep onset, TST = total sleep time, WT = wake time.
Data attrition and adherence
Among all participants, there were acceptable levels of missing data for sleep diary variables (3.49%) and wrist activity monitors (6.35%). Three participants, randomized to the eTRE group, did not adhere fully to the protocol. Two participants adhered to eTRE but did not attempt to sleep earlier as was in the instructions. Another participant’s understanding of English was not as fluent as conveyed and did not fully understand the instructions. Additionally, 2 eTRE participants were given permission to not adhere to the eTRE protocol on preselected nights due to the conditions of the coronavirus disease 2019 (COVID-19) pandemic. The first participant went to bed later on 2 nights for regularly scheduled video calls with family. The other participant stayed up later to attend and present at international conferences via video calls. Given the small sample size, we conducted 2 sets of analyses based on all participant data and compliant-only data.
Primary outcome: sleep onset
Relative to the control group, the self-reported timing of sleep onset in the eTRE group moved 34.5 ± 15.8 minutes earlier in all participants and 56.1 ± 18.1 minutes earlier in compliant participants (Table 3). Similarly robust differences were observed in sleep-onset timing in wrist activity monitor data, with sleep timing moving 52.3 ± 17.9 minutes earlier in all participants and 66.5 ± 18.7 minutes earlier in compliant participants (Table 3).
Table 3.
Sleep onset.
| eTRE Change | Control Change | Difference in Change | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimate (SE) | P | 95% CI | Estimate (SE) | P | 95% CI | Estimate (SE) | P | 95% CI | |
| Sleep diary | |||||||||
| Compliant only | −58.3 (12.9) min | <.0001 | −83.6, –32.9 min | −2.2 (12.7) min | .86 | −27.2, 22.8 min | −56.1 (18.1) min | .002 | −91.7, –20.5 min |
| All participants | −36.6 (10.1) min | .0003 | −56.4, –16.8 min | −2.1 (12.2) min | .86 | −26.4, 21.9 min | −34.5 (15.8) min | .03 | −65.6, –3.3 min |
| Activity monitor | |||||||||
| Compliant only | −43.1 (13.1) min | .001 | −68.9, –17.4 min | 23.4 (13.4) min | .08 | −3.0, 49.7 min | −66.5 (18.7) min | .001 | −103.4, –29.6 min |
| All participants | −28.9 (11.1) min | .01 | −50.8, –7.1 min | 23.4 (14.1) min | .10 | −4.3, 51.1 min | −52.3 (17.9) min | .004 | −87.6, –17.0 min |
Baseline and posttreatment means estimated from the linear mixed-effects models for eTRE and control groups for sleep diary– and activity monitor–derived sleep onset. CI = confidence interval, eTRE = early time-restricted eating.
Primary outcome: midsleep phase
Relative to the control group, the self-reported timing of sleep midpoint in the eTRE group moved 11.5 ± 5.5 minutes earlier in all participants and 19.5 ± 6.3 minutes earlier in compliant participants (Table 4). Similar differences were observed in MSP in wrist activity monitor data, with sleep timing moving 15.3 ± 5.7 minutes earlier in all participants and 21.9 ± 6.5 minutes earlier in compliant participants (Table 4).
Table 4.
Midsleep phase.
| eTRE Change | Control Change | Difference in Change | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimate (SE) | P | 95% CI | Estimate (SE) | P | 95% CI | Estimate (SE) | P | 95% CI | |
| Sleep diary | |||||||||
| Compliant only | −17.0 (4.5) min | .0002 | −25.8, –8.2 min | 2.6 (4.4) min | .56 | −6.1, 11.2 min | −19.5 (6.3) min | .002 | −31.9, –7.2 min |
| All participants | −9.0 (3.5) min | .01 | −15.8, –2.1 min | 2.6 (4.2) min | .55 | −5.8, 10.9 min | −11.5 (5.5) min | .04 | −22.3, –0.7 min |
| Activity monitor | |||||||||
| Compliant only | −13.3 (4.5) min | .004 | −22.2, –4.3 min | 8.6 (4.7) min | .07 | −0.5, 17.8 min | −21.9 (6.5) min | .001 | −34.7, –9.1 min |
| All participants | −6.6 (3.5) min | .06 | −13.6, 0.4 min | 8.6 (4.5) min | .06 | −0.2, 17.5 min | −15.3 (5.7) min | .01 | −26.6, –4.0 min |
Baseline and posttreatment means estimated from the linear mixed-effects models for eTRE and control groups for sleep diary– and activity monitor–derived midsleep phase. CI = confidence interval, eTRE = early time-restricted eating.
Primary outcome: wake time
Relative to the control group, the self-reported timing of wake in the eTRE group moved 42.2 ± 20.0 minutes earlier in compliant participants; the 26.6 ± 17.1–minute shift to an earlier wake time in all participants was not significant (Table 5). Similar differences were observed in the timing of wake in wrist activity monitor data, with wake timing moving 39.3 ± 19.3 minutes earlier in compliant participants; the 30.0 ± 17.4 minutes earlier in all participants was not significant (Table 5).
Table 5.
Wake time.
| eTRE Change | Control Change | Difference in Change | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimate (SE) | P | 95% CI | Estimate (SE) | P | 95% CI | Estimate (SE) | P | 95% CI | |
| Sleep diary | |||||||||
| Compliant only | −28.0 (14.3) min | .05 | −56.1, 0.00 min | 14.1 (14.0) min | .31 | −13.4, 41.6 min | −42.2 (20.0) min | .04 | −81.5, –2.9 min |
| All participants | −12.5 (10.9) min | .25 | −33.9, 8.9 min | 14.2 (13.2) min | .28 | −11.7, 40.1 min | −26.6 (17.1) min | .12 | −60.2, 7.0 min |
| Activity monitor | |||||||||
| Compliant only | −22.6 (13.5) min | .10 | −49.2, 4.0 min | 16.7 (13.8) min | .23 | −10.5, 43.8 min | −39.3 (19.3) min | .04 | −77.3, –1.3 min |
| All participants | −13.3 (10.8) min | .22 | −34.5, 7.9 min | 16.7 (13.6) min | .22 | −10.2, 43.8 min | −30.0 (17.4) min | .09 | −64.2, 4.3 min |
Baseline and posttreatment means estimated from the linear mixed-effects models for eTRE and control groups for sleep diary– and activity monitor–derived wake time. CI = confidence interval, eTRE = early time-restricted eating.
Secondary outcome: total sleep time
Relative to the control group, there were no significant differences in TST per either self-report or wrist activity monitor (Table 6).
Table 6.
Total sleep time.
| eTRE Change | Control Change | Difference in Change | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimate (SE) | P | 95% CI | Estimate (SE) | P | 95% CI | Estimate (SE) | P | 95% CI | |
| Sleep diary | |||||||||
| Compliant only | 28.5 (13.9) min | .04 | 1.2, 55.8 min | 17.4 (13.7) min | .20 | −9.5, 44.3 min | 11.2 (19.5) min | .57 | −27.2, 49.5 min |
| All participants | 25.9 (10.8) min | .02 | 4.6, 47.2 min | 17.3 (13.1) min | .19 | −8.5, 43.2 min | 8.6 (17.0) min | .61 | −24.9, 42.0 min |
| Activity monitor | |||||||||
| Compliant only | 16.5 (13.1) min | .21 | −9.4, 42.3 min | −3.0 (13.4) min | .82 | −29.4, 23.4 min | 19.4 (18.7) min | .30 | −17.5, 56.3 min |
| All participants | 12.9 (10.6) min | .23 | −8.1, 33.8 min | −3.0 (13.5) min | .83 | −29.5, 23.6 min | 15.9 (17.2) min | .36 | −18.0, 49.7 min |
Baseline and posttreatment means estimated from the linear mixed-effects models for eTRE and control groups for sleep diary– and activity monitor–derived total sleep time. CI = confidence interval, eTRE = early time-restricted eating.
DISCUSSION
As compared with an education control, a single 30-minute session on eTRE elicited an advance of sleep timing by approximately 1 hour in late sleepers. The advance in sleep timing was evident in both self-reported (log) and objective (activity monitor) modalities and encompassed parallel shifts in the timing of sleep onset, sleep offset, and sleep midpoint.
We noted a non–statistically significant increase in TST as measured by both actigraphy (19.4 minutes) and sleep diaries (11.2 minutes). Given the high degree of night-to-night variability in TST, especially among late sleepers,24 we were not powered to detect these differences. Post hoc power analyses indicated that we would have needed 53 participants in each group to detect such a difference. If these gains were to hold true, they would be clinically significant and on par with increases in TST generated by 6 to 8 weeks of cognitive behavioral treatment for insomnia, the gold-standard treatment for insomnia.25
There were several limitations. As noted, the small sample size did not allow for detection of clinically meaningful changes in TST. We also had one-third of the eTRE group failing to adhere to the protocol. As mentioned previously, these were seemingly unrelated to the protocol itself (2 failed to understand that they needed to follow the earlier sleep timing in addition to eTRE; 1 presented with significant language difficulties), although a larger sample may reveal otherwise. Next, early meal timing can be challenging to adhere to due to cultural, social, and work requirements. Additionally, late sleepers may have difficulties eating earlier due to a delay in the natural timing of their hunger/appetite. The sample was also more socioeconomically advantaged compared with the general public and thus our results may not be generalizable to the general public. Finally, we did not have long-term follow-up, so do not know whether the shift to an earlier time would be stable over an extended time.
Overall, we found that a brief, single-session intervention to teach about eTRE was sufficient to advance the sleep of late sleepers by 1 hour. The present study was the first to trial eTRE as a sleep intervention in a randomized controlled trial. It shows intriguing potential as a novel strategy for both advancing sleep timing as well as increasing sleep duration in late sleepers. Future research is required to validate this method on larger, more representative samples, elucidate the mechanistic aspects of this technique, and explore the potential of eTRE in related paradigms of circadian misalignment, such as shift work, jetlag, and circadian rhythm sleep-wake disorders.
DISCLOSURE STATEMENT
All authors have contributed to and approved the manuscript. Work for this study was performed at NYU Shanghai. Financial support was provided by NYU Shanghai. The authors report no conflicts of interest.
ACKNOWLEDGMENTS
The authors thank NYU Shanghai for providing funding to conduct this study and the participants for their time and effort. Additionally, they acknowledge the helpful assistance and consultation of Dr. Fiona Barwick in formulating this study.
ABBREVIATIONS
- EDE
Eating Disorder Examination questionnaire
- eTRE
early time-restricted eating
- GAD-7
7-item Generalized Anxiety Disorder
- MSP
midsleep phase
- PHQ-9
9-item Patient Health Questionnaire
- PSQI
Pittsburg Sleep Quality Index
- TST
total sleep time
REFERENCES
- 1. Richardson C , Gradisar M . Depressed mood and repetitive negative thinking in delayed sleep-wake phase disorder: treatment effects and a comparison with good sleepers . J Sleep Res. 2022. ; 31 ( 1 ): e13452 . [DOI] [PubMed] [Google Scholar]
- 2. Gau SS-F , Shang C-Y , Merikangas KR , Chiu YN , Soong WT , Cheng AT . Association between morningness-eveningness and behavioral/emotional problems among adolescents . J Biol Rhythms. 2007. ; 22 ( 3 ): 268 – 274 . [DOI] [PubMed] [Google Scholar]
- 3. Goldstein D , Hahn CS , Hasher L , Wiprzycka UJ , Zelazo PD . Time of day, intellectual performance, and behavioral problems in morning versus evening type adolescents: is there a synchrony effect? Pers Individ Dif. 2007. ; 42 ( 3 ): 431 – 440 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Depner CM , Melanson EL , Eckel RH , et al . Ad libitum weekend recovery sleep fails to prevent metabolic dysregulation during a repeating pattern of insufficient sleep and weekend recovery sleep . Curr Biol. 2019. ; 29 ( 6 ): 957 – 967.e4 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Depner CM , Stothard ER , Wright KP Jr . Metabolic consequences of sleep and circadian disorders . Curr Diab Rep. 2014. ; 14 ( 7 ): 507 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dijk DJ , Czeisler CA . Paradoxical timing of the circadian rhythm of sleep propensity serves to consolidate sleep and wakefulness in humans . Neurosci Lett. 1994. ; 166 ( 1 ): 63 – 68 . [DOI] [PubMed] [Google Scholar]
- 7. Sack RL , Auckley D , Auger RR , et al. American Academy of Sleep Medicine . Circadian rhythm sleep disorders: part I, basic principles, shift work and jet lag disorders. An American Academy of Sleep Medicine review . Sleep. 2007. ; 30 ( 11 ): 1460 – 1483 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Caliandro R , Streng AA , van Kerkhof LWM , van der Horst GTJ , Chaves I . Social jetlag and related risks for human health: a timely review . Nutrients. 2021. ; 13 ( 12 ): 4543 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chung SJ , An H , Suh S . What do people do before going to bed? A study of bedtime procrastination using time use surveys . Sleep. 2020. ; 43 ( 4 ): zsz267 . [DOI] [PubMed] [Google Scholar]
- 10. Stokkan KA , Yamazaki S , Tei H , Sakaki Y , Menaker M . Entrainment of the circadian clock in the liver by feeding . Science. 2001. ; 291 ( 5503 ): 490 – 493 . [DOI] [PubMed] [Google Scholar]
- 11. Reynolds NC Jr , Montgomery R . Using the Argonne diet in jet lag prevention: deployment of troops across nine time zones . Mil Med. 2002. ; 167 ( 6 ): 451 – 453 . [PubMed] [Google Scholar]
- 12. Sutton EF , Beyl R , Early KS , Cefalu WT , Ravussin E , Peterson CM . Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes . Cell Metab. 2018. ; 27 ( 6 ): 1212 – 1221, e3 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wilkinson MJ , Manoogian ENC , Zadourian A , et al . ten-hour time-restricted eating reduces weight, blood pressure, and atherogenic lipids in patients with metabolic syndrome . Cell Metab. 2020. ; 31 ( 1 ): 92 – 104.e5 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Thompson PD , Arena R , Riebe D , Pescatello LS ; American College of Sports Medicine . ACSM’s new preparticipation health screening recommendations from ACSM’s guidelines for exercise testing and prescription, ninth edition . Curr Sports Med Rep. 2013. ; 12 ( 4 ): 215 – 217 . [DOI] [PubMed] [Google Scholar]
- 15. Fairburn CG , Beglin SJ . Assessment of eating disorders: interview or self-report questionnaire? Int J Eat Disord. 1994. ; 16 ( 4 ): 363 – 370 . [PubMed] [Google Scholar]
- 16. Aardoom JJ , Dingemans AE , Slof Op’t Landt MC , Van Furth EF . Norms and discriminative validity of the Eating Disorder Examination Questionnaire (EDE-Q) . Eat Behav. 2012. ; 13 ( 4 ): 305 – 309 . [DOI] [PubMed] [Google Scholar]
- 17. Gu L , Chen J , Huang Y , et al . Validity and reliability of the Chinese version of the Eating Disorder Examination Questionnaire 6.0 in female patients with eating disorders . Chinese Mental Health Journal. 2017. ; 31 ( 5 ): 350 – 355 . [Google Scholar]
- 18. Buysse DJ , Reynolds CF 3rd , Monk TH , Berman SR , Kupfer DJ . The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research . Psychiatry Res. 1989. ; 28 ( 2 ): 193 – 213 . [DOI] [PubMed] [Google Scholar]
- 19. Kendzerska TB , Smith PM , Brignardello-Petersen R , Leung RS , Tomlinson GA . Evaluation of the measurement properties of the Epworth Sleepiness Scale: a systematic review . Sleep Med Rev. 2014. ; 18 ( 4 ): 321 – 331 . [DOI] [PubMed] [Google Scholar]
- 20. Kroenke K , Spitzer RL , Williams JB . The PHQ-9: validity of a brief depression severity measure . J Gen Intern Med. 2001. ; 16 ( 9 ): 606 – 613 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Spitzer RL , Kroenke K , Williams JB , Löwe B . A brief measure for assessing generalized anxiety disorder: the GAD-7 . Arch Intern Med. 2006. ; 166 ( 10 ): 1092 – 1097 . [DOI] [PubMed] [Google Scholar]
- 22. Carney CE , Buysse DJ , Ancoli-Israel S , et al . The consensus sleep diary: standardizing prospective sleep self-monitoring . Sleep. 2012. ; 35 ( 2 ): 287 – 302 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chinoy ED , Cuellar JA , Huwa KE , et al . Performance of seven consumer sleep-tracking devices compared with polysomnography . Sleep. 2021. ; 44 ( 5 ): zsaa291 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Burgess HJ , Park M , Wyatt JK , Rizvydeen M , Fogg LF . Sleep and circadian variability in people with delayed sleep-wake phase disorder versus healthy controls . Sleep Med. 2017. ; 34 : 33 – 39 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Scott H , Cheung JMY , Muench A , et al . Does total sleep time substantially increase after cognitive behavioral therapy for insomnia? J Clin Sleep Med. 2022. ; 18 ( 7 ): 1823 – 1829 . [DOI] [PMC free article] [PubMed] [Google Scholar]