Abstract
Study Objectives:
This meta-analysis aimed to systematically assess the effects of continuous positive airway pressure (CPAP) in secondary prevention of major cardiovascular events (MACEs) in patients with moderate-to-severe obstructive sleep apnea and coronary artery disease.
Methods:
PubMed, Embase, the Cochrane Library, and ClinicalTrials.gov were searched for observational studies and randomized controlled trials that compared CPAP with usual care in patients with moderate-to-severe obstructive sleep apnea with coronary artery disease. The primary outcomes were MACEs, all-cause death, acute coronary syndrome, rehospitalization for heart failure, repeat revascularization, and arrhythmia.
Results:
A total of 11 studies (5 randomized controlled trials and 6 observational studies) with 5,410 patients reported outcomes of MACEs. Treatment with CPAP was associated with a modest risk reduction in MACEs (risk ratio [RR] 0.87, 95% confidence interval [CI] 0.78 to 0.98; P = .02). Similarly, CPAP significantly reduced the risk of all-cause and cardiovascular death by 23% (RR 0.77, 95% CI 0.60 to 0.99; P = .04; I2 = 0%). Subgroup analysis revealed that CPAP adherence time ≥ 4 hours/night had a greater benefit on MACEs by 42% (RR 0.58, 95% CI 0.42 to 0.79; P < .001; I2 = 0%) and repeat revascularization by 44% (RR 0.56, 95% CI 0.34 to 0.92; P = .02; I2 = 0%). Also, CPAP had a positive effect on systolic and diastolic blood pressure.
Conclusions:
CPAP therapy might prevent subsequent MACEs and all-cause death among patients with moderate to severe obstructive sleep apnea and concomitant coronary artery disease. CPAP use exceeding 4 hours/night may add more benefits on MACEs, repeat revascularization, and blood pressure.
Clinical Trial Registration:
Registry: PROSPERO database; Name: Effects of Continuous Positive Airway Pressure on Cardiovascular Events and Metabolic Components in Patients with Obstructive Sleep Apnea and Coronary Artery Disease; URL: https://www.crd.york.ac.uk/prospero/display_record.php?ID= CRD42020213546; Identifier: CRD42020213546.
Citation:
Yang D, Li L, Dong J, Yang W, Liu Z. Effects of continuous positive airway pressure on cardiac events and metabolic components in patients with moderate to severe obstructive sleep apnea and coronary artery disease: a meta-analysis. J Clin Sleep Med. 2023;19(12):2015–2025.
Keywords: continuous positive airway pressure, obstructive sleep apnea, coronary artery disease, meta-analysis
BRIEF SUMMARY
Current Knowledge/Study Rationale: The use of continuous positive airway pressure (CPAP) for improving the clinical variables was correlated with its duration, indicating that the longer CPAP is used per night, the greater the benefits. We hypothesized that CPAP usage ≥ 4 hours/night would be associated with a reduction in risk of major cardiovascular events.
Study Impact: CPAP usage in patients with moderate to severe obstructive sleep apnea and concomitant coronary artery disease was associated with a reduced risk of major cardiovascular events and all-cause death. More CPAP use exceeding 4 hours/night may add more benefits related to major cardiovascular events, repeat revascularization, and blood pressure.
INTRODUCTION
Obstructive sleep apnea (OSA) is common sleep-disordered breathing characterized by complete or partial upper airway collapse resulting in recurrent episodes of sleep fragmentation and intermittent hypoxia. Compared with the general population, patients with coronary artery disease (CAD) have a higher prevalence of OSA, ranging from 38% to 65%.1,2 In addition, increasing evidence suggests that OSA is significantly associated with an increased risk of subsequent cardiovascular events in participants with/without previous CAD, independent of traditional risk factors (eg, arterial hypertension or metabolic syndrome).3
Continuous positive airway pressure (CPAP) is currently recommended for patients with moderate to severe OSA. Multiple studies have sought to demonstrate that CPAP usage improves cardiovascular events and metabolic components, such as hypertension, dyslipidemia, and diabetes in patients with OSA.4–7 Still, the results were not based on established CAD. A previous meta-analysis showed that the use of CPAP in patients with CAD and OSA might prevent subsequent cardiovascular events in observational studies but not in randomized controlled trials (RCTs).8 However, the heterogeneity across the observational studies was high, with an I2 of 66%, and only 2 RCTs were included in the analysis. It is possible that the negative outcomes in RCTs are due to the ineffective use of CPAP for less than 4 hours per night. It was demonstrated that the use of CPAP for improving clinical variables was correlated with its duration, indicating that the longer the CPAP therapy is used per night, the greater the benefits.9 In literature and clinical practice, 4 hours of daily CPAP usage is considered an adequate adherence to therapy.10–12
This led to a hypothesis that increasing the duration of CPAP use may have a positive impact on major adverse cardiovascular events (MACEs) and their components. Is it possible that using CPAP for at least 4 hours per night could reduce the risk of MACEs? Previous studies have shown inconsistent results of CPAP therapy in patients with OSA and CAD.13–15 And none included the Impact of Sleep Apnea Syndrome in the Evolution of Acute Coronary Syndrome. Effect of Intervention With Continuous Positive Airway Pressure (ISAACC study),16 a multicenter RCT, adding significant new data in this field of research. To update the state of knowledge in patients with moderate to severe OSA with CAD, we aimed to investigate the impact of effective CPAP therapy compared with control on cardiovascular events. In addition, to the best of our knowledge, there is no study available that evaluates the treatment of OSA on metabolic components in patients with CAD through meta-analysis. Therefore, we also assessed the role of CPAP therapy on metabolic features in patients with OSA with CAD.
METHODS
Search strategy
The protocol for this study was prospectively registered with the PROSPERO database of systematic reviews (CRD42020213546). This research protocol was based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.17 Specifically, we performed a systematic search of relevant articles in the following databases: PubMed, Embase, the Cochrane Library, and ClinicalTrials.gov, from inception through December 20, 2021. The search terms used were as follows: “continuous positive airway pressure” and (“myocardial infarction” or “ischemic heart disease*” or “coronary artery disease*” or “coronary heart disease*”). We only included research articles that involved human participants and were published in the English language. Also, we manually searched the reference lists of relevant articles.
Eligibility criteria
Eligible studies for the OSA and CAD groups in the present analysis were as follows: (1) randomized or observational design of adult (> 18 years) patients with vs without CPAP treatment published in the English language, (2) reporting on outcomes of MACEs (all-cause death, nonfatal myocardial infarction [MI], rehospitalization for heart failure, revascularization, and arrhythmia) and metabolic components (blood pressure/lipids/glucose/body weight), (3) patients with moderate to severe OSA (apnea-hypopnea index [AHI] ≥ 15 events/h) and CAD, and (4) more than 1 month of follow-up after CAD was recorded.
Outcome measures
The primary outcomes were taken as MACEs and their components from baseline to follow-up. An MACE is defined as all-cause death, nonfatal MI, rehospitalization for heart failure, revascularization, or arrhythmia. Changes in metabolic components (blood pressure/lipids/glucose) were considered the secondary outcomes.
Risk of bias
The potential risk of bias of randomized study quality was assessed using a risk scale as implemented in Review Manager (RevMan [computer program], version 5.4.1, The Cochrane Collaboration, 2020). The quality of observational studies was appraised using the Newcastle-Ottawa Scale (Ottawa, Canada: Ottawa Hospital Research Institute; 2009).18 The elements of the Newcastle-Ottawa Scale checklist were divided into 3 domains upon selection, comparability, and outcomes categories. The scale extended from 0 to 9 points. Low-quality studies were defined as ≤ 5 points, moderate-quality studies as 6 to 7 points, and high-quality studies as 8 to 9 points. Funnel plots were constructed to investigate possible publication bias, with Egger’s and Begg’s tests used to assess asymmetry.
Data extraction
Two investigators independently screened all studies and extracted the data using customized data-extraction forms. A third investigator resolved disagreements. The following data were extracted: first author, publication date, country, study design, sample size, patient baseline characteristics, baseline OSA assessment, CPAP usage, follow-up duration, clinical outcomes (MACEs, all-cause death, acute coronary syndrome [ACS], rehospitalization for heart failure, repeat revascularization, and arrhythmia) and metabolic components (blood pressure/lipids/glucose/body weight). Clinical trials with multiple publications and sequential follow-up durations were considered as a single study. Studies with CPAP use of ≥ 4 hours per night were included as an effective treatment.
Statistical analysis
Outcome data were analyzed using Review Manager (RevMan), version 5.4.1 (The Cochrane Collaboration, 2020). Fixed-effects models were preferred unless heterogeneity extended beyond the expected outcome by chance. For dichotomous data, MACEs are presented as risk ratios (RRs) with 95% confidence intervals (CIs). For continuous data, changes in systolic blood pressure (SBP) or diastolic blood pressure (DBP) are presented as weighted mean difference with 95% CIs if outcomes were measured in the same way. The I2 statistic was used to examine the heterogeneity within the different subgroups. Heterogeneity was defined as low (25–50%), moderate (50–75%), or high (> 75%).19 Subgroup analyses were conducted based on CPAP usage (≥ 4 h/night or < 4 h/night) and study design after CAD. In studies reporting the mean ± SE, mean with 95% CI, or mean ± SD at baseline and follow-up, changes in SD were calculated with a standardized formula. Sensitivity analysis and Egger’s and Begg’s tests were conducted using Stata version 12 (StataCorp LP, College Station, TX). A 2-sided P value < .05 was deemed statistically significant in all analyses.
RESULTS
Search results
In total, 1,442 records were identified through the search strategy, and 3 additional records were identified through manual search after reviewing the article references. After deleting the duplicates, the search resulted in a total of 992 records. One hundred seventy-three articles were considered relevant after the first level of evaluation. Further, we excluded 146 articles by full-text assessment, among which 82 studies were excluded as they did not specify patients with CAD, 30 studies did not focus on CPAP intervention, 29 studies did not report on the outcomes of interest, and 5 studies were excluded due to ongoing investigations or were in a language other than English. Additional exclusion criteria are presented in Figure 1. Overall, 14 studies were analyzed.
Figure 1. Flow diagram of the study selection process for meta-analysis.
Study characteristics
Of the 14 included studies, 6 studies were prospective cohort,20–25 2 studies were retrospective cohort,26,27 and 6 were RCTs.13–15,28,29 All studies enrolled patients with OSA and CAD. The number of evaluated patients who received CPAP therapy ranged from 9 to 1,346 patients and 22 to 1,341 patients for the control (usual-care) group. Follow-up duration ranged from 12 to 86 months for MACE outcomes. The average participant age in the 14 studies ranged from 54 to 71 years. Most participants were male. The mean age, sex, and baseline body mass index were similar between the treatment and control groups. The main characteristics of the studies are summarized in Table 1.
Table 1.
Characteristics of studies included in this analysis.
| Study | Country | Sample Size | Mean Age, y | Male, % | BMI, mean (SD), kg/m2 | Study Type | Follow-up Duration, mo | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CPAP | CO | CPAP | CO | CPAP | CO | CPAP | CO | ||||
| Capodanno et al20, 2014 | Italy | 17 | 112 | 70 | 68 | 77 | 81 | 29 | 27 | PC | 36 |
| Cassar et al26, 2007 | USA | 175 | 196 | 64 | 64 | 85 | 90 | 35.4 (7.1) | 33.0 (5.6) | RC | 60 |
| Huang et al15, 2015 | China | 37 | 36 | 63 | 62 | 87 | 78 | 27.5 (2.6) | 27.9 (3.6) | RCT | 36 (median) |
| Huang et al28, 2016 | China | 37 | 33 | 62 | 62 | 80 | 87 | 23.1 (1.1) | 22.9 (1.0) | RCT | 12 |
| Lee et al21, 2017 | Taiwan | 9 | 27 | 62 | 62 | 89 | 89 | 29.7 (7.3) | 29.7 (7.3) | PC | 12 |
| McEvoy et al13, 2016 | Australia et al | 1346 | 1341 | 61 | 61 | 81 | 81 | 29.0 (15.9) | 29.6 (16.4) | RCT | 44.4 (median) |
| Milleron et al22, 2004 | France | 25 | 29 | 58 | 57 | 96 | 100 | 28.4 (4.2) | 28.2 (3.4) | PC | 86 |
| Nakashima et al23, 2015 | Japan | 56 | 39 | 71 | 71 | 77 | 77 | NR | NR | PC | 50 (median) |
| Peker et al29, 2016 | Sweden et al | 122 | 122 | 66 | 67 | 82 | 86 | 28.4 (3.8) | 28.5 (3.5) | RCT | 57 (median) |
| Peker et al14, 2020 | Sweden et al | 86 | 85 | 65 | 65 | 79 | 87 | 28.4 (4.0) | 28.7 (3.6) | RCT | 56.7 (median) |
| Sánchez-de-la-Torre et al16, 2019 | Spain | 629 | 626 | 60 | 61 | 84 | 85 | 29.6 (4.7) | 29.4 (4.3) | RCT | 40.2 (median) |
| Wu et al27, 2015 | China | 128 | 167 | 54 | 56 | 83 | 86 | 30 | 30 | RC | 58 (median) |
| Yang et al24, 2013 | China | 22 | 22 | 60 | 61 | 91 | 91 | 26.7 (2.9) | 27.3 (2.3) | PC | 3 |
| Zhao et al25, 2012 | China | 24 | 24 | 69 | 59 | 88 | 92 | 27.0 (2.8) | 28.4 (2.9) | PC | 1 |
BMI = body mass index, CO = control, CPAP = continuous positive airway pressure, PC = prospective cohort, RC = retrospective cohort, RCT = randomized controlled trial.
Intervention characteristics
The diagnosis of OSA was based on polysomnography (PSG) in 7 studies, on validated portable devices in 6 studies, and on either PSG or portable device in 1 study. No significant difference was found in the OSA degree (defined by AHI, with AHI ≥ 15 events/h as the cutoff value) between the CPAP and control groups. In addition, 11 studies reported the duration of adherence to CPAP, 8 with CPAP device usage ≥ 4 hours/night, and 6 with CPAP device usage < 4 hours/night or not available. Details on CPAP intervention characteristics are reported in Table 2.
Table 2.
Characteristics of CPAP therapy interventions and outcomes.
| Study | CPAP Use, h/night | AHI, events/h | OSA Diagnosis | Outcomes | |
|---|---|---|---|---|---|
| CPAP | CO | ||||
| Capodanno et al20, 2014 | NR | >15 | >15 | PD | Death, MI, revascularization, stroke |
| Cassar et al26, 2007 | NR | ≥15 | ≥15 | PSG | Death, severe angina, MI, PCI, CABG |
| Huang et al15, 2015 | ≥4 | ≥15 | ≥15 | PD | Death, revascularization, MI, HF, SBP, DBP |
| Huang et al28, 2016 | 4.2 ± 1.1 | 28.5 ± 12.0 | 28.9 ± 12.2 | PD | TG, TC, LDL-C, HDL-C |
| Lee et al21, 2017 | ≥4 | 49.8 ± 31.3 | 49.8 ± 31.3 | PSG | Death, MI, revascularization, stroke |
| McEvoy et al13, 2016 | <4 | ≥15 | ≥15 | PD | Death, revascularization, MI, HF, UA, AF, SBP, DBP |
| Milleron et al22, 2004 | 5.7 ± 1.5 | 33.7 ± 16.8 | 29.0 ± 12.8 | PSG | Death, HF, PTCA, ACS |
| Nakashima et al23, 2015 | NR | >20 | >20 | PSG | Death, ACS, HF |
| Peker et al29, 2016 | ≥4 | ≥15 | ≥15 | PSG | Death, MI, revascularization |
| Peker et al14, 2020 | <4 | ≥15 | ≥15 | PSG | Death, MI, revascularization |
| Sánchez-de-la-Torre et al16, 2019 | <4 | ≥15 | ≥15 | PSG | Death, MI, HF, UA, AF |
| Wu et al27, 2015 | ≥4 | ≥15 | ≥15 | PSG/PD | Death, MI, revascularization, stent thrombosis |
| Yang et al24, 2013 | 5 ± 1 | 25 ± 13 | 30 ± 15 | PD | Glucose |
| Zhao et al25, 2012 | 5.1 ± 0.8 | 26.9 ± 11.9 | 28.1 ± 16.1 | PD | SBP, DBP |
ACS = acute coronary syndrome, AF = atrial fibrillation, AHI = apnea-hypopnea index, CABG = coronary artery bypass graft, CO = control, CPAP = continuous positive airway pressure, DBP = diastolic blood pressure, HDL-C = high-density-lipoprotein cholesterol, HF = heart failure, LDL-C = low-density-lipoprotein cholesterol, MI = myocardial infarction, NR = not reported, OSA, obstructive sleep apnea, PCI = percutaneous coronary intervention, PD = portable device, PSG = polysomnography, PTCA = percutaneous transluminal coronary angioplasty, SBP = systolic blood pressure, TC = total cholesterol, TG = triglycerides, UA = unstable angina.
Primary outcomes
Association of CPAP with MACEs
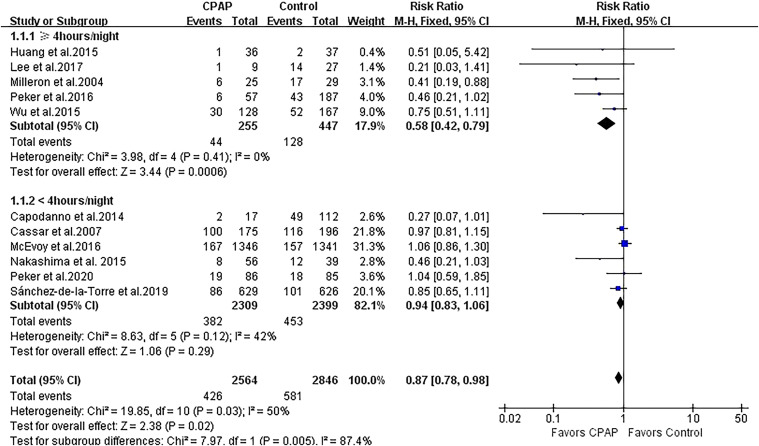
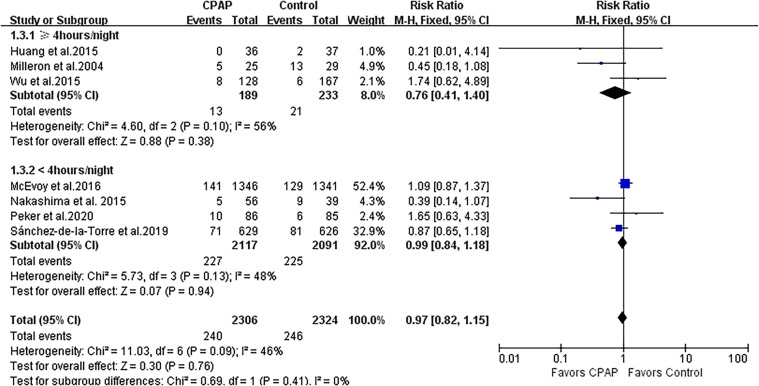
A total of 11 studies with 5,410 patients reported the outcome of MACEs. Treatment with CPAP was associated with a modest risk reduction in MACEs (RR 0.87, 95% CI 0.78 to 0.98; P = .02; Figure 2). There was evidence of statistical heterogeneity for the composite endpoint in the MACE studies (Q statistic P = .03; I2 = 50%). We further performed subgroup analysis by CPAP adherence and showed that the greater decreased risk of MACEs remained significant in 5 CPAP-compliant studies (RR 0.58, 95% CI 0.42 to 0.79; P < .001; I2 = 0%) but was not significant in 6 CPAP-noncompliant studies (RR 0.94, 95% CI 0.83 to 1.06; P = .29; I2 = 42%), and the heterogeneity was attenuated in both subgroups (Figure 2).
Figure 2. Forest plot showing the effect of CPAP vs control on MACEs.
CI = confidence interval, CPAP = continuous positive airway pressure, MACE = major adverse cardiovascular endpoint, M-H = Mantel-Haenszel.
Association of CPAP with all-cause and cardiovascular death
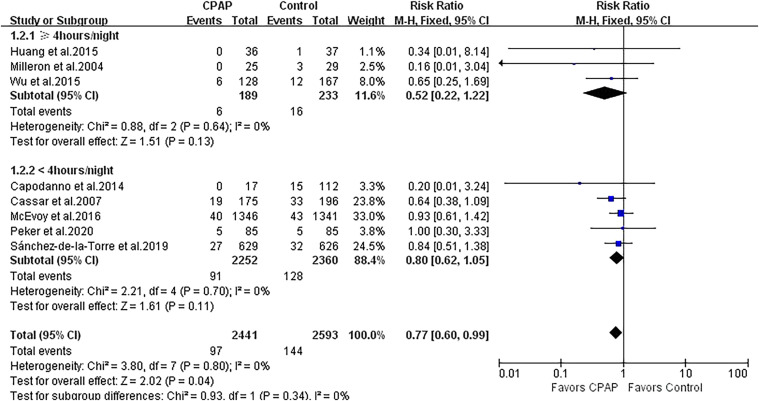
There were 8 studies (5,034 patients) that reported outcomes of all-cause and cardiovascular death. CPAP therapy significantly reduced the risk of all-cause and cardiovascular death by 23% (RR 0.77, 95% CI 0.60 to 0.99; P = .04; I2 = 0%; Figure 3). No heterogeneity was found among the studies.
Figure 3. Forest plot showing the effect of CPAP vs control on all-cause death.
CI = confidence interval, CPAP = continuous positive airway pressure, M-H = Mantel-Haenszel.
Association of CPAP with repeat revascularization
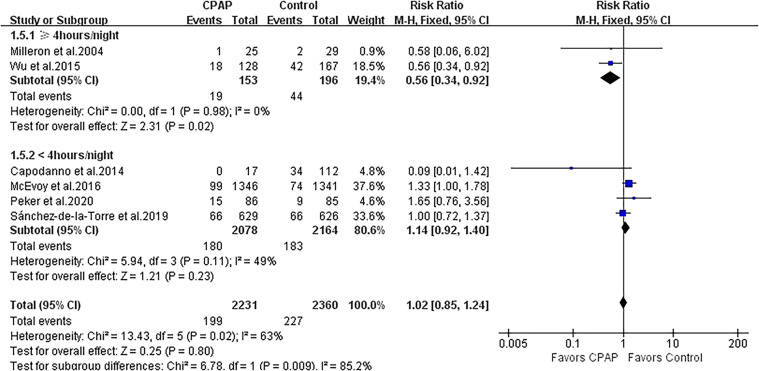
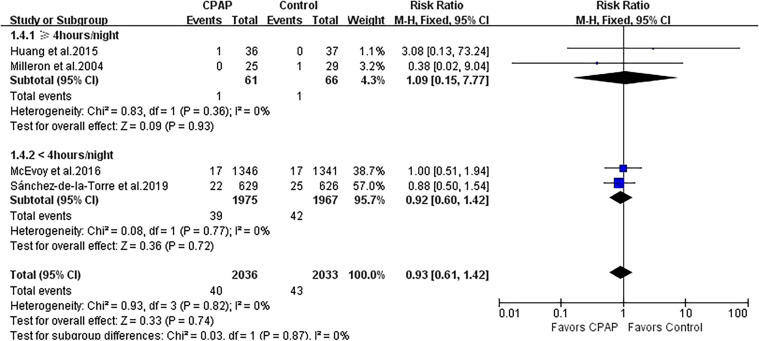
Repeat revascularization was evaluated in 6 studies with 4,591 patients. CPAP was not associated with the incidence of repeat revascularization, and moderate heterogeneity was observed (RR 1.02, 95% CI 0.85 to 1.24; P = .80; I2 = 63%; Figure 4). Subgroup analysis according to CPAP adherence showed the less risk of repeat revascularization in 2 studies with adherence ≥ 4 hours/night (RR 0.56, 95% CI 0.34 to 0.92; P = .02; I2 = 0%; Figure 4) but not in 4 studies with adherence < 4 hours/night (RR 1.14, 95% CI 0.92 to 1.40; P = .23; I2 = 49%; Figure 4). The heterogeneity substantially varied between both subgroups (I2 = 85.2%).
Figure 4. Forest plot showing the effect of CPAP vs control on repeat revascularization.
CI = confidence interval, CPAP = continuous positive airway pressure, M-H = Mantel-Haenszel.
Association of CPAP with other individual cardiac events
CPAP use was not associated with a reduction in ACS (RR 0.97, 95% CI 0.82 to 1.15; P = .76; I2 = 46%; Figure 5), rehospitalization for heart failure (RR 0.93, 95% CI 0.61 to 1.42; P = .74; I2 = 0%; Figure 6), or arrhythmia (RR 1.12, 95% CI 0.70 to 1.80; P = .64; I2 = 29%; Figure S1 (195.3KB, pdf) in the supplemental material). The effects of CPAP on ACS, rehospitalization for heart failure, and arrhythmia were quite consistent among various studies, with all 95% CIs of RRs crossing the null line of 1.00.
Figure 5. Forest plot showing the effect of CPAP vs control on ACS.
ACS = acute coronary syndrome, CI = confidence interval, CPAP = continuous positive airway pressure, M-H = Mantel-Haenszel.
Figure 6. Forest plot showing the effect of CPAP vs control on rehospitalization for heart failure.
CI = confidence interval, CPAP = continuous positive airway pressure, M-H = Mantel-Haenszel.
Secondary outcomes
Association of CPAP with blood pressure
Three studies involving 2,445 patients provided data for the meta-analyses of SBP and DBP. CPAP therapy was associated with significant improvement in both SBP (mean difference [MD] −1.35, 95% CI −2.63 to −0.08; P = .04; I2 = 53%; Figure S2 (195.3KB, pdf) ) and DBP (MD −1.01, 95% CI −1.86 to −0.16; P = .02; I2 = 43%; Figure S3 (195.3KB, pdf) ). These positive results were magnified when CPAP was used ≥ 4 hours/night (SBP: MD −4.47, 95% CI −7.76 to −1.19; P = .008; I2 = 0%; DBP: MD −3.21, 95% CI −6.11 to −0.31; P = .03; I2 = 9%; Figure S3 (195.3KB, pdf) ).
Association of CPAP with glucose, Homeostatic Model Assessment for Insulin Resistance, and lipids
One observational study from Yang et al11 that showed good adherence to CPAP (5 ± 1 h/night) over 3 months was associated with a positive impact on glucose (MD −0.45, 95% CI −0.85 to −0.05; P = .03) and Homeostatic Model Assessment for Insulin Resistance (MD −1.15, 95% CI −1.93 to −0.37; P = .004) in nondiabetic patients with OSA and CAD.
One randomized trial from Huang et al28 reported CPAP therapy with an adherence of 4.2 ± 1.1 hours/night had a nonsignificant effect on triglycerides (MD −0.04, 95% CI −0.67 to 0.59; P = .90), total cholesterol (MD 0.01, 95% CI −0.38 to 0.40; P = .96), low-density-lipoprotein cholesterol (MD 0.10, 95% CI −0.22 to 0.42; P = .54), or high-density-liproprotein cholesterol (MD −0.12, 95% CI −0.00 to −0.24; P = .06) in nonobese patients with OSA and CAD.
Risk-of-bias assessment
The methodological quality of the enrolled RCTs was assessed using key indicators. The 6 RCTs were open-label studies and did not include blinding participants and personnel to the intervention, but all did blind assessments concerning the analysis outcomes. All of the observational studies showed moderate-to-high quality (Newcastle-Ottawa Scale score >6). The pooled risk estimates did not materially change in the sensitivity analyses. Also, there was no evidence of publication bias based on either visual inspection of the funnel plots and Egger’s and Begg’s tests for the outcomes of all-cause death, ACS, rehospitalization for heart failure, and repeat revascularization (all P > .05). However, the funnel plot and Egger’s test suggested the presence of publication bias for the outcome of MACEs (P = .005). Subgroup funnel plots based on CPAP adherence indicated that the publication bias was not statistically significant (P = .13 and P = .14, respectively). This suggests that asymmetry is likely because of CPAP compliance.
DISCUSSION
In the present meta-analysis, we found that CPAP therapy was associated with a significant risk reduction in MACEs and all-cause death in patients with moderate to severe OSA with concomitant CAD at a mean follow-up of 4 years. Moreover, subgroup analysis showed that wearing CPAP ≥ 4 hours/night significantly alleviated MACEs and repeat revascularization. The meta-analysis also demonstrated significant effects of CPAP on either SBP or DBP in these patients. On the other hand, the meta-analysis showed no statistically significant impact of CPAP therapy, ACS, rehospitalization for heart failure, or arrhythmia in patients with OSA and CAD.
OSA is highly prevalent, and its association with increased incidence of cardiovascular events is well known. CPAP is effective in reversing upper airway obstruction, intermittent hypoxia, and intrathoracic pressure swings. However, in the RCTs13,14,16 and previous meta-analyses,8,30–33 except for the analysis from Wang et al,8 no significant beneficial effects of CPAP on MACEs or all-cause death were shown in patients with OSA with or without cardiovascular comorbidities. It is probably premature to conclude neutral outcomes due to the following reasons. First, the study population varied across studies from the general population to patients with different cardiovascular diseases (such as stroke, heart failure, ischemic heart disease), as well as OSA severity. The analysis conducted by Wang et al8 focused on a relatively homogenous group of patients with established CAD, suggesting that CPAP therapy was associated with a reduced risk of MACEs in the observational studies. But they observed significant statistical heterogeneity in MACEs in the observational studies, and the inconsistent ranges of AHI values across studies may be a confounder in addition to the study design. During our meta-analysis, we excluded 2 prospective studies that used AHI ≥ 5 events/h as the primary inclusion criterion.34,35 The prior meta-analysis only focused on literature related to “myocardial ischemia,” but we enhanced our retrieval strategy by incorporating additional terms like “myocardial infarction”, “ischemic heart disease*”, “coronary artery disease*”, and “coronary heart disease*”. This modification allowed us to obtain more accurate references for our research. Second, adherence to CPAP is crucial to its effectiveness, and the difference in CPAP adherence may contribute to the negative results of the RCTs and most prior meta-analyses. In the Continuous Positive Airway Pressure (CPAP) Treatment in Coronary Artery Disease and Sleep Apnea (RICCADSA) trial, adjusted on-treatment analysis exhibited better outcomes in patients who used CPAP for ≥ 4 hours/night. Similar results were reported in several meta-analyses by Abuzaid et al30 and Khan et al,32 where a significant improvement of > 30% in the primary composite MACE outcomes was observed in participants with CPAP adherence ≥ 4 hours/night. Our meta-analysis is the initial attempt to prove these benefits in a moderately consistent group of patients with moderate to severe OSA and established CAD. Subsequent studies should verify these findings.
Accumulating evidence has shown a clear association between OSA and the incidence of repeat revascularization. The results from Yang et al’s meta-analysis36 indicated that preexisting OSA increased a pooled 1.93-fold risk of repeat revascularization in patients with ACS. This review reported a nonsignificant risk reduction in repeat revascularization with a mean CPAP usage time of < 4 hours/night. CPAP also failed to significantly reduce ACS, rehospitalization for heart failure, and arrhythmia. There are several reasons that could explain the negative results. One possible explanation is the poor adherence to CPAP treatment, which may have mostly contributed to the null outcomes observed. Upon further analysis of subgroups, it was found that consistent use of CPAP for at least 4 hours per night had a positive effect on repeat revascularization. This discovery highlights the need for further research in this field. Additionally, the study included a wide range of individuals with different levels of risk, such as those with ACS, unstable angina, or MI, who had undergone revascularization procedures like percutaneous coronary intervention or coronary artery bypass grafting. As a result, the treatment effect was not as significant as expected. Therefore, it is essential to assess the effects of CPAP therapy on a more uniform patient population.
The favorable effects of CPAP on hypertension have been well established in the literature.37,38 Our meta-analysis suggested that CPAP was effective in lowering both SBP and DBP in patients with and CAD. Moreover, subgroup analysis showed that using CPAP therapy ≥ 4 hours/night might have a more significant benefit. Given the strong association of hypertension with cardiovascular events, the significant reductions in SBP and DBP can also result in better outcomes over time.32 However, the dose–response relationship between CPAP duration and its treatment effects on blood pressure or other metabolic factors need to be clarified in the future.
The current analysis has some limitations. First, most studies only included nonsleepy patients with OSA (Epworth Sleepiness Scale Score < 10) with a better prognosis when compared with patients with symptomatic OSA, which inevitably underestimates the risk of OSA on cardiovascular outcomes. Second, the definition of composite MACEs varied across studies, and the treatment effects of CPAP need to be further investigated in more homogeneous cardiovascular outcomes. Third, the average follow-up time was 4 years. The difference may be more significant in composite or individual cardiac events based on the mortality curves.
In conclusion, compared with medical therapy alone, the use of CPAP in patients with moderate to severe OSA and concomitant CAD was associated with a reduced risk of MACEs and all-cause death. Better treatment with CPAP may add more benefits to MACEs, repeat revascularization, and blood pressure. Future large RCTs with disease-specific populations are warranted to evaluate further the benefits of good CPAP compliance on the prevention of ACS, rehospitalization for heart failure, and arrhythmia.
DISCLOSURE STATEMENT
All authors have seen and approved this manuscript. The authors report no conflicts of interest.
ACKNOWLEDGMENTS
Author contributions: All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. D.Y. and L.L. served as principal authors. All authors contributed substantially to the study design, data analysis, interpretation, and writing of the manuscript.
ABBREVIATIONS
- ACS
acute coronary syndrome
- AHI
apnea-hypopnea index
- CAD
coronary artery disease
- CI
confidence interval
- CPAP
continuous positive airway pressure
- DBP
diastolic blood pressure
- OSA
obstructive sleep apnea
- MACE
major adverse cardiovascular event
- MD
mean difference
- MI
myocardial infarction
- PSG
polysomnography
- RCT
randomized controlled trial
- RR
risk ratio
- SBP
systolic blood pressure
REFERENCES
- 1. Ooi EL , Rajendran S . Obstructive sleep apnea in coronary artery disease . Curr Probl Cardiol. 2023. ; 48 ( 8 ): 101178 . [DOI] [PubMed] [Google Scholar]
- 2. Javaheri S , Barbe F , Campos-Rodriguez F , et al . Sleep apnea: types, mechanisms, and clinical cardiovascular consequences . J Am Coll Cardiol. 2017. ; 69 ( 7 ): 841 – 858 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lee CH , Sethi R , Li R , et al . Obstructive sleep apnea and cardiovascular events after percutaneous coronary intervention . Circulation. 2016. ; 133 ( 21 ): 2008 – 2017 . [DOI] [PubMed] [Google Scholar]
- 4. Jonas DE , Amick HR , Feltner C , et al . Screening for obstructive sleep apnea in adults: evidence report and systematic review for the US Preventive Services Task Force . JAMA. 2017. ; 317 ( 4 ): 415 – 433 . [DOI] [PubMed] [Google Scholar]
- 5. Fu Y , Xia Y , Yi H , Xu H , Guan J , Yin S . Meta-analysis of all-cause and cardiovascular mortality in obstructive sleep apnea with or without continuous positive airway pressure treatment . Sleep Breath. 2017. ; 21 ( 1 ): 181 – 189 . [DOI] [PubMed] [Google Scholar]
- 6. Iftikhar IH , Valentine CW , Bittencourt LR , et al . Effects of continuous positive airway pressure on blood pressure in patients with resistant hypertension and obstructive sleep apnea: a meta-analysis . J Hypertens. 2014. ; 32 ( 12 ): 2341 – 2350 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nadeem R , Singh M , Nida M , et al . Effect of CPAP treatment for obstructive sleep apnea hypopnea syndrome on lipid profile: a meta-regression analysis . J Clin Sleep Med. 2014. ; 10 ( 12 ): 1295 – 1302 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang X , Zhang Y , Dong Z , Fan J , Nie S , Wei Y . Effect of continuous positive airway pressure on long-term cardiovascular outcomes in patients with coronary artery disease and obstructive sleep apnea: a systematic review and meta-analysis . Respir Res. 2018. ; 19 ( 1 ): 61 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Campos-Rodriguez F , Peña-Griñan N , Reyes-Nuñez N , et al . Mortality in obstructive sleep apnea-hypopnea patients treated with positive airway pressure . Chest. 2005. ; 128 ( 2 ): 624 – 633 . [DOI] [PubMed] [Google Scholar]
- 10. Javaheri S , Martinez-Garcia MA , Campos-Rodriguez F , Muriel A , Peker Y . Continuous positive airway pressure adherence for prevention of major adverse cerebrovascular and cardiovascular events in obstructive sleep apnea . Am J Respir Crit Care Med. 2020. ; 201 ( 5 ): 607 – 610 . [DOI] [PubMed] [Google Scholar]
- 11. Yang D , Liu Z , Yang H . The impact of effective continuous positive airway pressure on homeostasis model assessment insulin resistance in non-diabetic patients with moderate to severe obstructive sleep apnea . Diabetes Metab Res Rev. 2012. ; 28 ( 6 ): 499 – 504 . [DOI] [PubMed] [Google Scholar]
- 12. Sawyer AM , Gooneratne NS , Marcus CL , Ofer D , Richards KC , Weaver TE . A systematic review of CPAP adherence across age groups: clinical and empiric insights for developing CPAP adherence interventions . Sleep Med Rev. 2011. ; 15 ( 6 ): 343 – 356 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McEvoy RD , Antic NA , Heeley E , et al. SAVE Investigators and Coordinators . CPAP for prevention of cardiovascular events in obstructive sleep apnea . N Engl J Med. 2016. ; 375 ( 10 ): 919 – 931 . [DOI] [PubMed] [Google Scholar]
- 14. Peker Y , Thunström E , Glantz H , Eulenburg C . Effect of obstructive sleep apnea and CPAP treatment on cardiovascular outcomes in acute coronary syndrome in the RICCADSA trial . J Clin Med. 2020. ; 9 ( 12 ): 4051 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Huang Z , Liu Z , Luo Q , et al . Long-term effects of continuous positive airway pressure on blood pressure and prognosis in hypertensive patients with coronary heart disease and obstructive sleep apnea: a randomized controlled trial . Am J Hypertens. 2015. ; 28 ( 3 ): 300 – 306 . [DOI] [PubMed] [Google Scholar]
- 16. Sánchez-de-la-Torre M , Sánchez-de-la-Torre A , Bertran S , et al. Spanish Sleep Network . Effect of obstructive sleep apnoea and its treatment with continuous positive airway pressure on the prevalence of cardiovascular events in patients with acute coronary syndrome (ISAACC study): a randomised controlled trial . Lancet Respir Med. 2020. ; 8 ( 4 ): 359 – 367 . [DOI] [PubMed] [Google Scholar]
- 17. Moher D , Shamseer L , Clarke M , et al. PRISMA-P Group . Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015 statement . Syst Rev. 2015. ; 4 ( 1 ): 1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wells G , Shea B , O’Connell D , et al . The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa, Canada: Ottawa Hospital Research Institute; 2009. .
- 19. Huedo-Medina TB , Sánchez-Meca J , Marín-Martínez F , Botella J . Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol Methods. 2006. ; 11 ( 2 ): 193 – 206 . [DOI] [PubMed] [Google Scholar]
- 20. Capodanno D , Milazzo G , Cumbo M , et al . Positive airway pressure in patients with coronary artery disease and obstructive sleep apnea syndrome . J Cardiovasc Med (Hagerstown). 2014. ; 15 ( 5 ): 402 – 406 . [DOI] [PubMed] [Google Scholar]
- 21. Lee MC , Shen YC , Wang JH , et al . Effects of continuous positive airway pressure on anxiety, depression, and major cardiac and cerebro-vascular events in obstructive sleep apnea patients with and without coronary artery disease . Ci Ji Yi Xue Za Zhi. 2017. ; 29 ( 4 ): 218 – 222 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Milleron O , Pillière R , Foucher A , et al . Benefits of obstructive sleep apnoea treatment in coronary artery disease: a long-term follow-up study . Eur Heart J. 2004. ; 25 ( 9 ): 728 – 734 . [DOI] [PubMed] [Google Scholar]
- 23. Nakashima H , Kurobe M , Minami K , et al . Effects of moderate-to-severe obstructive sleep apnea on the clinical manifestations of plaque vulnerability and the progression of coronary atherosclerosis in patients with acute coronary syndrome . Eur Heart J Acute Cardiovasc Care. 2015. ; 4 ( 1 ): 75 – 84 . [DOI] [PubMed] [Google Scholar]
- 24. Yang D , Liu ZH , Zhao Q , Luo Q . Effects of nasal continuous positive airway pressure treatment on insulin resistance and ghrelin levels in non-diabetic apnoeic patients with coronary heart disease . Chin Med J (Engl). 2013. ; 126 ( 17 ): 3316 – 3320 . [PubMed] [Google Scholar]
- 25. Zhao Q , Liu ZH , Luo Q , Zhao ZH , Zhang HL , Wang Y . Effects of continuous positive airway pressure on blood pressure and daytime sleepiness in obstructive sleep apnea patients with coronary heart diseases under optimal medications . Sleep Breath. 2012. ; 16 ( 2 ): 341 – 347 . [DOI] [PubMed] [Google Scholar]
- 26. Cassar A , Morgenthaler TI , Lennon RJ , Rihal CS , Lerman A . Treatment of obstructive sleep apnea is associated with decreased cardiac death after percutaneous coronary intervention . J Am Coll Cardiol. 2007. ; 50 ( 14 ): 1310 – 1314 . [DOI] [PubMed] [Google Scholar]
- 27. Wu X , Lv S , Yu X , Yao L , Mokhlesi B , Wei Y . Treatment of OSA reduces the risk of repeat revascularization after percutaneous coronary intervention . Chest. 2015. ; 147 ( 3 ): 708 – 718 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Huang Z , Liu Z , Zhao Z , Zhao Q , Luo Q , Tang Y . Effects of continuous positive airway pressure on lipidaemia and high-sensitivity C-reactive protein levels in non-obese patients with coronary artery disease and obstructive sleep apnoea . Heart Lung Circ. 2016. ; 25 ( 6 ): 576 – 583 . [DOI] [PubMed] [Google Scholar]
- 29. Peker Y , Glantz H , Eulenburg C , Wegscheider K , Herlitz J , Thunström E . Effect of positive airway pressure on cardiovascular outcomes in coronary artery disease patients with nonsleepy obstructive sleep apnea. The RICCADSA randomized controlled trial . Am J Respir Crit Care Med. 2016. ; 194 ( 5 ): 613 – 620 . [DOI] [PubMed] [Google Scholar]
- 30. Abuzaid AS , Al Ashry HS , Elbadawi A , et al . Meta-analysis of cardiovascular outcomes with continuous positive airway pressure therapy in patients with obstructive sleep apnea . Am J Cardiol. 2017. ; 120 ( 4 ): 693 – 699 . [DOI] [PubMed] [Google Scholar]
- 31. da Silva Paulitsch F , Zhang L . Continuous positive airway pressure for adults with obstructive sleep apnea and cardiovascular disease: a meta-analysis of randomized trials . Sleep Med. 2019. ; 54 : 28 – 34 . [DOI] [PubMed] [Google Scholar]
- 32. Khan SU , Duran CA , Rahman H , Lekkala M , Saleem MA , Kaluski E . A meta-analysis of continuous positive airway pressure therapy in prevention of cardiovascular events in patients with obstructive sleep apnoea . Eur Heart J. 2018. ; 39 ( 24 ): 2291 – 2297 . [DOI] [PubMed] [Google Scholar]
- 33. Labarca G , Dreyse J , Drake L , Jorquera J , Barbe F . Efficacy of continuous positive airway pressure (CPAP) in the prevention of cardiovascular events in patients with obstructive sleep apnea: systematic review and meta-analysis . Sleep Med Rev. 2020. ; 52 : 101312 . [DOI] [PubMed] [Google Scholar]
- 34. Garcia-Rio F , Alonso-Fernández A , Armada E , et al . CPAP effect on recurrent episodes in patients with sleep apnea and myocardial infarction . Int J Cardiol. 2013. ; 168 ( 2 ): 1328 – 1335 . [DOI] [PubMed] [Google Scholar]
- 35. Leão S , Conde B , Fontes P , Calvo T , Afonso A , Moreira I . Effect of obstructive sleep apnea in acute coronary syndrome . Am J Cardiol. 2016. ; 117 ( 7 ): 1084 – 1087 . [DOI] [PubMed] [Google Scholar]
- 36. Yang SH , Xing YS , Wang ZX , et al . Association of obstructive sleep apnea with the risk of repeat adverse cardiovascular events in patients with newly diagnosed acute coronary syndrome: a systematic review and meta-analysis . Ear Nose Throat J. 2021. ; 100 ( 4 ): 260 – 270 . [DOI] [PubMed] [Google Scholar]
- 37. Martínez-García MA , Capote F , Campos-Rodríguez F , et al. Spanish Sleep Network . Effect of CPAP on blood pressure in patients with obstructive sleep apnea and resistant hypertension: the HIPARCO randomized clinical trial . JAMA. 2013. ; 310 ( 22 ): 2407 – 2415 . [DOI] [PubMed] [Google Scholar]
- 38. Muxfeldt ES , Margallo V , Costa LM , et al . Effects of continuous positive airway pressure treatment on clinic and ambulatory blood pressures in patients with obstructive sleep apnea and resistant hypertension: a randomized controlled trial . Hypertension. 2015. ; 65 ( 4 ): 736 – 742 . [DOI] [PubMed] [Google Scholar]