Summary
In response to the scarcity of advanced in vitro models dedicated to human CNS white matter research, we present a protocol to generate neuroectoderm-derived embedding-free human brain organoids enriched with oligodendrocytes. We describe steps for neuroectoderm differentiation, development of neural spheroids, and their transferal to Matrigel. We then detail procedures for the development, maturation, and application of oligodendrocyte-enriched brain organoids. The presence of myelin-producing cells makes these organoids useful for studying human white matter diseases, such as leukodystrophy.
Subject areas: Neuroscience, Stem Cells, Organoids
Graphical abstract
Highlights
-
•
Step-by-step protocol to generate human oligodendrocyte-enriched brain organoids
-
•
Detailed steps to develop brain organoids free from embedding with Matrigel
-
•
Rapid induction of oligodendrocyte precursors over 9 days of brain organoids differentiation
-
•
Oligodendrocyte brain organoids are enriched with cortical neurons and astrocytes
Publisher’s note: Undertaking any experimental protocol requires adherence to local institutional guidelines for laboratory safety and ethics.
In response to the scarcity of advanced in vitro models dedicated to human CNS white matter research, we present a protocol to generate neuroectoderm-derived embedding-free human brain organoids enriched with oligodendrocytes. We describe steps for neuroectoderm differentiation, development of neural spheroids, and their transferal to Matrigel. We then detail procedures for the development, maturation, and application of oligodendrocyte-enriched brain organoids. The presence of myelin-producing cells makes these organoids useful for studying human white matter diseases, such as leukodystrophy.
Before you begin
Before initiating any experiment, make sure to obtain all required institutional permissions and approvals, particularly when working with human embryonic stem cells (ESCs) or induced pluripotent stem cells (iPSCs). Appropriate approvals from the respective Institutional Review Board (IRB), as well as Material Transfer Agreements (MTAs) for cell lines or materials obtained from external sources must be in place. These precautions are important for ensuring that the research is conducted ethically and in compliance with regulatory guidelines. In this study, all experiments were carried out in accordance with the ethical guidelines of the University of Queensland and with the approval by the University of Queensland Human Research Ethics Committee (Approval number-2019000159).
Several methods exist for the generation of brain organoids enriched in oligodendrocytes,1,2,3,4,5 each with its unique advantages and limitations. However, the protocol described in this manuscript offers several benefits over existing methods. First, it is embedding-free protocol. Unlike many existing protocols, our method generates brain organoids without the need for embedding in Matrigel, thereby reducing complexity and potential variability in the organoid formation process, collectively increases the reproducibility of our protocol and makes it cost effective. Second, we have emphasized the rapid enrichment in oligodendrocytes as a significant advantage of our protocol. Specifically, our method has been meticulously optimized to expedite the generation of organoids enriched with OPCs over 9 days of in vitro differentiation, manifesting a higher proportion of oligodendrocytes in a relatively shorter time frame.
To generating oligodendrocyte (OL)-enriched brain organoids, it is essential to prepare all necessary materials as previously described in Shaker et al.,6,7 and the specific differences will be highlighted in this protocol. The differences here mainly include the process of generating these oligodendrocytes brain organoids free from embedding with Matrigel. In the key resources table, we provide a comprehensive list of materials and required equipment. It is essential that all procedures are performed in a biological enclosure of a Class II biohazard hood, using standard sterilized equipment. The cells and brain organoids must be cultured in a tissue culture incubator with a 37°C temperature and a 5% CO2 atmosphere. The following protocol describes in detail how to generate oligodendrocyte enriched brain organoids from embryonic stem cells ESCs (G22 and Mel-1) and induced pluripotent stem cells iPSCs (WTC, HBSL3C, EU79) cell lines, with comparable results.
Note: if you choose to use reagents from diverse suppliers, be mindful to verify the quality of these reagents for organoid generation.
Institutional permissions
All experiments were carried out in accordance with the ethical guidelines of the University of Queensland and with the approval by the University of Queensland Human Research Ethics Committee (Approval number-2019000159).
Media preparation
Timing: 15–30 min
Our protocol requires the preparation of three different types of media. The first one is neural induction medium (see materials and equipment (Olig1)). Olig1 medium can be stored at 4°C for up to one month. The second medium is the neural expansion medium (Olig2 medium; see materials and equipment). The third medium is the differentiation and maintenance medium (Olig3 medium; see materials and equipment).
Note: It is recommended to prepare Olig2 and Olig3 medium fresh and avoid long-term storage at 4°C. Alternatively, they can be stored for long term at −20°C prior to the addition of 2-BME and insulin (semi-prepared medium). When adopting latter option, after thawing the Olig2- or Olig3 medium, freshly add 2-BME and insulin prior to feeding the organoids.
CRITICAL: if the medium contains 2-BME and/or insulin, do not freeze it, as these reagents lose potency once frozen.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| SOX1 (1:150) | R&D Systems | AF2018 |
| SOX2 (ICC [1:300], whole mount [1:150]) | Cell Signaling Technology | D9B8N |
| SOX10 (ICC [1:300], whole mount [1:150]) | Cell Signaling Technology | 89356 |
| OLIG2 (1:300) | In Vitro Technologies | RDSAF2418SP |
| GFAP (1:300) | Thermo Fisher Scientific | 71-1500 |
| MBP (1:300) | Cell Signaling Technology | 78896 |
| NANOG (1:300) | Cell Signaling Technology | 4893 |
| N-CADHERIN (ICC [1:300], whole mount [1:150]) | Cell Signaling Technology | 14215 |
| OCT4 (1:300) | Bio-Rad | mca5683 |
| CTIP2 (1:300) | Abcam | ab18465 |
| SATB2 (1:300) | Abcam | Ab51502 |
| ZO-1 (1:150) | Thermo Fisher Scientific | 33-9100 |
| HOXC9 (1:150) | DSHB | 5B5-2-s |
| Hoechst (1:1,000) | Abcam | AB228551-5ML |
| Chemicals, peptides, and recombinant proteins | ||
| Matrigel hESC-qualified matrix | Corning | 354277 |
| Neurobasal medium | Invitrogen | 21103049 |
| DMEM-F12 | Thermo Fisher Scientific | 11320033 |
| GlutaMAX supplement | Thermo Fisher Scientific | 35050061 |
| N-2 supplement | Thermo Fisher Scientific | 17502001 |
| B27 supplement without vitamin A | Invitrogen | 12587-010 |
| MEM non-essential amino acids solution (100×) | Thermo Fisher Scientific | 11140050 |
| Penicillin/Streptomycin (P/S) | Invitrogen | 15140122 |
| T3 | Sigma-Aldrich Pty Ltd. | T2877-250MG |
| NT3 | Capsugel | 450-03-100UG |
| Human IGF-I (1 mg) | Capsugel | 100-11-1000 |
| Human HGF | Capsugel | 100-39H-100 |
| FGF | In Vitro Technologies Pty Ltd. | RDS233FB01M |
| Biotin | Sigma-Aldrich Pty Ltd. | B4639-1G |
| PDGF-AA | Capsugel | 100-13A-100UG |
| CAMP | Sigma | D0627 |
| SB-431542 | Merck | US1616464-5MG |
| LDN193189 | Sigma | SML0559-5MG |
| 2-Mercaptoethanol | Thermo Fisher Scientific | 21985023 |
| Insulin | Sigma-Aldrich Pty Ltd. | I9278-5ML |
| ReLeSR | STEMCELL Technologies | 100-0484 |
| PBS | Thermo Fisher Scientific | 20012027 |
| HBSS | Thermo Fisher Scientific | 88284 |
| Dispase | Thermo Fisher Scientific | 17105041 |
| mTeSR Plus | STEMCELL Technologies | 100-0276 |
| Software and Algorithms | ||
| GraphPad Prism | https://www.graphpad.com/features | |
| Adobe Illustrator | https://www.adobe.com/products/illustrator.html | |
| Fiji | https://fiji.sc/ | |
| BeeNet v1 | https://honeycombbio.zendesk.com/hc/en-us/articles/4408704149147-BeeNet-v1-1-X-Installation-Video | |
| Seurat software | https://satijalab.org/seurat/ | |
| Experimental models: Cell lines | ||
| Human ESCs | G22, Mel-1 | A13777 |
| Human iPSCs | WTC, EU79, HBSL3i | EU798 and HBSL3i were generated in-house in the Wolvetang lab; WTC is a kind gift of Bruce Conklin |
| Other | ||
| Ultra-low attachment 24-well plate | In Vitro Technologies Pty Ltd. | COR3473 |
| U-bottom ultra-low attachment plates, 96 well | Sigma | CLS7007-24EA |
| Ultra-low attachment plates, 6 well | Sigma | CLS7007-24EA |
| 6-Well plate | Sigma-Aldrich Pty Ltd. | CLS3516-50EA |
| Scissors and forceps | N/A | |
Materials and equipment
Neural induction medium (Olig1)
| No. | Medium components | Concentration/Stock | 100 mL volume |
|---|---|---|---|
| 1 | DMEM F12 | 10× 500 mL | 47.45 mL |
| 2 | N2 Supplement 5 mL (100×) | Supplemented at 1% | 1 mL |
| 3 | B 27 Supplement 10 mL | Supplemented at 2% | 2 mL |
| 4 | MEM Non-Essential Amino Acids Solution (100×) | Supplemented at 1% | 1 mL |
| 5 | Penicillin-Streptomycin (10,000 U/mL) | Supplemented at 1% | 1 mL |
| 6 | 2-Mercaptoethanol k(1000×) | Supplemented at 0.1% | 0.1 mL |
Note: Avoid medium freezing if already added 2-mercaptoethanol to the medium.
Note: The medium is stable for 2–3 weeks at 4°C and for 6 months in −20°C.
Expansion medium (Olig2)
| No. | Medium components | Concentration/Stock | 100 mL volume |
|---|---|---|---|
| 1 | DMEM F12 Nutrient mix | 10× 500 mL | 95.44 mL |
| 2 | B27 without vitamin A | Supplemented at 2% | 2 mL |
| 3 | N2 | Supplemented at 1% | 1 mL |
| 4 | MEM Non-Essential Amino Acids Solution (100×) | Supplemented at 1% | 1 mL |
| 5 | Penicillin-Streptomycin (10,000 U/mL) | Supplemented at 1% | 1 mL |
| 6 | 10 ng/mL PDGF-AA | 50 μg/mL | 20 μL |
| 7 | 10 ng/mL IGF | 100 μg/mL | 10 μL |
| 8 | 10 ng/mL NT3 | 50 μg/mL | 20 μL |
| 9 | 60 ng/mL T3 | 600 μg/mL | 10 μL |
| 10 | 100 ng/mL biotin | 1 mg/mL | 10 μL |
| 11 | 10 μM cAMP | 100 mM | 1 μL |
| 12 | 10 ng/mL HGF | 50 μg/mL | 20 μL |
| 13 | 2-Mercaptoethanol 50 mL (1000×) | Supplemented at 0.1% | 0.035 mL |
Note: Avoid medium freezing if already added 2-mercaptoethanol to the medium.
Note: The medium is stable for 2–3 weeks at 4°C and for 6 months in −20°C.
CRITICAL: Please use B27 supplement without Vitamin A.
Differentiation and maturation medium (Olig3)
| No. | Olig3 medium components | Concentration/Stock | 100 mL volume |
|---|---|---|---|
| 1 | DMEM F12 Nutrient mix 10× 500 mL | 1:1 ratio of DMEM/F12 and Neurobasal medium | 47.67 mL |
| Neurobasal Medium | 47.67 mL | ||
| 2 | B27 without vitamin A | Supplemented at 1% | 1 mL |
| 3 | N2 | Supplemented at 0.5% | 0.5 mL |
| 4 | MEM Non-Essential Amino Acids | Supplemented at 1% | 1 mL |
| 5 | GlutaMAX | Supplemented at 1% | 1 mL |
| 6 | Pen/Strep | Supplemented at 1% | 1 mL |
| 7 | 10 ng/mL PDGF-AA | 50 μg/mL | 20 μL |
| 8 | 10 ng/mL IGF | 100 μg/mL | 10 μL |
| 9 | 10 ng/mL NT3 | 50 μg/mL | 20 μL |
| 10 | 60 ng/mL T3 | 600 μg/mL | 10 μL |
| 11 | 100 ng/mL biotin | 1 mg/mL | 10 μL |
| 12 | 10 μM cAMP | 100 mM | 1 μL |
| 13 | 10 ng/mL HGF | 50 μg/mL | 20 μL |
| 14 | 2-Mercaptoethanol (1000×) | 0.35 μL/mL | 35 μL |
| 15 | Insulin | 9.5–11.5 mg/mL | 25 μL |
Note: Avoid medium freezing if already added 2-mercaptoethanol and Insulin to the medium.
Note: The medium is stable for 2–3 weeks at 4°C and for 6 months in −20°C.
CRITICAL: Please use B27 supplement without Vitamin A.
Step-by-step method details
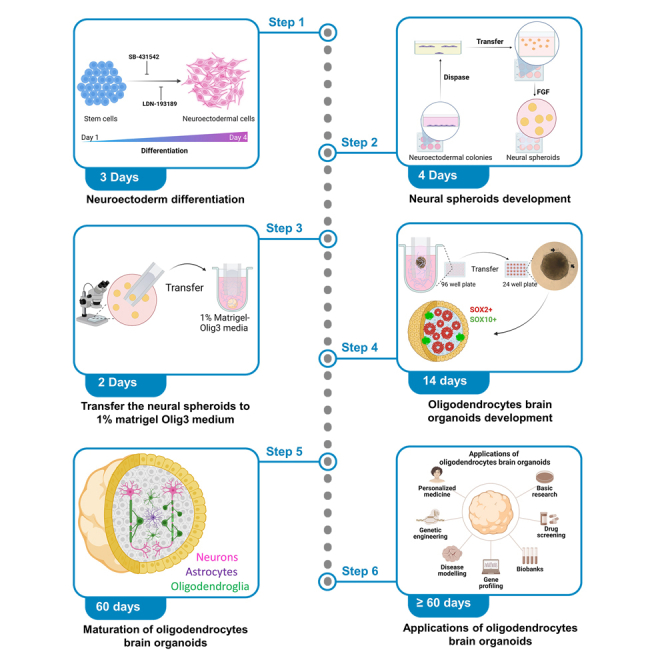
Neuroectodermal (NECT) colonies induction (days 1–3)
Timing: 3 days
Before initiating the neuroectodermal induction (NECT, See Figure 1A), prepare the experimental environment by culturing the stem cells on human embryonic stem cell (hESC)-qualified Matrigel, using the following steps.
-
1.
Prepare the basement membrane matrix by thawing a vial of 100% Matrigel on ice.
-
2.
Dilute the Matrigel for coating at a ratio of 1:50 in a standard DMEM/F12 medium.
-
3.Add 1 mL of DMEM/F12 medium with Matrigel per well of a 6-well plate.
-
a.Evenly spread across the well.
-
b.Let it coat the floor of the well at room temperature for an estimated 1-h or 30 min in the tissue culture incubator at 37°C temperature and 5% CO2.
-
a.
-
4.Once the well coating is done.
-
a.Carefully remove all the coating materials and avoid touching the surface of the coated wells.
-
b.Passage the stem cells using ReLeSR as described by STEMCELL Technologies.
-
a.
-
5.
Plate the stem cells into these Matrigel coated wells at a density between 20%–30% confluency per well. To achieve this density, transfer 60% confluent stem cell colonies from one well of a 6-well plate into three wells of another 6-well plate.
-
6.
Maintain the stem cells colonies for one day in mTeSR Plus medium prior to the differentiation.
-
7.Colony Assessment.
-
a.On the day of planned differentiation, inspect the stem cell colonies using a bright-field microscope at a magnification between 4× to 10×. It is best to photograph your colonies using phase optics for your records (e.g., See Figure 1A).
- b.
-
a.
-
8.
Prepare Olig1 medium by combining the reagents listed in neural induction medium recipe and allow it to reach room temperature prior to use.
-
9.
Remove the mTeSR Plus medium from the cultured plate with stem cells and add 2 mL of Olig1 medium to each well of a 6-well plate.
-
10.
Add the dual SMAD inhibitors, SB-431542 (10 μM) and LDN 193189 (0.1 μM) to each well containing Olig1 medium. Alternatively, add these inhibitors at appropriate concentrations to the required volume of Olig1 medium before adding it to the cells.
Note: To ensure even distribution of the inhibitors, gently rotate the plate or invert the tube containing the medium and the SMAD inhibitors 3–4 times.
-
11.
For the next 2 days, add fresh Olig1 medium containing 10 μM of SB-431542 and 0.1 μM of LDN 193189 daily.
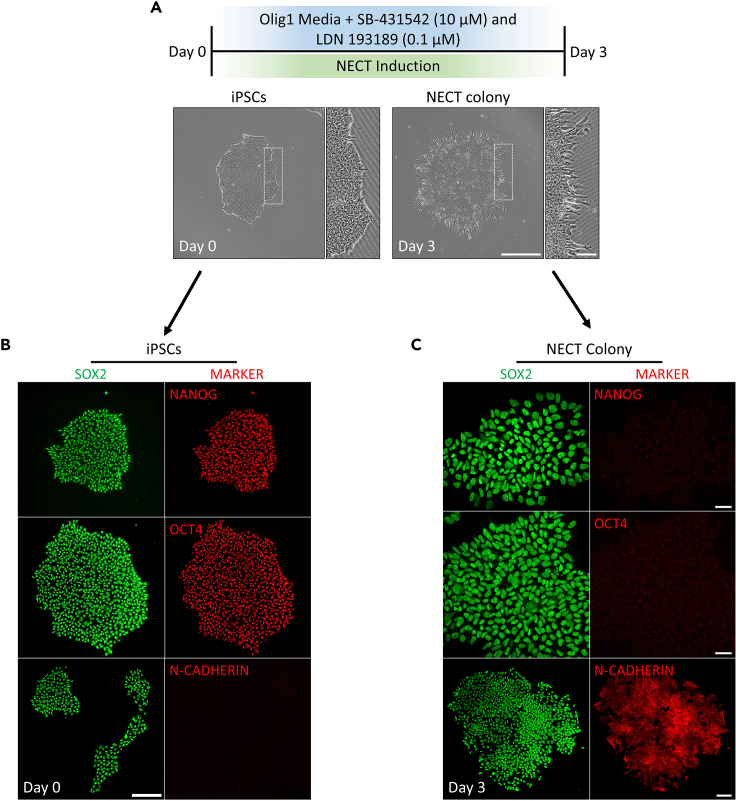
Note: Differentiated iPSC colonies towards neuroectodermal lineage should have a distinct shape compared to iPSC colonies, particularly at the colony's external borders. Namely, at day 3 one should see in these regions, a loss of cell contacts with fine cell membrane extensions (i.e., presence of filopodia and lamellipodia; Figure 1A, day 3). During this period (day 0–3), neuroectodermal colonies should lose pluripotent markers and express markers of neural cells, such as N-CADHERIN (Figures 1B and 1C).
Figure 1.
Characterization of iPSCs and neuroectodermal (NECT) colonies using immunocytochemistry
(A) Schematic diagram representing the protocol used to generate NECT colonies from stem cells. Representative bright-field-phase images showing the morphology of stem cells and NECT colonies in vitro are shown. Dotted white boxes indicate zoomed in regions. Scale bar = 500 μm, zoomed images scale bar = 100 μm.
(B) Immunocytochemistry staining of stem cell colonies marked with SOX2 (Green), NANOG (Red), OCT4 (Red), and N-CADHERIN (RED). Scale bar = 100 μm.
(C) Immunocytochemistry staining of NECT colonies marked with SOX2 (Green), NANOG (Red), OCT4 (Red), and N-CADHERIN (RED). Scale bar = 50 μm.
Generation/development of neural spheroids enriched in oligodendrocyte precursors (OPCs) (days 4–7)
Timing: 4 days
This phase of the protocol focuses on the preparation and enrichment of neural spheroids with oligodendrocyte precursors (OPCs). The following steps promote differentiation towards the oligodendrocytes lineage, fostering the proliferation of newly generated OPCs.
-
12.
Begin the procedure by preparing Olig2 medium using the reagents listed in Olig2 recipe and allow the medium to reach room temperature.
-
13.Colony detachment and Dispase treatment (Figure 2).
-
a.Remove Olig1 medium from each well of your 6 well plate and wash once with HBSS to ensure the total removal of Olig1 medium.Note: Ensure a complete removal of the Olig1 medium as it may potentially disrupt the enzymatic activity of Dispase, which could result in a failure to properly detach neuroectodermal colonies.
-
b.Remove the HBSS and add 1 mL of Dispase of 2.4 units/mL to detach NECT colonies.
-
c.Subject the tissue culture well to incubation at 37°C for a period ranging from 20 min to a maximum of 30 min and periodically monitor for colony detachment.Note: It's possible for smaller colonies to detach within the initial 20 min. After a 30-min period, all NECT colonies should be detached, and any colonies still attached should be discarded. Only detached colonies should be used for the next steps.
-
a.
-
14.NECT colonies handling (Figure 2).
-
a.After the Dispase incubation and detachment of the colonies, add 1 mL of Olig2 medium to each tissue culture well to halt the enzymatic activity of Dispase.
-
b.Use a wide-bore (cut) sterilized P1000 pipette tip (modified) to transfer the colonies into a 15 mL tube.
-
c.Allow the colonies to settle at the bottom of the tube by gravity, a process expected to take about 1 min (inspect by eye).
-
d.After the colonies have settled, cautiously remove the supernatant using a standard P1000 pipette tip and replacing it with 1 mL of fresh Olig2 medium. This step should be repeated three times to ensure a complete removal of Dispase from around the colonies.
-
a.
Note: The complete removal of Dispase is crucial, as any remaining Dispase may hinder the formation of neural spheroids and lead to significant cell death.
Note: To make the washing step cost effective, you can perform these washes using DMEM/F12 medium and only use the Olig2 medium on the last wash since Olig2 is significantly more expensive as compared to DMEM/F12 medium.
-
15.Colony Suspension and Seeding (Figure 2).
-
a.After the cell aggregates are sufficiently washed, resuspend them in 3 mL of Olig2 medium. This medium contains a cocktail of growth factors and small molecules that included thyroid hormone T3, neurotrophin NT3, hepatocytes growth factor (HGF), insulin growth factor (IGF), and platelet derived growth factor (PDGF-AA) to promote differentiation toward the oligodendroglial lineage and to foster the proliferation of newly generated OPCs.
-
b.Place the resuspended colonies in a single well of a 6-well plate and add 40 ng/mL of bFGF (Figure 3B).
-
a.
Note: If a large number of colonies have been detached, consider dividing them across multiple wells of a 6-well plate to prevent spheroids fusion. Monitor theses fusions over the next 24 h.
-
16.Maintain the spheroids in the same medium for the subsequent 3–4 days.
-
a.Supplement them with 40 ng/mL of bFGF daily to induce the proliferation of NECT cells and the self-organization activity that accompanies the neuroepithelia expansion (Figure 3B).
-
a.
Note: If the spheroids are plated at a higher density, a yellowing of the medium may occur, signaling a need to replace it every two days with fresh medium and 40 ng/mL of bFGF. To circumvent this issue, it's advised to keep a lower number of spheroids in each well.
Note: Between days 3 and 4, niches of SOX10+ cells, the precursors of oligodendrocytes, may begin to emerge. Use immunostaining procedures, to confirm their presence in organoids sections or intact (whole mount) organoids.
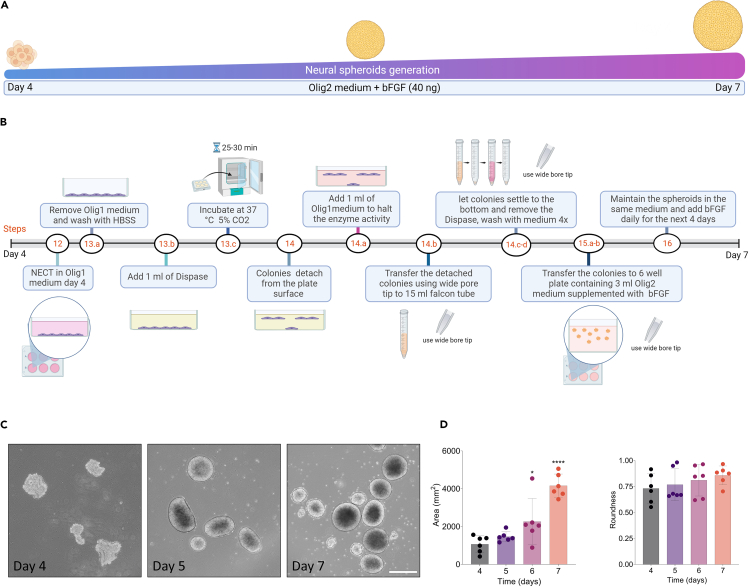
Figure 2.
Schematic diagram showing the process of neural spheroids generation enriched with oligodendrocyte progenitor cells from day 4 to day 7 of in vitro differentiation
Created with BioRender.com.
(A) Schematic diagram illustrates generation and characterization of neural spheroids.
(B) Schematic diagram showing the protocol used to culture the neural spheroids.
(C) Bright field images showing the progressive changes in morphology of neural spheroids during 4 days of proliferation. Scale bar = 400 μm.
(D) Bar graphs showing the quantification of neural spheroids area and roundness over 4 days of in vitro differentiation. Data are shown as the mean ± standard deviation; ∗p < 0.05, ∗∗∗∗p < 0.0001 via one-way ANOVA.
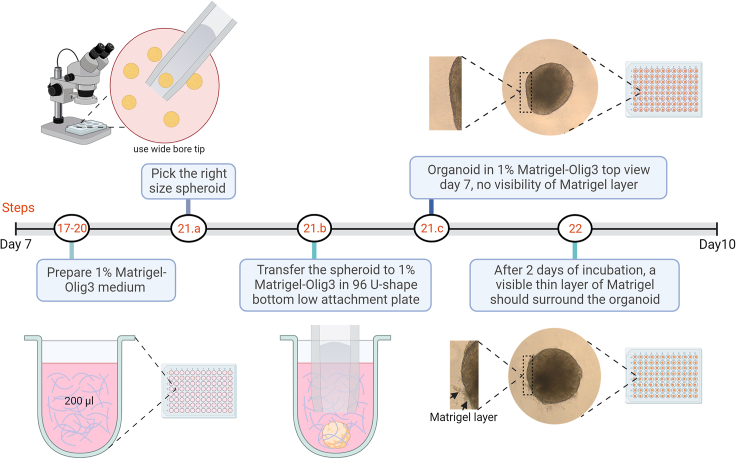
Figure 3.
Schematic diagram representing the detailed protocol of generating brain organoids enriched with oligodendrocyte precursors from day 7 to day 10
Created with BioRender.com.
Generation of brain organoids enriched in OPCs (day 8–10/11)
Timing: 3 days
This phase is dedicated to the process of generating brain organoids enriched with OPCs.
-
17.
Begin the procedure by preparing Olig3 medium using the reagents listed in Olig3 recipe and allow the medium to reach room temperature before use.
-
18.
Thaw a vial or aliquot of 100% Matrigel on ice.
-
19.Calculation and preparation of Matrigel and Olig3.
-
a.Calculate the required amounts of Olig3 medium and Matrigel to achieve 1% Matrigel. For instance, for 10 wells of 96 well plate, you will need 2.1 mL of Olig3 medium (including an extra 100 μL for pipetting error) and 21 μL of Matrigel.
-
b.Add the calculated amount of Matrigel to Olig3 medium in a 15 mL tube and mix well by inverting the tube vertically 4–5 times.
-
a.
-
20.
Add 200 μL of 1% Matrigel-Olig3 medium to each well of U-bottom low-attachment 96 well plate.
-
21.Selecting and transferring neural spheroids (Figure 3).
-
a.Use the stereomicroscope and select neural spheroids of similar size (around 500 μm) from the 6-well plate.
-
b.Use modified (cut tip) or wide bore pipette tip of 100 μL or 200 μL and carefully transfer neural spheroid one by one to each well of a 96-well plate (U-bottom low attachment).
-
a.
Note: Avoid using flat-bottom low attachment 96 well plate as it does not allow optimal intermingling of neural spheroids with the Matrigel containing medium.
-
22.
After 2 days of incubation in 1% Matrigel-Olig3 medium, the organoids should be intermingled with Matrigel, and a thin layer of Matrigel surrounding the organoid can be observed (Figure 3).
-
23.
Alternatively, if the interaction between organoids and Matrigel isn’t clear, the incubation in the 1% Matrigel-Olig3 medium can be extended for additional day.
Differentiation and maturation of brain organoid enriched oligodendrocytes (day 11/12–60)
Timing: ≥60 days
This segment of the protocol focuses on the extended differentiation and maturation of the brain organoid, specifically enriching it with mature oligodendrocytes.
-
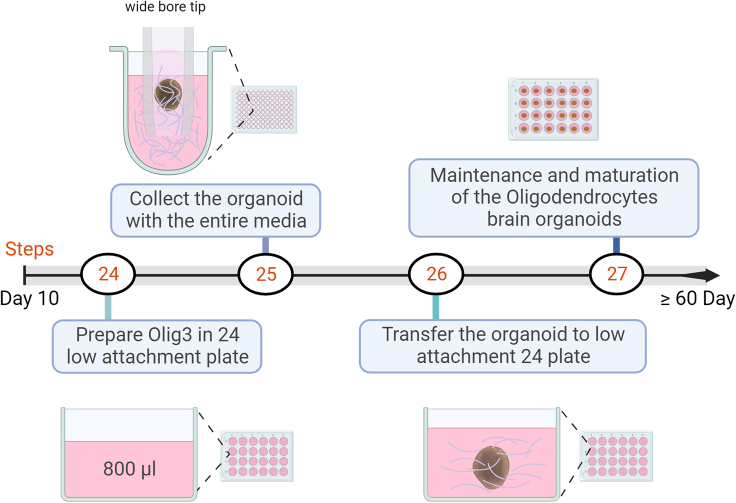
24.
Prepare 24 well low-attachment plates containing 800 μL of Olig3 medium per well (Figure 4).
-
25.
Collect the organoid with the entire medium using a wide bore tip.
-
26.
Transfer each organoid to a new 24-well low-attachment plate containing Olig3 medium to make 1 mL total volume.
Note: Always handle cells with care during the transfer to avoid damaging the organoids.
CRITICAL: There is a high probability of organoids fusing when culturing multiple organoids in the same well.
-
27.Maintenance and maturation of the oligodendrocytes brain Organoids
-
a.Maintain the differentiated oligodendrocyte brain organoids in Olig3 medium for more than six weeks to achieve mature oligodendrocytes.
-
b.Replace 0.5 mL of medium with 0.5 mL of fresh Olig3 medium in each well every 3 days during the first week.
-
c.After the first week of differentiation, medium can be changed every 2 days.
-
a.
CRITICAL: As organoid development progresses, nutrient consumption, and metabolic activity increases. Hence, it is recommended to visually inspect the medium for yellowing before the regular medium change (every 2–3 days). This is a crucial indicator for increasing the frequency of medium replacement.
Figure 4.
Schematic diagram representing the detailed protocol of generating brain organoids enriched with oligodendrocyte precursors from day 8 to day 10 or 11
Created with BioRender.com.
Expected outcomes
The primary outcome of the above protocol is the production of oligodendrocyte-enriched brain organoids, which are complex 3D in vitro models suited for human disease modeling.6 We have established a simplified and reproducible protocol to generate brain organoids enriched with oligodendrocytes. The steps include a fine-tuned medium cocktail of small molecules, allowing the progressive oligodendrocyte specification and maturation in addition to neurons and astrocytes. This model has applications for in vitro modeling of human white matter diseases such as hypomyelination and leukodystrophy.7
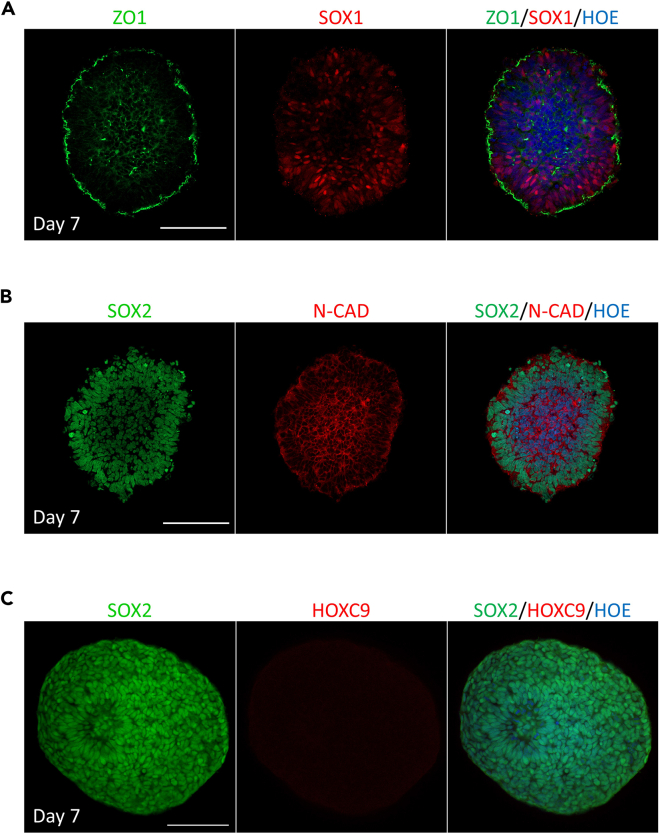
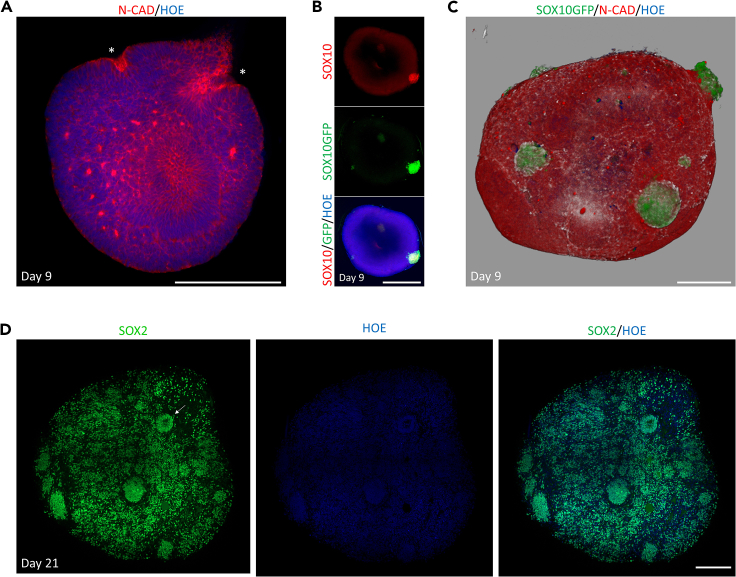
Organoids derived using the presented protocol contain multiple brain cell types, closely mimicking the human in vivo brain niche. At day 9 of neural expansion, the neural spheroids exhibit neural formation of rosettes and lack caudal identity (Figure 5). ZO1 labels the integrity of tight junctions at the apical side and reveals organized epithelial-like structures within the organoids. While the presence of SOX1 indicates a pool of early neural progenitors (Figure 5A), and SOX2 marks the definitive form of these proliferative progenitors within the organoid (Figure 5B). Additionally, we used HOX9 as an indicator for anterior-posterior patterning, emphasizing the segmental rostral identity and regional differentiation within the neural tissue (Figure 5C). Finally, N-Cadherin (NCAD) staining demonstrates cell-cell adhesion of neural cells (Figure 5B). Collectively, these markers offer a multi-dimensional validation of our organoid model, confirming their rostral identity and that it closely mimics complex human brain tissue both in cellular composition and organization as we previously demonstrated.8,9,10 At day 9 of differentiation, organoids exhibit neural tube folding (Figure 6A, white stars) as recently reported,11 and the emergence of SOX10+ cells in niches can be observed (Figure 6B).
Figure 5.
Characterization of neural spheroids at day 7 of in vitro differentiation
(A) Whole-mount immunostaining of neural spheroids stained with ZO1 (green) and SOX1 (red). All tissues were counterstained with Hoechst 33342 (blue). Scale bar = 100 μm.
(B) Whole-mount immunostaining of neural spheroids stained with SOX2 (green) and N-CADHERIN (red). All tissues were counterstained with Hoechst 33342 (blue). Scale bar = 100 μm.
(C) Whole-mount immunostaining of neural spheroids stained with SOX2 (green) and caudal spinal cord marker HOXC9 (red). All tissues were counterstained with Hoechst 33342 (blue). Scale bar = 100 μm.
Figure 6.
Characterization of differentiating neural spheroids in vitro
(A) Whole-mount immunostaining of differentiated neural spheroids at day 9 stained with N-CADHERIN (red) showing the neural rosettes and neural tube folding (white stars). All organoids were counterstained with Hoechst 33342 (blue). Scale bar = 50 μm.
(B) Whole-mount immunostaining of neural spheroids derived from WTC iPSCs-SOX10GFP reporter line, tissue was stained with SOX10 (red) and GFP (green). All organoids were counterstained with Hoechst 33342 (blue). Scale bar = 50 μm.
(C) Whole-mount 3D reconstruction images of day 9 neural spheroids derived from WTC iPSCs-SOX10GFP reporter line. The neural spheroid was immunostained with N-CADHERIN (red) and GFP (green). All organoids were counterstained with Hoechst 33342 (blue). Scale bar = 200 μm.
(D) Whole-mount staining for SOX2 (green) of oligodendrocyte brain organoids at day 21 of in vitro differentiation. Organoids were counterstained with Hoechst 33342 (blue). White arrow indicates a neural rosette. Scale bar = 200 μm.
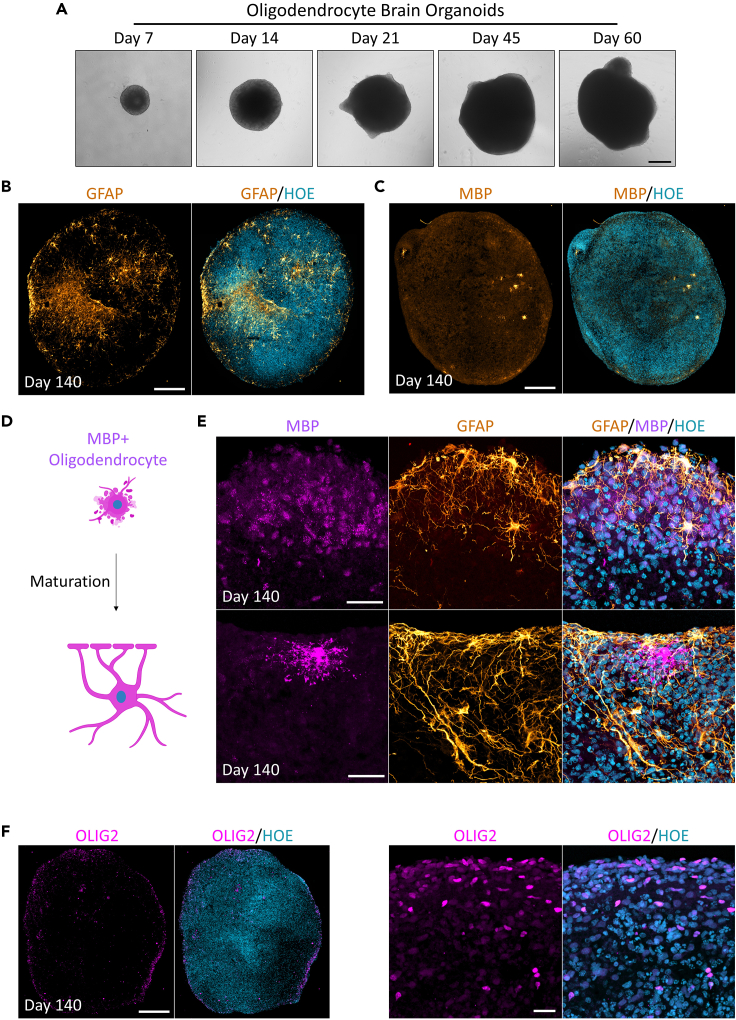
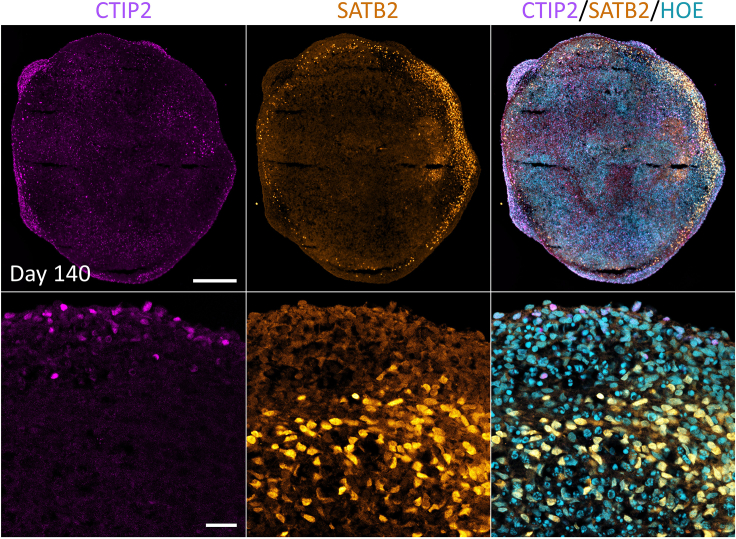
Whole-mount 3D reconstruction clearly shows multiple niches of SOX2+ cells were emerged (Figure 6C). The appearance of SOX10+ cells at day 9 of differentiation is a clear indicator of the onset of oligodendrocyte lineage specification within the developing organoids. SOX10 is a transcription factor critical for the development of OPCs. Its presence at this stage signifies that the oligodendrogenesis process is initiated within the organoids. This may enable researchers to generate and study the biology of early OPCs when using this 9-day protocol. Interestingly, these organoids still express a large proportion of rosettes SOX2+ cells at day 21 (Figure 6D, white arrow), suggesting that proliferation of neural progenitors is ongoing at this stage (Figure 7A). At day 120 of differentiation, organoids exhibit a substantial population of astrocytes and mature myelinating oligodendrocytes, as demonstrated by the presence of GFAP (Figure 7B) and myelin basic protein (MBP), respectively (Figure 7C). Interestingly, we observed early and late stages of MBP+ cells as marked by MBP staining (Figures 7D and 7E). We used anti-oligodendrocyte transcription factor (OLIG2 antibody) as a pan-molecular marker that labels oligodendroglial cells and found a large number of OLIG2+ cells in these oligodendrocytes brain organoids at day 120 (Figure 7F). We further confirmed the presence of cortical CTIP2 and SATB2 positive cortical neurons in these organoids (Figure 8). CTIP2 and SATB2 are neuronal transcription factors that can be used to identify subtypes of cortical neurons, and as development proceeds distinct classes of projecting neurons are being specified.10 Importantly, we noted that with our embedding free protocol described here, the development of a necrotic core is rarely observed (e.g., Figures 6, 7, and 8). This is in contrast to conventionally generated oligodendrocytes enriched brain organoids that often develop a necrotic core in the center of the organoid6 following months of in vitro culture.
Figure 7.
Characterization of oligodendrocyte brain organoids
(A) Bright-field images showing the developmental stages of oligodendrocytes brain organoids overtime in vitro. Scale bar = 400 μm.
(B) Analysis of immunostaining of organoid sections at day 140 showing expression of astrocyte marker GFAP (gold). The section was counterstained with Hoechst 33342 (cyan). Scale bar = 200 μm.
(C) Immunostaining of organoid sections on day 140 showing expression of the mature myelinating oligodendrocyte marker MBP (gold). The section was counterstained with Hoechst 33342 (cyan). Scale bar = 200 μm.
(D) Schematic diagram showing the different morphologies of MBP positive cells across organoid developmental stages.
(E) Examples of organoids sections at day 140 double immune-stained for astrocyte marker GFAP (gold) and the mature myelinating oligodendrocyte marker MBP (pink). All sections were counterstained with Hoechst 33342 (cyan). Scale bar = 25 μm.
(F) Immunostaining of organoids sections on day 120 showing expression of the pan-oligodendroglia marker OLIG2 (pink). The sections were counterstained with Hoechst 33342 (cyan). Scale bar = 200 μm. Zoomed images scale bar = 20 μm.
Figure 8.
Cortical Neuronal Characterization of oligodendrocyte-enriched brain organoids
Immunostaining of oligodendrocyte-enriched brain organoids sections at days 140 reveals cortical layers V neurons marked by CTIP2 (pink), and layer IV neurons marked by SATB2 (gold), respectively. All sections were counterstained with Hoechst 33342 cyan). Scale bar = 200 μm. Zoomed images scale bar = 25 μm.
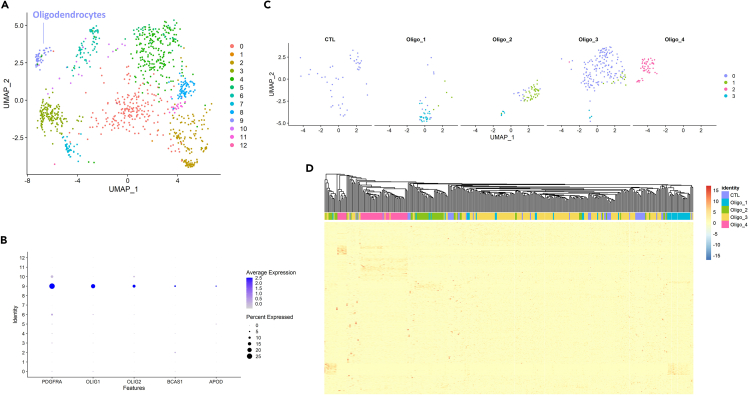
To compare the generated oligodendrocytes to those developed in-vivo in the human fetal brain, we performed single-cell RNA-sequencing of the oligodendrocyte brain organoid derived from HBSL3i using the HIVE platform. The generated fastq files were processed using BeeNet and Seurat v4.1.4 (https://satijalab.org/seurat/), yielding 13 clusters for 1028 cells that passed filtering criteria (Figure 9A). Cluster 9 (46 cells) expressed oligodendrocyte markers such as PDGFRA, OLIG1, and OLIG2 with some cells expressing mature oligodendrocyte markers APOD and BCAS1 (Figure 9B). This cluster was integrated using the “IntegrateData()” function into the oligodendrocyte population identified from fetal brain samples across gestational weeks 9 through 28.12 Re-clustering into 4 new clusters that largely matched the oligodendrocyte sub-populations from the original study showed that oligodendrocytes in brain organoids overlapped with Oligo_3 population (Figure 9C). Furthermore, marker genes for each of the five cell populations identified using “FindAllMarkers()” function based on the cutoff “p_val_adj < 0.05 & abs(avg_log2FC) > 0.25” also cluster these OPCs with Oligo_3 (Figure 9D). In conclusion, our protocol generates oligodendrocyte-enriched brain organoids that are highly reproducible in terms of size as well as cellular and molecular characteristics closely comparable to the developing human fetal oligodendrocytes, which is crucial for developing a reliable human-specific in vitro model for disease modeling and drug screening.
Figure 9.
Comparison of oligodendrocytes in brain organoids with fetal brain OPCs/oligodendrocytes
(A) UMAP of single-cell RNA-sequencing of oligodendrocyte brain organoids showing the oligodendrocyte population (cluster 9) in the generated brain organoids.
(B) Dot plot showing the expression of selected markers of oligodendrocytes. Dot size represents the percentage of clusters expressing a specific marker, while the intensity of color indicates the average expression level for that gene, in that cluster.
(C) Integration of cluster 9 with fetal brain OPCs and oligodendrocytes clusters the generated cells (marked as CTL) with Oligo_3 forming the new cluster 0, while Oligo_1, Oligo_2, and Oligo_4 form clusters 3, 1, and 2 respectively.
(D) Heatmap based on marker genes for each of the five populations (CTL, Oligo_1, Oligo_2, Oligo_3, Oligo_4) reveals similar overall gene expression of CTL and Oligo_3.
Limitations
Despite recent developments, the various organoid production techniques still need to be further optimized to recapitulate the cellular composition of the developing human brain reliably and consistently. The main limitation of the current protocol is that other brain cells such as microglia are not co-produced within the organoid, as they are formed from a non-neural lineage (i.e., outside the developing CNS). Microglia can however be separately produced and can seed brain organoids via co-culture if desired.13 Additionally, while our protocol captures the oligodendrocyte lineage’s transcriptional dynamics through single-cell RNA-sequencing, it currently lacks functional assessments like MEA recordings and electrophysiological studies, which are vital for understanding the functional maturity of these cells. As for the integration of functioning vasculature around and/or within brain organoids, this issue remains a significant challenge.14
Troubleshooting
Problem 1
Incomplete thawing of Matrigel can affect its consistency and functionality (related to step 1).
Potential solution
-
•
Ensure that the Matrigel is thoroughly thawed on ice before using.
Problem 2
Failure to detach neuroectodermal colonies with Dispase (related to step 13).
Potential solution
-
•
Make sure to wash thoroughly with HBSS to remove all traces of Olig1 medium, as it could interfere with Dispase activity.
-
•
Also, monitor the incubation period closely and discard any colonies that do not detach within 30 min.
Problem 3
Death of cells or failure to form neuroectodermal spheroids (related to step 14).
Potential solution
-
•
Ensure complete removal of Dispase after incubation, as any remaining Dispase may cause cell death or hinder spheroid formation.
Problem 4
Yellowing of the medium during neural spheroid expansion (related to step 16).
Potential solution
-
•
This could indicate that the spheroids are plated at too high a density. Try to keep a lower number of spheroids in each well and consider changing the medium more frequently.
Problem 5
Absence of a clear layer of Matrigel around the organoids (related to step 22 and 23).
Potential solution
-
•
Extend the incubation period for an extra day to allow more time for the Matrigel to form a layer around the organoids.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Mohammed R. Shaker (m.shaker@uq.edu.au).
Materials availability
All data reported in this paper will be shared by the lead contact upon request.
Data and code availability
This paper does not report original code.
Acknowledgments
B.A.-M. and S.D.M. are supported by the University of Queensland (UQ) Research Training Scholarship. B.A.-M. is also supported by the UQ Entrepreneurial PhD Top-up Scholarship. M.R.S. is supported by the Children’s Hospital Foundation (PCC0252021). E.J.W. is supported by the MRFF Leukodystrophy Flagship – Massimo’s Mission (EPCD000034). P.G.N. is supported by MNDRIA and NHMRC funding (IG 2326; #1188169). Bruce Conklin (Department of Medicine, Gladstone Institute of Cardiovascular Disease) is greatly acknowledged for the WTC iPSCs gift. The authors acknowledge the facilities, and the scientific and technical assistance, of the Microscopy Australia Facility at the School of Biomedical Sciences of the University of Queensland.
Author contributions
B.A. conceptualized the study, designed the experiments, and implemented, analyzed, and wrote the protocol; M.B.M. designed the experiments, provided data acquisition, and implemented the protocol; S.D.M., P.G., and M.A. performed additional experiments; P.G.N. supervised the study; and E.J.W. and M.R.S. conceived and supervised the study, interpreted results, and wrote the manuscript. The final version of the manuscript was approved by all authors.
Declaration of interests
The authors declare no competing interests.
Contributor Information
Ernst J. Wolvetang, Email: e.wolvetang@uq.edu.au.
Mohammed R. Shaker, Email: m.shaker@uq.edu.au.
References
- 1.Madhavan M., Nevin Z.S., Shick H.E., Garrison E., Clarkson-Paredes C., Karl M., Clayton B.L.L., Factor D.C., Allan K.C., Barbar L., et al. Induction of myelinating oligodendrocytes in human cortical spheroids. Nat. Methods. 2018;15:700–706. doi: 10.1038/s41592-018-0081-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marton R.M., Miura Y., Sloan S.A., Li Q., Revah O., Levy R.J., Huguenard J.R., Pașca S.P. Differentiation and maturation of oligodendrocytes in human three-dimensional neural cultures. Nat. Neurosci. 2019;22:484–491. doi: 10.1038/s41593-018-0316-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim H., Xu R., Padmashri R., Dunaevsky A., Liu Y., Dreyfus C.F., Jiang P. Pluripotent stem cell-derived cerebral organoids reveal human oligodendrogenesis with dorsal and ventral origins. Stem Cell Rep. 2019;12:890–905. doi: 10.1016/j.stemcr.2019.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yun W., Kim I.Y., Song G., You S. Rapid induction of gliogenesis in OLIG2 and NKX2. 2-expressing progenitors-derived spheroids. Stem Cells Transl. Med. 2020;9:1643–1650. doi: 10.1002/sctm.19-0455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.James O.G., Selvaraj B.T., Magnani D., Burr K., Connick P., Barton S.K., Vasistha N.A., Hampton D.W., Story D., Smigiel R., et al. iPSC-derived myelinoids to study myelin biology of humans. Dev. Cell. 2021;56:1346–1358.e6. doi: 10.1016/j.devcel.2021.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shaker M.R., Pietrogrande G., Martin S., Lee J.-H., Sun W., Wolvetang E.J. Rapid and Efficient Generation of Myelinating Human Oligodendrocytes in Organoids. Front. Cell. Neurosci. 2021;15:631548. doi: 10.3389/fncel.2021.631548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shaker M.R., Kahtan A., Prasad R., Lee J.-H., Pietrogrande G., Leeson H.C., Sun W., Wolvetang E.J., Slonchak A. Neural Epidermal Growth Factor-Like Like Protein 2 Is Expressed in Human Oligodendroglial Cell Types. Front. Cell Dev. Biol. 2022;10:803061. doi: 10.3389/fcell.2022.803061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Briggs J.A., Sun J., Shepherd J., Ovchinnikov D.A., Chung T.-L., Nayler S.P., Kao L.-P., Morrow C.A., Thakar N.Y., Soo S.-Y., et al. Integration-Free Induced Pluripotent Stem Cells Model Genetic and Neural Developmental Features of Down Syndrome Etiology. Stem Cell. 2013;31:467–478. doi: 10.1002/stem.1297. [DOI] [PubMed] [Google Scholar]
- 9.Shaker M.R., Aguado J., Chaggar H.K., Wolvetang E.J. Klotho inhibits neuronal senescence in human brain organoids. NPJ Aging Mech. Dis. 2021;7:18. doi: 10.1038/s41514-021-00070-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shaker M.R., Slonchak A., Al-mhanawi B., Morrison S.D., Sng J.D.J., Cooper-White J., Khromykh A., Wolvetang E.J. Choroid Plexus Defects in Down Syndrome Brain Organoids Enhance Neurotropism of SARS-CoV-2. bioRxiv. 2023 doi: 10.1101/2023.06.12.544552. Preprint at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee J.-H., Shin H., Shaker M.R., Kim H.J., Park S.-H., Kim J.H., Lee N., Kang M., Cho S., Kwak T.H., et al. Production of human spinal-cord organoids recapitulating neural-tube morphogenesis. Nat. Biomed. Eng. 2022;6:435–448. doi: 10.1038/s41551-022-00868-4. [DOI] [PubMed] [Google Scholar]
- 12.Fan X., Fu Y., Zhou X., Sun L., Yang M., Wang M., Chen R., Wu Q., Yong J., Dong J., et al. Single-cell transcriptome analysis reveals cell lineage specification in temporal-spatial patterns in human cortical development. Sci. Adv. 2020;6 doi: 10.1126/sciadv.aaz2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schafer S.T., Mansour A.A., Schlachetzki J.C.M., Pena M., Ghassemzadeh S., Mitchell L., Mar A., Quang D., Stumpf S., Ortiz I.S., et al. An in vivo neuroimmune organoid model to study human microglia phenotypes. Cell. 2023;186:2111–2126.e20. doi: 10.1016/j.cell.2023.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cakir B., Xiang Y., Tanaka Y., Kural M.H., Parent M., Kang Y.-J., Chapeton K., Patterson B., Yuan Y., He C.-S., et al. Engineering of human brain organoids with a functional vascular-like system. Nat. Methods. 2019;16:1169–1175. doi: 10.1038/s41592-019-0586-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This paper does not report original code.