Abstract
Background
People living with HIV (PLWH) have a higher risk of developing hypertension compared to HIV uninfected individuals. HIV assisted partner services (aPS), where PLWH are assisted by a healthcare provider to disclose their status to sexual and / or drug injecting partner(s), offers an opportunity for integrated HIV and hypertension screening. We evaluated the feasibility of the aPS model in supporting integrated HIV and hypertension screening at the Kenyatta National Hospital, Kenya.
Methods
Between August 2019 and December 2020, we conducted a pre-post intervention study. We enrolled women receiving HIV testing services (HTS) with confirmed hypertension (female index clients) and traced their male relatives for HIV and hypertension screening and reviewed management at 3-months. Hypertension was defined as systolic blood pressure (SBP) ≥ 140 mmHg, diastolic blood pressure (DBP) ≥ 90 mmHg, and/or use of antihypertensive medication.
Results
One hundred female index clients (median age: 55 years; interquartile range (IQR): 47–65) mentioned 165 male relatives (median: 49 years; IQR: 40–59) of whom 35% (n = 58/165) were enrolled. Of the male relatives, 29% had hypertension (n = 17/58), 34% had pre-hypertension (n = 20/58), and none were HIV-positive (n = 0/58). Among the female index clients, there was a statistically significant decline in SBP (pre: 156 mmHg, post: 133 mmHg, p-value: < 0.0001) and DBP (pre: 97 mmHg, post: 80 mmHg, p-value: < 0.0001), and increase in antihypertensive medication uptake (pre: 91%, n = 84/92; post: 98%, n = 90/92; X2: 4.3931, p-value: 0.036) relative to baseline. Among the male relatives, there was a statistically significant increase in antihypertensive medication uptake among those with hypertension (pre: 13%, n = 6/46; post: 17%, n = 8/46; X2: 32.7750, p-value: < 0.0001) relative to baseline.
Conclusion
HIV aPS holds promise for integrated HIV and hypertension screening among at-risk clients and their families. Twenty-nine percent of the male relatives had hypertension, higher than the national prevalence (24%), while one-third had pre-hypertension. We observed relatively high participant retention, reductions in blood pressure, and increase in antihypertensive medication uptake among those with confirmed hypertension. Future research expanding the aPS model to other non-communicable diseases through larger studies with longer follow-ups is required to better assess causal relationships and optimize integrated service delivery.
Keywords: HIV, Assisted partner services, Hypertension, Integration
Contributions to the literature
HIV assisted partner services (aPS) have been shown to be effective in HIV-case finding among sex partners to newly diagnosed HIV-positive individuals.
Many African countries are dealing with the double burden of communicable and non-communicable diseases.
We, therefore, assessed the feasibility of utilizing the aPS model to support integrated HIV and hypertension screening.
The high prevalence of hypertension and prehypertension among male relatives to women diagnosed with hypertension receiving HIV testing services is of grave concern.
The aPS model shows promise in providing family-based, integrated disease management.
Background
African countries are increasingly facing the double burden of communicable and non-communicable diseases (NCDs), especially cardiovascular diseases (CVDs) [1]. According to the World Health Organization (WHO), CVDs are the leading cause of death globally with over 75% of these deaths occurring in low and middle-income countries, particularly in Africa [2]. Hypertension is the leading risk factor for CVDs [2]. However, despite 24% of all adults in Kenya having hypertension, only 16% of them are aware of their hypertensive status [3]. HIV infection is also known to be a risk factor in the development of hypertension through derangement of lipid metabolism and immune activation [4]. In Kenya, the HIV prevalence is estimated at 5% with approximately 80% of persons living with HIV (PLWH) aware of their HIV-positive status [5].
Due to disparities in HIV and hypertension awareness, offering hypertension screening to individuals receiving HIV testing service (HTS) can potentially promote early diagnosis of hypertension among individuals with or at risk of HIV infection. One intervention to improve HIV screening that can be leveraged is HIV assisted partner services (aPS) - where consenting PLWH are assisted by a trained healthcare provider to disclose their status or to anonymously notify their sexual and / or drug injecting partner(s) of their potential exposure to HIV [6]. Several clinical trials and programmatic evaluations conducted in Africa have demonstrated the effectiveness of aPS in increasing HIV testing and linkage to care [7–13].
Data from several programs in Kenya, including the Healthy Heart Africa (HHA) initiative indicate that men are less likely than women to be screened for hypertension (36% versus 63%) and even less likely to link to care if diagnosed [14, 15]. Similar observations have been made in HIV programs in Kenya; despite numerous interventions to improve HIV testing in accordance with the UNAIDS 95-95-95 targets, 73% of men in Kenya are aware of their HIV status compared to 83% of women [5]. By leveraging HTS infrastructure, facilities can be used to offer both HIV and hypertension screening services. We, therefore, evaluated the feasibility of the HIV aPS model in supporting integrated HIV and hypertension screening in Nairobi, Kenya.
Methods
Study design and setting
We conducted a pre-and post-intervention study between August 2019 and December 2020 at the Kenyatta National Teaching and Referral Hospital (KNH) Voluntary Counselling and Testing (VCT) Center. KNH is the largest public facility in Kenya and was selected due to its diverse population of clients living with or without HIV [16].
Study participants
We recruited women with confirmed hypertension receiving HTS at KNH VCT who may or may not have been HIV-positive (female index clients). Eligible female index clients were at least 35 years of age, not pregnant, willing to provide informed consent and male relative information, and residing within 50 km of the study site. Male relatives, including husbands, sons, brothers, grandsons, fathers etc., were at least 35 years old and resided within 50 km of the study site.
Intervention
Female index clients were interviewed by study staff to obtain information on their male relatives. Study staff were HTS providers i.e., Ministry of Health certified facility-based lay workers with diplomas in social science or counseling psychology and certificate training on HTS. Male relatives were then traced on phone or in person at least 4 times over a 1 month duration by study staff. Those successfully traced were informed of the study, offered hypertension screening and HIV testing at the nearest hospital, home, or convenient venue, and referred for management, as appropriate. Participants testing HIV positive were referred to a HIV comprehensive care clinic, while those with confirmed hypertension were referred to a physician for management.
At baseline and on 3 month follow-up, enrolled participants received counselling on dietary and lifestyle management. At 3-months, participants with hypertension were reviewed for blood pressure control while those known to be HIV positive were reviewed for ART initiation and viral suppression. Participants with either poorly controlled blood pressure or had not initiated ART at follow-up were counselled and referred to the medical outpatient clinic or to the CCC for management, respectively. We have included the template for intervention description and replication (TIDieR) recommendations checklist.
Study procedures
Socio-demographic characteristics and anthropometric measurements (weight, height, waist, and hip circumference) were collected from enrolled participants at the baseline and 3-month follow-up visit. HIV infection was confirmed using rapid tests according to the national HTS protocol [17].
Blood pressure was measured using the automatic Omron M6 comfort (OMRON Healthcare, Japan) at three time points in the sitting position, 5 min apart. The Omron machines were calibrated daily following the manufacturer’s protocol to ensure accurate blood pressure readings. Hypertension was defined as a systolic blood pressure (SBP) ≥ 140 mm Hg and / or diastolic blood pressure (DBP) ≥ 90 mm Hg across three measurements on two occasions, and/or use of antihypertensive medication according to the Kenya guidelines [18]. Those with blood pressure levels above 140/90 mmHg were referred to the medical outpatient clinic for confirmation within 6 weeks before being confirmed as having hypertension. Pre-hypertension was defined as blood pressure of between 130/80 mmHg and 139/89 mmHg.
Weight was measured to the nearest ± 0.1 kg using ADE spring scale (1–150 kg). Height, waist, and hip circumferences were measured to the nearest ± 0.1 cm using a stadiometer SECA 213 portable stadiometer (SECA, German) or a mounted tape measure on a hard and level surface. Each week, the weight scale was calibrated using standard weights, while the height scale and mounted tape were calibrated using a standard one meter metallic rod. Body mass index (BMI) was calculated as weight/height2 (kg/m2) and the cut-off points were severely underweight < 16.5 kg/ m2, underweight: 16.5 to 18.5 kg/ m2, normal weight: 18.5 to 24.9 kg/ m2, overweight 25 to 29.9 kg/ m2, and obesity ≥ 30 kg/ m2 [18]. Waist-hip ratio (WHR) was calculated as waist circumference / hip circumference and the cut-off points were normal: male < 0.9, female < 0.85, and central obesity: male ≥ 0.9, female ≥ 0.85.
Data collection
Data was collected by study staff using interviewer-administered questionnaires programmed on mobile phones using the Open Data Kit platform before being uploaded onto a central server [12, 19]. This technology platform was programmed with validation checks to reduce data entry errors and improve efficiency in data management.
Data analysis
We described baseline sociodemographic characteristics and the prevalence of hypertension and HIV. We compared baseline to 3-month SBP, DBP, BMI, and WHR using paired sample t-tests, and baseline to 3-month antihypertensive medication uptake using Pearson’s chi-square test. Analysis was conducted using STATA version 17.
Ethical approval
This study received ethical approval from the KNH Ethical and Scientific Review Committee (P568/08/2018). All interviewees provided written informed consent prior to enrolment. For illiterate participants, informed consent to participate was taken from legal guardians.
Results
Female index client characteristics
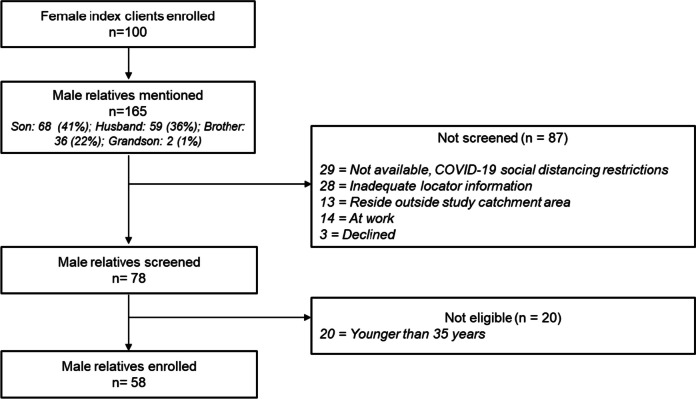
We enrolled 100 female index clients who had a median age of 55 years (interquartile range, IQR: 47–65) (Fig. 1, Table 1). Two-thirds were in married monogamous relationships (66%, n = 66/100), and none were HIV-positive (0%, n = 0/100). The median BMI and WHR were 29 kg/m2 and 0.86, respectively.
Fig. 1.
Flow diagram of participant screening and enrollment
Table 1.
Baseline demographic characteristics
| Variable | Details |
Female index clients n = 100 n (%), median (IQR) |
Overall male relatives n = 58 n (%), median (IQR) |
Husbands n = 27 n (%), median (IQR) |
Sons n = 18 n (%), median (IQR) |
Brothers n = 12 n (%), median (IQR) |
Grandson n = 1 n (%), median (IQR) |
|---|---|---|---|---|---|---|---|
| Age | Years | 55 (47–65) | 49 (40–59) | 56 (46–68) | 43 (39–51) | 49 (40–51) | 35 (35–35) |
| Education | Primary | 62 (62%) | 19 (33%) | 12 (44%) | 3 (17%) | 4 (33%) | - |
| Secondary | 34 (34%) | 29 (50%) | 12 (44%) | 10 (56%) | 6 (50%) | 1 (100%) | |
| Tertiary | 3 (3%) | 10 (17%) | 3 (11%) | 5 (27%) | 2 (17%) | - | |
| None | 1 (1%) | - | - | - | - | - | |
| Marital status | Married monogamous | 66 (66%) | 53 (91%) | 26 (96%) | 17 (94%) | 10 (83%) | - |
| Widow / widower | 20 (20%) | 1 (1%) | 1 (4%) | - | - | - | |
| Divorced / separated | 9 (9%) | 1 (1%) | - | - | 1 (8%) | - | |
| Single | 5 (5%) | 2 (3%) | - | - | 1 (8%) | 1 (100%) | |
| Married polygamous | - | 1 (1%) | - | 1 (6%) | - | - | |
| HIV status | Positive | - | - | - | - | - | - |
| Negative | 96 (96%) | 55 (95%) | 26 (96%) | 16 (89%) | 12 (100%) | 1 (100%) | |
| Declined testing | 4 (4%) | 3 (5%) | 1 (4%) | 2 (11%) | - | - | |
| Hypertension status | Hypertension | 100 (100%) | 17 (29%) | 8 (30%) | 7 (39%) | 2 (17%) | - |
| Pre-hypertension | - | 20 (34%) | 10 (37%) | 6 (33%) | 4 (33%) | - | |
| Blood pressure | Median mmHg | 154/95 (145/90–167/100) | 127/79 (120/76–139/85) | 130/79 (120/76–140/84) | 130/80 (125/77–140/90) | 120/77 (118/76–131/85) | 119/70 (119/70–119/70) |
| Body mass index | Kg/m2 | 29 (25–33) | 23 (22–26) | 24 (23–26) | 24 (22–26) | 23 (22–25) | 20 (20–20) |
| Waist/hip ratio | Ratio | 0.86 (0.83–0.90) | 0.92 (0.87–0.95) | 0.94 (0.87–0.97) | 0.92 (0.89–0.95) | 0.87 (0.86–0.89) | 0.94 (0.94–0.94) |
IQR Interquartile range, Hypertension Defined as systolic blood pressure ≥ 140 mm Hg and / or diastolic blood pressure ≥ 90 mm Hg and / or use of antihypertensive medication, Pre-hypertension Defined as blood pressure of between 130/80 mmHg and 139/89 mmHg. Of the 100 female index clients with hypertension, 92 (92%)were on antihypertensive medication. Of the 58 male relatives, 9 (16%) were on antihypertensive medications - all of whom had confirmed hypertension
Male relative characteristics
The female index clients mentioned 165 male relatives namely, brothers: 68 (41%), husbands: 59 (36%), sons: 36 (22%), and grandsons: 2 (1%) (Fig. 1). Of these, 35% (n = 58/165) were enrolled into the study. Main reasons for low male relative enrollment were COVID-19 social distancing restrictions at the time of the study, inadequate locator information, residing outside the study catchment area, and being younger than 35 years.
The 58 enrolled male relatives had a median age of 49 years (IQR: 40–59), 91% were in married monogamous relationships (n = 53/58), and none (0%, n = 0/58) were HIV positive (Table 1). About one-third of the male relative had pre-hypertension (34%, n = 20/58), while 29% had hypertension (n = 17/58) i.e., husbands (30%, n = 8/27), sons 39%, n = 7/18), brothers (17%, n = 2/12), grandsons (0%, n = 0/1). The median BMI and WHR were 23 kg/m2 and 0.92, respectively.
Female index client retention at the 3 month visit
Of the 100 enrolled female index clients, 92 (92%) returned for the 3-month visit. Of the eight who were lost to follow-up, the reasons included being out of town (63%, n = 5/8) and death (38%, n = 3/8). The three deaths, recorded from the death certificates presented by the relatives, occurred after hospitalization due to renal failure (n = 2) and septic shock after eye surgery in a client with hypertension and diabetes (n = 1). All deaths were reported to the KNH Ethical and Scientific Review Committee as per the study protocol.
Male relative retention at the 3-month visit
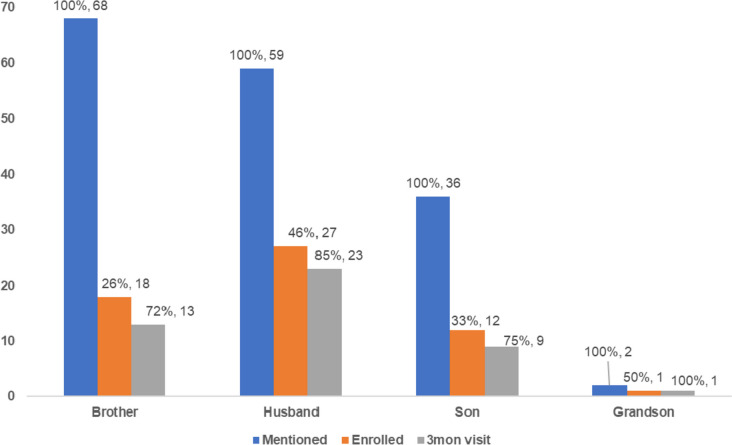
Of the 58 enrolled male relatives, 46 (79%) returned for the 3-month visit. Of the 12 who were lost to follow-up, the reasons included unreachability on phone and / or in-person (83%, n = 10/12), being out of town (8%, n = 1/12), and unavailability for clinic visit due to work (8%, n = 1/12). Less than half of the mentioned male relatives were enrolled in each category (husband: 46%, n = 27/59; son: 33%, n = 12/36; brother: 26%, n = 18/65; grandson: 50%, n = 1/2) (Fig. 2). After enrolment, retention among enrolled male relatives was relatively high across the categories (husband: 85%, n = 23/27; son: 75%, n = 9/12; brother: 72%, n = 13/18; grandson: 100%, n = 1/1).
Fig. 2.
Male relatives mentioned, enrolled, and retained at the 3-month visit
Changes in anthropometric measures and antihypertensive medication uptake
Among the 92 female index clients at the 3-month visit, there was a statistically significant decline in post-intervention SBP and DBP, and increase in antihypertensive medication uptake, relative to baseline (Tables 2 and 3). However, there was no difference between the baseline and 3-month BMI and WHR.
Table 2.
Changes in anthropometric measures between baseline and 3-month follow-up
| Baseline visit | 3-month visit | Mean difference | Lower 95% CI | Upper 95% CI | P-value | |
|---|---|---|---|---|---|---|
| Female index clients (n = 92) | ||||||
| Systolic BP | 156 | 133 | 23 | 19 | 26 | < 0.0001 |
| Diastolic BP | 97 | 80 | 17 | 14 | 19 | < 0.0001 |
| Body mass index | 29 | 29 | 0.1 | -0.4 | 0.5 | 0.7317 |
| Waist / hip ratio | 0.88 | 0.86 | 0.02 | -0.01 | 0.05 | 0.2569 |
| Male relatives (n = 46) | ||||||
| Systolic BP | 129 | 127 | 3 | -2 | 8 | 0.2697 |
| Diastolic BP | 81 | 78 | 2 | -2 | 6 | 0.2649 |
| Body mass index | 24 | 24 | -0.1 | -0.2 | 0.1 | 0.3936 |
| Waist / hip ratio | 0.91 | 0.91 | 0 | -0.01 | 0.01 | 0.6341 |
CI Confidence interval, BP Blood pressure
Table 3.
Changes in antihypertensive medication uptake between baseline and 3-month follow-up
| Baseline visit | 3-month visit | X2 | P-value | |
|---|---|---|---|---|
| Female index clients (n = 92) | ||||
| Hypertension status | ||||
| On anti-hypertensive medication | ||||
| Overall | 84 (91%) | 90 (98%) | 4.3931 | 0.036 |
| Hypertensive | 84 (91%) | 90 (98%) | ||
| Pre-hypertensive | 0 (0%) | 0 (0%) | ||
| Normal BP | 0 (0%) | 0 (0%) | ||
| Not on anti-hypertensive medication | ||||
| Overall | 8 (9%) | 2 (1%) | ||
| Hypertensive | 8 (9%) | 1 (1%) | ||
| Pre-hypertensive | 0 (0%) | 1 (1%) | ||
| Normal BP | 0 (0%) | 0 (0%) | ||
| Male relatives (n = 46) | ||||
| On anti-hypertensive medication | ||||
| Overall | 6 (13%) | 8 (17%) | 32.7750 | < 0.0001 |
| Hypertensive | 6 (13%) | 8 (17%) | ||
| Pre-hypertensive | 0 (0%) | 0 (0%) | ||
| Normal BP | 0 (0%) | 0 (0%) | ||
| Not on anti-hypertensive medication | ||||
| Overall | 40 (87%) | 38 (83%) | ||
| Hypertensive | 5 (11%) | 2 (4%) | ||
| Pre-hypertensive | 18 (39%) | 16 (35%) | ||
| Normal BP | 17 (37%) | 20 (43%) | ||
X2 Pearson chi-square statistic
Among the 46 male relatives at the 3-month visit, there was a statistically significant increase in post-intervention antihypertensive medication uptake among those with hypertension relative to baseline (Table 3). However, there was no statistically significant difference between pre- and post-intervention SBP, DBP, BMI, and WHR.
Discussion
In this study utilizing the aPS model to support integrated HIV and hypertension screening, female index clients mentioned not only their husbands, but also their brothers, sons, and grandsons, widening the reach to men who may otherwise have never received HIV and hypertension screening services. Despite low enrolment among the male relatives, 29% of those enrolled had hypertension, 34% had pre-hypertension, and none were HIV-positive. At the 3 month follow-up visit, we observed relatively high retention among participants with statistically significant increase in antihypertensive medication uptake among those with hypertension. We also observed significant reductions in SBP and DBP among female index clients, though no significant changes in blood pressure were observed among enrolled male relatives.
To our knowledge, this is the first study in Kenya showing spousal and maternal associations in hypertension risk, highlighting the potential of the aPS model to support targeted hypertension screening among families with shared genetic and lifestyle related risk factors. In a longitudinal cohort study evaluating spousal metabolic risk factors and incident hypertension in Iran, there was increased risk of hypertension from having a spouse with diabetes mellitus [20]. Although they did not evaluate the children of such couples, the study highlighted the potential of using NCD health data from one family member to evaluate risk and guide the screening of the other. Interestingly, no fathers to the female index clients were mentioned in our study, either because they were deceased or resided outside of study catchment area, indicating a missed opportunity for screening. Fathers, as well as other older relatives, are at a higher risk for hypertension and other age-related morbidities. They, together with their families, may benefit from targeted screening for HIV and hypertension among other NCDs.
Overall, there was low enrolment of male relatives due to COVID-19 social distancing restrictions which was largely unavoidable [21]. However, a significant proportion of those not enrolled had inadequate locator information which was surprising as these were close relatives to the female index clients. Inadequate locator information e.g., wrong phone numbers, residential / workplace addresses, names, has been a challenge in previous aPS studies [22, 23]. Healthcare providers offering aPS will require training and support in creating rapport and building trust with index clients as they elicit contact information.
Twenty-nine percent of the enrolled male relatives had hypertension, higher than the national hypertension prevalence in Kenya (24%) [3], while 34% of them had pre-hypertension. This is quite alarming and indicates the value of targeted hypertension screening among family members to individuals known to have hypertension. A similar approach may be used for other NCDs that are common among families, e.g., diabetes mellitus - whose risk factors include central obesity which we observed among our participants. In Tanzania, the prevalence of diabetes mellitus and renal failure among hypertensive PLWH was 9% and 29%, respectively, while 53% had an intermediate to high 10-year risk of an atherosclerotic cardiovascular disease (ASCVD) event [24], flagging the risk for comorbidities in this population group. As PLWH grow older, policymakers will need to integrate NCD management into HIV programs to avert such complications. This is all the more important given Kenya’s concerns over low HIV testing yields – defined as proportion of individuals newly testing HIV-positive out of total individuals tested for HIV. Kenya has a low national HIV testing yield of approximately ~1% similar to our study where none of the participants were HIV-positive [25]. The available HIV infrastructure can, therefore, be more effectively utilized to support integrated service delivery for both communicable and NCDs.
There was relatively high participant retention and anti-hypertensive medication uptake at 3-months, potentially due to the family-centered approach to screening. In our qualitative study, participants preferred such hypertension screening models due to the inbuilt family support systems [26]. Policymakers may need to adopt integrated service delivery models to PLWH with NCD related comorbidities [27]. One such example is multimonth dispensing of ART and antihypertensive medications which was shown to improve hypertension control, viral suppression, and retention to care among hypertensive PLWH in Uganda [28]. We also observed changes in anthropometric measures at the 3-month visit with statistically significant declines in blood pressure among female index clients, though this was not observed among the male relatives. In a pilot study in Tanzania, researchers observed similar blood pressure declines over a 4-week period when utilizing a community healthcare worker delivered educational intervention to support integrated hypertension care engagement in HIV programs [29]. Such people-centered care models hold promise in improving care and management of hypertension, and potentially other NCDs, within HIV care programs.
Our study had several strengths. First, we evaluated the feasibility of the aPS model in supporting integrated HIV and hypertension screening services, contributing to literature on its potential. Second, this study was conducted at KNH, the largest teaching and referral hospital in Kenya that receives patients from the entire country, improving the generalizability of study results. Third, our study design included a pre-post intervention assessment of our outcomes of interest allowing us to assess changes over time.
Among the limitations, we had a small sample size due to COVID-19 social distancing restrictions that limited participant access. Second, we had a 3-month follow-up duration that may not be sufficient in evaluating long-term blood pressure control and lifestyle management. Third, our study design lacked both random assignment and a control group. It is, therefore, challenging to assess whether the direction and magnitude of the changes would have been different from a placebo group or if they were due to natural maturation. We, however, believe that the findings from our study provide insights to integrated HIV and hypertension screening services.
Conclusion
The HIV aPS model holds promise in supporting targeted HIV and hypertension screening among at-risk clients and their families. This model can also be expanded to support widespread screening services for other non-communicable diseases e.g., targeted diabetes screening of at-risk relatives to individuals diagnosed with diabetes. Future research with larger studies involving longer follow-ups is required to better assess causal relationships and optimize integrated service delivery models.
Acknowledgements
The authors would like to acknowledge the contributions of the Kenyatta National Hospital, the study team, and study participants.
Abbreviations
- aPS
Assisted partner services
- BMI
Body mass index
- CVD
Cardiovascular disease
- DBP
Diastolic blood pressure
- HTS
HIV testing service
- IPV
Intimate partner violence
- IQR
Interquartile range
- KNH
Kenyatta National Teaching and Referral Hospital
- MOH
Ministry of Health
- NCD
Non-communicable disease
- PLWH
People living with HIV
- SBP
Systolic blood pressure
- TIDieR
Template for intervention description and replication
- VCT
Voluntary counselling and testing
- WHO
World Health Organization
- WHR
Waist hip ratio
Authors’ contributions
BW, CF, DB, and TT conceived and designed the study. BW, BS, and PM developed the data collection tools. BS, MN, and CM facilitated data collection. BS and PM extracted the data. BW and BS analyzed the data. BW drafted the manuscript. All authors reviewed and revised the manuscript. All authors approved the final draft for submission.
Funding
This study was funded by the joint WHO AFRO / Tropical Diseases Research / EDCTP small grants scheme. This project was conducted with the support of the Takemi Program in International Health at the Harvard T.H. Chan School of Public Health. BW received support from the Fogarty International Center: D43 TW009580, D43 TW009783 and D43 TW010905. The funders had no role in the study conception, design, data collection, analysis, decision to publish, or preparation of the article for publication.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study received ethical approval from the Kenyatta National Hospital Ethical and Scientific Review Committee (P568/08/2018). All interviewees provided written informed consent prior to participation. All methods were performed in accordance with the relevant guidelines and regulations.
All methods were performed in accordance with the relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Achwoka D, Waruru A, Chen TH, Masamaro K, Ngugi E, Kimani M, et al. Noncommunicable disease burden among HIV patients in care: a national retrospective longitudinal analysis of HIV-treatment outcomes in Kenya, 2003–2013. BMC Public Health. 2019;19(1):372. doi: 10.1186/s12889-019-6716-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO. Cardiovascular diseases (CVDs). WHO. Available from: http://www.who.int/mediacentre/factsheets/fs317/en/. Cited 2017 Oct 31.
- 3.Mohamed SF, Mutua MK, Wamai R, Wekesah F, Haregu T, Juma P, et al. Prevalence, awareness, treatment and control of hypertension and their determinants: results from a national survey in Kenya. BMC Public Health. 2018;18(3):1219. doi: 10.1186/s12889-018-6052-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gibellini D, Borderi M, Clò A, Morini S, Miserocchi A, Bon I, et al. HIV-related mechanisms in atherosclerosis and cardiovascular diseases. J Cardiovasc Med. 2013;14(11):780–790. doi: 10.2459/JCM.0b013e3283619331. [DOI] [PubMed] [Google Scholar]
- 5.National AIDS and STI Control Programme (NASCOP). Kenya Population-based HIV Impact Assessment (KENPHIA) 2018: final report. Nairobi; 2022. Available from: https://phia.icap.columbia.edu/wp-content/uploads/2022/08/KENPHIA_Ago25-DIGITAL.pdf.
- 6.WHO. Guidelines on HIV self-testing and partner notification. WHO. Available from: http://www.who.int/hiv/pub/vct/hiv-self-testing-guidelines/en/. Cited 2017 Jan 8.
- 7.Brown LB, Miller WC, Kamanga G, Nyirenda N, Mmodzi P, Pettifor A, et al. HIV partner notification is effective and feasible in sub-Saharan Africa: opportunities for HIV treatment and prevention. J Acquir Immune Defic Syndr. 2011;56(5):437–442. doi: 10.1097/QAI.0b013e318202bf7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rutstein SE, Brown LB, Biddle AK, Wheeler SB, Kamanga G, Mmodzi P, et al. Cost-effectiveness of provider-based HIV partner notification in urban Malawi. Health Policy Plan. 2014;29(1):115–126. doi: 10.1093/heapol/czs140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cherutich P, Golden MR, Wamuti B, Richardson BA, Ásbjörnsdóttir KH, Otieno FA, et al. Assisted partner services for HIV in Kenya: a cluster randomised controlled trial. Lancet HIV. 2017;4(2):e74–82. doi: 10.1016/S2352-3018(16)30214-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Myers RS, Feldacker C, Cesár F, Paredes Z, Augusto G, Muluana C, et al. Acceptability and effectiveness of assisted human immunodeficiency virus partner services in Mozambique: results from a pilot program in a public. Urban Clinic Sex Transm Dis. 2016;43(11):690–695. doi: 10.1097/OLQ.0000000000000529. [DOI] [PubMed] [Google Scholar]
- 11.Henley C, Forgwei G, Welty T, Golden M, Adimora A, Shields R, et al. Scale-up and case-finding effectiveness of an HIV partner services program in Cameroon: an innovative HIV prevention intervention for developing countries. Sex Transm Dis. 2013;40(12):909–914. doi: 10.1097/OLQ.0000000000000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cherutich P, Golden M, Betz B, Wamuti B, Maingi P, Macharia P, et al. Surveillance of HIV assisted partner services using routine health information systems in Kenya. BMC Med Inform Decis Mak. 2016;16(1):97. doi: 10.1186/s12911-016-0337-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wamuti BM, Erdman LK, Cherutich P, Golden M, Dunbar M, Bukusi D, et al. Assisted partner notification services to augment HIV testing and linkage to care in Kenya: study protocol for a cluster randomized trial. Implement Sci. 2015;10(1):23. doi: 10.1186/s13012-015-0212-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ogola E, Okello FO, Macgregor-skinner E, Jimenez J, Yonga G. [OP.3C.03] Blood pressure screening results from Healthy Heart Africa: screening locations, participant characteristics, and hypertension classification in Kenya. J Hypertens. 2017;35:e30. doi: 10.1097/01.hjh.0000523058.87062.ab. [DOI] [Google Scholar]
- 15.Van Gelder A, Jimenez J, Ogola E, Yonga G. Healthy Heart Africa - a coordinated program to increase awareness, screening and treatment of hypertension in Kenya through collaboration with local healthcare systems. J Hypertens. 2017;35:e95–e96. doi: 10.1097/01.hjh.0000523222.34110.19. [DOI] [Google Scholar]
- 16.Kenyatta National Teaching and Referral Hospital, Nairobi, Kenya | History. Available from: https://knh.or.ke/index.php/history/. Cited 2022 Jan 26.
- 17.National AIDS and STI Control Programme, Ministry of Health, Kenya. Guidelines for HIV testing services in Kenya. 2015.
- 18.Ministry of Health. Kenya national guidelines for cardiovascular diseases management. 2018.
- 19.ODK - collect data anywhere. Available from: https://getodk.org. Cited 2023 Mar 30.
- 20.Ramezankhani A, Guity K, Azizi F, Hadaegh F. Spousal metabolic risk factors and incident hypertension: a longitudinal cohort study in Iran. J Clin Hypertens. 2020;22(1):95–102. doi: 10.1111/jch.13783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lagat H, Sharma M, Kariithi E, Otieno G, Katz D, Masyuko S, et al. Impact of the COVID-19 pandemic on HIV testing and assisted partner notification services, Western Kenya. AIDS Behav. 2020;24(11):3010–3013. doi: 10.1007/s10461-020-02938-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu W, Wamuti BM, Owuor M, Lagat H, Kariithi E, Obong’o C, et al. “It is a process” – a qualitative evaluation of provider acceptability of HIV assisted partner services in western Kenya: experiences, challenges, and facilitators. BMC Health Serv Res. 2022;22(1):616. doi: 10.1186/s12913-022-08024-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goyette M, Muthoni W, Owuor M, Bukusi D, Mutiti M, Abuna O, et al. Understanding barriers to scaling up HIV assisted partner services in Kenya. AIDS Patient Care STDS. 2016;30(11):506–11. doi: 10.1089/apc.2016.0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manavalan P, Madut DB, Hertz JT, Thielman NM, Okeke NL, Mmbaga BT, et al. Hypertension among adults enrolled in HIV care in northern Tanzania: comorbidities, cardiovascular risk, and knowledge, attitudes and practices. Pan Afr Med J. 2022;41:285. doi: 10.11604/pamj.2022.41.285.26952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Cock KM, Barker JL, Baggaley R, El Sadr WM. Where are the positives? HIV testing in sub-Saharan Africa in the era of test and treat. AIDS. 2019;33(2):349. doi: 10.1097/QAD.0000000000002096. [DOI] [PubMed] [Google Scholar]
- 26.Wamuti B, Owuor M, Magambo C, Ndegwa M, Sambai B, Temu TM, et al. ‘My people perish for lack of knowledge’: barriers and facilitators to integrated HIV and hypertension screening at the Kenyatta National Hospital, Nairobi, Kenya. Open Heart. 2023;10(1):e002195. doi: 10.1136/openhrt-2022-002195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Godfrey C, Vallabhaneni S, Shah MP, Grimsrud A. Providing differentiated service delivery to the ageing population of people living with HIV. J Int AIDS Soc. 2022;25(S4):e26002. doi: 10.1002/jia2.26002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kimera ID, Namugenyi C, Schwartz JI, Musimbaggo DJ, Ssenyonjo R, Atukunda P, et al. Integrated multi-month dispensing of antihypertensive and antiretroviral therapy to sustain hypertension and HIV control. J Hum Hypertens. 2023;37(3):213–219. doi: 10.1038/s41371-022-00655-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manavalan P, Madut DB, Wanda L, Msasu A, Mmbaga BT, Thielman NM, et al. A community health worker delivered intervention to address hypertension among adults engaged in HIV care in northern Tanzania: outcomes from a pilot feasibility study. J Clin Hypertens. 2022;24(8):1095–1104. doi: 10.1111/jch.14518. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.