Abstract
Background
Arcobacter species are considered emerging foodborne pathogens that can potentially cause serious infections in animals and humans. This cross-sectional study determined the frequency of potentially pathogenic Arcobacter spp. in both commercial and smallholder farm animals in Ghana and Tanzania. A total of 1585 and 1047 (poultry and livestock) samples were collected in Ghana and Tanzania, respectively. Selective enrichment media, along with oxidase and Gram testing, were employed for isolation of suspected Arcobacter spp. and confirmation was done using MALDI-TOF MS. Antibiotic susceptibility was assessed through disk diffusion method and ECOFFs were generated, for interpretation, based on resulting inhibition zone diameters.
Results
The overall Arcobacter frequency was higher in Ghana (7.0%, n = 111) than in Tanzania (2.0%, n = 21). The frequency of Arcobacter in commercial farms in Ghana was 10.3% (n/N = 83/805), while in Tanzania, it was 2.8% (n/N = 12/430). Arcobacter was detected in only 3.6% (n/N = 28/780) of the samples from smallholder farms in Ghana and 1.5% (n/N = 9/617) of the samples from Tanzania. For commercial farms, in Ghana, the presence of Arcobacter was more abundant in pigs (45.1%, n/N = 37/82), followed by ducks (38.5%, n/N = 10/26) and quails (35.7%, n/N = 10/28). According to MALDI-TOF-based species identification, Arcobacter butzleri (91.6%, n/N = 121/132), Arcobacter lanthieri (6.1%, n/N = 8/132), and Arcobacter cryaerophilus (2.3%, n/N = 3/132) were the only three Arcobacter species detected at both study sites. Almost all of the Arcobacter from Ghana (98.2%, n/N = 109/111) were isolated during the rainy season. The inhibition zone diameters recorded for penicillin, ampicillin, and chloramphenicol allowed no determination of an epidemiological cut-off value. However, the results indicated a general resistance to these three antimicrobials. Multidrug resistance was noted in 57.1% (n/N = 12/21) of the Arcobacter isolates from Tanzania and 45.0% (n/N = 50/111) of those from Ghana. The type of farm (commercial or smallholder) and source of the sample (poultry or livestock) were found to be associated with multi-drug resistance.
Conclusions
The high levels of MDR Arcobacter detected from farms in both countries call for urgent attention and comprehensive strategies to mitigate the spread of antimicrobial resistance in these pathogens.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13099-023-00588-3.
Keywords: Arcobacter, Commercial farms, Smallholder farms, Antimicrobial resistance, Ghana, Tanzania, Arcobacter butzleri, Arcobacter lanthieri, Arcobacter cryaerophilus
Background
Arcobacter species are considered emerging foodborne pathogens that can potentially cause human infections [1, 2]. Arcobacter is closely related to Campylobacter in terms of taxonomy and clinical symptoms. Clinically important pathogenic Arcobacter species include Arcobacter butzleri, Arcobacter cryaerophilus, and Arcobacter skirrowii [3]. Of these, A. butzleri is the most frequently isolated and associated with septicemia and gastroenteritis in humans [4]. In animals, the bacterium is primarily transmitted horizontally from the environment or one animal to another and vertically from parents to progeny [5]. Humans mainly get infected through ingestion and handling of fresh or undercooked contaminated foods of animal origin. Most Arcobacter infections are self-limiting and, hence, do not require treatment with antibiotics. Currently, tetracyclines and fluoroquinolones are the recommended antibiotics for treating infections caused by Arcobacter spp. [6].
In sub-Saharan Africa (SSA), the emergence of Arcobacter spp. resistant to tetracycline, aminoglycosides, and fluoroquinolones can be attributed to the excessive use of antibiotics in human medicine and animal husbandry [7–9]. Studies conducted in different geographical locations in SSA have reported multidrug-resistant Arcobacter [10, 11]. So far, more than 50 genes associated with tetracycline resistance in Arcobacter isolates from environmental samples have been described [12, 13]. Also, fluoroquinolone resistance associated with mutations in gyrA has been observed in Arcobacter species [14]. The World Health Organization (WHO) recently classified fluoroquinolone-resistant Campylobacter-like organisms as part of the 12 antibiotic-resistant priority pathogens that pose the greatest threat to human health [15].
Isolation of Arcobacter from local and imported poultry meat has been reported in Ghana [9]. In Ghana and Tanzania, poultry and livestock meat products are largely consumed, and most rural and semi-urban households own poultry [8]. Consumers may be at risk if farm animals carry pathogenic Arcobacter species. Monitoring and characterising Arcobacter species along the food chain is essential for a more accurate estimation of the population at risk. So far, only a few studies have been conducted in SSA, of which most studies focused on commercially produced poultry but not on the smallholder farm level [9, 16, 17]. Therefore, this study aimed to determine the frequency and antimicrobial resistance of Arcobacter species in both commercial and smallholder farm animals in Ghana and Tanzania.
Results
Frequency and species distribution of Arcobacter in smallholder and commercial farms
In Ghana, we sampled 15 commercial farms and 62 smallholder farms, while in Tanzania, we sampled 31 commercial farms and 71 smallholder farms. In total, 1585 samples were collected from farms in Ghana and 1047 from farms in Tanzania. The majority of samples from Tanzania were collected from smallholder farms (58.9%, n = 617), while in Ghana, the number of samples collected from commercial (50.8%, n = 805) and smallholder (49.2%, n = 780) farms were approximately the same. In both countries, chicken samples were the most frequently collected, making up 76.7% (n = 1216) of samples from Ghana and 74.2% (n = 777) from Tanzania. However, in Ghana, samples were also collected from other poultry birds such as turkey (n = 27), duck (n = 26), and quail (n = 28). Livestock samples in both countries were collected from cows (n = 271), goats (n = 138), pigs (n = 121), and sheep (n = 28). In total, 189 (11.9%, n/N = 189/1585) presumptive Arcobacter spp. were recovered from the samples collected from Ghana. In contrast, only 49 (4.7%) presumptive Arcobacter spp. were recovered from the samples collected from Tanzania. During freeze-storage, 5.8% (n = 11) of the presumptive Arcobacter spp. from Ghana and 38.8% (n/N = 19) from Tanzania were lost.
The relative frequency of confirmed Arcobacter spp. in poultry and livestock samples was higher in Ghana (84.1%, n/N = 111/132) than in Tanzania (15.9%, n/N = 21/132). The majority of the presumptive Arcobacter spp. that were not confirmed as Arcobacter spp. turned out to be Campylobacter spp. and Comamonas spp. Also, the relative frequency of the confirmed Arcobacter was higher in commercial farms in Ghana (87.4%, n/N = 83/95) compared to Tanzania (12.6%, n/N = 12/95). A total of eight different poultry (n = 4) and livestock (n = 4) species were sampled from commercial farms located in Ghana, and the incidence of Arcobacter was highest in pigs (45.1%, n/N = 37/82), followed by ducks (38.5%, n/N = 10/26), quails (35.7%, n/N = 10/28) and sheep (13.3%, n/N = 2/15). The remaining farm animal species had Arcobacter frequencies of less than 10%. The frequency of Arcobacter in chicken samples from commercial (3.7%, n/N = 20/545) and smallholder farms (4.0%, n/N = 27/671) in Ghana was similar. Table 1 provides details on the frequency of Arcobacter spp. isolated from poultry and livestock faecal samples collected from commercial and smallholder farms in Ghana and Tanzania.
Table 1.
Frequency of Arcobacter spp. in commercial and smallholder farm animals in Ghana and Tanzania
| Sample type | Commercial, % (n/N) | Smallholder, % (n/N) | Total, % (n/N) | |||
|---|---|---|---|---|---|---|
| Ghana | Tanzania | Ghana | Tanzania | Ghana | Tanzania | |
| Chicken | 3.7 (20/545) | 3.2 (12/371) | 4.0 (27/671) | 1.2 (5/406) | 3.9 (47/1216) | 2.2 (17/777) |
| Turkey | 7.4 (2/27) | NA | NA | NA | 7.4 (2/27) | NA |
| Duck | 38.5 (10/26) | NA | NA | NA | 38.5 (10/26) | NA |
| Quail | 35.7 (10/28) | NA | NA | NA | 35.7 (10/28) | NA |
| Cow | 1.5 (1/65) | 0 (0/40) | NA | 1.8 (3/166) | 1.5 (1/65) | 1.5 (3/206) |
| Pig | 45.1 (37/82) | 0 (0/19) | NA | 0 (0/20) | 45.1 (37/82) | 0 (0/39) |
| Goat | 5.9 (1/17) | NA | 1.0 (1/98) | 4.3 (1/23) | 1.7 (2/115) | 4.3 (1/23) |
| Sheep | 13.3 (2/15) | NA | 0 (0/11) | 0 (0/2) | 7.7 (2/26) | 0 (0/2) |
| Total | 10.3 (83/805) | 2.8 (12/430) | 3.6 (28/780) | 1.5 (9/617) | 7.0 (111/1585) | 2.0 (21/1047) |
n number positive, N total samples collected, and NA Not Applicable (No samples collected)
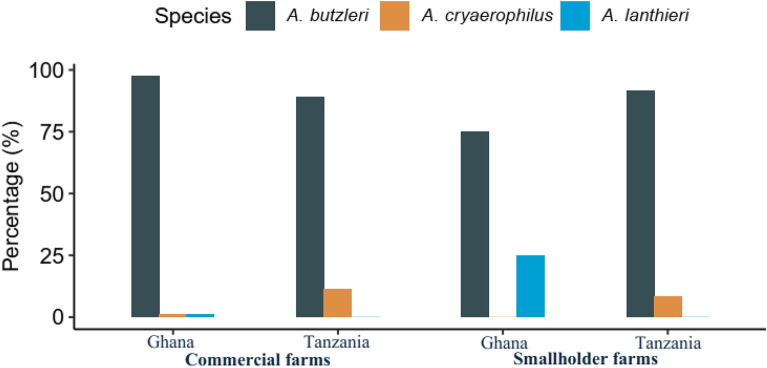
According to MALDI-TOF-based species identification, the majority of Arcobacter spp. isolated from both Ghana (91.9%, n/N = 102/111) and Tanzania (90.5%, n/N = 19/21) were identified as A. butzleri. The proportion of A. butzleri in commercial farms was similar to that of smallholder farms in Ghana and Tanzania. Three A. cryaerophilus were isolated, one from Ghana and two from Tanzania. All Arcobacter lanthieri (100%, n/N = 8/8) were isolated from chickens in Ghana, with the majority being isolated from smallholder farms (87.5%, n/N = 7/8) (Fig. 1).
Fig. 1.
Arcobacter species from commercial and smallholder farms in Ghana and Tanzania
Arcobacter frequencies by month
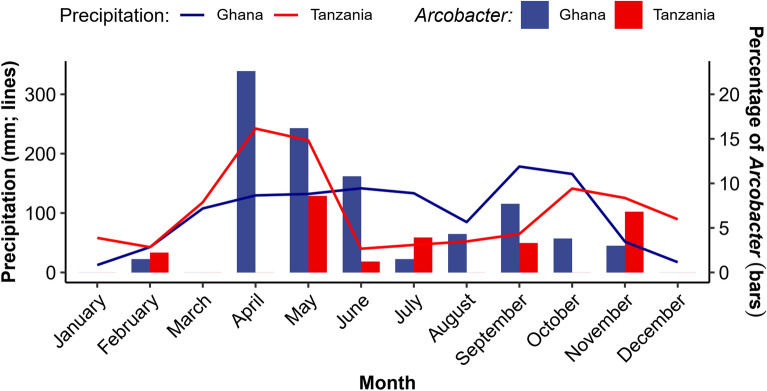
The monthly precipitation (lines) and percentage of Arcobacter isolated (bars) from Ghana and Tanzania are shown in Fig. 2. Unlike Ghana, where Arcobacter was isolated in nine out of the 12 months of the year, in Tanzania, it was isolated in six out of the 12 months. Arcobacter was not isolated in both countries in January, March, and December. The monthly frequency in Ghana ranged from 0% to 22.6% in April. In May, Tanzania recorded the highest monthly frequency of 8.6% (n/N = 3/35). Almost all Arcobacter from Ghana (98.2%, n/N = 109/111) and 38.1% (n/N = 8/21) from Tanzania were isolated during the rainy season. In Ghana, Arcobacter were 20 times (95% CI 5.0–80.5) more likely to be isolated in the rainy season than during the dry season, while in Tanzania, detection rates were similar in both seasons (PR = 1.1, 95% CI 0.4–2.5).
Fig. 2.
Monthly precipitation (line graph) and percentage of Arcobacter isolated (bar graph) from farms in Ghana and Tanzania. The monthly average precipitation data for the Tanga Region was acquired from (https://tcktcktck.org/tanzania/tanga/korogwe), whereas the data for the Ashanti Region was also obtained from (https://tcktcktck.org/ghana/ashanti)
Antimicrobial resistance in Arcobacter species
Epidemiological cut-off values (ECOFFs) were derived for all antibiotics tested (Additional file 1). None of the Arcobacter isolates from smallholder farms in either country was resistant to tetracycline and kanamycin (Table 2). In contrast, 41.7% (n/N = 5/12) and 15.7% (n/N = 13/83) of Arcobacter isolates from commercial farms in Tanzania and Ghana, respectively, were resistant to tetracycline. Commercial farms from both countries were 5.2 (95% CI 1.7–15.8) and 4.7 (95% CI 1.2–18.8) times more likely to harbour ciprofloxacin and streptomycin-resistant Arcobacter, respectively, than smallholder farms. Of the eight antibiotics tested, ciprofloxacin exhibited the fourth-highest resistance level among isolates from Ghana (30.6%, n/N = 34/111) and Tanzania (42.9%, n/N = 9/21). Arcobacter from commercial farms in Tanzania was 5.9 (95% CI 2.4–14.7) and 2.7 (95% CI 1.2–6.1) times more likely to be resistant to erythromycin and tetracycline, respectively, than isolates from Ghana. Except for erythromycin, which showed a higher degree of resistance in Tanzania than Ghana isolates (PR = 2.9, 95% CI 1.3–6.3), all other antibiotics tested showed comparable resistance frequencies (Table 2).
Table 2.
Antibiotic-resistant Arcobacter spp. isolated from commercial and smallholder farm animals in Ghana and Tanzania
| Antibiotic | Resistance rate in % (n) | |||||
|---|---|---|---|---|---|---|
| Commercial farm | Smallholder farm | Total | ||||
| Ghana (N = 83) | Tanzania (N = 12) | Ghana (N = 28) | Tanzania (N = 9) | Ghana (N = 111) | Tanzania (N = 21) | |
| Penicillin | 100 (83) | 100 (12) | 100 (28) | 100 (9) | 100 (111) | 100 (21) |
| Ampicillin | 100 (83) | 100 (12) | 100 (28) | 100 (9) | 100 (111) | 100 (21) |
| Chloramphenicol | 100 (83) | 100 (12) | 100 (28) | 100 (9) | 100 (111) | 100 (21) |
| Ciprofloxacin | 39.8 (33) | 58.3 (7) | 3.6 (1) | 22.2 (2) | 30.6 (34) | 42.9 (9) |
| Streptomycin | 26.5 (22) | 16.7 (2) | 7.1 (2) | 0 (0) | 21.6 (24) | 9.5 (2) |
| Erythromycin | 8.4 (7) | 50 (6) | 21.4 (6) | 11.1 (1) | 11.7 (13) | 33.3 (7) |
| Tetracycline | 15.7 (13) | 41.7 (5) | 0 (0) | 0 (0) | 11.7 (13) | 23.8 (5) |
| Kanamycin | 12.0 (10) | 8.3 (1) | 0 (0) | 0 (0) | 9.0 (10) | 4.7 (1) |
n number positive and N total samples collected
All A. lanthieri isolates (100%, n/N = 8/8) were susceptible to ciprofloxacin, erythromycin, tetracycline, and kanamycin, and the majority (87.5%, n/N = 7/8) were susceptible to streptomycin. The observed resistance rates of A. butzleri (N = 121) to ciprofloxacin, streptomycin, erythromycin, tetracycline, and kanamycin were 33.9% (n = 41), 19.0% (n = 23), 15.7% (n = 19), 13.2% (n = 16), and 8.3% (n = 10), respectively.
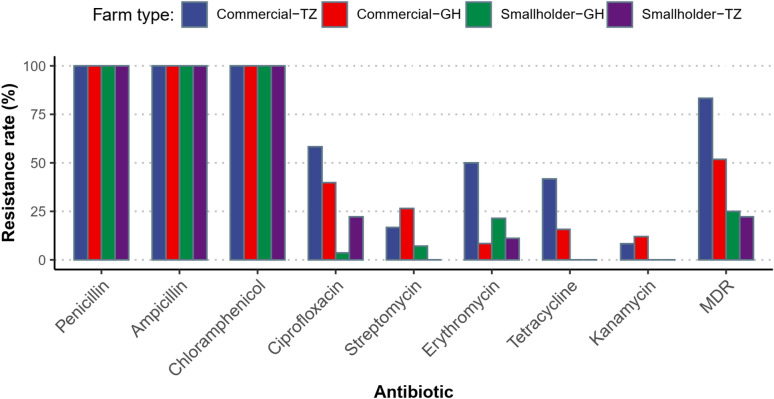
Figure 3 shows antibiotic resistance of Arcobacter isolates from commercial and smallholder farms in Tanzania and Ghana. In general, higher antibiotic resistance was observed in Arcobacter from commercial farms compared to smallholder farms in both countries. Also, more resistant isolates were observed in Arcobacter from commercial farms in Tanzania than in Ghana. Multi-drug resistance (MDR) was observed in 57.1% (n/N = 12/21) and 45.0% (n/N = 50/111) of Arcobacter isolates from Tanzania and Ghana, respectively.
Fig. 3.
Antibiotic resistance of Arcobacter isolates from commercial and smallholder farms in Tanzania and Ghana. TZ Tanzania, GH Ghana, MDR multi drug resistance
Multidrug resistance (MDR) was observed in 57.1% (n/N = 12/21) and 45.0% (n/N = 50/111) of the Arcobacter isolates from Tanzania and Ghana, respectively. Table 3 summarizes the factors associated with MDR in all Arcobacter isolates. The type of farm (commercial or smallholder) and source of the sample (poultry or livestock) were found to be associated with MDR (Table 3). In both countries combined, a higher prevalence of MDR Arcobacter was isolated from commercial farms (55.8%, n/N = 53/95) than from smallholder farms (24.3%, n/N = 9/37) (PR = 2.3, 95% CI 1.3–4.2). The adjusted PRs also indicate that poultry were 1.3 times (95% CI 1.1–2.6) more likely to have MDR Arcobacter strains than livestock. However, seasonal variation, the country from which samples were collected, and the particular Arcobacter species were not associated with MDR.
Table 3.
Associations with the frequency of multi drug-resistant Arcobacter
| Variable | Crude PR (95% CI) | Adjusted PR (95% CI) |
|---|---|---|
| Commercial vs. smallholder farm | 2.3 (1.3–4.2) | 2.7 (1.6–4.9) |
| Rainy vs. dry season | 1.3 (0.3–2.0) | 1.3 (0.3–1.8) |
| Poultry vs. livestock | 1.6 (0.9–1.5) | 1.3 (1.1–2.6) |
| Arcobacter butzleri vs. Other species | 1.8 (0.7–4.7) | 2.1 (0.8–5.5) |
| Ghana vs. Tanzania | 0.8 (0.5–1.2) | 0.9 (0.6–1.3) |
PR prevalence ratio, CI confidence interval
Discussion
The present study describes antibiotic-resistant Arcobacter species from commercial and smallholder farm animals in Ghana and Tanzania. The observed overall Arcobacter proportion in Ghana (7.0%) and Tanzania (2.0%) was much lower than what was described in a previous study with a focus on local and imported poultry meat in Kumasi, Ghana (26.5%) [9] and a study conducted in ostriches in South Africa (68%) [18], and in poultry abattoir effluents in Nigeria (14.0%) [17]. The differences in the current Arcobacter proportion compared to the few earlier studies conducted in similar geographical areas could be due to several factors. For instance, the types of samples analyzed, variations in the timing of sample collection throughout the year and the specific microbiological methods utilized. Among the different farm animals sampled in Ghana, pigs (45.1%), ducks (38.5%), and quails (35.7%) had the highest overall Arcobacter frequencies. While a few studies have reported similar findings [19, 20], other studies conducted in Asia and Africa have observed the highest Arcobacter frequencies in chicken [3, 11, 18]
In this study, Matrix-Assisted Laser Desorption Ionization Time-of-Flight Mass Spectrometry (MALDI-TOF MS) species identification revealed the presence of three types of Arcobacter spp.: A. butzleri, A. cryaerophilus, and A. lanthieri. The predominant species was A. butzleri, which is not uncommon in poultry and livestock [20–22] and is also most commonly implicated in human infections. Surprisingly, A. skirrowii was not found in this study, even though it is a known colonizer of poultry and livestock [11, 23]. The present study identified eight A. lanthieri from chicken in Ghana with the majority being isolated from smallholder farms. A. lanthieri was only recently described in 2015 [24] and since then, it has been isolated from pigs, dairy cattle manure, and humans [24–26]. The presence of A. lanthieri in farms in Ghana is concerning as it is known to encode many putative virulence genes [25].
In this study, the isolation rate for Arcobacter in Ghana was much higher in the rainy season than in the dry season, while in Tanzania, the detection rate was similar in both seasons. In temperate climates, there is no consensus on the differences in Arcobacter prevalence by season. A recent study observed varied frequencies according to season and poultry type [20]. Similarly, studies conducted in Japan and Italy recorded no significant difference in prevalence by season [3, 27]. However, in tropical settings, higher frequencies of enteric bacterial pathogens have been observed in the rainy season than in the dry season [28, 29]. The significantly higher contamination of farms in Africa by enteric bacterial pathogens during the rainy season has been attributed to open defecation practices, increased runoff, and more frequent overflowing of onsite septic tanks and sanitation systems [30]. In addition, the lower temperatures during the rainy seasons favour the survival of mesophilic foodborne pathogens such as Arcobacter.
Because no ECOFF values could be defined for penicillin, ampicillin and chloramphenicol due to the lack of discrimination of distinct susceptible or resistant isolate populations, all Arcobacter isolates tested in this study were considered resistant to these three antibiotics. This is in line with studies conducted in Turkey and Iran, where most Arcobacter isolates were found to be resistant to ampicillin and chloramphenicol, respectively. [10, 11]. Also, 32.6% and 19.7% of the Arcobacter isolates tested against ciprofloxacin and streptomycin, respectively, had inhibition zone diameters below the ECOFF values indicating resistance for both antimicrobials. A recent study on backyard chickens and retail poultry meat in Chile found lower rates of ciprofloxacin, tetracycline, and erythromycin resistance [31]. The increased resistance rate observed in this study could be due to differences in geographic location and misuse of antibiotics in commercial and smallholder farms in the current study areas [8, 32]. Not surprisingly, the resistance patterns of Campylobacter isolates from farms in the study area in Ghana showed similar results to those reported here [28, 33]. Nevertheless, it is reassuring that our study observed that all Arcobacter spp. from smallholder farms in the two countries were susceptible to both tetracycline and kanamycin. This could be due to the lower use of antibiotics in smallholder farms compared to commercial farms, as previously described in the same study area in Ghana [8].
A. butzleri was found to be generally more resistant to antibiotics than A. lanthieri. This correlates with findings from previous studies [34, 35]. Among all known Arcobacter spp., A. butzleri has been reported as the most significant clinical pathogen due to its high overall prevalence and pathogenicity [35]. We also identified multidrug-resistant Arcobacter spp. in this study. The inherent resistance of Campylobacteraceae to β-lactam antibiotics may explain the high resistance rate observed [2]. We observed more multidrug-resistant Arcobacter isolates in poultry than in livestock. A report from Tanzania suggests that antimicrobial misuse is widespread among farmers, with poultry farmers having higher rates of misuse than livestock farmers [36].
There were some limitations in our study. Sampling was limited to a single district in both countries, so the observed results may not reflect true nationwide prevalence in each country. The high number of presumptive isolates from Tanzania dying during freeze storage resulting in low Arcobacter frequencies, and the less variety of farm animals sampled from Tanzania, made it difficult to do a detailed comparison between the two countries but rather show trends only. In addition, the enrichment and selective medium used in this study disproportionately favour the isolation of A. butzleri, probably at the expense of other Arcobacter species. Despite the above limitations, this study is, to the best of our knowledge, the first to report on Arcobacter species in both commercial and smallholder farms in Ghana and Tanzania.
Conclusion
Our findings suggest that commercial and smallholder farm animals in Ghana and Tanzania are carriers and potential transmission reservoirs for Arcobacter species. All Arcobacter recovered from poultry and livestock were resistant to at least two or more antibiotic classes tested. The high levels of MDR Arcobacter detected call for immediate development and implementation of effective Arcobacter control strategies in commercial and smallholder farms to curb the proliferation of multidrug-resistant strains and safeguard animal and human health. Furthermore, our findings may inspire further research in SSA to comprehensively understand the prevalence, virulence, and pathogenicity of Arcobacter spp. across a broader range of geographic areas.
Methods
Study site
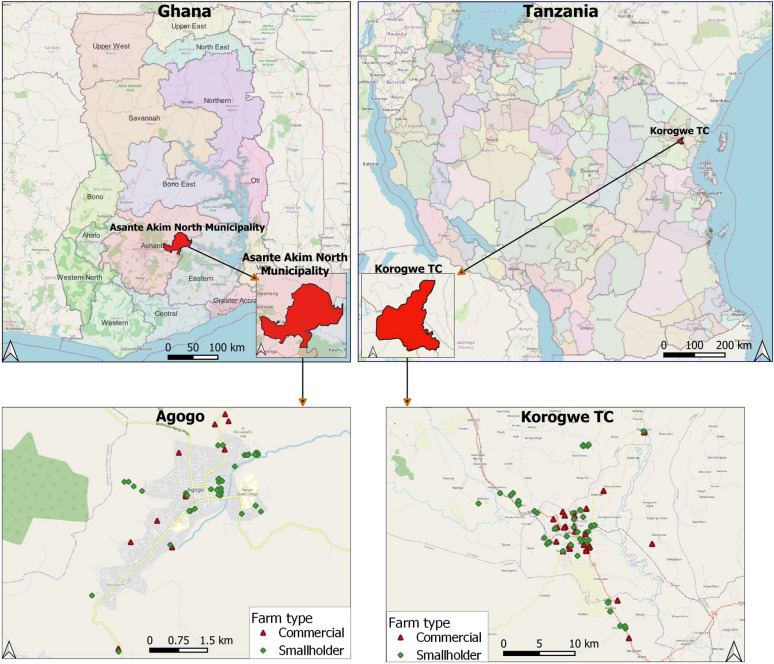
A cross-sectional study was conducted in two countries in SSA, Ghana and Tanzania. In Ghana, this study was conducted in Agogo, the capital of the Asante Akim North Municipality, located in the eastern part of the Ashanti Region (Fig. 4). Asante Akim North Municipality is a rural community with a population of 85,788 [37]. Almost half (42%) of the households in the municipality rear farm animals, and poultry accounts for 56% of the animals, with the remaining ones being livestock [37]. Ghana has a tropical climate with two main seasons. The rainy season extends from April to October, and the dry season from November to March.
Fig. 4.
Location of commercial and smallholder farms in Agogo, Ashanti Region, Ghana and Korogwe TC, Tanga Region, Tanzania that were included in the study. This map was created using the QGIS version 3.24.0-Tisler software (https://qgis.org/en/site/)
In Tanzania, this study was conducted in Korogwe Town Council (TC), located within the Tanga Region in northeastern Tanzania (Fig. 4). Based on preliminary results of the 2022 Tanzania population and housing census [38], Korogwe TC population is estimated at 73,464. Tanzania has a tropical Savannah climate with two rainy seasons. March to May is characterized by long rains, and November to mid-January by short and lighter rains. Most of the population resides in rural settings, mainly engaging in informal trade or subsistence farming (hereafter called smallholder farming).
Sample collection
Sampling took place between March 2019 and July 2020. A farm with an intensive housing system of caged poultry and/or livestock was considered commercial. Smallholder farms were households with free-roaming poultry (mainly indigenous breeds) and/or livestock with shelter provided by basic or temporary roofing. Before sampling, a list of all commercial farms within each study site was obtained from each country’s respective district office of the Ministry of Agriculture. In a community within the study site, we initially identified one or two households engaged in rearing free-range farm animals. We then requested those households to introduce us to another household that kept farm animals for possible sampling. Before sample collection, the farm was visited to ascertain the number of pen houses. Multiple pen house farms were visited more than once during sampling; nonetheless, each pen house was sampled only once throughout the study period. Faecal samples were collected from poultry and livestock in the commercial and smallholder farms. Poultry included chicken, duck, turkey, and quail, while livestock included sheep, goats, pigs, and cows. For each sample, approximately 2 g of freshly voided faecal droppings were collected using a sterile spatula and placed in a sterile plastic container without preservatives. All samples were transported in a cool box (4–8 ℃) and processed within 2–4 h at the Kumasi Centre for Collaborative Research in Tropical Medicine (KCCR) in Ghana or the National Institute for Medical Research (NIMR) in Korogwe, Tanzania.
Identification of Arcobacter
Arcobacter spp. was isolated using selective enrichment media as described by [10]. Suspected Arcobacter colonies were tested for the enzyme cytochrome oxidase and those that were positive were examined by Gram staining. Gram-negative spiral-rod-shaped colonies were stored, as presumptive Arcobacter isolates, at −80 ℃ using the Microbank™ system (Pro-Lab Diagnostics, Bromborough, UK). All isolates were shipped to Germany on dry ice and species confirmation was performed by MALDI-TOF MS using the VITEK® MS system (bioMérieux, Marcy-l'Étoile, France).
Antibiotic susceptibility testing
The Kirby Bauer disk diffusion method [39] was used to assess the antibiotic susceptibility of all confirmed Arcobacter isolates. Antibiotic disks (Oxoid, Hampshire, UK) were placed on Mueller–Hinton agar supplemented with 5% sheep blood and inoculated with Arcobacter for antibiotic susceptibility testing. Plates were incubated at 30 ℃ under microaerophilic conditions for 24 h. After 24 h, isolates with insufficient growth were further incubated, and the inhibition zone was read after a total of 40–48 h. Isolates were tested against ampicillin (10 µg), chloramphenicol (30 µg), ciprofloxacin (5 µg), streptomycin (25 µg), erythromycin (15 µg), tetracycline (30 µg) and kanamycin (30 µg). So far, the European Committee on Antimicrobial Susceptibility Testing (EUCAST) clinical breakpoints have not been determined for Arcobacter, therefore, ECOFFs were determined based on the frequency distribution of measured inhibition zone diameters (Additional file 1). Additional Arcobacter isolates obtained from children at the same study sites during the research period were included in the development of the ECOFFs. (Additional file 1). The procedure for developing ECOFFs has been described previously [40, 41]. The zone diameter measurements, indicating susceptibility (S) or resistance (R) for each antibiotic, are detailed in Table 4. Multidrug resistance (MDR) was defined as resistance to at least one agent in three or more antimicrobial categories.
Table 4.
Epidemiological Cut-Off Values (ECOFFs) used for Antimicrobial Resistance in Arcobacter spp
| Antibiotic (disk concentration) | Zone diameter (mm) | |
|---|---|---|
| S ≥ | R < | |
| Tetracycline (30 µg) | 18 | 18 |
| Ciprofloxacin (5 µg) | 18 | 18 |
| Streptomycin (25 µg) | 15 | 15 |
| Ampicillin (10 µg) | NA | NA |
| Chloramphenicol (30 µg) | NA | NA |
| Erythromycin (15 µg) | 11 | 11 |
| Kanamycin (30 µg) | 14 | 14 |
| Penicillin (10 µg) | NA | NA |
S susceptible, R resistant, NA not applicable—100% resistant
Data analysis
Descriptive statistics of categorical variables were calculated using absolute frequencies and corresponding percentages. Prevalence ratios (PRs) and their respective 95% confidence intervals (CIs) were computed to show associations between two categorical variables. Because of the explanatory nature of this study, p-values were not calculated. Poisson regression with robust standard errors was used to fit multivariable models for multiple drug resistance in Arcobacter isolates. The dependent variable in the Poisson regression was whether an Arcobacter isolate was multiple drug-resistant or not. The independent variables were whether the isolate was collected from a commercial or smallholder farm, during the rainy or dry season, from poultry or livestock samples, and coming from Ghana or Tanzania. R software (version 4.3.1) was used for all statistical analyses [42]. The epiR (2.0.19) package was used to calculate the PRs, and the sandwich package (version 3.0–0) was used to compute robust standard errors of the Poisson regression. A bar chart was created, using the R package ggplot2 (version 3.3.5), to show Arcobacter spp. with inhibition zone diameters below (resistant) and above (susceptible) the ECOFFs. Also, the line graph and bar chart showing Arcobacter frequency by month were plotted using the ggplot2 package (version 3.3.5). The line graph for the Tanga Region was plotted using the monthly average precipitation data obtained from https://tcktcktck.org/tanzania/tanga/korogwe, whereas the data for the Ashanti Region was also acquired from https://tcktcktck.org/ghana/ashanti/agogo. QGIS software, version 3.24 [43], was used to draw a map showing the geographical location of the farms sampled in Ghana and Tanzania.
Supplementary Information
Additional file 1. Epidemiological cut-off values (ECOFFs) determined based on the frequency distribution of measured inhibition zone diameters of all antibiotics tested against Arcobacter isolates
Acknowledgements
The authors wish to thank the farm owners/caretakers for granting access to their outlets. The authors gratefully acknowledge the support of Abdul Seidu Razak, Dennis Fosu and Cynthia Adu Kyerewaa, Kumasi Centre for Collaborative Research in Tropical Medicine (KCCR), for helping collect data from Ghana. We would also like to sincerely thank Britta Liedigk at the Bernhard Nocht Institute for Tropical Medicine for her exceptional technical laboratory assistance.
Author contributions
Conceptualization: DD, JM, JP AL, LAO and KOD.; methodology: EKP, CWA, AEZ, JM, JK, DTRM and SG; validation: ML, NAK and AJ; formal analysis: EKP and RK, data curation: AEZ, EKP, CWA, and JM; original draft preparation: EKP; writing, reviewing and editing: DD, RK, AEZ, LAO, KOD, EKP, CWA, DTRM, NAK, AJ and ML; supervision: DD, LAO, KOD, RK, AEZ, JP AL and JM; funding acquisition: DD, LAO, and JM. All authors read and approved the final manuscript.
Funding
This research was funded by the “German Research Foundation (DFG) within the project “Genetic adaptation of non-typhoid Salmonella within human and animal reservoirs in sub-Saharan Africa”, grant number 649070.
Data availability
The raw data supporting the conclusions of this article are included in the article or are available as supplementary data files.
Declarations
Ethics approval and consent to participate
The protocol of this study was reviewed and approved by the Committee on Human Research Publication and Ethics. Written informed consent was obtained from the farm owners for the participation of their animals in this study.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Score J, Phillips CA. Arcobacter species: an emerged or emerging pathogen? Food Safety; 2015. 235–263. 10.1016/B978-0-12-800245-2.00012-5
- 2.Ramees TP, Dhama K, Karthik K, Rathore RS, Kumar A, Saminathan M, Singh RK. Arcobacter: an emerging food-borne zoonotic pathogen, its public health concerns and advances in diagnosis and control–a comprehensive review. Vet Quart. 2017;37(1):136–161. doi: 10.1080/01652176.2017.1323355. [DOI] [PubMed] [Google Scholar]
- 3.Kabeya H, Maruyama S, Morita Y, Kubo M, Yamamoto K, Arai S, Izumi T, Kobayashi Y, Katsube Y, Mikami T. Distribution of Arcobacter species among livestock in Japan. Vet Microbiol. 2003;93(2):153–158. doi: 10.1016/s0378-1135(02)00312-7. [DOI] [PubMed] [Google Scholar]
- 4.Chieffi D, Fanelli F, Fusco V. Arcobacter butzleri: up-to-date taxonomy, ecology, and pathogenicity of an emerging pathogen. Compr Rev Food Sci Food Saf. 2020;19:2071–2109. doi: 10.1111/1541-4337.12577. [DOI] [PubMed] [Google Scholar]
- 5.Shange N, Gouws P, Hoffman LC. Campylobacter and Arcobacter species in food-producing animals: prevalence at primary production and during slaughter. World J Microbiol Biotechnol. 2019;35(9):146. doi: 10.1007/s11274-019-2722-x. [DOI] [PubMed] [Google Scholar]
- 6.Ferreira S, Luís Â, Oleastro M, Pereira L, Domingues FC. A meta-analytic perspective on Arcobacter spp. antibiotic resistance. J Globa Antimicrob Res. 2019;16:130–139. doi: 10.1016/j.jgar.2018.12.018. [DOI] [PubMed] [Google Scholar]
- 7.Azabo R, Dulle F, Mshana SE, Matee M, Kimera S. Antimicrobial use in cattle and poultry production on the occurrence of multidrug-resistant Escherichia coli. A systematic review with a focus on sub-Saharan Africa. Front Vet Sci. 2022;9:1000457. doi: 10.3389/fvets.2022.1000457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paintsil EK, Ofori LA, Akenten CW, Fosu D, Ofori S, Lamshöft M, May J, Danso KO, Krumkamp R, Dekker D. Antimicrobial usage in commercial and domestic poultry farming in two communities in the Ashanti region of Ghana. Antibiotics. 2021;10(7):800. doi: 10.3390/antibiotics10070800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dekker D, Eibach D, Boahen KG, Akenten CW, Pfeifer Y, Zautner AE, Mertens E, Krumkamp R, Jaeger A, Flieger A, Owusu-Dabo E, May J. Fluoroquinolone-resistant salmonella enterica, Campylobacter spp., and Arcobacter butzleri from local and imported poultry meat in Kumasi Ghana. Foodborne Pathog Dis. 2019;16(5):352–358. doi: 10.1089/fpd.2018.2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elmali M, Can HY. Occurrence and antimicrobial resistance of Arcobacter species in food and slaughterhouse samples. Food Sci Technol. 2017;37(2):280–285. doi: 10.1590/1678-457x.19516. [DOI] [Google Scholar]
- 11.Khodamoradi S, Abiri R. The incidence and antimicrobial resistance of Arcobacter species in animal and poultry meat samples at slaughterhouses in Iran. Iran J Microbiol. 2020;12(6):531–536. doi: 10.18502/ijm.v12i6.5027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang S, Gao X, Gao Y, Li Y, Cao M, Xi Z, Zhao L, Feng Z. Tetracycline resistance genes identified from distinct soil environments in China by functional metagenomics. Front Microbiol. 2017;8:1406. doi: 10.3389/fmicb.2017.01406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sciortino S, Arculeo P, Alio V, Cardamone C, Nicastro L, Arculeo M, Alduina R, Costa A. Occurrence and antimicrobial resistance of Arcobacter spp. recovered from aquatic environments. Antibiotics. 2021;10(3):288. doi: 10.3390/antibiotics10030288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferreira S, Correia DR, Oleastro M, Domingues FC. Arcobacter butzleri ciprofloxacin resistance: Point mutations in DNA gyrase A and role on fitness cost. Microbial Drug Resist. 2018 doi: 10.1089/mdr.2017.0295. [DOI] [PubMed] [Google Scholar]
- 15.Shrivastava S, Shrivastava P, Ramasamy J. World health organization releases global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. J Med Soc. 2018;32(1):76. doi: 10.4103/jms.jms_25_17. [DOI] [Google Scholar]
- 16.Adesiji YO, Coker AO, Oloke JK. Detection of Arcobacter in feces of healthy chickens in Osogbo Nigeria. J Food Prot. 2011;74(1):119–121. doi: 10.4315/0362-028X.JFP-10-231. [DOI] [PubMed] [Google Scholar]
- 17.Amisu KO, Coker AO, On SLW, Isokpehi RD. Arcobacter butzlieri strains from poultry abattoir effluent in Nigeria. East Afr Med J. 2003;80(4):218–222. [PubMed] [Google Scholar]
- 18.Shange N, Gouws PA, Hoffman LC. Prevalence of Campylobacter and Arcobacter species in ostriches from Oudtshoorn, South Africa. J Food Prot. 2020;83(4):722–728. doi: 10.4315/JFP-19-472. [DOI] [PubMed] [Google Scholar]
- 19.Merga JY, Williams NJ, Miller WG, Leatherbarrow AJH, Bennett M, Hall N, Ashelford KE, Winstanley C. Exploring the diversity of Arcobacter butzleri from cattle in the UK using MLST and whole genome sequencing. PLoS ONE. 2013;8(2):e55240. doi: 10.1371/journal.pone.0055240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Çelik E, Otlu S. Isolation of Arcobacter spp. and identification of isolates by multiplex PCR from various domestic poultry and wild avian species. Ann Microbiol. 2020 doi: 10.1186/s13213-020-01603-7. [DOI] [Google Scholar]
- 21.Van Driessche E, Houf K, Vangroenweghe F, De Zutter L, Van Hoof J. Prevalence, enumeration and strain variation of Arcobacter species in the faeces of healthy cattle in Belgium. Vet Microbiol. 2005;105(2):149–154. doi: 10.1016/j.vetmic.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 22.Jasim SA, Al-Abodi HR, Ali WS. Resistance rate and novel virulence factor determinants of Arcobacter spp., from cattle fresh meat products from Iraq. Microb Pathog. 2021;152(104649):104649. doi: 10.1016/j.micpath.2020.104649. [DOI] [PubMed] [Google Scholar]
- 23.Shah AH, Saleha AA, Zunita Z, Murugaiyah M, Aliyu AB, Jafri N. Prevalence, distribution and antibiotic resistance of emergent Arcobacter spp. from clinically healthy cattle and goats: emergent Arcobacter spp. In cattle and goats. Transbound Emerg Dis. 2013;60(1):9–16. doi: 10.1111/j.1865-1682.2012.01311.x. [DOI] [PubMed] [Google Scholar]
- 24.Whiteduck-Léveillée K, Whiteduck-Léveillée J, Cloutier M, Tambong JT, Xu R, Topp E, Arts MT, Chao J, Adam Z, André Lévesque C, Lapen DR, Villemur R, Talbot G, Khan IUH. Arcobacter lanthieri sp. Nov., isolated from pig and dairy cattle manure. Int J Syst Evolut Microbiol. 2015;65(8):2709–2716. doi: 10.1099/ijs.0.000318. [DOI] [PubMed] [Google Scholar]
- 25.Brückner V, Fiebiger U, Ignatius R, Friesen J, Eisenblätter M, Höck M, Alter T, Bereswill S, Heimesaat MM, Gölz G. Characterization of Arcobacter strains isolated from human stool samples: results from the prospective German prevalence study Arcopath. Gut Pathogens. 2020;12(1):3. doi: 10.1186/s13099-019-0344-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kerkhof P-J, On SLW, Houf K. Arcobacter vandammei sp. Nov., isolated from the rectal mucus of a healthy pig. Int J Syst Evolut Microbiol. 2021 doi: 10.1099/ijsem.0.005113. [DOI] [PubMed] [Google Scholar]
- 27.Traversa A, Gallina S, Martucci F, Boteva C, Baioni E, Maurella C, Chiavacci L, Benvenuto E, Ferrero I, Ferrero E, Giacometti F, Piva S, Chiesa F, Bianchi DM, Serraino A, Decastelli L. Arcobacter spp. in raw milk from vending machines in Piedmont and occurrence of virulence genes in isolates. Ital J Food Saf. 2019;8(4):7859. doi: 10.4081/ijfs.2019.7859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paintsil EK, Ofori LA, Akenten CW, Zautner AE, Mbwana J, Jaeger A, Lamshöft M, May J, Obiri-Danso K, Philipps RO, Krumkamp R, Dekker D. Antibiotic-resistant Campylobacter coli and Campylobacter jejuni in commercial and smallholder farm animals in the Asante Akim North Municipality of Ghana. Front Microbiol. 2022;13:983047. doi: 10.3389/fmicb.2022.983047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mahagamage MGYL, Pathirage MVSC, Manage PM. Contamination status of Salmonella spp., Shigella spp. and Campylobacter spp. in surface and groundwater of the Kelani River Basin Sri Lanka. Water. 2020;12(8):2187. doi: 10.3390/w12082187. [DOI] [Google Scholar]
- 30.Kapembo ML, Al Salah DMM, Thevenon F, Laffite A, Bokolo MK, Mulaji CK, Mpiana PT, Poté J. Prevalence of water-related diseases and groundwater (drinking-water) contamination in the suburban municipality of Mont Ngafula, Kinshasa (Democratic Republic of the Congo) J Environ Sci Health Part A. 2019;54(9):840–850. doi: 10.1080/10934529.2019.1596702. [DOI] [PubMed] [Google Scholar]
- 31.Vidal-Veuthey B, Jara R, Santander K, Mella A, Ruiz S, Collado L. Antimicrobial resistance and virulence genes profiles of Arcobacter butzleri strains isolated from backyard chickens and retail poultry meat in Chile. Lett Appl Microbiol. 2021;72(2):126–132. doi: 10.1111/lam.13404. [DOI] [PubMed] [Google Scholar]
- 32.Mdegela RH, Mwakapeje ER, Rubegwa B, Gebeyehu DT, Niyigena S, Msambichaka V, Nonga HE, Antoine-Moussiaux N, Fasina FO. Antimicrobial use, residues, resistance and governance in the food and agriculture sectors, Tanzania. Antibiotics. 2021;10(4):454. doi: 10.3390/antibiotics10040454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paintsil EK, Ofori LA, Adobea S, Akenten CW, Phillips RO, Maiga-Ascofare O, Lamshöft M, May J, Obiri Danso K, Krumkamp R, Dekker D. Prevalence and antibiotic resistance in Campylobacter spp. Isolated from humans and food-producing animals in West Africa: a systematic review and meta-analysis. Pathogens. 2022;11(2):140. doi: 10.3390/pathogens11020140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ünver A, Atabay HI, Sahi̇n M, Celebi̇ Ö. Antimicrobial susceptibilities of various Arcobacter species. Turk JMed Sci. 2013;43:548–552. doi: 10.3906/sag-1207-115. [DOI] [Google Scholar]
- 35.Ferreira S, Queiroz JA, Oleastro M, Domingues FC. Insights in the pathogenesis and resistance of Arcobacter: a review. Crit Rev Microbiol. 2016;42(3):364–383. doi: 10.3109/1040841X.2014.954523. [DOI] [PubMed] [Google Scholar]
- 36.Azabo R, Mshana S, Matee M, Kimera SI. Antimicrobial usage in cattle and poultry production in Dar es Salaam, Tanzania: pattern and quantity. BMC Vet Res. 2022;18(1):7. doi: 10.1186/s12917-021-03056-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ghana Statistical Service. Ghana 2021 Population and Housing Census: General Report Volume 3N. 2021. https://census2021.statsghana.gov.gh/. Accessed 9 Mar 2023
- 38.National Bureau of statistics - population and housing census. Nbs.Go.Tz. 2020. https://www.nbs.go.tz/index.php/en/census-surveys/population-and-housing-census. Accessed 9 Mar 2023.
- 39.Hudzicki J. Kirby-Bauer disk diffusion susceptibility test protocol. Am Soc Microbiol. 2009;15:55–63. [Google Scholar]
- 40.Bénéjat L, Sifré E, Domingues Martins C, Ducournau A, Buissonnière A, Bessède E, Mégraud F, Lehours P. Epidemiologic cutoff values to separate wild-type from non–wild-type Campylobacter fetus to ciprofloxacin. Diagn Microbiol Infect Dis. 2018;92(2):164–167. doi: 10.1016/j.diagmicrobio.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 41.Zautner AE, Riedel T, Bunk B, Spröer C, Boahen KG, Akenten CW, Dreyer A, Färber J, Kaasch AJ, Overmann J, May J, Dekker D. Molecular characterization of Arcobacter butzleri isolates from poultry in rural Ghana. Front Cell Infect Microbiol. 2023;13:1094067. doi: 10.3389/fcimb.2023.1094067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.R Core Team . R: a language and environment for statistical computing. Vienna: R foundation for statistical computing; 2020. [Google Scholar]
- 43.QGIS Development Team . QGIS Geographic Information System. Zurich: Open Source Geospatial Foundation Project; 2021. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Epidemiological cut-off values (ECOFFs) determined based on the frequency distribution of measured inhibition zone diameters of all antibiotics tested against Arcobacter isolates
Data Availability Statement
The raw data supporting the conclusions of this article are included in the article or are available as supplementary data files.