Abstract
Introduction
Metastatic pancreatic ductal adenocarcinoma (PDAC) carries a poor prognosis and significant morbidity from local tumor progression. We investigated outcomes among oligometastatic PDAC patients treated with stereotactic magnetic resonance image-guided ablative radiotherapy (SMART) to primary disease.
Methods
We performed a retrospective multi-institutional analysis of oligometastatic PDAC at diagnosis or with metachronous oligoprogression during induction chemotherapy treated with primary tumor SMART. Outcomes of interest included overall survival (OS), progression-free survival (PFS), freedom from locoregional failure (FFLRF), and freedom from distant failure (FFDF). Acute and late toxicity were reported and in exploratory analyses patients were stratified by the number of metastases, SMART indication, and addition of metastasis-directed therapy.
Results
From 2019 to 2021, 22 patients with oligometastatic PDAC (range: 1–6 metastases) received SMART to the primary tumor with a median follow-up of 11.2 months from SMART. Nineteen patients had de novo synchronous metastatic disease and three had metachronous oligoprogression. Metastasis location most commonly was liver only (40.9%), multiple organs (27.3%), lungs only (13.6%), or abdominal/pelvic nodes (13.6%). All patients received either FOLFIRINOX (64%) or gemcitabine/nab-paclitaxel (36%) followed by SMART (median 50 Gy, 5 fractions) for local control (77%), pain control (14%), or local progression (9%). Additionally, 41% of patients received other metastasis-directed treatments. The median OS from diagnosis and SMART was 23.9 months and 11.6 months, respectively. Calculated from SMART, the median PFS was 2.4 months with 91% of patients having distant progression, and 1-year local control was 68. Two patients (9%) experienced grade 3 toxicities, gastric outlet obstruction, and gastrointestinal bleed without grade 4 or 5 toxicity.
Conclusion
There was minimal morbidity of local disease progression after SMART in this cohort of oligometastatic PDAC. As systemic therapy options improve, additional strategies to identify patients who may derive benefits from local consolidation or metastasis-directed therapy are needed.
Keywords: oligometastatic pancreatic adenocarcinoma, MRI-guided radiotherapy, stereotactic magnetic resonance image-guided ablative radiotherapy, local control
Introduction
Pancreatic cancer is a highly aggressive disease and is projected to be the fourth leading cause of cancer-related deaths in the United States, with poor outcomes particularly for patients with metastatic disease.1,2 Systemic therapy is the mainstay of treatment for metastatic or inoperable disease with modern regimens consisting of FOLFIRINOX (leucovorin calcium/fluorouracil/irinotecan hydrochloride and oxaliplatin) or gemcitabine/nab-paclitaxel. Although these treatments have achieved progressive improvements in overall survival (OS), it is estimated that 30% to over 55% of patients with pancreatic cancer suffer from the morbidity and/or mortality of local disease progression.3,4 Local disease control provides important palliation for metastatic patients; however, a potential opportunity arises for patients with low-volume or biologically indolent pancreatic cancer to potentially derive further benefit from local therapy.
Oligometastatic disease was first defined by Hellman and Weichselbaum in 1995 to identify a subgroup of patients with limited metastatic burden who may benefit from metastasis-directed therapy or still possess potential for curative treatment. 5 However, outcomes for patients with metastatic pancreatic cancer have been limited due to disease aggressiveness and relatively poor systemic disease control. To date, the locoregional interventions for patients with metastatic pancreatic cancer have ranged in aggressiveness such as primary tumor resection with or without metastasectomy, radiofrequency ablation (RFA), irreversible electroporation (IRE), high-intensity focused ultrasound (HIFU), selective internal radiation (SITR), transarterial radioembolization (TARE), transarterial chemoembolization (TACE), and stereotactic body radiotherapy (SBRT). 6
For inoperable disease, stereotactic magnetic resonance image-guided ablative radiotherapy (SMART) is a non-invasive option that provides excellent 1-year local disease control approximating 84–90% in patients with only localized disease.7,8 Ablative doses with this radiation technique have historically been limited by nearby radiosensitive structures such as the stomach and duodenum; however, SMART provides superior soft tissue contrast than CT imaging and allows for real-time gating and adaptive replanning based on daily variations in patient anatomy. In early retrospective experiences, grade 3 or greater toxicities with this technique are less than 5% with high frequencies of such adapted fractions with plan optimization based on the anatomy of the day. 9 Recently, the results of the first prospective trial in borderline resectable and locally advanced PDAC treated with SMART demonstrated a 1-year survival of 94% and 9% grade 3+ toxicities; however, caution was recommended on treating patients with this technique who are surgical candidates particularly if there is a plan for vascular repair or reconstruction as there was a higher incidence of post-operative mortality. 8
We sought to report outcomes of patients with pancreatic cancer with limited disease burden who received treatment of the primary tumor with ablative doses using SMART. We hypothesize that as the major advancements in care for patients with metastatic disease have been systemically focused, there may be improved outcomes for some patients treated to prevent local disease progression. Exploratory analyses were performed based on clinical disease factors such as the number and location of metastases and the reason for radiation delivery to the primary tumor (pain control, local progression, or local control) to identify prognostic factors associated with improved outcomes with this treatment.
Methods and Materials
Patient Eligibility
A retrospective review was conducted on 22 pancreatic cancer patients who were either diagnosed with oligometastatic disease before treatment or experienced metachronous oligoprogression during induction chemotherapy. These patients received treatment using SMART between 2019 and 2021, and the data was obtained from 2 institutional review board-approved databases from Moffitt Cancer Center and Miami Cancer Institute. Relevant Equator guidelines were followed, and all patient details have been de-identified. 10 Inclusion criteria for patient selection included those from the above-listed centers with pre-treatment stage IV pancreatic cancer based on the American Joint Committee on Cancer, a metastatic disease with a limited burden of 6 or fewer metastases, treatment with neoadjuvant chemotherapy, and treatment using SMART to the primary lesion. All patients completed standardized pre-treatment staging imaging including CT chest/abdomen/pelvis (CT CAP) with pancreas protocol and PET/CT. Patients were eligible to also receive metastasis-directed therapy by radiation (MRI-guided or traditional SBRT) or liver-directed therapy by radioembolization, resection, or microwave ablation. The use of institutional databases from 2 different hospitals from different cities may lead to a decrease in the potential for institutional bias and therefore increase the external validity of the analysis.
Stereotactic Magnetic Resonance Image-Guided Ablative Radiotherapy Planning and Treatment
The protocols for SMART for pancreas cancer have been previously published.9,11 In brief, all patients were simulated after a 3-hour fast in the supine position in a .35 T MRIdian Linac (ViewRay Inc., Denver, CO) and imaged with a balanced steady-state free precession signal (TrueFISP) with respiratory gating. A breath-hold (17–30 seconds) cine sequence was obtained while the patient was performing a cycle of breath hold and free breathing maneuvers to ensure appropriate tracking and duty cycle for treatment delivery. They subsequently underwent conventional CT simulation. At one institution, patients were simulated with and without IV and oral contrast.
The gross tumor volume (GTV) was defined as the primary tumor as well as peripancreatic lymph nodes >1 cm in longest diameter. The GTV was targeted by CT or MRI and consisted of arterial or venous vasculature involved by the primary tumor and proximal soft tissue density on associated vasculature up to the celiac axis, aorta, or portal vein and was prescribed 40–50 Gy in 5 fractions as per physician discretion. Elective regional nodal irradiation to define a Clinical Target Volume (CTV) was performed as per discretion of the treating physician and encompassed a .5–1.0 cm expansion of the celiac axis, superior mesenteric vein, and superior mesenteric artery including the proximal 1.0 to 1.5 cm of vessels. Dose constraints to the stomach and small bowel had small variations per institutional protocols. Daily adaptive replanning was performed to normalize to organ at risk volumes if there was a constraint violation of any of the luminal gastrointestinal organs or if GTV coverage was inferior to the original treatment plan.
Patients were re-staged 4–6 weeks and then every 3 months after 5-fraction SMART using CT chest/abdomen/pelvis (CT CAP) with pancreas protocol. PET/CT and/or abdominal MRI were also obtained according to institutional preference. As such, variation in frequency and use of differing imaging modalities prevented the use of standardized RECIST criteria. All patients had received neoadjuvant chemotherapy with either FOLFIRINOX (64%) or gemcitabine/nab-paclitaxel (36%). A single patient underwent surgical resection of her metastatic and primary disease by distal pancreatectomy, and 41% of patients received other metastasis-directed therapy.
Statistical Analysis
Toxicity was prospectively evaluated and recorded at the time of patient follow-up and according to Common Terminology Criteria for Adverse Events (CTCAE), version 5.0. OS and progression-free survival (PFS) were evaluated using Kaplan–Meier analysis from the time of diagnosis and completion of SMART, respectively, to the most recent clinical follow-up. Freedom from local regional failure (FFLRF) was calculated from SMART until disease recurrence or progression at the primary tumor location or regional lymph node recurrence (peripancreatic, celiac, or peri-portal). Freedom from distant failure (FFDF) was calculated from SMART until evidence of disease progression in distant organs (lung and liver) and non-regional lymph nodes, or abdomen (mesentery, peritoneal disease, and malignant ascites). Progression was coded at the date of biopsy-proven pathological confirmation if available, otherwise, the date of radiographic progression with an elevation of tumor markers or treating oncologist clinical suspicion to initiate or alter systemic therapy was sufficient. Both TTLRF and TTDF were censored at the time of the most recent diagnostic restaging scan. For subgroup analyses, the number of metastases was grouped by median split into 1–2 vs 3–6 lesions and comparisons were made for patients treated for local control vs for local tumor oligoprogression. An exploratory analysis of oligometastatic disease as defined by Damanakis et al was performed on patients with limited disease defined as baseline CA19-9 <1000 U/mL, responsive or stable disease after chemotherapy, and <4 metastases in the liver or lung. 12 Clinical endpoints were calculated from the date of diagnosis with the Kaplan–Meier method with log-rank comparisons in SPSS version 28 (IBM Corp., Armonk, NY). Median follow-up from diagnosis and SBRT was calculated by reverse Kaplan–Meier estimate.
Results
A total of 22 patients with oligometastatic pancreatic adenocarcinoma were evaluated with characteristics summarized in Table 1. The median age at diagnosis was 66 years (range 49–75) with a slight male predominance (59.1%). The majority of patients had ECOG 0 (63.6%) or 1 (27.3%) performance status. Most had 1–2 (59%) vs 3–6 distant metastases (41%, n = 9). The location of the distant metastases included liver only (40.9%), lung only (13.6%), abdominal or pelvic lymph node(s) (13.6%), bone only (4.5%), or multiple organs (27.3%). At the time of diagnosis, the median CA19-9 was 149.9 U/mL (range: 0–36,114 U/mL). Median CA 19-9 levels before SMART and immediately following SMART were 59 U/mL (range: 0–16422 U/mL) and 154 U/mL (range: 0–55283 U/mL), respectively. Slightly above 80% of patients presented with de novo oligometastatic disease (n = 18), and a majority (86.4%) had synchronous disease vs metachronous (14%, n = 3).
Table 1.
Patient Characteristics (n = 22).
| No. of Patients | % | ||
|---|---|---|---|
| Age, years | |||
| Median | 65.5 | ||
| Range | 49–75 | ||
| Sex | |||
| Male | 13 | 59.1 | |
| Female | 9 | 40.9 | |
| ECOG | |||
| 0 | 14 | 63.6 | |
| 1 | 6 | 27.3 | |
| 2 | 2 | 9.1 | |
| Primary tumor location | |||
| Head/neck | 13 | 59.1 | |
| Body/tail | 9 | 40.9 | |
| Clinical T-stage | |||
| 1 | 2 | 9.1 | |
| 2 | 7 | 31.8 | |
| 3 | 4 | 18.2 | |
| 4 | 9 | 40.9 | |
| Clinical N-stage | |||
| 0 | 19 | 86.4 | |
| 1 | 2 | 9.1 | |
| 2 | 1 | 4.5 | |
| Presentation | |||
| Synchronous | 19 | 86.4 | |
| Metachronous | 3 | 13.6 | |
| Oligometastatic classification | |||
| De novo | 18 | 81.8 | |
| Induced | 4 | 18.2 | |
| Number of metastasis | |||
| 1–2 | 13 | 59.1 | |
| 3–4 | 7 | 31.8 | |
| 5–6 | 2 | 9.1 | |
| Location of metastasis | |||
| Solitary liver | 9 | 40.9 | |
| Solitary lung | 3 | 13.6 | |
| Solitary abdominal/pelvic node | 3 | 13.6 | |
| Solitary bone | 1 | 4.5 | |
| Multiple organs | 6 | 27.3 | |
| Tumor size (cc) | |||
| Median | 66 | ||
| Range | 49–75 | ||
| CA19-19 (U/mL) median (range) | |||
| At diagnosis | 149.9 (0–36,114) | ||
| Pre-RT | 59.2 (3.2–16,422) | ||
| Post-RT (4–6 wks) | 153 (2.1–25,306) | ||
ECOG, Eastern Cooperative Oncology Group; CA19-19, cancer antigen 19-19; RT, radiation therapy; wks, weeks.
Table 2 summarizes the treatment characteristics for this patient cohort. All patients received neoadjuvant chemotherapy within a median of 26 days from the time of diagnosis (range: 8–69 days), with 64% receiving FOLFIRINOX (median 13 cycles and 224 days), and the remaining 36% received gemcitabine/nab-paclitaxel (median 7 cycles and 220 days). The median time from diagnosis to SMART was 297 days (range: 135–722 days). The most common reason for SBRT was to improve local control in patients who had no disease progression on chemotherapy via consolidation (77%) followed by patients treated for progression or pain control (23%). In total, 41% of patients received any metastasis-directed therapy. SMART was delivered to the primary site to a median dose of 50 Gy (range: 40–50 Gy) in 5 fractions delivered daily (BED10 = 100 Gy; n = 18) with 41% of patients receiving chemotherapy post-SMART (n = 9). Dose constraints varied slightly according to institutional protocol as previously 9 described but are briefly summarized in Table 2.
Table 2.
Treatment Characteristics (n = 22).
| No. of Patients | % | ||
|---|---|---|---|
| Neoadjuvant chemotherapy | |||
| FOLFIRINOX | 14 | 63.6 | |
| Gemcitabine/nab-paclitaxel | 8 | 36.4 | |
| Neoadjuvant chemotherapy cycles | |||
| 3–6 | 5 | 22.7 | |
| 6–12 | 11 | 50.0 | |
| >12 | 6 | 27.3 | |
| Missing | 2 | 9.1 | |
| Neoadjuvant chemotherapy | |||
| Median no. of cycles | 9.5 | ||
| Median no. of days | 224 | ||
| Metastasis-directed therapy | |||
| Yes | 9 | 40.9 | |
| No | 13 | 59.1 | |
| Reason for SBRT administration | |||
| Consolidative | 17 | 77.3 | |
| Progression/pain control | 5 | 22.7 | |
| SIB Radiation prescription, gy (BED10) | |||
| 40–45 Gy (72.0–85.5 Gy) | 4 | 18.2 | |
| 50 Gy (100.0 Gy) | 18 | 81.8 | |
| Local RT to lymph nodes | |||
| Yes | 3 | 13.6 | |
| No | 19 | 86.4 | |
| Locoregional failure | |||
| Yes | 10 | 45.5 | |
| No | 12 | 54.5 | |
| Dose constraints | |||
| Miami Cancer Center | |||
| Stomach | V35 < .5 cc | ||
| Small bowel | V40 < .03 cc | ||
| Moffitt Cancer Center | |||
| Stomach | V32 < 2 cc | ||
| Small bowel | V35 < .5 cc | ||
SBRT, stereotactic body radiation therapy; SIB, simultaneous integrated boost; gy, gray; BED, biologically effective dose; RT, radiation therapy.
For the entire cohort, the median follow-up from diagnosis was 21.6 months (range: 9.6–33.4 months) and from SMART was 11.2 months (range: 1–23.8). The median FFLRF was 11.8 months and included patients with primary tumor progression (32%) and regional nodal failure (14%). Two patients expired (9%) secondary to local disease progression which occurred at 9.7 months and 3.7 months post-SMART, respectively. The median time to distant failure was 2.7 months (range: .4–15 months) following SMART. At the last follow-up, 19 patients (86%) had experienced distant progression and 13 (69%) had died. A single patient underwent a margin-negative distal pancreatectomy and metastasectomy of an irradiated retroperitoneal tumor implant and then experienced distant disease progression in the liver 13 months after SMART.
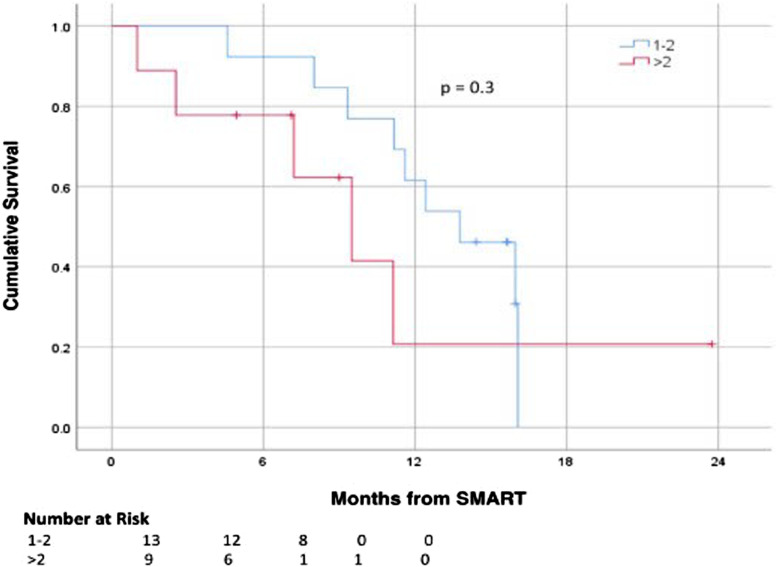
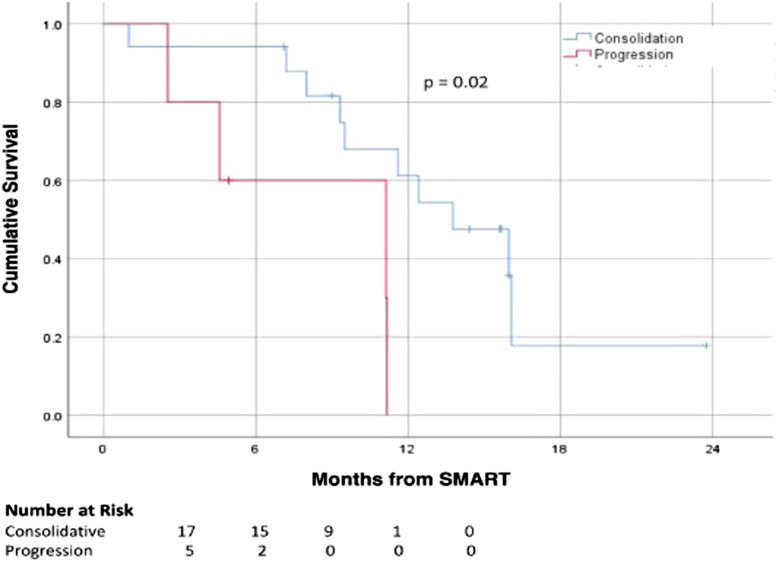
Subgroup analysis for number of metastases was performed comparing 1–2 metastases (n = 13, 59%) vs >2 metastases (n = 9, 41%) and demonstrated no statistically significant difference in median OS at 13.8 months vs 9.5 months, respectively (P = .3; Figure 1). In patients treated for local control vs for pain or tumor progression, a statistically significant difference was noted with a median OS of 13.8 months vs 11.1 months, respectively (P = .021; Figure 2). There was no difference in survival from SMART for patients with solitary vs multiple organ metastases (P = .5), lung vs non-lung metastases (P = .3), liver vs non-liver metastases (P = .3), use of any metastasis-directed therapy (P = .6), or ECOG 0 vs >0 (P = .2). A total of 8 patients (36.3%) met the Damanakis et al criteria 12 for limited oligometastatic disease; however, these patients did not have a statistically significant difference in survival from the remaining cohort at 11.2 vs 11.6 months (P = .3).
Figure 1.
Kaplan–Meier curves for survival outcomes stratified by number of metastases.
Figure 2.
Kaplan–Meier curves for survival outcomes stratified by SBRT intent.
Two patients (9%) experienced grade 3 toxicities. One patient with small-volume duodenal abutment at diagnosis who was on anticoagulation during SMART experienced a grade 3 gastrointestinal bleed at 69 days post-SMART and a portal vein pseudoaneurysm 168 days post-SMART. The second patient developed a grade 3 gastric outlet obstruction 430 days post-SMART. There were no grade 4 or 5 toxicities.
Discussion
To our knowledge, we are the first to report a multi-institutional experience using SMART for oligometastatic pancreatic adenocarcinoma. While rapid distant disease progression after SMART was common with 20 patients (91%) progressing at a median of 2.7 months after SMART, the median OS from diagnosis was 23.9 months and 11.6 months from SMART which reflects the favorable biology of the patient population to systemic therapy as well as the relatively limited oligometastatic disease burden criteria. Importantly, only 2 patients (9%) expired secondary to local disease progression, and local tumor control was 68% at a median follow-up of 15 months. These results are supported by a retrospective analysis of 62 patients with inoperable PDAC who were treated with chemotherapy and A-SMART. The study reported a 7.1% rate of death from locoregional progression after SMART in patients with localized disease. 13 Mitigating the local failure has proven to be difficult in pancreatic cancer as even after surgery upwards of 80% of patients may expire with evidence of locally recurrent disease. 14
The addition of local therapy may alleviate the morbidity of local failure which includes gastrointestinal bleeding, bowel perforation, small bowel obstruction, and biliary obstruction. 13 While the randomized LAP07 trial did not demonstrate an improvement in OS associated with conventionally fractionated chemoradiation for patients with locally advanced pancreatic cancer (LAPC), there was an improvement in local disease control. 15 In a small retrospective series, the authors indicate that local progression can be an important cause of death in the absence of palliative radiation (56% vs 29%, P = .12). 4 Additionally, it was noted that patients who received radiation had lower rates of GI bleed and extrahepatic biliary obstruction indicating that some of these potentially fatal comorbidities can be avoided. 4
Thus, shifting the pattern of failure and reducing the morbidity of local tumor progression may be achieved with primary site-directed radiation. Further benefits are suggested by a meta-analysis of over 60,000 patients with metastatic pancreatic adenocarcinoma which reported that local and metastasis-directed therapy was associated with improved OS compared to chemotherapy alone or best supportive care. 6 There may be some evidence supporting radiation dose escalation as palliative doses of SBRT for metastatic patients in the range of 25–30 Gy in 5 fractions have been associated with 1-year local control (LC) and OS rates of 43% and 53%, respectively. 3 A 2015 analysis by Su et al 16 reported on 25 patients treated with either 30–36 Gy (56%) in 3 fractions or 40–48 Gy (44%) in four fractions with CyberKnife SBRT. A third of this cohort was diagnosed with LAPC vs metastatic disease, but in contrast to our study, they were not screened for limited disease or oligometastatic criteria. They reported OS at 1 and 2 years as 37% and 18%, respectively. While local disease control was not reported, they found promising benefits in palliative care for pain control. Prior to the administration of SBRT, 80% (n = 20) of patients reported significant pain which decreased by 50% after 2 weeks post-radiation therapy and 3 patients were able to decrease analgesic use by 50%. Furthermore, SBRT in this study showed promising toxicity profiles with five patients (20%) experiencing grade 1 nausea and one patient experiencing a grade 2 toxicity (4%). No patients experienced any acute grade 3–5 toxicity. 16
A retrospective analysis by Ji et al 17 investigated outcomes of 89 patients with liver-only metastatic pancreatic adenocarcinoma treated with pancreas SBRT to a mean of 41.1 Gy (range 25–50) in 5–7 fractions plus chemotherapy (38%) or chemotherapy alone (62%). In propensity score-matched analysis, the addition of SBRT to chemotherapy decreased 12-month local progression from 53% to 14% and was associated with lower rates of abdominal pain. Comparable toxicities were noted between both groups with a single grade 3 toxicity consisting of duodenal ulcer and bleeding in a patient who received SBRT plus chemotherapy; however, this patient had a known history of a duodenal ulcer. 17 Another contemporary series sought to evaluate the role of consolidative SBRT to all oligometastatic pancreatic cancer sites defined as 1–5 metastases. 18 Patients who received radiation were matched to a cohort of patients who received chemotherapy alone. Their results demonstrated a 2-year LC of 83% for patients treated with pancreatic SBRT with improvements in time off chemotherapy, polyprogression (14 vs 40 months) as well as an OS benefit (18 vs 42 months). The use of consolidative SBRT to all active metastatic sites underlines a key difference from the current series as only 40% of our cohort received any metastasis-directed therapy (radiation, microwave ablation, and surgery).
Future investigations into optimal patient selection for patients who may benefit from local therapy are paramount. While there is no clearly established definition for oligometastatic pancreatic cancer, Damanakis et al 12 proposed the following criteria: limited disease, defined as the number of metastases in the liver and lung being 4 or fewer, CA19-9 below 1000 U/ml, and response to chemotherapy or stable disease on chemotherapy. Their analysis suggests that this subset population of pancreatic patients may benefit strongly from local treatments as their series identified 10 patients out of a cohort of 128 (8%) and showed a difference in survival of 7.2 to 19.4 months. While our data did not support a benefit in treating patients meeting this classification, our small patient numbers may have limited our ability to detect a difference. Ultimately, in our series disease biology appeared to be the primary driver of outcomes as patients treated after response or stable disease on chemotherapy had a small statistically significant survival advantage and there is a suggestion that patients with a lower metastatic burden may also derive a greater benefit; however, this difference was not statistically significant with our underpowered analysis.
In our series, the 1-year LC of 68% was lower than other series at using SMART in the non-metastatic setting ranging from 84% to 90%,7-9 which may be secondary to the more advanced disease and/or aggressive biology of our patient population as well as small patient numbers. Furthermore, routine elective nodal irradiation may mitigate the 14% of patients who experienced regional nodal failure as none of these patients also had local tumor progression after SMART. Distant progression occurred in 91% of our patients at a median of 2.7 months after SMART, which is consistent with previous studies demonstrating a 6-month PFS of 15% for patients with metastatic disease after local SBRT 19 and underlines the aggressive systemic nature of this disease. However, total oligometastatic consolidation with radiation may be a viable strategy in supplementing systemic disease control and provide patients with breaks from systemic therapy. 18
It is important to note that conventionally fractionated non-ablative radiotherapy or SBRT has not been demonstrated to impact OS for patients with metastatic disease; however, improving local control may help to reduce morbidity of local disease progression. 4 Encouraging results have been demonstrated in patients with LAPC with ablative dosing with 3-year OS rates of 35% and 5-year overall survival rates of 18%. 20 The use of SMART may allow these ablative dose regimens to be delivered over 5 treatments as opposed to 15 or 25 fractions, minimizing strain and financial burden on patients. In addition to use for local control, radiation plays a crucial role in palliation, particularly in the setting of pain control with half of the patients experiencing relief within 2 weeks of treatment and decrease in need for narcotic medications. 16 Celiac plexus blocks have historically provided further pain relief in patients receiving opioid therapy with continued uncontrolled pain. The use of palliative radiation may provide an additional option for patients experiencing inadequately controlled pain on opioid therapy alone. 21
Given the background of the literature showing benefit in terms of pain control, Pavic et al 22 are investigating this prospectively in a double-arm, parallel-group, randomized analysis. This study will examine the role of SMART of the primary tumor for pain control in patients with metastatic pancreatic ductal adenocarcinoma (mPDAC) from 3 institutions. It includes patients with stable metastatic disease, described as non-progressive for 8 weeks, receiving standard of care chemotherapy and randomly assigned to either receive MR-guided SBRT to the primary tumor or SoC-CT alone. Of note, the experimental group will receive non-ablative 33 Gy delivered in 5 fractions. Toxicity will be assessed, and pain control will be measured using the mean cumulative pain index (MCPI) and rated by the patients every four weeks with follow-ups continuing for 18 months. The results from this trial may help to inform clinicians about the use of palliative radiation for patients with metastatic pancreatic cancer, and the results may be indirectly compared to outcomes in our cohort treated with ablative doses.
Limitations of the current study include the retrospective nature as well as the limited sample size. Furthermore, treatment and follow-up of this cohort were heterogeneous between 2 multidisciplinary institutions which limited the use of standardized RECIST criteria for radiographic review and there was inconsistent use of consolidative therapies to metastatic sites. Finally, future inclusion of patients from multiple institutions of various states may further allow for increased external validity. Improved patient stratification is needed to support prospective studies investigating the potential impact of ablative SMART for patients with limited disease.
Conclusion
Ablative MRI-guided radiotherapy may offer promising LC for patients with oligometastatic pancreatic cancer, possibly limiting the morbidity and mortality of local disease progression. However, we are unable to firmly identify clinical factors for optimal patient selection for treatment due to our small sample size as well as limited survival. Further improvements in distant disease control and potentially complete disease consolidation with metastasis-directed therapy may provide further oncological benefits for this patient population.
Appendix.
Abbreviations
- CA19-9
Carbohydrate antigen 19-9
- CTCAE
Common terminology for adverse events
- CT CAP
CT chest/abdomen/pelvis
- ECOG
Eastern Cooperative Oncology Group
- FFDF
Freedom from distant failure
- FFLRF
Freedom from local regional failure
- FOLFIRINOX
Leucovorin calcium, fluorouracil, irinotecan hydrochloride, oxaliplatin
- GTV
Gross tumor volume
- HIFU
High-intensity focused ultrasound
- IRE
Irreversible electroporation
- LAPC
Locally advanced pancreatic cancer
- LC
Local control
- OS
Overall survival
- PFS
Progression-free survival
- RFA
Radiofrequency ablation
- SBRT
Stereotactic body radiotherapy
- SITR
Selective internal radiation
- SMART
Stereotactic MRI-guided ablative radiotherapy
- TACE
Transarterial chemoembolization
- TARE
Transarterial radioembolization
- TTDF
Time to distant failure
- TTLRF
Time to locoregional failure.
Footnotes
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: November 2022 and January 2023, travel expenses were partially supported by View Ray. October 2022, meal expense support from Galera Therapeutics.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical Statement
Ethical Approval
This study was conducted under institutional IRB-approved protocols with a data-sharing agreement. All patient data was de-identified.
ORCID iD
Russell F. Palm https://orcid.org/0000-0002-0448-8715
References
- 1.Fernandez-del Castillo C. Clinical manifestations, diagnosis, and staging of exocrine pancreatic cancer. In: TW Post, ed. UpToDate. Wolters Kluwer; 2023. Accessed 2022. https://www.uptodate.com/contents/clinical-manifestations-diagnosis-and-staging-of-exocrine-pancreatic-cancer?search=Clinicalmanifestations,diagnosis,andstagingofexocrinepancreaticcancer-UpToDate&source=search_result&selectedTitle=1%E2%88%BC150&usage_type=default&display_rank=12. [Google Scholar]
- 2.Pancreatic Cancer - Statistics. Cancer.net. Published June 25, 2012. Accessed November 29, 2023. https://www.cancer.net/cancer-types/pancreatic-cancer/statistics#:
- 3.Lischalk JW, Burke AM, Chew J, et al. Five-fraction stereotactic body radiation therapy (SBRT) and chemotherapy for the local management of metastatic pancreatic cancer. J Gastrointest Cancer. 2017;49(2):116-123. doi: 10.1007/s12029-016-9909-2. [DOI] [PubMed] [Google Scholar]
- 4.Seible D. The impact of local disease progression in pancreatic cancer at the end of life. International Journal of Radiation Oncology. 2015;93(3):E119. https://www.redjournal.org/article/S0360-3016(15)01581-3/fulltext# [Google Scholar]
- 5.Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol. 1995;13(1):8-10. doi: 10.1200/jco.1995.13.1.8. [DOI] [PubMed] [Google Scholar]
- 6.Timmer F, Geboers B, Nieuwenhuizen S, et al. Locoregional treatment of metastatic pancreatic cancer utilizing resection, ablation and embolization: a systematic review. Cancers. 2021;13(7):1608. doi: 10.3390/cancers13071608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hassanzadeh C, Rudra S, Bommireddy A, et al. Ablative five-fraction stereotactic body radiation therapy for inoperable pancreatic cancer using online MR-guided adaptation. Adv Radiat Oncol. 2021;6(1):100506. doi: 10.1016/j.adro.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parikh PJ, Lee P, Low DA, et al. A multi-institutional phase 2 trial of ablative 5-fraction stereotactic magnetic resonance–guided on-table adaptive radiation therapy for borderline resectable and locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2023;117(4):799-808. doi: 10.1016/j.ijrobp.2023.05.023. [DOI] [PubMed] [Google Scholar]
- 9.Chuong MD, Bryant J, Mittauer KE, et al. Ablative 5-fraction stereotactic magnetic resonance-guided radiation therapy (MRgRT) with on-table adaptive replanning and elective nodal irradiation for inoperable pancreas cancer. Pract Radiat Oncol. 2020;11(2):134-147. doi: 10.1016/j.prro.2020.09.005. [DOI] [PubMed] [Google Scholar]
- 10.von Elm E. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335(7626):344-349. doi: 10.1136/bmj.39386.490150.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bryant JM, Palm R, Liveringhouse C, et al. Surgical and pathologic outcomes of pancreatic adenocarcinoma (PA) after preoperative ablative stereotactic magnetic resonance image guided adaptive radiation therapy (A-SMART). Adv Radiat Oncol. 2022;7(6):101045. doi: 10.1016/j.adro.2022.101045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Damanakis AI, Ostertag L, Waldschmidt D, et al. Proposal for a definition of “Oligometastatic disease in pancreatic cancer”. BMC Cancer. 2019;19(1):1261. doi: 10.1186/s12885-019-6448-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chuong MD, Herrera R, Ucar A, et al. Causes of death among patients with initially inoperable pancreas cancer after induction chemotherapy and ablative 5-fraction stereotactic magnetic resonance image guided adaptive radiation therapy. Adv Radiat Oncol. 2023;8(1):101084. doi: 10.1016/j.adro.2022.101084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iacobuzio-Donahue CA, Fu B, Yachida S, et al. DPC4 gene status of the primary carcinoma correlates with patterns of failure in patients with pancreatic cancer. J Clin Oncol. 2009;27(11):1806-1813. doi: 10.1200/jco.2008.17.7188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hammel P, Huguet F, van Laethem JL, et al. Effect of chemoradiotherapy vs chemotherapy on survival in patients with locally advanced pancreatic cancer controlled after 4 months of gemcitabine with or without erlotinib: the LAP07 randomized clinical trial. JAMA. 2016;315(17):1844-1853. doi: 10.1001/jama.2016.4324. [DOI] [PubMed] [Google Scholar]
- 16.Su TS. Stereotactic body radiotherapy using CyberKnife for locally advanced unresectable and metastatic pancreatic cancer. World J Gastroenterol. 2015;21(26):8156. doi: 10.3748/wjg.v21.i26.8156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ji X, Zhao Y, He C, et al. Clinical effects of stereotactic body radiation therapy targeting the primary tumor of liver-only oligometastatic pancreatic cancer. Front Oncol. 2021;11. doi: 10.3389/fonc.2021.659987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elamir A. M., Karalis J. D., Sanford N. N., et al. Ablative radiation therapy in oligometastatic pancreatic cancer to delay polyprogression, limit chemotherapy, and improve outcomes. Int J Radiat Oncol Biol Phys. 2022;114(4):792-802. doi: 10.1016/j.ijrobp.2022.07.019. [DOI] [PubMed] [Google Scholar]
- 19.Chang DT, Schellenberg D, Shen J, et al. Stereotactic radiotherapy for unresectable adenocarcinoma of the pancreas. Cancer. 2009;115(3):665-672. doi: 10.1002/cncr.24059. [DOI] [PubMed] [Google Scholar]
- 20.Reyngold M, Parikh P, Crane CH. Ablative radiation therapy for locally advanced pancreatic cancer: techniques and results. Radiat Oncol. 2019;14(1):95. doi: 10.1186/s13014-019-1309-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Molnár I, Hegyi G, Zsom L, et al. Celiac plexus block increases quality of life in patients with pancreatic cancer. J Pain Res. 2019;12:307-315. doi: 10.2147/JPR.S186659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pavic M, Niyazi M, Wilke L, et al. MR-guided adaptive stereotactic body radiotherapy (SBRT) of primary tumor for pain control in metastatic pancreatic ductal adenocarcinoma (mPDAC): an open randomized, multicentric, parallel group clinical trial (MASPAC). Radiat Oncol. 2022;17(1):18. doi: 10.1186/s13014-022-01988-6. [DOI] [PMC free article] [PubMed] [Google Scholar]