Abstract
Purpose
The development of osteoarthritis (OA) has been linked to mechanical factors. Studies suggest that periodic mechanical stress (PMS) may be a factor contributing to cartilage repair and the onset of OA. Therefore, this study was designed to explore the effects and underlying mechanisms of PMS on OA development.
Patients and Methods
Firstly, surgery and interleukin (IL)-1β were used for the establishment of rat/cell models of OA, respectively. Subsequently, activating transcription factor (ATF) 3 expression was knocked down in OA rats, and OA chondrocytes were treated with different heights (0, 1, 2, 4, 8 cm) of PMS or si-ATF. Safranin O staining was used to observe the histological changes in the rat knee joint, and enzyme-linked immunosorbent assay (ELISA) was performed to detect levels of tumor necrosis factor (TNF)-α, IL-6, and IL-8 in vivo and in vitro. Further, the expression of extracellular matrix (ECM) proteins in the rat knee joint was assessed immunohistochemistry. Flow cytometry was used to evaluate chondrocyte apoptosis. Lastly, Western blot was performed to detect the expression of related proteins of the protein kinase B (Akt) signaling pathway and ECM.
Results
The OA rat model was successfully constructed. Further experiments indicated that the knockdown of ATF3 not only alleviated joint swelling, pain, inflammatory response and pathological damage, but also promoted ECM synthesis and the phosphorylation of Akt in OA rats. In vitro experiments showed that PMS (4 cm) effectively inhibited cell apoptosis, decreased the levels of TNF-α, IL-6 and IL-8, promoted ECM synthesis, and activated the Akt signaling pathway in osteoarthritic chondrocytes. However, ATF3 overexpression reversed the positive effects of PMS on osteoarthritic chondrocytes.
Conclusion
PMS can effectively inhibit the development of OA, and its protective effects may be attributed to the down-regulation of ATF3 expression and activation of the Akt signaling pathway.
Keywords: periodic mechanical stress, osteoarthritis, ATF3-Akt axis, joint damage, pain, apoptosis
Introduction
Osteoarthritis (OA) is a common degenerative joint disease affecting the elderly, causing joint pain and deformity, and may even lead to disability in severe cases.1 It has been reported that more than 240 million people are affected by OA worldwide.2 A large cohort study reported a prevalence of 13.5% and 18.7% for symptomatic and radiographic OA in men and women, respectively.3 OA not only severely affects the quality of life of patients but also imposes a heavy medical burden.4,5 OA is a whole joint disease and characterized by progressive articular cartilage degeneration, osteophyte formation, subchondral sclerosis, bone marrow injury, synovitis, inflammation and fibrosis of the infrapatellar fat pad and meniscal degeneration.6–8 In addition, risk factors closely related to the pathogenesis of OA includes genetic susceptibility, gender, age, joint location, joint malalignment, obesity, and trauma.9,10 Apart from genetic susceptibility and gender, other risk factors such as prolonged joint weight-bearing with age, increased joint weight-bearing due to obesity and weight-bearing asymmetry have been closely linked to mechanical factors caused by joint malalignment and trauma. Moreover, these risk factors can initiate the injury-repair process of various joint tissues, leading to the production of inflammatory mediators such as cytokines, chemokines and proteases, trigger degenerative cascade reactions, gradually result in the destruction of cartilage, bone and synovium, and ultimately leading to the characteristic pathological changes of OA.11,12 Although analgesics and non-steroidal anti-inflammatory drugs (NSAIDs) are the main drugs used to treat OA, they only provide pain relief and have no significant clinical impact on the prevention or improvement of OA.13,14 Hence, there is an urgent to explore more effective treatments for OA.
Cartilage degradation is a key factor in the development of OA.15 Cartilage comprises chondrocytes and extracellular matrix (ECM), rich in collagen and proteoglycans. Chondrocyte apoptosis is linked to cartilage degeneration and is considered a crucial event during the development of OA.16 The synthesis and degradation of the articular cartilage matrix are in a constant dynamic balance due to regulation by autocrine or paracrine signals involving cytokines, signaling molecules and transcription factors. As a load-bearing organ, joints are stimulated by various mechanical forces. And the biomechanical factors are the biological microenvironment of cartilage, which play a crucial role in the development, growth, injury, and repair regeneration of articular cartilage.17,18 A animal related study has shown that increasing joint load through moderate exercise can increase cartilage layer thickness, mechanical strength, and proteoglycan content in cartilage, while vigorous exercise may lead to cartilage damage.19 Therefore, mechanical load is like a “double-edged sword”, moderate mechanical stimulation regulates the normal physiological function of cartilage, while excessive mechanical load can lead to cartilage damage, indicating that mechanical signals can impact the synthesis and degradation of the articular cartilage matrix, leading to imbalances that contribute to the development of OA.20 However, the specific mechanical signal transduction pathway remains unclear.
Periodic mechanical stress (PMS) is a mechanical stimulation that simulates physiological mechanical conditions in vivo. Several studies have reported the promoting effect of PMS on the proliferation of chondrocytes and matrix synthesis.21–24 For example, van Valburg et al reported that joint traction provided mechanical stress to promote the repair of cartilage.25 Additionally, Gao et al discovered that PMS significantly induced the expression of extracellular matrix (ECM) Col-2A1 and aggrecan in nucleus pulposus (NP) cells.26 As OA development is reported to be closely related to chondrocyte damage and ECM degradation, we speculated that PMS might play a significant role in OA.
Activating transcription factor (ATF) 3, also known as activated transcription factor 3, is a member of the transcription factor family of ATF/cyclic adenosine monophosphate (cAMP)-response element binding proteins.27 It binds to with other transcription factors through its leucine zipper structure, by homodimerization or heterodimerization, in inflammation-related factor promoter regions and acts as an important inflammatory suppressor.28,29 Also, ATF3 is involved in many pathological conditions, including atherosclerosis,30 cancer,31,32 diabetes,33 acute kidney injury34 and so on. Studies have shown that ATF3 regulates the expression of inflammatory cytokines in chondrocytes and is involved in the occurrence and development of OA. Usually, with the feed-forward loop of inflammatory cytokines/NF-kB/ATF3 in chondrocytes is considered as a novel target for OA treatment.35 Additionally, Li et al found that the PR11-364P22.2/ATF3 protein interaction mediated IL-1β-induced catabolic effects in cartilage tissue and chondrocytes.36
Although a previous study37 by our team described the effect of PMS on OA, the specific mechanism is not fully understood. Additionally, a study by Shen et al on cell mechanical response revealed that ATF3 acted as an important signaling factor for cell mechanical force transduction.38 Therefore, we hypothesized the clinical relevance of investigating the relationship between ATF3 and PMS could further improve our understanding on the molecular mechanisms underlying the response of cells and tissues to mechanical stress, which could stimulate the development of novel therapeutic approaches for various disorders related to mechanical stress. For instance, mechanical stress can induce inflammation in various tissues, and ATF3 has been shown to play a role in regulating inflammation. Thus, studying the relationship between ATF3 and PMS could help understand the molecular mechanisms of stress-induced inflammation and identify potential therapeutic targets. Further, as it is known that mechanical stress is involved in tissue remodeling and repair processes, such as wound healing and bone formation, investigating the role of ATF3 in these processes under PMS could provide insights into the regulation of cell growth, differentiation, and apoptosis, leading to improved therapeutic strategies for tissue repair.
Therefore, models of OA in vivo and in vitro were constructed in this study to explore the role and molecular mechanism of PMS in OA. Further, the function of PMS in chondrocyte inflammation, ECM synthesis and chondrocyte apoptosis was evaluated in the OA models. Also, the molecular mechanism of PMS affecting OA was also further explored through in vivo and in vitro experiments. Collectively, the objective of this study was to provide valuable insights for the development of clinical interventions, including physical and drug treatments, for OA.
Materials and Methods
Model Establishment
Establishment of a Rat Model of OA
Forty healthy specific pathogen free (SPF) adult male Sprague-Dawley (SD) rats (6.8 ± 0.52 w) weighing 180 g - 220 g (188 ± 2.65 g) were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd and were caged in a laminar airflow cabinet under specific pathogen-free conditions. They were kept at 22 °C, and fed with sterilized water and food, and kept on a 12h light/dark cycle. We maintained all rats in the animal facility of Soochow University. The experimental protocol had been approved by the Institutional Animal Care and Use Committee of Soochow University (201708A105) and all studies followed ARRIVE guideline. Before the formal experiment, the rats were subject to adaptive feeding, 7 days later, they were randomly divided into four groups (10 rats/group). In the Sham group, only an incision was made on the medial side of the articular capsule to expose the anterior cruciate ligament in the rats. In the OA group, a rat model of OA was constructed through an anterior cruciate ligament transection and medial meniscectomy.39 The rats in the OA+si-NC group and OA+si-ATF group first underwent anterior cruciate ligament transection and medial meniscectomy to build the OA model, and then 50 nM si-NC and si-ATF3 were injected into the joint cavity of the corresponding group rats, respectively, twice a week. The si-NC and si-ATF3 were designed and synthesized by Shanghai GenePharma Co., Ltd (China). The specific siRNA sequences for ATF3 and control were as follows: si-NC: 5’-UUCUCCGAACGUGUCACGUTT-3’; si-ATF3: 5’-GCUGCAAAGUGCCGAAACATT-3’. Two rats in the four groups were fasted for 12 h, then euthanized by exposure to 100% CO2 in the empty seal chamber on the 2nd, 4th, and 8th week, respectively. Finally, the operated knee tissues of rats were collected for further experiments.
Establishment of a Cellular Model of OA with Chondrocytes
Articular chondrocytes were separated as previously described by Wang et al.40 Briefly, cartilage was collected from the knee joints of SD rats under sterile conditions, then washed five times with phosphate buffer solution (PBS, P1010, Solarbio, Beijing, China) and cut into small sections. Subsequently, the sections were digested in 0.25% trypsin- ethylene diamine tetra-acetic acid (EDTA) (C0203, Beyotime, Shanghai, China) solution. 30 min later, another digestion was performed in Dulbecco’s Modified Eagle Medium (DMEM) (Hyclone, UT, United States of America (USA) containing 0.2% collagenase type II (17101015, Thermo Fisher Scientific, MA, USA) for 4 h at 37 °C. Then, the cell suspension was centrifuged. After that, the chondrocytes were collected and cultured to confluence in DMEM medium with 10% fetal bovine serum (16140089, FBS, Gibco, CA, USA) and 1% penicillin-streptomycin (15140148, Gibco, CA, USA), followed by passages at a ratio of 1:3.
Subsequently, chondrocytes were treated by different intensities of PMS using a device mentioned in our previously published study.37 Specifically, chondrocytes (5 × 105/mL) were seeded in a 6-well plate and cultured until reaching 80% confluency. The chondrocytes were stimulated by IL-1β (10 ng/mL, P6245, Beyotime, Shanghai, China) for 24 hours to construct cell models of OA in vitro.41 OA cells were divided into five groups. Samples in group 0 were not administered PMS. In groups 1, 2, 4, and 8, samples were treated using PMS (0.15 Hz) at device heights of 1 cm, 2 cm, 4 cm and 8 cm, respectively. Cell supernatants were collected for subsequent Enzyme-linked immunosorbent assay (ELISA) assays at weeks 1, 2, 4, and 8 of PMS treatment, respectively. Cellular proteins were collected at week 8 for Western blot assay.
Chondrocytes (5 × 105/mL) were seeded in 6-well plates and divided into the following four groups. In the IL-1β group, the cells were first treated with IL-1β, followed by PMS application in vitro. The IL-1β + NC group was transfected with si-NC, treated with IL-1β and received PMS in vitro. The IL-1β + si-ATF3 group was transfected with si-ATF3, treated with IL-1β, and given PMS in vitro, and the IL-1β + ATF3 group was transfected with ATF3 lentiviral vector, treated with IL-1β and received PMS in vitro.
Determination of Joint Swelling and Weight-Bearing Test
The diameter of the knee joint was measured to determine the degree of joint swelling and assess the inflammation of the OA rat models. Specifically, the medial and lateral diameters of the knee were determined with a digital micrometer before modeling (week 0) and after modeling for 1 week (week 1), 2 weeks (week 2), 4 weeks (week 4) and 8 weeks (week 8). Pain behavior was measured according to as a weight-bearing asymmetry between the OA-induced and contralateral/ipsilateral limb described by Yu et al.42 In addition, an incapacitance meter test (IITC Life Sciences, Woodland Hills, CA, USA) was performed to assess weight-bearing capacity before and after modeling.
Safranin O Staining
The knee joint tissues of rats in each group were stained referring to the instructions of Safranin O staining solution (G1375, Solarbio, Beijing, China).43 Briefly, the tissues were fixed in 10% formalin (G2160, Solarbio, Beijing, China), then decalcified, embedded with paraffin and sectioned. Next, the section (6 µm) was stained with Weigert staining solution, followed by fast green staining and safranin staining. Subsequently, the samples were cleared using xylene and then mounted. Finally, the staining of tissue was observed under a microscope (Olympus, Tokyo, Japan) and photoed.
ELISA
The rat blood samples collected from four rats in each group were allowed to stand at ambient temperature for about 40 min. Then, the rat blood samples and chondrocyte culture supernatant were centrifuged at 3000 rpm and 4 °C for 10 minutes, and the upper serum and culture supernatant were collected for testing. Specifically, the levels of tumor necrosis factor (TNF)-α, interleukin (IL)-8 and IL-6 in the serum and chondrocyte culture supernatant of rats in each group were detected using an enzyme-linked immunosorbent assay (ELISA) kit (H052-1-2, H008-1-2, H007-1-2, Nanjing Jiancheng Bioengineering Institute, China) according to the manufacturer’s instructions, and sample absorbance (ABS) was measured at 450 nm using a Multiskan FC microplate reader (Thermo Fisher Scientific, Waltham, MA, USA) within 30 min.
Immunohistochemistry
Collagen II, aggrecan, and matrix metalloproteinase-13 (MMP-13) in knee tissues were stained through Immunohistochemistry, and their expression levels were assessed by the depth and area of staining. Tissue sections were deparaffinized and hydrated routinely. After that, they were blocked with 3% (volume fraction) of H2O2 and ethanol and digested with 0.1% trypsin (C0201, Beyotime, Shanghai, China). The digested sections were incubated with diluted primary antibodies (Collagen II Antibody, MA1-37493, 1:200; Aggrecan antibody, MA3-16888, 1:100; MMP-13 antibody, MA5-14238; 1:150; Invitrogen, Carlsbad, CA, USA) overnight at 4 °C. After washing three times with PBS, the section samples were incubated again with secondary antibodies (goat anti-mouse lgG, 1:500, Invitrogen, Carlsbad, CA, USA) for 30 minutes at ambient temperature. Subsequently, the samples were rinsed with PBS and incubated for 30 minutes in peroxidase anti-peroxidase complex at ambient temperature. Next, the sections were washed with PBS, stained with 3,3’-diaminobenzidine (DAB, P0203, Beyotime, Shanghai, China), staining solution, rinsed with distilled water, then stained with hematoxylin, rinsed with water, and differentiated using hydrochloric acid alcohol. Upon rinsing again with water, the treated sections were blued and dehydrated with gradient alcohol. After that, the sections were cleared with xylene and sealed.44 The staining was observed under a microscope, and the images were taken. Finally, average Optical Density (AOD) of immunohistochemistry images was determined by Image J V1.8 software (National Institutes of Health, Bethesda, MD, USA).
Flow Cytometry
Chondrocyte apoptosis rate was detected using an Annexin V- fluorescein isothiocyanate (FITC)/ propidium iodide (PI) apoptosis detection kit (556547, BD Pharmingen, CA, USA). Specifically, chondrocytes were washed twice with cold phosphate buffer solution (PBS), and the cell suspension (1 × 106 cells/mL) was prepared using 1× Binding Buffer. Then, 100 µL of cell suspension was placed in Falcon tubes and labeled. Next, the tubes were supplemented with 5 µL of FITC-Annexin and 10 µL of PI, placed at ambient temperature (20–25 °C) in the dark for 15 min, and then added with 400 µL of 1× Binding Buffer to each tube. Lastly, the apoptotic results were measured by FACScan flow cytometry system (Becton Dickinson, CA, USA) within 1 hour.37 FlowJo software (Version X; TreeStar, Ashland, OR, USA) was used to view and analyze data.
Western Blot
Total protein of cells or cartilage tissues was extracted with radio-immunoprecipitation assay (RIPA) lysate (R0010, Solarbio, Beijing, China) and protease inhibitor (PPC1010, Sigma-Aldrich, St. Louis, MO). Notably, the cartilage tissues were homogenized with a tissue homogenizer (JTM-48D, Shang Hai Jtone Electronic Co.Ltd., Shanghai, China), and the cells were broken in an ice bath with sonication. The protein supernatant was collected after centrifugation (10,000 rpm, 4 °C, 20 min), then the protein concentration was determined using a bicinchoninic acid (BCA) protein assay kit (P0010, Beyotime, Shanghai, China). Subsequently, 30 µg protein was separated using 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to Polyvinylidene Fluoride (PVDF, IPVH00010, Millipore, MA, USA) membranes. Next, the membranes were blocked in 5% Bovine Serum Albumin (SW3015, BSA, Solarbio, Beijing, China) blocking solution at ambient temperature. After 1–2 hours, the membranes were washed with TBST (triethanolamine-buffered saline +tween), then incubated overnight at 4 °C with primary antibodies (p-Akt antibody, #4060, 1:2000; Akt antibody, #4691, 1:1000; β-actin, #4970, 1:1000; Cell Signaling Technology, MA, USA. ATF3 antibody, NBP1-85816PEP, 1:1000, Novus Biologicals, Littleton, CO, USA. Collagen II Antibody, MA1-37493, 1:200; Aggrecan antibody, MA3-16888, 1:1000; MMP-13 antibody, MA5-14238; 1:200; Invitrogen, Carlsbad, CA, USA). After this, the membranes were washed twice with TBST, incubated with diluted secondary antibodies (ZB-2301, ZB2305, 1:5000, ZSGB-BIO, Beijing, China) for 1 hour at ambient temperature, and then washed again thrice for 10 min. Next, an enhanced chemiluminescence solution (P0018S, Beyotime, Shanghai, China) was added to develop the protein, and a chemiluminescence imager was used to assess the images. β-actin was used as the internal reference. The relative protein expression of the blots was analyzed using Image J V1.8 software (National Institutes of Health, Bethesda, MD, USA).
Statistical Analysis
All results that conformed to normal distribution are expressed as mean ± standard deviation (SD) and visualized by GraphPad Prism 9.0 (GraphPad Software, San Diego, CA, USA). The SPSS v22.0 (IBM, Armonk, NY, USA) software was used for statistical analysis. One-way analysis of variance (ANOVA) and two-way ANOVA followed by Tukey’s t-tests or two-tail unpaired Student’s t-tests were used to compare the means among groups. P < 0.05 was considered statistically significant.
Results
Establishment of a Rat Model of OA
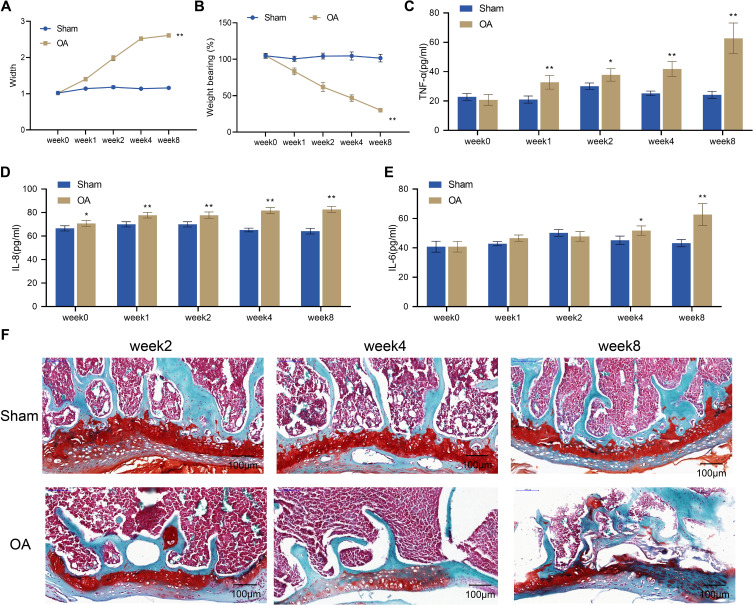
In this study, a rat model of OA was established by surgery. To evaluate the success of the model, we examined knee swelling, pain, inflammatory response, and histopathological changes in the knee joint of OA rats at 2, 4, and 8 weeks after surgery. The examination showed that at week 0, there was no significant difference in knee joint diameter and weight-bearing between the Sham group and the OA group. However, from week 1 to week 8, the OA group showed a gradual increase in knee joint diameter and a significant decrease in weight-bearing compared to the Sham group (P < 0.01, Figure 1A and B). The ELISA assay indicated a significant increase in TNF-α and IL-8 levels in the serum of rats in the OA group compared to the Sham group from week 1 to week 8 (P < 0.01, Figure 1C and D), while IL-6 expression was only significantly increased at week 4 to 8 (P < 0.01, Figure 1E). The results of safranin O staining showed that the articular surface of rats in the Sham group had smooth and intact cartilage with regularly arranged chondrocytes and smooth subchondral trabecular bone. However, rats in the OA group exhibited varying degrees of pathological damage to the articular cartilage. Specifically, at week 2, the articular cartilage surface of rats in the OA group was partially rough with an intact matrix, and the arrangement of chondrocytes in the superficial zone was uneven. At week 4, the articular cartilage surface was rough, with a reduction in the staining intensity of the matrix, and chondrocytes in the middle zone were disorganized and enlarged. By week 8, the knee joint exhibited chondrocyte death, intercellular matrix loss, and cartilage layer thinning or destruction (Figure 1F). Taken together, rat models of OA were successfully induced by surgery, as demonstrated by significant swelling, pain, inflammatory response, and histopathological damage in the knee joints of OA rats.
Figure 1.
Establishment of rat models of OA. (A) Width of the knee joints of rats in the Sham and OA groups; (B) Knee pain assessed by weight-bearing test in the Sham and OA groups; C-E, Serum levels of TNF-α (C), IL-8 (D) and IL-6 (E) measured by ELISA, n = 4; (F) Histopathological changes of the knee joint from rats (n = 2) observed by safranin O staining. Scale bar = 100 μm. Error bars are mean ± s.d. *P <0.05 and **P < 0.01 vs Sham group.
Abbreviations: OA, osteoarthritis; TNF, tumor necrosis factor; IL, interleukin.
Expression of Extracellular Matrix Proteins in Articular Cartilage of Rats with OA
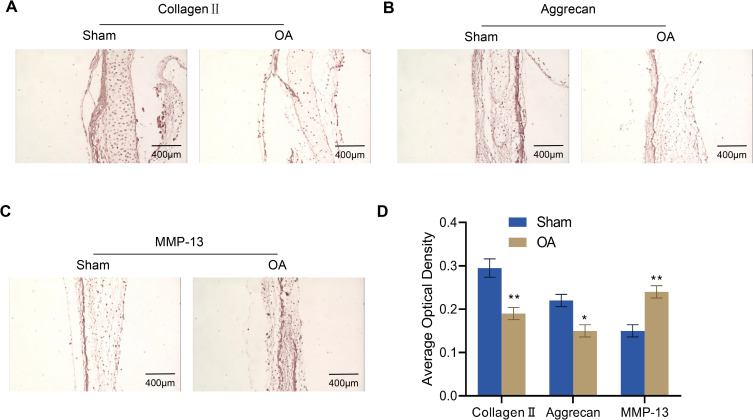
To investigate the changes in the articular cartilage of OA rats, we measured the levels of their ECM proteins (collagen II, aggrecan, and MMP-13) by immunohistochemistry. The outcomes revealed that the expression levels of collagen II and aggrecan in the knee joints of OA rats were much lower than those of the Sham group, while MMP-13 expression was significantly increased (Figure 2A–D).
Figure 2.
Immunohistochemical analysis for collagen II, aggrecan and MMP-13 expression in the articular cartilage of rats (n = 4). (A–D) Expression of collagen II (A), aggrecan (B) and MMP-13 (C) in the articular cartilage of osteoarthritis rats was assessed via immunohistochemistry and quantified by ImageJ software (D). Scale bar = 400 μm. Error bars are mean ± s.d. *P < 0.05; **P < 0.01 vs Sham group.
Abbreviation: MMP, matrix metalloproteinase.
Effect of Knockdown of ATF3 on Symptoms and Pathological Knee Joint Damage in Rats with OA
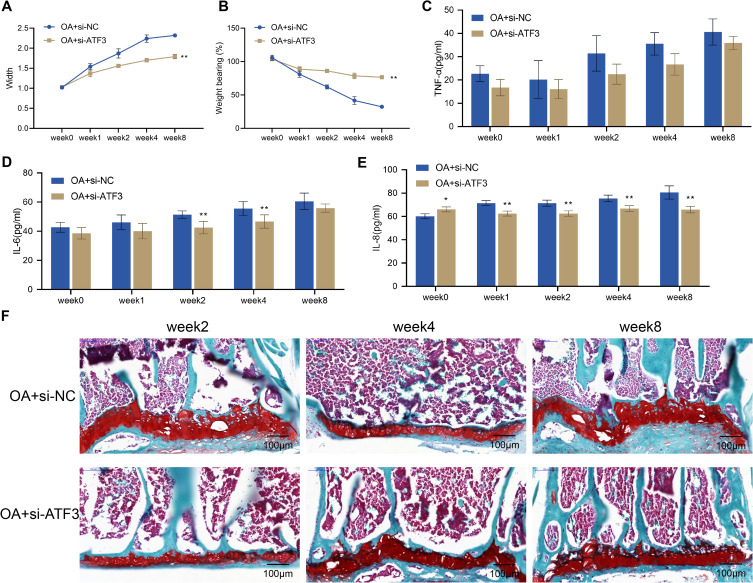
To clarify the role of ATF3 in OA, we examined the effects of ATF3 knockdown on knee joint swelling, pain, inflammation, and histopathological damage in OA rats. Prior to surgery, there was no significant difference in knee joint width or weight-bearing between the OA + si-NC and OA + si-ATF3 groups. However, compared with before surgery, the width and weight-bearing of the knee joint in both groups significantly increased over time from week 1 to week 8 after surgery. Notably, the OA + si-ATF3 group showed a significant decrease in knee joint width and a significant increase in weight-bearing compared to the OA + si-NC group (Figure 3A and B). Moreover, decreased levels of TNF-α, IL-8, and IL-6 in the OA + si-NC group indicated a reduction in inflammation, while safranin O staining revealed necrosis of articular chondrocytes, loss of matrix staining, and thinning or destruction of the cartilage layer in the OA + si-NC group rats (Figure 3C–E). In contrast, the OA + si-ATF3 group exhibited significantly reduced cartilage damage and increased matrix staining intensity (Figure 3F). These results suggested that the knockdown of ATF3 alleviated knee joint swelling, pain, inflammation, and histopathological damage in OA rats’ cartilage.
Figure 3.
Knockdown of ATF3 alleviates symptoms and pathological knee joint damage in rats with OA. (A) Width of the knee joints of rats. (B) Knee pain was assessed by weight-bearing test; (C–E) Serum levels of TNF-α (C), IL-8 (D) and IL-6 (E) were measured by ELISA (n = 4); (F) Histopathological changes of the knee joint from rats (n = 2) were observed by safranin O staining. Scale bar = 100 μm. Error bars are mean ± s.d. *P < 0.05 and **P < 0.01 vs OA+si-NC group.
Effect of Knockdown of ATF3 on Extracellular Matrix Proteins in Articular Cartilage of Rats with OA
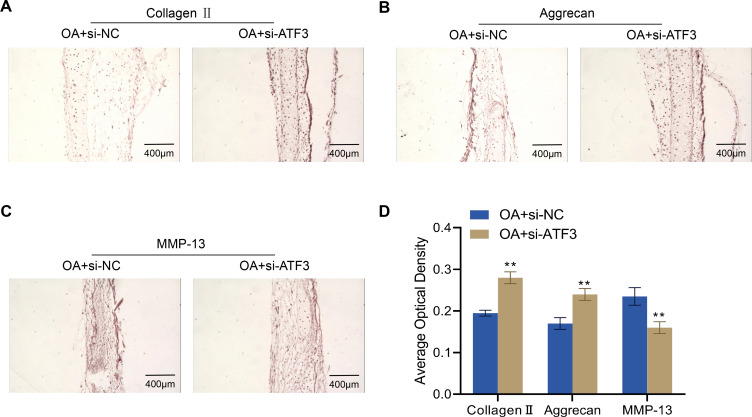
To further investigate the role of ATF3 in the ECM of articular cartilage in OA rats, we examined the expression levels of collagen II, aggrecan, and MMP-13 in the knee joints of OA + si-NC and OA + si-ATF3 group rats. Compared to the OA + si-NC group, the OA + si-ATF3 group exhibited significantly increased expression levels of collagen II and aggrecan, while MMP-13 expression was significantly decreased (Figure 4A–D). These findings suggest that the knockdown of ATF3 may have a protective role by regulating the expression of ECM-related proteins in the articular cartilages of OA rats.
Figure 4.
Effects of ATF3 knockdown on extracellular matrix proteins in the articular cartilage of rats with OA. (A–D), Expression of collagen II (A), aggrecan (B) and MMP-13 (C) in rat knee joints from rats (n=4) detected by immunohistochemistry and quantified by ImageJ software (D). Scale bar = 400 μm. Error bars are mean ± s.d. **P < 0.01 vs OA+si-NC group.
ATF3 Regulated Akt Signaling Pathway in OA
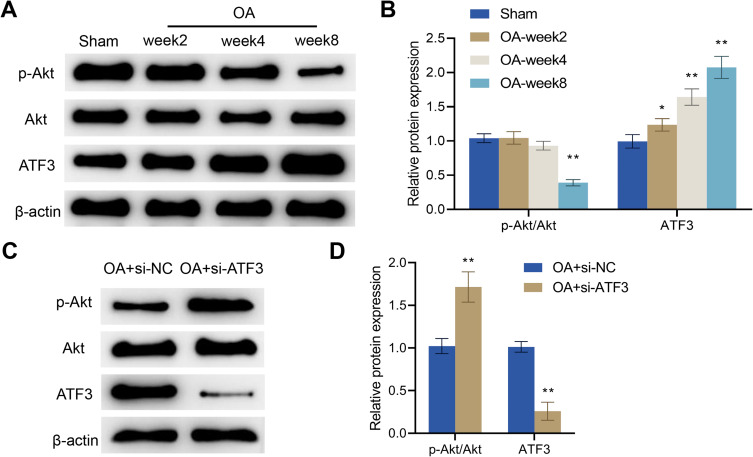
It has been reported that activation of the Akt signaling pathway can promote the anabolism of chondrocytes. Therefore, there is a close correlation between the activity of this pathway and the progression of OA.45 Herein, we examined Akt pathway-related proteins and ATF3 expression in the articular cartilage of rats and found that, compared with the Sham group, p-Akt expression in the OA group was significantly decreased, while the protein expression level of ATF3 was significantly increased. There was no significant difference in Akt expression between the two groups. Moreover, at weeks 2, 4, and 8, the phosphorylation levels of Akt gradually decreased while the expression levels of ATF3 increased in the articular cartilage of OA rats (Figure 5A and B). Overall, in comparison with the Sham group, the Akt signaling pathway was inhibited, while the ATF3 expression was increased with the progression of OA in the OA group.
Figure 5.
ATF3 regulates the Akt signaling pathway in OA rats. (A and B) Western blot was used to detect the protein levels of p-Akt, Akt and ATF3 in the articular cartilage in the Sham and OA groups, Error bars are mean ± s.d. *P < 0.05; **P < 0.01 vs Sham group; (C and D) Western blot was utilized to measure the protein levels of p-Akt, Akt and ATF3 in the articular cartilage in the OA + si-NC and OA + si-ATF3 groups, Error bars are mean ± s.d. **P < 0.01 vs OA + si-NC group, n = 4.
To further elucidate the role of ATF3 in regulating the Akt signaling pathway in OA rats, we examined ATF3 expression and Akt signaling pathway-related proteins in the articular cartilage. We observed significant increases in p-Akt expression and the ratio of p-Akt/Akt, as well as a decline in the protein expression levels of ATF3 after the knockdown of ATF3 in the OA + si-ATF3 group compared to the OA + si-NC group (Figure 5C and D). Consequently, ATF3 regulates the Akt signaling pathway and may play a key role in the development of OA through this pathway.
Effects of Periodic Mechanical Stress on Inflammation, Extracellular Matrix Proteins and the ATF3-Akt Axis in Osteoarthritic Chondrocytes
Several studies have reported that physiological mechanical stimulation (PMS) is beneficial in mimicking in vivo conditions and promoting mitosis and matrix synthesis in chondrocytes.21,22 Here, the chondrocytes were subjected to different degrees of PMS by adjusting the device height to 0, 1, 2, 4 and 8 cm, and then the effects of PMS on IL-1β-induced chondrocyte inflammation, matrix synthesis, and the ATF3-Akt axis were evaluated.
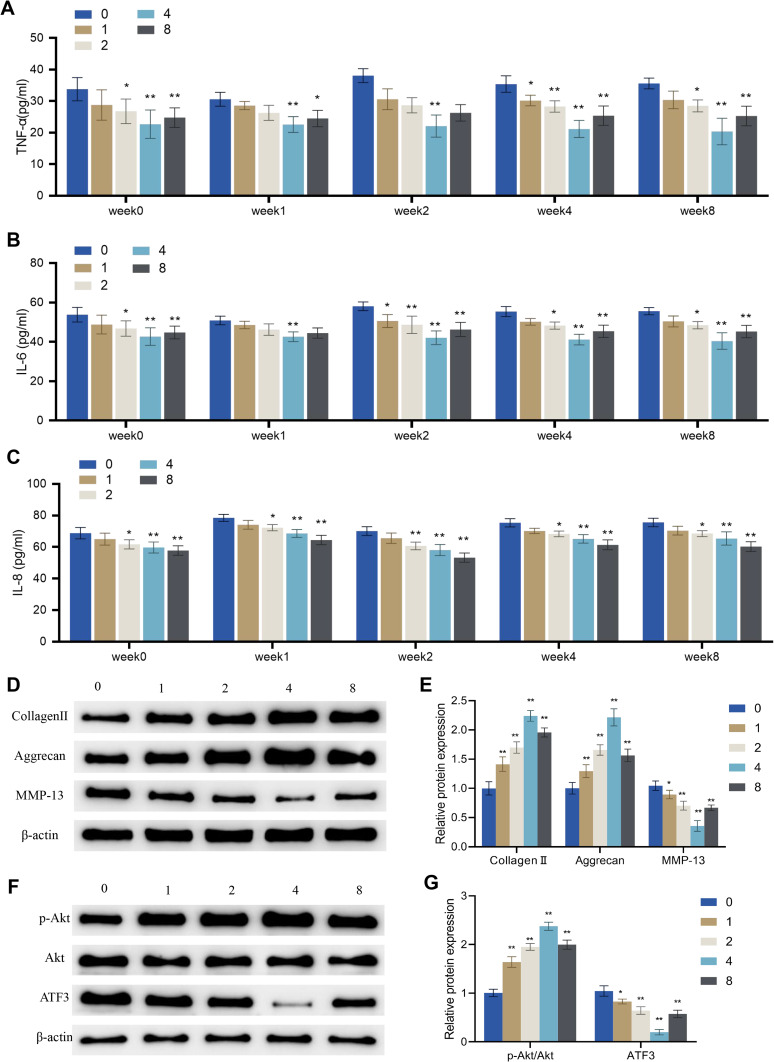
The results indicated that PMS at 2 cm, 4 cm, and 8 cm height inhibited the levels of TNF-α, IL-6, and IL-8 in IL-1β-induced chondrocytes, except for PMS at 2 cm on TNF-α and IL-6 in week 1. The inhibition of TNF-α and IL-6 in OA cells was strongest with PMS at 4 cm height, while the inhibition of IL-8 in OA cells was strongest with PMS at 8 cm height (P < 0.01, Figure 6A–C). Moreover, the expression levels of collagen II and aggrecan in OA cells of groups 1, 2, 4, and 8 at weeks 8 were significantly increased compared to those in group 0, while the expression levels of MMP-13 were significantly decreased. The most significant changes were observed in group 4 (Figure 6D–E). Additionally, compared to group 0, groups 1, 2, 4, and 8 showed marked increases in p-Akt expression and the ratio of p-Akt/Akt, as well as a decrease in the protein level of ATF3. The most significant changes were observed in group 4 (Figure 6F–G).
Figure 6.
Effects of periodic mechanical stress on inflammation, extracellular matrix proteins and ATF3-Akt axis in osteoarthritic chondrocytes. IL-1β-induced chondrocytes were treated without (group 0) or with PMS under a device height of 1 cm (group 1), 2 cm (group 2), 4 cm (group 4), and 8 cm (group 8). (A–C) Levels of TNF-α (A), IL-6 (B) and IL-8 (C) in the supernatant of OA cells measured by ELISA; (D and E) Protein expression levels of extracellular matrix proteins (collagen II, aggrecan, and MMP-13) in OA cells detected by Western blot; (F and G) Protein expression levels of p-Akt, Akt, and ATF3 in OA cells detected by Western blot. Error bars are mean ± s.d.*P < 0.05; **P < 0.01 vs Group 0.
Taken together, PMS with a device height of 4 cm exhibited the best inhibitory effect on inflammatory factors and the best-promoting effect on ECM protein expression and Akt signaling pathway activation in OA cells.
Up-Regulation of ATF3 Reverses the Effects of Periodic Mechanical Stress on Apoptosis, Inflammation and Extracellular Matrix Proteins Expression in Osteoarthritic Chondrocytes
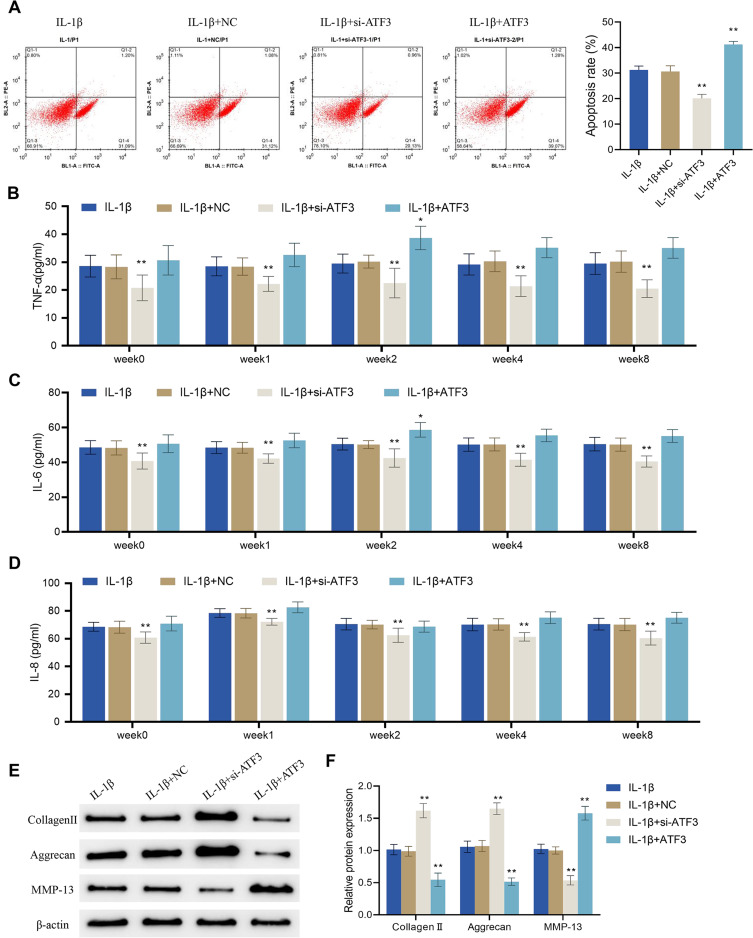
Although the application of PMS with a device height of 4 cm significantly reduced the protein expression of ATF3, it remained unclear whether its effects on osteoarthritic chondrocytes were achieved by acting on ATF3. Therefore, flow cytometry was used to assess the cell apoptotic rate in different treatment groups. The results showed that the cell apoptotic rate was significantly decreased in the IL-1β + si-ATF3 group compared to the IL-1β + NC group, while it was increased in the IL-1β + ATF3 group (which overexpressed ATF3) compared to the IL-1β + NC group (P < 0.01, Figure 7A). The levels of TNF-α, IL-8, and IL-6 in the supernatant of IL-1β-treated cells were markedly decreased after the knockdown of ATF3. Both TNF-α and IL-6 levels were significantly higher in the IL-1β + ATF3 group than in the corresponding IL-1β + NC group only at week 2, and there were no significant differences in the other groups (Figure 7B–D). Moreover, compared to the IL-1β + NC group, the expression levels of collagen II and aggrecan were significantly increased, and the expression levels of MMP-13 were notably decreased in the IL-1β + si-ATF3 group; nevertheless, opposite changes could be observed in the IL-1β + ATF3 group cells (Figure 7E and F). Collectively, the up-regulation of ATF3 reversed PMS-caused inhibition of apoptosis, inflammatory response and ECM protein expression in osteoarthritic chondrocytes.
Figure 7.
Up-regulation of ATF3 reverses the effects of periodic mechanical stress on apoptosis, inflammation and extracellular matrix proteins expression in osteoarthritic chondrocytes. (A and B) Apoptotic rate of OA cells was detected by flow cytometry; (B–D) Levels of TNF-α (B), IL-6 (C) and IL-8 (D) in OA cells were detected by ELISA; (E and F) Protein expression levels of collagen II, aggrecan and MMP-13 in OA cells were detected by Western blot. Error bars are mean ± s.d. *P < 0.05 and **P < 0.01 vs IL-1β + NC group.
Up-Regulation of ATF3 Reverses the Regulation of ATF3-Akt Axis by Periodic Mechanical Stress in Osteoarthritic Chondrocytes
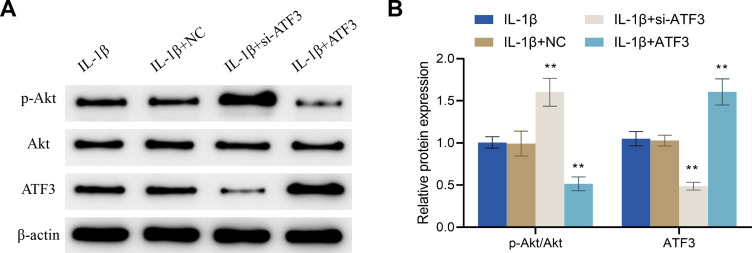
Further experimental results confirmed that, compared to the IL-1β + NC group, the IL-1β + si-ATF3 group showed a significant increase in the p-Akt expression and the p-Akt/Akt ratio, and a decrease in the protein expression level of ATF3, while the IL-1β + ATF3 group exhibited opposite changes (Figure 8A and B). All in all, up-regulation of ATF3 reversed the PMS-induced inhibition of ATF3 and promoted Akt phosphorylation in osteoarthritic chondrocytes.
Figure 8.
Up-regulation of ATF3 reverses the regulation of the ATF3-Akt axis by periodic mechanical stress in osteoarthritic chondrocytes. (A and B) Protein expression levels of p-Akt, ATF3 and Akt in osteoarthritic chondrocytes were detected by Western blot. Error bars are mean ± s.d. **P < 0.01 vs IL-1β + NC group.
Discussion
OA, characterized by the degradation and destruction of articular cartilage, is a progressive joint disease that affects millions of people worldwide.46 In this study, OA was successfully induced in rats by surgically anterior cruciate ligament transection and destabilizing medial meniscectomy, lowering collagen II and aggrecan expression levels and increasing MMP-13 expression in the knee joints of OA rats. The changes in the articular cartilage of OA rats were investigated by measuring the levels of ECM proteins (collagen II, aggrecan, and MMP-13) by immunohistochemistry. As major components of the ECM in articular cartilage, collagen II and aggrecan are closely associated with articular cartilage tissues’ structural integrity and mechanical properties. For instance, decreased expression levels of collagen II and aggrecan indicated reduced structural integrity and mechanical properties of articular cartilage tissue.47 Comparatively, MMP-13 is an enzyme that degrades collagen and other ECM proteins, leading to further cartilage breakdown and joint damage. These changes ultimately result in joint pain, stiffness, and loss of function in OA patients.
Previous studies suggested that ATF3 exerts its functions in various cancers by inhibiting Akt-related signaling pathways.48,49 Shi et al reported that the knockdown of ATF3 in human bronchial epithelial cells inhibited the malignant transformation of cells by suppressing the overproduction of IL-6, IL-8, and TNFα and promoting the phosphorylation of Akt.50 Other studies have shown that ATF3 plays an important regulatory role in the progression of osteoarthritis, and inhibiting ATF3 related signaling pathways can alleviate the progression of osteoarthritis.35 However, it is unclear whether PMS can regulate articular cartilage homeostasis and osteoarthritis progression through ATF3. Herein, we found that knockdown of ATF3 exerted protective functions in regulating the ECM-related proteins in the articular cartilages of the OA rat models via the Akt signaling pathway (ie, there was a significant decrease in p-Akt expression and an increase in ATF3 expression in the OA group compared with the Sham group), and PMS was found to effectively inhibit the development of OA through down-regulation of ATF3 expression and activation of the Akt signaling pathway, suggesting that targeting ATF3 could be a promising strategy for the treatment of OA, and PMS is an effective strategy for regulating ATF3 In OA. To further confirm the significance of ATF3 as a potential therapeutic target for OA, further studies can be conducted to evaluate the effects of pharmacological agents or other interventions that specifically target ATF3 or the Akt signaling pathway in preclinical and clinical settings. Besides, more experiments are needed to confirm the safety, efficacy, and optimal dosing of these interventions for OA treatment and determine whether the findings obtained from this study are also applicable to other OA models or to different stages of OA.
During joint motion, the articular surface is subject to mechanical stresses such as dynamic compression, stretching and shear forces. In addition, daily activities such as running and walking can rapidly increase stress within the joint cavity from zero to a certain intensity and then quickly return to zero.51,52 It is reported that such periodic mechanical changes can positively affect the lubrication of the cartilage surface and promote the absorption of nutrients by chondrocytes.53 IL-1β, TNF-α, and IL-6 are the most important inflammatory mediators in the pathogenesis of OA. They promote the progression of OA by activating different downstream signaling pathways, such as the mitogen-activated protein kinase (MAPK), nuclear factor-kappa B (NF-κB), activator protein-1 (AP-1), IL-6, or CCAAT/enhancer-binding protein (C/EBP) signaling pathways.54 In this present study, the application of PMS could significantly reduce the levels of TNF-α, IL-8, and IL-6 and increase the expression levels of collagen II and aggrecan, and decrease the expression levels of MMP-13 in OA cells, this indicates that PMS plays an important regulatory role in the anabolism and catabolism of articular chondrocytes, as well as in the inflammatory response of articular cartilage in osteoarthritis. However, the up-regulation of ATF3 could reverse the PMS-induced inhibition of ATF3 and promote Akt phosphorylation in osteoarthritic chondrocytes, based on previous research,49 it is shown that PSM regulates the anabolism and catabolism of articular chondrocytes, as well as the inflammatory response of osteoarthritis articular cartilage, through ATF3/AKT axis. This also indicates that ATF is a potential clinical treatment target for OA. Although no previous studies have investigated the regulation of inflammatory factors in OA by PMS, a study by Hortobagyi et al suggested that in vitro mechanical vibration downregulated pro-inflammatory signaling in human vocal fold fibroblasts.55 Several studies have shown that PMS can not only promote ECM expression and migration of rat nucleus pulposus cells26 but also promote matrix synthesis in chondrocytes.22
The findings that PMS of a device height of 4 cm improved inflammation, ECM protein expression and was associated with the activation of the Akt signaling pathway in OA cells could have promising clinical applications. These findings urged the need for further studies to optimize the PMS protocol in terms of frequency, duration, and intensity, as well as to establish the therapy’s safety and efficacy in human subjects before translation into clinical practice. One approach to improving these results could be to combine PMS with other existing OA therapies, such as NSAIDs, physical therapy, and weight loss. Additionally, further studies could investigate the long-term effects of PMS on OA progression and its ability to delay or prevent the need for surgical interventions like joint replacement. If deemed safe and effective, a similar PMS device could potentially be used in clinical practice to provide mechanical stimulation to the affected joint in OA patients, with device height adjusted to provide optimal results for each individual. Overall, the clinical significance of this finding is that PMS may provide a non-invasive, cost-effective, and low-risk therapy option for OA patients, especially those unsuitable for surgery or who wish to avoid it. However, further research is necessary to determine the appropriate protocol and duration of treatment to achieve optimal outcomes in humans.
Despite the promising results reported in this study, there were some limitations that should be further addressed. For instance, we only explored the role of PMS through the Akt signaling pathway and did not investigate other potential molecular transduction pathways. Meanwhile, the specific mechanism of PMS regulating the ATF3 and Akt signaling pathways remains unclear. In addition, the hypothesis of the relationship between ATF3 and PMS is weak, which is a defect in our experimental design. Therefore, further experimental verification is necessary to determine the efficacy and mechanism of PMS in OA treatment and the relationship between ATF3 and PMS. Moreover, the sample size of animals under each detection index is not sufficient, and there are differences among the comparison groups, but there may be accidental errors and individual differences. Hence, larger sample size will be enrolled in the future to improve the scientific rigor of our research results.
Conclusion
In summary, PMS can inhibit the inflammatory response and apoptosis of osteoarthritic chondrocytes and promote ECM synthesis by decreasing ATF3 expression and activating the Akt signaling pathway. This study can provided a reference to develop novel strategies for the clinical treatment of OA.
Funding Statement
This study was supported by the National Natural Science Foundation of China (No. 81902241).
Data Sharing Statement
The data used to support the findings of this study are available from the corresponding author upon request.
Ethical Statement
This study was approved by the Animal Ethics Committee of Soochow University (201708A105) and and carried out according to the National Institutes of Health protocol to laboratory animal care and use.
Disclosure
The authors declare that they have no competing interests.
References
- 1.Tang X, Wang S, Zhan S, et al. The prevalence of symptomatic knee osteoarthritis in China: results from the China Health and Retirement Longitudinal Study. Arthritis Rheumatol. 2016;68(3):648–653. doi: 10.1002/art.39465 [DOI] [PubMed] [Google Scholar]
- 2.Global Burden of Disease Study C. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386(9995):743–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jordan JM, Helmick CG, Renner JB, et al. Prevalence of knee symptoms and radiographic and symptomatic knee osteoarthritis in African Americans and Caucasians: the Johnston County Osteoarthritis Project. J Rheumatol. 2007;34(1):172–180. [PubMed] [Google Scholar]
- 4.Katz JN, Arant KR, Loeser RF. Diagnosis and treatment of hip and knee osteoarthritis: a review. JAMA. 2021;325(6):568–578. doi: 10.1001/jama.2020.22171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lespasio MJ, Piuzzi NS, Husni ME, Muschler GF, Guarino A, Mont MA. Knee osteoarthritis: a primer. Perm J. 2017;21(4):16–183. doi: 10.7812/TPP/16-183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abramson SB, Attur M, Yazici Y. Prospects for disease modification in osteoarthritis. Nat Clin Pract Rheumatol. 2006;2(6):304–312. doi: 10.1038/ncprheum0193 [DOI] [PubMed] [Google Scholar]
- 7.Poole AR. Osteoarthritis as a whole joint disease. HSS J. 2012;8(1):4–6. doi: 10.1007/s11420-011-9248-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Favero M, El-Hadi H, Belluzzi E, et al. Infrapatellar fat pad features in osteoarthritis: a histopathological and molecular study. Rheumatology. 2017;56(10):1784–1793. doi: 10.1093/rheumatology/kex287 [DOI] [PubMed] [Google Scholar]
- 9.Glyn-Jones S, Palmer AJ, Agricola R, et al. Osteoarthritis. Lancet. 2015;386(9991):376–387. doi: 10.1016/S0140-6736(14)60802-3 [DOI] [PubMed] [Google Scholar]
- 10.Sharma L, Kapoor D, Issa S. Epidemiology of osteoarthritis: an update. Curr Opin Rheumatol. 2006;18(2):147–156. doi: 10.1097/01.bor.0000209426.84775.f8 [DOI] [PubMed] [Google Scholar]
- 11.Jiang W, Chen H, Lin Y, et al. Mechanical stress abnormalities promote chondrocyte senescence - The pathogenesis of knee osteoarthritis. Biomed Pharmacother. 2023;167:115552. doi: 10.1016/j.biopha.2023.115552 [DOI] [PubMed] [Google Scholar]
- 12.Fang T, Zhou X, Jin M, Nie J, Li X. Molecular mechanisms of mechanical load-induced osteoarthritis. Int Orthop. 2021;45(5):1125–1136. doi: 10.1007/s00264-021-04938-1 [DOI] [PubMed] [Google Scholar]
- 13.Zeng C, Wei J, Persson MSM, et al. Relative efficacy and safety of topical non-steroidal anti-inflammatory drugs for osteoarthritis: a systematic review and network meta-analysis of randomised controlled trials and observational studies. Br J Sports Med. 2018;52(10):642–650. doi: 10.1136/bjsports-2017-098043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ding C. Do NSAIDs affect the progression of osteoarthritis? Inflammation. 2002;26(3):139–142. doi: 10.1023/A:1015504632021 [DOI] [PubMed] [Google Scholar]
- 15.Martel-Pelletier J, Boileau C, Pelletier JP, Roughley PJ. Cartilage in normal and osteoarthritis conditions. Best Pract Res Clin Rheumatol. 2008;22(2):351–384. doi: 10.1016/j.berh.2008.02.001 [DOI] [PubMed] [Google Scholar]
- 16.Lian LP, Xi XY. Long non-coding RNA XIST protects chondrocytes ATDC5 and CHON-001 from IL-1beta-induced injury via regulating miR-653-5p/SIRT1 axis. J Biol Regul Homeost Agents. 2020;34(2):379–391. doi: 10.23812/19-549-A-65 [DOI] [PubMed] [Google Scholar]
- 17.Uzieliene I, Bironaite D, Bernotas P, Sobolev A, Bernotiene E. Mechanotransducive biomimetic systems for chondrogenic differentiation in vitro. Int J Mol Sci. 2021;22(18). doi: 10.3390/ijms22189690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kurz B, Lemke AK, Fay J, Pufe T, Grodzinsky AJ, Schünke M. Pathomechanisms of cartilage destruction by mechanical injury. Ann Anat. 2005;187(5–6):473–485. doi: 10.1016/j.aanat.2005.07.003 [DOI] [PubMed] [Google Scholar]
- 19.Kiviranta I, Tammi M, Jurvelin J, Säämänen AM, Helminen HJ. Moderate running exercise augments glycosaminoglycans and thickness of articular cartilage in the knee joint of young beagle dogs. J Orthop Res. 1988;6(2):188–195. doi: 10.1002/jor.1100060205 [DOI] [PubMed] [Google Scholar]
- 20.Fitzgerald JB, Jin M, Grodzinsky AJ. Shear and compression differentially regulate clusters of functionally related temporal transcription patterns in cartilage tissue. J Biol Chem. 2006;281(34):24095–24103. doi: 10.1074/jbc.M510858200 [DOI] [PubMed] [Google Scholar]
- 21.Liang W, Zhu C, Liu F, et al. Integrin beta1 gene therapy enhances in vitro creation of tissue-engineered cartilage under periodic mechanical stress. Cell Physiol Biochem. 2015;37(4):1301–1314. doi: 10.1159/000430253 [DOI] [PubMed] [Google Scholar]
- 22.Liang W, Li Z, Wang Z, et al. Periodic mechanical stress INDUCES chondrocyte proliferation and matrix synthesis via CaMKII-mediated Pyk2 signaling. Cell Physiol Biochem. 2017;42(1):383–396. doi: 10.1159/000477483 [DOI] [PubMed] [Google Scholar]
- 23.Ren K, Tang J, Jiang X, et al. Periodic mechanical stress stimulates Cav-1-dependent IGF-1R mitogenic signals in rat chondrocytes through ERK1/2. Cell Physiol Biochem. 2018;48(4):1652–1663. doi: 10.1159/000492288 [DOI] [PubMed] [Google Scholar]
- 24.Ren K, Tang J, Jiang X, et al. Periodic mechanical stress stimulates GIT1-dependent mitogenic signals in rat chondrocytes through ERK1/2 activity. Cell Physiol Biochem. 2018;50(3):1015–1028. doi: 10.1159/000494513 [DOI] [PubMed] [Google Scholar]
- 25.van Valburg AA, van Roermund PM, Lammens J, et al. Can Ilizarov joint distraction delay the need for an arthrodesis of the ankle? A preliminary report. J Bone Joint Surg Br. 1995;77(5):720–725. doi: 10.1302/0301-620X.77B5.7559696 [DOI] [PubMed] [Google Scholar]
- 26.Gao G, Li H, Huang Y, et al. Periodic mechanical stress induces extracellular matrix expression and migration of rat nucleus pulposus cells through Src-GIT1-ERK1/2 signaling pathway. Cell Physiol Biochem. 2018;50(4):1510–1521. doi: 10.1159/000494650 [DOI] [PubMed] [Google Scholar]
- 27.Hai T, Wolfgang CD, Marsee DK, Allen AE, Sivaprasad U. ATF3 and stress responses. Gene Expr. 1999;7(4–6):321–335. [PMC free article] [PubMed] [Google Scholar]
- 28.Boespflug ND, Kumar S, McAlees JW, et al. ATF3 is a novel regulator of mouse neutrophil migration. Blood. 2014;123(13):2084–2093. doi: 10.1182/blood-2013-06-510909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou H, Guo H, Zong J, et al. ATF3 regulates multiple targets and may play a dual role in cardiac hypertrophy and injury. Int J Cardiol. 2014;174(3):838–839. doi: 10.1016/j.ijcard.2014.04.160 [DOI] [PubMed] [Google Scholar]
- 30.Wu J, Huang Y, Zhou X, et al. ATF3 and its emerging role in atherosclerosis: a narrative review. Cardiovasc Diagn Ther. 2022;12(6):926–942. doi: 10.21037/cdt-22-206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fu D, Wang C, Yu L, Yu R. Induction of ferroptosis by ATF3 elevation alleviates cisplatin resistance in gastric cancer by restraining Nrf2/Keap1/xCT signaling. Cell Mol Biol Lett. 2021;26(1):26. doi: 10.1186/s11658-021-00271-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Di Marcantonio D, Martinez E, Kanefsky JS, et al. ATF3 coordinates serine and nucleotide metabolism to drive cell cycle progression in acute myeloid leukemia. Mol Cell. 2021;81(13):2752–2764 e2756. doi: 10.3389/fcell.2021.618987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huo S, Wang Q, Shi W, et al. ATF3/SPI1/SLC31A1 signaling promotes cuproptosis induced by advanced glycosylation end products in diabetic myocardial injury. Int J Mol Sci. 2023;24(2):1667. doi: 10.3390/ijms24021667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu X, Qiu F, Jin X, Zhou J, Zang W. ATF3: a novel biomarker for the diagnosis of acute kidney injury after cardiac surgery. Ann Transl Med. 2021;9(22):1655. doi: 10.21037/atm-21-5231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iezaki T, Ozaki K, Fukasawa K, et al. ATF3 deficiency in chondrocytes alleviates osteoarthritis development. J Pathol. 2016;239(4):426–437. doi: 10.1002/path.4739 [DOI] [PubMed] [Google Scholar]
- 36.Li X, Li Y, Yang X, et al. PR11-364P22.2/ATF3 protein interaction mediates IL-1beta-induced catabolic effects in cartilage tissue and chondrocytes. J Cell Mol Med. 2021;25(13):6188–6202. doi: 10.1111/jcmm.16561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Song F, Wang Y, Jiang D, et al. Cyclic compressive stress regulates apoptosis in rat osteoblasts: involvement of PI3K/Akt and JNK MAPK signaling pathways. PLoS One. 2016;11(11):e0165845. doi: 10.1371/journal.pone.0165845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shen L, Zhou K, Liu H, et al. Prediction of mechanosensitive genes in vascular endothelial cells under high wall shear stress. Front Genet. 2021;12:796812. doi: 10.3389/fgene.2021.796812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chang HW, Sudirman S, Yen YW, Mao CF, Ong AD, Kong ZL. Blue Mussel (Mytilus edulis) water extract ameliorates inflammatory responses and oxidative stress on osteoarthritis in obese rats. J Pain Res. 2020;13:1109–1119. doi: 10.2147/JPR.S244372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang BW, Jiang Y, Yao ZL, Chen PS, Yu B, Wang SN. Aucubin protects chondrocytes against il-1beta-induced apoptosis in vitro and inhibits osteoarthritis in mice model. Drug Des Devel Ther. 2019;13:3529–3538. doi: 10.2147/DDDT.S210220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fei J, Liang B, Jiang C, Ni H, Wang L. Luteolin inhibits IL-1beta-induced inflammation in rat chondrocytes and attenuates osteoarthritis progression in a rat model. Biomed Pharmacother. 2019;109:1586–1592. doi: 10.1016/j.biopha.2018.09.161 [DOI] [PubMed] [Google Scholar]
- 42.Yu D, Liu F, Liu M, et al. The inhibition of subchondral bone lesions significantly reversed the weight-bearing deficit and the overexpression of CGRP in DRG neurons, GFAP and Iba-1 in the spinal dorsal horn in the monosodium iodoacetate induced model of osteoarthritis pain. PLoS One. 2013;8(10):e77824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang Y, Wang L, Jia XX, Lin XX, Zhang WX. Vitamin D alleviates airway remodeling in asthma by down-regulating the activity of Wnt/beta-catenin signaling pathway. Int Immunopharmacol. 2019;68:88–94. doi: 10.1016/j.intimp.2018.12.061 [DOI] [PubMed] [Google Scholar]
- 44.Gu XD, Wei L, Li PC, et al. Adenovirus-mediated transduction with Histone Deacetylase 4 ameliorates disease progression in an osteoarthritis rat model. Int Immunopharmacol. 2019;75:105752. doi: 10.1016/j.intimp.2019.105752 [DOI] [PubMed] [Google Scholar]
- 45.Li X, Ellman M, Muddasani P, et al. Prostaglandin E2 and its cognate EP receptors control human adult articular cartilage homeostasis and are linked to the pathophysiology of osteoarthritis. Arthritis Rheum. 2009;60(2):513–523. doi: 10.1002/art.24258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abramoff B, Caldera FE. Osteoarthritis: pathology, Diagnosis, and Treatment Options. Med Clin North Am. 2020;104(2):293–311. doi: 10.1016/j.mcna.2019.10.007 [DOI] [PubMed] [Google Scholar]
- 47.Ouyang Z, Dong L, Yao F, et al. Cartilage-related collagens in osteoarthritis and rheumatoid arthritis: from pathogenesis to therapeutics. Int J Mol Sci. 2023;24(12):9841. doi: 10.3390/ijms24129841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gao S, Gao L, Wang S, et al. ATF3 suppresses growth and metastasis of clear cell renal cell carcinoma by deactivating EGFR/AKT/GSK3beta/beta-catenin signaling pathway. Front Cell Dev Biol. 2021;9:618987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zu T, Wang D, Xu S, et al. ATF-3 expression inhibits melanoma growth by downregulating ERK and AKT pathways. Lab Invest. 2021;101(5):636–647. doi: 10.1038/s41374-020-00516-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shi Q, Hu B, Yang C, et al. ATF3 inhibits arsenic-induced malignant transformation of human bronchial epithelial cells by attenuating inflammation. Toxicology. 2021;460:152890. doi: 10.1016/j.tox.2021.152890 [DOI] [PubMed] [Google Scholar]
- 51.Jess R, Ling T, Xiong Y, Wright CJ, Zhao F. Mechanical environment for in vitro cartilage tissue engineering assisted by in silico models. Biomater Transl. 2023;4(1):18–26. doi: 10.12336/biomatertransl.2023.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Logerstedt DS, Ebert JR, MacLeod TD, Heiderscheit BC, Gabbett TJ, Eckenrode BJ. Effects of and Response to Mechanical Loading on the Knee. Sports Med. 2022;52(2):201–235. doi: 10.1007/s40279-021-01579-7 [DOI] [PubMed] [Google Scholar]
- 53.Fu S, Thompson CL, Ali A, et al. Mechanical loading inhibits cartilage inflammatory signalling via an HDAC6 and IFT-dependent mechanism regulating primary cilia elongation. Osteoarthritis Cartilage. 2019;27(7):1064–1074. doi: 10.1016/j.joca.2019.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Molnar V, Matisic V, Kodvanj I, et al. Cytokines and chemokines involved in osteoarthritis pathogenesis. Int J Mol Sci. 2021;22(17):9208. doi: 10.3390/ijms22179208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hortobagyi D, Grossmann T, Tschernitz M, et al. In vitro mechanical vibration down-regulates pro-inflammatory and pro-fibrotic signaling in human vocal fold fibroblasts. PLoS One. 2020;15(11):e0241901. doi: 10.1371/journal.pone.0241901 [DOI] [PMC free article] [PubMed] [Google Scholar]