Abstract
Study Design
A systematic review and meta-analysis.
Objective
Treatment of traumatic optic neuropathy (TON) has been a subject of debate for many decades due to the scarcity of evidence-based treatment protocols. This review compares surgical decompression (SD) and steroid therapy (ST) as treatment approaches in TON patients.
Methods
A PRISMA-guided systematic review using PubMed, Embase, Ovid and Scopus databases was performed till the last search date of July 31st 2021. The outcome of interest was an improvement in visual acuity. A meta-analysis of the odds ratio was performed using a random-effect model and sub-group analysis based upon criteria for assessment of improvement in visual acuity.
Results
Sixteen studies (including 1046 patients) were included in the review. The review could identify 590 patients treated with SD and 456 treated with ST. In addition, there was a second cohort of patients presenting with NLP (no light perception). A meta-analysis with a sub-group analysis revealed that there was statistically no significant difference between the two treatment approaches in terms of improvement in VA.
Conclusions
There is no difference in treatment results of SD or ST for TON. Several treatment protocols and different criteria for assessing visual acuity led to difficulty in generating evidence for selecting the correct treatment approach.
Keywords: traumatic optic neuropathy, surgical decompression, blindness , optic nerve injury , anterior cranial fossa
Introduction
Traumatic optic neuropathy (TON) has been historically documented as early as 500 BC by Hippocrates, who defined TON as the decreased vision from injury to the forehead region. 1 Later in 1845, Anton Nuhn described it as a lesion of the optic nerve resulting in post-traumatic amaurosis. 2 Another definition is impact injury to the optic nerve that results in complete or partial loss of function. 3 The incidence of vision loss after facial trauma is 2–5%. 2 Incidence of TON in head injury patients is less than 5% 4 or up to 6%, as reported in the case series of ZMC complex fractures. 5 The rare incidence of optic nerve injury makes it difficult to plan any randomised controlled trial (RCT) for its management. The treatment options of TON remain controversial and have evolved continuously with technology. However, the generally available steroids used in treatment have remained the same. The developing treatment protocols need regular updates regarding the recently reported results and outcome improvements. There is also a need to understand selection bias in treatment methods.
The clinical approach regarding the following is controversial with insufficient evidence.
(1) Type and indication of intervention.
(2) Timing of intervention. Assessment of post-treatment status.
(3) RCT trials available and their strength.
This review addresses the following research question – ‘which treatment approach is best for traumatic optic neuropathy: Surgical decompression or steroid therapy’.
Materials and Methods
The systematic review followed the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015 statement. 6 The search protocol was registered in Prospero (registration number: CRD42020202839).
Literature Search Strategy
A comprehensive search of PubMed, Embase, Ovid and Scopus databases was conducted without any time restriction till last search date – July 31st 2021. The search strategy is mentioned in Table 1.
Table 1.
Search Strategy Used in a Systematic Review.
| Database | Search strategy | Number of results |
|---|---|---|
| PubMed | (((‘Optic nerve injuries’ [MeSH Terms] OR ((‘optic’ [All Fields] AND ‘nerve’ [All Fields]) AND ‘injuries’ [All Fields])) OR ‘optic nerve injuries’ [All Fields]) | 3674 |
| Embase | ‘traumatic optic neuropathy’ [All Fields] | 1441 |
| Scopus | TITLE-ABS-KEY-AUTH (traumatic AND optic AND neuropathy) | 907 |
| Ovid | traumatic optic neuropathy.mp. [mp = ti, ab, tx, ct] | 1080 |
Keywords used for the search were as follows: 1. optic nerve injuries, 2. optic, 3. nerve, 4. injuries, 5. traumatic, 6. optic, 7. neuropathy and 8. traumatic optic neuropathy.
MeSH terms (PubMed) and Explosion (Embase) were used as tools to expand available articles for inclusion with the restriction of articles in the English language and humans as subjects of study.
Study Eligibility
Inclusion criteria
Following PICOS criteria were followed for inclusion criteria. Population(P): Patients with traumatic optic neuropathy due to craniomaxillofacial injuries. Participants/population: All patients with post-traumatic optic neuropathy associated with craniomaxillofacial injuries.
Intervention(I): All cases of post-traumatic optic neuropathy are managed surgically for optic nerve decompression.
Comparator(s)/control: All cases of post-traumatic optic neuropathy are managed non-surgically using steroid therapy.
Primary outcome (O): Improvement in visual acuity.
Studies included (S): Randomised control/clinical trials, controlled clinical trials, prospective cohort studies and case-controlled studies comparing surgical decompression to steroid therapy. Only English language papers were included in the review.
Exclusion criteria
Following exclusion criteria were used: 1. Review articles, 2. meta-analyses, 3. letters, 4. case reports, 5. opinion pieces, 6. case series of fewer than 10 cases and 7. studies comparing surgical decompression to only bed rest, head elevation and close observation without steroid therapy.
Data Extraction Process
The articles identified by search strategy were screened using the exclusion and inclusion criteria. These articles were used for data collection. The data were entered in a pre-piloted Excel sheet. All the papers included were reviewed by the first two authors (SP and GR); any disagreements were solved by the third author (AC). Fourth (AD) and fifth authors (BR) were involved in proofreading. Following data were extracted from the included studies: year, authors, country of origin, journal name, number of patients included, number of patients treated and improved with surgical optic nerve decompression and number of patients treated and improved with steroid therapy, the regimen of steroid therapy used and criteria used for assessment of improvement in visual acuity (VA).
Critical Appraisal of Included Studies
All non-randomised studies were assessed using the MINORS score, 7 where a maximum of 16 points were awarded for non-comparative studies, and 24 points were awarded for comparative studies and RCT if any were assessed by the Jadad scale. 8
Summary Measures and Synthesis of Results
The data were extracted, and the continuous variables were expressed as mean +/− standard deviation and the dichotomous variables were recorded as events of improvement in visual acuity. The extracted data were then subjected to meta-analysis. RevMan software 5 was used for the analysis. The heterogeneity of the studies was assessed with the Cochrane q and I2 values. If the I2 values were more than 50%, it suggested heterogeneity in the studies and a random-effects model was planned. If the I2 were less than 50%, then the fixed-effects model was planned. The bias among the studies was assessed using the funnel plot. If heterogeneity were found to be more than 50%, a sub-group analysis would be performed to assess the reasons.
The number of patients treated and improved by surgical decompression and steroid therapy was recorded. A meta-analysis (odds ratio) was conducted with the I2 test for heterogeneity to compare the two treatment protocols. Egger’s funnel plot was drawn to test publication bias. Any improvement in the vision status was taken as a positive response.
Results
Result of Search Strategy (Study Selection)
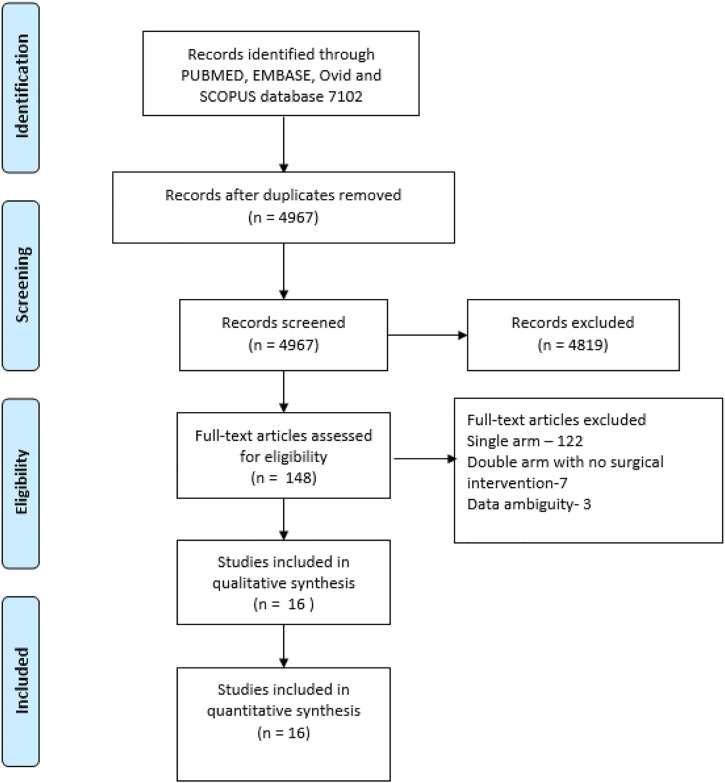
A total of 7102 abstracts were identified in the PubMed Embase, Ovid and Scopus databases (Table 1). After screening for duplicate articles, 4967 abstracts were shortlisted. Abstracts were screened based on inclusion criteria, and 4819 abstracts were excluded and remaining 148 articles were selected for full-text reading. Screening of full-text articles revealed 19 papers that were suitable for data extraction. During data extraction, three articles were excluded for ambiguity in data. A total of 16 articles were included (Table 2) in the master chart for data extraction (Figure 1 depicts the PRISMA flow chart).
Table 2.
Characteristics of Studies Included in the Systematic Review.
| Year | Author | Study design | Treated SD | Imp-SD | Treated-ST | Imp-ST | Tech for SD | VA | ST used | MS | JS | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1989 | Simmon et al. 9 | RS | 4 | 3 | 4 | 1 | TE | SC | ND | 9/16 | NA |
| 2 | 1992 | *Mauriello et al. 10 | RS | 7 | 4 | 16 | 9 | TCon | SC | ND | 11/16 | NA |
| 3 | 1993 | Mahapatra et al. 4 | NRPS | 45 | 17 | 50 | 25 | TE/TC | VEP | DMS | 13/16 | NA |
| 4 | 1996 | *P.I. Chou et al. 1 | RS | 25 | 15 | 23 | 13 | TE/TC | SC | PRS | 21/24 | NA |
| 5 | 1999 | *Levine et al. 11 | NRPS | 33 | 25 | 85 | 64 | MP | Log MAR SC | ND | 19/24 | NA |
| 6 | 1999 | *S.Mine et al. 12 | RS | 12 | 9 | 24 | 14 | TC | LR | DMS | 19/24 | NA |
| 7 | 2000 | Kountakis et al. 13 | RS | 17 | 14 | 34 | 11 | TS | SC | MPRS | 21/24 | NA |
| 8 | 2003 | Rajiniganth et al. 14 | NRPC | 30 | 15 | 18 | 10 | TS | SC | MPRS | 22/24 | NA |
| 9 | 2004 | Yang et al. 15 | RS | 24 | 10 | 18 | 8 | TC | Log MAR SC | MPRS | 12/24 | NA |
| 10 | 2004 | Goldenberg et al. 16 | RS | 4 | 3 | 11 | 4 | NM | ND | PRS | 19/24 | NA |
| 11 | 2006 | *Shibuya et al. 2 | RS | 10 | 3 | 28 | 18 | NM | Log MAR SC | MPRS | 13/16 | NA |
| 12 | 2008 | H Li et al. 17 | NRPS | 176 | 96 | 61 | 31 | TS | SC | DMS | 17/24 | NA |
| 13 | 2014 | William et al. 18 | RS | 91 | 75 | 24 | 10 | NM | SC | MPRS | 18/24 | NA |
| 14 | 2018 | Min Chen et al. 19 | RS | 26 | 17 | 26 | 0 | TS | SC | MPRS | 15/24 | NA |
| 15 | 2018 | Yu et al. 20 | RS | 62 | 34 | 29 | 21 | TE | SC | MPRS | 16/24 | NA |
| 16 | 2019 | *Chen et al. 21 | RCT | 24 | 11 | 5 | 3 | TC | Log MAR | MPRS | NA | 3/5 |
RS, retrospective study; NRCT, non-randomised controlled trial; NRPS, non-randomised prospective study; RCT, randomised controlled trial; TE, transethmoidal; TCon, transconjunctival; TE, transethmoidal; NM, not mentioned; TC, transcranial; MP, multiple protocols; SC, Snellen chart; VEP, visual-evoked potential; LR, Landolt’s ring; ND, not defined; DMS, dexamethasone; PRS, prednisolone; MPRS, methylprednisolone; MS, minors score; JS, Jadad score.
*studies included in NLP (no light perception) data analysis.
Figure 1.
Prisma flow chart of a systematic review. A total of 7102 articles were selected for screening, and 148 articles were shortlisted for full-text screening. Sixteen papers were included for data synthesis and analysis.
Description of studies
Included studies consisted of 11 retrospective studies. 1 RCT 16 and 5 non-randomised prospective studies (Table 2). Six out of eighteen studies provided details of treatment effects on patients with NLP (Table 3). In addition, nine studies described criteria for patient selection.
Table 3.
Studies With Data on Patients With NLP After TON.
Critical appraisal of included studies
MINORS score was used for 15 studies. Maximum MINORS score was 16 for 5 studies and 24 for rest of the 10 studies. Jadad score was used only for the RCT by Chen et al 16 included in the meta-analysis. The respective critical appraisal scores are presented in Table 2.
Improvement in Visual Acuity
The articles were assessed for the total number of cases treated by steroid therapy (ST) and the total number of cases treated by surgical decompression (SD). The authors noted different treatment protocols and scales for testing visual acuity. There were different criteria used for the assessment of improvement in visual acuity.
Synthesis of continuous data was not feasible, and dichotomous data were synthesized based upon the events of improvement in visual acuity as defined by different criteria used by authors.
A total of 1046 patients from 16 studies were included in the meta-analysis, of which 590 patients underwent surgical decompression with 343 events of improvement in visual acuity. Four hundred fifty-six patients underwent medical management with two hundred fifty-two events of improvement in visual acuity (Table 2).
Ten authors used Snellen chart (SC)–based criteria, three authors used Log MAR values (minimum angle of resolution) based on Snellen chart, one author used Landolt’s ring, one author used VEP and one author used percentage Log MAR values (Table 2).
The studies reported improvement in visual acuity based on different criteria. Hence, there was heterogeneity in the data, which mandated sub-group analysis based upon the criteria used.
Meta-analysis (odd ratio) was conducted, and a significant heterogeneity mandated a sub-group analysis based upon different criteria (mentioned below) used for reporting improvement in visual
(i) One line improvement in SC.
(ii) Two-line improvement in SC.
(iii) Three-line improvement in SC.
(iv) Log MAR values from SC.
(v) Landolt’s ring assessment (LR).
(vi) Percentage improvement in Log MAR values.
There was significant heterogeneity I2 = 66% among the studies which mandated a random-effect model. Meta-analysis of extracted data showed no significant difference in the overall visual improvement by SD or ST. A sub-group analysis of different criteria for visual improvement showed no statistically significant difference between the sub-groups with overall heterogeneity I2 = 37.3% (Figure 2). However, the sub-group using one-line improvement in SC as criteria for improvement showed treatment results in favour of ST (test for overall effect Z = 2.38, P = .02).
Figure 2.
Forest plot with sub-group analysis comparing surgical decompression to steroid therapy among all 16 studies.
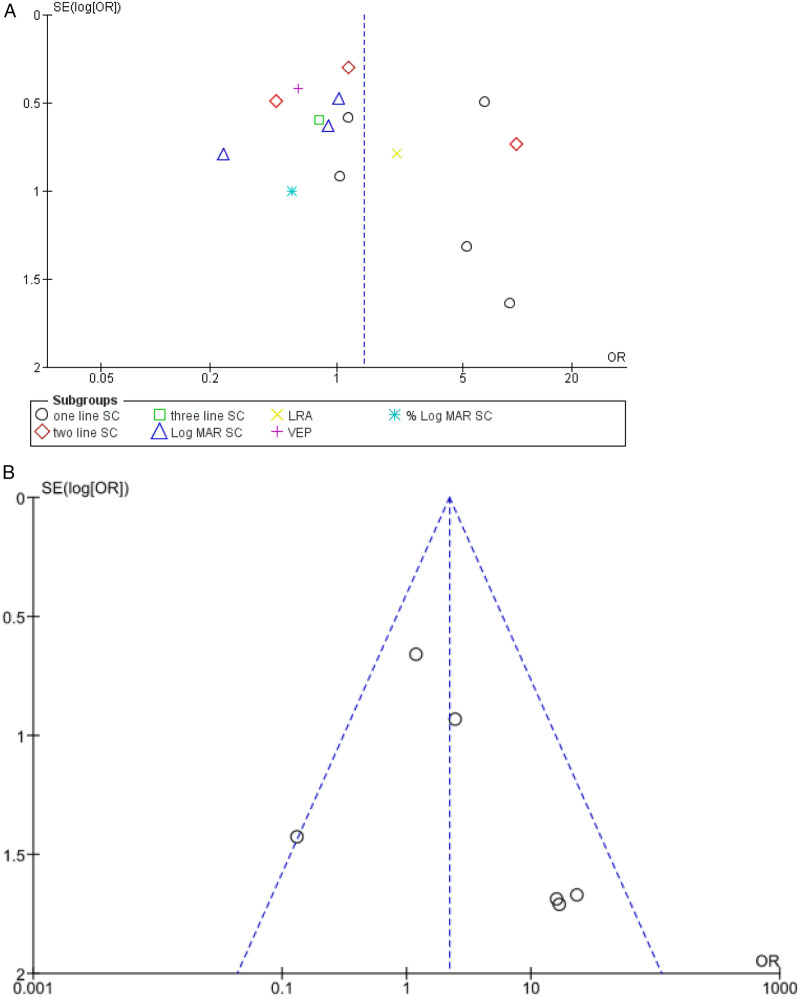
Test for publication bias is represented in Figure 3A, which suggests significant publication bias in included studies.
Figure 3.
A. funnel plot of all studies included in the meta-analysis. B. Funnel plot for six studies in the NLP cohort meta-analysis.
The patients in whom SC could not be used are assessed by a very basic method of assessment and grading as NLP (no light perception), PL (perception of light), HM (hand movement) and FC (finger counting). Any improvement in the visual acuity over and above NLP has been reported as an improvement by all authors. Six authors mentioned the treatment results separately in patients with NLP who have the worst possible vision after TON.
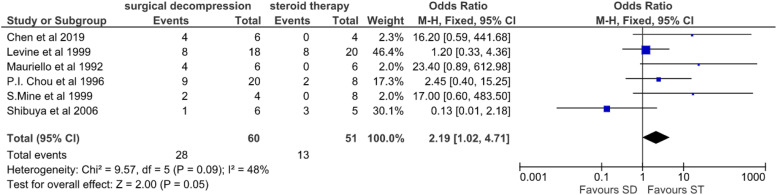
A second meta-analysis on patients with NLP was performed to compare the effect of surgical decompression (SD) and steroid therapy (ST). Here, any improvement in vision above and over NLP was taken as a positive event. There was a 46% (28/60) improvement in patients with NLP treated surgically compared to only 25% (13/51) who recovered from NLP when treated with steroid therapy (Table 3).
Meta-analysis of 6 studies in patients with baseline worst visual acuity of NLP (no light perception) on the first presentation revealed overall heterogeneity I2 = 48% (Figure 4).
Figure 4.
Forest plot comparing surgical decompression to steroid therapy among six studies involving patients with NLP.
The meta-analysis of the odds ratio revealed an overall heterogeneity I2 = 48%, which mandated a fixed-effect model suggesting statistically no significant difference between SD and ST (P = .05).
Funnel plots for studies included in the meta-analysis of patients with NLP represent no significant publication bias which was within acceptable limits (Figure 3B).
Analysis of specialities involved in management: An analysis of the specialities reported in the TON papers reveals the primary involvement of ophthalmology, neuro-ophthalmology and ophthalmic plastic surgery disciplines. There is also active involvement of neurosurgery, especially in an Indian population–based case series conducted by Mahapatra et al that includes 800 patients, 22 which has not been included in the review due to non-compliance with inclusion criteria. In addition, there is an increasing role in skull base surgeons, a sub-speciality of ENT surgeons involved by the virtue of their endoscopic surgery skills.
Discussion
Summary of evidence: The above presented systematic review highlights the meta-analysis using 16 studies involving 1046 patients of TON whose response to SD and ST was compared. The meta-analysis findings suggest no significant difference in the treatment results of TON patients treated with either SD or ST. A sub-group analysis has revealed no significant difference in the sub-groups based upon methods of visual acuity assessment. There was no significant difference in treatment outcomes in the group of patients with NLP either.
Sub-group analysis, however, reveals when a lower threshold criterion of one-line improvement in SC is used, treatment results favour ST. This result highlights the effect of improvement criteria with lower threshold on treatment results.
The findings of the study are in contrast to an earlier reported meta-analysis on the same topic by Rafael et al, 23 who concluded that surgical decompression is a better treatment modality in TON, and the earlier, the better results. However, the study included a lesser number of the patient as well as studies and failed to address heterogeneous methods of visual acuity assessment. This meta-analysis includes only well-designed RCT reported to date on the subject by Chen et al, 16 and our findings concur with Chen et al. that there is no statistically significant difference between the treatment strategy (SD/ST). In the light of the above findings, there is a need to further understand the mechanisms of both therapies and the possible consequences for the same. The selection of the best therapy can be guided by understanding the following important treatment aspects.
Steroid-Mechanism of Action and the Safe Dose
Several experimental studies have shown an anti-oxidant and neuroprotective role of high-dose corticosteroids in preventing injuries from free radicals that form after injury.24,25 As a result, the production of prostaglandins is reduced, and circulation is preserved, ultimately containing nerve cell death. However, the dose of steroids used has become a matter of concern in the backdrop of corticosteroid randomisation after a significant head injury trial in 2004 26 revealed increased mortality with high-dose corticosteroid-treated patients with a substantial head injury. Hence, there is a renewed debate over the safety of steroids since most patients with TON will have a head injury. Thus, the treatment’s safety for doing no further harm becomes a priority. When reviewing the dosage of the steroids used in our review, we found that 8 out of 16 authors have reported using methylprednisolone in their protocol (Table 2). In addition, the use of megadose is mentioned in 4 studies.2,27-29 Most importantly, three studies have reported the use of megadose even after a worldwide debate over the safety of megadose after a significant head injury trial in 2004.
The term ‘megadose’ is commonly used by ophthalmology which is equivalent to ‘high dose’ from neurology. These terms have been used to document the treatment protocol of intravenous methylprednisolone used in the management of acute spinal cord injury in second and third national spinal cord injury studies15,18 (NASCIS II and NASCIS III) – A loading dose of 30 mg/kg followed by a continuous intravenous infusion of 5.4 mg/kg per hour for 24 or 48 hours. 14
Animal model trials of different dosages have found the dose-dependent neuroprotective effect of methylprednisolone with interference in high doses. However, a 1 mg/kg dose did not interfere with the neuroprotective effect (14). The absence of data on a safe amount will not justify any human trial as it will be ethically not feasible.
The dosage duration is also suggestive of the observation time to assess the response to the treatment, which varied from 3 days to 3 weeks. 4 The lack of a reasonable level of evidence or difficulty in doing a randomised controlled trial can be explained by the lack of a defined dose in the international optic nerve trauma study published in 1999. Duration of 72 hours of observation after starting steroid therapy for improvement in vision was the most common protocol.10,11,21,28,29
Thus, the evidence for correctly titrated safe dose and its duration remains controversial as observed from trials comparing surgical decompression with steroid therapy. Nevertheless, the credible evidence of the MRC CRASH trial 13 should not be overlooked, and informed consent of increased mortality should be taken before starting a high dose of methylprednisolone. 14
Surgical Decompression Protocols and Techniques
The literature review is evident about the first use of steroids followed by surgery of patients not responding to medical management (Table 4). However, an RCT reported by Chen et al 16 in 2019 allotted patients to different treatment groups after due consent. However, the study did not rule out even post-surgery use of steroids. The most commonly used protocol is steroid therapy for three days, followed by reassessment and surgical decompression of non-responders to medical treatment. The improvement criteria are another factor that needs to be addressed and made homogeneous. The international collaboration failed in terms of uniformity of protocol or standards for improvement assessment. 12 While few published protocols dictate the terms of application in this treatment modality, the association of NLP at presentation has usually been considered a poor prognostic factor, as reported by Mauriello et al in 1992. 20
Table 4.
Criteria of Patient Selection for Surgical Decompression.
| 1989 | Simmon Lessel et al. 9 | No specific protocol |
| 1992 | Mauriello et al. 10 | 24–72 hours of steroid and then surgery |
| 1993 | Mahapatra et al. 4 | Three weeks of steroid therapy and then assessment |
| 1996 | P.I. Chou et al. 1 | No specific protocol was mentioned for case selection |
| 1999 | Levine et al. 11 | No specific protocol was mentioned for case selection |
| 1999 | S.Mine et al. 12 | No specific protocol was mentioned for case selection |
| 2000 | Kountakis et al. 13 | 48 hours of steroid therapy and then assessment |
| 2003 | Rajiniganth et al. 14 | I. 72 hours of a steroid without improvement |
| II. Progressive visual loss | ||
| III. Total blindness with CT evidence of nerve compression | ||
| 2004 | Yang et al. 15 | Three days of steroid if there is no improvement and then decompression |
| 2004 | Goldenberg-Cohen et al. 16 | No specific protocol was mentioned for case selection |
| 2006 | Shibuya et al. 2 | Steroid therapy and surgery for non-responding cases, but the observation threshold is not mentioned |
| 2008 | H Li et al. 17 | No specific protocol was mentioned for case selection |
| 2014 | William Marshal Guy et al. 18 | Three days of steroids and then assessment for surgery |
| 2018 | Min Chen et al. 19 | No specific protocol was mentioned for case selection |
| 2018 | Yu et al. 20 | Three days of steroid therapy and then re-assessment |
| 2019 | Chen et al. 21 | Upfront surgery after randomised treatment allotment |
Evolution of Surgical Decompression Techniques
The techniques and results have understandably evolved. The transcranial approach is less preferred than the endoscopic transethmoidal approach (Table 2). However, recent literature still finds mention of the transcranial approach as recently as 2019. 16 Irrespective of the approach, the treatment is based upon relieving annular strangulation and releasing the nerve from the bony confines of the optic canal to prevent haematoma or oedema from compressing the nerve. With the advent of the endoscopic minimally invasive technique, 27 surgery appears to be an appropriate treatment option in a polytrauma patient who has to be shifted to operative room for other complications. However, the decision to use surgical decompression cannot be justified until the true incidence of spontaneous visual recovery and the contributing factors are studied. 14 This systematic review of the literature suggests that a non-responsive or progressively deteriorating vision under steroid treatment has been subjected to surgical decompression. But, the choice of steroid therapy is controversial in presence of severe head injury.
Optic Canal Fracture and Treatment Protocol
The presence of direct evidence of optic canal fracture has been considered an important prognostic factor by many authors. 28 In contrast, the largest cohort of TON from the Indian population 22 has suggested optic canal fracture as a non-significant prognostic fracture. However, the radiographic evidence of optic canal fracture remains inconclusive because few studies have reported intra-operative findings of optic canal fracture without any radiological evidence; hence, this factor cannot be dependable. 9 The literature also finds mentions of the protocol of no intervention, which lacks support or evidence in the absence of any discussed treatment protocol or indication.
Indications for Surgical Decompression
Rajiniganth et al 28 has described three indications for surgical decompression as follows:
(I) 72 hours of steroid without improvement.
(II) Progressive visual loss.
(III) Total blindness with evidence of optic nerve compression on computed tomography.
The indications for surgical decompression remain widely debated (Table 4). However, the safety of steroid dosage in patients with significant head injury appears to make surgery a safer option, especially in patients with polytrauma who have to undergo surgery in GA (general anaesthesia) for other associated injuries. Nevertheless, the threshold of 3 days or 72 hours remains the most commonly followed protocol.17,20,28,29
Criteria for Assessment of Visual Acuity Improvement
The most significant factor that affected the data synthesis is the subjective nature of visual acuity assessment, especially in patients in the emergency room and their differing levels of consciousness. Mahapatra et al 4 have successfully demonstrated the use of visual-evoked potential in unconscious patients. Uncooperative children are also a challenge for clinicians to document the visual acuity (VA) upon presentation. Goldenberg and Cohen et al 30 have used relative afferent pupillary defects in uncooperative children. The most homogeneous data, which is common to all included studies, is the use of VA status as no light perception (NLP), perception of light (LP), hand movement (HM) and finger counting (FC). Hence, the data extracted in terms of NLP, LP, HM and FC becomes essential. However, it is disappointing that there is a scarcity of comprehensive data about patients in recently reported studies. Snellen’s chart remains the most commonly used visual chart, 31 with its fractions converted to Log MAR (Log minimum angle of resolution) values. Newer studies have used a ‘degree of improvement’ (calculated from Log MAR) as a reporting tool. 8
Limitations of Study and Scope of Improvements for Further Research
The review of literature could identify only one RCT; other studies are non-randomised prospective or case series. Assessment of primary outcome, that is, improvement in visual acuity, is difficult since different criteria are used. The authors also discovered that values of minimal vision in Snellen chart are not same for all the authors. The vision beyond 20/800 has been categorized in terms of CF, HM, PL and NLP by Shibuya et al, 2 where authors like William Marshal Guy et al 21 have used 20/400 as the minimal acuity beyond which vision was defined in terms of CF. The extraction of data, that is, improvement of vision, was reported in terms of criteria used by all the authors which was not uniform. The authors have thus used a separate meta-analysis of patients with NLP as baseline pre-intervention VA and assessed the effectiveness of ST and SD in this cohort. A sub-group analysis also investigated this heterogeneity in the study. The lack of uniformity and difficulty in generalising a single visual assessment method at the initial stage makes the comparison of studies difficult. We could identify that the data on severity of associated head injuries, that is, traumatic brain injury, sub-dural haematoma and anterior skull base fractures, were missing. The literature review lacks documentation of associated craniofacial fractures except for a few studies. The low incidence of this complication with the abovementioned limitations was reflected in the results of international collaboration optic nerve trauma study 12 with non-uniform protocols of ST and surgical intervention.
Conclusion
The meta-analysis concludes that there is no significant difference in SD and ST in terms of improvement in visual acuity. Patients with the worst pre-treatment vision of NLP also do not differ in their response to either SD or ST. The meta-analysis highlights the importance of uniform initial and follow-up visual assessment methods, the need for proper documentation of complications, the severity of associated head injuries and even the death of patients with an associated head injury. There is a need for proper speciality reference among various specialities and timely intervention to decrease the poor prognosis of vision in cases of TON (traumatic optic neuropathy).
Abbreviation
- TON
traumatic optic neuropathy
- VA
visual acuity
- NLP
no light perception
- LP
light perception
- HM
hand movement
- FC
finger counting
- SC
Snellen chart
- Log MAR
Log minimum angle of resolution.
Footnotes
Author Contributions: SP: Concept of study design, research question, data synthesis and interpretation. GR: Data synthesis and interpretation. AC: Data synthesis and interpretation. AD and BR: Proofreading.
The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
ORCID iD
Sameer Pandey https://orcid.org/0000-0002-7070-8237
References
- 1.Chou PI, Sadun AA, Chen YC, Su WY, Lin SZ, Lee CC. Clinical experiences in the management of traumatic optic neuropathy. Neuro Ophthalmol. 1996;16(6):325-336. [Google Scholar]
- 2.Shibuya TY, Feinberg SM, Mathog RH, et al. Visual risks of facial fracture repair in the setting of traumatic optic neuropathy. Arch Otolaryngol Neck Surg. 2006;132(3):258. [DOI] [PubMed] [Google Scholar]
- 3.Wang BH, Robertson BC, Girotto JA, et al. Traumatic optic neuropathy: A review of 61 patients. Plast Reconstr Surg. 2001;107(7):1655-1664. [DOI] [PubMed] [Google Scholar]
- 4.Mahapatra AK, Tandon DA. Traumatic optic neuropathy in children: a prospective study. Pediatr Neurosurg. 1993;19(1):34-39. [DOI] [PubMed] [Google Scholar]
- 5.Jamal BT, Pfahler SM, Lane KA, et al. Ophthalmic injuries in patients with zygomaticomaxillary complex fractures requiring surgical repair. J Oral Maxillofac Surg. 2009;67(5):986-989. [DOI] [PubMed] [Google Scholar]
- 6.Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ. 2015;350(1):g7647. [DOI] [PubMed] [Google Scholar]
- 7.Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (Minors): Development and validation of a new instrument. ANZ J Surg. 2003;73(9):712-716. [DOI] [PubMed] [Google Scholar]
- 8.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control Clin Trials. 1996;17(1):1-12. [DOI] [PubMed] [Google Scholar]
- 9.Mine S, Yamakami I, Yamaura A, et al. Outcome of traumatic optic neuropathy. Comparison between surgical and nonsurgical treatment. Acta Neurochir. 1999;141(1):27-30. [DOI] [PubMed] [Google Scholar]
- 10.Yu B, Ma Y, Tu Y, Wu W. The outcome of endoscopic transethmosphenoid optic canal decompression for indirect traumatic optic neuropathy with no-light-perception. J Ophthalmol. 2016;2016:1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen M, Jiang Y, Zhang J, Li N. Clinical treatment of traumatic optic neuropathy in children: Summary of 29 cases. Exp Ther Med. 2018;16(4):3562-3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levin LA, Beck RW, Joseph MP, Seiff S, Kraker R. The treatment of traumatic optic neuropathy: The international optic nerve trauma study. Ophthalmology. 1999;106(7):1268-1277. [DOI] [PubMed] [Google Scholar]
- 13.Edwards P, Arango M, Balica L, et al. Final results of MRC CRASH, a randomised placebo-controlled trial of intravenous corticosteroid in adults with head injury-outcomes at 6 months. Lancet. 1999;365(9475):1957-1959. [DOI] [PubMed] [Google Scholar]
- 14.Steinsapir KD, Goldberg RA. Traumatic optic neuropathy: An evolving understanding. Am J Ophthalmol. 2011;151(6):928-933. [DOI] [PubMed] [Google Scholar]
- 15.Bracken MB, Shepard MJ, Hellenbrand KG, et al. Methylprednisolone and neurological function 1 year after spinal cord injury. J Neurosurg. 1985;63(5):704-713. [DOI] [PubMed] [Google Scholar]
- 16.Chen HH, Lee MC, Tsai CH, Pan CH, Lin YT, Chen CT. Surgical decompression or corticosteroid treatment of indirect traumatic optic neuropathy: A randomized controlled trial. Ann Plast Surg. 2020;84(1S suppl 1):S80-S83. [DOI] [PubMed] [Google Scholar]
- 17.Yu B, Chen Y, Ma Y, Tu Y, Wu W. Outcome of endoscopic trans-ethmosphenoid optic canal decompression for indirect traumatic optic neuropathy in children. BMC Ophthalmol. 2018;18(1):152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bracken MB, Shepard MJ, Collins WF, et al. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury. N Engl J Med. 1990;322(20):1405-1411. [DOI] [PubMed] [Google Scholar]
- 19.Ben Simon GJ, Hovda DA, Harris NG, Gomez-Pinilla F, Goldberg RA. Traumatic brain injury induced neuroprotection of retinal ganglion cells to optic nerve crush. J Neurotrauma. 2006;23(7):1072-1082. [DOI] [PubMed] [Google Scholar]
- 20.Mauriello JA, DeLuca J, Krieger A, Schulder M, Frohman L. Management of traumatic optic neuropathy--a study of 23 patients. Br J Ophthalmol. 1992;76(6):349-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guy WM, Soparkar CNS, Alford EL, Patrinely JR, Sami MS, Parke RB. Traumatic optic neuropathy and second optic nerve injuries. JAMA Ophthalmol. 2014;132(5):567. [DOI] [PubMed] [Google Scholar]
- 22.Mahapatra A. Rationale of optic nerve decompression in optic nerve injury. Indian J Neurotrauma. 2004;1(1):33-36. [Google Scholar]
- 23.Martinez-Perez R, Albonette-Felicio T, Hardesty DA, Carrau RL, Prevedello DM. Outcome of the surgical decompression for traumatic optic neuropathy: A systematic review and meta-analysis. Neurosurg Rev. 2020;22. [DOI] [PubMed] [Google Scholar]
- 24.Hall ED. The neuroprotective pharmacology of methylprednisolone. J Neurosurg. 1992;76(1):13-22. [DOI] [PubMed] [Google Scholar]
- 25.Hall ED. Importance of pharmacologic considerations in the evaluation of new treatments for acute spinal cord injury. J Neurotrauma. 1992;9(2):173-176. [DOI] [PubMed] [Google Scholar]
- 26.Roberts I, Yates D, Sandercock P, et al. Effect of intravenous corticosteroids on death within 14 days in 10008 adults with clinically significant head injury (MRC CRASH trial): randomised placebo-controlled trial. Lancet. 2004;364(9442):1321-1328. [DOI] [PubMed] [Google Scholar]
- 27.Kountakis SE, Maillard AAJ, El-Harazi SM, Longhini L, Urso RG. Endoscopic optic nerve decompression for traumatic blindness. Otolaryngol Head Neck Surg. 2000;123(1):34-37. [DOI] [PubMed] [Google Scholar]
- 28.Rajiniganth MG, Gupta AK, Gupta A, Bapuraj JR. Traumatic optic neuropathy: visual outcome following combined therapy protocol. Arch Otolaryngol Head Neck Surg. 2003;129(11):1203-1206. [DOI] [PubMed] [Google Scholar]
- 29.Yang WG, Chen CT, Tsay PK, De Villa GH, Tsai YJ, Chen YR. outcome for traumatic optic neuropathy-surgical versus nonsurgical treatment. Ann Plast Surg. 2004;52(1):36-42. [DOI] [PubMed] [Google Scholar]
- 30.Goldenberg-Cohen N, Miller NR, Repka MX. Traumatic optic neuropathy in children and adolescents. J AAPOS. 2004;8(1):20-27. [DOI] [PubMed] [Google Scholar]
- 31.Li H, Zhou B, Shi J, Cheng L, Wen W, Xu G. Treatment of traumatic optic neuropathy: Our experience of endoscopic optic nerve decompression. J Laryngol Otol. 2008;122(12):1325-1329. [DOI] [PubMed] [Google Scholar]