Abstract
Study Design
A stereological and histopathological study in an animal model
Objective
This study explores the effects of the nerve growth factor and photobiomodulation therapy on the damaged nerve tissue and fracture healing.
Methods
A total of 24 rabbits were divided into 4 groups: control group (n = 5), nerve growth factor (NGF) group (n = 7), photobiomodulation (PBMT) group (n = 6), and nerve growth factor and photobiomodulation therapy (NGF+PBMT) group (n = 6). The vertical fracture was performed between the mental foramen and the first premolar, and the mental nerve was crushed for 30 seconds with a standard serrated clamp with a force of approximately 50 N in all groups. The control group received an isotonic solution (.02 mL, .09% NaCl) to the operation site locally. The NGF group received 1 μg human NGF-β/.9% .2 mL NaCl solution for 7 days locally. The PBMT group received PBMT treatment (GaAlAs laser, 810 nm, .3 W, 18 J/cm2) every 48 hours for 14 sessions following the surgery. The NGF+PBMT group received both NGF and PBMT treatment as described above. After 28 days, the bone tissues and mental nerves from all groups were harvested and histologically and stereologically analyzed.
Results
According to the stereological results, the volume of the new vessel and the volume of the new bone were significantly higher in the PBMT group than in other groups (P < .001). According to the histopathological examinations, higher myelinated axons were observed in experimental groups than in the control group.
Conclusions
As a result, PBMT has beneficial effects on bone regeneration. Based on the light microscopic evaluation, more regenerated axon populations were observed in the NGF group than in the PBMT and PBMT + NGF groups in terms of myelinated axon content.
Keywords: Nerve Growth Factor, Fracture Healing, Nerve Regeneration, Photobiomodulation
Introduction
The oral and maxillofacial region is the most unprotected part of the body and is often exposed to trauma. As a result of trauma, maxillofacial fractures can occur.1,2 Fracture healing is a complex and well-organized physiological process initiated in response to a bone injury. 3
Inferior alveolar nerve (IAN) is frequently damaged by trauma, dentoalveolar surgery, or orthognathic surgery.4,5 In most of these patients, sensory disorders can occur, including severe dysesthesia, permanent paresthesia, and pain. Patients may benefit from surgical treatments such as nerve grafting. These are difficult procedures and ineffective in crush-type nerve injuries, 6 and patients with nerve damage have a higher rate of fracture formation. Furthermore, changes in fracture healing and weaker calluses were observed in experimentally denervated rats. 7 It has been reported that alveolar bone remodeling is prevented in IAN transected rats. 8
Nerve growth factor (NGF), a neurotrophic factor, plays a critical role in peripheral nerve regeneration following injury. It increases the proliferation and differentiation of neurons and regulates the repair of damaged nerves.9–15 Several studies have shown that the local administration of NGF increases nerve healing.10,16,17 NGF promotes not only on neuronal cells but also on several non-neuronal cells. NGF was shown to have positive effects on angiogenesis, and angiogenesis plays a vital role in fracture healing and nerve regeneration. 18
In recent decades, bone healing has been stimulated using PBMT, low-intensity pulsed ultrasound, 19 and ozone therapy. 20 The effects of PBM are based on absorption of light by intracellular chromophores that activate the metabolic process.21–23 A systematic review concluded that PBMT may induce osteogenesis and that it has the ability to increase osteogenesis, fibroblast, and osteoblast proliferation. 24 When a peripheral nerve injury occurs, pro-inflammatory cytokine production increases, and they are released to the injury site. For nerve regeneration, neurotrophic factors such as NGF, brain-derived neurotrophic factor (BDNF), and growth factors such as vascular endothelial growth factor (VEGF) are released.25,26 A review of in vivo studies suggests that PBMT is an effective treatment option for peripheral nerve injury. 27 According to our investigation, there is no study in which both fracture and nerve healing are examined together.
In this study, we hypothesized that NGF and PBMT would enhance the regeneration of both nerve and bone tissue. This study aimed to reveal new information about NGF and PBMT applications in the treatment of bone and nerve injuries through stereological and histopathological analyses.
Materials & Methods
All experiments were authorized by the OMU Faculty of Medicine Experimental Animals Research Center (2012/51, Samsun, Turkey) and approved by the local ethics committee on animal experiments.
Animals
In this study, 28 female New Zealand rabbits weighing between 2.5 and 3 kg were used. Animals were obtained from the Experimental Animal Application and Research Center of Ondokuz Mayıs University. The experiments were performed at this center. The rabbits were housed in individual cages at 1 atm pressure, 25°C, and a 12-h light/dark cycle. They were allowed free access to food and water. All animals were fed with pellet chow and water throughout the experiments.
Experimental Groups
The rabbits were randomly divided into 1 control group and 3 study groups, with 7 rabbits per group. Vertical osteotomy was performed between the mental foramen and the first premolar on the right mandible. The right mental nerve was crushed for 30 seconds using a standard serrated clamp with a force of approximately 50 N in all groups.
Control Group (n = 7)
Control group animals were injected postoperatively with .9% .2 mL NaCl solution percutaneously around the mental foramen. The laser probe was also applied to the same area on the buccal skin of the rabbits, but the device was turned off.
PBMT Group (n = 7)
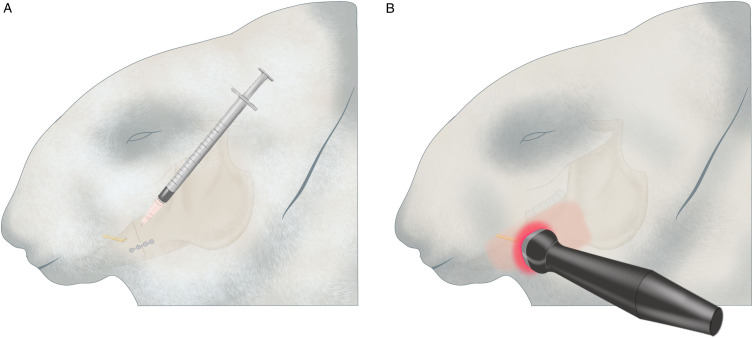
The PBMT group received PBMT treatment every 48 hours for 14 sessions following the surgery. 28 This PBMT consisted of a GaAlAs laser (CheeseTM, Wuhan Gigaa Optronics Technology Co, Ltd, China) with a wavelength of 810 nm, a power of .3 W, and an energy density of approximately 18 J/cm2. The probe was placed on the buccal skin of the rabbit in contact with the skin, including the site of injury, for 180 s. The crushed nerve and the fractured bone were in the same area. This area was then irradiated (Figure 1B).
Figure 1.
Images of application site: A. NGF application. B. PBMT application 1218x553 mm (96 x 96 DPI).
NGF Group (n = 7)
The animals received 1 μg human NGF-β (hNGF)/.9% .2 mL NaCl solution for 7 days percutaneously to the site of the injury (Figure 1A) as described by Wang L et al. 17 and Bao et al. 30 PBMT + NGF group (n = 7): The animals received 1 μg human NGF-β/.9% .2 mL NaCl solution for 7 days and PBMT treatment for 14 sessions. We applied NGF solution to the injury site first and then applied the PBMT (Figure 1).
NGF Preparation
A total of 100 μg of hNGF-β (Bio Vision, San Francisco, USA) was dissolved in .9% NaCl solution according to the manufacturer’s instructions. Subsequently, solutions containing 1 μg/.2 mL NaCl were prepared for single-dose administration and stored at −20° C. The solutions were applied at room temperature to the rabbits.
Surgical Procedure and Study Design
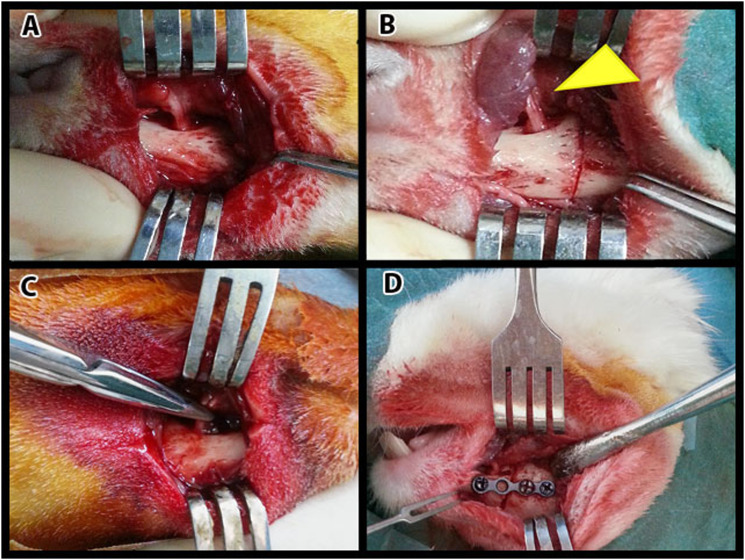
All surgical procedures were performed under sterile conditions. Animals were anesthetized by intramuscular injection of 50 mg/kg ketamine hydrochloride (Ketalar; Pfizer, Istanbul, Turkey) and 10 mg/kg xylazine hydrochloride (Rompun; Bayer, Istanbul, Turkey). Under general anesthesia, a skin incision was made at the inferior border of the right mandible. The bone tissue was exposed by dissecting the skin and subcutaneous tissues (Figure 2A). A vertical osteotomy was performed between the first premolar and mental foramen (Figure 2B). Osteotomies were completed with a fine chisel and performed carefully to avoid direct injury to the IAN. The lingual periosteum was not reflected. The mental nerve was crushed for 30 s using a serrated standard clamp with a force of approximately 50 N (Figure 2C). The fracture line was fixed with a four-hole miniplate and screws (7 × 1.6 mm) with a thickness of 1 mm (Figure 2D). The subcutaneous tissues and skin were closed with 3-0 Vicryl and 3-0 silk sutures. Tramadol (1 mg/kg; Contramal, Abdi İbrahim, İstanbul, Türkiye) and cefazolin sodium (500 mg/kg; Sefazol, M Nevzat, İstanbul, Türkiye) were administered twice for 4 days for postoperative pain and infection. All the rabbits were fed a soft diet for at least 7 days. Rabbits were frequently monitored for food intake and activity.
Figure 2.
Surgery: A. The exposure of the surgery area. B. The corticotomy of the mandibular bone. The yellow arrow tip shows the mental nerve. C. Mental nerve crush injury. D. The fractured bone is treated with screws and a titanium plaque. 162x121 mm (100 x 100 DPI).
Stereological and Histopathological Analyses
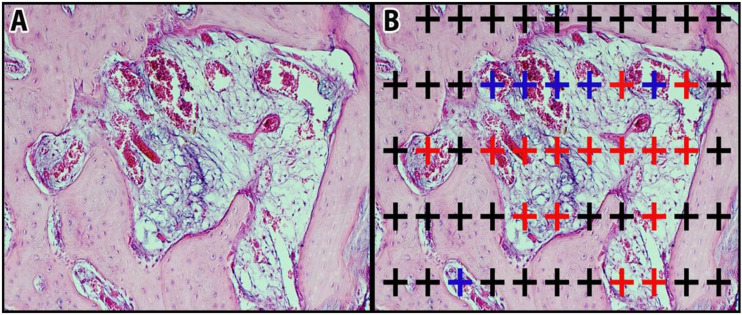
After 4 weeks, the animals were euthanized with an intravenous injection of 1% pentobarbital. The tissue samples were evaluated blindly by the histologist. Soft tissues on the mandible were removed, and the obtained samples included the entire fracture line and were decalcified in 5% formic acid for 21 days. After decalcification, 10% formaldehyde postfixation samples were gradually dehydrated with alcohol, cleaned with xylol, and prepared for histological examination under a light microscope. For this purpose, serial sections of paraffin-embedded samples with microtome were taken (Leica RM 2135; Leica Instruments, Nussloch, Germany) according to the systematic random sampling approach, which forms the basis of 7 μm thick stereological methods and examination methods. According to the pilot study, the appropriate systematic random sampling rate was 1/100. The selected sections were stained with hematoxylin-eosin (H&E) and photographed using a camera attachment (Olympus DP25) light microscope (Olympus BX 50). New bone tissue, new connective tissue, and new vessel volume were calculated stereologically using the Cavalier method. 30 According to the pilot study, the content and point density of the dotted area measurement scale selected for the method were determined to cover the entire fracture line in the cross-sections (Figure 3). In the calculations, the validity of the sampling in each subject was determined according to the appropriate error coefficient, and the validity of the sampling in each group was determined according to the coefficient of variation of Gundersen and Jensen. 30 The following formula was used to calculate the volume:
Figurr 3.
Representation of the point count method: A. Section view. B. Grid view, blue “+” shows blood vessels, red “+” shows connective tissue, and black “+” shows newly formed bone tissue. 207x93 mm (150 x 141 DPI).
Microscopic Evaluation
The mental nerves of the subjects were harvested. The samples were kept in 5% glutaraldehyde solution for 1 h. They were then washed with Millonig buffer for 4x15 min. After being washed with the buffer, they were kept in a dark environment for 1.5 hours in 1% osmium tetraoxide. Samples were washed again with Millonig buffer for 4x15 minutes and dehydrated in a series of graded alcohols. The tissues that were blocked in the silicon mold were placed in the oven at 45°C, and the temperature was increased by 5°C every 62 min to 62°C. The polymers were allowed to polymerize at 62°C for 48 h. Semi-thin sections were cut by microtome and stained with 1% toluidine blue. Histopathological evaluation of semi-thin-stained sections was performed using light microscopy.
Statistical Analysis
The data were analyzed using a statistical package program (IBM SPSS Statistics 21.0, Mac). Levene’s test was used to evaluate data homogeneity. After normality evaluation (Shapiro–Wilk, P ≤ .05), one-way ANOVA followed by Tukey’s test was performed to compare the groups, and .05 significance level (p) was used.
Results
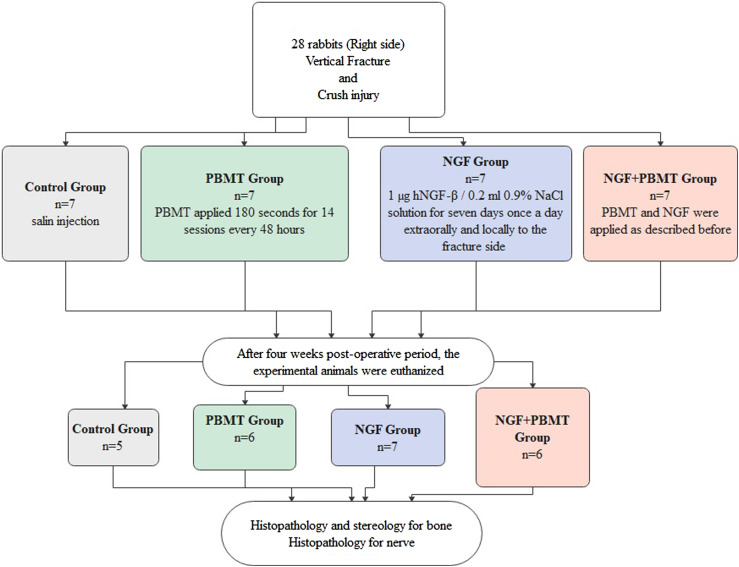
Two animals in the control group died due to excessive weight loss in the first week following surgery. One animal from the PBMT group and 1 animal from the PBMT + NGF group died of infection. A total of 24 rabbits were included in this study (Figure 4).
Figure 4.
Flow chart of the experimental design 209x160 mm (144 x 144 DPI).
Stereological analysis revealed the formation of new bone, connective tissue, and blood vessels in all the groups.
New Bone Volume
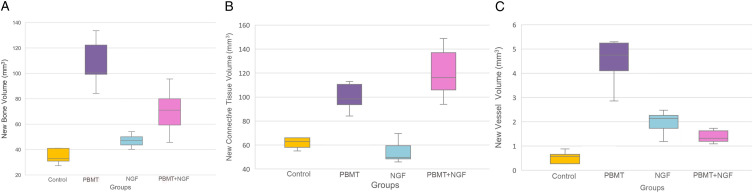
The total new bone volumes of the groups are shown in Figure 5A. The highest bone volume value was observed in the PBMT group (108 ± 19.4 mm3).
Figure 5.
A. Statistical analysis of the data obtained from each group according to the results of the representation of newly formed bone volumes. B. Statistical analysis of the data obtained from each group according to the results of the representation of connective tissue volume. C. Statistical analysis of the data obtained from each group according to the results of the representation of new vessel volumes 1109x262 mm (96 x 96 DPI).
In the control group, the new bone volume value (34.7 ± 6.7 mm3) was significantly lower than in the PBMT (108 ± 19.4 mm3) (P<.001) and PBMT + NGF groups (70.2 ± 18 mm3) (P = .02).
The new bone volume in the NGF group (47 ± 5.2 mm3) was lower than in the PBMT (108 ± 19.4 mm3) (P<.001) and PBMT + NGF groups (70.2 ± 18 mm3) (P = .03), and this difference was statistically significant.
The new bone volume values of the PBMT + NGF group (70.2 ± 18 mm3) were higher than the control (34.7 ± 6.7 mm3) (P = .02) and NGF group (47 ± 5.2 mm3) (P = .03), and the difference was statistically significant.
Connective Tissue Volume
The highest connective tissue volumes were observed in the PBMT + NGF group (120.3 ± 22 mm3), the PBMT group (105.2 ± 22 mm3), the control group (68 ± 17 mm3), and the NGF group (54.4 ± 9 mm3), respectively (Figure 5(B)).
There was a statistically significant difference between the PBMT and PBMT + NGF groups when compared with the NGF group (P <.001). When the PBMT + NGF group was compared with the other groups, a significant difference was found between the control group and the NGF group (P <.001).
New Vessel Volume
The highest vessel volume was found in the PBMT group (4.5 ± 1 mm3), and this difference was statistically significant (P <.001) (Figure 5(C)).
There was a significant difference between the control group (.5 ± .3 mm3) (P<.001) and the PBMT (4.5 ± 1 mm3) (P = .02) when compared with the NGF group (2 ± .4 mm3). When the PBMT + NGF group (2.2 ± 1.2 mm3) was compared with the other groups, a statistically significant difference was found in the PBMT group (4.5 ± 1 mm3) (P<.001).
Histopathological Findings of Bone Tissue
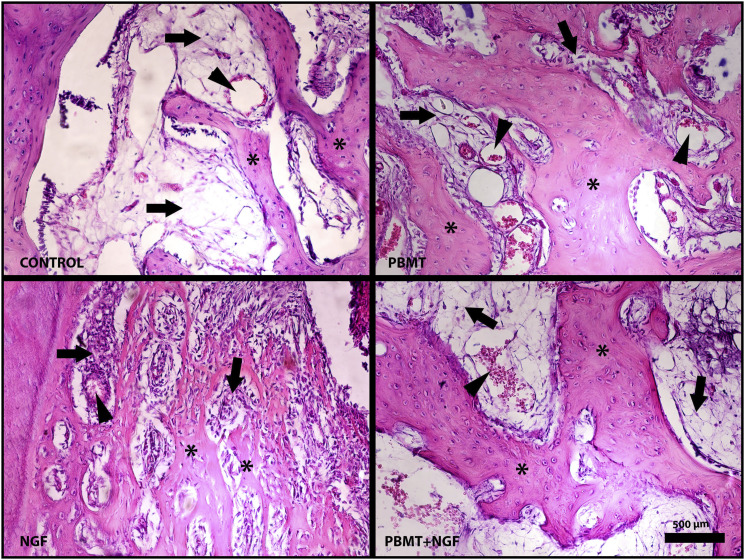
On the light microscopic examination of bone tissue samples, there was no significant difference in the new bone area between the control and the NGF samples. In the PBMT group, prominent new bone areas were observed as open-stained areas compared with the other groups. New bone areas were observed in the NGF + PBMT group than in the control and NGF groups. Particularly in the islets that were considered new ossification within the infiltration areas of connective tissue, clusters of osteocytes trapped in the lacuna attracted attention. In this group, these mature osteocyte formations in the bone islets indicate that bone maturation was more advanced than in the PBMT group. These osteocyte formations also showed new bone formation.
Connective tissue infiltration was observed in both the PBMT and NGF + PBMT groups. Regenerative connective tissue formation was more intense in these groups than that in the control and NGF groups. However, more vascularization was observed in the connective tissue areas of the PBMT group than in those of the other groups. Histopathological images of bone tissue are shown in Figure 6 and 7 according to the groups.
Figure 6.
Histopathological view of experimental groups. A. Control group. B. PBMT group. C. NGF group. D. PBMT+NGF group. “∗” shows new bone areas, “ → ” shows connective tissue areas, and “ ▽ “ shows newly formed vessels. Scale bar 500 μm (stained with hematoxylin-eosin) 846x635 mm (72 x 72 DPI).
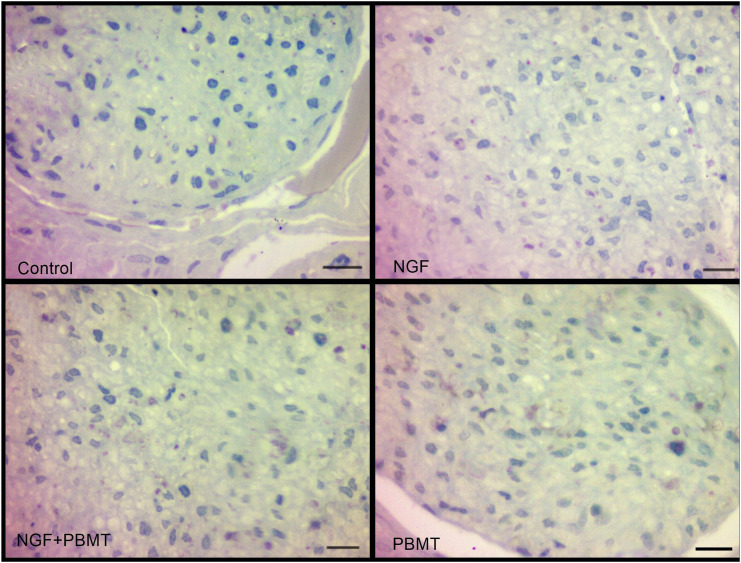
Figure 7.
Histopathological view of experimental groups. Scale bar of the control and PBMT groups 4μm. Scale bar of the NGF and NGF+PBMT groups 40 μm 561x424 mm (96 x 96 DPI).
Histopathological Examination of Nerve Tissue
Based on light microscopic evaluation, more regenerated axon populations were observed in the NGF group than in the PBMT and PBMT + NGF groups in terms of myelinated axon content. There was no difference between the PBMT and NGF + PBMT groups in terms of the number of myelinated axons. Although myelin sheath thickness was degenerated from time to time in myelin sheaths, in general, there was no difference between the NGF, PBMT, and NGF + PBMT groups, and the myelin structures were similar in appearance.
Discussion
This in vivo study tested the effects of NGF and PBMT on bone and nerve regeneration using a rabbit model. We observed that PBMT promoted fracture healing. PBMT can stimulate new bone formation by enhancing osteoblast-, fibroblast-, and osteogenesis-related factors. 23 In a systematic review, Hosseinpour et al 29 found that photo biomodulation administration demonstrated incentive results for bone regeneration. Son et al 31 used a diode laser (808 nm, 1 W, 15.38 J/cm2) on the rat tibial fracture model and found statistically significant differences in new bone formation between the control group and the laser-treated group at different time intervals. In accordance with the abovementioned literature, the results of stereological examination revealed that new bone volume and new vessel volume were highest in the laser-treated group. Contrary to our study, the authors observed no statistically significant differences in the radiological and histological parameters of the groups after 4 weeks. 31 This difference in our study may be attributed to our specific PBMT parameters, such as power and energy density.
NGF is expressed during early period of fracture healing and remains high during the healing process. NGF may promote fracture healing through both direct and indirect pathways. NGF may stimulate osteogenic cells directly and the healing of the sensory nerves carrying osteogenic factors. 32 Sang et al 33 analyzed the mechanism of the nerve growth factors in callus formation with tibial fracture and they concluded that NGF promoted cartilage differentiation, increased osteoclast formation, and tibial fracture healing. Grills et al 34 investigated the effect of the topical application of NGF on unsplinted fractured rat ribs and reported that NGF improved ossification and fracture healing. They reported that the callus healed quickly and the fracture site was strong. Liu et al 35 concluded that bone morphogenetic protein expression in fractures increased because of the intraperitoneal NGF application in rabbits with radial fractures; thus, NGF could be directly and indirectly beneficial in fracture healing. In a rabbit distraction osteogenesis model, locally applied NGF accelerated callus maturation. 10 In the current study, the new bone volume in the NGF group was lower than that in the other groups, which may be due to the indirect effect of NGF on enhancing bone maturation mentioned above or the rapid removal of NGF from the injection site.
PBMT also has positive effects on connective tissue. In an in vivo study; parallel and well organized connective tissue was observed in extensive areas with PBMT. 36 Yan et al 37 observed a higher Ca/P ratio and a large amount of newly formed alveolar bone in the rhβ-NGF-treated group compared to the 2 control groups. Large diameter collagen bundles were observed in the SEM images of the rhβ-NGF group. According to these results, rhβ-NGF may improve the quality of regenerated bone and stimulate bone formation. 37 Buchignani et al 38 evaluated the effect of PBMT in critical defects in rats created by treating with zoledronic acid. They observed newly formed trabecular bone with osteoblasts and organized connective tissue surrounded the bone with blood vessels in the PBMT group. Connective tissue volume was highest in the PBMT and NGF + PBMT groups. Although there was an increase in connective tissue volume in the NGF + PBMT group compared with that in the PBMT group, no statistically significant difference was found between these 2 groups. These results support the idea that laser application has a positive effect on bone regeneration by increasing connective tissue migration during the possible regeneration process.
Laser therapy induces fibro vascularization through the strong expression of VEGF. Park et al 39 applied a 980 nm GaAlAs diode laser to the rat extraction sockets and found that PBMT promoted VEGF expression. Briteño-Vázquez et al 40 applied PBMT for 10 days in a continuous fashion to the rat tibial fracture model. The fibroblast growth and proliferation, bone matrix, and newly formed vessels increased in the PBMT group. Góralczyk et al 41 observed that low-level laser therapy at 635 nm significantly increased the number of human umbilical vein endothelial cells. In our study, the PBMT group showed higher new vessel volume in accordance with the literature. In contrast to our results, it was reported that NGF could enhance endothelial cell proliferation, gene expression on different titanium surfaces, and neovascularization in chicken embryo chorioallantoic membrane. 18 The absence of a significant difference in the volume of new vessels in the PBMT + NGF group suggests that the laser may delay the maturation effect of the NGF and suppress its long-term effect.
PBMT is frequently used to accelerate nerve regeneration in clinical practice. In a systematic review, IAN sensory healing after sagittal split osteotomy was improved by PBMT. 42 Diker et al 43 reported that PBMT with the 808 nm wavelength stimulated IAN regeneration after nerve crush injury. Andreo et al 27 investigated the effects of PBMT on the treatment of peripheral nerve injury in experimental models and reported that PBMT using red or infrared light showed positive results for the treatment of peripheral nerve injury and is a viable phototherapeutic modality for the treatment. According to the histopathological results in this study, PBMT has positive effects on nerve regeneration in accordance with the literature. Contrary to our results, in the rat facial injury model, the results for the PBMT group were not statistically significant compared to those for the control group. The authors attributed this result to the distant position of the probe from the injury site. 44
NGF is an important growth factor in the survival, growth, and maintenance of certain types of neurons in the central and peripheral nervous systems. 9 Du et al 45 performed intramuscular NGF for 20 postoperative days after bilateral distraction osteogenesis in a rabbit study. They observed less myelin debris and more regenerated axons in the inferior alveolar nerve at the end of the second and fourth weeks than in the control group at the end of the distraction and at the first, second, and fourth weeks after consolidation. The myelinated axon density was found to be significantly higher than that in the control group, and NGF accelerated and IAN healing in distraction osteogenesis. Wang et al 46 showed that the local application of human NGF-modified mesenchymal stem cells accelerated nerve morphological recovery after mandibular distraction osteogenesis. The authors also reported no risk of injection-induced nerve injury. In this study, we used repeated injections of NGF locally, and most of the myelinated nerve fibers regenerated in the NGF-treated group and showed a normal appearance with significantly reduced axon diameter and myelin sheath thickness compared to the control group. Histopathological analysis of the mental nerve samples showed that there was no difference between the NGF, PBMT, and NGF + PBMT groups, and the myelin structures were observed to have a similar appearance. This could be attributed to the local delivery of NGF to the fracture site. Carriers can be used to maintain the NGF concentration. Because of the repeated punctures, the effect of NGF may not be observed due to infection.
This study has some limitations that should be highlighted. The most important limitation of this study is the lack of stereological analysis of nerve tissue during histopathological follow-up procedures, due to problems with nerve tissue fixation. However, the histopathological results obtained in this study were consistent with those of previous studies.
PBMT is thought to be a more useful method, especially in cases where bone and nerve injuries occur simultaneously. NGF may be recommended for healing nerve tissues after crush injury. Further studies using different laser parameters and treatment protocols are needed to establish the mechanism of action of the methods used in vivo. Additionally, different doses of NGF and appropriate injection sites or application methods can be used in further studies to understand the effect of NGF on bone healing.
Acknowledgments
Thanks to Ondokuz Mayis University Research Fund for supporting this study. Thanks to Dr Mert Nahir for illustrations to describe the application of PBMT and NGF.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was funded by Ondokuz Mayis University Research Fund (Grand Number PYO.DIS.1904.13.004).
Ethical Approval: All experimentation was authorized by the Experimental Animal Research Center of the OMU Faculty of Medicine (2012/51, Samsun, Turkey) and was approved by the institutional review board and the local ethics committee on animal experiments.
ORCID iD
Esengul Sen https://orcid.org/0000-0001-9273-0235
References
- 1.Oji C. Jaw fractures in Enugu, Nigeria, 1985–95. The British Journal of Oral Maxillofacial Surgery. 1999, 37(2):106-109. doi: 10.1054/BJOM.1997.0083 [DOI] [PubMed] [Google Scholar]
- 2.De Sousa A. Psychological issues in oral and maxillofacial reconstructive surgery. The British Journal of Oral Maxillofacial Surgery. 2008, 46(8):661-664. doi: 10.1016/J.BJOMS.2008.07.192 [DOI] [PubMed] [Google Scholar]
- 3.Dimitriou R, Tsiridis E, Giannoudis P. V. Current concepts of molecular aspects of bone healing. Injury. 2005, 36(12):1392-1404. doi: 10.1016/j.injury.2005.07.019 [DOI] [PubMed] [Google Scholar]
- 4.Guarini D, Gracia B, Ramírez-Lobos V, Noguera-Pantoja A, Solé-Ventura P. Laser biophotomodulation in patients with neurosensory disturbance of the inferior alveolar nerve after sagittal split ramus osteotomy: a 2-year follow-up study. Photomedicine and Laser Surgery. 2018, 36(1):3-9. doi: 10.1089/pho.2017.4312 [DOI] [PubMed] [Google Scholar]
- 5.Auyong TG, Le A. Dentoalveolar nerve injury. Oral and Maxillofacial Surgery Clinics of North America. 2011;23(3):395-400. doi: 10.1016/j.coms.2011.05.001 [DOI] [PubMed] [Google Scholar]
- 6.Thomas DA, Ren K, Besse D, Ruda MA, Dubner R. Application of nitric oxide synthase inhibitor, Nω-nitro-L-arginine methyl ester, on injured nerve attenuates neuropathy-induced thermal hyperalgesia in rats. Neuroscience Letters. 1996;210(2):124-126. doi: 10.1016/0304-3940(96)12670-7 [DOI] [PubMed] [Google Scholar]
- 7.Madsen JE, Hukkanen M, Aune AK, Basran I, Moller JF, Polak JM, Nordsletten L, Fracture healing and callus lnnervation after peripheral nerve resection in rats. Clinical Orthopeadics and Related Research. 1998;(351):230-240. doi: 10.1097/00003086-199806000-00028 [DOI] [PubMed] [Google Scholar]
- 8.Yamashiro T, Fujiyama K, Fujiyoshi Y, Inaguma N, Takano-Yamamoto T. Inferior alveolar nerve transection inhibits increase in osteoclast appearance during experimental tooth movement. Bone. 2000;26(6):663-669. doi: 10.1016/S8756-3282(00)00282-9 [DOI] [PubMed] [Google Scholar]
- 9.Levi-Montalcini R. The nerve growth factor 35 years later. Science. 1987;237(4819):1154-1162. doi: 10.1126/science.3306916 [DOI] [PubMed] [Google Scholar]
- 10.Wang L, Zhou S, Liu B, Lei D, Zhao Y, Lu C, Tan A, Locally applied nerve growth factor enhances bone consolidation in a rabbit model of mandibular distraction osteogenesis. Journal of Orthopaedic Research. 2006;24(12):2238-2245. doi: 10.1002/jor.20269 [DOI] [PubMed] [Google Scholar]
- 11.Savignat M, Vodouhe C, Ackermann A, Haikel Y, Lavalle P, Libersa P. Evaluation of early nerve regeneration using a polymeric membrane functionalized with nerve growth factor (NGF) after a crush lesion of the rat mental nerve 711. Journal of Oral Maxillofacial Surgery. 2008;66:711-717. doi: 10.1016/j.joms.2007.06.654 [DOI] [PubMed] [Google Scholar]
- 12.Savignat M, De-Doncker L, Vodouhe C, Garza J, Lavalle P, Libersa P. Rat nerve regeneration with the use of a polymeric membrane loaded with NGF. Journal of Dental Research. 2007;86(11):1051-1056. doi: 10.1177/154405910708601106 [DOI] [PubMed] [Google Scholar]
- 13.Nakagawa K, Takeda M, Tsuboi Y, Kondo M, Kitagawa J, Matsumoto S, Kobayashi A, Sessle BJ, Shinoda M, Iwata K, Alteration of primary afferent activity following inferior alveolar nerve transection in rats. Molecular Pain. 2010;6:1744-8069 doi:1 10.1186/1744-8069-6-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li K, Yang L, Qiao Y-J, Liang Y-J, Wang X, Liao G-Q. Risk factors and prognosis for the primary intraosseous carcinoma of the jaw. International Journal of Oral and Maxillofacial Surgery. 2019;48(2):157-162. doi: 10.1016/J.IJOM.2018.07.019 [DOI] [PubMed] [Google Scholar]
- 15.Sun W, Sun C, Lin H, Zhao H, Wang J, Ma H, Chen B, Xiao Z, Dai J, The effect of collagen-binding NGF-β on the promotion of sciatic nerve regeneration in a rat sciatic nerve crush injury model. Biomaterials. 2009;30(27):4649-4656. doi: 10.1016/j.biomaterials.2009.05.037 [DOI] [PubMed] [Google Scholar]
- 16.Eppley BL, Snyders RV, Winkelmann TM, Roufa DG. Efficacy of nerve growth factor in regeneration of the mandibular nerve: a preliminary report. Journal of Oral Maxillofacial Surgery. 1991;49(1):61-68. doi: 10.1016/0278-2391(91)90268-Q [DOI] [PubMed] [Google Scholar]
- 17.Wang L, Zhao Y, Cheng X, Yang Y, Liu G, Ma Q, Shang H, Tian L, Lei D, Effects of locally applied nerve growth factor to the inferior alveolar nerve histology in a rabbit model of mandibular distraction osteogenesis. International Journal of Oral Maxillofacial Surgery. 2009;38(1):64-69. doi: 10.1016/J.IJOM.2008.11.010 [DOI] [PubMed] [Google Scholar]
- 18.Guang M, Yao Y, Zhang L, Huang B, Xiang L, Jin J, Gong P, The effects of nerve growth factor on endothelial cells seeded on different titanium surfaces. International Journal of Oral Maxillofacial Surgery. 2015;44(12):1506-1513. doi: 10.1016/J.IJOM.2015.06.016 [DOI] [PubMed] [Google Scholar]
- 19.Bayat M, Virdi A, Jalalifirouzkouhi R, Rezaei F. Comparison of effects of LLLT and LIPUS on fracture healing in animal models and patients: a systematic review. Progress in Biophysics and Molecular Biology. 2018;132:3-22. doi: 10.1016/j.pbiomolbio.2017.07.004 [DOI] [PubMed] [Google Scholar]
- 20.Kazancioglu HO, Ezirganli S, Aydin MS. Effects of laser and ozone therapies on bone healing in the calvarial defects. Journal of Craniofacial Surgery. 2013;24(6):2141-2146. doi: 10.1097/SCS.0b013e3182a244ae [DOI] [PubMed] [Google Scholar]
- 21.Luger EJ, Rochkind S, Wollman Y, Kogan G, Dekel S. Effect of low-power laser irradiation on the mechanical properties of bone fracture healing in rats. Lasers in Surgery and Medicine. 1998;22(2):97-102. doi: [DOI] [PubMed] [Google Scholar]
- 22.Rochkind S, Ouaknine GE. New trend in neuroscience: low-power laser effect on peripheral and central nervous system (basic science, preclinical and clinical studies). Neurological Research. 1992;14(1):2-11. doi: 10.1080/01616412.1992.11740003 [DOI] [PubMed] [Google Scholar]
- 23.Friedmann H, Lubart R, Laulicht I, Rochkind S. A possible explanation of laser-induced stimulation and damage of cell cultures. Journal of Photochemistry Photobiology B Biology. 1991;11(1):87-91. doi: 10.1016/1011-1344(91)80271-I [DOI] [PubMed] [Google Scholar]
- 24.Kheiri A, Amid R, Kheiri L, Namdari M, Mojahedi M, Kadkhodazadeh M. Effect of low- level laser therapy on bone regeneration of critical-size bone defects: a systematic review of in vivo studies and meta-analysis. Archives of Oral Biology. 2020;117:104782. doi: 10.1016/j.archoralbio.2020.104782 [DOI] [PubMed] [Google Scholar]
- 25.Hsieh YL, Chou LW, Chang PL, Yang CC, Kao MJ, Hong CZ. Low-level laser therapy alleviates neuropathic pain and promotes function recovery in rats with chronic constriction injury: possible involvements in hypoxia-inducible factor 1α (HIF-1α). Journal of Comparative Neurology. 2012;520(13):2903-2916. doi: 10.1002/cne.23072 [DOI] [PubMed] [Google Scholar]
- 26.Gomes LEA, Dalmarco EM, André ES. The brain-derived neurotrophic factor, nerve growth factor, neurotrophin-3, and induced nitric oxide synthase expressions after low-level laser therapy in an axonotmesis experimental model. Photomedicine Laser and Surgery. 2012;30(11):642-647. doi: 10.1089/pho.2012.3242 [DOI] [PubMed] [Google Scholar]
- 27.Andreo L, Soldera CB, Ribeiro BG, de Matos PRV, Bussadori SK, Fernandes KPS, et al. Effects of photobiomodulation on experimental models of peripheral nerve injury. Lasers in Medical Science. 2017;32(9):2155-2165. doi: 10.1007/s10103-017-2359-7 [DOI] [PubMed] [Google Scholar]
- 28.Fazilat F, Ghoreishian M, Fekrazad R, Kalhori KAM, Khalili SD, Pinheiro ALB. Cellular effect of low-level laser therapy on the rate and quality of bone formation in mandibular distraction osteogenesis. Photomedicine and Laser Surgery. 2014;32(6):315-321. doi: 10.1089/pho.2013.3559 [DOI] [PubMed] [Google Scholar]
- 29.Hosseinpour S, Fekrazad R, Arany PR, Ye Q. Molecular impacts of photobiomodulation on bone regeneration: a systematic review. Progress in Biophysics and Molecular Biology. 2019;149:147-159. doi: 10.1016/j.pbiomolbio.2019.04.005 [DOI] [PubMed] [Google Scholar]
- 30.Odaci E, Sahin B, Sonmez OF, Kaplan S, Bas O, Bilgic S, et al. Rapid estimation of the vertebral body volume: a combination of the Cavalieri principle and computed tomography images. European Journal of Radiology. 2003;48(3):316-326. doi: 10.1016/S0720-048X(03)00077-9 [DOI] [PubMed] [Google Scholar]
- 31.Son J, Kim Y-B, Ge Z, Choi S-H, Kim G. Bone healing effects of diode laser (808 nm) on a rat tibial fracture model. In Vivo. 2012;26(4):703-709. [PubMed] [Google Scholar]
- 32.Sun S, Diggins NH, Gunderson ZJ, Fehrenbacher JC, White FA, Kacena MA. No pain, no gain? the effects of pain-promoting neuropeptides and neurotrophins on fracture healing. Bone. 2020;131:115109. doi: 10.1016/j.bone.2019.115109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sang XG, Wang ZY, Cheng L, Liu YH, Li YG, Qin T, et al. Analysis of the mechanism by which nerve growth factor promotes callus formation in mice with tibial fracture. Experimental and Therpeutic Medicine. 2017;13(4):1376-1380. doi: 10.3892/etm.2017.4108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grills BL, Schuijers JA, Ward AR. Topical application of nerve growth factor improves fracture healing in rats. Journal of Orthopaedic Research. 1997;15(2):235-242. doi: 10.1002/jor.1100150212 [DOI] [PubMed] [Google Scholar]
- 35.Liu Y, Zhao D, Wang W, Wang B, Liu Z, Zhang Y, et al. [Nerve growth factor modulates bone morphogenetic protein expression in rabbit fracture]. Zhonghua Yixue Zazhi. 2014;94(23):1825-1828. [PubMed] [Google Scholar]
- 36.Moreira G, Alves P, Esper L, Sbrana M, Dalben G, Neppelenbroek K, et al. Effect of low-level laser on the healing of bone defects filled with autogenous bone or bioactive glass: in vivo study. International Journal of Oral Maxillofacial Implants. 2018;33(1):169-174. doi: 10.11607/jomi.5900 [DOI] [PubMed] [Google Scholar]
- 37.Yan X-Z, Ge S-H, Sun Q-F, Guo H-M, Yang P-S. A pilot study evaluating the effect of recombinant human bone morphogenetic protein-2 and recombinant human beta-nerve growth factor on the healing of class iii furcation defects in dogs. Journal of Periodontology. 2010;81(9):1289-1298. doi: 10.1902/jop.2010.090655 [DOI] [PubMed] [Google Scholar]
- 38.Buchignani VC, Germano EJ, dos Santos LM, Gulinelli JL, Ishikiriama BLC, Orcini WA, et al. Effect of low-level laser therapy and zoledronic acid on bone repair process. Lasers Medical Science. 2019;34(6):1081-1088. doi: 10.1007/s10103-019-02810-8 [DOI] [PubMed] [Google Scholar]
- 39.Park JB, Ahn SJ, Kang YG, Kim EC, Heo JS, Kang KL. Effects of increased low-level diode laser irradiation time on extraction socket healing in rats. Lasers Medical Science. 2015;30(2):719-726. doi: 10.1007/s10103-013-1402-6 [DOI] [PubMed] [Google Scholar]
- 40.Briteño-Vázquez M, Santillán-Díaz G, González-Pérez M, Gallego-Izquierdo T, Pecos-Martin D, Plaza-Manzano G, et al. Low power laser stimulation of the bone consolidation in tibial fractures of rats: a radiologic and histopathological analysis. Lasers Medical Science. 2014;30(1):333-338. doi: 10.1007/s10103-014-1673-6 [DOI] [PubMed] [Google Scholar]
- 41.Góralczyk K, Szymańska J, Łukowicz M, Drela E, Kotzbach R, Dubiel M, et al. Effect of LLLT on endothelial cells culture. Lasers Medical Science. 2014;30(1):273-278. doi: 10.1007/s10103-014-1650-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mirzaei A, Saberi-Demneh A, Gutknecht N, Ramezani G. The effect of low-level laser radiation on improving inferior alveolar nerve damage after sagittal split osteotomy: a systematic review. Lasers Medical Science. 2019;34(5):865-872. doi: 10.1007/s10103-019-02718-3 [DOI] [PubMed] [Google Scholar]
- 43.Diker N, Aytac D, Helvacioglu F, Oguz Y. Comparative effects of photobiomodulation therapy at wavelengths of 660 and 808 nm on regeneration of inferior alveolar nerve in rats following crush injury. Lasers Medical Science. 35, 413, 420, 2019. doi: 10.1007/s10103-019-02838-w [DOI] [PubMed] [Google Scholar]
- 44.Yuca Y, Yucesoy T, Tok OE, Alkan A. The efficiency of ozone therapy and low-level laser therapy in rat facial nerve injury. Journal of Cranio-Maxillofacial Surgery 2020;48(3):308-314. doi: 10.1016/j.jcms.2020.01.017 [DOI] [PubMed] [Google Scholar]
- 45.Du ZJ, Wang L, Lei DL, Liu B, Cao J, Zhang P, et al. Nerve growth factor injected systemically improves the recovery of the inferior alveolar nerve in a rabbit model of mandibular distraction osteogenesis. British Journal of Oral Maxillofacial Surgery. 2011;49(7):557-561. doi: 10.1016/j.bjoms.2011.07.002 [DOI] [PubMed] [Google Scholar]
- 46.Wang L, Zhao Y, Cao J, Yang X, Lei D. Mesenchymal stem cells modified with nerve growth factor improve recovery of the inferior alveolar nerve after mandibular distraction osteogenesis in rabbits. British Journal of Oral Maxillofacial Surgery. 2015;53(3):279-284. doi: 10.1016/j.bjoms.2014.12.014 [DOI] [PubMed] [Google Scholar]