Abstract
The role of a component of the bacterial photosystem, the PufX protein, was examined by heterologous expression of the pufX gene from Rhodobacter capsulatus in a strain of R. sphaeroides that lacks the native pufX gene. The strain of R. sphaeroides containing the R. capsulatus PufX protein was capable of efficient transduction of light energy despite a low degree of sequence conservation between the PufX proteins from the two species. The organization of the hybrid reaction center/LH1 photosystem in strains of R. sphaeroides containing the R. capsulatus LH1 antenna complex was affected differently by the R. sphaeroides and R. capsulatus PufX proteins. We discuss the implications of our findings for the role of the PufX protein in organizing the bacterial photosystem for efficient transduction of light energy.
Photosynthetic organisms carry out the conversion of light energy into electrochemical energy in membrane-bound pigment-protein complexes termed reaction centers (5, 6, 10, 27). The efficiency of the photosynthetic process is greatly enhanced by the presence of light-harvesting pigment-protein complexes, which funnel excitation energy to the reaction center. In Rhodobacter sphaeroides, the reaction center is intimately associated with the LH1 antenna in a characteristic stoichiometry, forming the RC/LH1 core complex. Core complexes are surrounded and interconnected by a variable amount of a peripheral LH2 antenna (25). In Rhodopseudomonas acidophila, the LH2 complex has a ninefold symmetry, comprising a transmembrane cylinder of nine α polypeptides surrounded by a second cylinder of nine β polypeptides (22). These concentric cylinders encase rings of 18 B850 and 9 B800 bacteriochlorophylls (Bchls), as well as carotenoid molecules. It has been proposed that LH1 antenna complexes have a similar cylindrical structure and that the reaction center occupies the space in the center of the LH1 cylinder (4, 15, 28).
In addition to the reaction center and LH1 complex, the core complex of R. sphaeroides appears to contain one or more copies of a polypeptide of 82 amino acids that is the product of the pufX gene (7). This gene is part of a polycistronic message which includes both LH1 genes (pufAB), as well as two of the reaction center genes (pufLM) (18). The pufX gene has been identified unambiguously in only one other species of purple bacterium, namely, Rhodobacter capsulatus (29). These puf operon genes are transcribed in the order BALMX in both R. sphaeroides and R. capsulatus, and it is thought that the structure and organization of the photosynthetic apparatus encoded by these genes are very similar in the two bacteria (11, 30). In support of this, sequence comparisons have revealed a high degree of identity between the protein sequences of the reaction center L, M, and H subunits and the LH1 α and β polypeptides from the two species (∼75%, on average). The role of the PufX protein appears to be to facilitate photosynthetic growth in strains of bacteria that have normal, or near to normal, levels of the LH1 antenna complex (9, 16, 20, 23).
Given the similarities between the photosystems of R. sphaeroides and R. capsulatus, it has been assumed that the role played by the PufX protein is essentially the same in the two species. However, the sequence identity in alignments between the PufX proteins from the two species is very low (29%; see Discussion), with no clear regions of local high conservation. This study examined the functional properties of the PufX proteins from R. capsulatus and R. sphaeroides by heterologous expression of the R. capsulatus protein in a strain of R. sphaeroides that lacks the native PufX protein. The functional similarity of the photosystems of these two species was examined further by heterologous expression of the structural genes of the R. capsulatus LH1 complex in R. sphaeroides and coexpression of these genes with the pufX gene from R. capsulatus.
MATERIALS AND METHODS
Construction of plasmids for heterologous expression of the pufX gene.
Replacement of the R. sphaeroides pufX gene was achieved by modification of plasmid pRKEH10 (23). To avoid confusion, the pufX genes from R. sphaeroides and R. capsulatus will henceforth be referred to as pufXs and pufXc, respectively, and the corresponding gene products will be called PufXs and PufXc. The LH1 genes and complexes from the two species are described by a similar nomenclature, i.e., pufAsBs, pufAcBc, LH1s, and LH1c. Plasmid pRKEH10 is based on broad-host-range vector pRK415 and contains a 6.5-kb EcoRI-HindIII fragment of R. sphaeroides DNA encompassing the entire puf operon. For subcloning, plasmid pSKBH was constructed; this consisted of 3.2 kb of R. sphaeroides DNA from the BamHI site located between pufM and pufXs to the downstream HindIII site cloned into plasmid pBlueSK+ (Stratagene). To facilitate excision of the pufXs gene coding sequence, an EcoRI site was introduced at a position 2 bp downstream of the stop codon of the pufX gene by mismatch oligonucleotide mutagenesis. The template for mutagenesis was a 326-bp BamHI-SmaI restriction fragment encompassing the pufX gene cloned into plasmid pALTER-1 (Promega). After successful introduction of the EcoRI site, the mutated BamHI-SmaI restriction fragment was inserted into plasmid pSKBH, replacing the equivalent native BamHI-SmaI fragment and forming plasmid pSKBEH. The EcoRI site downstream of pufXs was then transferred to plasmid pRKEH10 as a 3.2-kb BamHI-HindIII fragment to give plasmid pRKEHXs. Replacement of the pufXs gene with the pufXc gene was achieved by using plasmid pSKBEH. The pufXc gene was isolated by PCR from a pUC13-based plasmid containing the entire R. capsulatus puf operon (pUC13::puf). The oligonucleotide primers used were designed such that only the pufX coding sequence would be changed in the final construct. The PCR product was digested with BamHI and EcoRI and inserted into plasmid pSKBEH in place of the 265-bp BamHI-EcoRI fragment that encompasses the pufXs gene. Successful replacement of the R. sphaeroides gene was confirmed by DNA sequencing. The hybrid R. sphaeroides pufBALM-R. capsulatus pufX operon was then constructed by transfer of the pufXc gene into plasmid pRKEHXs as a 3.2-kb BamHI-HindIII fragment to give plasmid pRKEHXc. The success of this procedure was confirmed by DNA sequencing. The pufX deletion construct used in this study was plasmid pRKEH10X− (23).
Construction of plasmids for heterologous expression of the R. capsulatus pufBA genes.
Replacement of the R. sphaeroides pufBsAs genes was achieved by a strategy similar to that described above for pufXs. The starting plasmid was pUCEBHNX, which consists of a 1.6-kb EcoRI-XbaI restriction fragment encompassing pufBsAs in pUC19. This restriction fragment had an engineered BamHI site located 1 bp upstream of the start codon of pufBs, an engineered HindIII restriction site located immediately upstream of the start codon for pufAs, and an engineered NruI restriction site located immediately downstream of the stop codon for pufAs. These engineered restriction sites do not affect expression of the R. sphaeroides puf operon (26). The R. capsulatus pufBA genes were isolated by PCR from plasmid pUC13::puf. The oligonucleotide primers were constructed such that only the coding sequences for pufBA, plus the 13-bp intervening region, would be changed in the final construct. The PCR product was digested with BamHI and NruI and inserted into plasmid pUCEBHNX in place of the 353-bp BamHI-NruI fragment that encompasses the pufBsAs genes. Successful replacement of the R. sphaeroides genes was confirmed by loss of the R. sphaeroides HindIII restriction site and subsequent DNA sequencing. The hybrid R. sphaeroides-R. capsulatus construct was then shuttled into plasmids pRKEHXs, pRKEHXc, and pRKEH10X− as an EcoRI-XbaI restriction fragment, generating plasmids pRKEHLH1cXs, pRKEHLH1cXc, and pRKEHLH1c10X−, respectively.
Construction of mutant strains.
The six plasmids described above were transferred to R. sphaeroides DD13 (13) by conjugation from Escherichia coli S17-1 (12). Transconjugant strains were selected on the basis of growth of single colonies on M22+ agar plates supplemented with tetracycline and kanamycin under semiaerobic conditions in the dark.
Bacterial growth and preparation of intracytoplasmic membranes.
R. sphaeroides strains were grown under dark, semiaerobic conditions at 34°C in M22+ medium as previously described (14). Photoheterotrophic growth was conducted in 15-ml screw-top test tubes filled with M22+ medium and supplemented with 0.1% Casamino Acids and kanamycin at 20 μg/ml. The inoculum consisted of cells harvested from a 70-ml culture grown under dark, semiaerobic conditions to an optical density at 680 nm (OD680) of approximately 1.5; harvested cells were added to the 15-ml screw-top tubes to give an initial OD680 of between 0.1 and 0.2. The tubes were incubated at 34°C in a glass-sided water bath illuminated by three 500-W tungsten floodlamps, giving an incident light intensity of approximately 40 W/m2. Growth was monitored at 680 nm with a Sherwood colorimeter. Intracytoplasmic membranes were prepared from cells grown under dark, semiaerobic conditions to late log phase as described previously (14).
Spectroscopy.
Absorbance spectra of cells and intracytoplasmic membranes were recorded in a Beckman DU640 spectrophotometer. Fluorescence emission spectra were recorded with a FluoroMax spectrofluorimeter (SPEX Industries Inc.). Excitation was in the Qx band of LH1 at 590 nm with 18-nm resolution. Emission was monitored between 850 and 950 nm with a resolution of 9 nm. Samples were normalized to a concentration of 0.2 absorbance unit cm−1 at the maximum of the LH1 Qy band.
RESULTS
Heterologous expression of the R. capsulatus pufX gene in R. sphaeroides.
Plasmids pRKEHXs, pRKEH10X−, and pRKEHXc (see Materials and Methods) were expressed in R. sphaeroides DD13 (13), which is devoid of RC/LH1 core complexes due to a deletion-insertion mutation in the genomic copy of the puf operon and is devoid of LH2 antenna complexes due to a similar mutation in the genomic copy of the puc operon. Transconjugant strains were selected as described previously (12) and were given the names RCLH1sXs, RCLH1sXd, and RCLH1sXc, where the lowercase letters indicate the origins of the LH1 antenna and PufX protein (s, R. sphaeroides; c, R. capsulatus; d, deletion of complex). The strains had an RC+ LH1+ LH2− phenotype.
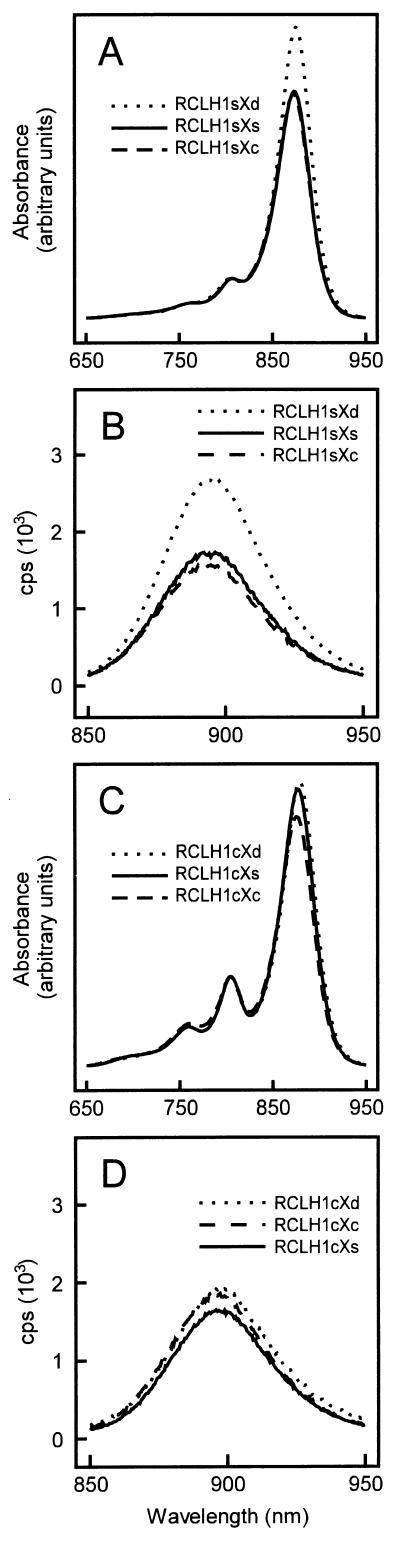
To assess the effects of the PufXc protein on the composition of the R. sphaeroides core complex, intracytoplasmic membranes were prepared from cells grown under semiaerobic conditions in the dark and examined by absorbance spectroscopy (Fig. 1A). As seen in a previous study (23, 24), deletion of the pufXs gene resulted in an increase in the amount of LH1 per reaction center (Fig. 1A; dotted and solid curves), seen as an increase in the amplitude of the LH1 Qy band (at approximately 875 nm) relative to the reaction center Qy band (at approximately 802 nm). The position of the absorbance maximum of the LH1 Qy band was not affected significantly by removal of PufXs (23, 24). Experiments with samples that had been normalized to contain approximately the same concentration of LH1 showed that the amount of LH1 fluorescence from membranes of strain RCLH1sXd was ∼60% greater than that from membranes of control strain RCLH1sXs (Fig. 1B, cf. dotted and solid curves). The emission maximum was at 895 nm for membranes from both strains. These fluorescence data are in good agreement with observations on R. capsulatus (21) which showed that strains that lack the PufX polypeptide use harvested light energy less effectively. For comparison, the amount of fluorescence emission was also measured for membranes from an LH1-only strain (i.e., a strain which lacks the reaction center, LH2, and PufX). For samples normalized to the same concentration of LH1 Bch1, emission was approximately 3.7-fold greater in membranes from the LH1-only strain than from strain RCLH1sXs (data not shown).
FIG. 1.
Room temperature absorbance (A and C) and fluorescence emission (B and D) spectra of intracytoplasmic membranes. For comparison, absorbance spectra were corrected for background scatter between 650 and 950 nm and normalized to the same absorbance at 802 nm, approximating the same concentration of reaction centers. For fluorescence emission spectra, membranes were normalized to the same absorbance (0.2 absorbance unit cm−1) at the maximum of the LH1 Qy band.
Assembly of the core complex was not impaired by replacement of the pufXs gene by the pufXc gene in strain RCLH1sXc. The level of LH1 per reaction center seen in the presence of the PufXc protein was essentially the same as that seen in the presence of PufXs and was quite distinct from the elevated level of LH1 seen in strain RCLH1sXd (Fig. 1A, dashed curve). The LH1 Qy band was blue shifted by 2 nm relative to that seen in strains RCLH1sXs and RCLH1sXd. Membranes of the RCLH1sXc strain showed a level of LH1 fluorescence slightly below that seen in membranes from the RCLH1sXs strain and much lower than that seen in the RCLH1sXd strain (Fig. 1B; dashed curve). This indicated that the presence of the PufXc protein improved the effectiveness with which LH1 excitation energy was used by the reaction center, relative to core complexes which lacked either PufX protein. Taken together, therefore, the absorption and fluorescence data demonstrate a direct interaction between the PufXc protein and the R. sphaeroides core complex.
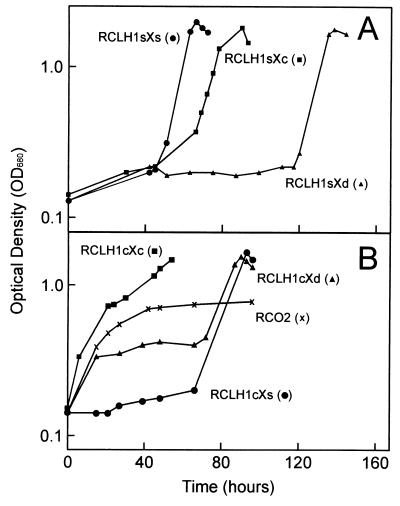
Photosynthetic growth of strain RCLH1sXc was similar to that exhibited by control strain RCLH1sXs (Fig. 2A) in that appreciable photosynthetic growth was observed after a lag of 50 to 60 h. This lag has been attributed to a period of adaptation from semiaerobic, dark growth conditions to photosynthetic growth that is peculiar to strains with an RC+ LH1+ LH2− phenotype and which have normal, or near to normal, levels of the LH1 antenna complex (23, 24). Experiments involving cycles of semiaerobic, dark growth and photosynthetic growth showed that the growth exhibited by strains RCLH1sXs and RCLH1sXc after the 60-h lag did not occur in response to suppressor mutations (data not shown). Growth of strain RCLH1sXc was quite distinct from that exhibited by PufX-deficient strain RCLH1sXd, which only grew after a very long lag phase (if at all), presumably in response to one or more suppressor mutations (1, 19). The PufXc protein, therefore, was competent in supporting the photosynthetic growth of R. sphaeroides.
FIG. 2.
Photosynthetic growth of strains containing the LH1s complex (A) and the LH1c complex (B). Growth was monitored by measuring OD680 and is plotted on a log10 scale. For each strain, the inoculum consisted of cells taken from a culture that had been grown under semiaerobic conditions in the dark. In B, the growth of antenna-deficient strain RCO2 is shown for comparison.
Heterologous expression of the pufBA genes from R. capsulatus in R. sphaeroides.
The functional identity between core complex proteins from the two species of purple bacteria was investigated further by heterologous expression of the pufBA genes from R. capsulatus in R. sphaeroides. The pufBcAc genes were expressed in R. sphaeroides DD13 in tandem with the pufXs gene (strain RCLH1cXs) and in the absence of either pufX gene (strain RCLH1cXd).
Room temperature absorption spectra showed that the LH1c complex was successfully expressed in R. sphaeroides but that the level of expression relative to that of the reaction center was 50 to 60% of that of the native LH1s complex, regardless of the presence or absence of the PufXs protein (Fig. 1C, solid and dotted curves). This can be seen by comparing the relative heights of the LH1 Qy band at 875 to 878 nm and the reaction center Qy band at 802 nm in Fig. 1A and C. The maximum of the LH1c Qy band in strain RCLH1cXs was located 2 nm to the red of the corresponding band of native LH1s (877 versus 875 nm) and was red shifted by a further 1 nm in strain RCLH1cXd. The amount of fluorescence emission from membranes of strain RCLH1cXd (Fig. 1D, dotted curve) was similar to that of strain RCLH1cXs (Fig. 1D, solid curve), in contrast to the significant difference shown in Fig. 1B for the analogous strains containing the LH1s complex. The level of fluorescence in both strains was comparable to that seen for control strain RCLH1sXs (Fig. 1B, solid curve) and was therefore well below the level expected for RC-free LH1 (see above).
To examine whether the relatively low level of LH1c expression in R. sphaeroides was attributable to the lack of the PufXc protein, plasmid pRKEHLH1cXc was constructed to allow coexpression of the pufBcAc genes with the pufXc gene (see Materials and Methods). As can be seen in Fig. 1C (dashed curve), the absorption spectrum of membranes from the resulting strain (RCLH1cXc) was very similar to those of strains RCLH1cXd and RCLH1cXs, showing that the relatively low level of LH1c in these membranes was not a consequence of the absence of the PufXc protein. Fluorescence emission from membranes of strain RCLH1cXc was similar to that shown by strains RCLH1cXs and RCLH1cXd and control strain RCLH1sXs (Fig. 1D).
All three of the LH1c-containing strains were capable of photosynthetic growth (Fig. 2B). Strain RCLH1cXs grew following a 60-h lag (Fig. 2B, circles) in a manner similar to that of control strain RCLH1sXs (Fig. 2A, circles) and PufXc-containing strain RCLH1sXc (Fig. 2A, squares). In contrast, strain RCLH1cXc grew without the initial lag (Fig. 2B, squares) in a manner similar to that of antenna-deficient strain RCO2 (Fig. 2B, crosses), although the final OD reached was significantly greater than that achieved by strain RCO2, being comparable to that seen in the other strains with an LH1 antenna. Finally, strain RCLH1cXd showed both appreciable growth with no lag and a second phase of growth after ∼70 h (Fig. 2B, triangles). It should be noted that each of these patterns of photosynthetic growth was distinctive and highly reproducible, despite the fact that the absorbance and fluorescence properties of the three strains were very similar, over and above some minor differences in the positions of absorbance and fluorescence emission maxima.
DISCUSSION
Sequence analysis of PufXs and PufXc and implications for the role of PufX.
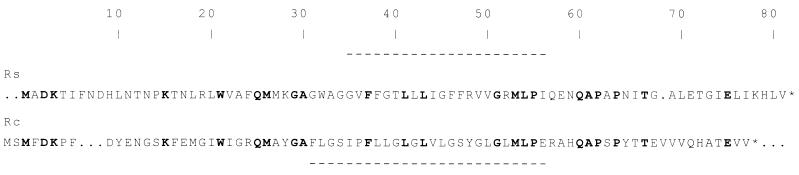
Alignments of the amino acid sequences of PufXs and PufXc, such as that shown in Fig. 3, reveal a maximum of 29% identity between the two proteins. This low sequence identity is in marked contrast to the high degree of identity seen in alignments between the reaction center and LH1 polypeptides from R. sphaeroides and R. capsulatus (between 70 and 80%). Part of this discrepancy may arise from the requirement of the LH1 and reaction center complexes to bind carotenoid and Bchl pigments, although such binding does not appear to require well-conserved and extensive structural motifs. As can be seen from the alignment shown in Fig. 3, such identity that exists between the two PufX proteins is not focused on one or two areas of high conservation but rather is randomly distributed along the entire length of the two polypeptide chains. The fact that the PufXc protein is very effective in substituting for the PufXs protein suggests that the primary sequence of a large part of the PufX protein is not particularly important for function, raising the possibility that the main requirement for PufX activity is a small number of residues, perhaps widely distributed along the protein sequence. Alternatively, the principal requirement could be the overall shape of the molecule or some other structural property that can be provided by more than one specific amino acid sequence. Hydrophobicity plots (Kyte-Doolittle algorithm) (17) performed with the PufXs and PufXc protein sequences suggested a stretch of hydrophobic amino acids in both proteins that would, in principle, be sufficiently long to span a lipid bilayer membrane as an alpha helix (Fig. 3).
FIG. 3.
Alignment of the PufX polypeptide sequences from R. sphaeroides (Rs) and R. capsulatus (Rc). The alignment gives 29% identical residues and 49% similar residues. Residue numbering is for R. sphaeroides. Identical residues are indicated by bold type. Horizontal dashes indicate probable transmembrane regions, as indicated by hydrophobicity plots. As discussed in reference 8, the best alignment is achieved with two gaps, one in each sequence.
Organization of the Rhodobacter photosystem by PufX.
The data presented in this report show that the PufX protein is a key factor in the proper functional organization of the LH1/RC core complex in R. sphaeroides and R. capsulatus. Two findings, in particular, point to this role. The first is the observation that the restoration to photosynthetic competence seen on expression of the pufXc gene in place of the pufXs gene (in strain RCLH1sXc) was accompanied by restoration of the LH1:RC ratio to the value seen in control strain RCLH1sXs and restoration of the level of LH1 fluorescence to that seen in the control strain. It has been proposed that the increase in the number of LH1 Bchls per reaction center seen on removal of the PufX polypeptide reflects an increase in the aggregation state of the LH1 complex surrounding the reaction center, the result of which is to interfere with the rate of ubiquinone-ubiquinol exchange at the QB site through steric constraints (2, 3, 20, 21, 23, 24). The effects of expression of the pufXc gene in R. sphaeroides on the optical properties of the core complex and the photosynthetic competence of the bacterium revealed in this study lend further support to this model, in which PufX defines the proper organization of LH1 in the core complex.
The second finding is the distinctive patterns of photosynthetic growth exhibited by the three LH1c-containing strains, cells and membranes of which had very similar absorbance and fluorescence properties. We believe that this observation shows that while PufX is not necessarily the main determinant of the relative levels of reaction center and LH1 in the membrane, it does affect the organization of the components of the core complex and that this, in turn, determines the ability of the bacterium to grow under photosynthetic conditions. The origin of the differences in membrane organization that underlie the markedly different patterns of photosynthetic growth exhibited by strains RCLH1cXs, RCLH1cXd, and RCLH1cXc is the subject of ongoing investigations.
ACKNOWLEDGMENTS
This work was supported by the Biotechnology and Biological Sciences Research Council of the United Kingdom. M.R.J. is a BBSRC Advanced Research Fellow.
We thank C. N. Hunter, A. R. Crofts, and W. Westerhuis for helpful discussions.
REFERENCES
- 1.Barz W P, Oesterhelt D. Photosynthetic deficiency of a pufX deletion mutant of Rhodobacter sphaeroides is suppressed by point mutations in the light-harvesting complex genes pufB or pufA. Biochemistry. 1994;33:9741–9752. doi: 10.1021/bi00198a045. [DOI] [PubMed] [Google Scholar]
- 2.Barz W P, Francia F, Venturoli G, Melandri B A, Verméglio A, Oesterhelt D. Role of PufX protein in photosynthetic growth of Rhodobacter sphaeroides. 1. PufX is required for efficient light-driven electron transfer and photophosphorylation under anaerobic conditions. Biochemistry. 1996;34:15235–15247. doi: 10.1021/bi00046a032. [DOI] [PubMed] [Google Scholar]
- 3.Barz W P, Verméglio A, Francia F, Venturoli G, Melandri B A, Oesterhelt D. Role of PufX protein in photosynthetic growth of Rhodobacter sphaeroides. 2. PufX is required for efficient ubiquinone/ubiquinol exchange between the reaction center QB site and the cytochrome bc1 complex. Biochemistry. 1996;34:15248–15258. doi: 10.1021/bi00046a033. [DOI] [PubMed] [Google Scholar]
- 4.Boonstra A F, Germeroth L, Boekema E J. Structure of the light harvesting antenna from Rhodospirillum molischianum studied by electron microscopy. Biochim Biophys Acta. 1994;1184:227–234. [Google Scholar]
- 5.Deisenhofer J, Epp O, Miki K, Huber R, Michel H. Structure of the protein subunits in the photosynthetic reaction centre of Rhodopseudomonas viridis at 3 Å resolution. Nature. 1985;318:618–624. doi: 10.1038/318618a0. [DOI] [PubMed] [Google Scholar]
- 6.Deisenhofer J, Epp O, Sinning I, Michel H. Crystallographic refinement at 2.3 Å resolution and refined model of the photosynthetic reaction center from Rhodopseudomonas viridis. J Mol Biol. 1995;246:429–457. doi: 10.1006/jmbi.1994.0097. [DOI] [PubMed] [Google Scholar]
- 7.Farchaus J W, Barz W P, Grünberg H, Oesterhelt D. Studies on the expression of the pufX polypeptide and its requirement for photoheterotrophic growth in Rhodobacter sphaeroides. EMBO J. 1992;11:2779–2788. doi: 10.1002/j.1460-2075.1992.tb05345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farchaus J W, Gruenberg H, Gray K A, Wachtveitl J, DeHoff B, Kaplan S, Oesterhelt D. The puf B, A, L, M genes are not sufficient to restore the photosynthetic minus phenotype to a puf L, M, X deletion strain. In: Drews G, Dawes E A, editors. Molecular biology of membrane-bound complexes in phototrophic bacteria. New York, N.Y: Plenum Press; 1990. pp. 65–76. [Google Scholar]
- 9.Farchaus J W, Gruenberg H, Oesterhelt D. Complementation of a reaction center-deficient Rhodobacter sphaeroides pufLMX deletion strain with pufBALM does not restore the photosynthesis-positive phenotype. J Bacteriol. 1990;172:977–985. doi: 10.1128/jb.172.2.977-985.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fleming G R, van Grondelle R. The primary steps of photosynthesis. Phys Today. 1994;47:48–55. [Google Scholar]
- 11.Foloppe N, Ferrand M, Breton J, Smith J C. Structural model of the photosynthesis reaction center of Rhodobacter sphaeroides. Proteins Struct Funct Genet. 1995;22:226–244. doi: 10.1002/prot.340220304. [DOI] [PubMed] [Google Scholar]
- 12.Hunter C N, Turner G. Transfer of genes coding for apoproteins of reaction centre and light-harvesting LH1 complexes to Rhodobacter sphaeroides. J Gen Microbiol. 1988;134:1471–1480. [Google Scholar]
- 13.Jones M R, Fowler G J S, Gibson L C D, Grief G G, Olsen J D, Crielaard W, Hunter C N. Mutants of Rhodobacter sphaeroides lacking one or more pigment-protein complexes and complementation with reaction-center, LH1, and LH2 genes. Mol Microbiol. 1992;6:1173–1184. doi: 10.1111/j.1365-2958.1992.tb01556.x. [DOI] [PubMed] [Google Scholar]
- 14.Jones M R, Heer-Dawson M, Mattioli T A, Hunter C N, Robert B. Site-specific mutagenesis of the reaction centre from Rhodobacter sphaeroides studied by Fourier-transform Raman spectroscopy: mutations at tyrosine M210 do not affect the electronic structure of the primary donor. FEBS Lett. 1994;339:18–24. doi: 10.1016/0014-5793(94)80376-5. [DOI] [PubMed] [Google Scholar]
- 15.Karrasch S, Bullough P A, Ghosh R. The 8.5 Å projection map of the light-harvesting complex I from Rhodospirillum rubrum reveals a ring composed of 16 subunits. EMBO J. 1995;4:631–638. doi: 10.1002/j.1460-2075.1995.tb07041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klug G, Cohen S N. Pleiotropic effects of localized Rhodobacter capsulatus puf operon deletions on production of light-absorbing pigment-protein complexes. J Bacteriol. 1988;170:5814–5821. doi: 10.1128/jb.170.12.5814-5821.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kyte J, Doolittle R F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982;157:105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- 18.Lee J K, DeHoff B S, Donohue T J, Gumport R I, Kaplan S. Transcriptional analysis of puf operon expression in Rhodobacter sphaeroides 2.4.1 and an intercistronic transcription terminator mutant. J Biol Chem. 1989;264:19354–19365. [PubMed] [Google Scholar]
- 19.Lilburn T G, Beatty J T. Suppressor mutants of the photosynthetically incompetent pufX deletion mutant Rhodobacter capsulatus ΔRC6(pTL2) FEMS Microbiol Lett. 1992;100:155–160. [Google Scholar]
- 20.Lilburn T G, Haith C E, Prince R C, Beatty J T. Pleiotropic effects of pufX gene deletion on the structure and function of the photosynthetic apparatus of Rhodobacter capsulatus. Biochim Biophys Acta. 1992;1100:160–170. doi: 10.1016/0005-2728(92)90077-f. [DOI] [PubMed] [Google Scholar]
- 21.Lilburn T G, Prince R C, Beatty J T. Mutation of the Ser2 codon of the light-harvesting B870 α polypeptide of Rhodobacter capsulatus partially suppresses the pufX phenotype. J Bacteriol. 1995;177:4593–4600. doi: 10.1128/jb.177.16.4593-4600.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McDermott G, Prince S M, Freer A A, Hawthornthwaite-Lawless A M, Papiz M Z, Cogdell R J, Isaacs N W. Crystal structure of an integral membrane light-harvesting complex from photosynthetic bacteria. Nature. 1995;374:517–521. [Google Scholar]
- 23.McGlynn P, Hunter C N, Jones M R. The Rhodobacter sphaeroides PufX protein is not required for photosynthetic competence in the absence of a light harvesting system. FEBS Lett. 1994;349:349–353. doi: 10.1016/0014-5793(94)00701-2. [DOI] [PubMed] [Google Scholar]
- 24.McGlynn P, Westerhuis W H J, Jones M R, Hunter C N. Consequences for the organisation of reaction center/LH1 core complexes of Rhodobacter sphaeroides arising from deletion of amino acid residues from the C terminus of the LH1 α polypeptide. J Biol Chem. 1996;271:3285–3292. doi: 10.1074/jbc.271.6.3285. [DOI] [PubMed] [Google Scholar]
- 25.Monger T G, Parson W W. Singlet-triplet fusion in Rhodopseudomonas sphaeroides. Biochim Biophys Acta. 1977;460:393–407. doi: 10.1016/0005-2728(77)90080-9. [DOI] [PubMed] [Google Scholar]
- 26.Olsen J D. Ph.D. thesis. Sheffield, United Kingdom: University of Sheffield; 1995. [Google Scholar]
- 27.Parson W W. Chlorophyll functions: reaction centers. In: Scheer H, editor. Chlorophylls. Boca Raton, Fla: CRC Press, Inc.; 1991. pp. 1153–1180. [Google Scholar]
- 28.Walz T, Ghosh R. Two-dimensional crystallisation of the light-harvesting 1-reaction center photounit from Rhodospirillum rubrum. J Mol Biol. 1997;265:107–111. doi: 10.1006/jmbi.1996.0714. [DOI] [PubMed] [Google Scholar]
- 29.Youvan D C, Bylina E J, Alberti M, Begusch H, Hearst J E. Nucleotide and deduced polypeptide sequences of the photosynthetic reaction center, B879 antenna, and flanking polypeptides from R. capsulata. Cell. 1984;37:949–957. doi: 10.1016/0092-8674(84)90429-x. [DOI] [PubMed] [Google Scholar]
- 30.Zilsel J, Lilburn T G, Beatty J T. Formation of functional inter-species hybrid photosynthetic complexes in Rhodobacter capsulatus. FEBS Lett. 1989;253:247–252. doi: 10.1016/0014-5793(89)80969-x. [DOI] [PubMed] [Google Scholar]