Abstract
TNF plays a crucial role in inflammation and bone resorption in various inflammatory diseases, including rheumatoid arthritis (RA). However, its direct ability to drive macrophages to differentiate into osteoclasts is limited. Although RBP-J is recognized as a key inhibitor of TNF-mediated osteoclastogenesis, the precise mechanisms that restrain TNF-induced differentiation of macrophages into osteoclasts are not fully elucidated. Here, we identified that the Notch ligand Jagged1 is a previously unrecognized RBP-J target. The expression of Jagged1 is significantly induced by TNF mainly through RBP-J. The TNF-induced Jagged1 in turn functions as a feedback inhibitory regulator of TNF-mediated osteoclastogenesis. This feedback inhibition of osteoclastogenesis by Jagged1 does not exist in RANKL-induced mouse osteoclast differentiation, since RANKL does not induce Jagged1 expression. The Jagged1 level in peripheral blood monocytes/osteoclast precursors is decreased in RA compared to non-erosive inflammatory disease systemic lupus erythematosus (SLE), suggesting a mechanism that contributes to increased osteoclast formation in RA. Moreover, recombinant Jagged1 suppresses human inflammatory osteoclastogenesis. Our findings identify Jagged1 as an RBP-J direct target that links TNF and Notch signaling pathways and restrains TNF-mediated osteoclastogenesis. Given that Jagged1 has no effect on TNF-induced expression of inflammatory genes, its use may present a new complementary therapeutic approach to mitigate inflammatory bone loss with little impact on the immune response in disease conditions.
Introduction
The skeleton is a complex and dynamic organ that continuously adapts to mechanical and physiological changes through remodeling. This process is largely controlled by the balanced regulation of bone resorption and formation. Osteoclasts, derived from the myeloid/macrophage cell lineage, are specialized cells that are responsible for bone resorption. RANKL is an osteoclastogenic cytokine that acts together with M-CSF to induce osteoclast differentiation from macrophages. Osteoclasts not only play a critical role in bone development and physiological remodeling, but also in inflammatory bone destruction, such as in rheumatoid arthritis (RA) and periodontitis(1–7). While anti-RANKL/RANK treatments like denosumab have been effective in treating excessive bone resorption, long-term side effects have been reported from the blockade of RANKL/RANK signaling. These include bone remodeling defects, strongly inhibited osteoclast formation leading to bone repair failure, risks of atypical femoral fractures and osteonecrosis of the jaw (8, 9). Discontinuation of denosumab treatment has also been associated with rapid rebound bone resorption and increased fracture risk (10). Furthermore, standard antiresorptive therapies have limited efficacy against inflammatory bone resorption (11–17), indicating the existence of uncharacterized pathogenic mechanisms that contribute to this process.
TNF is a key cytokine that induces inflammation and stimulates bone erosion in many inflammatory diseases, such as RA, psoriatic arthritis and periodontitis. However, despite belonging to the same superfamily, TNF displays little direct ability to stimulate macrophages to differentiate into osteoclasts in contrast to RANKL, which has been a puzzle in bone field (6, 18–23). The underlying mechanisms for the weak direct osteoclastogenic capacity of TNF are largely unclear. We identified RBP-J as a critical inhibitory transcription factor that restrains TNF-mediated osteoclastogenesis (23, 24). RBP-J was originally identified as a master transcription factor in canonical Notch signaling pathway. Binding of Notch ligands, including Jagged1 (Jag1), Jagged 2 (Jag2), Delta-like (Dll) 1, 3 and 4, to Notch receptors triggers the cleavage of Notch intracellular domains, which then translocate to nucleus and activate RBP-J activity (25). Despite the fact that RBP-J is implicated in both TNF and Notch pathways, it remains unclear how RBP-J regulates the interplay of these two critical pathways and the biological significance of this regulation.
This study presents interesting findings. We have discovered that TNF induces the expression of the Notch ligand Jagged1, which is primarily dependent on RBP-J. Our results also demonstrate that Jagged1 is a direct target of RBP-J and plays a significant role in suppressing TNF-mediated osteoclastogenesis in both mouse and human cell cultures. As a result, the RBP-J-Jagged1 axis links the TNF and Notch signaling pathways. The TNF-induced Jagged1 serves as an important feedback inhibitory mechanism by which TNF limits its osteoclastogenic capacity.
Materials and Methods
Animals
We generated mice with myeloid/macrophage-specific deletion of Rbpj by crossing Rbpjf/f mice (26) with a lysozyme M promoter-driven Cre transgene on the C57BL/6 background (known as LysMcre; The Jackson Laboratory, Stock No. 004781), referred to as RbpjΔM/ΔM. We also generated mice with myeloid/macrophage-specific deletion of Jag1 by crossing LysMcre mice with B6.129S-Jag1tm2Grid/SjJ, which are floxed mutant mice possessing loxP sites flanking exon 4 of the Jag1 gene (The Jackson Laboratory Stock No. 031272), referred to as Jag1ΔM/ΔM. We generated myeloid/macrophage-specific Jag1 overexpression mice by crossing R26-LSL-JAG1 mice expressing JAG1 after exposure to Cre recombinase (The Jackson Laboratory Stock No. 030173) with the LysMcre mice, referred to as Jag1mTg. Gender- and age-matched mice with LysMcre(+) genotype were used as wild type controls (referred to as Ctrl) for experiments. All animal procedures were approved by the Hospital for Special Surgery Institutional Animal Care and Use Committee (IACUC), and Weill Cornell Medical College IACUC.
Reagents
Murine or human M-CSF, murine or human TNFα, human TGFβ1, and human RANKL were purchased from PeproTech. Murine TGFβ1 was purchased from R&D systems. Human recombinant Jagged1 was purchased from R&D Systems.
Cell culture
For cultures of mouse bone marrow macrophages (BMMs), bone marrow cells were harvested from tibiae and femora of age and gender-matched mutant and control mice and cultured for 3 days in α-MEM medium (Gibco, Thermo Fisher Scientific) with 10% fetal bovine serum (FBS) (Atlanta Biologicals, S11550), glutamine (2.4 mM, Thermo Fisher Scientific, 25030164), Penicillin–Streptomycin (Thermo Fisher Scientific, 15070063), and L929 supernatant (condition medium, CM), which contained the equivalent of 20 ng/ml of rM-CSF and was used as a source of M-CSF (27), with or without mouse TGFβ priming (1 ng/ml, Thermo Fisher Scientific, 14–8342-62). The attached BMMs were scraped and cultured in α-MEM medium with 10% FBS, glutamine and CM for overnight. The cells were then treated without or with the optimized concentration of TNFα (40 ng/ml) (PeproTech, 315–01A) or RANKL (40ng/ml) (PeproTech, 315–01C) in the presence of CM for times indicated in the figure legends. Culture media was exchanged every three days.
Human osteoclast cultures were performed as described previously (28). Briefly, peripheral blood mononuclear cells (PBMCs) from whole blood of healthy volunteers were isolated by density gradient centrifugation using Ficoll (Invitrogen Life Technologies). CD14(+) monocytes were purified from fresh PBMCs using anti-CD14 magnetic beads (Miltenyi Biotec, 130–050-201) as recommended by the manufacturer. Human CD14(+) monocytes were seeded at a density of 12.5 × 104/cm2 and cultured in α-MEM medium with 10% FBS in the presence of human M-CSF (20 ng/ml; PeproTech, 300–25) with or without human TGFβ1 (10 ng/ml, PeproTech, 100–21) for 3 days to obtain monocyte-derived macrophages with or without TGFβ1 priming. The cells were then washed with neat α-MEM medium to remove TGFβ1, and further cultured with human TNFα (40 ng/ml, PeproTech, 300–01A) and M-CSF (20 ng/ml) in α-MEM medium for different times as indicated in figure legends, and in the absence or presence of recombinant human Jagged1 (200 ng/ml, R&D Systems,1277-JG-050).
TRAP staining was performed with an acid phosphatase leukocyte diagnostic kit (Sigma-Aldrich, 387A) in accordance with the manufacturer’s instructions. TRAP-positive multinucleated cells (MNCs) containing 3 or more nuclei were counted as osteoclasts and quantified as total number or calculated at percentage of total TRAP-positive MNCs.
Mineral resorption pit assay
The mineral resorption activity of osteoclasts was examined using 96-well Corning Osteo Assay Surface Plates (Sigma-Aldrich). Frozen BMMs were thawed and seeded at a density of 6.25 × 104/cm2 in Osteo Assay Surface Plate and cultured in the presence of CM and TNF (40 ng/ml) for fourteen days. Culture medium was exchanged every two days. Cells were then removed twice with 10% bleach solution for 5 min at room temperature (RT), followed by washing with distilled water. The minerals were stained with Von Kossa to visualize the formation of resorptive pits.
Immunoblot analysis
Total cellular extracts were obtained using lysis buffer containing 150 mM Tris-HCl (pH 6.8), 6% SDS, 30% glycerol, and 0.03% Bromophenol Blue, with 10% 2-Mercaptoethanol added immediately before harvesting cells. Cell lysates were fractionated on 7.5% SDS-PAGE, transferred to Immobilon-P membranes (0.45 μm, Millipore), and incubated with specific antibodies. Western Lightning Plus-ECL (PerkinElmer) was used for detection. Jagged1 antibody (70109, 1:1000) was obtained from Cell Signaling Technology; NFATc1 antibody (556602, 1:1000) was from BD Biosciences; Blimp1 (sc-47732, 1:1000), c-Fos (sc-52, 1:1000), GAPDH (sc-25778, 1:1000) and p38α (sc-535, 1:3000) antibodies were from Santa Cruz Biotechnology.
Reverse transcription and real-time PCR
DNA-free RNAs were isolated from cells with the RNeasy MiniKit (Qiagen, 74104) with DNase treatment, and total RNA was reverse-transcribed with random hexamers using the RevertAid RT Kit (Thermo Fisher Scientific, K1691) according to the manufacturer’s instructions. Real-time PCR was done in triplicate with the QuantStudio 5 Real-time PCR system (Applied Biosystems; A28138) and Fast SYBR® Green Master Mix (Thermo Fisher Scientific; 4385612) with 500 nM primers. mRNA amounts were normalized relative to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA. The mouse primers for real-time PCR used were as follows: Jag1: 5’- TGCCTGCCGAACCCCTGTCATAAT-3’ and 5’- CCGATACCAGTTGTCTCCGTCCAC-3’; Jag2: 5’- TCCTCCTGCTGCTTTGTGAT-3’ and 5’- TGTCAGGCAGGTCCCTTG-3’; Dll1: 5’-ACAGAGGGGAGAAGATGTGC-3’ and 5’- CCCTGGCAGACAGATTGG-3’; Dll3: 5’- TCGTACGTGTGCCCTTCC-3’ and 5’- TGCTCTCTCCAGGTTTCAATG-3’; Dll4: 5’- AGGTGCCACTTCGGTTACAC-3’ and 5’- GGGAGAGCAAATGGCTGATA-3’; Mx1: 5’-GGCAGACACCACATACAACC-3’ and 5’-CCTCAGGCTAGATGGCAAG-3’; Ifit1: 5’-CTCCACTTTCAGAGCCTTCG-3’ and 5’-TGCTGAGATGGACTGTGAGG-3’; Ifit2: 5’-AAATGTCATGGGTACTGGAGTT-3’ and 5’-ATGGCAATTATCAAGTTTGTGG-3’; Il6: 5’-TACCACTTCACAAGTCGGAGGC-3’ and 5’-CTGCAAGTGCATCATCGTTGTTC-3’; Il1b: 5’-AGCTTCCTTGTGCAAGTGTCT-3’ and 5’-GACAGCCCAGGTCAAAGGTT-3’; Tnf: 5’-CCCTCACACTCAGATCATCTTCT-3’ and 5’-CTTTGAGATCCATGCCGTTG-3’; Jag1 (exon 4–6): 5’- TGTGACCAGAACGGCAACAA-3’ and 5’- CACCTGCAGTCACCTGGAAG −3’; Irf8: 5’-AATGCAAGCTGGGCGTGGCA-3’ and 5’-CCTGCACTGGGCTGCTGGAC-3’; Mafb: 5’-AACGGTAGTGTGGAGGAC-3’ and 5’-TCACAGAAAGAACTCAGGA-3’; Rbpj: 5’-CGGCCTCCACCCAAACGACT-3’ and 5’-TCCAACCACTGCCCATAAGATACA-3’; Gapdh: 5’-ATCAAGAAGGTGGTGAAGCA-3’ and 5’-AGACAACCTGGTCCTCAGTGT-3’. The human primers for real-time PCR used were as follows: JAG1: 5’- AATGGCTACCGGTGTGTCTG-3’ and 5’- CCCATGGTGATGCAAGGTCT-3’; ACP5: 5′-TGGCTTTGCCTATGTGGA-3′ and 5′-CCTGGTCTTAAAGAGGGACTT-3′; CTSK: 5′-CTCTTCCATTTCTTCCACGAT-3′ and 5′-ACACCAACTCCCTTCCAAAG-3′; GAPDH: 5’-ATCAAGAAGGTGGTGAAGCA-3’ and 5’-GTCGCTGTTGAAGTCAGAGGA-3’.
ChIP assay
Bone marrow macrophages (107 cells per condition) derived from WT control mice were cultured in the absence or presence of TNFα for 24h. Cells were crosslinked for 10 minutes at room temperature with 1% formaldehyde solution followed by 5-minutes quenching with 125 mM glycine. Nuclei preparation and chromatin digestion were performed using the SimpleChIP® Enzymatic Chromatin IP Kit (Cell Signaling Technology, 9003) according to manufacturer’s instructions. Digested chromatin was sonicated using the Bioruptor® Pico sonication device (Diagenode, NJ, USA) for 6 cycles with 30 seconds on/30 seconds off. Analysis of chromatin digestion and concentration were analyzed on agarose gel and measured using Nanodrop, respectively. The chromatin lysates were immunoprecipitated with ChIP-grade Protein G magnetic beads (Cell Signaling Technology, 9006) for 6 hours at 4°C after incubation with anti-RBP-J antibody (Cell Signaling Technology, 5313, 1:20) or equivalent amount of normal rabbit IgG (Cell Signaling Technology, 2729) as the negative isotype control overnight at 4°C. Chromatin DNA was purified using QIAquick PCR Purification kit (Qiagen, 28104) after cross-link reversal by overnight incubation at 65°C, with the addition of 0.2M NaCl and treatment with Proteinase K (Cell Signaling Technology, 9003; 20 mg/ml). DNA was analyzed by qPCR and normalized relative to total input. The qPCR primers used in the ChIP assay were as follows: Jag1 promoter locus 1: 5’-GCTCCCTGACCCTGACTTTT-3’ and 5’-CAACCTGGTTTGGGGGCATA-3’; Jag1 promoter locus 2: 5’-ACACACCGACAGAGTCGAAC-3’ and 5’- CCACCCAGAATGGAAGACCC-3’; Jag1 promoter locus 3: 5’-CTCAAGGAGTATCAGTCCCGC-3’ and 5’-GAAGGTGTTACCCCCGATGA-3’; Jag1 promoter locus 4: 5’-GTCATCGGGGGTAACACCTT-3’ and 5’-CGTTCGACTCTGTCGGTGT-3’.
Analysis of Gene Expression in PBMCs from RA and SLE patients
Microarray raw data were extracted from GSE110169 (29). We analyzed the microarray data using the affy and limma package in R (30, 31). Normalized signaling intensity values of Jag1 were input to Graphpad Prism® software for statistical analysis.
Statistical analysis
Statistical analysis was performed using Graphpad Prism® software. Two-tailed Student’s t test was applied when there were only two groups of samples. In the case of more than two groups of samples, one-way ANOVA will be used with one condition, and two-way ANOVA was used with more than two conditions. ANOVA analysis was followed by post hoc Bonferroni’s correction for multiple comparisons. p < 0.05 was taken as statistically significant. Data are presented as the mean ± SD as indicated in the figure legends.
Results
TNF induces Jagged1 expression that is dependent on RBP-J
To explore the connection between TNF and Notch signaling pathways, we wondered whether TNF affects Notch ligand expression. The expression of Notch ligands in macrophages or osteoclast precursors is generally low at a basal level (Fig. 1A). Surprisingly, TNF induces a high expression level of Jagged1, but not the expression of other Notch ligands (Fig. 1C). This exclusive induction of Jagged1 by TNF is largely dependent on RBP-J, as RBP-J deficiency drastically abrogated the Jagged1 mRNA expression and almost completely abolished its protein production by TNF stimulation (Fig. 1A, B, Supplementary Fig. 1). These results indicate that proinflammatory cytokine TNF stimulation can enhance Notch signaling in macrophages via increasing Notch ligand Jagged1 expression.
Figure 1. TNF-induced Jagged1 expression is mainly dependent on RBP-J.
A) Immunoblot analysis of Jagged1 expression in the WT control (Ctrl) and RbpjΔM/ΔM BMMs treated with TNFα for the indicated time periods. p38 was blotted as the loading control. B, C, D) Quantitative real-time PCR (qPCR) analysis of mRNA expression of the indicated genes after TNFα stimulation for 0h, 4h, and 24h in Ctrl and RbpjΔM/ΔM BMMs (B, C) or RANKL stimulation for 0h, 6h and 24h in the WT control cells (D), displayed relative to % GAPDH. Data in A, B and D are representative of three independent experiments. Data are mean ± SD. *p < 0.05.
Since RANKL is a master inducer of osteoclastogenesis, especially in physiological conditions, we also tested whether RANKL regulates Jagged1 expression. In contrast to TNF, RANKL did not induce expression of Jagged1, but rather led to its decrease (Fig. 1D). These results suggest that TNF and RANKL have different effects on Jagged1 expression and presumably its mediated Notch signaling.
Jagged1 is an RBP-J target
Since RBP-J is a transcription factor, we next examined whether RBP-J directly targets Jag1 locus. We employed ChIP assay, and found that RBP-J binds to multiple locations at Jag1 locus regardless of TNF treatment (Fig. 2), indicating that Jagged1 is an RBP-J target. As RBP-J deficiency decreases Jagged1 expression (Fig. 1), RBP-J acts as a transcriptional activator of Jagged1 expression. These results identify Jagged1 as a previously unrecognized RBP-J target. Different from canonical Notch targets that are suppressed by RBP-J at basal level, Jagged1 is transcriptionally activated by RBP-J. Enhanced Jagged1 expression is able to further accelerate Notch signaling, as Jagged 1 is a Notch ligand. Thus, RBP-J-Jagged1 is a feed-forward regulatory axis for Notch signaling.
Figure 2. RBP-J binds to Jagged1 locus.
A) Diagram depicting four putative RBP-J binding motifs in the mouse Jag1 promoter region. B) ChIP analysis of RBP-J occupancy at the indicated Jag1 loci in BMMs stimulated or not with TNFα (40 ng/ml) for 24 h. Data in B are representative of two independent experiments. Data are mean ± SD. *p < 0.05; **p < 0.01; n.s., not statistically significant.
Loss of Jagged1 enables TNF to induce macrophages to differentiate to osteoclasts
Both TNF and RANKL belong to the TNF super family. However, the osteoclastogenic capacity of TNF is far weaker than RANKL. It has been a long-standing mystery in terms of the low osteoclastogenic capacity of TNF. RBP-J has been identified as a key inhibitory regulator in restraining TNF-mediated osteoclastogenesis (23, 24). However, the downstream targets of RBP-J that are responsible for osteoclastic inhibition are not well understood. Since we found that Jagged1 is an RBP-J target, we asked how Jagged1 regulates TNF-mediated osteoclastogenesis. To this end, we generated Jag1 conditional knock out (KO) mice, in which Jag1 is specifically deleted in myeloid lineage macrophages/osteoclast precursors by crossing Jag1flox/flox mice with LysMcre mice (Jag1f/f;LysMCre; hereafter referred to as Jag1ΔMΔM). LysMcre+ littermates served as wild type controls (hereafter referred to as Ctrl) (Fig. 3A). TNF only induced a small number of TRAP+ multinucleated osteoclasts in the control cells (Fig. 3B), which is as expected (28). In contrast, more than 3 times of TRAP+ multinucleated osteoclasts were induced by TNF in Jag1ΔMΔM BMM cell cultures (Fig. 3B). These TNF-induced osteoclasts possess resorptive ability (Fig. 3C). Moreover, the transcription factors that drive osteoclastogenesis, including Blimp1, NFATc1 and c-Fos, were more highly expressed in Jag1ΔMΔM cell cultures than the controls stimulated by TNF during osteoclast differentiation (Fig. 3D). Taken together, these results suggest that Jagged1, induced by TNF, serves as an autocrine feedback inhibitor for TNF-mediated osteoclastogenesis (Fig. 3E).
Figure 3. Jagged1 deficiency enhances TNF-mediated osteoclastogenesis.
A) qPCR analysis of Jag1 expression in BMMs of the WT control (Ctrl) and Jag1ΔM/ΔM mice, B) Osteoclast differentiation using BMMs (3.125 × 104/cm2) derived from WT control (Ctrl) and Jag1ΔM/ΔM mice was stimulated with and without TNFα for 5 days. Left: TRAP staining. Scale bar: 200 μm. Right: TRAP-positive multinucleated cells (MNCs) (≥3 nuclei/cell) per well (n=4/group). C) Von Kossa staining of osteoclast differentiation cultures induced by TNF for 14 days. Mineralized area: black; resorption area: white. D) Immunoblot analysis of Blimp1, Nfatc1, c-Fos expression in the Ctrl and Jag1ΔM/ΔM BMMs with TNFα treatments for the indicated time periods. p38 was blotted as the loading control. The relative density of each cFos band to its corresponding loading control p38 band was calculated by Image J software, and then was normalized to the WT controls at time 0 (the 1st lane). E) Schematic of TNF-induced feedback inhibition of osteoclast differentiation of macrophages.
Interestingly, Jagged1 deficiency does not affect RANKL-induced osteoclastogenesis (Supplementary Fig. 2), presumably because RANKL does not induce Jagged1 expression.
Overexpression of Jagged1 suppresses TNF-mediated inflammatory osteoclastogenesis
Our previous study (28) has identified a TGFβ/TNF-driven inflammatory osteoclastogenic program, which is independent of RANKL and allows TNF to efficiently induce osteoclast differentiation from TGFβ primed macrophages. In the present study, we wondered whether overexpression of Jagged1 suppresses osteoclastogenesis in inflammatory conditions. We first generated Jag1 conditional transgenic (Tg) mice, in which Jag1 is specifically overexpressed in myeloid lineage macrophages/osteoclast precursors by crossing R26-LSL-JAG1 mice with LysMcre mice (hereafter referred to as Jag1mTg). LysMcre+ littermates served as wild type controls (hereafter referred to as Ctrl). Consistent with our prior study (28), after TGFβ priming, TNF induced many giant multinucleated osteoclasts in the WT control BMM cells (Fig. 4A). However, Jagged1 overexpression in Jag1mTg cell cultures abolished this osteoclast differentiation mediated by TGFβ/TNF (Fig. 4A). Furthermore, TNF-induced Jagged1 expression was drastically abrogated by TGFβ priming/TNF stimulation (Fig. 4B). TGFβ priming enabled TNF to effectively induce the expression of osteoclastogenic transcription factors, such as Blimp1 and NFATc1, which corroborated the enhanced osteoclast differentiation by TGFβ priming/TNF stimulation. Jagged1 overexpression in Jag1mTg cell cultures, however, reversed TGFβ priming/TNF effect on the expression of Blimp1 and NFATc1, resulting in an almost undetectable Blimp1 level and a very low expression level of NFATc1 (Fig. 4B). These data collectively support the fact that Jagged1 suppresses TNF-mediated inflammatory osteoclastogenesis. The negative regulators of osteoclast formation, such as IRF8 and Mafb, were not influenced by Jagged1 in BMMs stimulated by TNF (Supplementary Fig. 3).
Figure 4. Overexpression of Jagged1 suppresses inflammatory osteoclastogenesis.
A) Osteoclast differentiation using BMMs (6.25 × 104/cm2) derived from WT control (Ctrl) and Jag1mTg mice were stimulated with TNFα for 4 days with or without TGFβ priming for 3 days. Left: TRAP staining. Scale bar: 200 μm. Right: TRAP-positive MNCs (≥3 nuclei/cell) per well (n=4/group). B) Immunoblot analysis of Jagged1 and Blimp1 (top), and Nfatc1 (bottom) expression in Ctrl and Jag1mTg BMMs with TNFα treatments for the indicated time periods, with or without TGFβ priming. p38 was blotted as the loading control.
Jagged1 does not impact TNF-induced inflammatory gene expression in macrophages
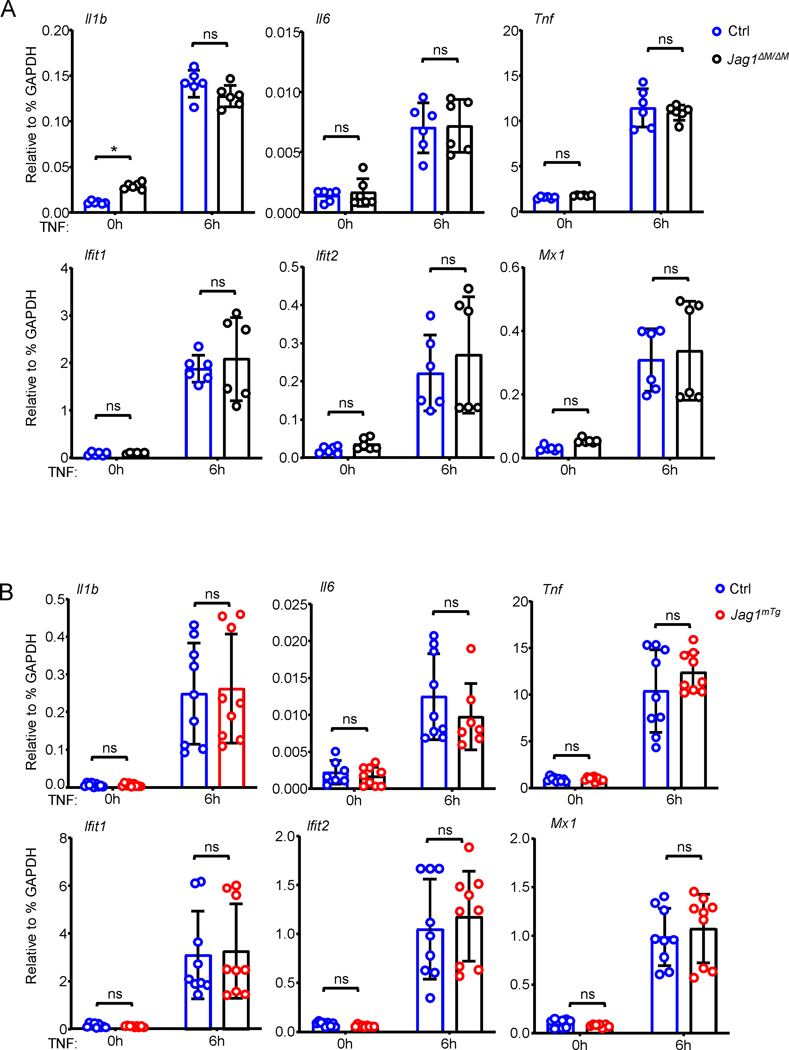
Besides as a mediator of osteoclastogenesis, TNF is an important proinflammatory cytokine in inflammatory diseases. We thus asked whether Jagged1 affects TNF-induced inflammatory gene expression in macrophages. Surprisingly, in contrast to its significant regulation of osteoclastogenesis, neither deficiency (Fig. 5A) nor overexpression (Fig. 5B) of Jagged1 influenced the expression of inflammatory cytokine genes, such as Il1b, Il6 and Tnf, and interferon stimulated genes (ISGs), such as Mx1, Ifit1 and Ifit2, in macrophages treated with TNF. These results suggest that Jagged1 does not affect TNF-induced inflammatory response in macrophages, but selectively regulates TNF-mediated osteoclast differentiation of macrophages.
Figure 5. TNF-induced inflammatory gene expression is not regulated by Jagged1.
qPCR analysis of mRNA expression of the indicated genes in the Ctrl and Jag1ΔM/ΔM BMMs (A) or the Ctrl and Jag1mTg BMMs (B) after TNFα stimulation for the indicated times. Data in A are replicates combined from two independent experiments, and data in B are replicates combined from three independent experiments. Data are mean ± SD. n.s., not statistically significant.
Recombinant Jagged1 inhibits human inflammatory osteoclastogenesis
We next sought to investigate the role of Jagged1 in human inflammatory osteoclastogenesis. Since the TGFβ/TNF-driven inflammatory osteoclastogenic program is present in RA (28), we used TGFβ priming and TNF stimulation to induce human inflammatory osteoclastogenesis to first examine Jagged1 expression in human system. As shown in Fig. 6A, B, Jagged1 is highly induced by TNF at both mRNA and protein levels in human CD14+ macrophages. The induction of Jagged1 by TNF was abolished by TGFβ priming (Fig. 6A, B). Along with the diminishment of Jagged1 induction, the expression of osteoclast marker genes, such as ACP5 (encoding TRAP) and CTSK (encoding Cathepsin K), was significantly enhanced by TGFβ priming and TNF stimulation (Fig. 6C).
Figure 6. Jagged1 level is decreased in RA PBMCs and recombinant Jagged1 inhibits human inflammatory osteoclastogenesis.
A) Immunoblot analysis of Jagged1 expression in human CD14(+) monocytes primed with or without TGFβ for 3 days, followed by TNFα stimulation for the indicated time periods. GAPDH was blotted as the loading control. B, C) qPCR analysis of JAG1 (B), and ACP5 and CTSK (C) expression from human CD14(+) monocytes primed with or without TGFβ for 3 days, followed by TNFα stimulation for the indicated time periods (n=12/group). D) Normalized Signal Intensity of JAG1 in SLE and RA PBMCs obtained from microarray data. n=82 for SLE patients and n=84 for RA patients. E) Osteoclast differentiation derived from human CD14(+) monocytes primed with or without TGFβ for 3 days, followed by TNFα in the presence or absence of recombinant human Jagged1 (200 ng/ml) for 7 days. Left: TRAP staining. Scale bar: 200 μm. Right: Quantification of TRAP-positive MNCs displayed as % of total TRAP+ MNCs. Data are mean ± SD. *p < 0.05; **p < 0.01; n.s., not statistically significant.
Inflammatory bone erosion driven by excessive osteoclastogenesis is a serious consequence of RA disease and a challenging clinic problem. Current treatment of inflammatory osteoclast formation is still limited with undesired side effects (8, 9). Therefore, there is a clinical unmet need to identify new therapeutic targets for osteoclast inhibition in inflammatory conditions. The inhibition of TNF-mediated osteoclastogenesis by Jagged1 inspired us to look at Jagged1 level in inflammatory diseases associated with bone erosion. We analyzed a recently published dataset (29), in which the genome-wide gene expression of peripheral blood monocytes (PBMCs) from cross-sectional cohorts, including 82 SLE (Systemic lupus erythematosus) patients and 84 RA patients, was obtained. PBMCs correspond to circulating osteoclast precursors (32, 33). RA and SLE are distinct rheumatic diseases; one important distinguishing feature is that RA patients often develop joint erosion with aggressive osteoclast formation/activity, whereas SLE arthropathy is usually non-erosive (34, 35). Thus, the gene sets from SLE and RA cohorts in this published study appeared to be optimal for us to compare Jagged1 levels between inflammatory diseases with (RA) or without (SLE) osteoclastic bone erosion. Results show that Jagged1 expression level is significantly lower in RA patients than SLE (Fig. 6D). Taken together with the results demonstrating that Jagged1 suppressed inflammatory osteoclastogenesis (Fig. 3, 4), the data from patients suggest a potential link between the decreased Jagged1 level and enhanced inflammatory bone erosion in RA. Based on these findings, we asked whether using recombinant Jagged1 can suppress inflammatory osteoclastogenesis in human culture system. Similar to the mouse culture system, TNF alone showed a very weak ability to induce human osteoclast differentiation (Fig. 6E). TGFβ priming and TNF stimulation markedly enhanced human osteoclastogenesis (Fig. 6E), in parallel to diminished Jagged1 expression and enhanced osteoclast marker gene expression (Fig. 6A, B, C). When recombinant Jagged1 was added to the culture, the osteoclastogenesis was suppressed to below 50% of the level induced by TGFβ priming and TNF stimulation (Fig. 6E). Our results indicate that the administration of recombinant Jagged1 has the potential to effectively suppress human osteoclastogenesis in inflammatory conditions, making it a promising therapeutic candidate.
Discussion
Inflammatory bone resorption is a serious consequence of many inflammatory diseases, such as RA, psoriatic arthritis and periodontitis. Given that inflammatory bone erosion is often refractory to standard anti-resorption therapy, it is a clinical challenge to inhibit excessive bone resorption while maintaining bone remodeling in inflammatory conditions. Moreover, currently available treatments are not sufficient to suppress inflammatory bone loss without side effects. Osteoclasts are the key cell type that is specialized to resorb bone. Thus, appropriate control of osteoclastogenesis in inflammatory conditions is of importance to impede bone loss. This study identified Jagged1 as a TNF-induced feedback inhibitor of TNF-mediated inflammatory osteoclastogenesis. Furthermore, Jagged1 level is decreased in PBMCs/osteoclast precursors in RA, and recombinant Jagged1 significantly suppresses TNF-mediated human osteoclast formation. Based on these findings, recombinant Jagged1 may hold potential as a therapeutic agent for suppressing human osteoclastogenesis in inflammatory conditions.
The function of Jagged1 in macrophages appears to be highly dependent on the environmental conditions. Specifically, this study discovered that Jagged1 plays an inhibitory role in TNF-mediated inflammatory osteoclastogenesis. However, in a breast cancer bone metastasis model, tumor-derived Jagged1 was found to strongly accelerate osteoclast formation and osteolysis by stimulating osteoblasts to release Il6, a known promoter of osteoclastogenesis (36). Jagged1 was also found to increase Il6 production in macrophages that were stimulated by LPS with IFNγ priming (37). Interestingly, in macrophages treated with TNF, we did not find that Jagged1 had any significant effect on inflammatory gene expression, including Il6. These differing biological effects of Jagged1 in various settings are likely due to the presence of distinct signaling pathways. The interaction between Jagged1-mediated Notch signaling pathway and other pathways, such as those mediated by TNF, LPS and Il6, leads to diverse biological functions of Jagged1. Given this context-dependent nature of Jagged1’s interactions with various signaling pathways, it is crucial to investigate the function of Jagged1 in different settings to fully understand its role in disease pathogenesis and guide the development of targeted therapeutic strategies for specific disease conditions.
This study has identified Jagged1 as a previously unrecognized target of RBP-J, whose expression is mainly dependent on RBP-J. Therefore, Jagged1 is not only a Notch ligand but also a target of Notch signaling, which could enable Jagged1 to auto-amplify the activity of Notch signaling. Additionally, TNF stimulation strongly induces the expression of Jagged1, at least partially through RBP-J, providing the first evidence for the activation of the Notch signaling pathway by TNF. However, TNF-induced Jagged1 acts as a feedback inhibitor that restrains TNF-mediated osteoclast differentiation. These findings demonstrate the crosstalk between TNF and Notch signaling in macrophages and its biological significance in regulating osteoclastogenesis in inflammatory conditions. It’s worth noting that RANKL does not induce Jagged1 expression and Jagged1 does not affect RANKL-induced osteoclast formation; therefore, Jagged1-mediated feedback inhibition does not exist in RANKL-induced osteoclastogenesis. The distinct regulation of Jagged1 between TNF and RANKL contributes to their different osteoclastogenic abilities.
The level of Jagged1 in PBMCs/osteoclast precursors in RA was found to be much lower than that in SLE. RA is an inflammatory disease associated with excessive osteoclastogenesis and bone erosion, while SLE is not (34, 35). Therefore, the reduced Jagged1 expression level in RA osteoclast precursors may contribute to heightened osteoclastogenesis observed in this disease. Interestingly, despite the fact that TNF induces Jagged1 expression in macrophages, this induction appears to be compromised by the complex inflammatory conditions present in RA. As a result, the overall level of Jagged1 in macrophages is lower in RA, which facilitates osteoclastogenesis.
In this study, it has been demonstrated that Jagged1 selectively inhibits TNF-mediated osteoclastogenesis, while having minimal effect on TNF-induced inflammatory gene expression. This finding is consistent with previous research on the specific regulation of osteoclastogenesis but not inflammation by RBP-J in inflammatory arthritis settings (23, 24). These results highlight Jagged1 and RBP-J as specific negative regulators of inflammatory osteoclastogenesis. According to previous literature, NF-κB p100 (38) and IRF1 (28) have also been found to selectively restrict TNF-induced osteoclast formation and bone resorption, while B-Myb (28) enhances these processes. This indicates the presence of a distinct group of regulators that primarily influence TNF-mediated osteoclastogenesis rather than RANKL-induced osteoclastogenesis. Current treatments for inflammatory diseases, such as TNF inhibitors used for RA, inhibit inflammation and joint erosion, but their long-term use can have immunosuppressive side effects, including common and opportunistic infections, and reactivation of latent tuberculosis (39). The use of Jagged1 may offer a novel therapeutic strategy to suppress inflammatory bone loss without or with minimal impact on the immune response in disease settings, making it an attractive potential treatment option.
Supplementary Material
Key points:
Jagged1 is an RBP-J target, and TNF activates RBP-J-Jagged1 axis in macrophages.
TNF-induced Jagged1 is a feedback inhibitor of inflammatory osteoclastogenesis.
RANKL does not induce Jag1, which does not affect RANKL-induced osteoclastogenesis.
ACKNOWLEDGEMENTS
We thank Ruoxi Yuan, Bikash Mishra, Chao Yang, Yong Du and Vidyanath Chaudhary for sharing the de-identified human CD14+ monocytes, Ting Zheng for providing cDNAs from BMMs stimulated with RANKL and Ruge Chen for technical assistance. We are grateful to the lab members from Dr. Baohong Zhao’s laboratory for their helpful discussions and assistance.
This work was supported by grants from the National Institutes of Health (AR06897, AR071463 and AR078212 to BZ) and by support for the Rosensweig Genomics Center at the Hospital for Special Surgery from The Tow Foundation. The content of this manuscript is solely the responsibilities of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Competing Interests statement
The authors have no conflict of interests.
References
- 1.Goldring SR 2003. Pathogenesis of bone and cartilage destruction in rheumatoid arthritis. Rheumatology 42 Suppl 2: ii11–16. [DOI] [PubMed] [Google Scholar]
- 2.Novack DV, and Teitelbaum SL 2008. The osteoclast: friend or foe? Annu Rev Pathol 3: 457–484. [DOI] [PubMed] [Google Scholar]
- 3.Sato K, and Takayanagi H. 2006. Osteoclasts, rheumatoid arthritis, and osteoimmunology. Current opinion in rheumatology 18: 419–426. [DOI] [PubMed] [Google Scholar]
- 4.Boyce BF, Yao Z, Zhang Q, Guo R, Lu Y, Schwarz EM, and Xing L. 2007. New roles for osteoclasts in bone. Ann N Y Acad Sci 1116: 245–254. [DOI] [PubMed] [Google Scholar]
- 5.Schett G, and Gravallese E. 2012. Bone erosion in rheumatoid arthritis: mechanisms, diagnosis and treatment. Nat Rev Rheumatol 8: 656–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Teitelbaum SL 2006. Osteoclasts; culprits in inflammatory osteolysis. Arthritis Res Ther 8: 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi Y, Arron JR, and Townsend MJ 2009. Promising bone-related therapeutic targets for rheumatoid arthritis. Nat Rev Rheumatol 5: 543–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aspenberg P. 2014. Denosumab and atypical femoral fractures. Acta orthopaedica 85: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boquete-Castro A, Gomez-Moreno G, Calvo-Guirado JL, Aguilar-Salvatierra A, and Delgado-Ruiz RA 2016. Denosumab and osteonecrosis of the jaw. A systematic analysis of events reported in clinical trials. Clinical oral implants research 27: 367–375. [DOI] [PubMed] [Google Scholar]
- 10.Popp AW, Zysset PK, and Lippuner K. 2016. Rebound-associated vertebral fractures after discontinuation of denosumab-from clinic and biomechanics. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA 27: 1917–1921. [DOI] [PubMed] [Google Scholar]
- 11.Gravallese EM, and Goldring SR 2000. Cellular mechanisms and the role of cytokines in bone erosions in rheumatoid arthritis. Arthritis Rheum 43: 2143–2151. [DOI] [PubMed] [Google Scholar]
- 12.Eggelmeijer F, Papapoulos SE, van Paassen HC, Dijkmans BA, Valkema R, Westedt ML, Landman JO, Pauwels EK, and Breedveld FC 1996. Increased bone mass with pamidronate treatment in rheumatoid arthritis. Results of a three-year randomized, double-blind trial. Arthritis Rheum 39: 396–402. [DOI] [PubMed] [Google Scholar]
- 13.Sileghem A, Geusens P, and Dequeker J. 1992. Intranasal calcitonin for the prevention of bone erosion and bone loss in rheumatoid arthritis. Annals of the rheumatic diseases 51: 761–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rehman Q, and Lane NE 2001. Bone loss. Therapeutic approaches for preventing bone loss in inflammatory arthritis. Arthritis research 3: 221–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cohen G, Gossec L, Dougados M, Cantagrel A, Goupille P, Daures JP, Rincheval N, and Combe B. 2007. Radiological damage in patients with rheumatoid arthritis on sustained remission. Annals of the rheumatic diseases 66: 358–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Molenaar ET, Voskuyl AE, Dinant HJ, Bezemer PD, Boers M, and Dijkmans BA 2004. Progression of radiologic damage in patients with rheumatoid arthritis in clinical remission. Arthritis Rheum 50: 36–42. [DOI] [PubMed] [Google Scholar]
- 17.Coury F, Peyruchaud O, and Machuca-Gayet I. 2019. Osteoimmunology of Bone Loss in Inflammatory Rheumatic Diseases. Frontiers in immunology 10: 679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boyce BF, Schwarz EM, and Xing L. 2006. Osteoclast precursors: cytokine-stimulated immunomodulators of inflammatory bone disease. Current opinion in rheumatology 18: 427–432. [DOI] [PubMed] [Google Scholar]
- 19.Lam J, Takeshita S, Barker JE, Kanagawa O, Ross FP, and Teitelbaum SL 2000. TNF-alpha induces osteoclastogenesis by direct stimulation of macrophages exposed to permissive levels of RANK ligand. The Journal of clinical investigation 106: 1481–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schett G, and Teitelbaum SL 2009. Osteoclasts and arthritis. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 24: 1142–1146. [DOI] [PubMed] [Google Scholar]
- 21.Kim N, Kadono Y, Takami M, Lee J, Lee SH, Okada F, Kim JH, Kobayashi T, Odgren PR, Nakano H, Yeh WC, Lee SK, Lorenzo JA, and Choi Y. 2005. Osteoclast differentiation independent of the TRANCE-RANK-TRAF6 axis. J Exp Med 202: 589–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kobayashi K, Takahashi N, Jimi E, Udagawa N, Takami M, Kotake S, Nakagawa N, Kinosaki M, Yamaguchi K, Shima N, Yasuda H, Morinaga T, Higashio K, Martin TJ, and Suda T. 2000. Tumor necrosis factor alpha stimulates osteoclast differentiation by a mechanism independent of the ODF/RANKL-RANK interaction. J Exp Med 191: 275–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao B. 2020. Intrinsic Restriction of TNF-Mediated Inflammatory Osteoclastogenesis and Bone Resorption. Frontiers in endocrinology 11: 583561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao B. 2017. TNF and Bone Remodeling. Curr Osteoporos Rep 15: 126–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kopan R, and Ilagan MX 2009. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell 137: 216–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tanigaki K, Han H, Yamamoto N, Tashiro K, Ikegawa M, Kuroda K, Suzuki A, Nakano T, and Honjo T. 2002. Notch-RBP-J signaling is involved in cell fate determination of marginal zone B cells. Nat Immunol 3: 443–450. [DOI] [PubMed] [Google Scholar]
- 27.Li S, Miller CH, Giannopoulou E, Hu X, Ivashkiv LB, and Zhao B. 2014. RBP-J imposes a requirement for ITAM-mediated costimulation of osteoclastogenesis. The Journal of clinical investigation 124: 5057–5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xia Y, Inoue K, Du Y, Baker SJ, Reddy EP, Greenblatt MB, and Zhao B. 2022. TGFbeta reprograms TNF stimulation of macrophages towards a non-canonical pathway driving inflammatory osteoclastogenesis. Nature communications 13: 3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu Y, Carman JA, Holloway D, Kansal S, Fan L, Goldstine C, Lee D, Somerville JE, Latek R, Townsend R, Johnsen A, Connolly S, Bandyopadhyay S, Shadick N, Weinblatt ME, Furie R, and Nadler SG 2018. Development of a Molecular Signature to Monitor Pharmacodynamic Responses Mediated by In Vivo Administration of Glucocorticoids. Arthritis & rheumatology 70: 1331–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gautier L, Cope L, Bolstad BM, and Irizarry RA 2004. affy--analysis of Affymetrix GeneChip data at the probe level. Bioinformatics 20: 307–315. [DOI] [PubMed] [Google Scholar]
- 31.Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, and Smyth GK 2015. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic acids research 43: e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sorensen MG, Henriksen K, Schaller S, Henriksen DB, Nielsen FC, Dziegiel MH, and Karsdal MA 2007. Characterization of osteoclasts derived from CD14+ monocytes isolated from peripheral blood. Journal of bone and mineral metabolism 25: 36–45. [DOI] [PubMed] [Google Scholar]
- 33.Murata K, Fang C, Terao C, Giannopoulou EG, Lee YJ, Lee MJ, Mun SH, Bae S, Qiao Y, Yuan R, Furu M, Ito H, Ohmura K, Matsuda S, Mimori T, Matsuda F, Park-Min KH, and Ivashkiv LB 2017. Hypoxia-Sensitive COMMD1 Integrates Signaling and Cellular Metabolism in Human Macrophages and Suppresses Osteoclastogenesis. Immunity 47: 66–79 e65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Santiago MB, and Galvao V. 2008. Jaccoud arthropathy in systemic lupus erythematosus: analysis of clinical characteristics and review of the literature. Medicine 87: 37–44. [DOI] [PubMed] [Google Scholar]
- 35.Pipili C, Sfritzeri A, and Cholongitas E. 2008. Deforming arthropathy in systemic lupus erythematosus. European journal of internal medicine 19: 482–487. [DOI] [PubMed] [Google Scholar]
- 36.Sethi N, Dai X, Winter CG, and Kang Y. 2011. Tumor-derived JAGGED1 promotes osteolytic bone metastasis of breast cancer by engaging notch signaling in bone cells. Cancer cell 19: 192–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Foldi J, Chung AY, Xu H, Zhu J, Outtz HH, Kitajewski J, Li Y, Hu X, and Ivashkiv LB 2010. Autoamplification of Notch signaling in macrophages by TLR-induced and RBP-J-dependent induction of Jagged1. Journal of immunology 185: 5023–5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yao Z, Xing L, and Boyce BF 2009. NF-kappaB p100 limits TNF-induced bone resorption in mice by a TRAF3-dependent mechanism. The Journal of clinical investigation 119: 3024–3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kalliolias GD, and Ivashkiv LB 2016. TNF biology, pathogenic mechanisms and emerging therapeutic strategies. Nat Rev Rheumatol 12: 49–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.