Abstract
Background:
Physical activity (PA) has emerged as a promising approach to delay Alzheimer’s disease and related dementias, but the optimal intensity of PA to improve cognitive health remains unknown.
Objective:
To evaluate the association between duration and intensity of PA and cognitive domains (executive function, processing speed, and memory) in aging Americans.
Methods:
Linear regressions in hierarchical blocks for variable adjustment and the size of effect (η2) were analyzed by using the data of 2,377 adults (age = 69.3 ± 6.7 years) from the NHANES 2011–2014.
Results:
Participants with 3–6 h/week of vigorous- and > 1 h/week of moderate-intensity PA scored significantly higher in executive function and processing speed domains of cognition compared to inactive peers (η2 = 0.005 & 0.007 respectively, p < 0.05). After adjustment, the beneficial effects of 1–3 h /week of vigorous-intensity PA became trivial for delayed recall memory domain test scores (β = 0.33; 95%CI: −0.01,0.67; η2 = 0.002; p = 0.56). There was no linear dose-response relationship between the cognitive test scores and weekly moderate-intensity of PA. Interestingly, higher handgrip strength and higher late-life body mass index were associated with a higher performance across all cognitive domains.
Conclusion:
Our study supports habitual PA with superior cognition health in some but not all domains among older adults. Furthermore, increased muscle strength and higher late-life adiposity may also impact cognition.
Keywords: Alzheimer’s disease, body mass index, cognitive function, executive function, handgrip strength, physical activity
INTRODUCTION
Aging is not only related to normal decline in fluid cognition [1, 2] and its domains (executive function, processing speed, language ability, and memory) [3], but also it increases the risk of severe cognitive impairment like dementia and Alzheimer’s disease (AD) [4, 5], a major cause of disability and dependency among older adults [6]. More than 6 million aging Americans are suffering from AD in 2021 and are projected to grow 13.8 million by 2060 [6], generating more than threefold increase in government and individual spending on healthcare and long-term care, costing the nation 1.1 trillion US dollars [6]. Thus, the role of non-pharmacological interventions to maintain, enhance or reverse declines in cognitive performance (CP) has attracted attention among researchers.
Physical activity (PA) is a non-pharmacological intervention that has documented benefits to slow cognitive decline [7] and reduce AD-risk by exerting neuroprotection and slowing neuropathological changes [8]. Furthermore, PA modifies the lifestyle risk factors (obesity, hypertension, diabetes, late-life depression, social isolation, etc.) associated with dementia and AD [9]. Although several studies linked PA to enhanced global CP [10, 11], these findings are not universally supported. Several studies have only demonstrated the beneficial effect of PA on select domains of cognition in older adults [12–14]. In addition, despite extensive research to explore the optimal PA dosages (duration and intensity) to improve cognitive health, the optimal intensity and duration of PA remains elusive [15, 16]. Several studies reported a positive dose-response effect of PA on cognition [17, 18], but others reported selective [19] or no dose-response relationship [20]. Since these inconsistent results may be due to several methodological inconsistencies and confounding factors such as socio-demographic and individual characteristics [15]. Previous research has documented the link between socio-demographic characteristics (such as age, sex, education, marital status, etc.) and individual characteristics (such as body mass index (BMI), disease condition (hypertension, diabetes, depression), etc.) with both PA and CP [15, 21]. Therefore, research investigating the relationship between individual domains of fluid cognition and PA is warranted for clarification.
Hence, the purposes of our study were three folds: 1) to examine possible associations between different durations and intensities of PA and CP across individual domains, 2) to investigate dose-response association between PA and cognitive domains, and 3) to determine the association between other socio-demographics and cognitive domains among aging Americans by using a national database.
METHODS
Data source and analytic sample
We analyzed the publicly available data from two cycles of the National Health and Nutrition Examination Survey (NHANES) (2011–2012, 2013–2014). This survey was designed to evaluate the health status of a nationally representative sample of non-institutionalized U.S. civilians and consisted of in-home interview and standardized health examinations in the mobile examination centers. Among the 19,931 people enrolled in NHANES surveys in 2011–2014, data from 2,377 adults who were 60 years or older with complete information for all CP tests, PA questionnaires, and covariates were analyzed. Participants who answered “Refused” or “Don’t know” to any questions or had missing data for any of the CP tests, PA questionnaires, and covariates were excluded from the analysis. All the data collection procedures performed in NHANES were carried out in accordance with the Health Statistics Research Ethics Review Board. The participants provided informed written consent in accordance with the Declaration of Helsinki [22]. Additional details can be found online (https://www.cdc.gov/nchs/nhanes/index.htm).
Assessment of CP
Three CP assessments were conducted by trained interviewers. Consortium to Establish a Registry for AD (CERAD) word learning subtest, assesses immediate and delayed learning ability for new verbal information (memory sub-domain) [23]. The test includes three consecutive immediate recall trials (CERAD.IR), and a single delayed recall trial (CERAD.DR). The Animal Fluency Test (AFT) that examines categorical verbal fluency (a measure of executive function [24], and language ability [25]). The Digit Symbol Substitution test (DSS), a component of the Wechsler Adult Intelligence Scale III [26], assessed processing speed, sustained attention, and working memory.
Self-reported PA
Participants self-reported their PA pattern by completing the Global PA Questionnaire to assess moderate (MPA) and vigorous-intensity PA (VPA) [27]. The frequency and duration of MPA and VPA in a typical week was used to calculate weekly PA. Participants were categorized based on their total minutes of weekly activity. For VPA, less than 1 hour (VPA < 1 h), 1–3 hours (VPA 1–3 h), 3–6 hours (VPA 3–6 h), and more than 6 hours (VPA 6 + h) per week. MPA was categorized as no MPA (No activity), less than 1 hour (MPA < 1 h), 1–3 hours (MPA 1–3 h), 3–6 hours (MPA 3–6 h), 6–9 hours (MPA 6–9 h), 9–12 hours (MPA 9–12 h), and more than 12 hours (MPA 12 + h).
Covariates
The study takes into consideration several socio-demographic information and physical attributes and disease conditions: Age (years, continuous), Gender (male or female), Self-reported race [Hispanic (Mexican American and other Hispanic), Non-Hispanic white, Non-Hispanic black, or Other (Non-Hispanic Asian and Other race-including multi-racial)], Education status [< 9th grade, 9–11th grade (includes 12th grade with no diploma), High school graduate/GED or equivalent, some college or AA degree, College graduate or above], Marital status (lives alone or living with someone). BMI (calculated as kg/m2), physician-diagnosed hypertension (Yes or No) and diabetes status (Yes, No, Borderline), high-density lipoprotein (HDL), categorized depressive symptoms by Patient Health Questionnaire (0–4 “none or minimum”, 5–9 “mild”, 10–14 “moderate”, 15–19 “moderately severe”, and 20–27 “severe”) was included. As comorbid conditions can affect the level of PA [28] and cognitive ability [29], we included seven chronic conditions such as chronic cardiovascular diseases (coronary artery disease, stroke, congestive heart failure, heart attack), chronic musculoskeletal disease (arthritis), and chronic respiratory diseases (emphysema and chronic bronchitis). The total score ranged from 0–7 (one point each comorbid condition). As muscular strength is associated with performing PA [30], handgrip strength in kg, measured with a digital handgrip dynamometer (Takei Dynamometer Model T.K.K.5401; Akiha-Ku, Japan), was included.
Statistical analyses
Descriptive statistics and contrast between genders were computed. A t-test for continuous variables and Fisher’s exact test for categorical variables were performed. Hierarchical linear regression analyses were computed to examine the associations of levels of PA (VPA and MPA) and CP test scores. Models were computed separately for each CP test. In each model, VPA or MPA was the main independent variable. For all models, the most physically inactive group was considered as the reference group and the coefficients (95% CI), and effect size (η2) were calculated. The unadjusted model represents the bivariate relationship between CP test scores and PA (VPA/ MPA) that did not control for covariates. In the minimally adjusted models, the greatest change in the β-coefficients was observed. The fully adjusted model included all the covariates discussed above. Finally, surface analysis plots were computed by the weighted inverse of the variance of each data point to explore the relationship between CP, education status, and VPA. The analyses were conducted using statistical software package R (R foundation, version 4.0.3). All analyses were two-tailed and statistical significance was established as a nominal alpha of 0.05.
RESULTS
Descriptive characteristics
Among the analytic sample of 2,377 older adults, 1,209 (50.86%) were female. Most participants were physically inactive, 82.71% (n = 1966) engaged in VPA for less than an hour and 35.59% of participants (n = 846) did not engage in any weekly MPA. Characteristics of study participants are shown in Table 1.
Table 1.
Demographic, lifestyle, and health characteristics of participants included in analyses from NHANES (2011–2014), by Gender
| Variable | Male (n =1,168) | Female (n =1,209) | Total (n = 2,377) |
|---|---|---|---|
|
| |||
| Age (mean (SD)) | 69.32 (6.78) | 69.28 (6.69) | 69.30 (6.73) |
| Race (%) | |||
| Non- Hispanic White | 562(48.1) | 627 (51.9) | 1,189 (50.0) |
| Hispanic | 216 (18.5) | 225 (18.6) | 441 (18.6) |
| Non-Hispanic Black | 275 (23.5) | 259 (21.4) | 534 (22.5) |
| Other | 115 (9.8) | 98(8.1) | 213 (9.0) |
| Education** | |||
| < 9th grade | 135 (11.6) | 110(9.1) | 245 (10.3) |
| 9–11th grade | 149 (12.8) | 165 (13.6) | 314 (13.2) |
| High school grad/ GED | 261 (22.3) | 302 (25.0) | 563 (23.7) |
| College | 301 (25.8) | 383 (31.7) | 684 (28.8) |
| College Grad and above | 322 (27.6) | 249 (20.6) | 571 (24.0) |
| Marital status* =Live with someone (%) | 838 (71.7) | 561 (46.4) | 1,399 (58.9) |
| BMI (mean (SD))* | 28.56 (5.48) | 29.58 (6.92) | 29.08 (6.28) |
| Grip Strength (mean (SD))* | 75.52 (16.38) | 48.15 (10.32) | 61.60 (19.32) |
| HDL (mean (SD))* | 49.55 (14.67) | 59.28 (16.5) | 54.50 (16.36) |
| Cognitive performance assessment | |||
| CERAD.IR (mean (SD))* | 18.30(4.36) | 19.98 (4.52) | 19.16(4.52) |
| CERAD.DR (mean (SD))* | 5.65 (2.23) | 6.44 (2.21) | 6.05 (2.25) |
| AFT (mean (SD)) | 17.07 (5.55) | 16.84 (5.40) | 16.95 (5.47) |
| DSS (mean (SD))* | 44.31 (16.00) | 49.82 (17.44) | 47.11 (16.97) |
| Physical activity | |||
| Total minutes of vigorous-intensity activity/week (mean (SD))* | 122.48 (367.60) | 47.00 (217.58) | 84.08 (303.09) |
| Vigorous-intensity activity.** | |||
| < 1 h | 903 (77.3) | 1,063 (87.9) | 1,966 (82.7) |
| 1–3 h | 86 (7.4) | 73 (6.0) | 159 (6.7) |
| 3–6 h | 78 (6.7) | 28 (2.3) | 106 (4.5) |
| 6 + h | 101 (8.6) | 45 (3.7) | 146 (6.1) |
| Total minutes of moderate-intensity activity/week (mean (SD))* | 353.18 (578.87) | 268.68 (497.11) | 310.20(540.38) |
| Moderate-intensity activity** | |||
| No activity | 379 (32.4) | 467 (38.6) | 846 (35.6) |
| <1 h | 75 (6.4) | 67 (5.5) | 142 (6.0) |
| 1–3 h | 207 (17.7) | 241 (19.9) | 448 (18.8) |
| 3–6 h | 184 (15.8) | 182(15.1) | 366 (15.4) |
| 6–9 h | 84 (7.2) | 81 (6.7) | 165 (6.9) |
| 9–12h | 66 (5.7) | 52 (4.3) | 118(5.0) |
| 12 + h | 173 (14.8) | 119 (9.8) | 292 (12.3) |
| Depression status (%)** | |||
| Minimum | 949 (81.2) | 832 (68.8) | 1,781 (74.9) |
| Mild | 143 (12.2) | 233 (19.3) | 376 (15.8) |
| Moderate | 44 (3.8) | 86 (7.1) | 130 (5.5) |
| Moderate Severe | 18 (1.5) | 42 (3.5) | 60 (2.5) |
| Severe | 14 (1.2) | 16(1.3) | 30(1.3) |
| Physician-diagnosed Hypertension = Yes (%)* | 682 (58.4) | 770 (63.7) | 1,452(61.1) |
| Physician-diagnosed Diabetes | |||
| No | 838 (71.7) | 902 (74.6) | 1,740 (73.2) |
| Borderline | 54 (4.6) | 57 (4.7) | 111 (4.7) |
| Yes | 276 (23.6) | 250 (20.7) | 526(22.1) |
| Comorbidity score (mean (SD))* | 0.85 (1.03) | 0.95 (0.97) | 0.90(1.00) |
Unweighted sample size
t-test, p < 0.05,
Fisher’s exact test, p < 0.05. CERAD.IR, immediate recall memory test; CERAD.DR, delayed recall memory test; AFT, animal fluency test; DSS, digit symbol substitution test.
CERAD.IR scores ranged from 0 to 30 with mean ± SD of 19.16 ± 4.52 and CERAD.DR scores ranged from 0 to 10 with a mean ± SD of 6.05 ± 2.25 (Right-skewed distribution, Fig. 2A, B). AFT scores ranged from 3 to 40 with a median of 16 (Leftskewed distribution Fig. 2C). DSS scores ranged from 0 to 105 with a mean ± SD of 47.11 ± 16.97, were normally distributed (Fig. 2D). Except for AFT, females scored significantly higher in all the CP assessment tests (all p < 0.01) (Table 1).
Fig. 2.
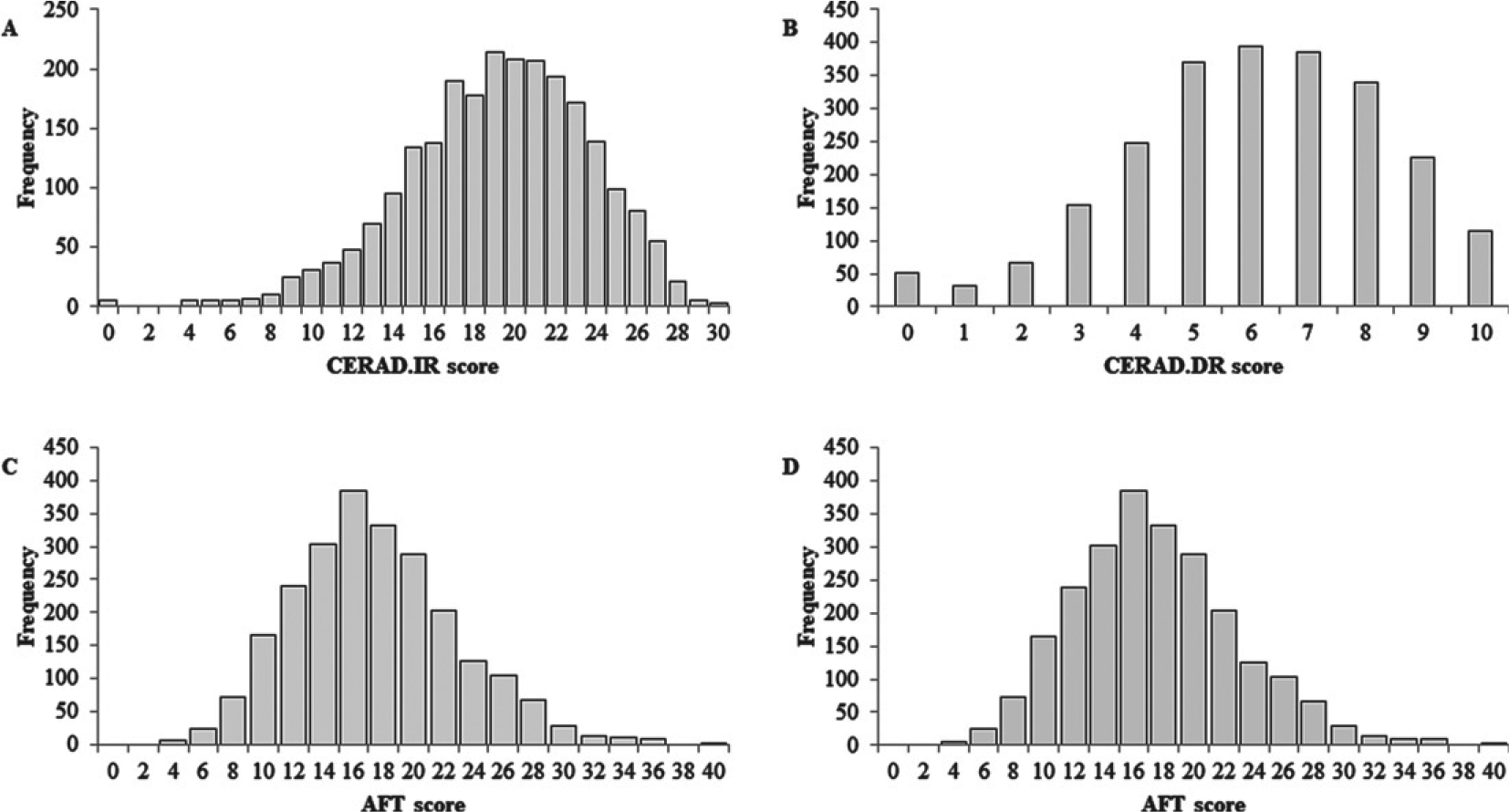
Cognitive performance assessment scores. Immediate recall memory test (CERAD.IR), delayed recall memory test (CERAD.DR), animal fluency test (AFT), digit symbol substitution test (DSS).
VPA and CP
CP assessment scores across different categories of VPA is shown in Table 2.
Table 2.
Cognitive performance assessment scores across different categories vigorous-intensity physical activity (n = 2,377). Mean ± SD
| VPA |
||||
|---|---|---|---|---|
| <1 h (n = 1,966) | 1–3 h(n =159) | 3–6 h (n = 106) | 6 + h (n = 146) | |
|
| ||||
| Age (y) | 69.8 ± 6.81 | 67.01 ± 5.91 | 67.17 ± 5.84 | 66.55 ± 5.53 |
| CERAD.IR | 18.95 ± 4.58 | 20.53 ± 3.95 | 20.22 ± 3.77 | 19.64 ± 4.45 |
| CERAD.DR | 5.96 ± 2.28 | 6.80 ± 2.15 | 6.56 ± 1.96 | 6.20 ± 2.08 |
| AFT | 16.51 ± 5.33 | 19.20 ± 5.86 | 19.75 ± 5.93 | 18.38 ± 5.26 |
| DSS | 45.79 ± 16.79 | 55.22 ± 16.62 | 55.27 ± 16.05 | 50.08 ± 16.03 |
CERAD.IR, immediate recall memory test; CERAD.DR, delayed recall memory test; AFT, animal fluency test; DSS, digit symbol substitution test.
The hierarchical regression analyses evaluated the association between the CP assessment scores and VPA are shown in Table 3.
Table 3.
Regression analyses examining the association between cognitive performance assessment scores andvigorous-intensity physical activity (n = 2,377)
| β (95% CI) | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Model | Unadjusted | Minimally adjusted | Fully adjusted | |||
| CERAD.IR | ||||||
| VPA<1 h | Ref | Ref | Ref | |||
|
| ||||||
| VPA 1–3 h | 1.58 (0.86, 2.31)*** | 0.56(−0.12, 1.23)^ | 0.41 (−0.26, 1.08) | |||
| VPA 3–6 h | 1.26(0.39,2.14)** | 0.55 (−0.26, 1.36) | 0.42 (−0.39, 1.23) | |||
| VPA 6 + h | 0.69 (−0.06, 1.45)^ | 0.42 (−0.27, 1.12) | 0.30 (−0.40, 0.99) | |||
| R-squared | 0.01 | 0.188 | 0.197 | |||
|
| ||||||
| Partial Eta-squared | VPA | 0.011 | VPA | 0.002 | VPA | 0.001 |
| Gender | 0.041 | Gender | 0.033 | |||
| Age | 0.068 | Age | 0.044 | |||
| Race | 0.009 | Race | 0.007 | |||
| Education | 0.066 | Education | 0.056 | |||
| BMI | 0.001 | |||||
| Handgrip | 0.004 | |||||
|
| ||||||
| CERAD.DR | ||||||
| VPA< 1 h | Ref | Ref | Ref | |||
|
| ||||||
| VPA 1–3 h | 0.84 (0.48, 1.20)*** | 0.37 (0.03, 0.71)* | 0.33 (−0.01, 0.67)^ | |||
| VPA 3–6 h | 0.60 (0.16, 1.04)** | 0.28 (−0.13, 0.69) | 0.23 (−0.18, 0.64) | |||
| VPA 6 + h | 0.24 (−0.13, 0.62) | 0.07 (−0.28, 0.42) | 0.04 (−0.31, 0.39) | |||
| R-squared | 0.01 | 0.172 | 0.18 | |||
|
| ||||||
| Partial Eta-squared | VPA | 0.011 | VPA | 0.003 | VPA | 0.002 |
| Gender | 0.036 | Gender | 0.025 | |||
| Age | 0.079 | Age | 0.051 | |||
| Race | 0.013 | Race | 0.014 | |||
| Education | 0.039 | Education | 0.034 | |||
| BMI | 0.005 | |||||
| Handgrip | 0.002 | |||||
|
| ||||||
| AFT | ||||||
| VPA< 1 h | Ref | Ref | Ref | |||
|
| ||||||
| VPA 1–3 h | 2.69 (1.82, 3.56)*** | 1.01 (0.21, 1.82)* | 0.76 (−0.04, 1.56)^ | |||
| VPA 3–6 h | 3.23 (2.18, 4.28)*** | 1.57 (0.60, 2.53)** | 1.27 (0.31, 2.24)** | |||
| VPA 6 + h | 1.86(0.96, 2.77)*** | 1.03 (0.20, 1.86)* | 0.83 (0.00, 1.66)^ | |||
| R-squared | 0.031 | 0.208 | 0.226 | |||
|
| ||||||
| Partial Eta-squared | VPA | 0.032 | VPA | 0.008 | VPA | 0.005 |
| Age | 0.067 | Age | 0.043 | |||
| Race | 0.070 | Race | 0.069 | |||
| Education | 0.074 | Education | 0.061 | |||
| BMI | 0.003 | |||||
| Handgrip | 0.007 | |||||
|
| ||||||
| DSS | ||||||
| VPA< 1 h | Ref | Ref | Ref | |||
|
| ||||||
| VPA 1–3 h | 9.43(6.73,12.13)*** | 2.75 (0.72, 4.78)** | 1.81 (−0.18, 3.80)^ | |||
| VPA 3–6 h | 9.48(6.22,12.74)*** | 3.68 (1.22, 6.13)** | 2.81 (0.41, 5.22)* | |||
| VPA 6 + h | 4.28 (1.47, 7.09)** | 2.03 (−0.07, 4.13)^ | 1.16 (−0.90, 3.22) | |||
| R-squared | 0.031 | 0.473 | 0.499 | |||
|
| ||||||
| Partial Eta-squared | VPA | 0.033 | VPA | 0.007 | VPA | 0.003 |
| Gender | 0.047 | Gender | 0.055 | |||
| Age | 0.129 | Age | 0.113 | |||
| Race | 0.116 | Race | 0.120 | |||
| Education | 0.246 | Education | 0.224 | |||
| BMI | 0.001 | |||||
| Handgrip | 0.015 | |||||
Minimally adjusted model included the covariates age, gender, race, and education Fully adjusted model included the covariates age, gender, race, education, marital status, BMI, handgrip strength, hypertension status, diabetes status, depression status, comorbid score, and serum HDL level.
p<0.001,
p<0.01,
p<0.05.
p<0.1 Ref, reference category; CERAD.IR, immediate recall memory test; CERAD.DR, delayed recall memory test; AFT, animal fluency test; DSS, digit symbol substitution test. Effect size is represented by Partial Eta-squared.
After fully adjusting (socio-demographic, lifestyle, and health characteristics), the association between VPA and CERAD.IR scores were attenuated (Table 3). Across all models, the most active older adults (weekly VPA 6 + h) tend to score lower in all the CP tests relative to the ones who performed weekly VPA 1–3 h and VPA 3–6 h. Therefore, a linear dose-response association was not evident.
Notably, after adjusting for socio-demographic characteristics (age, gender, race, education status), the greatest change in the β-coefficients was observed, suggesting their major role in determining CP. Indeed, education status showed highest influence on cognition (Table 3).
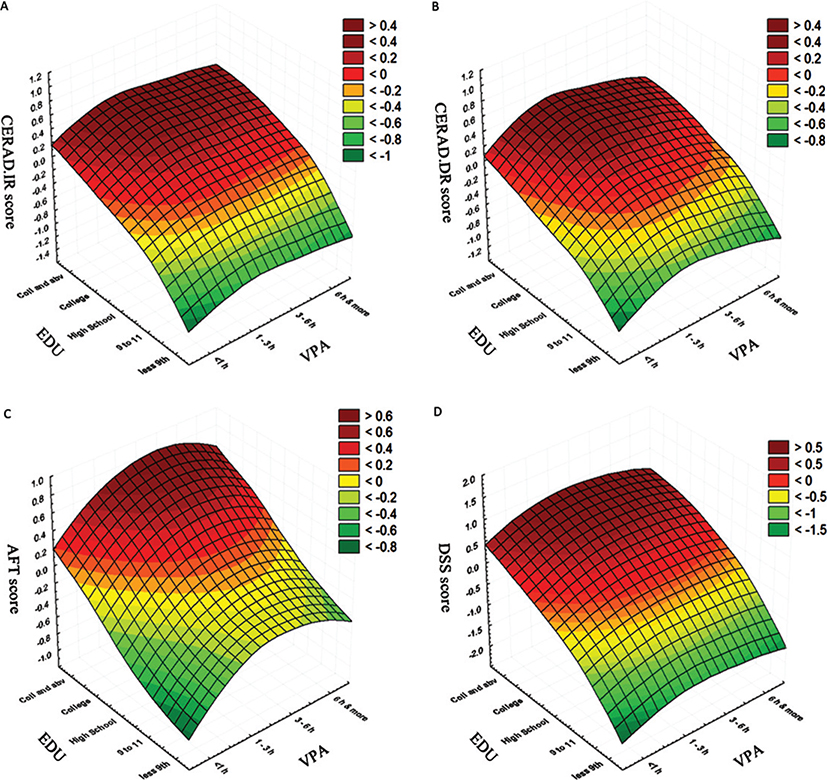
The surface plots demonstrating the relationship between education status and VPA and the CP assessment scores of the older adults are shown in Fig. 3. As expected, the highest values of all the assessment scores correspond with higher education status. Interestingly, an increase in weekly VPA, from VPA<1h to VPA 1–3 h, increased the AFT score, even in participants with lower education status. Similar increase in scores was also noticed in participants in VPA 3–6 h group. However, beyond this duration, a decrease in the AFT score is noticed, indicating most active older adults (weekly VPA 6 + h) scored lower compared to the ones who performed weekly VPA 1–3 h and VPA 3–6 h. Although not as profound as the changes observed in the AFT scores, a similar pattern was noticeable in other CP tests as well.
Fig. 3.
Relationship between education status, vigorous-intensity physical activity and cognitive performance assessment scores. A) Education (EDU) versus vigorous physical activity (VPA) versus Immediate recall memory test (CERAD.IR), B) EDU versus VPA versus delayed recall memory test (CERAD.DR), C) EDU versus VPA versus animal fluency test (AFT), D) EDU versus VPA versus digit symbol substitution test (DSS).
MPA and CP
Table 4 shows the mean CP test scores by category of MPA.
Table 4.
Cognitive performance assessment scores by moderate-intensity physical activity category (n = 2,377). Mean ± SD
| MPA |
||||||||
|---|---|---|---|---|---|---|---|---|
| No activity (n = 846) | <1 h (n =142) | 1–3 h (n = 448) | 3–6 h (n = 366) | 6–9 h (n = 165) | 9–12 h (n =118) | 12 + h (n = 292) | ||
|
| ||||||||
| Age (y) | 70.02 ± 6.90 | 70.01 ± 6.74 | 69.29 ± 6.69 | 69.27 ± 6.90 | 68.84 ± 6.21 | 67.99 ± 6.10 | 67.72 ± 6.27 | |
| CERAD.IR | 18.78 ± 4.72 | 18.92 ± 5.03 | 19.49 ± 4.50 | 19.31 ± 4.30 | 19.55 ± 3.82 | 19.27 ± 4.09 | 19.39 ± 4.46 | |
| CERAD.DR | 5.82 ± 2.36 | 6.16 ± 2.21 | 6.23 ± 2.22 | 6.10 ± 2.25 | 6.38 ± 1.93 | 6.08 ± 2.32 | 6.16 ± 2.13 | |
| AFT | 15.91 ± 5.35 | 16.61 ± 5.23 | 17.43 ± 5.45 | 17.47 ± 5.38 | 17.30 ± 5.59 | 17.20 ± 5.64 | 18.44 ± 5.45 | |
| DSS | 43.89 ± 16.94 | 48.91 ± 16.38 | 49.16 ± 17.03 | 49.35 ± 16.37 | 48.62 ± 18.12 | 49.24 ± 16.00 | 47.90 ± 16.40 | |
CERAD.IR, immediate recall memory test; CERAD.DR, delayed recall memory test; AFT, animal fluency test; DSS, digit symbol substitution test.
The hierarchical regression analyses evaluating the association between the CP assessments and MPA is shown in Table 5. The fully adjusted model shows no association between MPA and CERAD.IR and CERAD.DR scores. However, MPA was non-linearly associated with AFT and DSS test scores. Older adults engaging in highest weekly MPA (MPA12 + h) showed highest association to AFT test score (β = 1.39; 95% CI: 0.73, 2.05; η2 = 0.009; p < 0.001), compared to their inactive peers. However, participants performing MPA 1–3 h (β = 0.90; 95% CI: 0.34, 1.46; η2 = 0.009; p < 0.01) and MPA 3–6 h (β = 0.79; 95% CI: 0.18, 1.39; η2 = 0.009; p < 0.05) also scored significantly higher compared to the reference group. Participants engaging in weekly MPA<1h (β = 3.36; 95% CI: 1.22, 5.50; η2 = 0.007; p < 0.01) and MPA 1–3 h (β = 2.06, 95% CI: 0.67, 3.45; η2 = 0.007; p < 0.01) showed significantly higher DSS score compared to those who did not engage in any MPA. Furthermore, older adults engaging in weekly MPA 3–6 h showed a trend to score slightly higher in DSS test (β = 1.38; 95% CI: −0.13, 2.88; η2 = 0.007, p = 0.73), compared to those engaging in weekly MPA No activity. Therefore, an inconsistent dose-response relationship was observed between the executive function and processing speed performance and duration of weekly MPA.
Table 5.
Regression analyses examining the association between cognitive performance assessment scores and moderate-intensity physical activity (n = 2,377)
| β (95% CI) | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Model | Unadjusted | Minimally adjusted | Fully adjusted | |||
| CERAD.IR | ||||||
| MPA No activity | Ref | Ref | Ref | |||
| MPA<1h | 0.14 (−0.66, 0.94) | −0.07 (−0.80, 0.66) | −0.12 (−0.85, 0.60) | |||
| MPA 1–3 h | 0.71 (0.19, 1.23)** | 0.34 (−0.13, 0.81) | 0.23 (−0.25, 0.70) | |||
| MPA 3–6 h | 0.53 (−0.03, 1.08)^ | 0.06 (−0.44, 0.57) | −0.06 (−0.57, 0.45) | |||
| MPA 6–9 h | 0.76(0.01, 1.52)* | 0.37 (−0.32, 1.05) | 0.22 (−0.47, 0.90) | |||
| MPA 9–12 h | 0.49 (−0.38, 1.36) | 0.09 (−0.70, 0.88) | −0.06 (−0.85, 0.73) | |||
| MPA 12 + h | 0.61 (0.00, 1.21)* | 0.25 (−0.30, 0.80) | 0.04 (−0.51, 0.60) | |||
| R-squared | 0.002 | 0.187 | 0.196 | |||
|
| ||||||
| Partial Eta-squared | MPA | 0.005 | MPA | 0.001 | MPA | 0.001 |
| Gender | 0.039 | Gender | 0.032 | |||
| Age | 0.072 | Age | 0.046 | |||
| Race | 0.010 | Race | 0.007 | |||
| Education | 0.068 | Education | 0.058 | |||
| Handgrip | 0.004 | |||||
| CERAD.DR | ||||||
|
| ||||||
| MPA No activity | Ref | Ref | Ref | |||
| MPA< 1 h | 0.34 (−0.06, 0.74)^ | 0.28 (−0.08, 0.65) | 0.25 (−0.11, 0.62) | |||
| MPA 1–3 h | 0.40(0.15,0.66)** | 0.23 (−0.01, 0.46)* | 0.20 (−0.04, 0.44) | |||
| MPA 3–6 h | 0.27 (0.00, 0.55)^ | 0.06 (−0.19, 0.31) | 0.05 (−0.21, 0.30) | |||
| MPA 6–9 h | 0.55 (0.18,0.93)** | 0.34 (0.00, 0.68)* | 0.31 (−0.04, 0.66) | |||
| MPA 9–12 h | 0.25 (−0.18,0.69) | 0.03 (−0.37, 0.43) | 0.00 (−0.40, 0.40) | |||
| MPA 12 + h | 0.33 (0.03, 0.63)* | 0.15 (−0.12, 0.43) | 0.10 (−0.18, 0.38) | |||
| R-squared | 0.004 | 0.171 | 0.180 | |||
|
| ||||||
| Partial Eta-squared | MPA | 0.007 | MPA | 0.003 | MPA | 0.003 |
| Gender | 0.036 | Gender | 0.023 | |||
| Age | 0.083 | Age | 0.054 | |||
| Race | 0.013 | Race | 0.014 | |||
| Education | 0.041 | Education | 0.034 | |||
| BMI | 0.005 | |||||
| Handgrip | 0.003 | |||||
|
| ||||||
| AFT | ||||||
| MPA No activity | Ref | Ref | Ref | |||
| MPA< 1 h | 0.70 (−0.026, 1.66) | 0.33 (−0.54, 1.19) | 0.26 (−0.60, 1.12) | |||
| MPA 1–3 h | 1.52(0.90, 2.14)*** | 1.05 (0.49, 1.60)*** | 0.90 (0.34, 1.46)** | |||
| MPA 3–6 h | 1.56(0.90, 2.22)*** | 0.95 (0.35, 1.55)** | 0.79(0.18, 1.39)* | |||
| MPA 6–9 h | 1.38 (0.48,2.29)** | 0.83 (0.02, 1.64)* | 0.61 (−0.20, 1.43) | |||
| MPA 9–12 h | 1.29 (0.25, 2.33)* | 0.78 (−0.17, 1.72)^ | 0.62 (−0.32, 1.55) | |||
| MPA 12 + h | 2.52(1.80, 3.24)*** | 1.66(1.01,2.32)*** | 1.39(0.73,2.05)*** | |||
| R-squared | 0.023 | 0.212 | 0.228 | |||
|
| ||||||
| Partial Eta-squared | MPA | 0.025 | MPA | 0.013 | MPA | 0.009 |
| Age | 0.071 | Age | 0.046 | |||
| Race | 0.072 | Race | 0.071 | |||
| Education | 0.076 | Education | 0.063 | |||
| BMI | 0.004 | |||||
| Handgrip | 0.007 | |||||
|
| ||||||
| DSS | ||||||
| MPA No activity | Ref | Ref | Ref | |||
| MPA< 1 h | 5.02 (2.03, 8.01)** | 3.57 (1.37, 5.76)** | 3.36(1.22, 5.50)** | |||
| MPA 1–3 h | 5.27 (3.34, 7.19)*** | 2.82 (1.40, 4.23)*** | 2.06 (0.67, 3.45)** | |||
| MPA 3–6 h | 5.46 (3.40, 7.52)*** | 2.23 (0.71, 3.75)** | 1.38 (−0.13, 2.88)^ | |||
| MPA 6–9 h | 4.73 (1.92, 7.53)*** | 1.82 (−0.24, 3.88)^ | 0.74 (−1.29, 2.77) | |||
| MPA 9–12 h | 5.35 (2.11, 8.59)** | 2.67 (0.29, 5.06)* | 1.83 (−0.51, 4.16) | |||
| MPA 12 + h | 4.01 (1.78, 6.25)*** | 1.38 (−0.28, 3.04)^ | 0.13 (−1.51, 1.77) | |||
| R-squared | 0.018 | 0.474 | 0.501 | |||
|
| ||||||
| Partial Eta-squared | MPA | 0.021 | MPA | 0.009 | MPA | 0.007 |
| Gender | 0.044 | Gender | 0.053 | |||
| Age | 0.173 | Age | 0.119 | |||
| Race | 0.120 | Race | 0.124 | |||
| Education | 0.247 | Education | 0.225 | |||
| BMI | 0.001 | |||||
| Handgrip | 0.015 | |||||
Minimally adjusted model included the covariates age, gender, race, and education Fully adjusted model included the covariates age, gender, race, education, marital status, BMI, handgrip strength, hypertension status, diabetes status, depression status, comorbid score, and serum HDL level.
p <0.001,
p<0.01,
p<0.05,
p <0.1 Ref, reference category; CERAD.IR, immediate recall memory test; CERAD.DR, delayed recall memory test; AFT, animal fluency test; DSS, digit symbol substitution test. Effect size is represented by Partial Eta-squared.
The covariates with significant negative associations with all the cognitive test scores included age, some racial ethnicity, education level, severity of depression (all p < 0.05). Additionally, hypertension demonstrated significant negative association with AFT scores (p < 0.05). Diabetes (p < 0.01) and chronic comorbidities (p < 0.05) were negatively associated with DSS test scores. Whereas, female gender, higher educational attainment, higher handgrip strength demonstrated significant positive association with cognitive test scores. Interestingly, a higher BMI and HDL level was positively associated with higher AFT score. A higher BMI was also associated with higher CERAD.DR score.
DISCUSSION
The findings of this study provide evidence that PA (both VPA and MPA) is associated with better performance in measures of executive function, and processing speed but not memory. Secondly, VPA (not MPA) is associated with enhancing memory-specific cognitive ability (delayed recall memory), suggesting an intensity-specific cognitive health-related outcome. Thirdly, PA may be effective in promoting CP in a non-linear dose-response manner. Fourthly, higher handgrip strength was associated with a higher CP across all domains (memory, executive function, and processing speed). Lastly, a higher BMI at late-life may provide protective benefits against cognitive dysfunction.
In this cross-sectional analysis of a national sample of community-dwelling older adults in the US, bivariate analysis suggested that CP was preserved among older adults who engaged in regular PA compared to their less-active counterparts. However, the magnitude of the association was diminished for delayed memory, verbal fluency, executive function, and processing speed, and was completely absent for immediate memory when we controlled for confounding factors (socio-demographic and physical attributes).
Even though PA has been linked to improved memory performance [31, 32] and several others claimed a global betterment in CP following PA [10, 11], the findings from the present study indicated that PA (both VPA and MPA) correlated with significantly better performance on the measures of executive function and processing speed but not memory. This result provide support for the “selective improvement hypothesis” introduced by Kramer and collaborators that proposed PA induced improvement in cardiorespiratory fitness brings about selective, rather than generalized, improvement in CP [14]. A similar finding was also observed in numerous other studies [12, 33, 34].
The results of this study suggest that VPA (not MPA) may provide delayed memory-enhancing benefits in older adults. This is in line with a recent meta-analysis [35] and researchers have suggested that VPA-induced heightened physical arousal facilitates learning and information consolidation in long-term memory stores [36] perhaps explained by post-exercise increase in catecholamines [36, 37]. PA also increased lactate in the hippocampal region of the brain [38] and BDNF (a key molecule related to learning and memory) that enhances neuroplasticity via different pathways [39].
Older adults who engaged in the highest duration weekly VPA tend to perform lower in all the CP assessments relative to the ones who performed moderate-duration weekly VPA. This finding is in alignment with a meta-analysis that evaluated 18 interventional studies and reported that moderate-duration PA sessions improved CP among older adults more effectively than long-duration PA sessions [40]. However, other studies has observed non-linear or curvilinear duration-response [20, 41] and also significantly positive duration-response of PA with cognitive assessment scores [17, 18] and a lower risk of AD and related dementias [42]. Although it is difficult to explain why lower scores on executive function and processing speed in various duration of MPA, it can be due to higher variance of scores among the participants and relatively lower number of participants among these groups. Furthermore, the difference in intensity of MPA performed can be additional source of variance.
The findings of this study recommend a minimum of 3 h of VPA per week is necessary to significantly improve cognitive health in older individuals. Performing more than 3 h of VPA up to 6 h per week may result in a similar benefit. The highest gain is noticeable in the executive function domain with similar improvement in other domains however in a lower magnitude. Even though performing more than 6 h of weekly VPA seemed to have some negative impact on cognition, various duration and intensity of PA can be beneficial for other physiological systems.
Even though several previous studies found no association between handgrip strength and dementia risk [43, 44], our recent analysis showed that greater handgrip strength was related to higher CP across all domains in aging Americans. This result is comparable to a cross-sectional study that reported increased handgrip strength was significantly correlated with increased CP (r = 0.42; p < 0.01) in elderly participants (n = 70) [45]. Furthermore, another large-scale longitudinal investigation [46] reported that every 5 kg loss in handgrip strength was associated with 10% increased odds for poor CP and 18% increased odds of severe cognitive dysfunction. Since handgrip strength test is a low-cost non-invasive viable screening tool for determining sarcopenia [47], it may be useful in detecting impaired cognition and aid healthcare practitioners in recognizing the development and progression of cognitive impairment in clinical and epidemiological settings. The finding illustrates the relationship between age-related loss of skeletal muscle strength, motor impairment, and cognitive decline. Previous research has identified reduced muscle strength as a potential risk factor for cognitive deficits [48] and linked the age-related decrease in the motor system functioning to the onset of cognitive impairment [49]. The finding also sheds light on the potential aspects of muscle strengthening exercise programs in improving cognitive health-related outcomes.
Our study shows that older participants with higher BMI displayed higher cognitive ability. The finding is consistent with multiple epidemiological studies which illustrates increased adiposity (overweight and obesity) in late life is associated with decreased dementia risk [50, 51]. Research implies that adiposity has a “bimodal” influence on CP [52]. A greater BMI in midlife appears to enhance risk of AD and dementia [53–55], whereas, higher BMI in later life have a favorable influence on retaining CP [56, 57]. Leptin has been proposed as a plausible mechanism for the obesity-cognition protective link by modulating hippocampal synaptic plasticity and amyloid processing [58], improving neuronal survival and proliferation [59]. We speculate that midlife adiposity may result in less sensitive leptin receptors in the brain in later life and unable to provide neuroprotection, but late-life adiposity may boost leptin signaling, resulting in neuroprotection and better cognition.
Limitations
This study is not without limitations. Firstly, the use of an analytic cross-sectional study can be considered as a limitation. Older adults included in the analytic sample with various levels of existing cognitive impairment may also be less likely to engage in weekly PA. Secondly, tests administered to assess CP were chosen for ease of administration [60], but they may not be as sensitive to variations in PA level. Thirdly, the subjective assessment of PA can also be considered as a limitation as participants tend to provide an inflated estimate of PA. Fourthly, participants’ history of PA engagement, which alters the level of benefits and overall health, was not known or determined. Finally, lack of information regarding the location of residency of the participants could be an additional source of variation, as PA in different environmental settings (i.e. climate, altitude) results in different outcomes [61].
In conclusion, this study provides evidence delineating positive association between PA and CP across different domains in a national sample of aging Americans. It also indicates a non-linear dose-response association between PA and cognition, recommending 3–6 h of weekly VPA as an optimal range of PA for improving cognitive health. It also provides support regarding how individual characteristics like (hand-grip strength and late-life adiposity) may relate to CP.
Fig. 1.
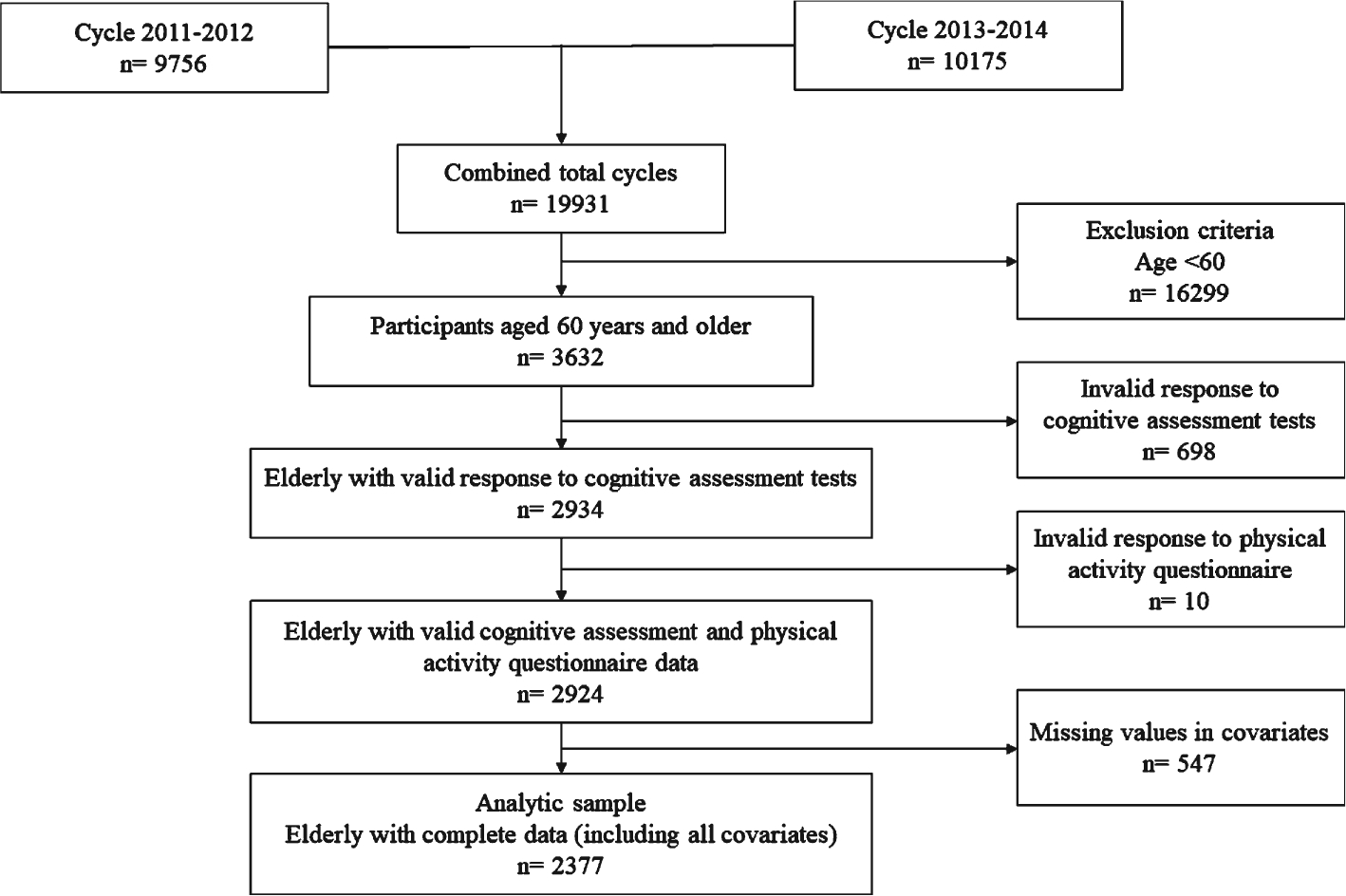
Flow diagram of analytic sample selection from NHANES 2011–2014 dataset based on inclusion and exclusion criteria.
ACKNOWLEDGMENTS
We thank the National Center of Health Statistics at for the availability of NHANES survey data and acknowledge the staffs who design, collect, administer, and release data for public use.
FUNDING
Research reported in this publication was supported by the National Institute on Aging of the National Institutes of Health under Award Number DP1AG069870. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
DATA AVAILABILITY
Data and respective datasets are displayed at the NHANES website: https://www.cdc.gov/nchs/nhanes/Index.htm
REFERENCES
- [1].Hedden T, Gabrieli JDE (2004) Insights into the ageing mind: A view from cognitive neuroscience. Nat Rev Neurosci 5, 87–96. [DOI] [PubMed] [Google Scholar]
- [2].Salthouse T (2012) Consequences of age-related cognitive declines. Ann Rev Psychol 63, 201–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Harada CN, Love MCN, Triebel KL (2013) Normal cognitive aging. Clin Geriatr Med 29, 737–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Brayne C, Gill C, Paykel ES, Huppert F, O’Connor DW (1995) Cognitive decline in an elderly population–a two wave study of change. Psychol Med 25, 673–683. [DOI] [PubMed] [Google Scholar]
- [5].Seeley WW, Miller BL (2018) Dementia. In Harrison’s Principles of Internal Medicine, 20e, Jameson JL, Fauci AS, Kasper DL, Hauser SL, Longo DL, Loscalzo J, eds. McGraw-Hill Education, New York, NY. [Google Scholar]
- [6].Alzheimer’s Association (2021) 2021 Alzheimer’s disease facts and figures. Alzheimers Dement 17, 327–406. [DOI] [PubMed] [Google Scholar]
- [7].Muscari A, Giannoni C, Pierpaoli L, Berzigotti A, Maietta P, Foschi E, Ravaioli C, Poggiopollini G, Bianchi G, Magalotti D, Tentoni C, Zoli M (2010) Chronic endurance exercise training prevents aging-related cognitive decline in healthy older adults: A randomized controlled trial. Int J Geriatr Psychiatry 25, 1055–1064. [DOI] [PubMed] [Google Scholar]
- [8].Müller S, Preische O, Sohrabi HR, Gräber S, Jucker M, Ringman JM, Martins RN, McDade E, Schofield PR, Ghetti B, Rossor M, Fox NN, Graff-Radford NR, Levin J, Danek A, Vöglein J, Salloway S, Xiong C, Benzinger T, Buckles V, Masters CL, Sperling R, Bateman RJ, Morris JC, Laske C, Dominantly Inherited Alzheimer Network (DIAN) (2018) Relationship between physical activity, cognition, and Alzheimer pathology in autosomal dominant Alzheimer’s disease. Alzheimers Dement 14, 1427–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kirk-Sanchez NJ, McGough EL (2014) Physical exercise and cognitive performance in the elderly: Current perspectives. Clin Interv Aging 9, 51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Aarsland D, Sardahaee FS, Anderssen S, Ballard C, the Alzheimer’s Society Systematic Review g (2010) Is physical activity a potential preventive factor for vascular dementia? A systematic review. Aging Mental Health 14, 386–395. [DOI] [PubMed] [Google Scholar]
- [11].Kivipelto M, Rovio S, Ngandu T, Kåreholt I, Eskelinen M, Winblad B, Hachinski V, Cedazo-Minguez A, Soininen H, Tuomilehto J, Nissinen A (2008) Apolipoprotein E ε4 magnifies lifestyle risks for dementia: A population-based study. J Cell Mol Med 12, 2762–2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Netz Y, Dwolatzky T, Zinker Y, Argov E, Agmon R (2011) Aerobic fitness and multidomain cognitive function in advanced age. Int Psychogeriatr 23, 114–124. [DOI] [PubMed] [Google Scholar]
- [13].Frederiksen KS, Verdelho A, Madureira S, Bäzner H, O’Brien JT, Fazekas F, Scheltens P, Schmidt R, Wallin A, Wahlund LO, Erkinjunttii T, Poggesi A, Pantoni L, Inzitari D, Waldemar G (2015) Physical activity in the elderly is associated with improved executive function and processing speed: The LADIS Study. Int J Geriatr Psychiatry 30, 744–750. [DOI] [PubMed] [Google Scholar]
- [14].Kramer AF, Hahn S, Cohen NJ, Banich MT, McAuley E, Harrison CR, Chason J, Vakil E, Bardell L, Boileau RA, Colcombe A (1999) Ageing, fitness and neurocognitive function. Nature 400, 418–419. [DOI] [PubMed] [Google Scholar]
- [15].Panza GA, Taylor BA, MacDonald HV, Johnson BT, Zaleski AL, Livingston J, Thompson PD, Pescatello LS (2018) Can exercise improve cognitive symptoms of Alzheimer’s disease? J Am Geriatr Soc 66, 487–495. [DOI] [PubMed] [Google Scholar]
- [16].Kovacevic A, Fenesi B, Paolucci E, Heisz JJ (2020) The effects of aerobic exercise intensity on memory in older adults. Appl Physiol Nutr Metab 45, 591–600. [DOI] [PubMed] [Google Scholar]
- [17].Weuve J, Kang JH, Manson JE, Breteler MM, Ware JH, Grodstein F (2004) Physical activity, including walking, and cognitive function in older women. JAMA 292, 1454–1461. [DOI] [PubMed] [Google Scholar]
- [18].Xu L, Jiang CQ, Lam TH, Zhang WS, Thomas GN, Cheng KK (2011) Dose-response relation between physical activity and cognitive function: Guangzhou Biobank Cohort Study. Ann Epidemiol 21, 857–863. [DOI] [PubMed] [Google Scholar]
- [19].Vidoni ED, Johnson DK, Morris JK, Van Sciver A, Greer CS, Billinger SA, Donnelly JE, Burns JM (2015) Dose-response of aerobic exercise on cognition: A community-based, pilot randomized controlled trial. PLoS One 10, e0131647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Etnier JL, Nowell PM, Landers DM, Sibley BA (2006) A meta-regression to examine the relationship between aerobic fitness and cognitive performance. Brain Res Rev 52, 119–130. [DOI] [PubMed] [Google Scholar]
- [21].Liyanage SI, Santos C, Weaver DF (2018) The hidden variables problem in Alzheimer’s disease clinical trial design. Alzheimers Dement (N Y) 4, 628–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Johnson CL, Dohrmann SM, Burt VL, Mohadjer LK (2014) National health and nutrition examination survey: Sample design, 2011–2014. Vital Health Stat 2, pp. 1–33. [PubMed] [Google Scholar]
- [23].Morris J, Heyman A, Mohs R, Hughes J, van Belle G, Fillenbaum G, Mellits E, Clark C (1989) The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part I. Clinical and neuropsychological assesment of Alzheimer’s disease. Neurology 39, 1159–1159. [DOI] [PubMed] [Google Scholar]
- [24].Strauss E, Sherman E, Spreen O (2006) A compendium of neuropsychological tests, Oxford University Press, New York. [Google Scholar]
- [25].Whiteside DM, Kealey T, Semla M, Luu H, Rice L, Basso MR, Roper B (2016) Verbal fluency: Language or executive function measure? Appl Neuropsychol Adult 23, 29–34. [DOI] [PubMed] [Google Scholar]
- [26].Wechsler (1997) WAIS Manual, Psychological Corporation, New York. [Google Scholar]
- [27].Hallal PC, Andersen LB, Bull FC, Guthold R, Haskell W, Ekelund U, Group LPASW (2012) Global physical activity levels: Surveillance progress, pitfalls, and prospects. Lancet 380, 247–257. [DOI] [PubMed] [Google Scholar]
- [28].Peng X, Bao X, Xie Y, Zhang X, Huang J, Liu Y, Cheng M, Liu N, Wang P (2020) The mediating effect of pain on the association between multimorbidity and disability and impaired physical performance among community-dwelling older adults in southern China. Aging Clin Exp Res 32, 1327–1334. [DOI] [PubMed] [Google Scholar]
- [29].Cai H, Li G, Hua S, Liu Y, Chen L (2017) Effect of exercise on cognitive function in chronic disease patients: A meta-analysis and systematic review of randomized controlled trials. Clin Interv Aging 12, 773–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Liu C-j Shiroy DM, Jones LY Clark DO (2014) Systematic review of functional training on muscle strength, physical functioning, and activities of daily living in older adults. Eur Rev Aging Phys Activity 11, 95–106. [Google Scholar]
- [31].Sabia S, Kivimaki M, Kumari M, Shipley MJ, Singh-Manoux A (2010) Effect of Apolipoprotein E ε4 on the association between health behaviors and cognitive function in late midlife. Mol Neurodegener 5, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Vercambre M-N, Grodstein F, Manson JE, Stampfer MJ, Kang JH (2011) Physical activity and cognition in women with vascular conditions. Arch Intern Med 171, 1244–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Wilbur J, Marquez DX, Fogg L, Wilson RS, Staffileno BA, Hoyem RL, Morris MC, Bustamante EE, Manning AF (2012) The relationship between physical activity and cognition in older Latinos. J Gerontol B Psychol Sci Soc Sci 67, 525–534. [DOI] [PubMed] [Google Scholar]
- [34].Smiley-Oyen AL, Lowry KA, Francois SJ, Kohut ML, Ekkekakis P (2008) Exercise, fitness, and neurocognitive function in older adults: The “selective improvement” and “cardiovascular fitness” hypotheses. Ann Behav Med 36, 280–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Chang YK, Labban JD, Gapin JI, Etnier JL (2012) The effects of acute exercise on cognitive performance: A meta-analysis. Brain Res 1453, 87–101. [DOI] [PubMed] [Google Scholar]
- [36].Winter B, Breitenstein C, Mooren FC, Voelker K, Fobker M, Lechtermann A, Krueger K, Fromme A, Korsukewitz C, Floel A, Knecht S (2007) High impact running improves learning. Neurobiol Learn Mem 87, 597–609. [DOI] [PubMed] [Google Scholar]
- [37].Cahill L, Alkire MT (2003) Epinephrine enhancement of human memory consolidation: Interaction with arousal at encoding. Neurobiol Learn Mem 79, 194–198. [DOI] [PubMed] [Google Scholar]
- [38].Suzuki A, Stern SA, Bozdagi O, Huntley GW, Walker RH, Magistretti PJ, Alberini CM (2011) Astrocyte-neuron lactate transport is required for long-term memory formation. Cell 144, 810–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Muller P, Duderstadt Y, Lessmann V, Muller NG (2020) Lactate and BDNF: Key mediators of exercise induced neuroplasticity? J Clin Med 9, 1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Colcombe S, Kramer AF (2003) Fitness effects on the cognitive function of older adults: A meta-analytic study. Psychol Sci 14, 125–130. [DOI] [PubMed] [Google Scholar]
- [41].Sofi F, Valecchi D, Bacci D, Abbate R, Gensini GF, Casini A, Macchi C (2011) Physical activity and risk of cognitive decline: A meta-analysis of prospective studies. J Intern Med 269, 107–117. [DOI] [PubMed] [Google Scholar]
- [42].Laurin D, Verreault R, Lindsay J, MacPherson K, Rockwood K (2001) Physical activity and risk of cognitive impairment and dementia in elderly persons. Arch Neurol 58, 498–504. [DOI] [PubMed] [Google Scholar]
- [43].Sattler C, Erickson KI, Toro P, Schröder J (2011) Physical fitness as a protective factor for cognitive impairment in a prospective population-based study in Germany. J Alzheimers Dis 26, 709–718. [DOI] [PubMed] [Google Scholar]
- [44].Gray SL, Anderson ML, Hubbard RA, LaCroix A, Crane PK, McCormick W, Bowen JD, McCurry SM, Larson EB (2013) Frailty and incident dementia. J Gerontol A Biol Sci Med Sci 68, 1083–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Ramnath U, Rauch L, Lambert EV, Kolbe-Alexander TL (2018) The relationship between functional status, physical fitness and cognitive performance in physically active older adults: A pilot study. PLoS One 13, e0194918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].McGrath R, Robinson-Lane SG, Cook S, Clark BC, Herrmann S, O’Connor ML, Hackney KJ (2019) Handgrip strength is associated with poorer cognitive functioning in aging Americans. J Alzheimers Dis 70, 1187–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Shaughnessy KA, Hackney KJ, Clark BC, Kraemer WJ, Terbizan DJ, Bailey RR, McGrath R (2020) A narrative review of handgrip strength and cognitive functioning: Bringing a new characteristic to muscle memory. J Alzheimers Dis 73, 1265–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Carson RG (2018) Get a grip: Individual variations in grip strength are a marker of brain health. Neurobiol Aging 71, 189–222. [DOI] [PubMed] [Google Scholar]
- [49].Seidler RD, Bernard JA, Burutolu TB, Fling BW, Gordon MT, Gwin JT, Kwak Y, Lipps DB (2010) Motor control and aging: Links to age-related brain structural, functional, and biochemical effects. Neurosci Biobehav Rev 34, 721–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Hughes TF, Borenstein AR, Schofield E, Wu Y, Larson EB (2009) Association between late-life body mass index and dementia: The Kame Project. Neurology 72, 1741–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Atti AR, Palmer K, Volpato S, Winblad B, De Ronchi D, Fratiglioni L (2008) Late-life body mass index and dementia incidence: Nine-year follow-up data from the Kungsholmen Project. J Am Geriatr Soc 56, 111–116. [DOI] [PubMed] [Google Scholar]
- [52].Naderali EK, Ratcliffe SH, Dale MC (2009) Obesity and Alzheimer’s disease: A link between body weight and cognitive function in old age. Am J Alzheimers Dis Other Demen 24, 445–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Gustafson DR, Luchsinger JA (2013) High adiposity: Risk factor for dementia and Alzheimer’s disease? Alzheimers Res Ther 5, 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Stewart R, Masaki K, Xue QL, Peila R, Petrovitch H, White LR, Launer LJ (2005) A 32-year prospective study of change in body weight and incident dementia: The Honolulu-Asia Aging Study. Arch Neurol 62, 55–60. [DOI] [PubMed] [Google Scholar]
- [55].Gustafson D, Bäckman K, Joas E, Waern M, Östling S, Guo X, Skoog I (2012) A 37-year longitudinal follow-up of body mass index and dementia in women. J Alzheimers Dis 28, 162–171. [Google Scholar]
- [56].Sun Z, Wang ZT, Sun FR, Shen XN, Xu W, Ma YH, Dong Q, Tan L, Yu JT (2020) Late-life obesity is a protective factor for prodromal Alzheimer’s disease: A longitudinal study. Aging (Albany NY) 12, 2005–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Besser LM, Gill DP, Monsell SE, Brenowitz W, Meranus DH, Kukull W, Gustafson DR (2014) Body mass index, weight change, and clinical progression in mild cognitive impairment and Alzheimer disease. Alzheimer Dis Assoc Disord 28, 36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Anjum I, Fayyaz M, Wajid A, Sohail W, Ali A (2018) Does obesity increase the risk of dementia: A literature review. Cureus 10, e2660–e2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Morrison CD (2009) Leptin signaling in brain: A link between nutrition and cognition? Biochim Biophys Acta 1792, 401–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Brody DJ, Kramarow EA, Taylor CA, McGuire LC (2019) Cognitive Performance in adults aged 60 and over: National Health and Nutrition Examination Survey, 2011–2014. Natl Health Stat Report, pp. 1–23. [PubMed] [Google Scholar]
- [61].Rogerson M, Gladwell VF, Gallagher DJ, Barton JL (2016) Influences of green outdoors versus indoors environmental settings on psychological and social outcomes of controlled exercise. Int J Environ Res Public Health 13, 363. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data and respective datasets are displayed at the NHANES website: https://www.cdc.gov/nchs/nhanes/Index.htm