Abstract
Little is known about exposure determinants of acrylamide (AA), a genotoxic food-processing contaminant, in Europe. We assessed determinants of AA exposure, measured by urinary mercapturic acids of AA (AAMA) and glycidamide (GAMA), its main metabolite, in 3157 children/adolescents and 1297 adults in the European Human Biomonitoring Initiative. Harmonized individual-level questionnaires data and quality assured measurements of AAMA and GAMA (urine collection: 2014–2021), the short-term validated biomarkers of AA exposure, were obtained from four studies (Italy, France, Germany, and Norway) in children/adolescents (age range: 3–18 years) and six studies (Portugal, Spain, France, Germany, Luxembourg, and Iceland) in adults (age range: 20–45 years). Multivariable-adjusted pooled quantile regressions were employed to assess median differences (β coefficients) with 95% confidence intervals (95% CI) in AAMA and GAMA (µg/g creatinine) in relation to exposure determinants. Southern European studies had higher AAMA than Northern studies. In children/adolescents, we observed significant lower AA associated with high socioeconomic status (AAMA:β = − 9.1 µg/g creatinine, 95% CI − 15.8, − 2.4; GAMA: β = − 3.4 µg/g creatinine, 95% CI − 4.7, − 2.2), living in rural areas (AAMA:β = − 4.7 µg/g creatinine, 95% CI − 8.6, − 0.8; GAMA:β = − 1.1 µg/g creatinine, 95% CI − 1.9, − 0.4) and increasing age (AAMA:β = − 1.9 µg/g creatinine, 95% CI − 2.4, − 1.4; GAMA:β = − 0.7 µg/g creatinine, 95% CI − 0.8, − 0.6). In adults, higher AAMA was also associated with high consumption of fried potatoes whereas lower AAMA was associated with higher body-mass-index. Based on this large-scale study, several potential determinants of AA exposure were identified in children/adolescents and adults in European countries.
Subject terms: Epidemiology, Predictive markers, Environmental monitoring, Nutrition
Introduction
Acrylamide (AA) is a genotoxic food-processing contaminant classified as probably carcinogenic to humans (group 2A) by the International Agency for Research on Cancer (IARC)1. It is mainly formed in commonly consumed food, existing in high content in starch e.g., coffee, crisps, fried potatoes, biscuits and cereals, when processed at temperatures above 120 °C under low moisture conditions2. AA can be also formed from acrolein, exists in smoking tobacco and, in the occupational setting, it is used as a chemical for the production of polyacrylamides3. However, the latter source of AA exposure is considered of less concern4.
In vivo and in vitro studies have shown that AA and its main metabolite glycidamide (GA) are carcinogenic, neurotoxic, reprotoxic and toxic for the developmental system3. Emerging evidence also links AA exposure to several other diseases5–8. In humans, the association between AA and cancer risk, investigated in epidemiological studies, remains unclear9. Recently, the European Food Safety Agency (EFSA) reported additional evidence of its genotoxic and non-genotoxic effects and concluded that a risk associated to dietary intake of AA cannot be discarded10.
Despite the adoption of mitigation, monitoring and regulatory measures at European level to reduce AA content in food11,12, dietary exposure to AA still remains widespread representing a global concern in the general population3.
Identification of determinants of exposure might be of great importance as a first step to implement effective measures to reduce AA exposure, especially in vulnerable population groups13. Also, it might help to explain the high variability in AA exposure across different populations and countries14.
Current knowledge on determinants of AA exposure in the general European population is limited, especially in children15–17. Human biomonitoring studies could be useful tools to identify potential factors of exposure to chemical pollutants in children and adults by measuring them and/or their metabolites (biomarkers) in biological samples, such as urine, blood, hair, together with the use of questionnaires18,19. For AA, the validated biomarkers are AA and its metabolite GA measured as hemoglobin adducts in blood, including cord blood20, and as mercapturic acids in urine samples. These biomarkers are used as long- and short-term exposure biomarkers to this compound, respectively14. In addition, evidence suggests that acrylamide levels could also be measured in other human samples, such as breast milk and placenta21.
Hence, we aim to assess potential determinants of exposure to AA measured via its urinary biomarkers, AAMA (N-acetyl-S-(2-carbamoylethyl)-L-cysteine) and GAMA (N-acetyl-S-(2-carbamoyl-2-hydroxyethyl)-L-cysteine), in children/adolescents and adults using harmonized data from the European Human Biomonitoring Initiative HBM4EU participating studies, covering different European regions.
Materials and methods
Study design, data sources and data collection
The present study was based on the participating studies in the HBM4EU initiative (https://www.hbm4eu.eu/), where newly harmonized, individual-level data, were produced within the so-called HBM4EU Aligned Studies sampled between 2014 and 2021. The HBM4EU survey leveraged existing European capacity by incorporating both new/ongoing and recently conducted studies establishing the first large-scale joint effort to align and harmonize ongoing European HBM initiatives. Key factors contributing to the alignment of these studies include target populations, biomarker analysis quality assurance/control program, data handling, and statistical procedures, all implemented through standardized protocols22–24. This unique material enhances inter-study/country comparability. Despite efforts in aligning and harmonizing data, variations persisted in certain variables, such as time sampling (confined to the period 2014–2020), biological matrices, and questionnaires. These differences were acknowledged, and strategies were employed to minimize them, including the use of standardized variables retrieved by the questionnaire and conversion factors for different matrices22,25. The complete sampling scheme for the inclusion, combination and data harmonization of HBM4EU Aligned Studies is fully described in Gilles et al.22. Two additional HBM studies which generated individual-level data on urinary AA biomarkers outside the HBM4EU initiative were included and harmonized following the same procedure. That is, the German Environmental Survey, 2014–2017, (GerES V)17 on children/adolescents (3–18 years old, only a subset there of being part of the Aligned Studies), and the BETTERMILK study (2015)26 on adult women (20–45 years old).
The criteria for inclusion of studies were: samples in European children/adolescents (3–18 years old) or adults (20–45 years old), in which AA biomarkers were measured in urines collected between 2014 and 2021, and providing questionnaire data to be used for the investigation of exposure determinants.
The full description of data access permissions and ethical considerations has been described elsewhere22. In brief, all the participating studies followed methods in accordance with national and European guidelines and ethics regulation. Each of the country's Ethics Committees approved the study (The Regional Committees for Medical and Health Research Ethics in Norway; the Ethics Committees of the University of Udine and the Institute for Maternal and Child Health—IRCCS Burlo Garofolo, Trieste, Italy; Ile-de-France Protection, The French Data Protection Agency, French Advisory Committee on Information Processing for Research and The French National Agency for Medicines and Health Products' Safety, France; the Ethics Commission of the Berlin Chamber of Physicians, the Medical Association Westfalen-Lippe, the Medical Faculty of the University of Münster and Medical Association of the Saarland and the Federal Officer for Data Protection and Freedom of Information, Germany; The National Bioethics Committee, Iceland; the National Ethical committee of Luxembourg, Luxembourg; the Ethical Committees of the National Institute of Health Doutor Ricardo Jorge, the Regional Health Administrations of North, Center, Lisbon, Tagus Valey, Alentejo, Algarve, the Health Service of the Autonomous Region of Madeira and of the Hospital of Horta, Portugal; the Clinical Research Ethics Committee of the Public Health Directorate, the Center for Public Health Research of the Valencian Government, and the Biomedical Scientific Ethic Committee of the University and Polytechnic Hospital “La Fe”, Spain). Written informed consent was obtained from all participants. For children, the written consent was obtained by legal tutors. In general, it was the child's parents who signed the consent. However, there may be differences in the exact procedure between the participating studies i.e. in some countries approval of one parent was sufficient whereas in other countries consent from both parents may be needed22. Each study also confirmed that informed consent and approval were in place for secondary use of the collected data.
In total, 4 studies for children/adolescents (n = 3157) and 6 studies for adults (n = 1297) were included.
Determinants of exposure
Determinants of exposure were selected based on either prior knowledge (e.g., smoking, BMI, and dietary factors) and/or a non-hypothesis-based approach. The selection was limited due to the availability of data in each participating study since questionnaires differed between some surveys. A full description of the variables considered in this study, together with the harmonization, as well as the treatment as continuous or categorical variables in the association with AA urinary biomarkers, is presented in Supplementary Table S1.
Urinary levels of AA biomarkers: chemical analysis
Urine sampling strategies across studies were either 24-h (n = 1 study for adults), first-morning spot (n = 2 studies for children/adolescents and 3 studies for adults) or random spot (n = 2 studies for children/adolescents and 2 studies for adults) urine samples. The individual urinary concentrations of AAMA and GAMA obtained from each study were generated using different analytical methods, but their comparability was generally guaranteed by the HBM4EU quality assurance/quality control (QA/QC) programme (further details in Supplementary Information SI-1). The AAMA and GAMA urinary levels were standardized for creatinine (µg/g creatinine) for children/adolescents and adults, to account for variation in dilution and to increase comparability of the data.
Statistical analysis
Descriptive summary statistics of AAMA and GAMA urinary levels (mean, standard deviation, 10th, 25th, 50th, 75th and 90th percentiles) were calculated for children/adolescents and adults, and by European geographical region if available. As quantification frequencies (QFs) of AAMA and GAMA in urine were > 92% in all the studies, those concentration values reported as < LoQ were excluded from the analysis (n = 2 for AAMA, and n = 2 for GAMA in children; n = 14 for AAMA, and n = 17 for GAMA in adults). This decision was supported by experts in the field and statisticians of the HBM4EU, concluding that this exclusion ensured an improvement of homogeneity and accuracy of the data for analysis. Median regression models were employed to investigate the association between continuous urinary levels of AAMA and GAMA and potential exposure determinants in children/adolescents and adults. This statistical approach allows to regress any percentile of the outcome distribution. As the urinary biomarkers of AA tend to have a skewed distribution, the median regression might be considered a better summary measure than the mean and thus preferable to the classical linear regression. Since results from the 10th, 25th, 50th, 75th, and 90th percentile did not show different results across the percentiles of the distribution, we decided to present only those based on the median regression (50th percentile). The results, expressed as beta coefficients and 95% confidence intervals (95% CI), need to be interpreted as median differences in AAMA and/or GAMA in relation to the corresponding predictor, adjusted for all other factors included in the model. To account for potential heterogeneity among studies, we included in the regression model a study-specific fixed effect. The selection of determinants to be included in the multiple regression models was based on data availability, prior findings, knowledge of the field, and collinearity. To retain statistical power, missing values in categorical predictors were treated as a separate category (known as missing indicator method or “dummy variable adjustment”). Because cigarette smoking is known to increase the levels of AA3 by 3–4 times27, main analyses were presented excluding active smokers (n = 39 in children/adolescents, and n = 238 in adults).
Additional analyses were performed including both smokers and non-smokers and by participating studies.
STATA software (STATA version 12.1, Corp, College Station, TX, USA) was used to perform all statistical analyses.
Results
The HBM studies providing data on individual AAMA and GAMA levels in urine of European children/adolescents (median age: 10, IQR: 7–13 years old) and adults (median age: 33, IQR: 28–37 years old) are summarized in Table 1. In total, urinary data on AA metabolites of 3157 children/adolescents and 1297 adults were collected from different countries including European studies from Northern (Norway and Iceland), Southern (Italy, Spain and Portugal) and Western Europe (France, Germany and Luxembourg). No studies on AA urinary levels from Eastern European countries were available and/or agreed to participate. Out of the total number of children/adolescents, 81% were based on studies from Western countries (France and Germany), 9.5% from the North (Norway), and 9.5% from the South (Italy). In adults, the participating studies were more equally distributed within the European regions, with 53% of the individuals being from the West (France, Germany, Luxembourg), 31% from the South (Spain and Portugal), and 16% from the North (Iceland). Sampling years ranged from 2014 to 2017 in studies on children/adolescents, and from 2014 to 2021 in those on adults. Distribution of exposure determinants by geographical area in children and adults, respectively, are shown in Supplementary Table S2.
Table 1.
Characteristics of the HBM4EU participating studies with urinary levels of AA in children/adolescents and adults.
| Population group (total number) median age (IQR, years) | Geographical region | Country | Study acronyma | Age years median (IQR) | Sampling year | Type of urine sample for AA analysis | N | HBM4EU QA/QC labelb |
|---|---|---|---|---|---|---|---|---|
| Children/adolescents (3158*) 10 (7–13) | North | Norway | NEBII-NO | 10 (9–11) | 2016–2017 | Random spot urine | 299 | A |
| South | Italy | NACII-IT | 7 (7–7) | 2014–2016 | Random spot urine | 300 | A | |
| West | France | ESTEBAN-FR | 9 (7–10) | 2014–2016 | First-morning spot urine | 300* | A | |
| Germany | GerESV-DE | 11 (7–14) | 2015–2017 | First-morning spot urinec | 2,259 | B | ||
| Adults (1298**) 33 (28–37) | North | Iceland | Diet_HBM-IS | 31 (26–35) | 2019–2021 | Random spot urine | 203** | A |
| South | Spain | BETTERMILK | 33.5 (31–37) | 2015 | First-morning spot urine | 117 | C | |
| Portugal | INSEF-ExpoQuim-PT | 35 (32–37) | 2019–2020 | First-morning spot urine | 294** | A | ||
| West | France | ESTEBAN-FR | 34 (29–37) | 2014–2016 | First-morning spot urine | 300 | A | |
| Germany | ESB-DE | 23 (21–24.5) | 2015, 2019, 2021 | 24-h urine | 180 | A | ||
| Luxembourg | Oriscav-Lux2-LU | 34 (31–37) | 2016–2018 | Random spot urine | 204 | A |
IQR: Interquartile range; AA: acrylamide.
*One sample was removed due to missing creatinine value in the ESTEBAN study (299 samples with creatinine value), so finally 3157 urine samples of children/adolescents were included in the present research.
**There were 17 urine samples with missing values for GAMA, and one urine sample with missing values for AAMA, 3 of them from INSEF and 14 from Diet_HBM, therefore, a total number of 1297 and 1281 urine samples of adults were considered for AAMA and GAMA analysis, respectively.
aNEBII-NO: “the Norwegian Environmental Biobank II”, Norway (Norwegian Institute of Public Health, NIPH); NACII-IT: "the Northern Adriatic cohort II", Italy (Section of Hygiene and Epidemiology within the Department of Medical and Biological Sciences of the University of Udine, EPIUD); ESTEBAN-FR: "the Etude de santé sur l’environnement, la biosurveillance, l’activité physique et la nutrition", France (Agence Nationale De Sante Publique, ANSP); GerESV-DE: "the German Environmental Survey 2014–2017", Germany (German Environment Agency, UBA); Diet_HBM-IS: "the Icelandic National Dietary Survey", Iceland (University of Iceland, UI); BETTERMILK: "Exposure to persistent and emerging contaminants in infants through breast milk. Characterization of associated environmental and dietary factors and risk assessment, Spain (Foundation for the Promotion of Health and Biomedical Research of the Valencian Region, FISABIO); INSEF-ExpoQuim-PT: the "Exposure of the Portuguese Population to Environmental Chemicals: a study nested in the National Health Examination Survey", Portugal (National Institute of Health Dr. Ricardo Jorge, INSA); ESB-DE: "the Environmental Specimen Bank", Germany (UBA); Oriscav-Lux2-LU: "the Observation of cardiovascular risk factors in Luxembourg", Luxembourg (Laboratoire national de santé, LNS).
bLabels of the HBM4EU QA/QC programme: Label A = "Biomarker data quality assured by HBM4EU QA/QC programme"; Label B = "Biomarker data generated before HBM4EU QA/QC programme but deemed comparable by HBM4EU Quality Assurance Unit (QAU)"; Label C = "Biomarker data generated before HBM4EU QA/QC programme but comparability not guaranteed by HBM4EU Quality Assurance Unit (QAU)" (see further details in Supplementary SI-1).
cSome samples were defined as random spot urines, since they differed from the standard definition of first-morning urine sample provided in the HBM4EU protocol (e.g. sampled too early in the morning or too late).
Descriptive data on urinary AAMA and GAMA in µg/g creatinine in children/adolescents and adults, and by participating studies are summarized in Table 2. Quantifiable levels of AA metabolites were identified in almost all urine samples, with quantification frequencies ranging from 99 to 100% in children/adolescents, and from 92 to 100% in adults for AAMA and GAMA, respectively. AAMA and GAMA concentrations were fairly similar in children/adolescents and adults, but with a slight tendency of being higher in the youngest age group (Table 2).
Table 2.
AAMA and GAMA urinary levels (µg/g creatinine) in European children/adolescents and adults (including smokers), by European geographical regions and by participating studies.
| Population group | Metabolite | European region | Study acronym | N samples | LOQ | N samples > LOQ | QF (%) | Mean (SD) | P10 | P25 | P50 | P75 | P90 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Children/adolescents | AAMA | South | NACII-IT | 300 | 3.2 | 298 | 99.3 | 100.2 (94.8) | 37.8 | 55.7 | 78.7 | 119.4 | 181.0 |
| West | ESTEBAN-FR | 299 | 3.2 | 299 | 100 | 110.2 (114.9) | 42.0 | 57.1 | 83.9 | 126.5 | 193.0 | ||
| GerESV-DE | 2259 | 1 | 2259 | 100 | 74.6 (72.7) | 30.1 | 40.1 | 57.3 | 84.8 | 128.6 | |||
| Total | 2558 | – | 2558 | 100 | 78.8 (79.6) | 30.6 | 41.8 | 59.9 | 89.8 | 137.7 | |||
| North | NEBII-NO | 299 | 1 | 299 | 100 | 62.9 (56.4) | 27.6 | 34.5 | 51.4 | 75.1 | 102.4 | ||
| Total | All regions | 3157 | – | 3155 | 99.9 | 79.3 (79.7) | 30.4 | 41.9 | 60.7 | 90.8 | 139.2 | ||
| GAMA | South | NACII-IT | 300 | 1 | 298 | 99.3 | 34.5 (19.9) | 16.6 | 22.4 | 30.7 | 40.0 | 55.4 | |
| West | ESTEBAN-FR | 299 | 1 | 299 | 100 | 14.6 (10.2) | 6.8 | 9.0 | 11.9 | 17.7 | 23.1 | ||
| GerESV-DE | 2259 | 1 | 2257 | 99.9 | 14.7 (10.6) | 6.5 | 8.7 | 12.3 | 17.6 | 25.0 | |||
| Total | 2558 | – | 2556 | 99.9 | 14.7 (10.6) | 6.6 | 8.7 | 12.2 | 17.6 | 24.9 | |||
| North | NEBII-NO | 299 | 1 | 299 | 100 | 9.5 (5.3) | 5.2 | 6.6 | 8.3 | 10.9 | 14.2 | ||
| Total | All regions | 3157 | – | 3153 | 100 | 16.1 (13.0) | 6.5 | 8.6 | 12.5 | 19.2 | 29.3 | ||
| Adults | AAMA | South | INSEF-ExpoQuim-PT | 294 | 3.2 | 294 | 100 | 94.0 (80.8) | 35.1 | 47.1 | 72.1 | 111.8 | 182.9 |
| BETTERMILK | 117 | 0.25 | 117 | 100 | 89.8 (76.2) | 33.2 | 46.6 | 70.3 | 113.3 | 161.5 | |||
| Total | 411 | – | 411 | 100 | 92.8 (79.4) | 34.9 | 46.7 | 70.5 | 112.5 | 170.9 | |||
| West | ESTEBAN-FR | 300 | 3.2 | 300 | 100 | 162.5 (211.3) | 39.5 | 54.9 | 91.7 | 195.7 | 342.6 | ||
| ESB-DE | 180 | 10 | 166 | 92 | 39.9 (25.8) | 19.3 | 25.7 | 34.6 | 48.4 | 69.5 | |||
| Oriscav-Lux2-LU | 204 | 1 | 204 | 100 | 42.9 (45.7) | 16.1 | 20.6 | 28.8 | 46.2 | 88.3 | |||
| Total | 684 | – | 670 | 98 | 94.6 (154.8) | 19.8 | 28.9 | 48.3 | 94.3 | 211.5 | |||
| North | Diet_HBM-IS | 202 | 2 | 202 | 100 | 63.8 (59.7) | 24.4 | 33.0 | 47.3 | 74.7 | 105.5 | ||
| Total | All regions | 1297 | – | 1283 | 99 | 89.2 (123.7) | 22.7 | 34.3 | 55.1 | 95.6 | 188.3 | ||
| GAMA | South | INSEF-ExpoQuim-PT | 291 | 1 | 291 | 100 | 28.1 (15.5) | 14.8 | 18.8 | 25.0 | 32.9 | 42.3 | |
| BETTERMILK | 117 | 0.25 | 117 | 100 | 17.2 (10.2) | 8.1 | 10.4 | 14.3 | 22.1 | 29.5 | |||
| Total | 408 | – | 408 | 100 | 25.0 (15.0) | 10.6 | 15.3 | 22.4 | 30.2 | 40.7 | |||
| West | ESTEBAN-FR | 300 | 1 | 299 | 99.7 | 17.3 (21.3) | 5.2 | 7.2 | 11.2 | 20.8 | 34.3 | ||
| ESB-DE | 180 | 1 | 179 | 99.4 | 7.6 (3.3) | 4.3 | 5.5 | 7.1 | 8.9 | 11.4 | |||
| Oriscav-Lux2-LU | 204 | 1 | 204 | 100 | 8.0 (6.2) | 3.7 | 4.7 | 6.0 | 8.2 | 14.9 | |||
| Total | 684 | – | 682 | 99.7 | 12.0 (15.3) | 4.3 | 5.6 | 7.8 | 12.8 | 22.3 | |||
| North | Diet_HBM-IS | 189 | 3 | 174 | 92 | 8.4 (6.8) | 2.2 | 4.9 | 7.5 | 10.3 | 15.5 | ||
| Total | All regions | 1281 | – | 1264 | 97.9 | 15.6 (15.7) | 4.5 | 6.5 | 10.4 | 21.0 | 31.2 |
LOQ: Limit of quantification; QF: quantification frequency (% samples > LOQ); SD: standard deviation; P: percentile.
AAMA and GAMA levels varied by region and were mainly highest in the Southern region compared to Western and Northern countries. The pooled crude and multivariable adjusted median differences in AAMA and GAMA by geographical region for non-smoking children/adolescents and adults are shown in Table 3 (including smokers in Supplementary Table S3). The multi-adjusted results were produced in a model in which the fixed effect of participating studies was omitted due to collinearity with the indicators of geographical area. Higher median AAMA and GAMA levels in both age groups were observed in the Southern region as compared to those observed in Northern Europe. Similar findings were observed after multivariable adjustment for several dietary and non-dietary factors. Higher levels of AAMA and GAMA were also noted in the studies on children/adolescents from the Western region (vs North), even after multivariable adjustment. By contrast, lower levels of AAMA and GAMA were observed in adults from the West compared to the Northern region.
Table 3.
Pooled crude and multivariable-adjusted median differences in AAMA and GAMA urinary levels (beta coefficients with 95% CI) in relation to European geographical regions (North vs. South and West). Results are presented for non-smoking children/adolescents and adults, respectively.
| Population group | Model | AA urinary biomarkers | North reference | European geographical region | N | |
|---|---|---|---|---|---|---|
| South β (95% CI) (µg/g creatinine) | West β (95% CI) (µg/g creatinine) | |||||
| Children/Adolescents | Crude | AAMA | Ref | 27.3 | 7.9 | 2895 |
| (20.3, 34.3) | (2.7, 13.1) | |||||
| GAMA | – | 22.4 | 3.9 | 2895 | ||
| (21.2, 23.7) | (3.9, 4.8) | |||||
| Multivariable-adjusteda | AAMA | – | 22.9 | 26.6 | 2958 | |
| (− 17.5, 63.3) | (3.0, 50.2) | |||||
| GAMA | – | 25.2 | 7.1 | 2958 | ||
| (18.2, 32.1) | (3.1, 11.2) | |||||
| Adults | Crude | AAMA | – | 13.3 | − 6.9 | 937 |
| (6.3, 20.4) | (− 13.3, − 0.5) | |||||
| GAMA | – | 12.6 | − 0.3 | 925 | ||
| (11.7, 13.5) | (− 1.1, 0.6) | |||||
| Multivariable-adjustedb | AAMA | – | 14.8 | − 21.0 | 877 | |
| (2.4, 27.3) | (− 36.9, − 5.0) | |||||
| GAMA | – | 6.3 | − 2.0 | 867 | ||
| (4.3, 8.2) | (− 4.4, 0.4) | |||||
aModel adjusted for the following variables: sex, age, passive smoking, sampling year, place of residence (cities, town and rural areas), highest educational level in the household, body mass index (BMI), physical activity, frequency of consumption of seafood, fish, meat, cereals, fried potatoes, bread, fast food, sugar drinks and tea/coffee.
bModel adjusted for the following variables: sex, sampling year, age, place of residence (cities, town and rural areas), educational level, physical activity, BMI, frequency of consumption of seafood, fried potatoes, fruits/vegetables, coffee and alcohol.
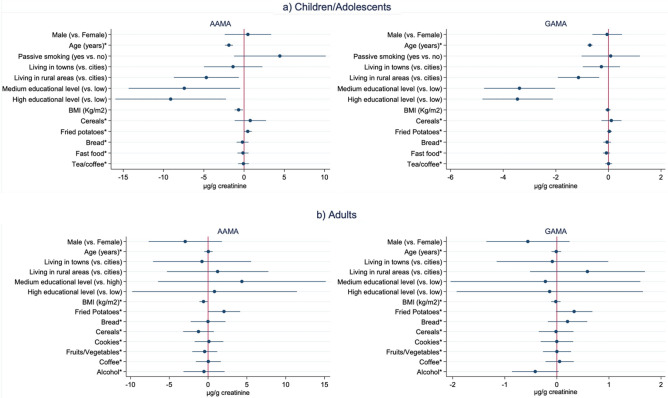
Results from multivariable-adjusted pooled median regression analysis considering both dietary and non-dietary factors (but not region), excluding smokers in children/adolescents and adults are shown in Fig. 1. In both children/adolescents and adults, lower levels of AAMA were associated with participants having higher BMI (β = − 0.7, 95% CI − 1.2, − 0.2 µg/g creatinine; and β = − 0.6, 95% CI − 1.1, − 0.1 µg/g creatinine, respectively). For children/adolescents, lower levels of AAMA and GAMA were observed in relation to increasing age (β = − 1.9, 95% CI − 2.4, − 1.4 µg/g creatinine; and β = − 0.7, 95% CI − 0.8, − 0.6 µg/g creatinine, respectively), higher education (β = − 9.1, 95% CI − 15.8, − 2.4 µg/g creatinine; and β = − 3.4, 95% CI − 4.7, − 2.2 µg/g creatinine, respectively) and living in rural areas (β = − 4.7, 95% CI − 8.6, − 0.8 µg/g creatinine; and β = − 1.1, 95% CI − 1.9, − 0.4 µg/g creatinine, respectively). In children/adolescents, none of the dietary factors were associated with levels of AAMA and/or GAMA, whereas in adults, higher levels of AAMA were observed in participants that reported a high consumption of fried potatoes (β = 2.1, 95% CI 0.2, 4.0 µg/g creatinine).
Figure 1.
Pooled multivariable-adjusted1 median differences in AAMA and GAMA urinary levels (beta coefficients and 95% CI in µg/g creatinine) in relation to non-dietary and dietary determinants. Results are presented for non-smoking children/adolescents (a) and adults (b), respectively2. Educational level classified according to ISCED: international standard classification for education (for children/adolescents referred to the maximum reported in the household); BMI: body mass index. 1In children/adolescents, adjustments were made for variables shown in Figure (a) plus participating study, sampling year, physical activity, and consumption of seafood, fish, meat and sugar drinks; in adults, adjustments were made for variables shown in Figure (b) plus participating study, sampling year, physical activity, vegetarian diet, and consumption of seafood, fish, meat and milk. 2For a better readability of the results, raw data of the following variables were multiplied by a factor of 25: cereals, fried potatoes, bread and fast food in children/adolescents; and cereals, fruit/vegetables and coffee in adults. *Age, BMI and dietary variables (frequency of consumption) treated as continuous (unit increase).
Results from multivariable-adjusted analysis including smoking children/adolescents and adults are shown in Supplementary Fig. S1. These results were, in general, observed to be in the same direction as the results obtained for the non-smokers.
Results from additional analyses investigating crude and multivariable-adjusted associations between each of the considered determinants and levels of AAMA and GAMA by participating studies (data not shown) pointed out that smoking (active) was clearly associated with higher levels of AAMA and GAMA in all the participating studies when available in the corresponding study dataset.
Discussion
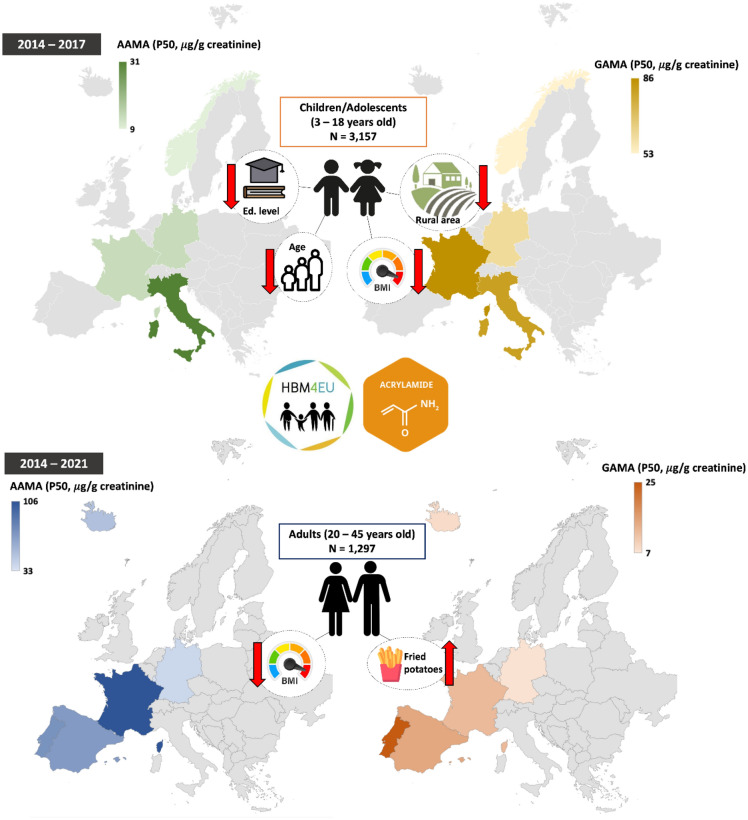
Based on AA data obtained from this large cross-sectional study performed in 3157 children/adolescents from 4 different European countries (Norway, Germany, France and Italy) and 1297 adults from 6 different European countries (Iceland, Germany, France, Luxembourg, Portugal and Spain), several determinants potentially relevant to AA exposure, measured though urinary biomarkers, were identified (Fig. 2).
Figure 2.
Schematic representation of the results by participating studies (colored countries) in children/adolescents (top) and adults (bottom). The figure depicts the median levels of AAMA (left side) and GAMA (right side) (µg/g creatinine), measured in urine samples collected between 2014 and 2017 in children, and 2014–2021 in adults. Various shades of colors differentiate the levels in both age groups. Exposure determinants associated with AAMA and GAMA levels are depicted on the left and right sides, respectively. Red arrows indicate direction of observed associations with urinary levels of AA biomarkers: an up arrow shows an increase, while a down arrow shows a decrease.
In particular, for the first time, our study showed that AA exposure, measured through urinary biomarkers, was higher in Southern European countries compared to those from the North, independently of several dietary and non-dietary factors. In comparison, a previous study based on the European Prospective Investigation into Cancer and Nutrition (EPIC) performed in 9 European countries (Sweden, Denmark, France, United Kingdom, The Netherlands, Germany, Spain, Italy, and Greece) including around 30 adults per country (age range 41–60 years old), noted that AA exposure differ by countries, with higher levels in Western countries 28, including United Kingdom and The Netherlands, than the remaining countries included in the study. Compared to our data, the EPIC study was performed in a smaller sample size, which may impact the uncertainty of their findings; different European countries were included and haemoglobin adducts of AA in blood were measured. Our observed South-North geographical differences did not seem to be fully explained by dietary and non-dietary determinants investigated here. However, these differences in AA exposure might be still due to unmeasured determinants, i.e., specific regional dietary habits, as well as environmental factors29, variability of AA metabolism across populations30–32, and the presence of gene-environmental interactions and/or a combination of these33.
In children/adolescents, our results of decreasing urinary levels of AA biomarkers in relation to increasing age agree with two previous HBM studies16,34 but disagree with most of previous HBM studies17,35–37. Also, our findings of decreasing urinary levels of AA in relation to high education level and living in rural areas disagree with earlier HBM studies16,17,35–37. These discrepancies might be due to differences between our study and the previous HBM studies in terms of design, study characteristics, and used methodologies, i.e., different urine collection methods, covered age ranges, sampling years and statistical methods. The findings related to age were also confirmed by the higher average levels of AAMA and GAMA in children/adolescents compared to adults observed in our study but also in Poteser et al.38. In support of that, European young children have been observed to have higher dietary intake relative to body weight (0.5–1.9 µg/kg bw/day) compared to adolescents and adults (0.4–0.9 µg/kg bw/day), and consume more food containing higher concentrations of AA, such as snacks, biscuits, and cereal-based products, than older population groups3,29. In addition, it is worth mentioning that children´s interindividual variation in the activity and polymorphisms of the enzymes involved in the process of AA detoxification has been suggested to be different from adults39,40. Regarding the observed association between high urinary AA biomarkers in children/adolescents and low educational level and living in urban areas, some potential explanations can be provided. Firstly, children/adolescents living in a household of low educational level may consume more processed food (more likely to have high levels of AA) than those living in a high educational level environment since they and/or their parents may have little awareness of healthy dietary habits, including cooking methods, which are known to affect AA exposure41,42. Secondly, as previously suggested43, rural environments are considered greenness locations associated with low exposure to volatile organic compounds present in polluted areas, such as AA. Furthermore, children/adolescents living in urban areas may have easier access to processed and fast food than those living in rural areas44, which may easily find local and home-grown food products, such as vegetables and fruits, leading to a lower exposure to AA45.
Interestingly, the association between higher BMI and lower AAMA found here for both population groups (children/adolescents and adults) is in line with two previous HBM studies46,47 but not with all16,26,36,37,39,48,49. The main methodical differences, potentially explaining the divergent results, may be that most of the latter studies were performed in Asian populations which may have different genetics and lifestyle, affecting both BMI, dietary intake and metabolism of AA50,51. It has been proposed that body composition can play an important role affecting the metabolism of AA and also its excretion efficiency in humans48,52,53. In addition, high BMIs have been associated with increased oxidative stress, deficiency of antioxidant mechanisms and higher cytochrome 2E1 (CYP2E1) activity54,55, which may induce lower AAMA and higher production of GA. However, in our study no association between GAMA and BMI was observed.
In general, increased concentrations of urinary biomarkers of AA in relation to higher consumption of fried potatoes in adults confirmed results from most previous HBM studies16,17,26,36,39,42,46,48,56,57. The weak or no associations observed between specific dietary determinants and urinary biomarkers of AA, especially in children/adolescents, has previously been reported in several studies16,17,26,36,56. One possible explanation for these findings may be that the employment of self-reported questionnaires (as in this study), especially food frequency questionnaires that assess intake of long-term AA exposure (weeks/months/years), may not be captured by urinary AA metabolites, which are known to be short-term biomarkers of dietary AA exposure (half-life ≈ 3.3 h)39.
Finally, our results confirmed that smoking is a strong determinant of AA exposure estimated by urinary levels in adults, as previously reported14,38. However, it is important to highlight that in our study even though smoking status slightly affected the magnitude of the associations, the overall direction of the associations between urinary AA levels and potential determinants did not. Although the reason is not fully clear, some scientific evidence suggest that smokers may have different activity of the enzymes involved in AA metabolism e.g., CYP2E19,58.
Strengths and limitations of the study
This study is based on unique data comprising large harmonized high-quality data on urinary levels of AA biomarkers and several exposure determinants from a wide spectrum of European geographical areas. Despite the large effort made to harmonize the data, some heterogeneity across studies could not be avoided, for instance differences in how urine samples were collected and study characteristics. Also, since all the determinants investigated were assessed through self-reported data from questionnaires, bias related to misclassification of exposure determinants cannot be ruled out. Another study limitation is that we might have failed to identify some associations with long-term exposure determinants since urinary AA metabolites are short-term biomarkers. In general, this would most likely cause the estimated coefficients to be biased towards the null due to dilution effects. In addition, these results may not be generalizable to other non- and/or European population groups since the study base of the included HBM4EU participating studies was not representative of the corresponding region/country/European area. Furthermore, the presence of overfitting may have inflated the standard errors making it difficult to achieve statistically significant results.
Conclusions
In this large high-quality and harmonized data including a wide spectrum of European areas, several potential determinants of AA exposure were identified in both children/adolescents (socioeconomic status, living in rural areas, age, and BMI) and adults (fried potatoes intake and BMI). Our findings provide additional scientific knowledge on exposure determinants of AA, which may form basis to decrease the burden of AA exposure in the European population. Overall, our findings strengthen the evidence that children/adolescents might be a vulnerable group, and, generally, encourage the continuation of monitoring levels of AA biomarkers among the European citizens. In addition, further research is needed to investigate potential determinants, dietary and non-dietary, using well-aligned questionnaires, including genetics and their interactions behind the observed European geographic differences in AA exposure.
Supplementary Information
Acknowledgements
The authors thank all the partners of the HBM4EU project. Fernández S.F. acknowledges the CIBEFP/2021/51 funding provided by the Valencian Government (Spain) and the European Social Fund. We also thank Frumento P and Discacciati A. for their statistical support.
Author contributions
All authors contributed substantively to this work. Laguzzi F. conceptualized the study and performed the statistical analyses. Govart E., Rodriguez Martin L and Gilles L, collected and harmonized the data. Fernández S.F. and Laguzzi F. drafted the manuscript. All authors were involved in the interpretation of results and the editing of the manuscript. All authors approved the final manuscript.
Funding
Open access funding provided by Karolinska Institute. This work was supported by the European Union Horizon-2020 Research and Innovation Programme under Grant Agreement No. 733032 (HBM4EU project). The funding of the German data by the German Ministry for the Environment, Nature Conservation, Nuclear Safety and Consumer Protection is gratefully acknowledged. The Norwegian Institute of Public Health (NIPH) has contributed to funding of the Norwegian Environmental Biobank (NEB). The laboratory measurements have partly been funded by the Research Council of Norway through research Projects (275903 and 268465).
Data availability
The data that support the findings of this study are available from the Personal Exposure and Health (PEH) Data Platform (https://hbm.vito.be/peh-data-platform) but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available upon reasonable request and with permission of the Data Request Access Committee (DRAC) following the procedures for data access as described here: https://hbm.vito.be/peh-data-platform/data-access-procedure.
Competing interests
The authors declare no competing interests.
Footnotes
The original online version of this Article was revised: The original version of this Article contained an error in the name of the author Federica Laguzzi, which was incorrectly given as Laguzzi Federica.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
1/29/2024
A Correction to this paper has been published: 10.1038/s41598-024-52775-0
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-48738-6.
References
- 1.IARC. List of Classifications—IARC Monographs on the Identification of Carcinogenic Hazards to Humans (accessed 19 May 2022); https://monographs.iarc.who.int/list-of-classifications
- 2.ECHA. Acrylamide: Brief Profile (accessed 19 May 2022); https://echa.europa.eu/sv/brief-profile/-/briefprofile/100.001.067
- 3.EFSA Scientific opinion on acrylamide in food. EFSA J. 2015;13(6):4104. doi: 10.2903/j.efsa.2015.4104. [DOI] [Google Scholar]
- 4.ATSDR. Toxicological Profile for Acrylamide (2012). [PubMed]
- 5.Liang J, et al. Total cholesterol: A potential mediator of the association between exposure to acrylamide and hypertension risk in adolescent females. Environ. Sci. Pollut. Res. 2022;29(25):38425–38434. doi: 10.1007/s11356-021-18342-0. [DOI] [PubMed] [Google Scholar]
- 6.Hsu CN, Hou CY, Lu PC, Chang-Chien GP, Lin S, Tain YL. Association between acrylamide metabolites and cardiovascular risk in children with early stages of chronic kidney disease. Int. J. Mol. Sci. 2020;21(16):1–12. doi: 10.3390/ijms21165855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Z, Sun J, Zhang D. Association between acrylamide hemoglobin adduct levels and depressive symptoms in US adults: NHANES 2013–2016. J. Agric. Food Chem. 2021;69(46):13762–13771. doi: 10.1021/acs.jafc.1c04647. [DOI] [PubMed] [Google Scholar]
- 8.Quan W, et al. Effect of dietary exposure to acrylamide on diabetes-associated cognitive dysfunction from the perspectives of oxidative damage, neuroinflammation, and metabolic disorders. J. Agric. Food Chem. 2022;70(14):4445–4456. doi: 10.1021/acs.jafc.2c00662. [DOI] [PubMed] [Google Scholar]
- 9.Filippini T, et al. Dietary acrylamide exposure and risk of site-specific cancer: A systematic review and dose-response meta-analysis of epidemiological studies. Front. Nutr. 2022;9:875607. doi: 10.3389/fnut.2022.875607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.EFSA, Benford, D., Bignami, M., Chipman, J. K. & Ramos Bordajandi, L. Assessment of the genotoxicity of acrylamide. EFSA J.20 (5), e07293 (2022). 10.2903/j.efsa.2022.7293. [DOI] [PMC free article] [PubMed]
- 11.European Commission. Commission regulation (EU) 2017/2158 of 20 November 2017: Establishing mitigation measures and benchmark levels for the reduction of the presence of acrylamide in food. Off. J. Eur. Union, L304/24–L304/44 (2017).
- 12.European Commission, Commission Recommendation (EU) 2019/1888 of 7 November 2019 on the monitoring of the presence of acrylamide in certain foods. Off. J. Eur. Union L290/31–L290/33 (2019).
- 13.Burdorf A. Identification of determinants of exposure: Consequences for measurement and control strategies. Occup. Environ. Med. 2005;62(5):344–350. doi: 10.1136/oem.2004.015198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Albiach-Delgado A, Esteve-Turrillas FA, Fernández SF, Garlito B, Pardo O. Review of the state of the art of acrylamide human biomonitoring. Chemosphere. 2022;295:133880. doi: 10.1016/j.chemosphere.2022.133880. [DOI] [PubMed] [Google Scholar]
- 15.Mojska H, Gielecińska I, Winiarek J, Sawicki W. Acrylamide content in breast milk: The evaluation of the impact of breastfeeding women’s diet and the estimation of the exposure of breastfed infants to acrylamide in breast milk. Toxics. 2021;9(11):298. doi: 10.3390/toxics9110298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fernández SF, Pardo O, Coscollà C, Yusà V. Risk assessment of the exposure of Spanish children to acrylamide using human biomonitoring. Environ. Pollut. 2022;305:119319. doi: 10.1016/j.envpol.2022.119319. [DOI] [PubMed] [Google Scholar]
- 17.Schwedler G, et al. Benzene metabolite SPMA and acrylamide metabolites AAMA and GAMA in urine of children and adolescents in Germany: Human biomonitoring results of the German Environmental Survey 2014–2017 (GerES V) Environ. Res. 2021;192:110295. doi: 10.1016/j.envres.2020.110295. [DOI] [PubMed] [Google Scholar]
- 18.WHO. Human Biomonitoring: Facts and Figures (2015); http://www.euro.who.int/__data/assets/pdf_file/0020/276311/Human-biomonitoring-facts-figures-en.pdf
- 19.WHO Regional Office for Europe. Human Biomonitoring: Assessment of Exposure to Chemicals and Their Health Risks: Summary for Decision Makers (Accessed 20 November 2023); https://www.who.int/europe/publications/i/item/WHO-EURO-2023-7574-47341-69480
- 20.Törnqvist M, Fred C, Haglund J, Helleberg H, Paulsson B, Rydberg P. Protein adducts: Quantitative and qualitative aspects of their formation, analysis and applications. J. Chromatogr B. 2002;778(1):279–308. doi: 10.1016/S1570-0232(02)00172-1. [DOI] [PubMed] [Google Scholar]
- 21.Sörgel F, et al. Acrylamide: Increased concentrations in homemade food and first evidence of its variable absorption from food, variable metabolism and placental and breast milk transfer in humans. Chemotherapy. 2002;48(6):267–274. doi: 10.1159/000069715. [DOI] [PubMed] [Google Scholar]
- 22.Gilles L, et al. HBM4EU combines and harmonises human biomonitoring data across the EU, building on existing capacity: The HBM4EU survey. Int. J. Hyg. Environ. Health. 2021;237:113809. doi: 10.1016/j.ijheh.2021.113809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gilles L, et al. Harmonization of human biomonitoring studies in Europe: Characteristics of the HBM4EU-aligned studies participants. Int. J. Environ. Res. Public Health. 2022;19:6787. doi: 10.3390/ijerph19116787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Esteban-López M, et al. The European human biomonitoring platform: Design and implementation of a laboratory quality assurance/quality control (QA/QC) programme for selected priority chemicals. Int. J. Hyg. Environ. Health. 2021;234:113740. doi: 10.1016/j.ijheh.2021.113740. [DOI] [PubMed] [Google Scholar]
- 25.Govarts E, et al. Harmonized human biomonitoring in European children, teenagers and adults: EU-wide exposure data of 11 chemical substance groups from the HBM4EU Aligned Studies (2014–2021) Int. J. Hyg. Environ. Health. 2023;249:114119. doi: 10.1016/j.ijheh.2023.114119. [DOI] [PubMed] [Google Scholar]
- 26.Fernández SF, Pardo O, Coscollà C, Yusà V. Exposure assessment of Spanish lactating mothers to acrylamide via human biomonitoring. Environ. Res. 2021;203:111832. doi: 10.1016/j.envres.2021.111832. [DOI] [PubMed] [Google Scholar]
- 27.Mojska H, Gielecińska I, Cendrowski A. Acrylamide content in cigarette mainstream smoke and estimation of exposure to acrylamide from tobacco smoke in Poland. Ann. Agric. Environ. Med. 2016;23(3):456–461. doi: 10.5604/12321966.1219187. [DOI] [PubMed] [Google Scholar]
- 28.Vesper HW, et al. Cross-sectional study on acrylamide hemoglobin adducts in subpopulations from the European Prospective Investigation into Cancer and Nutrition (EPIC) study. J. Agric. Food Chem. 2008;56(15):6046–6053. doi: 10.1021/jf703750t. [DOI] [PubMed] [Google Scholar]
- 29.Timmermann CAG, et al. A review of dietary intake of acrylamide in humans. Toxics. 2021;9(7):1–24. doi: 10.3390/toxics9070155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li D, Wang P, Liu Y, Hu X, Chen F. Metabolism of acrylamide: Interindividual and interspecies differences as well as the application as biomarkers. Curr. Drug Metab. 2016;17(4):317–326. doi: 10.2174/1389200216666151015115007. [DOI] [PubMed] [Google Scholar]
- 31.Huang YF, et al. The modifying effect of CYP2E1, GST, and mEH genotypes on the formation of hemoglobin adducts of acrylamide and glycidamide in workers exposed to acrylamide. Toxicol. Lett. 2012;215(2):92–99. doi: 10.1016/j.toxlet.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 32.Poteser M, et al. Time trends of acrylamide exposure in Europe: Combined analysis of published reports and current HBM4EU studies. Toxics. 2022;10(8):481. doi: 10.3390/TOXICS10080481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miranda-Filho A, Piñeros M, Ferlay J, Soerjomataram I, Monnereau A, Bray F. Epidemiological patterns of leukaemia in 184 countries: A population-based study. Lancet Haematol. 2018;5(1):e14–e24. doi: 10.1016/S2352-3026(17)30232-6. [DOI] [PubMed] [Google Scholar]
- 34.Hartmann EC, Boettcher MI, Schettgen T, Fromme H, Drexler H, Angerer J. Hemoglobin adducts and mercapturic acid excretion of acrylamide and glycidamide in one study population. J. Agric. Food Chem. 2008;56(15):6061–6068. doi: 10.1021/jf800277h. [DOI] [PubMed] [Google Scholar]
- 35.Brisson B, et al. Relation between dietary acrylamide exposure and biomarkers of internal dose in Canadian teenagers. J. Expo. Sci. Environ. Epidemiol. 2014;24(2):215–221. doi: 10.1038/jes.2013.34. [DOI] [PubMed] [Google Scholar]
- 36.Choi SY, Ko A, Kang HS, Hwang MS, Lee HS. Association of urinary acrylamide concentration with lifestyle and demographic factors in a population of South Korean children and adolescents. Environ. Sci. Pollut. Res. 2019;26(18):18247–18255. doi: 10.1007/s11356-019-05037-w. [DOI] [PubMed] [Google Scholar]
- 37.Lin C, Lee H, Chen Y, Lien G, Lin L. Positive association between urinary levels of 8-hydroxydeoxyguanosine and the acrylamide metabolite N-acetyl-S-(propionamide)-cysteine in adolescents and young adults. J. Hazard Mater. 2013;261:372–377. doi: 10.1016/j.jhazmat.2013.06.069. [DOI] [PubMed] [Google Scholar]
- 38.Poteser M, et al. Trends of exposure to acrylamide as measured by urinary biomarkers levels within the HBM4EU biomonitoring aligned studies (2000–2021) Toxics. 2022;10:443. doi: 10.3390/toxics10080443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bjellaas T, et al. Urinary acrylamide metabolites as biomarkers for short-term dietary exposure to acrylamide. Food Chem. Toxicol. 2007;45(6):1020–1026. doi: 10.1016/j.fct.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 40.Kocadağli T, Gökmen V. Metabolism of Acrylamide in Humans and Biomarkers of Exposure to Acrylamide. Amsterdam: Elsevier; 2016. [Google Scholar]
- 41.WHO. Environment and Health Risks: A Review of the Influence and Effects of Social Inequalities (2010).
- 42.Lee JH, Lee KJ, Ahn R, Kang HS. Urinary concentrations of acrylamide (AA) and N-acetyl-S-(2-carbamoylethyl)-cysteine (AAMA) and associations with demographic factors in the South Korean population. Int. J. Hyg. Environ. Health. 2013;217(7):751–757. doi: 10.1016/j.ijheh.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 43.Yeager R, et al. Association between residential greenness and exposure to volatile organic compounds. Sci. Total Environ. 2020;707:135435. doi: 10.1016/j.scitotenv.2019.135435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vilar-Compte M, et al. Urban poverty and nutrition challenges associated with accessibility to a healthy diet: A global systematic literature review. Int. J. Equity Health. 2021;20:1–19. doi: 10.1186/s12939-020-01330-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morton LW, Bitto EA, Oakland MJ, Sand M. Accessing food resources: Rural and urban patterns of giving and getting food. Agric. Hum. Values. 2008;25(1):107–119. doi: 10.1007/s10460-007-9095-8. [DOI] [Google Scholar]
- 46.Lee J, Lee K, Kang H. Estimation of the daily human intake of acrylamide (AA) based on urinary N-acetyl-S-(2-carbamoylethyl)-cysteine (AAMA) and the contribution of dietary habits in South Korean adults. J. Environ. Health Sci. 2016;42:235–245. doi: 10.5668/JEHS.2016.42.4.235. [DOI] [Google Scholar]
- 47.Ji K, et al. Science of the total environment urinary levels of N-acetyl-S-(2-carbamoylethyl)-cysteine(AAMA), an acrylamide metabolite, in Korean children and their association with food consumption. Sci. Total Environ. 2013;456–457:17–23. doi: 10.1016/j.scitotenv.2013.03.057. [DOI] [PubMed] [Google Scholar]
- 48.Wang Q, Chen X, Ren Y, Chen Q, Meng Z, Cheng J. Toxicokinetics and internal exposure of acrylamide: New insight into comprehensively profiling mercapturic acid metabolites as short-term biomarkers in rats and Chinese adolescents. Arch. Toxicol. 2017;91(5):2107–2118. doi: 10.1007/s00204-016-1869-6. [DOI] [PubMed] [Google Scholar]
- 49.Huang CCJ, Li CM, Wu CF, Jao SP, Wu KY. Analysis of urinary N-acetyl-S-(propionamide)-cysteine as a biomarker for the assessment of acrylamide exposure in smokers. Environ. Res. 2007;104(3):346–351. doi: 10.1016/j.envres.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 50.Kotemori A, et al. Validity of a self-administered food frequency questionnaire for the estimation of acrylamide intake in the Japanese population: The JPHC FFQ validation study. J. Epidemiol. 2018;28(12):482–487. doi: 10.2188/JEA.JE20170186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.The WHO expert consultation Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363(9403):157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 52.Hays SM, Aylward LL, Blount BC. Variation in urinary flow rates according to demographic characteristics and body mass index in NHANES: Potential confounding of associations between health outcomes and urinary biomarker concentrations. Environ. Health Perspect. 2015;123(4):293–300. doi: 10.1289/ehp.1408944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chu PL, Lin LY, Chen PC, Su TC, Lin CY. Negative association between acrylamide exposure and body composition in adults: NHANES, 2003–2004. Nutr. Diabetes. 2017;7(3):e246. doi: 10.1038/nutd.2016.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marseglia L, et al. Oxidative stress in obesity: A critical component in human diseases. Int. J. Mol. Sci. 2015;16(1):378–400. doi: 10.3390/ijms16010378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lucas D, Farez C, Bardou LG, Vaisse J, Attali JR, Valensi P. Cytochrome P450 2E1 activity in diabetic and obese patients as assessed by chlorzoxazone hydroxylation. Fundam. Clin. Pharmacol. 1998;12(5):553–558. doi: 10.1111/J.1472-8206.1998.TB00985.X. [DOI] [PubMed] [Google Scholar]
- 56.Heudorf U, Hartmann E, Angerer J. Acrylamide in children: Exposure assessment via urinary acrylamide metabolites as biomarkers. Int. J. Hyg. Environ. Health. 2009;212(2):135–141. doi: 10.1016/j.ijheh.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 57.Goerke K, et al. Biomonitoring of nutritional acrylamide intake by consumers without dietary preferences as compared to vegans. Arch. Toxicol. 2019;93(4):987–996. doi: 10.1007/s00204-019-02412-x. [DOI] [PubMed] [Google Scholar]
- 58.Van Vleet TR, Bombick DW, Coulombe RA. Inhibition of human cytochrome P450 2E1 by nicotine, cotinine, and aqueous cigarette tar extract in vitro. Toxicol. Sci. 2001;64(2):185–191. doi: 10.1093/TOXSCI/64.2.185. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the Personal Exposure and Health (PEH) Data Platform (https://hbm.vito.be/peh-data-platform) but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available upon reasonable request and with permission of the Data Request Access Committee (DRAC) following the procedures for data access as described here: https://hbm.vito.be/peh-data-platform/data-access-procedure.