Abstract
BACKGROUND:
With the increasing age of lung transplant candidates, we studied waitlist and post-transplant outcomes of candidates ≥70 years during the Lung Allocation Score era.
METHODS:
Adult lung transplant candidates from 2005-2020 in the United Network for Organ Sharing database were included and stratified based on age at listing into: 18-59 years old, 60-69 years old, and ≥70 years old. Baseline characteristics, waitlist outcomes, and post-transplant outcomes were assessed.
RESULTS:
A total of 37,623 candidates were included (52.3% aged 18-59, 40.6% aged 60-69, 7.1% aged ≥70 years). Candidates ≥70 years were more likely than younger candidates to receive a transplant (81.9% vs. 72.7% [aged 60-69] vs. 61.6% [aged 18-59]) and less likely to die or deteriorate on the waitlist within one year (9.1% vs. 10.1% [aged 60-69] vs. 12.2% [aged 18-59], p<0.001). Donors for older recipients were more likely to be extended criteria (75.7% vs. 70.1% [aged 60-69] vs. 65.7% [aged 18-59], p<0.001). Recipients ≥70 years were found to have lower rates of acute rejection (6.7% vs. 7.4% [aged 60-69] vs. 9.2% [aged 18-59], p<0.001) and prolonged intubation (21.7% vs. 27.4% [aged 60-69] vs. 34.5% [aged 18-59], p<0.001). Recipients aged ≥70 years had increased 1- (aHR [95%CI]: 1.19 [1.06-1.33]; p<0.001), 3- (aHR [95%CI]: 1.28 [1.18-1.39]; p<0.001) and 5-year mortality (aHR [95%CI]: 1.29 [1.21-1.38]; p<0.001) compared to recipients aged 60-69.
CONCLUSIONS:
Candidates ≥70 years had favorable waitlist and perioperative outcomes, despite increased use of extended criteria donors. Careful candidate selection and post-operative surveillance may improve post-transplant survival in this population.
Graphical Abstract

The Lung Allocation Score (LAS) was implemented in 2005, creating an allocation system based on waitlist urgency instead of time accrued. The impetus of this change was to decrease waitlist mortality.[1] This system increased access to transplant for candidates with high waitlist urgency, including older candidates.[2] As a result, the median age of candidates has steadily increased over the last 15 years, with candidates ≥65 years comprising 30.1% of total candidates in 2017.[3]
While the International Society for Heart & Lung Transplantation guidelines consider age ≥65 years as a relative contraindication to lung transplant,[4] it has become increasingly common to transplant recipients aged ≥70.[5] A previously published United Network for Organ Sharing (UNOS) study of recipients from 1995 to 2009 found that age ≥70 years was not a risk factor for 1-year mortality in the LAS era.[6] A more recent study from 2000 to 2013 found that short-term survival after lung transplant in recipients ≥70 years have improved over time and are comparable to outcomes in younger recipients, but 3-year and 5-year survival remain significantly worse in recipients ≥70 years.[7]
Given the increasing age of the lung transplant candidate population, further evidence is needed to evaluate whether listing and transplanting older candidates is a safe and effective practice. Evidence on waitlist and post-transplant outcomes in a contemporary cohort of candidates ≥70 years is limited. This study examined the U.S. experience with lung transplant candidates ≥70 years at listing during the LAS era.
MATERIAL AND METHODS
Study Population
Adult (≥18 years) first-time lung-only transplant candidates listed between 2005 and 2020 in the UNOS database were included. The start date of this study period reflects the year that the LAS was implemented. Candidates were stratified into three groups based on age at initial listing: 18-59 years, 60-69 years, and ≥70 years. The cutoff of ≥70 years was chosen given that the literature on this population in particular is limited. A comparison group of candidates aged 60-69 was chosen given that (1) this group would represent a more similar population in terms of comorbidities and indications; and (2) previous studies have demonstrated excellent outcomes in recipients aged 60-69.[8] For post-transplant analyses, the study population was further limited to candidates who received a lung transplant prior to December 31, 2020 to allow ≥1 year of follow-up time for all candidates. Recipients were followed to outcome of interest or last known follow-up date. This study was deemed exempt for the need for institutional review board approval by the Johns Hopkins Institutional Review Board.
Baseline characteristics
Normality of all variables were evaluated using Shapiro-Wilk testing and histogram visualization. Parametric continuous variables were compared using one-way ANOVA and reported as mean (standard deviation). Nonparametric continuous variables were compared using Kruskal Wallis testing and reported as median (interquartile range [IQR]). Categorical variables were assessed using Chi-squared testing and reported as number (percentage). Trends in characteristics by era were assessed using Cuzick tests. Extended criteria donor was defined as donation after circulatory death (DCD) age >55, abnormal chest x-ray, >20 pack-year smoking history, or PaO2/FiO2 ratio <300.
Waitlist analysis
Competing risk analysis using Fine-Gray subdistribution hazard models[9] was used to evaluate the cumulative incidence of death/deterioration on the waitlist within one year with transplant considered a competing risk. Candidate waitlist cause of death was compared among age groups using Chi-squared testing.
Post-transplant outcomes
Predischarge acute rejection, post-operative requirement for dialysis, prolonged intubation (≥72 hours), extracorporeal membrane oxygenation (ECMO) support at 72 hours, and acute rejection within 1 year were compared using Chi-squared testing. For post-transplant survival, time-to-event analysis was performed, and outcomes were visualized using Kaplan-Meier curves. Univariate and multivariable Cox regression were used to compare post-transplant survival at 1, 3, and 5 years post-transplant by age group. Models were adjusted for baseline recipient, donor, and transplant characteristics with p<0.2 on univariate analysis in order to isolate potentially significant confounding variables to include in the multivariable model. Recipient post-transplant cause of death was compared among age groups using Chi-squared testing. A subgroup analysis of bilateral versus single transplants was performed in recipients ≥70 years of age. All statistics were performed using StataSE 17 (StataCorp, College Station, Texas).
RESULTS
Waitlist candidate characteristics
A total of 37,623 lung transplant candidates were included in this study, of which 52.3% were aged 18-59, 40.6% were aged 60-69, and 7.1% were ≥70 years. For candidates ≥70 years, the median age at listing was 71 years (IQR 70-73) and the oldest candidate was 83 years. Candidates ≥70 years were more likely to have restrictive disease (79.6% vs. 58.9% [aged 60-69] vs. 40.7% [aged 18-59], p<0.001) and less likely to be supported on a ventilator (1.5% vs. 2.2% [aged 60-69] vs. 5.1% [aged 18-59], p<0.001) or ECMO (0.6% vs. 0.9% [aged 60-69] vs. 3.1% [aged 18-59], p<0.001) at listing (Table 1).
Table 1:
Baseline characteristics of lung transplant candidates, 2005-2020.
| Variable, n (%) | 18-59 years N=19,667 |
60-69 years N=15,275 |
≥70 years N=2,681 |
p-value |
|---|---|---|---|---|
| Age at listing (years), median (IQR) | 50.0 (37.0-56.0) | 64.0 (62.0-66.0) | 71.0 (70.0-73.0) | <0.001 |
| Male sex | 9,700 (49.3%) | 9,359 (61.3%) | 1,989 (74.2%) | <0.001 |
| Ethnicity | <0.001 | |||
| White | 14,620 (74.3%) | 12,869 (84.2%) | 2,388 (89.1%) | |
| Black | 2,574 (13.1%) | 993 (6.5%) | 81 (3.0%) | |
| Hispanic | 1,834 (9.3%) | 1,013 (6.6%) | 137 (5.1%) | |
| Other | 639 (3.2%) | 400 (2.6%) | 75 (2.8%) | |
| Diagnosis | <0.001 | |||
| Obstructive Disease | 4,749 (24.1%) | 5,270 (34.5%) | 468 (17.5%) | |
| Pulmonary Vascular Disease | 1,707 (8.7%) | 418 (2.7%) | 47 (1.8%) | |
| Cystic Fibrosis | 3,906 (19.9%) | 65 (0.4%) | 0 (0.0%) | |
| Restrictive Disease | 8,012 (40.7%) | 8,996 (58.9%) | 2,135 (79.6%) | |
| Other | 1,293 (6.6%) | 526 (3.4%) | 31 (1.2%) | |
| Disease severity | ||||
| Pre-transplant ventilation | 998 (5.1%) | 333 (2.2%) | 39 (1.5%) | <0.001 |
| Pre-transplant ECMO | 613 (3.1%) | 137 (0.9%) | 15 (0.6%) | <0.001 |
| LAS at listing, median (IQR) | 37.1 (33.5-44.4) | 36.7 (33.2-44.4) | 37.9 (34.0-45.5) | <0.001 |
| Comorbidities | ||||
| BMI (kg/m2), median (IQR) | 24.5 (20.3-28.7) | 26.7 (23.4-29.5) | 26.3 (23.8-29.0) | <0.001 |
| History of smoking | 8,927 (45.4%) | 11,000 (72.0%) | 1,811 (67.5%) | <0.001 |
| Diabetes | 3,683 (18.7%) | 2,454 (16.1%) | 435 (16.2%) | <0.001 |
| History of malignancy | 938 (4.8%) | 1,541 (10.1%) | 499 (18.6%) | <0.001 |
| Prior cardiac surgery | 530 (2.7%) | 787 (5.2%) | 211 (7.9%) | <0.001 |
IQR, interquartile range; BMI, body mass index; ECMO, extracorporeal membrane oxygenation; LAS, lung allocation score.
Candidate characteristics by era
When comparing characteristics of candidates ≥70 years in the most recent era (2015-2020) to the earliest era (2005-2009), there was an increase in the percentage of candidates with restrictive disease (81.4% vs. 75.6%, p=0.02) and a decrease in median LAS at listing (37.3 [33.8-43.1] vs. 39.2 [33.5-48.7], p<0.001; Supplemental Table 1). A similar proportion of candidates in the most recent era compared to the earliest era were supported on a ventilator (1.2% vs. 0.9%, p=0.35) or ECMO (0.7% vs 0.0%, p=0.14) pre-transplant, or had prior history of smoking (66.5% vs 67.6%, p=0.25), diabetes (16.4% vs. 15.1%, p=0.69), malignancy (19.2% vs. 17.3%, p=0.34), or cardiac surgery (7.6% vs. 9.8%, p=0.29).
Waitlist outcomes
Candidates greater than ≥70 years were more likely to receive a transplant (81.9% vs. 72.7% [aged 60-69] vs. 61.6% [aged 18-59]; Figure 1) and less likely to die/deteriorate on the waitlist (9.1% vs. 10.1% [aged 60-69] vs. 12.2% [aged 18-59], p<0.001; Table 2) within one year of listing. Candidates ≥70 years had shorter median time to transplant compared to candidates aged 60-69 or 18-59 (30 [10-83] vs. 52 [16-150] vs. 68 [19-207] days, p<0.001).
Figure 1:
Waitlist outcomes of lung transplant candidates 2005-2020, by age. (A) Cumulative incidence of waitlist death/delisting within one year. (B) Cumulative incidence of transplant within one year.
Table 2:
Waitlist outcomes of lung transplant candidates, 2005-2020.
| Variables, n (%) | 18-59 years N=19,667 |
60-69 years N=15,275 |
≥70 years N=2,681 |
p-value |
|---|---|---|---|---|
| Outcome within 1 year | <0.001 | |||
| Transplanted | 12,119 (61.6%) | 11,111 (72.7%) | 2,197 (81.9%) | |
| Died or deteriorated | 2,406 (12.2%) | 1,542 (10.1%) | 243 (9.1%) | |
| Improved | 142 (0.7%) | 68 (0.4%) | 11 (0.4%) | |
| Other | 919 (4.7%) | 458 (3.0%) | 77 (2.9%) | |
| Remains on waitlist | 4,081 (20.8%) | 2,096 (13.7%) | 153 (5.7%) | |
| Cause of death on waitlist | 0.013 | |||
| Pulmonary | 1,033 (54.1%) | 602 (57.9%) | 86 (67.2%) | |
| Cardiovascular | 252 (13.2%) | 153 (14.7%) | 16 (12.5%) | |
| Hemorrhage | 286 (15.0%) | 150 (14.4%) | 15 (11.7%) | |
| Infection | 130 (6.8%) | 68 (6.5%) | 6 (4.7%) | |
| Cerebrovascular | 22 (1.2%) | 9 (0.9%) | 1 (0.8%) | |
| Malignancy | 7 (0.4%) | 3 (0.3%) | 0 (0.0%) | |
| Renal Failure | 4 (0.2%) | 2 (0.2%) | 0 (0.0%) | |
| Other | 175 (9.2%) | 52 (5.0%) | 4 (3.1%) | |
| Days on waitlist before transplant, median (IQR) | 68 (19-207) | 52 (16-150) | 30 (10-83) | <0.001 |
| Days on waitlist before death, median (IQR) | 94 (22-323) | 94 (19-310) | 81 (16-207) | 0.009 |
IQR, interquartile range.
Recipient characteristics
Among the 27,958 lung transplant recipients, 47.3% were 18-59 years old, 43.9% were 60-69 years old, and 8.8% were ≥70 years. For recipients ≥70 years at listing, the median age at transplant was 71 years (IQR 70-73) and the oldest recipient was 84 years at transplant. The proportion of recipients aged ≥70 increased from 2.2% in 2005 to 14.3% in 2020 (p<0.001, Figure 2) and the number of centers performing transplants in recipients ≥70 years increased from 8 (15.3%) to 52 (85.2%) over the study period (p<0.001). Median LAS at time of transplant was 40.5 for recipients ≥70 years, 39.3 for recipients aged 60-69, and 41.3 for recipients aged 18-59 (p<0.001; Supplemental Table 2).
Figure 2:
Trends in transplanting lung transplant candidates ≥70 years. (A) Percent of recipients ≥70 years at listing, by transplant year. (B) Number of total lung transplant centers (solid line) and centers performing ≥1 transplant in lung transplant candidates ≥70 years (dashed line).
Donor characteristics
Recipients ≥70 years, compared to recipients aged 60-69 or 18-59, received organs from donors who were older (34 [24-48] vs. 33 [23-47] vs. 32 [22-45] years, p<0.001; Supplemental Table 3). Donors for older recipients were also more likely to be extended criteria (75.7% vs. 70.1% [aged 60-69] vs. 65.7% [aged 18-59], p<0.001) and be designated as Public Health Service “Increased Risk” donors (20.2% vs. 18.2% [aged 60-69] vs. 16.6% [aged 18-59], p<0.001)
Perioperative outcomes
Recipients ≥70 years were found to have lower likelihood of pre-discharge acute rejection (6.7% vs. 7.4% [aged 60-69] vs. 9.2% [aged 18-59], p<0.001), post-operative dialysis (5.2% vs. 6.0% [aged 60-69] vs. 7.6% [aged 18-59], p<0.001), and intubation ≥72 hours (21.7% vs. 27.4% [aged 60-69] vs. 34.5% [aged 18-59], p<0.001), and less likely to be treated for acute rejection within 1 year of transplant (21.7% vs. 23.6% [aged 60-69] vs. 26.7% [aged 18-59], p<0.001; Table 3). Older recipients were also found to have shorter median hospital length of stay compared to recipients aged 18-59 (16 [11-27] vs. 17 [12-29] days, p<0.001).
Table 3:
Post-transplant outcomes and causes of death of lung transplant recipients, 2005-2020.
| Variable, n (%) | 18-59 years N=13,228 |
60-69 years N=12,282 |
≥70 years N=2,448 |
p-value |
|---|---|---|---|---|
| ECMO within 72 hours | 557 (9.0%) | 374 (5.7%) | 79 (4.9%) | <0.001 |
| Acute rejection | 1,213 (9.2%) | 914 (7.4%) | 163 (6.7%) | <0.001 |
| Post-operative dialysis | 1,006 (7.6%) | 738 (6.0%) | 127 (5.2%) | <0.001 |
| Hospital LOS (days), median (IQR) | 17 (12-29) | 16 (11-28) | 16 (11-27) | <0.001 |
| Intubation at 72 hours | 2,144 (34.5%) | 1,814 (27.4%) | 353 (21.7%) | <0.001 |
| Treated for rejection within 1 year | 3,054 (26.7%) | 2,467 (23.6%) | 442 (21.7%) | <0.001 |
| Cause of death by 1 year | <0.001 | |||
| Infection | 345 (24.5%) | 469 (28.4%) | 102 (25.6%) | |
| Hemorrhage | 209 (14.8%) | 257 (15.6%) | 76 (19.1%) | |
| Pulmonary | 212 (15.0%) | 253 (15.3%) | 66 (16.6%) | |
| Cardiovascular | 136 (9.6%) | 180 (10.9%) | 49 (12.3%) | |
| Malignancy | 34 (2.4%) | 63 (3.8%) | 25 (6.3%) | |
| Graft Failure – other | 129 (9.1%) | 104 (6.3%) | 18 (4.5%) | |
| Cerebrovascular | 101 (7.2%) | 100 (6.1%) | 14 (3.5%) | |
| Graft Failure – rejection | 74 (5.2%) | 61 (3.7%) | 11 (2.8%) | |
| Renal Failure | 2 (0.1%) | 13 (0.8%) | 4 (1.0%) | |
| Other | 169 (12.0%) | 151 (9.1%) | 33 (8.3%) | |
| Cause of death by 5 years | <0.001 | |||
| Infection | 785 (18.7%) | 1,050 (22.3%) | 210 (18.9%) | |
| Pulmonary | 907 (21.6%) | 857 (18.2%) | 210 (18.9%) | |
| Hemorrhage | 569 (13.5%) | 694 (14.7%) | 189 (17.0%) | |
| Malignancy | 233 (5.5%) | 499 (10.6%) | 161 (14.5%) | |
| Cardiovascular | 246 (5.8%) | 369 (7.8%) | 107 (9.6%) | |
| Graft Failure – rejection | 745 (17.7%) | 588 (12.5%) | 97 (8.7%) | |
| Graft Failure – other | 210 (5.0%) | 171 (3.6%) | 34 (3.1%) | |
| Cerebrovascular | 151 (3.6%) | 156 (3.3%) | 26 (2.3%) | |
| Renal Failure | 36 (0.9%) | 62 (1.3%) | 14 (1.3%) | |
| Other | 326 (7.7%) | 262 (5.6%) | 66 (5.9%) |
ECMO, extracorporeal membrane oxygenation; LOS, length of stay; IQR, interquartile range.
Post-transplant survival
Unadjusted survival at one year post-transplant was 89.3% for recipients aged 18-59, 86.5% for recipients aged 60-69, and 83.6% for recipients aged ≥70 (p<0.001). Unadjusted survival at three years post-transplant was 74.2% for recipients aged 18-59, 69.5% for recipients aged 60-69, and 61.1% for recipients aged ≥70 (p<0.001). Unadjusted survival at five years post-transplant was 63.2% for recipients aged 18-59, 54.2% for recipients aged 60-69, and 42.2% for recipients aged ≥70 (p<0.001, Figure 3). By era, unadjusted one-year post-transplant survival was 79.7% for recipients transplanted 2005-2009, 81.1% for recipients transplanted 2010-2014, and 85.4% for recipients transplanted 2015-2020 (p=0.02, Figure 4) for those ≥70 years of age.
Figure 3:
5-year unadjusted Kaplan Meier survival curves for lung transplants by age, 2005-2020.
Figure 4:
1-year unadjusted Kaplan Meier survival curves in candidates ≥70 years by transplant era.
On multivariable analysis, recipients aged ≥70 years had increased 1- (aHR [95%CI]: 1.19 [1.06-1.33]; p<0.001; Supplemental Table 4), 3- (aHR [95% CI]: 1.28 [1.18-1.39]; p<0.001; Supplemental Table 5), and 5-year mortality (aHR [95%CI]: 1.29 [1.21-1.38]; p<0.001; Supplemental Table 6) compared to recipients aged 60-69. By one and five years post-transplant, recipients ≥70 years were less likely than younger recipients to die of graft rejection and failure and more likely to die of malignancy and cardiovascular causes (p<0.001, Table 3).
Bilateral versus single transplant
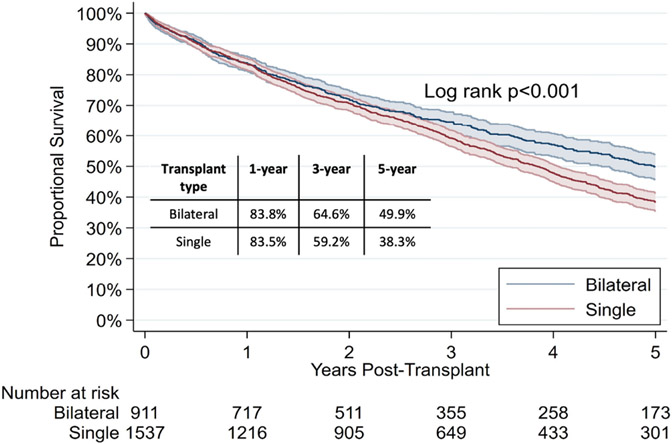
In recipients ≥70 years, bilateral lung recipients were more likely to have restrictive disease pathology (82.6% vs. 70.1%, p<0.001; Supplemental Table 7). On unadjusted analysis, single lung transplant recipients were found to have similar 1-year (83.5% vs. 83.8%, p=0.9) and 3-year (59.2% vs. 64.6%, p=0.07) survival, but worse 5-year post-transplant survival (38.3% vs. 49.9%, p<0.001; Figure 5). On adjusted analysis, single lung transplant recipients were still found to have similar mortality at 1 year (aHR [95% CI]: 0.99 [0.80-1.23]; p=0.95) and 3 years (aHR [95% CI]: 1.13 [0.97-1.31]; p=0.11), but increased mortality at 5 years post-transplant (aHR [95% CI]: 1.25 [1.10-1.43]; p=0.001).
Figure 5:
5-year unadjusted Kaplan Meier survival curves in candidates ≥70 years by transplant type (bilateral versus single).
COMMENT
In this national retrospective analysis, we found that almost all centers now perform lung transplants in candidates ≥70 years. Candidates ≥70 years were more likely to be transplanted with shorter time on the waitlist and less likely to die/deteriorate on the waitlist. Recipients ≥70 years had superior perioperative outcomes but inferior post-transplant survival. Older recipients were less likely to die of rejection and graft failure; their cause of death was more likely to be malignancy.
Our finding of a significant increase in the percentage of lung transplants in candidates ≥70 years is consistent with previous studies showing an increase in median age at transplant and idiopathic pulmonary fibrosis (IPF) as an indication for transplant.[3,10,11] Prior to the LAS era, chronic obstructive pulmonary disease was the leading indication for lung transplant, as these patients were the most likely to survive long enough to receive a transplant.[2,10] With the LAS, the sickest candidates were prioritized, and IPF has since become the leading indication for lung transplant. We found no differences in the comorbidities of older candidates listed in previous eras versus more recent era, but we observed a decrease in median LAS at listing among candidates ≥70 years. This likely reflects a shift towards earlier referral of candidates with IPF, as underscored in the 2014 International Society for Heart & Lung Transplantation consensus document,[12] while still maintaining stringency in the types of older candidates being considered for transplant. We also observed that candidates ≥70 years were more likely to be male and white, which may reflect sex and race differences in underlying disease etiology. Previous studies have shown that IPF has a male predominance and that white patients with IPF live with the disease longer than those of other races.[13,14] Whether these differences reflect organic disease progression or systemic biases should be further explored.
Despite transplant from more extended criteria donors, we found that, by many metrics, older recipients had superior perioperative outcomes compared to younger recipients; they were less likely to require post-operative dialysis, prolonged intubation, and post-operative ECMO support. Our survival data demonstrate that older recipients have inferior but overall acceptable 1-, 3-, and 5-year post-transplant survival. Our 1-year survival result is in contrast with a previously published study by Hayanga et al.,[7] who demonstrated no differences in 1-year post-transplant survival. Our analysis included a larger sample size and therefore greater power to detect a difference, as well as a more contemporary population. Promisingly, our results demonstrate superior 1-year post-transplant survival in candidates transplanted during the most recent era (2015-2020) compared to earlier era, with the most recent era having a 1-year survival rate of 85.4%. Improvements in outcomes in this population mirror improvements in lung transplant outcomes across other age groups.[15] These improvements can be attributed to a number of changes, such as adjustments in patient selection, donor management, perioperative care, nursing care, and immunosuppression regimens. Additionally, the average survival for patients with idiopathic pulmonary fibrosis has previously been shown to be 3-5 years since diagnosis, with patients over the age of 70 having worse survival than younger patients.[16,17] Those who qualify for transplant candidacy have severe disease prognosis, with an expected 1-year mortality rate of 25-40%.[18,19] Although a direct comparison is not feasible, the post-transplant survival rates found in our older recipient population compare favorably to the predicted survival rates without transplant.
When looking at causes of post-transplant mortality, we found that older recipients were less likely to die from rejection and graft failure and more likely to die from malignancy. Older recipients were also less likely to experience acute rejection pre-discharge or within one year of transplant. These results mirror findings in other solid organ transplantation, such as heart[20] and kidney.[21] We hypothesize that these findings reflect the well-established age-related decline in immune function, attributable to a number of processes, including decreased production of naïve T cells due to thymic involution and impairments in naïve CD4 T cell function.[22] Overall, these findings highlight the positive graft-related post-transplant outcomes in older recipients and suggest a role for further investigation into additional candidate selection criteria, age-based tailoring of immunosuppression, and additional post-operative surveillance to address non-graft-related mortality.
Single lung transplants were more commonly performed for older recipients relative to younger recipients. Kilic et al.[6] previously reported an increase in the percentage of bilateral lung transplants being performed for recipients ≥70 years following implementation of the LAS. In their cohort of recipients transplanted between 2005 and 2009, 22.1% of the transplants were bilateral. Our results build upon these findings and demonstrate that 37.2% of transplants in recipients ≥70 years were bilateral, suggesting that there has been increased comfort with performing bilateral transplants in older recipients. Single lung transplants are preferentially performed over bilateral lung transplants in older recipients in part due to early studies demonstrating worse short-term outcomes following bilateral lung transplantation in older recipients.[23] However, our results demonstrate that there are no longer differences in short-term mortality between single and bilateral lung transplants. This could reflect improved recipient selection and perioperative management of these recipients. In fact, our results show that bilateral lung transplantation may lead to improved long-term survival in these recipients, even after adjusting for baseline characteristics.
The LAS will be replaced in early 2023 with the Lung Composite Allocation Score (CAS).[24] This new allocation framework has notable differences from the LAS, including elimination of strict geographic boundaries, doubling of the weight of post-transplant outcomes, and potentially including long-term post-transplant outcomes.[25,26] Given our data showing high waitlist urgency, as well as worse post-transplant survival in recipients ≥70 years, older candidates might be disadvantaged under this new system. In the field of transplantation, justice, or access to treatment for patients with medical urgency, and utility, or achieving greatest net societal gain, are often at odds.[27] We must continue to reevaluate the ethics of our allocation systems, taking into account new evidence and evolving outcomes.
Our study was limited by its retrospective nature and the data available in the UNOS database. Specifically, data on additional comorbidities (hypertension, congestive heart failure, chronic kidney disease, etc.) was not available. We were also unable to study patients who were determined not suitable for transplantation. As a result, these findings may not be generalizable to the entire cohort of older patients with end-stage lung disease. However, even with this, the number of older patients qualifying for and receiving transplant has increased. Lastly, there are reports of bilateral transplants being performed in a staged fashion weeks to months between contralateral sides to minimize perioperative risks.[28] We identified only nine similar cases in recipients ≥70 years of age (three of which had over a year in between the two transplants). Given the small sample size and UNOS data policy, we are unable to further analyze this group’s data but believe further investigations are warranted should the practice continue.
In conclusion, we found that transplant in candidates ≥70 years make up >14% of all lung transplants and is associated with favorable waitlist and perioperative outcomes. Post-transplant survival in this population remains inferior relative to younger recipients but is improving over time. Careful candidate selection and post-operative surveillance may address non-graft-related mortality and further improve post-transplant survival in this population. This will be of particular importance as the CAS takes effect.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: None
Disclosures: None
IRB: This study was deemed exempt for the need for institutional review board approval by the Johns Hopkins Institutional Review Board.
Meeting presentation: Accepted for oral presentation at the Southern Thoracic Surgical Association 2022 conference.
REFERENCES
- 1.Egan TM, Murray S, Bustami RT, et al. Development of the New Lung Allocation System in the United States. American Journal of Transplantation. 2006;6(5p2):1212–1227. [DOI] [PubMed] [Google Scholar]
- 2.Benvenuto LJ, Arcasoy SM. The new allocation era and policy. J Thorac Dis. 2021;13(11):6504–6513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Valapour M, Lehr CJ, Skeans MA, et al. OPTN/SRTR 2017 Annual Data Report: Lung. American Journal of Transplantation. 2019;19(S2):404–484. [DOI] [PubMed] [Google Scholar]
- 4.Leard LE, Holm AM, Valapour M, et al. Consensus document for the selection of lung transplant candidates: An update from the International Society for Heart and Lung Transplantation. The Journal of Heart and Lung Transplantation. 2021;40(11):1349–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arjuna A, Olson MT, Walia R. Current trends in candidate selection, contraindications, and indications for lung transplantation. Journal of Thoracic Disease. 2021;13(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kilic A, Merlo CA, Conte JV, Shah AS. Lung transplantation in patients 70 years old or older: have outcomes changed after implementation of the lung allocation score? J Thorac Cardiovasc Surg. 2012;144(5):1133–1138. [DOI] [PubMed] [Google Scholar]
- 7.Hayanga AJ, Aboagye JK, Hayanga HE, et al. Contemporary analysis of early outcomes after lung transplantation in the elderly using a national registry. The Journal of Heart and Lung Transplantation. 2015;34(2):182–188. [DOI] [PubMed] [Google Scholar]
- 8.Vadnerkar A, Toyoda Y, Crespo M, et al. Age-specific complications among lung transplant recipients 60 years and older. J Heart Lung Transplant. 2011;30(3):273–281. [DOI] [PubMed] [Google Scholar]
- 9.Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association. 1999;94(446):496–509. [Google Scholar]
- 10.Yusen RD, Shearon TH, Qian Y, et al. Lung Transplantation in the United States, 1999–2008. American Journal of Transplantation. 2010;10(4p2):1047–1068. [DOI] [PubMed] [Google Scholar]
- 11.Elgharably H, Ayyat KS, Okamoto T, et al. Evolution of Recipient Characteristics Over 3 Decades and Impact on Survival After Lung Transplantation. Transplantation. 2021;105(12):e387. [DOI] [PubMed] [Google Scholar]
- 12.Weill D, Benden C, Corris PA, et al. A consensus document for the selection of lung transplant candidates: 2014—An update from the Pulmonary Transplantation Council of the International Society for Heart and Lung Transplantation. The Journal of Heart and Lung Transplantation. 2015;34(1):1–15. [DOI] [PubMed] [Google Scholar]
- 13.Sesé L, Nunes H, Cottin V, et al. Gender Differences in Idiopathic Pulmonary Fibrosis: Are Men and Women Equal? Front Med (Lausanne). 2021;8:713698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swigris JJ, Olson AL, Huie TJ, et al. Ethnic and racial differences in the presence of idiopathic pulmonary fibrosis at death. Respir Med. 2012;106(4):588–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chambers DC, Perch M, Zuckermann A, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-eighth adult lung transplantation report — 2021; Focus on recipient characteristics. The Journal of Heart and Lung Transplantation. 2021;40(10):1060–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strongman H, Kausar I, Maher TM. Incidence, Prevalence, and Survival of Patients with Idiopathic Pulmonary Fibrosis in the UK. Adv Ther. 2018;35(5):724–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koo SM, Uh ST, Kim DS, et al. Relationship between survival and age in patients with idiopathic pulmonary fibrosis. Journal of Thoracic Disease. 2016;8(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laporta Hernandez R, Aguilar Perez M, Lázaro Carrasco MT, Ussetti Gil P. Lung Transplantation in Idiopathic Pulmonary Fibrosis. Med Sci (Basel). 2018;6(3):68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.du Bois RM, Weycker D, Albera C, et al. Ascertainment of Individual Risk of Mortality for Patients with Idiopathic Pulmonary Fibrosis. Am J Respir Crit Care Med. 2011;184(4):459–466. [DOI] [PubMed] [Google Scholar]
- 20.Wever-Pinzon O, Edwards LB, Taylor DO, et al. Association of recipient age and causes of heart transplant mortality: Implications for personalization of post-transplant management—An analysis of the International Society for Heart and Lung Transplantation Registry. The Journal of Heart and Lung Transplantation. 2017;36(4):407–417. [DOI] [PubMed] [Google Scholar]
- 21.Ahn JB, Bae S, Chu NM, et al. The Risk of Postkidney Transplant Outcomes by Induction Choice Differs by Recipient Age. Transplant Direct. 2021;7(7):e715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palmer D. The Effect of Age on Thymic Function. Frontiers in Immunology. 2013;4. Accessed August 1, 2022. https://www.frontiersin.org/articles/10.3389/fimmu.2013.00316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meyer DM, Bennett LE, Novick RJ, Hosenpud JD. Single vs bilateral, sequential lung transplantation for end-stage emphysema: influence of recipient age on survival and secondary end-points. J Heart Lung Transplant. 2001;20(9):935–941. [DOI] [PubMed] [Google Scholar]
- 24.Continuous distribution - lung - OPTN. Accessed October 15, 2022. https://optn.transplant.hrsa.gov/policies-bylaws/a-closer-look/continuous-distribution/continuous-distribution-lung/
- 25.Lehr CJ, Wey A, Skeans MA, Lease ED, Valapour M. Impact of incorporating long-term survival for calculating transplant benefit in the US lung transplant allocation system. The Journal of Heart and Lung Transplantation. 2022;41(7):866–873. [DOI] [PubMed] [Google Scholar]
- 26.Valapour M, Lehr CJ, Wey A, Skeans MA, Miller J, Lease ED. Expected effect of the lung Composite Allocation Score system on US lung transplantation. American Journal of Transplantation. n/a(n/a). [DOI] [PubMed] [Google Scholar]
- 27.Fazal MS, Gordon EJ, Humbyrd CJ. Current Bioethical Issues in Geriatric Organ Transplantation. Curr Transpl Rep. 2022;9(2):55–62. [Google Scholar]
- 28.Hartwig M, Ganapathi A, Osho A, et al. Staging of Bilateral Lung Transplantation For High-Risk Patients with Interstitial Lung Disease: One lung at a time. Am J Transplant. 2016;16(11):3270–3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.