Abstract
Metastatic breast cancer is responsible for 90% of mortalities among women suffering from various types of breast cancers. Traditional cancer treatments such as chemotherapy and radiation therapy can cause significant side effects and may not be effective in many cases. However, recent advances in nanomedicine have shown great promise in the treatment of metastatic breast cancer. For example, nanomedicine demonstrated robust capacity in detection of metastatic cancers at early stages (i.e., before the metastatic cells leave the initial tumor site), which gives clinicians a timely option to change their treatment process (for example, instead of endocrine therapy they may use chemotherapy). Here recent advances in nanomedicine technology in the identification and treatment of metastatic breast cancers are reviewed.
Keywords: metastatic breast cancer, nanomedicine, early detection, biomarker, women health
1. Introduction
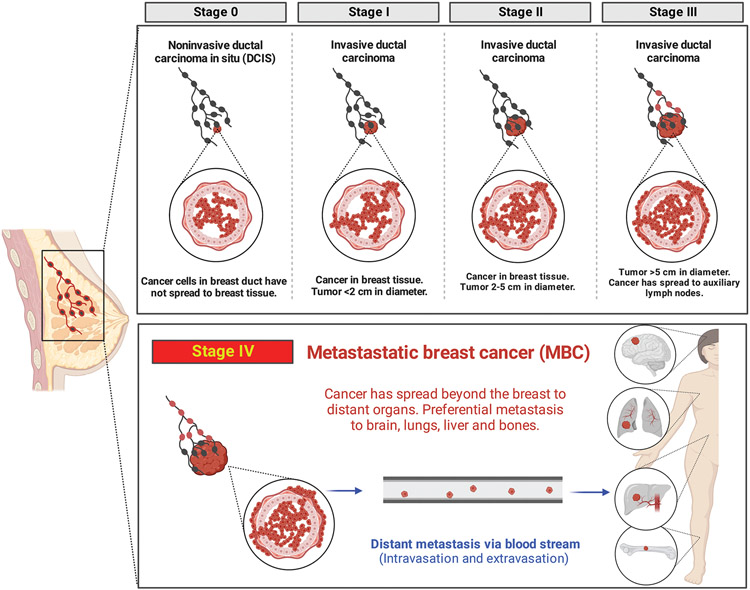
Breast cancer (BC) is the most common cancer type in women in the United States, accounting for 31% of all female cancers,[1] which translated to more than 2.3 million cases of newly diagnosed female BC worldwide in 2020.[2] BC tumors are defined by their types and stages (Figure 1). There are two categories (i.e., noninvasive and invasive) and five different stages of BC recognized among specialists.[3] About 16% of all BC diagnoses are ductal carcinoma in situ (DCIS); this is noninvasive stage 0 in which cancer cells have not broken through the basement membrane. The 5-year survival rate for DCIS cases in almost 100%.[4] The remainder of cases are invasive BC in which cancer cells have escaped the anatomically defined ductal tree structure, spread through the breast tissue, and migrate toward the vascular and lymphatic systems. Stages 1–3 are defined by the size of the primary tumor and the extent of its local and regional spread (lymph node involvement). Stage 4 or metastatic breast cancer (MBC) is defined by the spread of cancer cells to distant organs (e.g., bones, brain, lung, and/or liver)[5] regardless of the size of the primary tumor. MBC is of crucial importance and presents a clinical challenge due to its high mortality rate (i.e., < 30% 5-year survival).[6-8] MBC patients account for up to 90% of all BC-associated deaths.[1] MBC is a heterogenous disease in terms of the genetic background of patients, the anatomical location of distant organ spread, and the challenge of treating contemporaneous MBC to primary BC versus refractory MBC after multiple rounds of previous treatments.[9] Both incidence and mortality rates of MBC are higher among black women compared to other races.[5] Consequently, current treatments for MBC are not curative for most women, but instead prevent or delay disease progression and/or provide palliative care by reducing pain and other comorbidities.[10]
Figure 1.
Various stages of BC. Schematic illustration of different stages/types and specifications of BC including MBC.
Here, we review advances in multidisciplinary approaches in medicine (e.g., nanotechnology and proteomics) that have recently revolutionized the possibility of cancer detection and treatment at early stages.[11-17] In general, nanomedicine has demonstrated substantial potential to improve the quality and efficacy of healthcare.[18-20] Various nanomedicine products, such as nanoparticles (NPs), have gained significance in diagnosis, treatment, and monitoring of MBC.[21-23] Nanoparticle platforms offer much more functionality, compared to the current clinical approaches, for detection of multiple biomarkers in the diagnostic setting, e.g., through proteomics and circulating genome fragments. Additionally, compared to the current clinical approaches, they offer much more versatility and loading capacity of payloads in the therapeutic setting, as exemplified by the Food and Drug Administration (FDA)-approved albumin-bound paclitaxel nanoparticle (Abraxene) and PEGylated liposomal doxorubicin nanoparticle (CAELYX).[24,25]
2. Pathophysiology of MBC
BC metastasis takes place through several steps, including invasion of nearby healthy cells, penetration into the circulatory or lymph system, migration through circulation, lodging in capillaries (i.e., at a specific location they stop moving and migrate into the surrounding tissue), and formation of smaller tumors (also called micrometastases).[26]
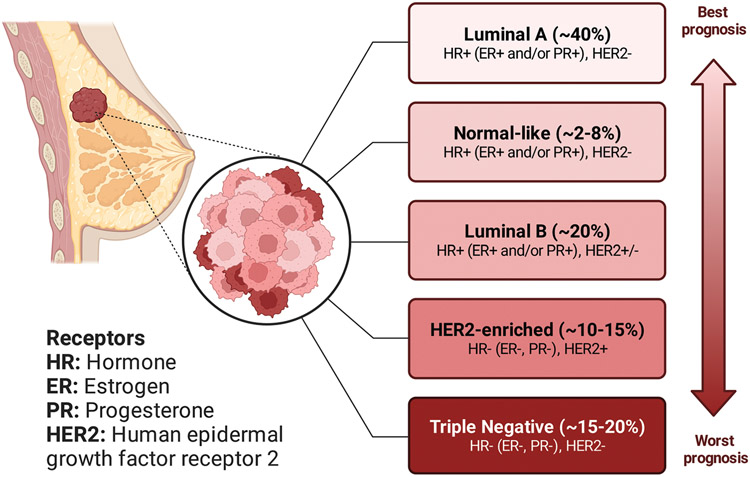
Hormone-sensitive BC cells contain some proteins called hormone receptors (HR) that become activated when hormones bind to them, leading to changes in the expression of specific genes and consequently stimulation of cell growth (Figure 2).[28] For example, when the tumor cells contain estrogen receptors, the cancer is called estrogen receptor positive (i.e., ER+), and when the tumor cells contain progesterone receptors, the cancer is called progesterone receptor positive (PR+).[29] Although most ER+ BCs are also PR+, BC is called HR+ when tumor cells have both estrogen and progesterone receptors. Human epidermal growth factor receptor 2 (HER2) is a protein that helps BC cells grow quickly, and HER2+ BC tests positive for this protein.[30] BCs that lack ERs are called ER−, those that lack PRs are called PR−, and if they lack both ERs and PRs are called HR−. According to the American Joint Committee on Cancer (AJCC) and the Surveillance, Epidemiology, and End Results (SEER) statistics, the prevalence of various subtypes of BC in descending order is: HR+/HER2− > HR−/HER2− > HR+/HER2+ > HR−/HER2+ (Figure 3).[31] HR+/HER2− is the most common subtype of BC, approximately six times more frequent than HR−/Her2−BC. When none of the receptors mentioned above (i.e., ER, PR, and HER2) is present in BC tumor cells, as determined by lack of expression on immunohistochemistry and in situ hybridization molecular testing, it is called triple-negative breast cancer (TNBC).[32]
Figure 2.
Molecular classifications of BC. Various intrinsic or molecular subtypes of BC and their corresponding prognosis. Some parts of the figure were reproduced with permission.[27] Copyright 2023, American Chemical Society.
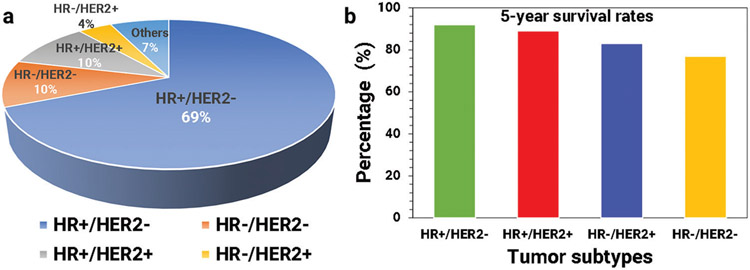
Figure 3.
Distribution and survival rates of BC subtypes. a) Prevalence of various subtypes of BC and their percentages, and b) the 5-year survival rates of various subtypes of BC (all stages).
Although the prevalence of particular subtypes of BC is higher (e.g., HR+/HER2−), compared to the other subtypes, survival rates associated with various subtypes differ widely. The 5-year survival rate is an important metric, showing the percentage of people who are alive five years after BC diagnosis. The overall 5-year survival rates for various tumor subtypes are illustrated in Figure 3b (based on the most recent data from SEER).[33]
3. Current standard MBC treatment strategies
Although we have recently witnessed remarkable advancements in the treatment of MBC, its treatment is still considered noncurative.[10] There are five main conventional strategies for the treatment of MBC: hormonal therapy, targeted therapy, chemotherapy, radiation therapy, and surgery[27]; each associated with unique side effects and rates of relapse.
As for primary BC, with MBC patient management and treatment selection are based mainly on expression of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor-2 (HER2).[28,34-36] For example, when cancer cells express ER, the cancer is called estrogen receptor positive (i.e., ER+).[29] Most ER+ MBCs are also PR+ and are called HR+ and most ER− BCs are also PR− and called HR−. According to the AJCC and SEER statistics, the prevalence of various subtypes of MBC in descending order is: HR+/HER2− 69%, HR−/HER2− 10%, HR+/HER2+ 10%, unknown 7%, and HR−/HER2+ 4%.[33] The 5-year survival rate of any BC subtype is > 90% when the tumor is localized. However, when regional or distant metastatic lesions are present, there is a marked difference between HR+/HER2 and other subtypes, especially TNBC.[37] The 5-year survival rate for the HR+/HER2− subtype with regional metastasis is 90.3% and with distant metastasis is 34%, in stark contrast to 66.2% and 12.8% (respectively) for the TNBC subtype.[4,28,33,37]
The HR+ subtype is treated with hormonal therapy. ERs and their corresponding ligands play a crucial role in the development and progression of MBC through their involvement in direct modulation of gene expression associated with cell growth. Hormonal therapy blocks/modulates ER activity using tamoxifen, raloxifene and toremifene or downregulates selective ER receptors using fulvestrant.[38] Moreover, the addition of cyclin-dependent kinase (CDK)4/6 inhibitors is considered an efficient approach, targeting the cell cycle machinery and interrupting the intracellular and mitogenic hormone signals that stimulate proliferation of cancer cells.[38-40] HER2+ is treated with targeted therapy. Trastuzumab is an antibody targeting the extracellular domain of HER2 that upon binding blocks HER2 signaling as well as antibody-dependent cellular cytotoxicity.[41] Together, these approaches lead to the death of cancer cells overexpressing HER2. The specificity of HER2 targeting by trastuzumab has been further exploited to enhance cancer cell-killing by conjugation to small-molecule cytotoxic agents, including the microtubule inhibitor mertansine (trastuzumab emtansine) and the topoisomerase I inhibitor exatecan (trastuzumab deruxtecan). The high specificity and potency of these antibody drug conjugates may allow their use to be extended to HR+ cases with weak HER2 expression.[42]
TNBC is treated mainly with chemotherapy, but immunotherapy and antibody drug conjugates are considered for specific cases. Lack of ER and HER2 expression renders the above-mentioned hormonal and targeted therapies ineffective in TNBC tumors.[43] However, a subset of TNBC tumors express other receptors (e.g., Trop-2 and epidermal growth factor receptor) or ligands (e.g., PD-L1) that can inform other personalized treatments. Immunotherapy with pembrolizumab increases survival in TNBC patients whose tumors express high levels of immune checkpoint programmed death ligand 1 (PD-L1).[44] Targeted therapy with an antibody-drug conjugate (sacituzumab govitecan) that consists of an antibody-targeting trophoblast cell-surface antigen 2 and a payload of topoisomerase I inhibitor SN-38 (Trop-2) increases progression-free and overall survival compared to single-agent chemotherapy.[45]
TNBC and HR−/HER2+ are initially diagnosed with evidence of regional or distant metastasis more frequently than the HR+/HER2− subtype, whose prognosis is more favorable.[8] The propensity of cancer cells to metastasize to distant organs (i.e., bone, brain, liver, lung) varies per subtype. HR+/HER2− MBC cases present predominantly with bone metastasis, HR−/HER2+ with liver metastasis, and TNBC with lung metastasis.[8] The survival rate of MBC is correlated with both the subtype and the distant site of metastasis. Within the same subtype, patients with only bone metastasis have longer survival than those with any other single site or multiple sites of metastasis. TNBC cases with multiple sites of metastasis have the worst clinical outcomes.[8]
One of the main challenges of MBC treatment is drug resistance.[46,47] Since most types of MBC are treated with a variety of drugs and/or combinations, there is a high chance of multidrug resistance. Despite recent advancements in drug development and various options of drug combinations, most MBC patients will develop resistance to drug treatment through intracellular and extracellular mechanisms.[48] Intracellular mechanisms of drug resistance are associated with drug metabolism and efflux, target modulations and damage restoration, while extracellular mechanisms are attributable to the crosstalk between tumor cells and environmental factors. However, the exact mechanisms underlying BC multidrug resistance in the vast majority of patients remains unknown.[49]
4. Standard Diagnostic Procedures versus Nanotechnology Approches
4.1. Current Standard MBC Diagnostic Approaches
Breast cancer statistics clearly reveal the crucial importance of early detection.[50] Just in the US, ≈250 000 (1 in 8 women) new cases of BC are reported each year and around 40 000 women die.[51] If BC remains contained to the breast at the time of diagnosis, average 5-year survival is ≈98%.[52] If the BC is confined to the breast and the lymph nodes, average 5-year survival decreases to 84%. However, with metastasis, the average 5-year survival significantly decreases to 25%. The earlier the detection and treatment, the better the chance of curing the cancer.[53] Moreover, early detection using regular screenings (e.g., mammograms) not only reduces the amount of treatment necessary but also improves survival. In addition, early screening and detection reduce the chances of additional treatments after surgery (e.g., chemotherapy) and recurrence.[54,55]
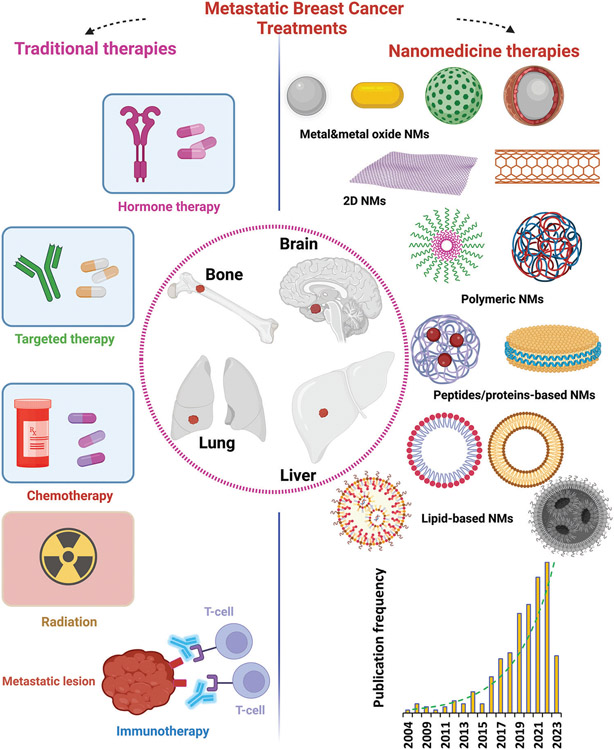
Similar to other types of cancer, early detection of BC is critical to improve the overall survival rate. Conventional BC diagnostic routines include combination of several complicated approaches, including clinical and physical examinations, histopathology, imaging, mammography, ultrasound, magnetic resonance imaging (MRI), Xray, computed tomography (CT), positron emission tomography (PET), cytology, and biopsy.[56-58] However, the sensitivity of current imaging modalities and testing is insufficient to detect small lesions in distant organs.[59] The most common sites of distant metastasis for BC are bone, lung, brain, and liver, and about 10% of patients with metastatic disease will have lesions at multiple sites (Figure 4).[60]
Figure 4.
MBC treatment strategies. Schematic illustration of conventional therapies and emerging nanomedicine treatments to combat MBC. There is growing use of various nanomaterials against MBC, ranging from metals and metal oxides to proteins and lipid-based nanostructures, reflected by a significant rise in the number of publications in recent years. Data is extracted from the Scopus database (date of search April 28, 2023), reflecting publications from 2004 to April 2023 with the words “nano” and “breast cancer” in the title.
Detection of exosomes in body fluids (e.g., blood) is also considered a noninvasive means to monitor the progression of MBC and identification of new therapeutic targets. Exosomes are small extracellular vesicles released by many cell types, including cancer cells.[61] They are involved in cell-to-cell communication and carry various biomolecules, such as proteins, lipids, and nucleic acids. Exosomes have been shown to play a critical role in the progression of cancer, including MBC, by promoting tumor growth, invasion, and metastasis.[62,63] In MBC, tumor cells release exosomes that contain unique molecules such as proteins and microRNAs that are distinct from those found in non-MBC or normal cells.[62,63] These biomolecules can serve as biomarkers for the early detection of MBC. Exosomes have several advantages over traditional biomarkers, including superior stability, abundance, and specificity. Several studies have shown that exosomes can be isolated from the blood of BC patients for use as noninvasive tools for detecting MBC.[62,63] For example, it was reported that exosomal protein biomarkers can distinguish between patients with MBC and those with non-MBC with high accuracy.[62] However, the isolation and purification of exosomes is challenging.[64] In addition, exosomes derived from drug-resistant BC cells can transfer drug efflux pumps and other molecules to sensitive cells, further decreasing drug sensitivity.[65] In fact, extracellular vesicles play a relatively complex role in BC, and a deeper understanding of their function will pave the way for development of new therapeutic strategies for BC.[66,67]
For example, different types of NPs have been developed and tested for their ability to isolate and purify exosomes from complex biological fluids.[68,69] In most cases, NPs are coated with a protein or antibody that can bind specifically to exosomes (e.g., anti-CD63 antibody). The exosome-bound NPs can then be isolated using centrifugation, allowing for the purification of exosomes from complex biological fluids. This technique has shown high specificity and sensitivity and has potential for clinical use. The use of NPs for exosome purification offers several advantages over more-traditional methods, including high specificity, efficiency, and the potential for clinical applications.[68,70,71]
4.2. Nanomedicine Approaches for Diagnosis of MBC
Although current technologies and approaches are sophisticated and successful to some extent, some are also invasive, expensive, time-consuming, plagued by intense side effects, and require advanced laboratories and infrastructure.[72] Therefore, there is a critical need for development of new approaches that are simple, noninvasive, low-risk, and accurate for screening or diagnosis of MBC.[73,74] Recent advancements in BC diagnosis include development of new tests (e.g., Oncotype DX and MammaPrint) capable of screening multiple genes and mRNA levels using tumor tissue samples.[75]
Due to their unique physicochemical properties and characteristics, nanomedicines have demonstrated substantial potential to improve the quality and efficacy of healthcare.[18-20] Advances in nanotechnology and proteomics have recently introduced the possibility of identification and treatment of early-stage cancers.[11-17] Various nanomedicine products have already gained significance in diagnosis, treatment, and monitoring of MBC (Figure 4).[21-23]
Nanomedicine technologies have been extensively studied over the past few decades for detection and treatment of a wide range of cancers including MBC.[76,77] A wide range of nanomaterials, from metals and metal oxides to polymeric and lipid NPs, have been used for diagnosis of MBC (Figure 4).[76,78-81] A detailed survey of the literature from 2004 to the end of April 2023 using specific keywords “nano” and “breast cancer” in the title in the Scopus database reveals an increasing trend in publications discussing BC diagnosis and/or treatment using various nanoscale platforms (Figure 4). Compared with traditional procedures for MBC detection, nanoscale materials provide more-specific targeting, higher sensitivity, and better capability for multiple-target measurements due to increased surface-to-volume ratio and higher interactions within the tumor microenvironment (TME).[82] For example, nanobiosensors have shown great promise as nanoscale platforms for detection of a wide range of biomarkers including alpha fetoprotein (AFP), cancer antigen 125 (CA125), cancer antigen 153 (CA15-3), cancer antigen 19-9 (CA19-9), carcinoembryonic antigen (CEA), and interleukins (ILs), in addition to the most common BC biomarkers, with very low limits of detection (e.g., ≈fg mL−1).[83]
5. Nanobiosensors in MBC Detection
Nanobiosensors and nanoscale devices/chips have shown exciting potential for detection of a wide range of biomarkers, circulating tumor cells (CTCs), and biomolecules that may be released from the TME into the blood during the development of different types of BC.[84-87] The TME contains a wide range of biomolecules including genes, DNA and RNA, proteins, lipids and other metabolites, that are originated from various TME associated cells, that indicate the biological and pathological states of the disease, which can be found in several sources of biological fluids including urine, serum, and cerebrospinal fluid.[88] Nanobiosensors are analytical devices that use nanomaterials for detection of such biological elements (e.g., cell receptors and enzymes) utilizing an analyte and transform that to a measurable signal using various physiochemical mechanisms (e.g., optical and/or electrochemical).[89-93] One of the main challenges in the early detection of BC is the low concentration (e.g., 1 ng mL−1) and short half-life (e.g., 10 min) of such biomarkers/biomolecules in biological fluids. In this regard, highly sensitive nanoscale materials/devices containing unique physicochemical properties have been developed over recent decades for specific and selective detection of various biomarkers and small molecules released during the development and progression of the BC.[94,95]
Multifunctional magnetic nanowires can detect CTCs in the blood of patients with nonmetastatic early-stage BC.[79] The o1D pencil-like geometry of magnetic nanowires greatly increases the rate of contact with CTCs.[79] In addition to the enhancement of cell-to-nanowire attachments, the 1D structure of nanowires provides multivalent binding sites for targeting ligands. Using one of the proteins (i.e., Smoothened receptor) of the MBC cells as a biomarker and graphene NPs, an assay was developed for detection of even small numbers of MBC cells in tumor sites, thereby helping predict metastatic potential in the early stages of BC.[96] Although CTCs of MBC are found at low concentration and are challenging to detect, nanomaterials-based biosensors are able to amplify the low-intensity signals of these biomarkers, enabling robust and reliable detection of MBC cells.[97-99] For instance, a DNA hybridization chain reaction was used for detection of rare HER2+ CTCs using a DNA nanotechnology approach.[100,101] Since HER2 is highly expressed in BC tumor cells, a biotin-streptavidin system was used to link trastuzumab (anti-HER2 antibody) to the DNA amplification system for detection of MBC CTCs.[102] Although the detection limit was far from the common levels of CTCs in patients (1:106–108 cells), the proposed approach detected one cancer cell among 200 noncancer cells.[100]
One promising strategy for collection and detection of MBC cancer biomarkers is the use of protein corona (i.e., a layer that forms at the surface of NPs upon their interactions with biological fluids).[103,104] While the composition of circulating plasma proteins, nucleic acids, and other biomolecules is dynamic over the spectrum of health and disease, there is an unacceptable tradeoff between depth of coverage and sample throughput, given the vast dynamic range and high complexity of the plasma proteome.[105-107] The protein corona that forms on the surface of NPs is unique in its potential to overcome several limitations in the global discovery of plasma proteomics.[103,108-112] A unique feature of the protein corona is that the constituent proteins rarely correspond to the most abundant proteins in plasma.[103,111,112] In other words, NPs can provide a novel type of proteomics data (in terms of the number of proteins and their concentrations) that is significantly different from similar data on plasma proteins. The composition of the protein corona formed on the NPs’ surface reflects the composition of human plasma in health and disease[103,104]; using NPs we can obtain unique signatures of human conditions.[113-115] Protein corona analysis of NPs, after interactions with blood plasma of healthy and BC individuals, revealed substantial discrimination between oncological patients and healthy individuals related to cellular and molecular aspects of BC.[116]
5.1. Electrochemical, Fluorescence, and Colorimetric Nanobiosensors
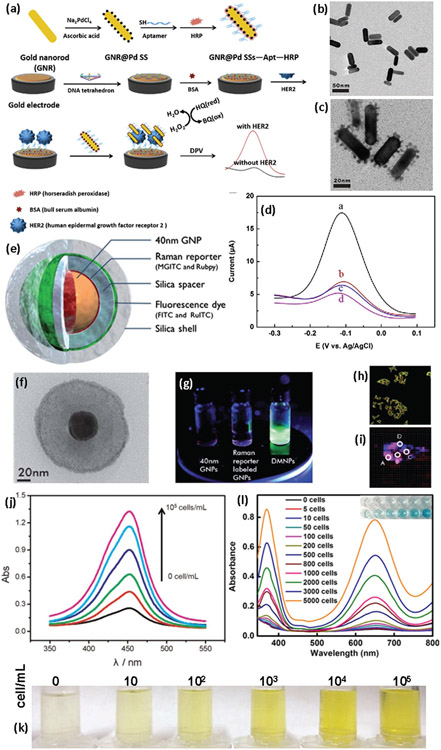
The main strategies for detection of BC cells using nanobiosensors include electrochemical, fluorescence, chemiluminescence, and colorimetric approaches. Electrochemical nanobiosensors are the most common devices capable of detecting electrical signals generated after binding of specific biomolecules (e.g., antibody and enzyme) on the surface of nanomaterials for detection of BC biomarkers.[56,117-120] For example, through conjugation of gold nanorods and palladium NPs, an electrochemical aptamer nanobiosensor was developed for detection of HER2 BC (Figure 5a-d). By immobilizing a DNA tetrahedron with expanded aptamer on the gold electrode, the prepared nanobiosensor was capable of specific and efficient detection of HER2 BC.[121,122] The prepared highly linear (i.e., from 10 to 200 ng mL−1) nanobiosensor revealed a very low limit of detection about 0.15 ng mL−1 for detection of HER2 and exciting potential for BC diagnosis.
Figure 5.
Nanobiosensors based on nanomaterials for BC diagnosis. a) Schematic illustration of fabrication of gold nanorods@Pd aptamer nanobiosensor for HER2 detection of BC, b) TEM image of gold nanorods, c) TEM image of gold nanorods@Pd, d) differential pulse voltammetry signals obtained from HER2, epidermal growth factor receptor, VEGF165 and blank control at identical concentration (from a to d, respectively). Reproduced with permission.[121] Copyright 2019, Elsevier, e) schema showing the various layers of the SERS-fluorescence dual-mode nanoprobe and a corresponding TEM image (f), g) photographs showing the fluorescence emission of GNP colloid, Raman reporter-labeled GNP colloid, and DMNP colloid, h) fluorescence, and i) SERS overlay images of CD24 and CD44 BC biomarkers. Reproduced with permission.[128] Copyright 2012, RSC Publishing, j) optical absorption changes of PtAuNP colorimetric sensor as a function of HR+/HER2+ MCF-7 BC cell numbers (i.e., 0, 10, 102, 103, 104, 105 cells/mL), k) photographs showing the color changes of the nanostructures in the presence of BC cells. Reproduced with permission.[129] Copyright 2015, Elsevier, and l) optical absorption and visual color changes in the presence of liposome-gold NPs and different numbers of SKBR3 BC cells. Reproduced with permission.[130] Copyright 2017, Ivyspring International Publisher.
On the other hand, fluorescence-based nanobiosensors commonly utilize florescent nanomaterials such as quantum dots and 2D nanomaterials for BC biomarker detection due to their size-tunable fluorescence properties and high fluorescent quantum yields.[100,123,124] For example, a fluorescence nanoprobe composed of rare-earth-doped albumin nanocomposites was developed for diagnosis of two BC models, 4175 and MCF-7. It is noteworthy that MCF-7 and 4175 have different metastatic potentials in terms of their tissue tropisms and aggressiveness. The highly aggressive cell line 4175 is specific to the lung.[125] Ligand-functionalized nanoprobes increased cellular uptake and accumulation in targeted BC cells compared with untargeted nanoprobes and enabled both in vivo imaging of biomarkers in mice bearing bilateral tumors and tumor detection.[125] Moreover, fluorescence-based nanobiosensors are of crucial importance in diagnosis of MBC, since fluorescence microscopy is capable of showing the distribution of specific proteins within cancer cells.[126,127] Dual-mode (SERS)-fluorescence nanoprobes containing gold NPs, Raman reporters, a silica spacer, a silica shell, and fluorescent dye (Figure 5e,f) were developed for both SERS and fluorescence diagnosis of BC using CD24 and CD44 coexpressed in MDA-MB-231 TNBC human cells. It has been suggested that the synergistic effect of SERS imaging and dual mode nanoprobes is a robust approach to identify colocalization of CD24 and CD44 biomarkers in BC cells (Figure 5h,i).[128]
Colorimetric sensors are another class of nanobiosensors that simply utilize optical absorption changes of materials for sensing and detection.[131-135] In this regard, plasmonic nanomaterials and particularly noble metal NPs (e.g., silver and gold nanostructures) are among the most widely used nanomaterials for diagnostic applications based on colorimetric sensors.[136-140] More specifically, colloidal gold NPs exhibit a wide range of colors in the visible region of the electromagnetic spectrum, which enables detection of various analytes/biomarkers through probing the red/blue shifts of the plasmonic peaks that are produced by their binding to specific biomolecules.[141-143] In addition, gold nanozymes exhibit more enzymatic efficiency than natural enzymes due to intrinsic properties such as high biocompatibility and stability, which provides the conditions necessary for design of advanced nanoscale colorimetric sensors. Based on this concept, a liposome-gold NPs hybrid nanostructure was developed and conjugated with anti-HER2 for colorimetric detection of HER2+ BC cells in human serum and BC tissue samples (Figure 5j,k).[130] Using the high catalytic activity of noble metal NPs (i.e., gold and platinum), a hybrid nanostructure (PtAuNP) was constructed for detection ofHR+/HER2+ MCF-7 human BC cells.[129] It was found that the color of the prepared nanostructures changes remarkably in the presence of very small numbers of BC cells (i.e., 10 cells mL−1), which is visually observable even by the naked eye. The limit of detection of the prepared colorimetric nanobiosensor was reported to be as low as 10 cells mL−1, with detection linear range from 10 to 105 cells mL−1 in human serum samples (Figure 5l).
6. Surface-Enhanced RAMAN Scattering-based detection
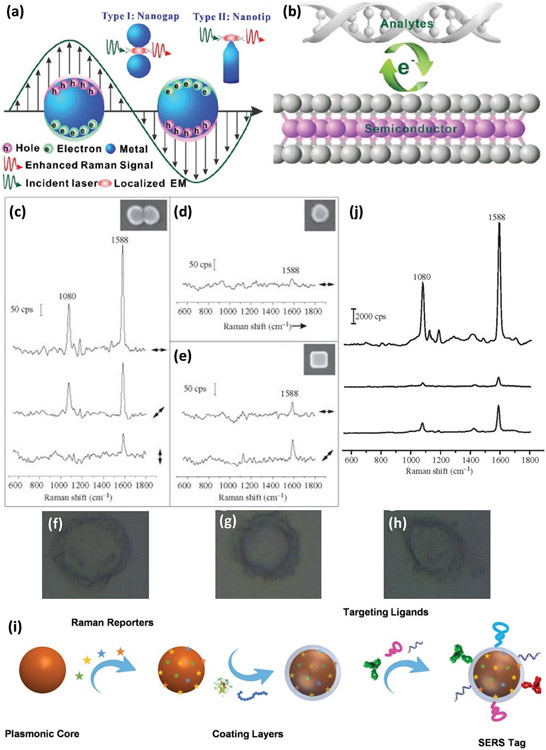
There is increasing use of the surface-enhanced Raman scattering (SERS) characteristics of nanomaterials, particularly noble metallic nanostructures (e.g., silver and gold) and 2DNMs (e.g., graphene), for diagnosis of various diseases including MBC.[144-146] SERS-based disease detection is a very promising approach, since the Raman signal can be enhanced by several orders of magnitude (i.e., 103–108) when using nanoscale materials, due to differences in their optical and chemical properties compared with bulk-scale materials (Figure 6).[147,148] The conventional Raman scattering technique is based on the vibration energy of molecules; however, because all molecules have their own unique vibrational spectrum, Raman scattering is fundamentally a relatively low-sensitivity technique.[149] In the other hand, when molecules are adsorbed onto a particular surface (i.e., noble metal nanostructures), the Raman signals increase by several orders of magnitude through two main mechanisms: electromagnetic and chemical enhancement.[150,151] Electromagnetic enhancement usually occurs in metallic nanostructures through the localized surface plasmon resonance (LSPR) of free oscillating charges at the surface of the noble metals (Figure 6a). Most wavelengths, from UV to the visible region of the electromagnetic spectrum, can excite the LSPR effect of metallic nanostructures, while this is not applicable to semiconductor nanomaterials.[152,153] On the other hand, chemical enhancement is the main driver of SERS enhancement in semiconductor nanomaterials, which depends on the energy levels and electronic structure (i.e., density of states, bandgap energy, highest occupied molecular orbital, and lowest unoccupied molecular orbital) of the corresponding semiconductor (Figure 6b).[150]
Figure 6.
Using SERS in BC detection. Schematic illustration of (a) and (b) two main fundamental mechanisms involved in enhancement of Raman scattering signals. Reproduced with permission.[150] Copyright 2021, RSC Publishing, Raman spectra and the corresponding SEM images (inside) of various shapes of SERS tags on glass substrates: c) dimer tags, d) sphere tags, e) cube tags in various directions of laser polarization relative to the particle orientation, f-h) SERS mapping of SKBR3 human BC cells by detecting HER2 using dimer tags, sphere tags, and cube tags (from top to bottom). Reproduced with permission.[160] Copyright 2013, The Royal Society Publishing, i) scheme showing the various components and preparation steps of noble metal NPs SERS tags. Reproduced with permission.[158] Copyright 2022, Ivyspring International Publisher, and j) Raman spectra of various shapes of SERS tags in aqueous solution (dimer, sphere, and cube, from top to bottom). Reproduced with permission.[160] Copyright 2013, The Royal Society Publishing.
In general, there are two direct and indirect SERS approaches for detection of biomolecules/biomarkers using nanomaterials; however, direct detection (also known as label-free) using SERS is challenging due to the small inherent Raman cross-sections of most biomolecules.[154] Another important issue with direct detection using SERS is the formation of the protein corona, which severely limits the Raman signal intensity, selectivity, and circulation time.[155-157] Therefore, researchers have been interested in indirect SERS approaches (i.e., SERS tags), in which various organic molecules are used as Raman reporters on the surface of noble metal NPs and targeting ligands on the protective shell for indirect detection of proteins, DNA, and other biomarkers (Figure 6i).[144,158] In indirect SERS detection, the target is identified by the targeting moieties attached to the nanomaterials, followed by binding of the SERS tag to the molecular target and enhancement of the Raman signals of the reporter.[147,159]
The physicochemical properties of noble metallic nanostructures have a significant influence on factors enhancing SERS signals and enable detection of several biomolecular signals in heterogeneous BC.[161] It was found that dimer-like silver SERS tags provide more enhanced Raman signals due to formation of hot spots at the junction of two silver nanospheres compared with single silver nanospheres and nanocubes (Figure 6c-e). For example, the enhancement factor of a dimer tag on a glass substrate can increase up to 4.3 × 106 but drops to 2.8 × 105 and 8.7 × 105 when using silver nanospheres and nanocubes of similar size, respectively (Figure 6j). Using dimer-shaped SERS tags, BC cells overexpressing HER2 receptors were imaged with high sensitivity and selectivity (Figure 6f-h).[160]
7. Metal Nanostructures’s Shape in Diagnosis OF MBC
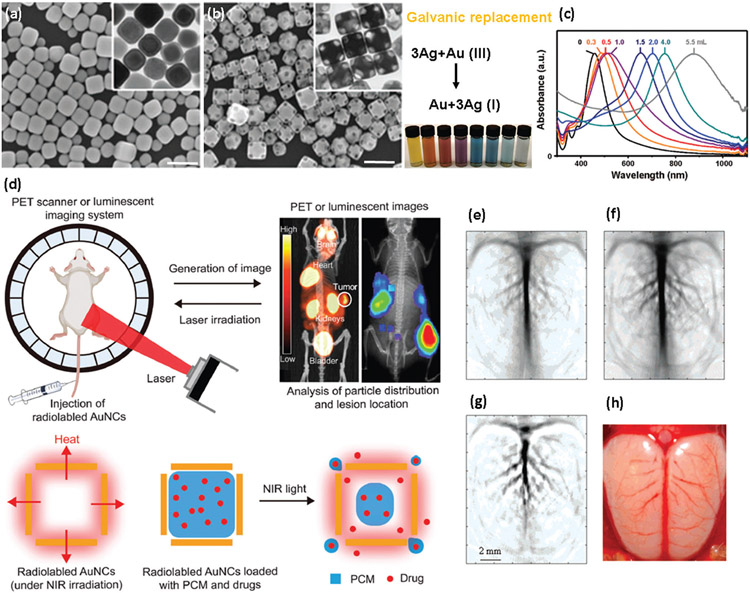
Shape-controlled noble metal nanostructures are a novel class of multifunctional nanomaterials with potential theranostics applications (e.g., imaging and contrast agents) due to their particular geometry and biocompatibility.[149,162-164] It is well documented that the shape/geometry of nanomaterials can significantly change their properties and consequently their performance in diagnostic applications.[165,166] One of the unique characteristics of shape-controlled noble metal nanostructures is their plasmonic property as a result of collective oscillation of the free electrons in the metal under appropriate light irradiation (also known as surface plasmon resonance or SPR) (Figure 7).[167-169] For instance, through the well-controlled galvanic replacement reaction, the plasmonic peak of gold nanocages (AuNCs) can be specifically tuned from visible to near infrared (NIR) regions, where the damping of light by blood and soft tissue is remarkably low, making them a potential contrast agent for optical coherence tomography (OCT) (Figure 7b,c).[170,171] Pharmacokinetic studies suggest that a particular class of hollow gold nanostructures (i.e., gold nanocages) with smaller edge sizes have greater biodistribution, and higher tumor uptake but lower hepatic and splenic uptake than larger nanocages, in a murine EMT-6 BC model. Quantitative PET imaging studies revealed rapid accumulation and retention of gold nanocages inside the tumors, which make these nanostructures very promising for diagnostic applications.[172,173]
Figure 7.
Shape-controlled metallic nanostructures in BC imaging. SEM and TEM (inset) of a) silver nanocubes and b) gold nanocages (after galvanic replacement; scale bars are 200 nm), c) optical absorption spectroscopy showing the tuning of the plasmonic peak of gold nanocages (AuNCs) from visible to NIR region, d) scheme showing the potential application of gold nanocages in imaging, photothermal therapy, and controlled release. Reproduced with permission.[167] Copyright 2008, American Chemical Society; Reproduced with permission.[174] Copyright 2021, John Wiley and Sons. Photoacoustic tomography of a rat’s cerebral cortex without (e) and with (f) gold nanocages, g) a pixelwise differential image of (e) and (f), h) optical image of rat’s cerebral cortex showing the vasculature features after interactions with gold nanocages. Reproduced with permission.[175] Copyright 2007, American Chemical Society.
In addition to the significant therapeutic effect of gold nanocages, discussed in detail below, when gold nanocages are labeled with a suitable radionuclide (e.g., 64Cu), they offer a multifunctional image-guided therapy platform capable of optimized treatment and improved outcomes (Figure 7d).[174] Photoacoustic tomography is a promising technique in BC diagnosis and treatment; however, it is usually challenging due to the heterogeneity of TME and the presence of various malignant cells in the tumor. In this regard, nanomaterials (e.g., gold nanocages) can serve as appropriate photoacoustic contrast agents to distinguish various BC tumor subtypes.[176] Smaller gold nanocages (i.e., <50 nm) have been shown to be more effective NIR contrast agents for photoacoustic tomography compared with larger-sized and/or other shapes of gold nanostructures due to their higher optical absorption and wavelengths of plasmonic peaks (Figure 7e-h).[175]
8. Therapeutic Nanomaterials against MBC
The tumor microenvironment (TME) plays a crucial role in treatment and therapeutic responses of BC.[177] BC metastasis to distant organs strongly depends on the TME characteristics. The vascular structure and blood vessels of the TME are significantly different from normal healthy tissues. In fact, the proliferation of cancer cells causes rapid growth of blood vessels, incomplete branching patterns, and irregular vascularization.[88,178,179] Consequently, it takes longer for oxygen to pass through the tumor vasculature. which results in oxygen deficiency (hypoxia) in the TME and high interstitial fluid pressure. Regardless of the therapeutic approach, all drugs must i) enter the blood vessels of the TME, ii) cross the vessel walls, and iii) migrate through the interstitium.[177,180] Although the blood vessels in the TME are generally more permeable to macromolecules than normal vessels, the interstitial fluid pressure often acts against the molecular movement of drugs. However, unlike conventional treatments for MBC, nanomaterials can be specifically engineered to overcome these barriers.[181-183] For example, there is a direct relationship between the molecular weight of the polymeric NPs and the permeability of the TME vessels. It has been reported that increasing the molecular weight of polymeric NPs enhances the accumulation of macromolecules within the solid tumors but substantially decreases the vascular permeability, since macromolecules concentrate at the vascular surface and cannot penetrate deeper into the core of the tumor, which is critical for efficacious therapy.[184,185] It is also well documented that modifications of the physicochemical properties of nanomaterials (e.g., size and surface chemistry) elicits desirable cellular uptake and penetration of nanodrugs into the thick and fibrous mucus barriers of solid tumors.[165,186,187] Therefore, novel nanoscale materials-based approaches (e.g., nano drug delivery systems) offer unique opportunities to increase the efficacy of anticancer therapies.
8.1. Metal and Metal Oxide Nanomaterials
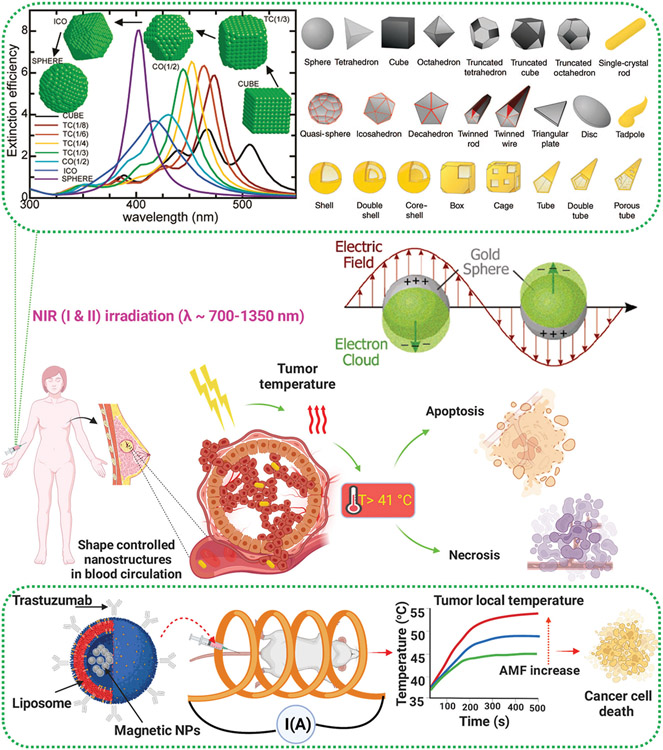
Metallic nanostructures (e.g., silver and gold) are an interesting class of nanomaterials with exciting potential in photothermal BC therapy due to their plasmonic characteristics.[188-190] Plasmons are the oscillations of free electrons in metallic NPs, and when the frequency (wavelength) of an incident light matches the frequency (wavelength) of the oscillating electrons, absorption of light increases, resulting in a plasmonic resonance.[191] What distinguishes metallic nanostructures from other nanomaterials is the possibility of controlling their shape/geometry during the synthesis process, producing a wide range of geometries with various properties and applications (Figure 8).[18] Different shapes of metallic nanostructures have different symmetries, which affects the polarizations of these nanostructures and consequently the number of plasmonic peaks.[189,192]
Figure 8.
Metal and metal oxide nanostructures employed against MBC using photothermal therapy (PTT) and hyperthermia. (Top panel) Shape-dependent plasmonic resonance of metallic nanostructures scheme showing plasmonic resonance in gold spherical NPs as an example, and shape-selective synthesis of silver and gold nanostructures. Reproduced with permission.[193] Copyright 2007, American Chemical Society, (middle panel) possible PTT mechanisms of shape-controlled nanostructures against MBC, and (bottom panel) illustration showing magnetic hyperthermia using magnetic NPs to combat MBC.
Spherical metallic NPs (i.e., the most symmetric shape) show just one plasmonic peak, since a sphere can be polarized only in dipole mode, while other geometries of metallic nanostructures (e.g., rods, cubes, and triangles) can be polarized in higher modes (e.g., dipole and quadrupole) and therefore have more than one plasmonic peak.[191,194-196] This unique property has made them a focus of research in many applications including photothermal BC therapy, due to their strong light absorption in the NIR region. In other words, through controlling the geometry of metallic nanostructures their plasmonic properties can be specifically tuned toward distant NIR regions, with practical applications in photothermal BC therapy.[189]
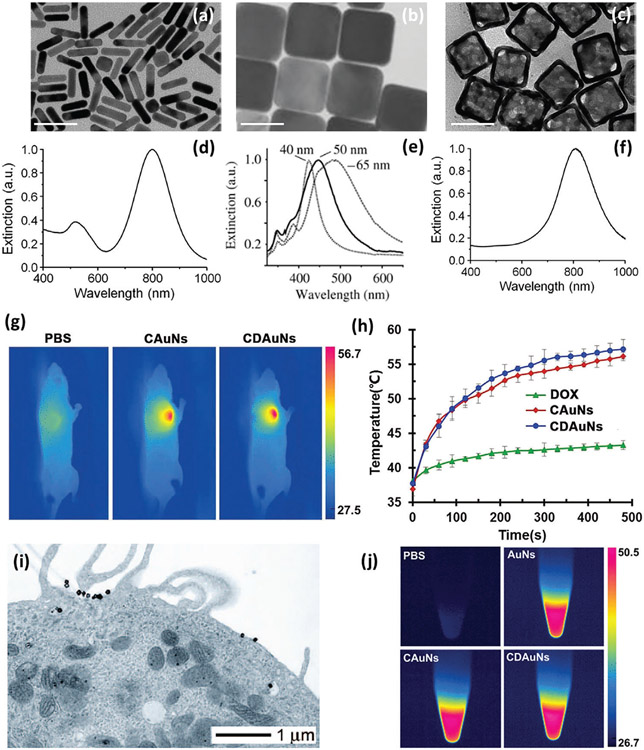
A quantitative study of the photothermal effect of gold nanostructures with various shapes revealed that gold nanocages and nanorods are more effective light absorbers (Figure 9) than other geometries (e.g., spheres and shells) and appropriate photothermal agents again invasive BC.[198,199] Gold nanocages (average edge length 65±7 nm) have a plasmonic peak centered around 800 nm, which is irradiated with a pulsed NIR laser at various power densities and exposure times to optimize treatment conditions for efficient destruction of HER2+ BC cells.[198] A similar study, using doxorubicin (DOX)-loaded gold nanocages (AuNs) and surface-coated DOX-incorporated AuNs (CDAuNs), showed that CDAuNs enabled hyperthermia-triggered drug release under NIR laser irradiation (λ = 808 mn); the combination of chemotherapy and PTT revealed higher therapeutic benefits on the tumor volume and metastatic nodules in MBC (4T1 orthotopic mammary tumor models).[197]
Figure 9.
SPR-mediated temperature increase and tumor ablation of MBC cells. TEM images of a) gold nanorods, b) silver nanocubes, c) gold nanocages (scale bars are 50 nm) and d-f) the corresponding plasmonic peaks. Reproduced with permission.[189] Copyright 2013, American Chemical Society, g) NIR thermographic images of MBC (4T1) after NIR irradiation, and h) the corresponding temperature increase at tumor site. Reproduced with permission.[197] Copyright 2017, John Wiley and Sons, i) TEM image of a human HER2+ BC cell (SKBR3) conjugated with gold nanocages shows decoration and internalization of gold nanocages by the cell. Reproduced with permission.[198] Copyright 2008, American Chemical Society, and j) infrared thermal photos of control sample and gold nanostructures. Reproduced with permission.[197] Copyright 2017, John Wiley and Sons.
One of the most relevant examples of metal oxide therapeutic NPs being used against BC are magnetic NPs (e.g., iron oxide). Magnetic NPs are able to induce local tumor hyperthermia and temperature increase (>41 °C) when an external alternating magnetic field (AMF) is applied, which results in energy transfer through hysteresis loss and consequently local heating and damage to the tumor (Figure 8, bottom panel).[200,201] Local changes in temperature of the tumor can be easily controlled by altering the strength and duration of the external magnetic field.[202] In addition the local temperature of the tumor can be tuned by altering the physicochemical properties of magnetic NPs (e.g., size and surface charge), which supports the applicability of magnetic NPs-based hyperthermia.[203,204] Both magnetic NPs and metallic nanostructures produce heat and cause hyperthermia at the tumor site, by different mechanisms (i.e., external magnetic fields and light irradiation, respectively), increasing the temperature and causing cancer cell death.[197] One of the main advantages of nanoscale materials is that they can be highly functionalized with desired characteristics for cancer-specific treatments. However, one concern is the focusing of heat generation at the tumor site without damaging the surrounding normal cells.[205] This issue has been addressed by developing Herceptin-conjugated liposomes containing magnetite NPs to improve the targeting capabilities of the prepared magneto-liposomes (HMLs) against both BT474 (high HER2 expression) and SKOV3 (low HER2 expression) BC cells in mice.[201] This particular type of HMLs exhibited improved adsorption and incorporation into BC cells, compared to other NPs, of nude mice and enabled cancer-specific hyperthermia. It has been shown that HMLs can specifically bind to HER2-overexpressing BC tumors, and all injected magnetic NPs were retained in the tumor 48 h after injection, while magnetic NPs were virtually cleared in the same period of time in control samples (i.e., HER2-negative tumors).[201]
8.2. 2D Nanomaterials
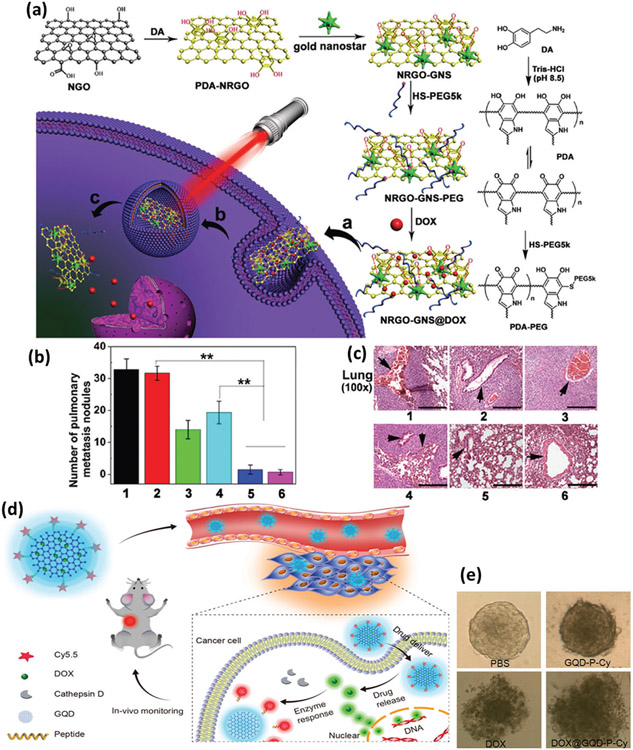
2D nanomaterials (2DNMs) are ultrathin nanostructures with an approximate thickness of one atom.[206] 2DNMs include a very broad range of materials from graphene to hexagonal boron nitride (h-BN) and transitionmetal dichalcogenides with high therapeutic potential owing to their unique optoelectronic properties, unusual electrical conductivity, high biocompatibility, and extraordinary thermal and mechanical properties.[207,208] Since the discovery of graphene in 2004, 2DNMs have attracted much attention in theragnostic applications to various types of cancer including BC due to their particular planar structure, which serves as a unique carrier for different types of drugs, including chemotherapeutic agents, genes, and small interfering RNAs (siRNAs).[209,210] Most 2DNMs show strong optical absorption in the NIR region, which makes them a potential photosensitizing agent in PTT and photodynamic therapy against BC.[211] Compared to traditional approaches to combat MBC, multifunctional 2DNMs are not only able to deliver different chemotherapies but also have photothermal and photodynamic impacts on target sites such as fluidizing TME and surpassing the high oncotic pressure of tumor interstitial fluid, due to their strong light absorption.[212] Based on these concepts, a nanoscale platform consisting of polydopamine-functionalized nanosized reduced graphene oxide (NRGO), gold nanostars (GNS), and doxorubicin (DOX) (denoted NRGO-GNS@DOX) was developed for combination chemotherapy and PTT treatment of MBC (Figure 10a). It was found that when irradiated with NIR laser light (λ = 655 nm), NRGO-GNS@DOX nanocomposites produce substantial cytotoxicity in aggressive 4T1 TNBC cells (mouse model) due to the cumulative therapeutic effect of NRGO-GNS-elicited hyperthermia and DOX-induced cytotoxicity. The results revealed a substantial higher therapeutic effect of NRGO-GNS+laser or NRGO-GNS@ DOX+laser compared with the PBS or NRGO-GNS group, indicating the potential of the NRGO-GNS@DOX nanoscale platform in combination with PTT to suppress MBC (Figure 10b,c).[213] Due to the oxygen-rich functional groups on their surface and efficient fluorescence properties, 2DNMs are ideal platforms for loading BC drugs and detectable delivery of chemotherapeutic drugs into BC cells without additional fluorescence labeling.[214] Based on these characteristics, a novel theranostic agent was developed by loading DOX onto the surface of graphene quantum dots and conjugating Cy5.5 dye to the dots through a cathepsin D-responsive peptide. The results against mouse 4T1 TNBC cells demonstrate improved therapeutic efficacy both in vitro and in vivo due to enhanced tissue penetration and cellular uptake (Figure 10d,e).[214]
Figure 10.
Nanomaterials in combinational BC therapies. a) Schematic illustration of the preparation of NRGO-GNS@DOX nanocomposite for combined photothermal and chemotherapy of BC, including internalization, entrapment inside the lysosome, and cytosol release of DOX under NIR irradiation (inside (a), (b), and (c), respectively), b) numbers of lung metastasis nodules, and c) staining of the lung in mice treated with various samples (i.e., 1. PBS, 2. NRGO-GNS, 3. DOX, 4. NRGO-GNS@DOX, 5. NRGO-GNS+laser, 6. NRGO-GNS@DOX+laser) (**p < 0.01). Reproduced with permission.[213] Copyright 2016, John Wiley and Sons, d) illustration of graphene quantum dot-based theranostic agent for probing anticancer drug delivery, release, and response, and e) images of multicellular 3D tumor spheroids. Reproduced with permission.[214] Copyright 2017, American Chemical Society.
8.3. Polymeric Nanomaterials
Polymeric nanomaterials are a promising class of materials that have been used to treat MBC, as they are highly tunable in order to increase the kinetic and thermodynamic stability of their drug cargo as well as biodistribution and biocompatibility.[182,215] For instance, traceable poly(ethylene oxide)-poly(ester) micelles conjugated to poly(ester) with poly(ϵ-caprolactone) (PCL) or poly(a-benzyl carboxylate-ϵ-caprolactone) (PBCL) cores were developed using click chemistry approaches and used against aggressive MDA-MB-231 TNBC cells (human model).[216]
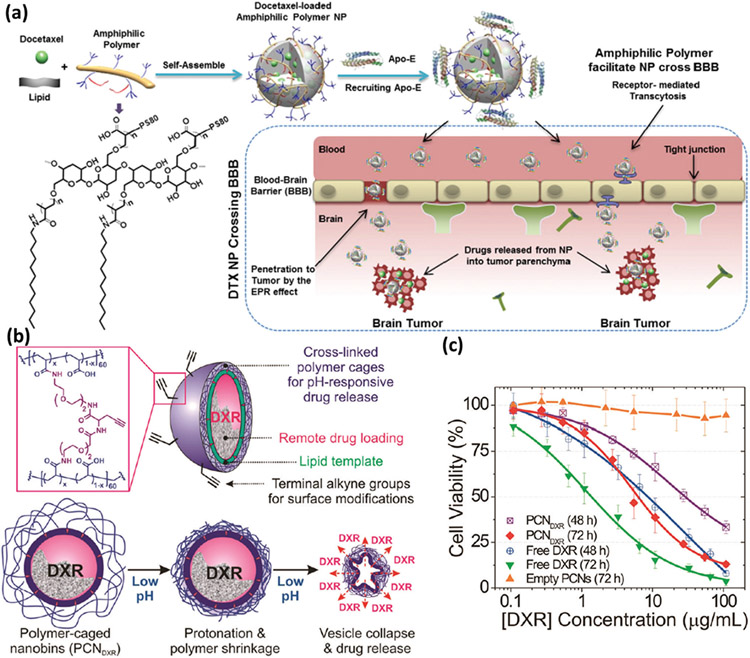
The findings suggest that stabilization of nanodrug due to the presence of PBCL over PCL cores significantly affects nanocarrier accumulation in primary BC tumor.[216,219] Although traditional chemotherapies are effective to some extent against MBC, they are not efficient against brain metastasis, mainly due to the blood–brain barrier (BBB). This issue has been addressed by developing BBB-penetrating amphiphilic polymer-lipid NPs that can effectively deliver the antimitotic drug docetaxel (DTX) for the treatment of brain metastasis of TNBC in a human model. Biodistribution, brain accumulation, pharmacokinetics and efficacy studies against TNBC demonstrate release of DTX-NP from brain micro-vessels and accumulation in micro-metastasis lesions in the brain. The DTX-NPs platform increased the blood distribution of DTX by 5.5-fold compared with Taxotere (a clinically used DTX formulation), reduced tumor growth by 11-fold, and increased median survival of tumor-bearing mice by 94% compared with Taxotere in similar experimental conditions (Figure 11a).[217]
Figure 11.
a) Schematic showing formation of DTXx02010NPs from the amphiphilic terpolymer, recruitment of Apo-E, and proposed enhanced mechanism for crossing the bloodx02010brain barrier (BBB). Reproduced with permission.[217] Copyright 2017, Elsevier, b) Schematic illustration of DXRx02010encapsulated polymerx02010caged nanobins, and c) in vitro cytotoxicity of DXRx02010encapsulated polymer-caged nanobins, empty PCN vehicles, and free DXR against human TNBC cells. Reproduced with permission.[218] Copyright 2010, American Chemical Society.
The acidic nature of the TME is considered a hallmark of MBC tumors and plays a critical role in tumor progression.[220] In this regard, pH-responsive nanomaterials (i.e., containing a core-shell structure that responds to external pH) have shown promise for MBC drug delivery. For example, doxorubicin-loaded polymer-caged nanobins are dual-function cross-linked polymers that protect the drug payload and serve as a pH-responsive trigger to improve drug release in the acidic TME. One of the main advantages of such polymeric nanoscale systems is the ability to tune the degree of cross-linking to increase the in vivo blood distribution of the nanocarriers against the invasive TNBC (Figure 11b,c).[218]
8.4. Peptide- and Protein-Based Nanomaterials
Nanostructures functionalized with peptides and proteins are a particular class of nanodrugs with enhanced killing of cancer cells for MBC therapeutic use. It has been reported that protein- and peptide-conjugated nanomaterials can increase tumor homing of nanomaterials and improve cellular penetration to promote MBC cytotoxicity while reducing side effects in healthy tissues.[221] For example, Abraxane (the NP formulation of Paclitaxel/PTX) was one of the first nanoscale-based materials for the treatment of MBC, approved by FDA in 2005 due to its longer plasma half-life and reduced toxicity.[222] PTX is one of the drugs most commonly used against various types of advanced-stage cancers including MBC. However, due to its poor solubility, PTX requires the use of a lipid-based solvent (e.g., polyoxyl castor oil) to maximize its effect. In addition, PTX can cause severe hypersensitivity reactions, drug resistance, and other significant side effects particularly at higher dosages.[223-225] Thus, researchers became interested in modifying Abraxane to make it more efficient against MBC. In this regard, various peptide- and protein-based Abraxane have been developed to increase solubility and biocompatibility.[24,226,227]
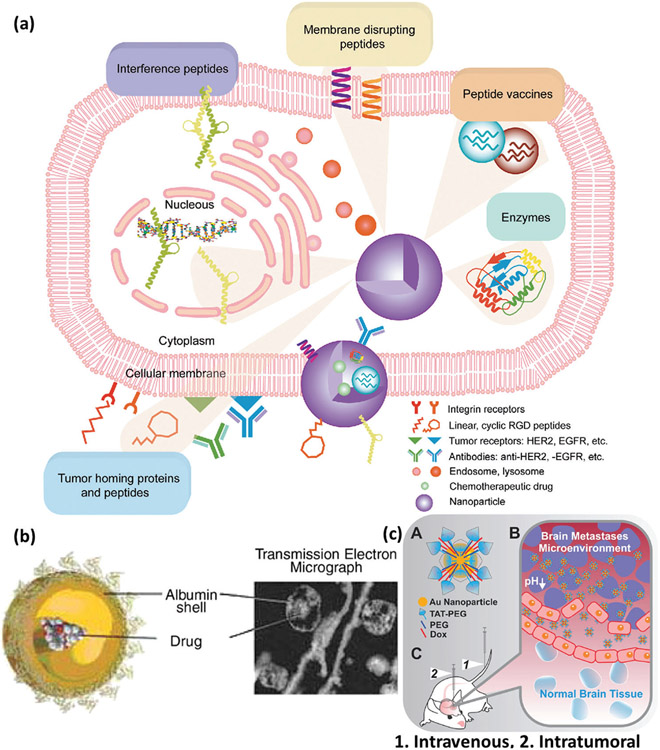
Peptide- and protein-based NPs are an essential therapeutic nanomaterial against MBC, since they can target several membrane receptors such as HER2 and epidermal growth factor receptor (Figure 12).[221] Regarding composition, albumin-based nanomaterials are the most common type of NPs incorporating proteins or peptides against MBC.[222] For example, NP albumin-bound PTX (nab-PTX), which is PTX conjugated to albumin NPs (≈130 nm), is a suspension of albumin NPs designed to increase the therapeutic efficacy of PTX through receptor-mediated transport (also known as transcytosis) and to reduce its toxicity and side effects (Figure 12a). Albumin-based nanodrugs are biocompatible, biodegradable, and easy to be functionalized.[221] Due to the presence of a permeable vasculature and absence of lymphatic drainage in cancerous tissue, albumin generally achieves high penetration. The size of albumin is another important feature, i.e., small enough to be released from tumor blood vessels into the tumor tissue but large enough (67 kDa) to be maintained in the tumor interstitium (unlike <40 kDa macromolecules), making it a highly desirable MBC treatment (Figure 12b).[228] For example, it has been shown that DOX-loaded albumin NPs targeted with Trastuzumab (HER2 antibody) against SKBR3 (HER2+) have higher uptake due to increased tumor homing and penetration alongside lower clearance (Figure 12a). SKBR3 (also known as SK-BR-3) is a human BC cell line derived from a pleural effusion due to an adenocarcinoma originating in a 43-year-old Caucasian female, isolated by the Memorial Sloan – Kettering Cancer Center in 1970, that is used in therapeutic research, especially in the context of HER2 targeting.[229] The conjugation of nanomaterials and biomolecules including peptides/proteins is highly controllable, making it a suitable approach for particular applications like MBC treatment. For instance, cell-penetrating peptides have proven a promising platform against MBC due to increased tissue penetration and crossing of the BBB.[230] It is reported that trans-activating transcriptional activator (TAT) cell-penetrating peptide conjugated to gold NPs results in drug delivery increase to brain MBC. Intravenous administration of TAT peptide-modified gold NPs (≈23 nm total diameter) carrying DOX causes high accumulation of TAT-gold NPs and, therefore, improved survival in an intracranial MDA-MB-231-Br xenograft mouse model (Figure 12c).[230]
Figure 12.
a) Schema showing protein- and peptide-based nanomaterials for improved tumor homing of nanomaterials, increased cancer cell disruption and penetration, and attacking particular genetic and biochemical processes responsible for MBC development. Reproduced with permission.[221] Copyright 2011, American Chemical Society, b) Schema and transmission electron microscope (TEM) image of a 130 nm nab-PTX showing conjugation of albumin and PTX. Reproduced with permission.[228] Copyright 2008, Elsevier, and c) schematic representation of conjugation of TAT to gold NPs and Dox (A), illustration showing the possible mechanism by which TAT-Au-Dox reaches the leaky tumor vasculature, where particles penetrate cancer cells and are retained at the tumor site, secondary to the enhanced permeability and retention effect and release of drug due to the low-pH TME (B), and intravenous and intratumoral delivery modes (C). Reproduced with permission.[230] Copyright 2016, American Chemical Society.
8.5. Lipid-Based Nanomaterials
Lipid-based NPs such as liposomes, solid lipid NPs, lipid nanocarriers, etc., are emerging classes of nanoscale materials for MBC targeting and/or treatment.[231,232] Liposomes, which are one of the most frequently used lipid NPs, are spherical vesicles composed of phospholipid bilayers and constitute an important class of lipid-based nanomaterials.[233] The size of liposomes can be readily modified, from a few nm to a few μm, making them an excellent platform for drug delivery systems due to their ability to trap a wide size range of hydrophilic and lipophilic drugs, as well as their biocompatibility, biodegradability, low toxicity and low immunogenicity.[233] In addition, the surface chemistry of liposomes is highly tunable through covalent and noncovalent surface functionalization (e.g., by using polyethylene glycol/PEG), which increases their biodistribution and tumor targeting and penetration (e.g., by using antibodies and peptides).[234]
Drug delivery using nanomaterials/nanocarriers such as liposomes has been shown to have several advantages over traditional chemotherapy approaches because of lower toxicity to normal tissues and fewer severe side effects.[235] Further, liposomes and micelles have been shown to be more effective drug delivery platforms compared with conventional chemotherapies for several reasons, including increased biodistribution, deeper penetration into tumor cells, and enhanced biocompatibility.[233] Liposome-based nanocarriers are capable of protecting healthy tissues from the toxic effects of drugs due to their phospholipid bilayer. In addition, the phospholipid bilayer enables PE-Gylation of liposomes, which increases biodistribution time and consequently increases the concentration of the drug delivered to the tumor site.[235] However, inefficient drug transfer from liposome nanocarriers to tumor cells is one of the major challenges of these types of nontargeted drug delivery systems. One possible approach to address such inefficiencies is targeted liposome-based chemotherapeutic drug delivery or development of smart drugs for localization in the tumor cells. It was demonstrated that nano-immunotherapy, by reprogramming cancer-associated fibroblasts (CAFs), enhances therapeutic delivery and immune-stimulation and improves BC outcomes.[182,236,237] Utilizing the CAF-reprogramming capabilities of tranilast, they combined tranilast-loaded micelles and epirubicin-micelles or Doxil with immunotherapy, which resulted in an increase in T-cell infiltration and curing of mice bearing immunotherapy-resistant BC.[182] There is growing evidence of MBC resistance to traditional chemotherapies due to the presence of cancer stem cells (CSC) and dormant cells. Combinations of various therapeutic approaches elicit better MBC outcomes by rendering CSC and dormant cells more sensitive to active drugs.[238-240] In other words, the combination of chemotherapy and immunotherapy enhances the action of cytotoxic T-lymphocytes and leads to more durable responses, and when further combined with traditional chemotherapies, prolongs the lifespan of tumor-bearing mice.[241]
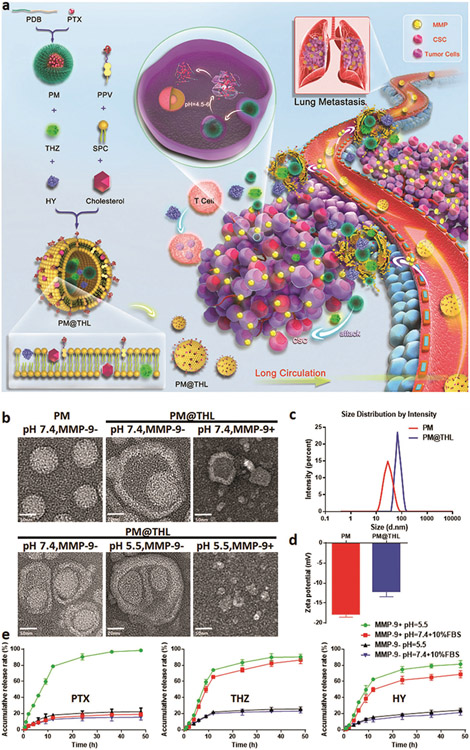
Combining chemotherapy and immunotherapy, Lan and co-workers proposed a novel approach against MBC using a cocktail strategy, in which traditional chemotherapy against MBC cells and CSC and immune checkpoint blockade therapy are integrated into one drug delivery platform.[241] They incorporated PTX as the chemotherapeutic agent, thioridazine (THZ) as the anti-CSC agent, and the PD-1/PD-L1 inhibitor HY19991 (HY) into an enzyme/pH dual-sensitive NP with a micelle–liposome double-layer structure, and named this nanodevice PM@THL (Figure 13).[241] Their findings suggest that this combination therapy increases intratumoral drug concentrations due to shrinkage of NPs and exhibits significant efficacy against mouse BC, with a tumor inhibition rate of 93.45% and lung metastasis suppression rate of 97.64%.
Figure 13.
a) A combination of chemotherapy and immunotherapy approaches against MBC using drug-loaded micelle-liposome double-layer nanostructures (i.e., PM@THL), b) TEM images of PTX-micelle (PM) and PM@THL under different experimental conditions, c,d) size distribution and zeta potential of PM and PM@THL at pH 7.4, and e) PTX, THZ, and HY release curves from PM@THL under different experimental conditions. Reproduced with permission.[241] Copyright 2019, John Wiley and Sons.
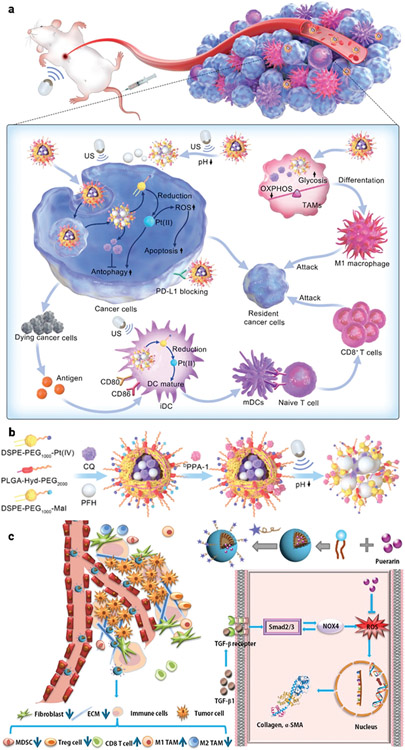
Among various types of BC, TNBC behaves more aggressively, is more likely to recur, and has higher mortality due to its particular pathological characteristics, making it very difficult to diagnose and treat.[242] Although current immunotherapy approaches have shown promise in BC therapy, they are not efficient against MBC or more advanced stages of BC (e.g., TNBC) due to the interaction of the tumor cells and the suppressive TME, with its high lactic acid metabolism and antioxidant levels.[243] Cisplatin (Pt(II)) is a common first-line treatment for TNBC, upregulating genes related to inflammation and increasing the ratio of dendritic cells (DCs)/macrophages to induce a more favorable TME with immune checkpoint.[244] Using a combination of Pt(II), chloroquine (CQ), and perfluorohexane (PFH) contrast agent, a nanoscale platform was designed to improve the ratio of mature dendritic cells (mDCs) and proinflammatory macrophages by reprogramming the metabolism of immature DCs (iDCs) and tumor-associated macrophages (TAMs) (Figure 14a,b).[245] This nanoscale platform increases the intracellular reactive oxygen species (ROS) and the ratio of mDCs and apoptotic tumor cells, which cooperatively enhance antitumor chemoimmunotherapy by suppressing tumor cell autophagy and reprogramming immunocyte metabolism.
Figure 14.
a) Schematic showing Pt(IV)/CQ/PFH NPs-DPPA-1-mediated ultrasound for chemoimmunotherapy against TNBC, b) the corresponding preparation steps of Pt(IV)/CQ/PFH NPs. Reproduced with permission.[245] Copyright 2022, American Chemical Society, and c) illustration of TME remodulation by targeted puerarin delivery. Reproduced with permission.[246] Copyright 2020, Elsevier.
Using a similar strategy Xu and coworker developed a puerarin nano emulsion (nanoPue) to increase the solubility and biodistribution of puerarin.[246] Their findings reveal that the nanoPue was able to effectively modify the TME and reduce the physical barrier to particle and cell penetration of the 4T1 murine TNBC tumor model, which significantly accelerated the chemotherapeutic effect, e.g., ≈6-fold reduction of tumor-associated fibroblasts in nanoPue-treated mice compared with the PBS control, p < 0.0001 (Figure 14c).
A quick search (www.clinicaltrials.gov) of clinically approved nanomaterials for MBC treatment shows that liposomes are critical, since a major portion of the completed clinical trials against MBC.[231,232] A recent review of cancer nanomedicine by He and co-workers reveals that liposome-based nanodrugs are among the MBC nanomedicines most likely to receive clinical approval, including but not limited to PEGylated liposomal doxorubicin (Doxil/Caelyx), liposomal doxorubicin (Myocet), liposomal paclitaxel (Lipusu), albumin-bound paclitaxel (Abraxane), paclitaxel micellar (Genexol-PM), and paclitaxel nanosuspension (PICN).[247] More specifically, Abraxane/PTX, which utilizes liposomes in its structure, is among the nanodrugs most commonly used against MBC.[248,249] Previous clinical trials of Abraxane demonstrated an enhanced response rate for women with MBC compared with other drugs such as Taxanes (e.g., Taxol and Taxotere), which cause hypersensitivity reactions in many patients.[250,251] The notable presence of liposomes among clinical nanodrugs against MBC underlines the high potential of liposome-based nanodrugs to combat MBC and their importance in the future of MBC nanomedicine.
9. Conclusion
In this review, we summarized various types of MBC and their pathophysiology. We then provided details of current clinical approaches to identification of MBC together with their benefits and shortcomings. We then focused our attention on the potential of the emerging field of nanomedicine in identification and treatment of MBC. While the field of nanomedicine is still developing, the potential impact on detection and treatment of women’s metastatic BC is significant.[252] By providing more effective and targeted treatments with fewer side effects, nanomedicine has the potential to improve the lives of millions of women worldwide.[253] However, it is important to note that further research and clinical trials are needed to fully evaluate the safety and efficacy of these new treatments, and to ensure that they are accessible and affordable to all women who need them.[254] Although nanomaterials have shown great promise in the diagnosis and treatment of MBC, there are challenges that need to be considered to successfully translate nanomedicine discoveries to the clinic. These include our incomplete understanding of the effect of the personalized protein corona and its complexity on the safety and therapeutic efficacy of nanomedicine technologies, substantial unwanted targeting of nanoparticles, limited penetration of tumor tissues to reach cancer cells, and regulatory challenges.[255-259] Fully addressing these considerations is likely to delay the clinical availability of nanomedicine treatments.
Acknowledgements
This work was supported, in part, by the National Institutes of Health DK131417 (to MM) and CA258314 (to LS). Figures 1, 2, 4, and 8 as well as the TOC figure were created with BioRender.com.
Biography

Morteza Mahmoudi is an Associate Professor at the Department of Radiology and Precision Health Program at Michigan State University. He is also a member of the Connors Center for Women’s Health & Gender Biology, at Brigham and Women’s Hospital, Harvard Medical School. His research is focused on investigating i) overlooked factors in nanomedicine and ii) academic bullying and harassment. He received several graduate and postgraduate trainings from Sharif University of Technology (Iran), University College Dublin (Ireland), École Polytechnique Fédérale de Lausanne (Switzerland), the University of Illinois at Urbana Champaign, and Stanford School of Medicine. Priorto coming Michigan State University, he was an Assistant Professor at Harvard University. He is among 2018, 2020, 2021, and 2022 highly cited researchers as reported by Clarivate Analytics.
Footnotes
Conflict of Interest
M.M. discloses that: i) he is a cofounder and director of the Academic Parity Movement (www.paritymovement.org), a nonprofit organization dedicated to addressing academic discrimination, violence and incivility; ii) he has also conflict of interest with Partner at Partners in Global Wound Care (PGWC), NanoServ Corp. and Targets Tip Corp.; and iii) he receives royalties/honoraria for his published books, plenary lectures, and licensed patent
The ORCID identification number(s) for the author(s) of this article can be found under https://doi.org/10.1002/smll.202301385
Contributor Information
Ali Akbar Ashkarran, Department of Radiology and Precision Health Program, Michigan State University, East Lansing, MI48824, USA.
Zijin Lin, Department of Radiology and Precision Health Program, Michigan State University, East Lansing, MI48824, USA.
Jatin Rana, Division of Hematology and Oncology, Michigan State University, East Lansing, MI48824, USA.
Harvey Bumpers, Department of Surgery, Michigan State University, East Lansing, MI48824, USA.
Lorenzo Sempere, Department of Radiology and Precision Health Program, Michigan State University, East Lansing, MI48824, USA.
Morteza Mahmoudi, Department of Radiology and Precision Health Program, Michigan State University, East Lansing, MI48824, USA; Connors Center for Women’s Health & Gender Biology, Brigham & Women’s Hospital, Harvard Medical School, Boston, MA02115, USA.
References
- [1].Siegel RL, Miller KD, Fuchs HE, Jemal A, Ca - Cancer J. Clin 2022, 72, 7. [DOI] [PubMed] [Google Scholar]
- [2].Luo C, Li N, Lu B, Cai J, Lu M, Zhang Y, Chen H, Dai M, Chin. Med. J 2022, 135, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Weigelt B, Geyer FC, Reis-Filho JS, Mol. Oncol 2010, 4, 192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Weigelt B, Reis-Filho JS, Nat. Rev. Clin. Oncol 2009, 6, 718. [DOI] [PubMed] [Google Scholar]
- [5].Arnedos M, Vicier C, Loi S, Lefebvre C, Michiels S, Bonnefoi H, Andre F, Nat. Rev. Clin. Oncol 2015, 12, 693. [DOI] [PubMed] [Google Scholar]
- [6].Mariotto AB, Etzioni R, Hurlbert M, Penberthy L, Mayer M, Cancer Epidemiol., Biomarkers Prev 2017, 26, 809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Zardavas D, Baselga J, Piccart M, Nat. Rev. Clin. Oncol 2013, 10, 191. [DOI] [PubMed] [Google Scholar]
- [8].Guo Y, Arciero CA, Jiang R, Behera M, Peng L, Li X, Asian Pac. J. Cancer Prevent.: APJCP 2020, 21, 3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kertmen N, Babacan T, Keskin O, Solak M, Sarici F, Akin S, Arik Z, Aslan A, Ates O, Aksoy S, Ozisik Y, Altundag K, J. BUON 2015, 20, 35. [PubMed] [Google Scholar]
- [10].Miglietta F, Bottosso M, Griguolo G, Dieci MV, Guarneri V, ESMO Open 2022, 7, 100409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ferrari M, Nat. Rev. Cancer 2005, 5, 161. [DOI] [PubMed] [Google Scholar]
- [12].Choi Y-E, Kwak J-W, Park JW, Sensors 2010, 10, 428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Zheng G, Patolsky F, Cui Y, Wang WU, Lieber CM, Nat. Biotechnol 2005, 23, 1294. [DOI] [PubMed] [Google Scholar]
- [14].Kawasaki ES, Player A, Nanomed.: Nanotechnol., Biol. Med 2005, 1, 101. [Google Scholar]
- [15].Heath JR, Davis ME, Annu. Rev. Med 2008, 59, 251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bakhtiary Z, Saei AA, Hajipour MJ, Raoufi M, Vermesh O, Mahmoudi M, Nanomed.: Nanotechnol., Biol. Med 2016, 12, 287. [DOI] [PubMed] [Google Scholar]
- [17].Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R, Nat. Nanotechnol 2007, 2, 751. [DOI] [PubMed] [Google Scholar]
- [18].Kinnear C, Moore TL, Rodriguez-Lorenzo L, Rothen-Rutishauser B, Petri-Fink A, Chem. Rev 2017, 117, 11476. [DOI] [PubMed] [Google Scholar]
- [19].Kumari Y, Kaur G, Ayinkamiye C, Singh SK, Kumar R, Yadav AK, Jha CB, Verma S, Arch. Phytopathol. Pflanzenschutz 2019, 19, 2195. [Google Scholar]
- [20].Zaib S, Areeba BS, Nehal Rana BS, Wattoo JI, Alsaab HO, Alzhrani RM, Awwad NS, Ibrahium HA, Khan I, ChemistrySelect 2022, 7, 10.1002/slct.202104553. [DOI] [Google Scholar]
- [21].Yu W, Hu C, Gao H, Adv. Drug Delivery Rev 2021, 178, 113909. [DOI] [PubMed] [Google Scholar]
- [22].Pal AK, Nandave M, Gautam RK, Advanced Drug Delivery Systems in the Management of Cancer, (Eds: Dua Kamal, Mehta Meenu, de Jesus Andreoli Pinto Terezinha, Pont Lisa G, Williams Kylie A., Rathbone Michael J.), Academic Press, 2021, 387–407, https://www.sciencedirect.com/book/9780323855037/advanced-drug-delivery-systems-in-the-management-of-cancer. [Google Scholar]
- [23].Thorat ND, Bauer J, Nanomed. Breast Cancer Theranostics 2020. 3, https://www.sciencedirect.com/book/9780128200162/nanomedicines-for-breast-cancer-theranostics. [Google Scholar]
- [24].Miller K, Wang M,Gralow J, Dickler M, Cobleigh M, Perez EA, Shenkier T, Cella D, Davidson NE, N. Engl. J. Med 2007, 357, 2666. [DOI] [PubMed] [Google Scholar]
- [25].Schmid P, Rugo HS, Adams S, Schneeweiss A, Barrios CH, Iwata H, Diéras V, Henschel V, Molinero L, Chui SY, Maiya V, Husain A, Winer EP, Loi S, Emens LA, Lancet Oncol. 2020, 21, 44. [DOI] [PubMed] [Google Scholar]
- [26].Welch HG, Gorski DH, Albertsen PC, N. Engl. J. Med 2015, 373, 1685. [DOI] [PubMed] [Google Scholar]
- [27].Kitsios K, Sharifi S, Mahmoudi M, ACS Pharmacol. Transl. Sci 2023, 10.1021/acsptsci.3c00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Eckhardt BL, Francis PA, Parker BS, Anderson RL, Nat. Rev. Drug Discovery 2012, 11, 479. [DOI] [PubMed] [Google Scholar]
- [29].Suva LJ, Brander BE, Makhoul I, Nat. Rev. Endocrinol 2011, 7, 380. [DOI] [PubMed] [Google Scholar]
- [30].Wang Y, Chen J, Irudayaraj J, ACS Nano 2011, 5, 9718. [DOI] [PubMed] [Google Scholar]
- [31].Shannon CE, ACM SIGMOBILE Mobile Comput. Commun. Rev 2001, 5, 3. [Google Scholar]
- [32].Cao L, Niu Y, Cancer Biol Med 2020, 17, 293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33]. https://seer.cancer.gov/statfacts/html/breast-subtypes.html.
- [34].Moy B, Rumble RB, Come SE, Davidson NE, Leo AD, Gralow JR, Hortobagyi GN, Yee D, Smith IE, Chavez-MacGregor M, Nanda R, McArthur HL, Spring L, Reeder-Hayes KE, Ruddy KJ, Unger PS, Vinayak S, Jr WJI, Armaghani A, Danso MA, Dickson N, Turner SS, Perkins CL, Carey LA, J. Clin. Oncol 2021, 39, 3938. [DOI] [PubMed] [Google Scholar]
- [35].Giordano SH, Franzoi MAB, Temin S, Anders CK, Chandarlapaty S, Crews JR, Kirshner JJ, Krop IE, Lin NU, Morikawa A, Patt DA, Perlmutter J, Ramakrishna N, Davidson NE, J. Clin. Oncol 2022, 40, 2612. [DOI] [PubMed] [Google Scholar]
- [36].Gennari A, André F, Barrios CH, Cortés J, de Azambuja E, DeMichele A, Dent R, Fenlon D, Gligorov J, Hurvitz SA, Im SA, Krug D, Kunz WG, Loi S, Penault-Llorca F, Ricke J, Robson M, Rugo HS, Saura C, Schmid P, Singer CF, Spanic T, Tolaney SM, Turner NC, Curigliano G, Loibl S, Paluch-Shimon S, Harbeck N, Ann. Oncol 2021,32, 1475. [DOI] [PubMed] [Google Scholar]
- [37].Hinestrosa MC, Dickersin K, Klein P, Mayer M, Noss K, Slamon D, Sledge G, Visco FM, Nat. Rev. Cancer 2007, 7, 309. [DOI] [PubMed] [Google Scholar]
- [38].Watt AC, Goel S, Breast Cancer Res. 2022, 24, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Dean JL, Thangavel C, McClendon AK, Reed CA, Knudsen ES, Oncogene 2010, 29, 4018. [DOI] [PubMed] [Google Scholar]
- [40].Scott SC, Lee SS, Abraham J, Semin. Oncol 2017, 44, 385. [DOI] [PubMed] [Google Scholar]
- [41].Hudis CA, N. Engl. J. Med 2007, 357, 39. [DOI] [PubMed] [Google Scholar]
- [42].Ferraro E, Drago JZ, Modi S, Breast Cancer Res. 2021, 23, 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Esteva FJ, Hubbard-Lucey VM, Tang J, Pusztai L, Lancet Oncol. 2019, 20, e175. [DOI] [PubMed] [Google Scholar]
- [44].Modi S, Jacot W, Yamashita T, Sohn J, Vidal M, Tokunaga E, Tsurutani J, Ueno NT, Prat A, Chae YS, Lee KS, Niikura N, Park YH, Xu B, Wang X, Gil-Gil M, Li W, Pierga J-Y, Im S-A, Moore HCF, Rugo HS, Yerushalmi R, Zagouri F, Gombos A, Kim S-B, Liu Q, Luo T, Saura C, Schmid P, Sun T, et al. , N. Engl. J. Med 2022, 387, 9.35665782 [Google Scholar]
- [45].Bardia A, Hurvitz SA, Tolaney SM, Loirat D, Punie K, Oliveira M, Brufsky A, Sardesai SD, Kalinsky K, Zelnak AB, Weaver R, Traina T, Dalenc F, Aftimos P, Lynce F, Diab S, Cortés J, O’Shaughnessy J, Diéras V, Ferrario C, Schmid P, Carey LA, Gianni L, Piccart MJ, Loibl S, Goldenberg DM, Hong Q, Olivo MS, Itri LM, Rugo HS, N. Engl. J. Med 2021, 384, 1529. [DOI] [PubMed] [Google Scholar]
- [46].Füredi A, Szebényi K, Tóth S, Cserepes M, Hámori L, Nagy V, Karai E, Vajdovich P, Imre T, Szabó P, Szüts D, Tóvári J, Szakács G, J. Controlled Release 2017, 261, 287. [DOI] [PubMed] [Google Scholar]
- [47].Yuan Y, Cai T, Xia X, Zhang R, Chiba P, Cai Y, Drug Delivery 2016, 23, 3350. [DOI] [PubMed] [Google Scholar]
- [48].Yu DD, Wu Y, Shen HY, Lv MM, Chen WX, Zhang XH, Zhong SL, Tang JH, Zhao JH, Cancer Sci. 2015, 106, 959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Portman N, Milioli HH, Alexandrou S, Coulson R, Yong A, Fernandez KJ, Chia KM, Halilovic E, Segara D, Parker A, Haupt S, Haupt Y, Tilley WD, Swarbrick A, Caldon CE, Lim E, Breast Cancer Res. 2020, 22, 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Siegel RL, Miller KD, Fuchs HE, Jemal A, CA - Cancer J. Clin 2022, 72, 7. [DOI] [PubMed] [Google Scholar]
- [51].Lu WL, Jansen L, Post WJ, Bonnema J, Van De Velde JC, De Bock GH, Breast Cancer Res. Treat 2009, 114, 403. [DOI] [PubMed] [Google Scholar]
- [52].Arnold M, Morgan E, Rumgay H, Mafra A, Singh D, Laversanne M, Vignat J, Gralow JR, Cardoso F, Siesling S, Soerjomataram I, Breast 2022, 66, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F, CA - Cancer J Clin. 2021, 71, 209. [DOI] [PubMed] [Google Scholar]
- [54].Asiago VM, Alvarado LZ, Shanaiah N, Gowda GAN, Owusu-Sarfo K, Ballas RA, Raftery D, Cancer Res. 2010, 70, 8309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Nothacker M, Duda V, Hahn M, Warm M, Degenhardt F, Madjar H, Weinbrenner S, Albert US, BMC Cancer 2009, 9, 335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Şahin S, Caglayan MO, Üstündağ Z, Microchim. Acta 2020, 187, 10.1007/s00604-020-04526-x. [DOI] [PubMed] [Google Scholar]
- [57].Ali MA, Mondal K, Singh C, Dhar Malhotra B, Sharma A, Nanoscale 2015, 7, 7234. [DOI] [PubMed] [Google Scholar]
- [58].Garrido-Cano I, Pla L, Santiago-Felipe S, Simón S, Ortega B, Bermejo B, Lluch A, Cejalvo JM, Eroles P, Martínez-Máñez R, ACS Sens. 2021, 6, 1022. [DOI] [PubMed] [Google Scholar]
- [59].Mu Q, Wang H, Zhang M, Expert Opin. Drug Delivery 2017, 14, 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Pesapane F, Downey K, Rotili A, Cassano E, Koh DM, Insights Imaging 2020, 11, 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Pegtel DM, Gould SJ, Annu. Rev. Biochem 2019, 88, 487. [DOI] [PubMed] [Google Scholar]
- [62].Wang J, Zhang Q, Zhou S, Xu H, Wang D, Feng J, Zhao J, Zhong S, Epigenomics 2019, 11, 411. [DOI] [PubMed] [Google Scholar]
- [63].He C, Zheng S, Luo Y, Wang B, Theranostics 2018, 8, 237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Tian F, Zhang S, Liu C, Han Z, Liu Y, Deng J, Li Y, Wu X, Cai L, Qin L, Chen Q, Yuan Y, Liu Y, Cong Y, Ding B, Jiang Z, Sun J, Nat. Commun 2021, 12, 10.1038/s41467-021-22913-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Feng Q, Zhang C, Lum D, Druso JE, Blank B, Wilson KF, Welm A, Antonyak MA, Cerione RA, Nat. Commun 2017, 8, 10.1038/ncomms14450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Keklikoglou I, Cianciaruso C, Güç E, Squadrito ML, Spring LM, Tazzyman S, Lambein L, Poissonnier A, Ferraro GB, Baer C, Cassará A, Guichard A, Iruela-Arispe ML, Lewis CE, Coussens LM, Bardia A, Jain RK, Pollard JW, De Palma M, Nat. Cell Biol 2019, 21, 190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Rontogianni S, Synadaki E, Li B, Liefaard MC, Lips EH, Wesseling J, Wu W, Altelaar M, Commun. Biol 2019, 2, 10.1038/s42003-019-0570-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Jiang Z, Liu G, Li J, Chem. Asian J 2020, 15, 3973. [DOI] [PubMed] [Google Scholar]
- [69].Chang M, Chang Y-J, Chao PY, Yu Q, PLoS One 2018, 13, e0199438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Ayala-Mar S, Donoso-Quezada J, Gallo-Villanueva RC, Perez-Gonzalez VH, González-Valdez J, Electrophoresis 2019, 40, 3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Wang K, Kumar US, Sadeghipour N, Massoud TF, Paulmurugan R, ACS Nano 2021, 15, 18327. [DOI] [PubMed] [Google Scholar]
- [72].Pisano ED, Gatsonis C, Hendrick E, Yaffe M, Baum JK, Acharyya S, Conant EF, Fajardo LL, Bassett L, D’Orsi C, Jong R, Rebner M, N. Engl. J. Med 2005, 353, 1773. [DOI] [PubMed] [Google Scholar]
- [73].Pilling MJ, Henderson A, Gardner P, Anal. Chem 2017, 89, 7348. [DOI] [PubMed] [Google Scholar]
- [74].Tsutsui H, Mochizuki T, Inoue K, Toyama T, Yoshimoto N, Endo Y, Todoroki K, Min JZ, Toyo’oka T, Anal. Chem 2013, 85, 11835. [DOI] [PubMed] [Google Scholar]
- [75].Zeng C, Zhang J, Transl. Cancer Res 2022, 11, 897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Corsi F, De Palma C, Colombo M, Allevi R, Nebuloni M, Ronchi S, Rizzi G, Tosoni A, Trabucchi E, Clementi E, Prosperi D, Small 2009, 5, 2555. [DOI] [PubMed] [Google Scholar]
- [77].Zhai LY, Li MX, Pan WL, Chen Y, Li MM, Pang JX, Zheng L, Chen JX, Duan WJ, ACS Appl. Mater. interfaces 2018, 10, 39478. [DOI] [PubMed] [Google Scholar]
- [78].Ghosh M, Mandal S, Ta S, Das D, Sens. Actuators, B 2017, 249, 339. [Google Scholar]
- [79].Hong W, Lee S, Chang HJ, Lee ES, Cho Y, Biomaterials 2016, 106, 78. [DOI] [PubMed] [Google Scholar]
- [80].Lee S, Chon H,Lee J,Ko J, Chung BH, Lim DW,Choo J, Biosens. Bioelectron 2014, 51, 238. [DOI] [PubMed] [Google Scholar]
- [81].Lim EK, Kim HO, Jang E, Park J, Lee K, Suh JS, Huh YM, Haam S, Biomaterials 2011, 32, 7941. [DOI] [PubMed] [Google Scholar]
- [82].Yang X, Hu C, Tong F, Liu R, Zhou Y, Qin L, Ouyang L, Gao H, Adv. Funct. Mater 2019, 29, 1901896. [Google Scholar]
- [83].Laraib U, Sargazi S, Rahdar A, Khatami M, Pandey S, Int. J. Biol. Macromol 2022, 195, 356. [DOI] [PubMed] [Google Scholar]
- [84].Yankaskas CL, Thompson KN, Paul CD, Vitolo MI, Mistriotis P, Mahendra A, Bajpai VK, Shea DJ, Manto KM, Chai AC, Varadarajan N, Kontrogianni-Konstantopoulos A, Martin SS, Konstantopoulos K, Nat. Biomed. Eng 2019, 3, 452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Manak MS, Varsanik JS, Hogan BJ, Whitfield MJ, Su WR, Joshi N, Steinke N, Min A, Berger D, Saphirstein RJ, Dixit G, Meyyappan T, Chu H-M, Knopf KB, Albala DM, Sant GR, Chander AC, Nat. Biomed. Eng 2018, 2, 761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Tabata M, Liu X, Khamhanglit C, Kotaki S, Miyahara Y, J. Am. Chem. Soc 2022, 144, 16545. [DOI] [PubMed] [Google Scholar]
- [87].Loeian MS, Mehdi Aghaei S, Farhadi F, Rai V, Yang HW, Johnson MD, Aqil F, Mandadi M, Rai SN, Panchapakesan B, Lab Chip 2019, 19, 1899. [DOI] [PubMed] [Google Scholar]
- [88].Allinen M, Beroukhim R, Cai L, Brennan C, Lahti-Domenici J, Huang H, Porter D, Hu M, Chin L, Richardson A, Schnitt S, Sellers WR, Polyak K, Cancer Cell 2004, 6, 17. [DOI] [PubMed] [Google Scholar]
- [89].Nikshoar MS, Khayamian MA, Ansaryan S, Sanati H, Gharooni M, Farahmand L, Rezakhanloo F, Majidzadeh-A K, Hoseinpour P, Dadgari S, Kiani-M L, Saqafi M, Gity M, Abdolahad M, Nat. Commun 2017, 8, 2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Cai S, Pataillot-Meakin T, Shibakawa A, Ren R, Bevan CL, Ladame S, Ivanov AP, Edel JB, Nat. Commun 2021, 12, 3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Bekmurzayeva A, Ashikbayeva Z, Myrkhiyeva Z, Nugmanova A, Shaimerdenova M, Ayupova T, Tosi D, Sci. Rep 2021, 11, 19583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Ranjan P, Parihar A, Jain S, Kumar N, Dhand C, Murali S, Mishra D, Sanghi SK, Chaurasia JP, Srivastava AK, Khan R, Anal. Biochem 2020, 610, 113996. [DOI] [PubMed] [Google Scholar]
- [93].Daemi S, Ashkarran AA, Bahari A, Ghasemi S, Sens. Actuators, B 2017, 245, 55. [Google Scholar]
- [94].Wong XY, Quesada-González D, Manickam S, New SY, Muthoosamy K, Merkoçi A, Sci. Rep 2021, 11, 2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Anker JN, Hall WP, Lyandres O, Shah NC, Zhao J, Van Duyne RP, Nat. Mater 2008, 7, 442. [DOI] [PubMed] [Google Scholar]
- [96].Li H, Huang Y, Yu Y, Li W, Yin Y, Li G, Anal. Chem 2015, 87, 9251. [DOI] [PubMed] [Google Scholar]
- [97].Jin L, Zhao W, Zhang J, Chen W, Xie T, Wang L, Fan W, Xie S, Shen J, Zheng H, Hu W, Wei Q, Dong M, Wang Q, Shen J, Liu Y, Cancer Med. 2020, 9, 1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Deutsch TM, Riethdorf S, Fremd C, Feisst M, Nees J, Fischer C, Hartkopf AD, Pantel K, Trumpp A, Schütz F, Schneeweiss A, Wallwiener M, Breast Cancer Res. Treat 2020, 182, 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Wang C, Mu Z, Chervoneva I, Austin L, Ye Z, Rossi G, Palazzo JP, Sun C, Abu-Khalaf M, Myers RE, Zhu Z, Ba Y, Li B, Hou L, Cristofanilli M, Yang H, Breast Cancer Res. Treat 2017, 161, 83. [DOI] [PubMed] [Google Scholar]
- [100].Rafıee SD, Kocabey S, Mayer M, List J, Rüegg C, ChemMedChem 2020, 15, 661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Davis AA, Zhang Q, Gerratana L, Shah AN, Zhan Y, Qiang W, Finkelman BS, Flaum L, Behdad A, Gradishar WJ, Platanias LC, Cristofanilli M, Breast Cancer Res. 2019, 21, 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Ignatiadis M, Litieère S, Rothe F, Riethdorf S, Proudhon C, Fehm T, Aalders K, Forstbauer H, Fasching PA, Brain E, Vuylsteke P, Guardiola E, Lorenz R, Pantel K, Tryfonidis K, Janni W, Piccart M, Sotiriou C, Rack B, Pierga JY, Ann. Oncol 2018, 29, 1777. [DOI] [PubMed] [Google Scholar]
- [103].Monopoli MP, Åberg C, Salvati A, Dawson KA, Nat. Nanotechnol 2012, 7, 779. [DOI] [PubMed] [Google Scholar]
- [104].Mahmoudi M, Lynch I, Ejtehadi MR, Monopoli MP, Bombelli FB, Laurent S, Chem. Rev 2011, 111, 5610. [DOI] [PubMed] [Google Scholar]
- [105].Keshishian H, Burgess MW, Specht H, Wallace L, Clauser KR, Gillette MA, Carr SA, Nat. Protoc 2017, 12, 1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Etzioni R, Urban N, Ramsey S, McIntosh M, Schwartz S, Reid B, Radich J, Anderson G, Hartwell L, Nat. Rev. Cancer 2003, 3, 243. [DOI] [PubMed] [Google Scholar]
- [107].Cuzick J, Thorat MA, Andriole G, Brawley OW, Brown PH, Culig Z, Eeles RA, Ford LG, Hamdy FC, Holmberg L, Ilic D, Key TJ, Vecchia CL, Lilja H, Marberger M, Meyskens FL, Minasian LM, Parker C, Parnes HL, Perner S, Rittenhouse H, Schalken J, Schmid H-P, Schmitz-Dräger BJ, Schröder FH, Stenzl A, Tombal B, Wilt TJ, Wolk A, Lancet Oncol. 15, e484, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Hajipour MJ, Laurent S, Aghaie A, Rezaee F, Mahmoudi M, Biomater. Sci 2014, 2, 1210. [DOI] [PubMed] [Google Scholar]
- [109].Hajipour MJ, Raheb J, Akhavan O, Arjmand S, Mashinchian O, Rahman M, Abdolahad M, Serpooshan V, Laurent S, Mahmoudi M, Nanoscale 2015, 7, 8978. [DOI] [PubMed] [Google Scholar]
- [110].Tenzer S, Docter D, Kuharev J, Musyanovych A, Fetz V, Hecht R, Schlenk F, Fischer D, Kiouptsi K, Reinhardt C, Nat. Nanotechnol 2013, 8, 772. [DOI] [PubMed] [Google Scholar]
- [111].Lundqvist M, Stigler J, Cedervall T, Berggård T, Flanagan MB, Lynch I, Elia G, Dawson K, ACS Nano 2011, 5, 7503. [DOI] [PubMed] [Google Scholar]
- [112].Schleh C, Semmler-Behnke M, Lipka J, Wenk A, Hirn S, Schäffler M, Schmid G, Simon U, Kreyling WG, Nanotoxicology 2012, 6, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Caracciolo G, Safavi-Sohi R, Malekzadeh R, Poustchi H, Vasighi M, Chiozzi RZ, Capriotti AL, Laganà A, Hajipour M, Di Domenico M, Di Carlo A, Caputo D, Aghaverdi H, Papi M, Palmieri V, Santoni A, Palchetti S, Digiacomo L, Pozzi D, Suslick KS, Mahmoudi M, Nanoscale Horiz. 2019, 4, 1063. [Google Scholar]
- [114].Digiacomo L, Jafari-Khouzani K, Palchetti S, Pozzi D, Capriotti AL, Laganà A, Zenezini Chiozzi R, Caputo D, Cascone C, Coppola R, Flammia G, Altomare V, Grasso A, Mahmoudi M, Caracciolo G, Nanoscale 2020, 12, 16697. [DOI] [PubMed] [Google Scholar]
- [115].Hajipour MJ, Ghasemi F, Aghaverdi H, Raoufı M, Linne U, Atyabi F, Nabipour I, Azhdarzadeh M, Derakhshankhah H, Lotfabadi A, Bargahi A, Alekhamis Z, Aghaie A, Hashemi E, Tafakhori A, Aghamollaii V, Mashhadi MM, Sheibani S, Vali H, Mahmoudi M, J. Alzheimers Dis 2017, 59, 1187. [DOI] [PubMed] [Google Scholar]
- [116].Digiacomo L, Jafari-Khouzani K, Palchetti S, Pozzi D, Capriotti AL, Laganà A, Chiozzi RZ, Caputo D, Cascone C, Coppola R, Nanoscale 2020, 12, 16697. [DOI] [PubMed] [Google Scholar]
- [117].Yang Y, Fu Y, Su H, Mao L, Chen M, Biosens. Bioelectron 2018, 122, 175. [DOI] [PubMed] [Google Scholar]
- [118].Haldavnekar R, Venkatakrishnan K, Tan DB, Biosens. Bioelectron 2021. 190, 113407. [DOI] [PubMed] [Google Scholar]
- [119].Hasanzadeh M, Shadjou N, de la Guardia M, Trends Analyt. Chem 2017, 91, 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Ashkarran AA, Hosseini A, Loloee R, Perry G, Lee KB, Lund M, Ejtehadi MR, Mahmoudi M, J. Colloid Interface Sci 2022, 606, 2038. [DOI] [PubMed] [Google Scholar]
- [121].Chen D, Wang D, Hu X, Long G, Zhang Y, Zhou L, Sens. Actuators, B 2019, 296, 126650. [Google Scholar]
- [122].Arya SK, Zhurauski P, Jolly P, Batistuti MR, Mulato M, Estrela P, Biosens. Bioelectron 2018, 102, 106. [DOI] [PubMed] [Google Scholar]
- [123].Jin Y, Du N, Huang Y, Shen W, Tan Y, Chen YZ, Dou W-T, He X-P, Yang Z, Xu N, Tan C, ACS Sens. 2022, 7, 1524. [DOI] [PubMed] [Google Scholar]
- [124].Rana S, Le NDB, Mout R, Saha K, Tonga GY, Bain RES, Miranda OR, Rotello CM, Rotello VM, Nat. Nanotechnol 2015, 10, 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Kantamneni H, Barkund S, Donzanti M, Martin D, Zhao X, He S, Riman RE, Tan MC, Pierce MC, Roth CM, Ganapathy V, Moghe PV, BMC Cancer 2020, 20, 1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Jensen-Smith H, Currall B, Rossino D, Tiede L, Nichols M, Hallworth R, Methods Mol. Biol 2009, 493, 369. [DOI] [PubMed] [Google Scholar]
- [127].Hoffman RM, Nat. Rev. Cancer 2005, 5, 796. [DOI] [PubMed] [Google Scholar]
- [128].Lee S, Chon H, Yoon S-Y, Lee EK, Chang S-I, Lim DW, Choo J, Nanoscale 2012, 4, 124. [DOI] [PubMed] [Google Scholar]
- [129].Wang K, Fan D, Liu Y, Wang E, Biosens. Bioelectron 2015, 73, 1. [DOI] [PubMed] [Google Scholar]
- [130].Tao Y, Li M, Kim B, Auguste DT, Theranostics 2017, 7, 899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Xiao L, Zhu A, Xu Q, Chen Y, Xu J, Weng J, ACS Appl. Mater. Interfaces 2017, 9, 6931. [DOI] [PubMed] [Google Scholar]
- [132].Ranganathan V, Srinivasan S, Singh A, DeRosa MC, Anal. Biochem 2020, 588, 113471. [DOI] [PubMed] [Google Scholar]
- [133].Teng Y, Shi J, Pong PWT, ACS Appl. Nano Mater 2019, 2, 7421. [Google Scholar]
- [134].Kaur B, Kumar S, Kaushik BK, Biosens. Bioelectron 2022, 197, 113805. [DOI] [PubMed] [Google Scholar]
- [135].Ashkarran AA, Estakhri S, Nezhad MRH, Eshghi S, Phys. Procedia 2013, 40, 76. [Google Scholar]
- [136].Wang H, Wu T, Li M, Tao Y, J. Mater. Chem. B 2021, 9, 921. [DOI] [PubMed] [Google Scholar]
- [137].Tang L, Li J, ACS Sens. 2017, 2, 857. [DOI] [PubMed] [Google Scholar]
- [138].Cathcart N, Chen JIL, Anal. Chem 2020, 92, 7373. [DOI] [PubMed] [Google Scholar]
- [139].Lindner R, Sullivan C, Offor O, Lezon-Geyda K, Halligan K, Fischbach N, Shah M, Bossuyt V, Schulz V, Tuck DP, PLoS One 2013, 8, e71915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Ashkarran AA, Curr. Appl. Phys 2010, 10, 1442. [Google Scholar]
- [141].Wang Z, Chen Q, Zhong Y, Yu X, Wu Y, Fu F, Anal. Chem 2020, 92, 1534. [DOI] [PubMed] [Google Scholar]
- [142].Kang J-H, Asami Y, Murata M, Kitazaki H, Sadanaga N, Tokunaga E, Shiotani S, Okada S, Maehara Y, Niidome T, Hashizume M, Mori T, Katayama Y, Biosens. Bioelectron 2010, 25, 1869. [DOI] [PubMed] [Google Scholar]
- [143].Zhang Y, Wang D, Yue S, Lu Y, Yang C, Fang J, Xu Z, ACS Sens. 2019, 4, 3210. [DOI] [PubMed] [Google Scholar]
- [144].De Silva Indrasekara AS, J. Appl. Phys 2021, 129, 231102. [Google Scholar]
- [145].Chauhan P, Bhargava A, Kumari R, Ratre P, Tiwari R, Kumar Srivastava R, Goryacheva IY, Kumar Mishra P, Drug Discovery Today 2022. 27, 2121. [DOI] [PubMed] [Google Scholar]
- [146].Meng S, Chen R, Xie J, Li J, Cheng J, Xu Y, Cao H, Wu X, Zhang Q, Wang H, Biosens. Bioelectron 2021, 190, 113470. [DOI] [PubMed] [Google Scholar]
- [147].Wilson AJ, Willets KA, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol 2013, 5, 180. [DOI] [PubMed] [Google Scholar]
- [148].Xie Y, Su X, Wen Y, Zheng C, Li M, Nano Lett. 2022, 22, 7910. [DOI] [PubMed] [Google Scholar]
- [149].Wang ZY, Li W, Gong Z, Sun PR, Zhou T, Cao XW, New J Chem. 2019, 43, 1733. [Google Scholar]
- [150].Wang H, Liu Y, Rao G, Wang Y, Du X, Hu A, Hu Y, Gong C, Wang X, Xiong J, Analyst 2021, 146, 5008. [DOI] [PubMed] [Google Scholar]
- [151].Zhao X, Campbell S, El-Khoury PZ, Jia Y, Wallace GQ, Claing A, Bazuin CG, Masson JF, ACS Sens. 2021, 6, 1649. [DOI] [PubMed] [Google Scholar]
- [152].Gorji MB, Ashkarran AA, Hamidinezhad H, Colloids Surf. A 2020, 591, 124532. [Google Scholar]
- [153].Shahini P, Ashkarran AA, Ceram. Int 2017, 43, 8655. [Google Scholar]
- [154].Fabris L, ChemNanoMat 2016, 2, 249. [Google Scholar]
- [155].Ashkarran AA, Gharibi H, Grunberger JW, Saei AA, Khurana N, Mohammadpour R, Ghandehari H, Mahmoudi M, ACS Biol. Med. Chem. Au 2022, 3, 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Ashkarran AA, Gharibi H, Voke E, Landry MP, Saei AA, Mahmoudi M, Nat. Commun 2022, 13, 6610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Sheibani S, Basu K, Farnudi A, Ashkarran A, Ichikawa M, Presley J, Bui K, Ejtehadi MR, Vali H, Mahmoudi M, Nat. Commun 2021, 12, 573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Liu H, Gao X, Xu C, Liu D, Theranostics 2022, 12, 1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Zeng L, Pan Y, Wang S, Wang X, Zhao X, Ren W, Lu G, Wu A, ACS Appl. Mater. Interfaces 2015, 7, 16781. [DOI] [PubMed] [Google Scholar]
- [160].Xia X, Li W, Zhang Y, Xia Y, Interface Focus 2013, 3, 20120092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Lee JU, Kim WH, Lee HS, Park KH, Sim SJ, Small 2019, 15, 1804968. [DOI] [PubMed] [Google Scholar]
- [162].Chen J, Saeki F, Wiley BJ, Cang H, Cobb MJ, Li ZY, Au L, Zhang H, Kimmey MB, Li X, Xia Y, Nano Lett. 2005, 5, 473. [DOI] [PubMed] [Google Scholar]
- [163].Skrabalak SE, Chen J, Au L, Lu X, Li X, Xia Y, Adv. Mater 2007, 19, 3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Ashkarran AA, Daemi S, Plasmonics 2016, 11, 1011. [Google Scholar]
- [165].Sun T, Zhang YS, Pang B, Hyun DC, Yang M, Xia Y, Angew. Chem. – Int. Ed 2014, 53, 12320. [DOI] [PubMed] [Google Scholar]
- [166].Zhao Y, Pang B, Luehmann H, Detering L, Yang X, Sultan D, Harpstrite S, Sharma V, Cutler CS, Xia Y, Liu Y, Adv. Healthcare Mater 2016, 5, 928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Skrabalak SE, Chen J, Sun Y, Lu X, Au L, Cobley CM, Xia Y, Acc. Chem. Res 2008, 41, 1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Ashkarran AA, Opt. Mater 2016, 58, 454. [Google Scholar]
- [169].Ashkarran AA, Derakhshi M, J. Cluster Sci 2015, 26, 1901. [Google Scholar]
- [170].Skrabalak SE, Au L, Lu X, Li X, Xia Y, Nanomedicine 2007, 2, 657. [DOI] [PubMed] [Google Scholar]
- [171].Daemi S, Ashkarran AA, Bahari A, Ghasemi S, J. Colloid Interface Sci 2017, 494, 290. [DOI] [PubMed] [Google Scholar]
- [172].Hassis ME, Niles RK, Braten MN, Albertolle ME, Witkowska HE, Hubel CA, Fisher SJ, Williams KE, Anal. Biochem 2015, 478, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Yavuz MS, Cheng Y, Chen J, Cobley CM, Zhang Q, Rycenga M, Xie J, Kim C, Song KH, Schwartz AG, Wang LV, Xia Y, Nat. Mater 2009, 8, 935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Qiu J, Liu Y, Xia Y, Adv. Healthcare Mater 2021, 10, 2002031. [Google Scholar]
- [175].Yang X, Skrabalak SE, Li ZY, Xia Y, Wang LV, Nano Lett. 2007, 7, 3798. [DOI] [PubMed] [Google Scholar]
- [176].Li L, Patil D, Petruncio G, Harnden KK, Somasekharan JV, Paige M, Wang LV, Salvador-Morales C, ACS Nano 2021, 15, 2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Tekpli X, Lien T, Røssevold AH, Nebdal D, Borgen E, Ohnstad HO, Kyte JA, Vallon-Christersson J, Fongaard M, Due EU, Svartdal LG, Sveli MAT, Garred Ø, Børresen-Dale AL, Schlichting E, Sauer T, Geisler J, Hofvind S, Bathen TF, Engebråten O, Geitvik GA, Langerød A, Kåresen R, Mælandsmo GM, Lingjærde OC, Skjerven HK, Park D, Fritzman B, Frigessi A, Sahlberg KK, et al. , Nat. Commun 2019, 10, 5499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Lim B, Woodward WA, Wang X, Reuben JM, Ueno NT, Nat. Rev. Cancer 2018, 18, 485. [DOI] [PubMed] [Google Scholar]
- [179].Ward C, Langdon SP, Mullen P, Harris AL, Harrison DJ, Supuran CT, Kunkler IH, Cancer Treat. Rev 2013, 39, 171. [DOI] [PubMed] [Google Scholar]
- [180].Mpekris F, Panagi M, Voutouri C, Martin JD, Samuel R, Takahashi S, Gotohda N, Suzuki T, Papageorgis P, Demetriou P, Pierides C, Koumas L, Costeas P, Kojima M, Ishii G, Constantinidou A, Kataoka K, Cabral H, Stylianopoulos T, Adv. Sci 2021, 8, 2001917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Martin JD, Panagi M, Wang C, Khan TT, Martin MR, Voutouri C, Toh K, Papageorgis P, Mpekris F, Polydorou C, Ishii G, Takahashi S, Gotohda N, Suzuki T, Wilhelm ME, Melo VA, Quader S, Norimatsu J, Lanning RM, Kojima M, Stuber MD, Stylianopoulos T, Kataoka K, Cabral H, ACS Nano 2019, 13, 6396. [DOI] [PubMed] [Google Scholar]
- [182].Panagi M, Mpekris F, Chen P, Voutouri C, Nakagawa Y, Martin JD, Hiroi T, Hashimoto H, Demetriou P, Pierides C, Samuel R, Stylianou A, Michael C, Fukushima S, Georgiou P, Papageorgis P, Papaphilippou PC, Koumas L, Costeas P, Ishii G, Kojima M, Kataoka K, Cabral H, Stylianopoulos T, Nat. Commun 2019. 13, 7165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Tang S, Meng Q, Sun H, Su J, Yin Q, Zhang Z, Yu H, Chen L, Chen Y, Gu W, Li Y, Adv. Funct. Mater 2016, 26, 6033. [Google Scholar]
- [184].Dreher MR, Liu W, Michelich CR, Dewhirst MW, Yuan F, Chilkoti A, Natl J. Cancer Inst. 2006, 98, 335. [DOI] [PubMed] [Google Scholar]
- [185].Price LSL, Stern ST, Deal AM, Kabanov AV, Zamboni WC, Sci. Adv 2020, 6, 10.1126/sciadv.aay9249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Kumar DN, Chaudhuri A, Aqil F, Dehari D, Munagala R, Singh S, Gupta RC, Agrawal AK, Cancers 2022, 14, 1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Xu J, Sun J, Ho PY, Luo Z, Ma W, Zhao W, Rathod SB, Fernandez CA, Venkataramanan R, Xie W, Yu AM, Li S, Biomaterials 2019, 210, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Derakhshi M, Ashkarran AA, Bahari A, Bonakdar S, Nanotechnology 2018, 29, 285102. [DOI] [PubMed] [Google Scholar]
- [189].Wang Y, Black KCL, Luehmann H, Li W, Zhang Y, Cai X, Wan D, Liu SY, Li M, Kim P, Li ZY, Wang LV, Liu Y, Xia Y, ACS Nano 2013, 7, 2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Wang Y, Shim MS, Levinson NS, Sung HW, Xia Y, Adv. Funct. Mater 2014, 24, 4206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Zhang H, Jin M, Xia Y, Angew. Chem. – Int. Ed 2012, 51, 7656. [DOI] [PubMed] [Google Scholar]
- [192].Wan D, Xia X, Wang Y, Xia Y, Small 2013, 9, 3111. [DOI] [PubMed] [Google Scholar]
- [193].Noguez C,J. Phys. Chem. C 2007, 111, 3806. [Google Scholar]
- [194].Derakhshi M, Ashkarran AA, Bahari A, Bonakdar S, Colloids Surf. A 2018, 545, 101. [DOI] [PubMed] [Google Scholar]
- [195].Xia Y, Gilroy KD, Peng HC, Xia X, Angew. Chem. – Int. Ed 2017, 56, 60. [DOI] [PubMed] [Google Scholar]
- [196].Xia Y, Xia X, Peng HC, J. Am. Chem. Soc 2015, 137, 7947. [DOI] [PubMed] [Google Scholar]
- [197].Sun H, Su J, Meng Q, Yin Q, Chen L, Gu W, Zhang Z, Yu H, Zhang P, Wang S, Li Y, Adv. Funct. Mater 2017, 27, 1604300. [Google Scholar]
- [198].Au L, Zheng D, Zhou F, Li ZY, Li X, Xia Y, ACS Nano 2008, 2, 1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Feng B, Xu Z, Zhou F, Yu H, Sun Q, Wang D, Tang Z, Yu H, Yin Q, Zhang Z, Li Y, Nanoscale 2015, 7, 14854. [DOI] [PubMed] [Google Scholar]
- [200].Andreu I, Natividad E, Solozábal L, Roubeau O, ACS Nano 2015, 9, 1408. [DOI] [PubMed] [Google Scholar]
- [201].Kikumori T, Kobayashi T, Sawaki M, Imai T, Breast Cancer Res. Treat 2009, 113, 435. [DOI] [PubMed] [Google Scholar]
- [202].Datta NR, Krishnan S, Speiser DE, Neufeld E, Kuster N, Bodis S, Hofmann H, Cancer Treat. Rev 2016, 50, 217. [DOI] [PubMed] [Google Scholar]
- [203].Nemati Z, Alonso J, Martinez LM, Khurshid H, Garaio E, Garcia JA, Phan MH, Srikanth H, J. Phys. Chem. C 2016, 120, 8370. [Google Scholar]
- [204].Du Y, Liu X, Liang Q, Liang XJ, Tian J, Nano Lett. 2019, 19, 3618. [DOI] [PubMed] [Google Scholar]
- [205].Wu H, Liu L, Song L, Ma M, Gu N, Zhang Y, ACS Nano 2019, 13, 14013. [DOI] [PubMed] [Google Scholar]
- [206].Derakhshi M, Daemi S, Shahini P, Habibzadeh A, Mostafavi E, Ashkarran AA, J Funct Biomater 2022, 13, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Cui G, Wu J, Lin J, Liu W, Chen P, Yu M, Zhou D, Yao G, J. Nanobiotechnol 2021, 19, 211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Tade RS, Patil PO, ACS Biomater. Sci. Eng 2020, 6, 5987. [DOI] [PubMed] [Google Scholar]
- [209].Orecchioni M, Cabizza R, Bianco A, Delogu LG, Theranostics 2015, 5, 710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Zheng J, Meng D, Zheng X, Zhang Y, Chen H, Int. J. Pharm 2021, 603, 120644. [DOI] [PubMed] [Google Scholar]
- [211].Robinson JT, Tabakman SM, Liang Y, Wang H, Sanchez Casalongue H, Vinh D, Dai H,J. Am. Chem. Soc 2011, 133, 6825. [DOI] [PubMed] [Google Scholar]
- [212].Dolatkhah M, Hashemzadeh N, Barar J, Adibkia K, Aghanejad A, Barzegar-Jalali M, Omidi Y, Colloids Surf., B 2020, 193, 111104. [DOI] [PubMed] [Google Scholar]
- [213].Wang F, Sun Q, Feng B, Xu Z, Zhang J, Xu J, Lu L, Yu H, Wang M, Li Y, Zhang W, Adv. Healthcare Mater 2016, 5, 2227. [DOI] [PubMed] [Google Scholar]
- [214].Ding H, Zhang F, Zhao C, Lv Y, Ma G, Wei W, Tian Z, ACS Appl. Mater. Interfaces 2017, 9, 27396. [DOI] [PubMed] [Google Scholar]
- [215].He Y, Huang Y, Huang Z, Jiang Y, Sun X, Shen Y, Chu W, Zhao C, J. Controlled Release 2017, 264, 76. [DOI] [PubMed] [Google Scholar]
- [216].Mahdaviani P, Bahadorikhalili S, Navaei-Nigjeh M, Vafaei SY, Esfandyari-Manesh M, Abdolghaffari AH, Daman Z, Atyabi F, Ghahremani MH, Amini M, Lavasanifar A, Dinarvand R, Mater. Sci. Eng. C 2017, 80, 301. [DOI] [PubMed] [Google Scholar]
- [217].He C, Cai P, Li J, Zhang T, Lin L, Abbasi AZ, Henderson JT, Rauth AM, Wu XY, J. Controlled Release 2017, 246, 98. [DOI] [PubMed] [Google Scholar]
- [218].Lee SM, Ahn RW, Chen F, Fought AJ, Ohalloran TV, Cryns VL, Nguyen ST, ACS Nano 2010, 4, 4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Garg SM, Paiva IM, Vakili MR, Soudy R, Agopsowicz K, Soleimani AH, Hitt M, Kaur K, Lavasanifar A, Biomaterials 2017, 144, 17. [DOI] [PubMed] [Google Scholar]
- [220].Cabral H, Matsumoto Y, Mizuno K, Chen Q, Murakami M, Kimura M, Terada Y, Kano MR, Miyazono K, Uesaka M, Nishiyama N, Kataoka K, Nat. Nanotechnol 2011, 6, 815. [DOI] [PubMed] [Google Scholar]
- [221].Sorolla A, Sorolla MA, Wang E, Ceña V, Expert Opin. Drug Delivery 2020, 17, 1597. [DOI] [PubMed] [Google Scholar]
- [222].Moreno-Aspitia A, Perez EA, Clin. Breast Cancer 2005, 6, 361. [DOI] [PubMed] [Google Scholar]
- [223].Yuan H, Guo H, Luan X, He M, Li F, Burnett J, Truchan N, Sun D, Mol. Pharmaceutics 2020, 17, 2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [224].Roy V, Laplant BR, Gross GG, Bane CL, Palmieri FM, Ann. Oncol 2009, 20, 449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Sparano JA, Wang M, Martino S, Jones V, Perez EA, Saphner T, Wolff AC, Sledge GW Jr, Wood WC, Davidson NE, N. Engl. J. Med 2008, 358, 1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [226].Feng B, Niu Z, Hou B, Zhou L,Li Y, Yu H, Adv. Funct. Mater 2020, 30, 1906605. [Google Scholar]
- [227].Mittendorf EA, Zhang H, Barrios CH, Saji S, Jung KH, Hegg R, Koehler A, Sohn J, Iwata H, Telli ML, Ferrario C, Punie K, Penault-Llorca F, Patel S, Duc AN, Liste-Hermoso M, Maiya V, Molinero L, Chui SY, Harbeck N, Lancet 2020, 396, 1090. [DOI] [PubMed] [Google Scholar]
- [228].Kratz F, J. Controlled Release 2008, 132, 171. [DOI] [PubMed] [Google Scholar]
- [229].Anhorn MG, Wagner S, Kreuter J, Langer K, Von Briesen H, Bio-conjugate Chem. 2008, 19, 2321. [DOI] [PubMed] [Google Scholar]
- [230].Morshed RA, Muroski ME, Dai Q, Wegscheid ML, Auffinger B, Yu D, Han Y, Zhang L, Wu M, Cheng Y, Lesniak MS, Mol. Pharmaceutics 2016, 13, 1843. [DOI] [PubMed] [Google Scholar]
- [231].Du J, Shao Y, Hu Y, Chen Y, Cang J, Chen X, Pei W, Miao F, Shen Y, Muddassir M, Zhang Y, Zhang J, Teng G, ACS Appl. Mater. Interfaces 2021, 13, 23396. [DOI] [PubMed] [Google Scholar]
- [232].Feng B, Zhou F, Lu W, Wang D, Wang T, Luo C, Wang H, Li Y, Yu H, Biomater. Sci 2017, 5, 1522. [DOI] [PubMed] [Google Scholar]
- [233].Park JW, Breast Cancer Res. 2002, 4, 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [234].Giulimondi F, Digiacomo L, Pozzi D, Palchetti S, Vulpis E, Capriotti AL, Chiozzi RZ, Laganà A, Amenitsch H, Masuelli L, Mahmoudi M, Screpanti I, Zingoni A, Caracciolo G, Nat. Commun 2019, 10, 3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [235].Khan DR, Webb MN, Cadotte TH, Gavette MN, Breast Cancer: Basic Clin. Res 2015, 9, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [236].Gener P, Gouveia LP, Sabat GR, de Sousa Rafael DF, Fort NB, Arranja A, Fernández Y, Prieto RM, Ortega JS, Arango D, Abasolo I, Videira M, Schwartz S, Nanomed.: Nanotechnol., Biol., Med 2015, 11, 1883. [DOI] [PubMed] [Google Scholar]
- [237].Hashemzadeh N, Dolatkhah M, Adibkia K, Aghanejad A, Barzegar-Jalali M, Omidi Y, Barar J, Life Sci. 2021, 271, 119110. [DOI] [PubMed] [Google Scholar]
- [238].Coley HM, Cancer Treat. Rev 2008, 34, 378. [DOI] [PubMed] [Google Scholar]
- [239].Arab A, Robati RY, Nicastro J, Slavcev R, Behravan J, Curr. Pharm. Des 2018, 24, 1195. [DOI] [PubMed] [Google Scholar]
- [240].Bahreyni A, Mohamud Y, Luo H, J. Nanobiotechnol 2020, 18, 180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [241].Lang T, Liu Y, Zheng Z, Ran W, Zhai Y, Yin Q, Zhang P, Li Y, Adv. Mater 2019, 31, 1806202. [DOI] [PubMed] [Google Scholar]
- [242].Ghosh S, Javia A, Shetty S, Bardoliwala D, Maiti K, Banerjee S, Khopade A, Misra A, Sawant K, Bhowmick S, J. Controlled Release 2021, 337, 27. [DOI] [PubMed] [Google Scholar]
- [243].Li Y, Xiao Y, Lin HP, Reichel D, Bae Y, Lee EY, Jiang Y,Huang X, Yang C, Wang Z, Biomaterials 2019, 188, 160. [DOI] [PubMed] [Google Scholar]
- [244].Mayer IA, Zhao F, Arteaga CL, Symmans WF, Park BH, Burnette BL, Tevaarwerk AJ, Garcia SF, Smith KL, Makower DF, Block M, Morley KA, Jani CR, Mescher C, Dewani SJ, Tawfık B, Flaum LE, Mayer EL, Sikov WM, Rodler ET, Wagner LI, DeMichele AM, Sparano JA, Wolff AC, Miller KD,J. Clin. Oncol 2021, 39, 2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [245].Yang X, Zhao M, Wu Z, Chen C, Zhang Y, Wang L, Guo Q, Wang Q, Liang S, Hu S, Duan Y, Sun Y, ACS Nano 2022, 16, 3417. [DOI] [PubMed] [Google Scholar]
- [246].Xu H, Hu M, Liu M, An S, Guan K, Wang M, Li L, Zhang J, Li J, Huang L, Biomaterials 2020, 235, 119769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [247].He H, Liu L, Morin EE, Liu M, Schwendeman A, Acc. Chem. Res 2019, 52, 2445. [DOI] [PubMed] [Google Scholar]
- [248].Gianni L, Mansutti M, Anton A, Calvo L, Bisagni G, Bermejo B, Semiglazov V, Thill M, Chacon JI, Chan A, Morales S, Alvarez I, Plazaola A, Zambetti M, Redfern AD, Dittrich C, Dent RA, Magazzu D, De Fato R, Valagussa P, Tusquets I, JAMA Oncol 2018, 4, 302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [249].Miele E, Spinelli GP, Miele E, Tomao F, Tomao S, Int. J. Nanomed 2009, 4, 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [250].Dranitsaris G, Yu B, Wang L, Sun W, Zhou Y, King J, Kaura S, Zhang A, Yuan P,J Oncol Pharm Pract 2016, 22, 205. [DOI] [PubMed] [Google Scholar]
- [251].Yuan H, Guo H, Luan X, He M, Li F, Burnett J, Truchan N, Sun D, Mol. Pharm 2020, 17, 2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [252].Hajipour MJ, Aghaverdi H, Serpooshan V, Vali H, Sheibani S, Mahmoudi M, Nat. Commun 2021, 12, 2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [253].Ashkarran AA, Swann J, Hollis L, Mahmoudi M, Trends Biotechnol. 2021, 39, 425. [DOI] [PubMed] [Google Scholar]
- [254].Sharifı S, Caracciolo G, Pozzi D, Digiacomo L, Swann J, Daldrup-Link HE, Mahmoudi M, Adv. Drug Delivery Rev. 2021, 174, 337. [DOI] [PubMed] [Google Scholar]
- [255].Mahmoudi M, Landry MP, Moore A, Coreas R, Nat. Rev. Mater 2023, 10.1038/s41578-023-00552-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [256].Liz-Marzán LM, Nel AE, Brinker CJ, Chan WCW, Chen C, Chen X, Ho D, Hu T, Kataoka K, Kotov NA, Parak WJ, Stevens MM, ACS Nano 2022, 16, 13257. [DOI] [PubMed] [Google Scholar]
- [257].Sindhwani S, Chan WCW, J. Intern. Med 2021, 290, 486. [DOI] [PubMed] [Google Scholar]
- [258].Sharifı S, Reuel N, Kallmyer N, Sun E, Landry MP, Mahmoudi M, ACS Nano 2023, 17, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [259]. https://www.nature.com/articles/s41467-021-27643-4 .