Abstract
Complementation analysis of a polyhydroxyalkanoate (PHA)-negative mutant of Aeromonas caviae proved that ORF3 in the pha locus (a 402-bp gene located downstream of the PHA synthase gene) participates in PHA biosynthesis on alkanoic acids, and the ORF3 gene is here referred to as phaJAc. Escherichia coli BL21(DE3) carrying phaJAc under the control of the T7 promoter overexpressed enoyl coenzyme A (enoyl-CoA) hydratase, which was purified by one-step anion-exchange chromatography. The N-terminal amino acid sequence of the purified hydratase corresponded to the amino acid sequence deduced from the nucleotide sequence of phaJAc except for the initial Met residue. The enoyl-CoA hydratase encoded by phaJAc exhibited (R)-specific hydration activity toward trans-2-enoyl-CoA with four to six carbon atoms. These results have demonstrated that (R)-specific hydration of 2-enoyl-CoA catalyzed by the translated product of phaJAc is a channeling pathway for supplying (R)-3-hydroxyacyl-CoA monomer units from fatty acid β-oxidation to poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) biosynthesis in A. caviae.
Polyhydroxyalkanoates (PHA) occur naturally in a wide variety of bacteria as a material for storage of carbon and energy from renewable carbon resources under conditions of restricted growth. They have recently attracted industrial attention because of their potential properties as biodegradable thermoplastics. A great deal of research has been undertaken with the aim of understanding the mechanism of PHA biosynthesis (1, 7), and numerous advances have been made from recent molecular analysis of PHA biosynthesis genes (29, 30).
In Alcaligenes eutrophus, two molecules of acetyl coenzymeA (acetyl-CoA) derived from various carbon sources are converted to (R)-3-hydroxybutyryl-CoA [(R)-(3HB)-CoA] via dimerization catalyzed by β-ketothiolase and subsequent (R)-specific reduction catalyzed by NADPH-acetoacetyl-CoA reductase. The resultant (R)-3HB-CoA molecules are then polymerized into poly(3-hydroxybutyrate) [P(3HB)] by the function of PHA synthase. The genes encoding the three enzymes are organized in a single operon as phbCAB, consisting of the genes for PHA synthase, thiolase, and reductase, respectively (21, 25, 28). This operon has been introduced into several prokaryotes and eukaryotes, resulting in P(3HB) accumulation (18, 22, 29, 34). Pseudomonads belonging to rRNA homology group I accumulated PHA consisting of medium-chain-length (C5 to C14) 3-hydroxyalkanoate (3HA) units from simple carbon sources, such as sugars (13, 15, 32) or n-alkanoic acids, n-alkanols, or n-alkanes (4, 12, 14, 16). In the production of PHA from alkanoic acids with six to nine carbon atoms by Pseudomonas oleovorans, the major monomer unit in PHA has the same chain length as the alkanoic acids fed as a carbon source, but monomer units with more or fewer carbon atoms than the acid fed are also generally present in the PHA formed. It has been proposed that acyl-CoA intermediates of the β-oxidation pathway are channeled to PHA biosynthesis (6a, 9); however, few details have been elucidated.
Aeromonas caviae isolated from soil has been reported to produce a random copolyester of 3HB and (R)-3-hydroxyhexanoate [(R)-3HHx], P(3HB-co-3HHx), from alkanoic acids with even carbon numbers or from plant oils (8, 26). On the basis of the fact that this bacterium does not accumulate any PHA from sugars, C4 and C6 (R)-3HA units were proposed to be supplied from the β-oxidation intermediates, similar to the PHA biosynthesis pathway in pseudomonads on alkanoic acids (8). Recently, we have cloned and analyzed the PHA biosynthesis genes of A. caviae and have suggested that ORF3 located downstream of the PHA synthase gene (phaCAc) encodes (R)-specific enoyl-CoA hydratase (11). In this paper, we report direct evidence that ORF3 is essential for PHA biosynthesis from alkanoic acids in A. caviae. Furthermore, ORF3, referred to as phaJAc, is overexpressed in Escherichia coli, and characteristics of the translated product are investigated.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The bacterial strains and plasmids used in this study are listed in Table 1. A. caviae strains were cultivated at 30°C in a nutrient-rich medium containing 10 g of meat extract, 10 g of polypeptone, and 2 g of yeast extract in 1 liter of distilled water, and E. coli strains were grown at 37°C on Luria-Bertani (LB) medium (23). Kanamycin (50 mg/liter) or ampicillin (50 mg/liter) was added to the medium when necessary.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Description | Source or reference(s) |
|---|---|---|
| A. caviae | ||
| FA440 | Wild type | FERM P-3432; 8, 26 |
| AC004 | PHA-negative mutant of FA440 | This study |
| E. coli | ||
| DH5α | deoR endA1 gyrA96 hsdR17(rK− mK+) recA1 relA1 supE44 thi-1 Δ(lacZYA-argFV169) φ80 ΔlacZΔM15 F− r− | Clontech |
| S17-1 | recA and tra genes of plasmid RP4 integrated into chromosome; auxotrophic for proline and thiamine | 27 |
| BL21(DE3) | F−ompT hsdSB(rB− mB−) gal dcm (DE3) | Novagen |
| pJRD215 | Cosmid; KmrSmr RSF1010 replicon; Mob+ | 5 |
| pJRDEE32 | 3.2-kbp insert (EcoRI fragment containing phaCAc, ORF1, and ORF3 [phaJAc]) in pJRD215 | 11 |
| pJRDEE32d1 | 2.8-kbp insert (EcoRI fragment containing phaCAc and ORF3 [phaJAc]) in pJRD215 | 11 |
| pJRDEE32d3 | 2.8-kbp insert (EcoRI fragment containing phaCAc and ORF1) in pJRD215 | 11 |
| pJRDEE32d13 | 2.4-kbp insert (EcoRI fragment containing phaCAc) in pJRD215 | 11 |
| pJRDG13 | 1.4-kbp insert (EcoRI fragment containing ORF1 and ORF3 [phaJAc]) in pJRD215 | This study |
| pEE32 | 3.2-kbp insert (EcoRI fragment containing phaCAc, ORF1, and ORF3 [phaJAc]) in pUC18 | 11 |
| pET-3a | Apr; contains a T7 promoter with start codon and designed ribosome binding site | Novagen |
| pETNB3 | 0.4-kbp insert (PCR-amplified NdeI-BamHI fragment of ORF3 [phaJAc]) in pET-3a | This study |
Chemical mutagenesis and isolation of a PHA-negative mutant of A. caviae.
A. caviae FA440 was treated with N-methyl-N′-nitro-N-nitrosoguanidine (50 μg/ml) and then inoculated on MM agar medium composed of 3 g of K2HPO4, 7 g of KH2PO4, 1 g of (NH4)2SO4, 0.1 g of yeast extract, 5 g of glucose, and 15 g of agar in 1 liter of distilled water (pH 7.0). Colonies grown at 30°C for 5 days were replicated on MPA agar medium composed of 7 g of K2HPO4, 3 g of KH2PO4, 1 g of (NH4)2SO4, 0.1 g of MgSO4 · 7H2O, 0.1 g of yeast extract, 1.5 g of palmitic acid, 10 ml of Triton X-100, and 15 g of agar in 1 liter of distilled water (pH 7.0). The small amount of yeast extract (0.1 g/liter) was added to enhance cell growth. PHA-negative mutants, which were not stained with Sudan Black B (24) on MPA agar medium, were isolated.
DNA manipulation.
Basic recombinant DNA techniques, such as preparation and purification of plasmid DNA, restriction endonuclease digestion, agarose gel electrophoresis, and transformation of E. coli, were carried out as described by Sambrook et al. (23). Transconjugation of A. caviae with E. coli S17-1 harboring broad-host-range plasmids was performed as described by Friedrich et al. (10).
Construction of pJRDG13.
Two BglII sites across phaCAc were created on pEE32 (11) by site-directed mutagenesis with the unique-site elimination procedure (6) using mutagenic primers M2 (5′-GACGCTACGGGCTAGATCTCGCCTCGGGTGTG-3′) and M5 (5′-GCGGCTCAACCCAGATCTTGCCTGCCCAACAG-3′), which corresponded to the sequences from nucleotides 2647 to 2678 and 4430 to 4461, respectively (11) (the created BglII sites are underlined). The resultant plasmid was digested with BglII and self-ligated to delete the coding region of phaCAc. The EcoRI restriction fragment excised from the phaCAc-deleted plasmid was inserted into pJRD215 (5) to form pJRDG13, harboring ORF1 and phaJAc (ORF3) of A. caviae.
Production and analysis of PHA.
A. caviae strains were first cultivated in 100 ml of nutrient-rich medium on a reciprocal shaker (130 strokes/min) at 30°C for 12 h. Then harvested and washed cells were transferred into 100 ml of nitrogen-free mineral salt medium (pH 7.0), which was composed of 0.9 g of Na2HPO4 · 12H2O, 0.15 g of KH2PO4, 0.02 g of MgSO4 · 7H2O, and 0.1 ml of trace element solution (15), and incubated at 30°C for 48 h. Sodium dodecanoate (1%) was added as a carbon substrate for PHA biosynthesis. For maintenance of broad-host-range plasmids in A. caviae, kanamycin was added to the medium at a concentration of 50 mg/liter. Cellular PHA contents were determined by gas chromatography after methanolysis of dried cells in the presence of 15% sulfuric acid, as described previously (15).
Construction of expression plasmid pETNB3.
To construct a plasmid for the overexpression of phaJAc, an NdeI site was introduced into the proposed translational start codon of phaJAc by PCR. The 427-bp fragment was amplified with primers P3N (5′-GCCATATGAGCGCACAATCCCTGGAAGTAG-3′) and P3C (5′-CTGGGATCCGCCGGTGCTTAAGGCAGCTTG-3′), corresponding to the sequences from nucleotides 4470 to 4499 and 4867 to 4896 (complementary sequence), respectively (11) (underlined sequences show an NdeI site in P3N and a BamHI site in P3C). The PCR product was purified, digested with NdeI and BamHI, and subcloned into pET-3a. The resultant plasmid was termed pETNB3.
Enzyme assay.
Enoyl-CoA hydratase activity was assayed by the hydration of crotonyl-CoA (19, 31). A 10-μl volume of enzyme solution was added to 290 μl of 50 mM Tris-HCl (pH 8.0) containing 0.25 mM crotonyl-CoA (Sigma) in quartz cuvettes with a 0.1-cm light path, and the decrease in absorbance at 263 nm was measured at 30°C. The ɛ263 of the enoyl-thioester bond is taken to be 6.7 × 103 M−1 cm−1 (31). Other C5, C6, and C8 trans-2-enoyl-CoA substrates used for the hydratase assay were synthesized from a lithium salt of CoA and the corresponding trans-2-alkenoic acids (Tokyo Kasei) based on a mixed-anhydride method and were purified with a Sep-Pak C18 column (Waters), as described by Valentin and Steinbüchel (33). Protein concentrations were determined by the method of Bradford (2) by using Bio-Rad assay solution and bovine serum albumin as the standard.
Expression of phaJAc in E. coli.
The expression plasmid pETNB3 was transformed into E. coli BL21(DE3). A 1-ml overnight culture of the cells was inoculated into 100 ml of LB medium containing 100 mg of ampicillin per liter and cultivated at 30°C. Isopropyl-β-d-thiogalactopyranoside (IPTG) was added to a final concentration of 0.4 mM when the absorbance at 600 nm reached 0.6, and cultivation was continued for an additional 2 h at 30°C. The cells grown in four 100-ml cultures were harvested, sonicated in buffer A (20 mM HEPES, pH 7.2), and subjected to centrifugation (20,000 × g, 30 min, 4°C). The obtained soluble protein fraction was loaded directly onto a HiLoad Q-Sepharose HP 16/10 column (Pharmacia) equilibrated with buffer A and was eluted with a linear gradient (250 ml) of 0 to 1.0 M NaCl (2.5 ml/min). The enoyl-CoA hydratase assay was done for each fraction (5 ml) with crotonyl-CoA used as a substrate, and the active fractions were combined, concentrated by ultrafiltration with a Centriplus 10 (Amicon), and desalted with a Sephadex G-25 column (Pharmacia). The purified enzyme was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (17). The N-terminal amino acid sequence of the enzyme was determined with a protein sequencer (model 610A; Perkin-Elmer) as instructed by the manufacturer.
Determination of molecular mass.
The molecular mass of the native enzyme was evaluated by gel filtration chromatography with a Superdex 75 HR 10/30 column (Pharmacia) in buffer A containing 0.15 M NaCl. The molecular mass of the subunit was determined by SDS-PAGE and matrix-assisted laser desorption ionization–time-of-flight mass spectrometry (MALDI-TOFMS).
RESULTS
Complementation analysis of A. caviae PHA-negative mutants.
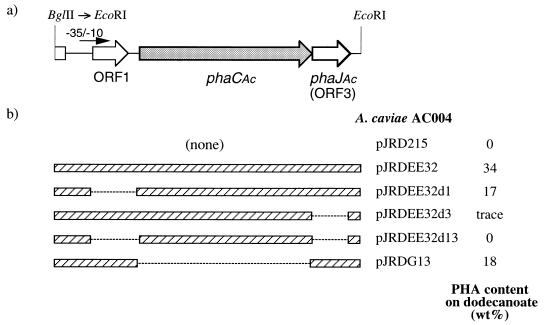
Chemical mutagenesis was carried out to generate PHA-negative mutants of A. caviae FA440, and five mutants incapable of accumulating PHA on an MPA agar plate containing palmitic acid were isolated after 104 colonies were screened. One such mutant, A. caviae AC004, was used for further analysis. The wild-type strain of A. caviae produced P(3HB-co-3HHx), up to 29 wt% of the dry cell weight from dodecanoate as a carbon substrate by two-step fermentation, whereas mutant AC004 could not synthesize any PHA under the same condition. As shown in Fig. 1b, the PHA− phenotype of AC004 was able to revert to PHA+ by introduction of pJRDEE32, harboring a 3.2-kbp fragment of A. caviae genomic DNA (ORF1, phaCAc, and ORF3) (11), indicating that AC004 contains a mutation within the 3.2-kbp region in its chromosome. Various deletion clones of pJRDEE32 were then introduced into AC004, and the ability of the recombinant strains to synthesize PHA was investigated to identify the mutation. The transconjugants of AC004 harboring pJRDEE32d3 or pJRDEE32d13 did not accumulate any PHA or accumulated only a trace amount. In contrast, both pJRDEE32d1 and pJRDG13, harboring ORF3, could complement the PHA-negative mutation of AC004. These results have revealed that AC004 does not lack the PHA synthase encoded by phaCAc but contains a mutation within the ORF3 region. This is clear evidence that ORF3 (phaJAc) is essential for PHA biosynthesis on alkanoic acids by A. caviae.
FIG. 1.
(a) Schematic drawing of a 3.2-kbp EcoRI fragment containing ORF1, phaCAc, and phaJAc (ORF3) with a putative promoter region from A. caviae FA440. (b) The ability of PJRDEE32 and its deleted clones to complement a PHA-negative mutant of A. caviae (AC004). PHA production was carried out on 1% dodecanoate by two-step fermentation, as described in the text.
Expression of phaJAc in E. coli.
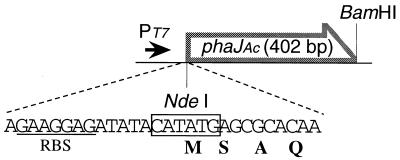
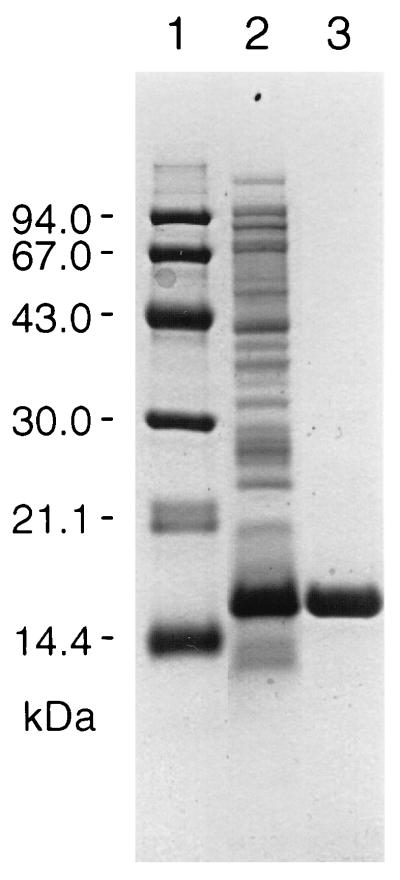
When phaJAc (ORF3) was expressed in E. coli DH5α together with phaCAc under the control of the native promoter, a higher enoyl-CoA hydratase activity was detected in the supernatant of the recombinant strain than in the control strain (11), suggesting that phaJAc is a gene encoding enoyl-CoA hydratase. For further investigation of the translated product of phaJAc, we constructed an expression plasmid, pETNB3, in which phaJAc is oriented in the T7 promoter and designed ribosome binding site of pET-3a (Fig. 2). E. coli BL21(DE3) was then transformed with pETNB3 and cultured until mid-log phase at 30°C. After the addition of 0.4 mM IPTG and further cultivation for 2 h, a very high enoyl-CoA hydratase activity (1.7 × 103 U/mg) was detected in the soluble protein fraction without formation of an inclusion body. This activity was more than 103-fold higher than that in strain DH5α carrying phaCAc and phaJAc under the control of the native promoter region. A protein of 15.5 kDa, which is in reasonable agreement with the approximate mass of the predicted phaJAc product (14.1 kDa), was observed in the soluble fraction from BL21(DE3)/pETNB3 as determined by SDS-PAGE analysis (Fig. 3, lane 2).
FIG. 2.
Construction of plasmid pETNB3 for overexpression of phaJAc in E. coli BL21(DE3). A designed ribosome binding site (RBS) from pET-3a is indicated (underlined). PT7, T7 promoter in pET-3a.
FIG. 3.
SDS-PAGE analysis of enoyl-CoA hydratase from E. coli BL21(DE3)/pETNB3. Lanes: 1, molecular mass standard proteins, with the masses indicated on the left (from top to bottom, phosphorylase b, bovine serum albumin, ovalbumin, carbonic anhydrase, and soybean trypsin inhibitor); 2, crude extract proteins of E. coli BL21(DE3)/pETNB3; 3, purified enoyl-CoA hydratase after chromatography on Q-Sepharose.
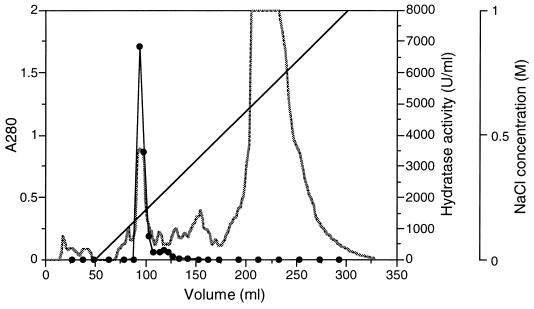
The soluble protein fraction prepared from the cells grown in four 100-ml cultures was loaded onto a Q-Sepharose column, and the enoyl-CoA hydratase activity was eluted from a linear gradient of NaCl (Fig. 4). SDS-PAGE analysis revealed that the combined active fraction was electrophoretically homogeneous (Fig. 3, lane 3). The hydratase activity could be recovered with a high yield (65% of total activity), and the purified enzyme showed a threefold-higher specific activity than the crude one, as given in Table 2. When this protein was subjected to Edman degradation, an N-terminal amino acid sequence (SAQSLEVGQKARLSKRFGAA) which corresponded to the amino acid sequence deduced from the nucleotide sequence of phaJAc (11) except for the initial Met residue was obtained.
FIG. 4.
Elution profile of enoyl-CoA hydratase from E. coli BL21(DE3)/pETNB3. The soluble protein fraction from the cells grown in four 100-ml cultures was applied to a Q-Sepharose column. ░⃞, absorbance at 280 nm; ——, concentration of NaCl; •, enoyl-CoA hydratase activity toward crotonyl-CoA.
TABLE 2.
Purification of enoyl-CoA hydratase from E. coli BL21(DE3)/pETNB3
| Purification step | Vol (ml) | Protein
|
Total activitya (U) | Sp acta (U/mg) | Yield (%) | Purification (fold) | |
|---|---|---|---|---|---|---|---|
| mg | mg/ml | ||||||
| None (crude enzyme) | 10 | 101 | 10.1 | 1.7 × 105 | 1.7 × 103 | ||
| Q-Sepharoseb | 5 | 21.6 | 4.3 | 1.1 × 105 | 5.1 × 103 | 65 | 3 |
Enoyl-CoA hydratase activity toward crotonyl-CoA (0.25 mM).
Enzyme was concentrated and desalted after elution from a Q-Sepharose column.
Characteristics of the phaJAc product.
The molecular mass of the purified enoyl-CoA hydratase was determined as 13,963 Da by MALDI-TOFMS analysis, and the obtained value was in good agreement with a value (13,954 Da) calculated from the deduced amino acid sequence in which the initial Met was ignored. The N-terminal Met was deleted probably due to posttranslational modification in E. coli cells. The native molecular mass of the hydratase was estimated as 31,000 Da by gel filtration chromatography, indicating the formation of a homodimer.
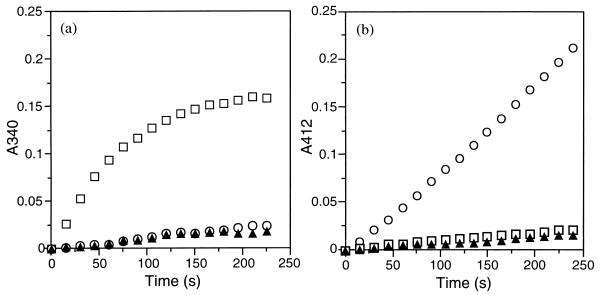
The stereospecificity of the enoyl-CoA hydratase encoded by phaJAc was evaluated by two procedures. One procedure is hydration of crotonyl-CoA by the purified hydratase coupled with oxidation of the resulting 3HB-CoA catalyzed by (S)-specific 3-hydroxyacyl-CoA dehydrogenase from porcine heart (Sigma). The formation of NADH linked with the dehydrogenation of (S)-3HB-CoA was followed by monitoring the absorbance at 340 nm (19). Almost no difference in the increase of the absorbance was observed between the reactions in which the phaJAc-derived hydratase was present and absent, while rapid formation of NADH was detected when crotonase [(S)-specific enoyl-CoA hydratase] from bovine liver (Sigma) was added instead of the hydratase from phaJAc (Fig. 5a). For the other procedure, a crude extract of A. eutrophus H16 (25) was added to the hydratase assay mixture as a crude PHA synthase solution together with 5,5′-dithio-bis(2-nitrobenzoic acid) (DTNB) (33). The release of CoA-SH linked with the polymerization of (R)-3HB-CoA to P(3HB) by the function of the PHA synthase could be measured as an increase in the absorbance at 410 nm with time in the presence of DTNB. As shown in Fig. 5b, release of CoA-SH depending on the phaJAc-derived hydratase and crotonyl-CoA was observed, in contrast to only the small change in absorbance caused by the addition of crotonase. From these results, it was evident that the enoyl-CoA hydratase translated from phaJAc has (R) specificity.
FIG. 5.
Evaluation of stereospecificity of enoyl-CoA hydratase encoded by phaJAc. (a) Hydration of crotonyl-CoA coupled with (S)-specific dehydrogenation of 3HB-CoA catalyzed by (S)-3HA-CoA dehydrogenase. The reaction mixture was composed of 0.25 mM crotonyl-CoA, 0.5 mM NAD+, 6 mU of (S)-3HA-CoA dehydrogenase, and 1 U of hydratase in 400 μl of 50 mM Tris-HCl (pH 8.0). (b) Hydration of crotonyl-CoA coupled with (R)-specific polymerization of 3HB-CoA catalyzed by crude PHA synthase. The reaction mixture was composed of 0.25 mM crotonyl-CoA, 10 mM DTNB, 1.2 mU of PHA synthase (a crude extract of A. eutrophus H16 containing 10 μg of protein), and 1 U of hydratase in 400 μl of 125 mM potassium phosphate (pH 7.2). The crude extract of A. eutrophus H16 was prepared from cells grown on fructose for 30 h at 30°C, as described previously (25). Symbols: ○, addition of phaJAc-derived enoyl-CoA hydratase; □, addition of crotonase [(S)-specific enoyl-CoA hydratase]; ▴, no addition of enoyl-CoA hydratase.
The apparent equilibrium constant Keq ([3HB-CoA]/[crotonyl-CoA]) for the reaction catalyzed by the phaJAc-derived hydratase was determined to be 2.2. The hydratase exhibited normal Michaelis-Menten kinetics, and Table 3 gives the Km and Vmax values for the hydratase-catalyzed reactions toward trans-2-enoyl-CoA with four, five, six, and eight carbon atoms. The Km values were within 29 to 50 μM and were nearly constant, independent of the chain length of the substrates. In contrast, the Vmax values decreased with increased carbon chain length of 2-enoyl-CoA. The Vmax for crotonyl-CoA was 6.2 × 103 U/mg, and the hydratase showed a still-high activity for 2-hexenoyl-CoA (Vmax = 1.8 × 103 U/mg). However, the value for 2-octenoyl-CoA was much lower than those for the shorter substrates. The (R)-specific enoyl-CoA hydratase encoded by phaJAc showed a high activity toward C4 to C6 2-enoyl-CoA, which is consistent with the composition of PHA produced by A. caviae from alkanoic acids with even numbers of carbon atoms or from plant oils (8).
TABLE 3.
Substrate specificity of enoyl-CoA hydratase encoded by phaJAc
| Substrate | Km (μM) | Vmax (U/mg) |
|---|---|---|
| Crotonyl-CoA | 29 | 6.2 × 103 |
| 2-Pentenoyl-CoA | 36 | 2.8 × 103 |
| 2-Hexenoyl-CoA | 34 | 1.8 × 103 |
| 2-Octenoyl-CoA | 50 | 2.5 |
DISCUSSION
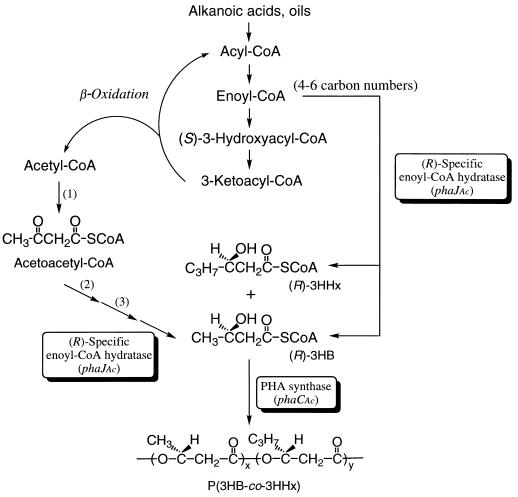
As the biosynthetic route to (R)-3HA-CoA from β-oxidation intermediates for PHA biosynthesis, three candidates have been proposed: (R)-specific hydration of 2-enoyl-CoA, (R)-specific reduction of 3-ketoacyl-CoA, or epimerization of (S)-3HA-CoA (6a, 9, 29). The results described here clearly indicate that (R)-specific enoyl-CoA hydratase encoded by phaJAc (ORF3) is involved in A. caviae PHA biosynthesis on alkanoic acids. The 2-enoyl-CoA intermediates with four to six carbon atoms derived from the β-oxidation of longer alkanoic acids can be converted to corresponding (R)-3HA-CoA by the hydratase. The resultant C4 to C6 (R)-3HA-CoA molecules are acceptable as substrates for PHA synthase of A. caviae encoded by phaCAc, and then they are polymerized to P(3HB-co-3HHx), as shown in Fig. 6. This is the first study to prove that the (R)-specific hydration of 2-enoyl-CoA is a channeling pathway for supplying (R)-3HA-CoA monomer units for PHA biosynthesis through the fatty acid β-oxidation pathway. The location of the gene encoding the (R)-hydratase within the pha locus is also a new finding.
FIG. 6.
Proposed pathway of P(3HB-co-3HHx) biosynthesis by A. caviae from alkanoic acids or oils. 1, β-ketothiolase; 2, NADH-acetoacetyl-CoA dehydrogenase; 3, crotonase [(S)-specific enoyl-CoA hydratase].
Rhodospirillum rubrum is known to synthesize PHA consisting of short- and medium-chain-length 3HA (C4 to C6), like A. caviae (3), and the presence of two stereospecific [(R)- and (S)-specific] enoyl-CoA hydratases in R. rubrum has been reported previously (19). As to their function in P(3HB) biosynthesis from acetate, it has been proposed that the (R)-3HB unit is supplied from two acetyl-CoA molecules via four-step reactions catalyzed by β-ketothiolase, NADH-acetoacetyl-CoA dehydrogenase, crotonase [(S)-specific enoyl-CoA hydratase], and (R)-specific enoyl-CoA hydratase. This pathway may function to supply the (R)-3HB unit from alkanoic acids in A. caviae together with the channeling route from the β-oxidation (Fig. 6), since a small fraction (3 to 5 mol%) of the 3HB unit was incorporated into copolyesters synthesized from alkanoic acids with odd numbers of carbon atoms (8). The activity toward 2-hexenoyl-CoA of the (R)-specific enoyl-CoA hydratase from R. rubrum was approximately one-third of the activity toward crotonyl-CoA (19), which is similar to the property of the enzyme encoded by phaJAc (Table 3). The (R)-specific hydratase of R. rubrum may also play an important role in PHA biosynthesis on alkanoic acids. However, further comparison of the properties of the (R)-specific hydratases of A. caviae and R. rubrum could not be made because of only a partial purification of the enzyme of R. rubrum. Methylobacterium rhodesianum, which is able to grow and to synthesize P(3HB) from methanol, has also been reported to possess two distinct crotonyl-CoA hydratases (20); however, the properties of the purified (R)-specific enzyme from M. rhodesianum (sigmoidal kinetics and a relatively high Km) are quite different from those of the phaJAc-derived hydratase. The participation of the (R)-hydratase in P(3HB) biosynthesis through the serine pathway in M. rhodesianum is still unclear.
PHA biosynthesis genes of A. caviae have been introduced into a PHA-negative mutant of A. eutrophus, and the ability of the transconjugants to synthesize PHA has been investigated (11). In some cases, the deletion of phaJAc resulted in an increase of the 3HHx fraction in P(3HB-co-3HHx) synthesized by the recombinant strains of A. eutrophus. This phenomenon was probably due to the higher hydration activity of the phaJAc product toward crotonyl-CoA than 2-hexenoyl-CoA. Expression of phaJAc in host cells may result in a higher concentration of (R)-3HB-CoA than of (R)-3HHx-CoA, which is reflected in the lower 3HHx unit content in the PHA produced. This result may suggest the possibility of changing the 3HHx fraction in bacterial polyesters by controlled expression of phaJAc.
The phaJAc product has been suggested to have another function in the accumulation of PHA granules in cells, and ORF1 of A. caviae located upstream of phaCAc may also take part in PHA biosynthesis and accumulation (11). Further study will be done to elucidate the characteristics and functions of the genes surrounding phaCAc.
ACKNOWLEDGMENT
This work was supported by CREST (Core Research for Evolutional Science and Technology) of Japan Science and Technology Corporation (JST).
REFERENCES
- 1.Anderson A J, Dawes E A. Occurrence, metabolic role, and industrial uses of bacterial polyhydroxyalkanoates. Microbiol Rev. 1990;54:450–472. doi: 10.1128/mr.54.4.450-472.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 3.Brandl H, Knee E J, Fuller R C, Gross R A, Lenz R W. Ability of the phototrophic bacterium Rhodospirillum rubrumto produce various poly(β-hydroxyalkanoates): potential source for biodegradable polyesters. Int J Biol Macromol. 1989;11:49–55. doi: 10.1016/0141-8130(89)90040-8. [DOI] [PubMed] [Google Scholar]
- 4.Brandl H, Gross R A, Lenz R W, Fuller R C. Pseudomonas oleovoransas a source of poly(3-hydroxyalkanoates) for potential application as biodegradable polyesters. Appl Environ Microbiol. 1988;54:1977–1982. doi: 10.1128/aem.54.8.1977-1982.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davison J, Heusterspreute M, Chevalier N, Ha-Thi V, Brunel F. Vectors with restriction site banks. pJRD215, a wide-host-range cosmid vector with multiple cloning sites. Gene. 1987;51:275–280. doi: 10.1016/0378-1119(87)90316-7. [DOI] [PubMed] [Google Scholar]
- 6.Deng W P, Nickoloff J A. Site-directed mutagenesis of virtually any plasmid by eliminating a unique site. Anal Biochem. 1992;200:81–88. doi: 10.1016/0003-2697(92)90280-k. [DOI] [PubMed] [Google Scholar]
- 6a.de Waard P, van der Wal H, Huijberts G N M, Eggink G. Heteronuclear NMR analysis of unsaturated fatty acids in poly(3-hydroxyalkanoates) J Biol Chem. 1993;268:315–319. [PubMed] [Google Scholar]
- 7.Doi Y. Microbial polyesters. New York, N.Y: VCH Publishers; 1990. [Google Scholar]
- 8.Doi Y, Kitamura S, Abe H. Microbial synthesis and characterization of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) Macromolecules. 1995;28:4822–4828. [Google Scholar]
- 9.Eggink G, de Waard P, Huijberts G N M. The role of fatty acid biosynthesis and degradation in the supply of substrates for poly(3-hydroxyalkanoate) formation in Pseudomonas putida. FEMS Microbiol Rev. 1992;103:159–164. [Google Scholar]
- 10.Friedrich B, Hogrefe C, Schlegel H G. Naturally occurring genetic transfer of hydrogen-oxidizing ability between strains of Alcaligenes eutrophus. J Bacteriol. 1981;147:198–205. doi: 10.1128/jb.147.1.198-205.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fukui T, Doi Y. Cloning and analysis of the poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) biosynthesis genes of Aeromonas caviae. J Bacteriol. 1997;179:4821–4830. doi: 10.1128/jb.179.15.4821-4830.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gross R A, DeMello C, Lenz R W, Brandl H, Fuller R C. Biosynthesis and characterization of poly(β-hydroxyalkanoates) produced by Pseudomonas oleovorans. Macromolecules. 1989;22:1106–1115. [Google Scholar]
- 13.Huijberts G N M, Eggink G, de Waard P, Huisman G W, Witholt B. Pseudomonas putidaKT2442 cultivated on glucose accumulates poly(3-hydroxyalkanoates) consisting of saturated and unsaturated monomers. Appl Environ Microbiol. 1992;58:536–544. doi: 10.1128/aem.58.2.536-544.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huisman G W, Leeuw O, Eggink G, Witholt B. Synthesis of poly-3-hydroxyalkanoates is a common feature of fluorescent pseudomonads. Appl Environ Microbiol. 1989;55:1949–1954. doi: 10.1128/aem.55.8.1949-1954.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kato M, Bao H J, Kang C K, Fukui T, Doi Y. Production of a novel copolyester of 3-hydroxybutyric acids and medium-chain-length 3-hydroxyalkanoic acid by Pseudomonassp. 61-3 from sugars. Appl Microbiol Biotechnol. 1996;45:363–370. [Google Scholar]
- 16.Kato M, Fukui T, Doi Y. Biosynthesis of polyester blends by Pseudomonassp. 61-3 from alkanoic acids. Bull Chem Soc Jpn. 1996;69:515–520. [Google Scholar]
- 17.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 18.Leaf T A, Peterson M S, Stoup S K, Somers D, Srienc F. Saccharomyces cerevisiaeexpressing bacterial PHB synthase produces poly-3-hydroxybutyrate. Microbiology (Reading) 1996;142:1169–1180. doi: 10.1099/13500872-142-5-1169. [DOI] [PubMed] [Google Scholar]
- 19.Moskowitz G J, Merrick J M. Metabolism of poly-β-hydroxybutyrate. Enzymatic synthesis of d-(−)-β-hydroxybutyryl coenzyme A by an enoyl hydrase from Rhodospirillum rubrum. Biochemistry. 1969;8:2748–2755. doi: 10.1021/bi00835a009. [DOI] [PubMed] [Google Scholar]
- 20.Mothes G, Babel W. Methylobacterium rhodesianumMB 126 possesses two stereospecific crotonyl-CoA hydratases. Can J Microbiol. 1995;41:68–72. [Google Scholar]
- 21.Peoples O P, Sinskey A J. Poly-β-hydroxybutyrate biosynthesis in Alcaligenes eutrophus H16. Identification and characterization of the PHB polymerase gene (phbC) J Biol Chem. 1989;264:15298–15303. [PubMed] [Google Scholar]
- 22.Poirier Y, Nawrath C, Somerville C. Production of polyhydroxyalkanoates, a family of biodegradable plastics and elastomers, in bacteria and plants. Bio/Technology. 1995;13:142–150. doi: 10.1038/nbt0295-142. [DOI] [PubMed] [Google Scholar]
- 23.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 24.Schlegel H G, Lafferty R, Krauss I. The isolation of mutants not accumulating poly-β-hydroxybutyric acid. Arch Mikrobiol. 1970;71:283–294. doi: 10.1007/BF00410161. [DOI] [PubMed] [Google Scholar]
- 25.Schubert P, Steinbüchel A, Schlegel H G. Cloning of the Alcaligenes eutrophus poly-β-hydroxybutyrate synthetic pathway and synthesis of PHB in Escherichia coli. J Bacteriol. 1988;170:5837–5847. doi: 10.1128/jb.170.12.5837-5847.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shimamura E, Kasuya K, Kobayashi G, Shiotani T, Shima Y, Doi Y. Physical properties and biodegradability of microbial poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) Macromolecules. 1994;27:878–880. [Google Scholar]
- 27.Simon R, Priefer U, Pühler A. A broad host range mobilization system for in vivo genetic engineering. Transposon mutagenesis in gram negative bacteria. Bio/Technology. 1983;1:784–791. [Google Scholar]
- 28.Slater S C, Voige W H, Dennis D E. Cloning and expression in Escherichia coli of the Alcaligenes eutrophusH16 poly-β-hydroxybutyrate biosynthetic pathway. J Bacteriol. 1988;170:4431–4436. doi: 10.1128/jb.170.10.4431-4436.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steinbüchel A. PHB and other polyhydroxyalkanoic acids. In: Rehm H J, Reed G, editors. Biotechnology. Weinheim, Germany: VCH Publishers; 1996. pp. 403–464. [Google Scholar]
- 30.Steinbüchel A, Hustede E, Liebergesell M, Pieper U, Timm A, Valentin H. Molecular basis for biosynthesis and accumulation of polyhydroxyalkanoic acids in bacteria. FEMS Microbiol Rev. 1992;103:217–230. doi: 10.1111/j.1574-6968.1992.tb05841.x. [DOI] [PubMed] [Google Scholar]
- 31.Stern J R, Del Campillo A, Raw I. Enzymes of fatty acid metabolism. I. General introduction; crystalline crotonase. J Biol Chem. 1955;218:971–983. [PubMed] [Google Scholar]
- 32.Timm A, Steinbüchel A. Formation of polyesters consisting of medium-chain-length 3-hydroxyalkanoic acids from gluconate by Pseudomonas aeruginosaand other fluorescent pseudomonads. Appl Environ Microbiol. 1990;56:3360–3367. doi: 10.1128/aem.56.11.3360-3367.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Valentin H E, Steinbüchel A. Application of enzymatically synthesized short-chain-length hydroxy fatty acid coenzyme A thioesters for assay of polyhydroxyalkanoic acid synthases. Appl Microbiol Biotechnol. 1994;40:699–709. [Google Scholar]
- 34.Williams M D, Rahn J A, Sherman D H. Production of a polyhydroxyalkanoate biopolymer in insect cells with a modified eucaryotic fatty acid synthase. Appl Environ Microbiol. 1996;62:2540–2546. doi: 10.1128/aem.62.7.2540-2546.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]