Abstract
Anesthesia with isoflurane prior to carbon dioxide euthanasia is recommended as a refinement, but vaporizer access can be limited. An alternative to vaporizers is the ‘drop’ method, introducing a fixed volume of isoflurane into the induction chamber. Previous work suggests that isoflurane administered at a concentration of 5% via the drop method is effective but aversive to mice; lower concentrations have not been tested. We assessed mouse behavior and insensibility with induction using the drop method for isoflurane concentrations below 5%. Male Crl:CD-1 (ICR) mice (n = 27) were randomly allocated to one of three isoflurane concentrations: 1.7%, 2.7%, and 3.7%. During induction, measures of insensibility and stress-related behaviors were recorded. All mice reached a surgical plane of anesthesia, and mice exposed to higher concentrations did so more quickly; as concentrations increased from 1.7 to 2.7 and 3.7%, the time to recumbency (Least squares means ± SE: 120.5 s ± 8.1, 97.9 s ± 8.1, and 82.8 s ± 8.1, respectively), loss of righting reflex (149.1 s ± 8.5, 127.7 s ± 8.5, and 100.7 s ± 8.5, respectively), and loss of pedal withdrawal reflex (214.5 s ± 8.3, 172.2 s ± 8.3, and 146.4 s ± 8.3, respectively) all declined. Rearing was the most frequently performed stress-related behavior, and was most pronounced immediately following isoflurane administration for all treatments. Our results indicate that the drop method can be used to effectively anesthetize mice with isoflurane concentrations as low as 1.7%; future work should assess mouse aversion.
Keywords: Euthanasia, induction, anesthetic, rodent, refinement
Anesthésie par isoflurane de souris à l'aide de la méthode «open drop» Résumé
L’anesthésie à l’isoflurane avant l'euthanasie au dioxyde de carbone est recommandée comme méthode de raffinement, mais l'accès au vaporisateur peut être limité. La méthode «open drop», qui introduit un volume fixe d'isoflurane dans la chambre d'induction, constitue une alternative aux vaporisateurs. Des travaux antérieurs suggèrent que l'isoflurane administré à une concentration de 5% par la méthode «open drop» est efficace mais aversif pour les souris; des concentrations plus faibles n'ont pas été testées. Nous avons évalué le comportement et l'insensibilité de la souris par induction en utilisant la méthode «open drop» à des concentrations d'isoflurane inférieures à 5%. Des souris mâles Crl:CD-1 (ICR) (n=27) ont été affectées au hasard à l'une des trois concentrations d'isoflurane suivantes: 1,7%, 2,7% et 3,7%. Au cours de l'induction, des mesures de l'insensibilité et des comportements liés au stress ont été enregistrées. Toutes les souris ont atteint un plan chirurgical d'anesthésie, les souris exposées à des concentrations plus élevées l'ayant fait plus rapidement; au fur et à mesure que les concentrations augmentaient de 1,7 à 2,7 et à 3,7%, le temps de la position allongée (moyenne des moindres carrés ± ET: 120,5 s ± 8,1, 97,9 s ± 8,1 et 82,8 s ± 8,1, respectivement), la perte du réflexe de redressement (149,1 s ± 8,5, 127,7 s ± 8,5 et 100,7 s ± 8,5, respectivement) et la perte du réflexe de retrait (214,5 s ± 8,3, 172,2 s ± 8,3, et 146,4 s ± 8,3, respectivement) ont tous diminué. Le recul était le comportement lié au stress le plus fréquent et il était le plus prononcé immédiatement après l'administration d'isoflurane pour tous les traitements. Nos résultats indiquent que la méthode de la goutte peut être utilisée pour anesthésier efficacement des souris avec des concentrations d'isoflurane aussi faibles que 1,7%; des travaux futurs devraient évaluer l'aversion de la souris.
Isofluran-Narkose bei Mäusen mittels Tropfenmethode Abstract
Die Anästhesie mit Isofluran vor der Euthanasie mit Kohlendioxid wird als Refinement empfohlen, allerdings kann der Zugang zu Verdampfern eingeschränkt sein. Eine Alternative zu Verdampfern ist die "Tropfenmethode", bei der ein festgelegtes Volumen Isofluran in die Induktionskammer gegeben wird. Frühere Arbeiten deuten darauf hin, dass Isofluran, das in einer Konzentration von 5% über die Tropfenmethode verabreicht wird, wirksam, aber für Mäuse aversiv ist; niedrigere Konzentrationen wurden nicht getestet. Wir haben das Verhalten und die Empfindungsfähigkeit von Mäusen bei der Induktion mit der Tropfenmethode bei Isofluran-Konzentrationen unter 5% untersucht. Männliche Crl:CD-1 (ICR)-Mäuse (n = 27) wurden nach dem Zufallsprinzip einer von drei Isofluran-Konzentrationen zugewiesen: 1,7%, 2,7% und 3,7%. Während der Induktion wurden die Empfindungsfähigkeit und stressbedingte Verhaltensweisen erfasst. Alle Mäuse erreichten eine chirurgische Ebene der Anästhesie, und bei Mäusen, die höheren Konzentrationen ausgesetzt waren, erfolgte dies schneller. Beim Anstieg der Konzentrationen von 1,7 auf 2,7 und 3,7% verringerte sich die Zeit bis zur Liegeposition (LS-Mittelwerte ± SE: 120,5 s ± 8,1, 97,9 s ± 8. 1 bzw. 82,8 s ± 8,1), der Verlust des Aufrichtungsreflexes (149,1 s ± 8,5, 127,7 s ± 8,5 bzw. 100,7 s ± 8,5) und der Verlust des Rückzugsreflexes (214,5 s ± 8,3, 172,2 s ± 8,3 bzw. 146,4 s ± 8,3) sanken durchweg. Aufrichten war das am häufigsten beobachtete stressbedingte Verhalten und war bei sämtlichen Behandlungen unmittelbar nach der Verabreichung von Isofluran am stärksten ausgeprägt. Unsere Ergebnisse legen nahe, dass die Tropfenmethode zur wirksamen Betäubung von Mäusen mit Isofluran-Konzentrationen von nur 1,7% verwendet werden kann. Künftige Forschung sollte sich auf die Aversion der Mäuse richten.
Anestesia con isoflurano en ratones mediante el método de la gota Resumen
La anestesia con isoflurano antes de la eutanasia con dióxido de carbono es recomendada como refinamiento, pero el acceso al vaporizador puede ser limitado. Una alternativa a los vaporizadores es el método de la "gota", que introduce un volumen fijo de isoflurano en la cámara de inducción. Estudios anteriores sugieren que el isoflurano administrado a una concentración del 5% mediante el método de la gota es eficaz pero aversivo para los ratones; no han podido probarse concentraciones inferiores. Evaluamos el comportamiento de los ratones y su insensibilidad con la inducción utilizando el método de la gota para concentraciones de isoflurano inferiores al 5%. Los ratones machos Crl:CD-1 (ICR) (n = 27) fueron asignados aleatoriamente a una de las tres concentraciones de isoflurano: 1,7%, 2,7%, y 3,7%. Durante la inducción, se registraron medidas de insensibilidad y comportamientos relacionados con el estrés. Todos los ratones alcanzaron un plano quirúrgico de anestesia, haciéndolo más rápidamente los ratones expuestos a concentraciones más elevadas; a medida que las concentraciones aumentaban de 1,7 a 2,7 y 3,7%, el tiempo hasta el decúbito (medias LS ± SE: 120.5 s ± 8,1, 97,9 s ± 8,1 y 82,8 s ± 8,1, respectivamente), la pérdida del reflejo de giro a la derecha (149,1 s ± 8,5, 127,7 s ± 8,5 y 100,7 s ± 8,5, respectivamente) y la pérdida del reflejo de retirada del pedal (214,5 s ± 8,3, 172,2 s ± 8,3 y 146,4 s ± 8,3, respectivamente) disminuyeron. La cría fue el comportamiento relacionado con el estrés más frecuentemente realizado y fue más pronunciado inmediatamente después de la administración de isoflurano para todos los tratamientos. Nuestros resultados indican que el método de la gota puede utilizarse para anestesiar eficazmente a ratones con concentraciones de isoflurano tan bajas como el 1,7%; los trabajos futuros deberán evaluar la aversión de los ratones.
Introduction
Isoflurane prior to euthanasia is considered by some as a refinement over conscious exposure to CO21–6 and is a preferred inhalation anesthetic among animal users owing to the rapid onset of anesthesia.2,3,7
Isoflurane is typically administered with a vaporizer. Vaporizers are designed to safely anesthetize patients, slowly introducing the agent, typically in a hyperoxic environment. Vaporizers allow for precise control of gas flow and thus anesthetic depth, but these features are unnecessary (and perhaps counterproductive) when the intention is to kill the animal. The use of oxygen as a carrier gas may be detrimental to the animal’s experience during the euthanasia procedure by prolonging time to loss of consciousness. 8 Moreover, access to a vaporizer can be a practical and economic barrier. When surveyed, two thirds of laboratory animal users responded that access to equipment was a barrier to refining CO2 euthanasia methods. 9
An alternative way to administer isoflurane is the “drop method”, by which liquid anesthetic is diffused directly into an induction box. This method relies upon the rapid vaporization of the volatile liquid, increasing anesthetic concentration more rapidly than is possible using a vaporizer. 10 Field researchers have used the drop method to anesthetize wildlife for many years (e.g. Potvin et al., 11 Parker et al. 12 ). The drop method can induce anesthesia in laboratory mice, 13 but the ideal concentration for induction has not been determined. Past work suggests that rapid induction with the drop method (to achieve a concentration of 5% isoflurane) can be aversive to mice; when isoflurane was administered in this way in the dark compartment of a light–dark box, mice withdrew more quickly than when exposed to the same concentration from a vaporizer. 14 Anesthetic concentrations ranging from 1.7% to 3.7% are thought to reduce aversion, but have only been assessed using a vaporizer. 15
The present study aimed to assess mouse behavior and loss of consciousness in response to isoflurane exposure using the drop method, testing how these measures changed in response to varying isoflurane concentrations within this lower range. Three progressive measures of insensibility (onset of recumbency, loss of righting reflex, and loss of pedal withdrawal reflex) were used to assess the depth of anesthesia in adult mice. We also assessed defecation, rearing, grooming, and freezing, as these are sometimes considered indicators of anxiety in mice. 16 We hypothesized that lower concentrations of isoflurane would slow the onset of anesthesia and decrease behaviors associated with fear and distress.
Methods
All animal procedures were approved by the University of British Columbia’s Animal Care Committee (protocol A21-0227).
Animals and housing
We used 27 male Crl:CD-1 (ICR) mice (Charles River Laboratories, Raleigh, USA) designated as surplus after being involved in an unrelated, non-invasive study. Our sample size (n = 9 per treatment) was based on that used in previous studies assessing anesthesia in rodents (n = 8–13).12,13,15 We used males as these were available to us as surplus animals. At the time of testing, mice were 18 weeks old and were housed in trios in ventilated rat cages measuring 39.4 cm long, 30.0 cm wide, and 19.4 cm high (Allentown, LLC, New Jersey, USA). Cages contained aspen chip bedding (Jamieson’s Pet Food Distributors Ltd, Delta, Canada), nesting material (Enviropak nesting material, Datesand, Stockport, UK; cotton nestlet, Ancare, Bellmore, USA), and three polycarbonate huts (Bio-Serv, New Jersey, USA). Mice were provided ad libitum food (Lab Diet Rodent Chow 2918) and reverse osmosis tap water. Cages were changed every two weeks. The animal room was kept on a 12 h cycle, with lights off at 09:00 h. Mice were housed at a (mean ± SD) room temperature of 21.4 ± 0.06°C and relative humidity of 52.0 ± 0.01%.
Experimental design and apparatus
One mouse from each cage was tested at each of the three treatment concentrations: 1.7%, 2.7%, and 3.7% isoflurane. 15 Treatment order within each cage was assigned in advance using a Latin-square design. Upon opening the home cage before transport to the procedure room, the mouse closest to the handler was selected for the current trial. The volume of isoflurane injected into the induction apparatus was calculated using the ideal gas law (PV = nRT; P as pressure, V as volume, n as number of moles of isoflurane, R (8.314) as the universal gas constant, and T as temperature) with 21°C as the room temperature, resulting in volumes of isoflurane of 0.45, 0.70, and 1.00 ml.
A transparent plastic container (12.7 cm long, 12.7 cm wide, and 22.7 cm high; Snaplock, Montreal, Canada) was used as the test apparatus (Figure 1). A hole was cut into one side and a rubber glove sealed into the opening. This allowed an experimenter’s hand to be placed into the box to monitor level of anesthesia without opening the lid. 14 Accounting for the space filled by the gloved hand, the total volume of the container was 3.5 L. A small hole was drilled in the container lid to allow for the insertion of the tip of a 1 ml syringe. Directly below, a piece of plastic mesh was secured against the underside of the lid with an open slot to slide in a compressed cotton pad (Quo Beauty, Toronto, Canada) to absorb the isoflurane dispensed from the syringe.
Figure 1.
Diagram of the experimental apparatus, depicting the induction box with an attached glove. The cotton pad and plastic mesh are located directly below a 1 ml syringe.
Experimental procedure
Habituation and test trials took place in a dark procedure room lit by a red light. All trials took place within a biological safety cabinet. Mice were habituated to the procedure room and testing apparatus in a 5-min habituation session one day before the experiment. During the habituation session, mice were transported individually to the procedure room in a transfer box and then placed into the induction apparatus. Mice were transferred between cages using a tunnel or their overturned hut. The lid of the apparatus was secured and an experimenter placed their hand inside the glove of the induction box, forming a fist and remaining motionless throughout the session. Following habituation, each mouse was placed into a holding cage inside of the animal housing room until all mice from the same home cage had undergone the procedure.
During test sessions, each mouse was transported to the procedure room one at a time. The apparatus was cleaned with 70% isopropyl alcohol between trials and a fresh cotton pad was placed into the mesh plastic on the apparatus lid. Mice were handled using a tunnel and placed into the prepared apparatus before the experimenter secured the lid and placed one hand into the apparatus glove. Mice were recorded in the apparatus for 2 min before a syringe with the pre-measured quantity of isoflurane was inserted in the apparatus lid and injected onto the cotton pad. The experimenter was blind to the isoflurane volume administered. Their hand remained in a fist until the mouse became recumbent. Following the onset of recumbency, loss of righting reflex was assessed by gently rolling the mouse onto his back. Response to this assessment, including the ability to self-right and leg paddling, resulted in the experimenter waiting 5 s before re-assessing loss of righting reflex. 16 Once unable to self-right, a toe pinch was performed on alternating hind paws four consecutive times, with 10 s between each pinch. If any response was observed, alternating toe pinches were continued until there was no response to four consecutive pinches, at which point the trial ended. As euthanasia was the intended endpoint, the mouse was then immediately transferred to a chamber pre-filled with 80% CO2 for a minimum of 5 min, following UBC guidelines. 17 Following this period, the mouse was removed from the induction chamber and cervical dislocation was performed as a secondary method of euthanasia.
All trials were video recorded (Canon VIXIA HF W10, Tokyo, Japan). Video observations were scored for rearing, freezing, grooming, and defecation, ataxia, onset of recumbency, loss of righting reflex, and loss of the pedal withdrawal reflex (Table 114,18). The observer was blinded to treatment and mouse identity. Interobserver reliability was calculated using a Pearson correlation from 30% of the videos scored by a second blinded observer. For stress-related behaviors (rearing, freezing, grooming, and defecation), r values ranged from 0.81 to 0.94. For the signs of insensibility, r values varied from 0.80 for ataxia, to 0.87 for recumbency, to 0.99 for both loss of righting reflex and loss of pedal withdrawal reflex.
Table 1.
Ethogram of behaviors scored following isoflurane administration (adapted from Moody and Weary, 14 Niel and Weary 18 ).
| Parameter | Definition |
|---|---|
| Rearing | Mouse raises his upper body while standing on hind paws. May include paw contact with the walls and lid of the container. |
| Freezing | Mouse is immobile and rigid or appears to be ‘frozen’. |
| Grooming | Mouse is licking or scratching himself. |
| Defecation | Mouse drops a single fecal bolus. |
| Ataxia | Loss of motor function or balance; mouse is stumbling or leaning on the walls of the induction box or gloved hand for support. |
| Recumbency | Mouse is lying prone with his head resting on the floor. Head and body are motionless and there is a loss of muscle tone for 5 s. |
| Loss of righting reflex | Mouse is unable to self right when placed on his back. Leg paddling or other attempts to roll over are absent. |
| Loss of pedal withdrawal reflex | The first of three consecutive non-responses to alternating hind toe pinches. |
Statistical analysis
Statistical analyses were carried out using SAS OnDemand for Academics (version 9.4, SAS Institute Inc.). An identical linear mixed model was used to test the effect of increasing isoflurane concentration on times to ataxia, recumbency, loss of righting reflex, and loss of pedal withdrawal reflex following administration of isoflurane. All models specified cage as a random factor. Plots were generated in R Studio (R version 4.0.1) using the ggplot2 package. Residual distributions were visually scrutinized for each analyzed behavior. One outlier at the 1.7% isoflurane concentration was identified for time to recumbency; this value was double checked and retained in analysis to display individual variation within treatments. Stress-related behaviors were scored as frequencies and binned in 15 s intervals for data visualization.
On average, mice started to become ataxic after 30 s of isoflurane exposure, and the first mouse to become recumbent did so a little more than 30 s after onset of isoflurane exposure. As this may have influenced their ability to express stress-related behaviors, we only statistically compared 30 s of baseline behaviors with the first 30 s after isoflurane exposure. Grooming, freezing, and defecation occurred too infrequently for meaningful analysis but are reported in the Supplementary Materials online (Table S1).
Results
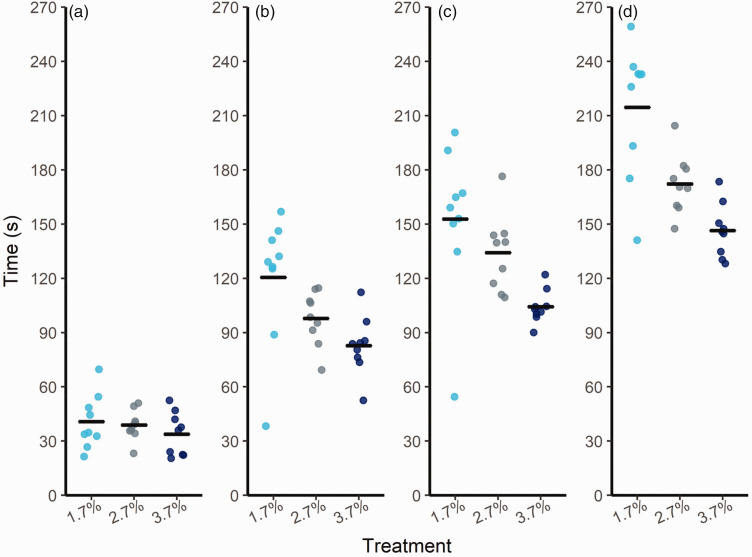
All mice reached a surgical plane of anesthesia following administration of isoflurane. Within treatment, mice showed individual variation in time taken to show signs of insensibility, particularly at the 1.7% concentration (Figure 2). Despite this variation within treatment, higher concentrations of isoflurane resulted in shorter times taken to reach recumbency, loss of righting reflex, and loss of pedal withdrawal reflex. As isoflurane concentration increased from 1.7% to 3.7%, the average time to reach recumbency decreased (120 s, 98 s, 83 s, respectively; F2,17 = 11.1, p < 0.01), time to reach loss of righting reflex decreased (149 s, 128 s, 101 s, respectively; F2,17 = 16.76, p = <0.001), and pedal withdrawal reflex decreased (215 s, 172 s, 146 s, respectively; F2,17 = 34.02, p = <0.0001). There was no effect of treatment on time to ataxia (F2,17 = 2.76, p = 0.12).
Figure 2.
Time (s) taken to reach (a) ataxia, (b) recumbency (p < 0.01), (c) loss of righting reflex (p = <0.001), and (d) loss of pedal withdrawal reflex (p = <0.0001) after administration of isoflurane. Points illustrate the results of individual mice (n = 9 per treatment) and black bars show the treatment mean.
Behavioral indicators of stress (rearing, freezing, grooming, and defecation) were observed following isoflurane administration. All behaviors were performed by mice in each treatment, except for freezing in the 3.7% treatment and grooming in the 2.7% treatment. The only behavior expressed with enough frequency to allow for meaningful analysis was rearing (Figure 3). Across treatments the number of rears in the 30 s before induction averaged 8.0 ± 0.9; this value doubled (to 16.1 ± 0.9 rears) in the 30 s after the isoflurane was introduced into the chamber (F1,40 = 37.24, p < 0.001), with no effect of treatment or interaction between phase and treatment.
Figure 3.
Treatment means ± SE (n = 9 mice per treatment) for rates of rearing (/15 s) following isoflurane administration. Time 0 shows average baseline values in the 30 s before administration.
Discussion
This study is the first to assess mouse anesthesia with isoflurane using the drop method at concentrations below 5%. All mice reached a surgical plane of anesthesia, and thus induction was more rapid at higher isoflurane concentrations. On average, mice exposed to a concentration of 3.7% became recumbent at about 80 s; this value is comparable to the induction time (60–90 s) achieved with the use of a vaporizer administering isoflurane at a concentration of 5%. 8
We found considerable variation within treatment in the times to reach all measures of insensibility, such as an exceptionally fast onset of recumbency (40 s) in one mouse in the 1.7% isoflurane treatment. Variation in sensitivity to volatile anesthetics is known in humans to be related to genetic differences, 19 a result that is consistent with known strain differences in the anesthetic potency of isoflurane. 20 The use of outbred (ICR) mice in the current study may have increased the variability in reactions to isoflurane. Further, male mice can show increased sensitivity to isoflurane. 21 As the present study assessed only one strain and sex with remaining questions about the mouse’s experience during exposure, we cannot yet recommend this technique as a refinement over the use of a vaporizer. However, the current results demonstrate that this technique is effective at achieving a surgical plane of anesthesia using small quantities of isoflurane, and may be appropriate for use prior to euthanasia. We encourage future work to investigate strain and sex differences in these results.
We had expected that lower concentrations of isoflurane would decrease stress-related behaviors, but freezing, grooming, and defecation occurred too infrequently to draw inferences. When mice were moved into the induction box, some reacted in a way that may indicate they were stressed, as evidenced by their baseline behavior (Figure 3). We gave the mice one trial of habituation to the apparatus, but additional exposures may have reduced their reaction to the novelty of this environment. Compared with the 30 s prior to isoflurane exposure, rearing doubled during the 30 s after exposure. Rearing persisted for a longer period when mice were exposed to lower isoflurane concentrations, consistent with the slower onset of anesthesia. On the basis of the area under the curve of these response profiles, one might conclude that higher concentrations are preferable, but we suggest caution in adopting this conclusion. Previous work using the light–dark paradigm found that induction with isoflurane at a concentration of 5% using the drop method was aversive to mice, 14 and increased concentration and rate of administration of isoflurane can heighten aversion through irritation (e.g. of the airway). 22
While there is some evidence that rearing can be an anxiety-related behavior, 23 it could also be linked to exploration, an excitatory response to isoflurane, 24 escape attempts, or attempts to breathe in fresh air as the isoflurane settled at the bottom of the induction box; therefore we cannot draw strong conclusions about affective states based upon this behavior. To better understand the welfare impacts of isoflurane induction using the drop method, future work should assess aversion using aversion-avoidance, approach-avoidance, 25 or conditioned place aversion tests. 26
Several considerations should be made before adopting this technique in future studies, such as the health and safety of personnel and suitability for recovery procedures. This protocol likely carries the same level of risk as refilling a vaporizer with isoflurane. To minimize human exposure, work should be conducted in a biological safety cabinet with appropriate backdraft and scavenging of waste gases, and the syringe and cotton pad must be properly disposed of. We are not recommending this method over the use of a vaporizer; rather, the drop method may reduce barriers to anesthetic use (e.g. in facilities that have not purchased a vaporizer, or in wildlife field work where this equipment is unavailable). Further, this method of anesthetic exposure is likely only appropriate in circumstances where the animal is going to be killed. A vaporizer is ideal for recovery procedures where the concentration of anesthetic needs to be precisely controlled and the goal is for the animal to survive the procedure. Isoflurane administration via the drop method can result in peak volatile concentrations of almost twice that of a vaporizer. 13 In the present study we lacked the necessary equipment to analyze changes in gas concentration during induction; this is a limitation of the study. The drop method relies upon a sealed chamber; over a prolonged period we would expect changes within the chamber in O2, CO2, temperature, and humidity, all likely to be aversive to the animals. In the current study animals were rapidly anesthetized, but future work should monitor these changes within the chamber.
The present study has the potential to refine current rodent euthanasia methods, particularly in labs that lack access to anesthetic equipment. Our results show that mice can be anesthetized with isoflurane via the drop method using concentrations as low as 1.7%; lower concentrations may be less aversive to rodents than a concentration of 5%. 14 At all concentrations tested, mice reared more after isoflurane was introduced to the cage; this result may indicate that mice found exposure aversive, but additional research is needed to assess the welfare impacts of these lower concentrations.
Conclusions
All mice in the present study reached a surgical plane of anesthesia at concentrations of 1.7%, 2.7%, and 3.7% isoflurane administered via the drop method. Measures of insensibility were reached more quickly at higher concentrations. Future studies should assess aversion to this induction method at concentrations below 5%.
Supplemental Material
Supplemental material, sj-pdf-1-lan-10.1177_00236772231169550 for Mouse isoflurane anesthesia using the drop method by Maya J Bodnar, Anna S Ratuski and Daniel M Weary in Laboratory Animals
Acknowledgements
We would like to thank Dr. Bernard MacLeod and Dr. Cathy Schuppli for their helpful input when planning this experiment. Thanks also to Dave Mazaraki and Alexi Thompson for technical assistance, and to our facility technicians for their assistance with animal care.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Sciences and Engineering Research Council of Canada (NSERC) (Discovery Grant RGPIN-2016-0462). ASR was supported by an NSERC Doctoral award and MJB was supported by an Undergraduate Student Research Award from NSERC.
Data availability
All data and code used for the statistical analysis are available on the UBC dataverse (https://doi.org/10.5683/SP3/OEC7QY).
References
- 1.Canadian Council on Animal Care. CCAC revised guidance on euthanasia using carbon dioxide, https://ccac.ca/Documents/Standards/Guidelines/CCAC_Revised_Guidance_on_Euthanasia_Using_Carbon_Dioxide.pdf (2020, accessed 7 October 2022).
- 2.Leach MC, Bowell VA, Allan TF, et al. Degrees of aversion shown by rats and mice to different concentrations of inhalational anaesthetics. Vet Rec 2002; 150: 808–815. [DOI] [PubMed] [Google Scholar]
- 3.Wong D, Makowska IJ, Weary DM. Rat aversion to isoflurane versus carbon dioxide. Biol Lett 2013; 9: 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marquardt N, Feja M, Hünigen H, et al. Euthanasia of laboratory mice: Are isoflurane and sevoflurane real alternatives to carbon dioxide? PLoS One 2018; 13: 1–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Makowska IJ, Weary DM. Rat aversion to induction with inhalant anaesthetics. Appl Anim Behav Sci 2009; 119: 229–235. [Google Scholar]
- 6.Makowska IJ, Vickers L, Mancell J, et al. Evaluating methods of gas euthanasia for laboratory mice. Appl Anim Behav Sci 2009; 121: 230–235. [Google Scholar]
- 7.Stokes EL, Flecknell PA, Richardson CA. Reported analgesic and anaesthetic administration to rodents undergoing experimental surgical procedures. Lab Anim 2009; 43: 149–154. [DOI] [PubMed] [Google Scholar]
- 8.American Veterinary Medical Association. AVMA guidelines for the euthanasia of animals: 2020 ed., https://www.avma.org/sites/default/files/2020-02/Guidelines-on-Euthanasia-2020.pdf (2020, accessed 2 May 2022).
- 9.Brunt MW, Améndola L, Weary DM. Attitudes of laboratory animal professionals and researchers towards carbon dioxide euthanasia for rodents and perceived barriers to change. Lab Anim 2021; 55: 531–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hawkins P, Prescott MJ, Carbone L, et al. A good death? Report of the second Newcastle meeting on laboratory animal euthanasia. Animals 2016; 6: 1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Potvin F, Breton L, Patenaude R. Field anesthesia of American martens using isoflurane. In: Harrison DJ, Fuller AK, Proulx G. (eds) in Martens and fishers (Martes) in human-altered environments. 1st ed. Boston: Springer, 2005, pp.265–273. [Google Scholar]
- 12.Parker WT, Muller LI, Gerhardt RR, et al. Field use of isoflurane for safe squirrel and woodrat anesthesia. J Wildl Manage 2008; 72: 1262–1266. [Google Scholar]
- 13.Risling TE, Caulkett NA, Florence D. Open-drop anesthesia for small laboratory rodents. Can Vet J 2012; 53: 299–302. [PMC free article] [PubMed] [Google Scholar]
- 14.Moody CM, Weary DM. Mouse aversion to isoflurane versus carbon dioxide gas. Appl Anim Behav Sci 2014; 158: 95–101. [Google Scholar]
- 15.Leach MC, Bowell VA, Allan TF, et al. Measurement of aversion to determine humane methods of anaesthesia and euthanasia. Anim Welf 2004; 13: S77–86. [Google Scholar]
- 16.Moody CB. Current methods of mouse euthanasia: Refinement and aversion. MSc Thesis, University of British Columbia, Canada, 2013.
- 17.University of British Columbia. UBC animal care guidelines SOP: Euthanasia of adult rodents, https://animalcare.ubc.ca/sites/default/files/documents/SOP%20INHALANT%20EUTHANASIA%20FOLLOWED%20BY%20CARBON%20DIOXIDE.pdf (2012, accessed 19 December 2022).
- 18.Niel L, Weary DM. Behavioural responses of rats to gradual-fill carbon dioxide euthanasia and reduced oxygen concentrations. Appl Anim Behav Sci 2006; 100: 295–308. [Google Scholar]
- 19.Wei Y, Zhang D, Zuo Y. Whole-exome sequencing reveals genetic variations in humans with differential sensitivity to sevoflurane: A prospective observational study. Br Paramed J 2022; 148: 1–8. [DOI] [PubMed] [Google Scholar]
- 20.Sonner JM, Gong D, Eger EI. Naturally occurring variability in anesthetic potency among inbred mouse strains. Anesth Analg 2000; 91: 720–726. [DOI] [PubMed] [Google Scholar]
- 21.Mogil JS, Smith SB, O’Reilly MK, et al. Influence of nociception and stress-induced antinociception on genetic variation in isoflurane anesthetic potency among mouse strains. Anesthesiology 2005; 103: 751–758. [DOI] [PubMed] [Google Scholar]
- 22.Cervin A, Lindberg S. Changes in mucociliary activity may be used to investigate the airway-irritating potency of volatile anaesthetics. Br J Anaesth 1998; 80: 475–480. [DOI] [PubMed] [Google Scholar]
- 23.Carola V, D’Olimpio F, Brunamonti E, et al. Evaluation of the elevated plus-maze and open field tests for the assessment of anxiety-related behaviour in inbred mice. Behav Brain Res 2002; 134: 49–57. [DOI] [PubMed] [Google Scholar]
- 24.Ma J, Shen B, Stewart LS, et al. The septohippocampal system participates in general anesthesia. J Neurosci 2002; 22: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boulanger Bertolus J, Nemeth G, Makowska IJ, et al. Rat aversion to sevoflurane and isoflurane. Appl Anim Behav Sci 2015; 164: 73–80. [Google Scholar]
- 26.Tzschentke TM. Conditioned place preference and aversion. In: Stolerman IP. (ed.) Encyclopedia of psychopharmacology. 1st ed. Berlin: Springer Berlin Heidelberg, 2014, pp.1–7. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-lan-10.1177_00236772231169550 for Mouse isoflurane anesthesia using the drop method by Maya J Bodnar, Anna S Ratuski and Daniel M Weary in Laboratory Animals
Data Availability Statement
All data and code used for the statistical analysis are available on the UBC dataverse (https://doi.org/10.5683/SP3/OEC7QY).