Abstract
The laboratory mouse is used extensively for human disease modeling and preclinical therapeutic testing for efficacy, biodistribution, and toxicity. The variety of murine models available, and the ability to create new ones, eclipses all other species, but the size of mice and their organs create challenges for many in vivo studies. For pulmonary research, improved methods to access murine airways and lungs, and track substances administered to them, would be desirable. A nonsurgical endoscopic system with a camera, effectively a bronchoscope, coupled with a cryoimaging fluorescence microscopy technique to view the lungs in 3D, is described here that allows visualization of the procedure, including the anatomical location at which substances are instilled and fluorescence detection of those substances. We have applied it to bacterial infection studies to characterize better and optimize a chronic lung infection murine model in which we instill bacteria-laden agarose beads into the airways and lungs to extend the duration of the infection and inflammation. The use of the endoscope as guidance for placing a catheter into the airways is simple and quick, requiring only momentary sedation, and reduces post-procedural mortality compared with our previous instillation method that includes a trans-tracheal surgery. The endoscopic method improves speed and precision of delivery while reducing the stress on animals and the number of animals generated and used for experiments.
Keywords: Bronchoscopy, ethics and welfare, lung infection, reduction, refinement
Approche bronchoscopique mini-invasive pour l'administration directe aux voies respiratoires murines et application aux modèles d'infection pulmonaire Résumé
La souris de laboratoire est largement utilisée pour la modélisation des maladies humaines et les tests précliniques et thérapeutiques d'efficacité, de biodistribution et de toxicité. La variété de modèles de souris disponibles, et la capacité de créer de nouveaux modèles, éclipse toutes les autres espèces, mais la taille des souris et de leurs organes créent des défis pour de nombreuses études in vivo. Dans le cas de la recherche pulmonaire, il serait souhaitable d'améliorer les méthodes d'accès aux voies aériennes et aux poumons de la souris et le suivi des substances qui leur sont administrées. Nous décrivons ici un système endoscopique non chirurgical doté d’une caméra, à savoir un bronchoscope, couplé à une technique de cryo-microscopie à fluorescence, pour visualiser les poumons en 3D et visualiser la procédure, notamment l'emplacement anatomique où les substances sont instillées et la détection par fluorescence de ces substances. Nous l'avons appliqué aux études d'infection bactérienne pour mieux caractériser et optimiser un modèle murin d'infection pulmonaire chronique dans lequel nous instillons des billes d'agarose chargées de bactéries dans les voies respiratoires et les poumons pour prolonger la durée de l'infection et de l'inflammation. L'utilisation de l'endoscope pour guider le cathéter dans les voies respiratoires est simple et rapide, ne nécessitant qu'une sédation momentanée, et réduit la mortalité post-intervention par rapport à notre méthode d'instillation précédente qui inclut une chirurgie trans-trachéale. La méthode endoscopique améliore la vitesse et la précision d'administration tout en réduisant le stress des animaux et le nombre d'animaux générés et utilisés pour les expériences.
Minimalinvasives bronchoskopisches Konzept für die direkte Verabreichung an die Atemwege von Mäusen und Anwendung auf Modelle von Lungeninfektionen Abstract
Die Labormaus wird in großem Umfang für die Modellierung menschlicher Krankheiten und für vorklinische Therapieversuche für Wirksamkeit, Biodistribution und Toxizität verwendet. Die Vielfalt der verfügbaren Mausmodelle und die Möglichkeit, neue Modelle zu entwickeln, stellt alle anderen Tierarten in den Schatten, aber die Größe der Maus und ihrer Organe erschwert viele In-vivo-Studien. Für die Lungenforschung wären bessere Methoden für den Zugang zu den Atemwegen und der Lunge der Maus und für die Verfolgung der verabreichten Substanzen wünschenswert. Vorliegend wird ein nicht-chirurgisches, endoskopisches System mit einer Kamera, im Grunde ein Bronchoskop, beschrieben, das mit Kryo-Bildgebungs- und Fluoreszenzmikroskopietechnik für die 3D-Betrachtung der Lunge gekoppelt ist, und das die Visualisierung des Verfahrens ermöglicht, einschließlich der anatomischen Stelle, an der die Substanzen eingebracht werden, und der Fluoreszenzdetektion dieser Substanzen. Wir haben diese Technik bei Versuchen zu bakteriellen Infektionen eingesetzt, um ein Mausmodell für chronische Lungeninfektionen besser zu charakterisieren und zu optimieren, bei dem wir mit Bakterien gekoppelte Agarosekügelchen in die Atemwege und die Lunge einbringen, um die Dauer der Infektion und Entzündung zu verlängern. Der Einsatz des Endoskops als Orientierungshilfe für die Platzierung eines Katheters in die Atemwege ist einfach und schnell, erfordert nur eine kurze Sedierung und verringert die Sterblichkeit nach dem Eingriff im Vergleich zu unserer bisherigen Instillationsmethode, die mit einem trans-trachealen Eingriff verbunden ist. Die endoskopische Methode ermöglicht eine schnellere und präzisere Verabreichung und reduziert gleichzeitig die Belastung der Tiere und die Anzahl der für die Versuche benötigten Tiere.
Un enfoque broncoscópico mínimamente invasivo para la administración directa en las vías respiratorias murinas y su aplicación en modelos de infección pulmonar Resumen
El ratón de laboratorio se utiliza ampliamente para el modelado de enfermedades humanas y las pruebas terapéuticas preclínicas de eficacia, biodistribución y toxicidad. La variedad de modelos de ratón disponibles, y la capacidad de crear otros nuevos, eclipsa a todas las demás especies, pero el tamaño de los ratones y sus órganos suponen retos para muchos estudios in vivo . Para la investigación pulmonar, sería deseable disponer de métodos mejorados para acceder a las vías respiratorias y los pulmones de los ratones, y realizar un seguimiento de las sustancias que se les administran. Aquí se describe un sistema endoscópico no quirúrgico con una cámara, en realidad un broncoscopio, acoplado a una técnica de criomicroscopía de fluorescencia para ver los pulmones en 3D, que permite la visualización del procedimiento, incluida la localización anatómica en la que se instilan las sustancias y la detección por fluorescencia de estas. Hemos aplicado este sistema en estudios de infección bacteriana para caracterizar y optimizar mejor un modelo de ratón de infección pulmonar crónica en el que instilamos microesferas de agarosa cargadas de bacterias en las vías respiratorias y los pulmones para prolongar la duración de la infección y la inflamación. El uso del endoscopio como guía para colocar un catéter en las vías respiratorias es sencillo y rápido, tan solo requiere sedación momentánea y reduce la mortalidad posprocedimiento en comparación con nuestro método de instilación anterior que incluye una cirugía transtraqueal. El método endoscópico mejora la velocidad y la precisión de la administración mientras reduce el estrés de los animales y el número de animales criados y utilizados para los experimentos.
Introduction
The laboratory mouse is used extensively in biomedical research due to the anatomical and physiological similarities to humans. For pulmonary biology and therapeutic development and testing, murine airways and lungs are challenging because of their size and accessibility. For bacterial exposure in rodent models, a variety of methods have been published, 1 including aerosolization,2,3 intranasal aspiration, 4 intratracheal, 5 and transtracheal via a tracheostomy.6,7 Disorders that involve infection and colonization of the lungs and airways, such as cystic fibrosis (CF), would benefit from models that best reflect the human disease etiology and pathophysiology. While some bacterial species may spontaneously infect and colonize CF murine airways, 8 others do not and are quickly cleared by mice. For these, methods have been developed to retain bacteria in the lungs, such as embedding bacteria in agarose beads. 9 This manipulation provides a longer duration of bacterial presence and the stimuli the bacteria produce, but the effects of the delivery method are still to be determined.
A variety of systems have been used to deliver bacteria physically to murine airways. Aerosolization is a noninvasive method, but measuring the amount of material delivered to the airways is difficult and imprecise. Aerosols are also often ingested, as they deposit on the fur of the animal and are consumed during grooming. Aspiration, where material is dripped into the nares, is also noninvasive but suffers from imprecise delivery to the lungs, with some portion being swallowed or otherwise not reaching the airways. Intratracheal delivery, either by aspiration through the oropharynx 10 or by catheter or gavage needle inserted through the mouth and into the trachea, 5 is mildly invasive but requires substantial skill to maneuver into the trachea and not the esophagus. Transtracheal administration ensures delivery into the trachea. It is used extensively at our institution to model lung infections6,11–18 and involves an incision into the trachea through which a catheter or gavage needle is inserted and through which relatively precise quantities of material can be delivered. While more accurate in terms of placement and precise in quantitative terms than the other methods described here, the surgery makes it an invasive and stressful procedure for the animal and hence results in a higher mortality rate. Consequently, there is room for improvement, particularly with regard to the effects of the various procedures on animal mortality, as well as experimental variability because of the ambiguity about where substances are delivered. If these issues could be addressed, it should reduce the numbers of animals needed for a study and improve the statistical power and robustness of experimental results.
A small endoscopic system has been described that can be used in a fashion analogous to a human bronchoscope, inserting a catheter with an exploring fiber optic into the trachea. 19 The small diameter of the catheter allows one to reach as far as the second generation of bronchi in adult mice. The lighted fiber optic and lens relay images to a computer screen, allowing the user to see exactly where the end of the catheter is located and allowing for precise delivery of substances with little to no adverse effects from the procedure on the animal.
The procedure is quick (less than two minutes), allowing acute anesthesia with isoflurane to suffice in immobilizing the animal yet achieving rapid recovery. Here, we have optimized the use of the bronchoscope by adding a larger outer catheter so that delivery of larger compounds can be accomplished, and we use 3D fluorescence imaging 20 to visualize where compounds deposit after instillation in the trachea. We developed the system using fluorescent latex beads and then compared the bronchoscopic procedure to the transtracheal method in retrospective studies where an inoculum of bacteria, embedded in agarose beads, was delivered. 21 The results demonstrate the bronchoscopic procedure causes minimal distress to animals undergoing the procedure.
Methods
Mice
C57BL/6J mice were reared in-house and weaned at three weeks of age onto Teklad 7904 rodent chow (Envigo, Indianapolis, IN) and sterile water with osmotic laxative, Colyte (mix of PEG-3350, Na2SO4, NaHCO3, KCl, and NaCl, all from Thermo Fisher Scientific, Waltham, MA). Animals were maintained on a 12-hour/12-hour light/dark schedule at a mean ambient temperature of 22°C and were housed in standard polysulfone microisolator cages (maximum five mice per cage) in ventilated units with corncob bedding, shelter, and nesting material. After instillation of agarose spheres, the mice were kept in a separate room in cages with filter tops and were observed daily (weight, activity, appearance, etc.). Mice undergoing transtracheal surgery were changed to Alpha-Dri™ bedding material instead of corncob. Mice were euthanized following the AVMA panel’s 2020 guidelines and the recommendations for euthanization22,23 using gradual displacement with CO2 (locked at 6 psi for a 60% displacement) in an 8″ × 10″ × 16″ chamber for five minutes. Animals were toe-pinched to make sure they were euthanized/deeply anaesthetized before exsanguination by opening up the thoracic cavity and drawing heart blood. Animal facilities are PHS assured and AAALAC accredited, and the Institutional Animal Care and Use Committee of Case Western Reserve University approved the animal protocols 2016-0066 and 2019-0020. We used the ARRIVE 2.0 reporting guidelines when writing our report. 24
Bronchoscopic administration procedure
The endoscopic system (#PD-DS-1086) was purchased from PolyDiagnost (Hallbergmoos, Germany) and is a semi-rigid micro-endoscope with an LED optical fiber and a 3000-pixel imaging system connected to a monitor. Due to the small diameter of the endoscope (0.45 mm), the endoscope does not have an insertion canal. Instead, we attached a Polyshaft (#PD-DS-1030) to the endoscope to make it rigid and then added a loosely attached catheter (#26741; Exelint international, Redondo Beach, CA) over the polyshaft and endoscope (Figure 1(a)). The endoscope was used for precise placement of the catheter in the lung and was withdrawn before administering substances through the catheter. With the catheter in a main bronchus, we were able to use a larger volume dose (150 µL) without blocking the whole lung, which could have asphyxiated the mouse. The mouse was anaesthetized in a chamber with 3.5% isoflurane mixed with O2. When the mouse was anesthetized, artificial tears are applied to its eyes before placing the mouse in a supine position on a tilting stand at a 45° angle, restrained by a tether to its upper incisors. The mouse was kept under 3.5% isoflurane anesthesia via a nose cone that did not cover its mouth, which was enough to keep the mouse fully anesthetized during the quick procedure. With a flat tweezer, the tongue was gently pulled aside and was thereafter gently held between thumb and index finger. The endoscope with catheter was gently inserted into the mouse's mouth (Figure 1(c)), and the epiglottis (Figure 1.b1) and vocal cords (Figure 1.b2) were identified. The gas anesthesia lowered the breathing pattern without causing arrest. Hence, the vocal cords still opened and closed rhythmically. By following the opening rhythm and entering from underneath (following the roof of the mouth), we were able to enter under the epiglottis slowly and gently into the trachea through the vocal cords. The best position for placing the catheter was then identified. The endoscope was removed, and the syringe with substances to be delivered and a polyshaft catheter attached were gently inserted while securing the catheter with the other hand. A volume of 50 µL into the trachea, or up to 150 µL in the right or left main bronchus, was then instilled, and the catheter and syringe were withdrawn four to five seconds later. The mouse was then placed in a clean cage on a heating pad where it quickly regained consciousness from the isoflurane inhalation anesthesia. Mice were monitored daily for adverse effects of treatment/instilled compounds.
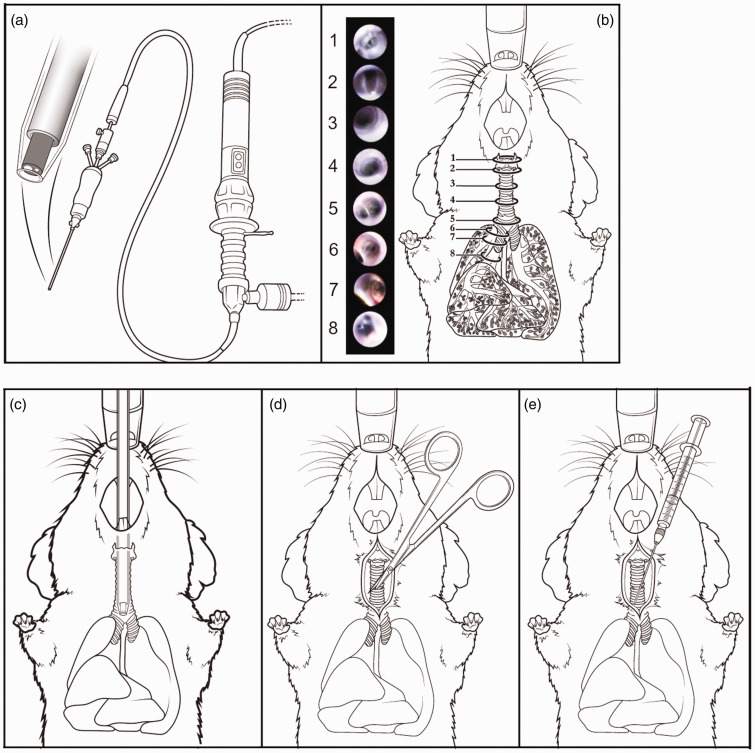
Figure 1.
The endoscopic system for accessing the airways compared with the transtracheal procedure. (a) The bronchoscope contains a fiber optic to guide the user through the airway. Images obtained from the fiber optic are shown in pictures 1–8, and the sites corresponding to the images are shown in (b). The tip is introduced through the mouth and proceeds into the trachea. The vocal cords and larynx are observed first (1), and upon opening of the vocal cords (2), the catheter proceeds into the trachea (3 and 4) to the carina (5) and into the right main stem bronchus (6 and 7) where the next generation of bronchioles are visualized (8) but are too small for the catheter to enter. (c) The endoscope sheathed with a removable catheter is inserted through the mouth of the mouse and enters the trachea through the epiglottis and the vocal cords. (d) and (e) The transtracheal involves a 0.5-cm incision in the skin to expose the trachea and a subsequent small incision between the cartilage rings through which a gavage needle is inserted to administer substances.
Tracheal administration procedures
The surgical tracheostomy procedures were carried out as described previously 21 and shown schematically in Figure 1(d) and (e) for comparison with the endoscopic method (Figure 1(c)).
Administration of latex beads
As a test cargo to represent imaging compounds instilled in the lungs of mice, F8819 beads (Invitrogen™ FluoSpheres latex beads, #F8819, 1 µm, Nile red (ex535/em575); Thermo Fisher Scientific) were used as a sterile noninflammatory surrogate for bacteria in four mice. F8819-laden agarose beads were prepared as described in Rosenjack et al., 21 but 10 mL of 1:100 F8819 was added to the warm agarose instead of bacteria. For free instillation, a 1:100 dilution of F8819 latex beads in phosphate-buffered saline (PBS) was used. The sizes of the agarose beads were measured, and fluorescence was confirmed before usage (Figure 2). Sizes ranged from 39 to 699 µm, with an average size of 298 µm, and the beads were filtered through a 500-µm pluriStrainer mesh (43-50500-03; pluriSelect, Leipzig, Germany), since the polyshaft’s inner diameter is only 0.65 mm. Sizes after filtering ranged from 39 to 551 µm, with an average size of 277 µm. F8819-laden agarose beads (two mice) and free F8819 latex beads (two mice) were instilled using the bronchoscope into the right main bronchus (see Table 1 for study groups). Mice were euthanized two hours post instillation of F8819, and no mice were excluded.
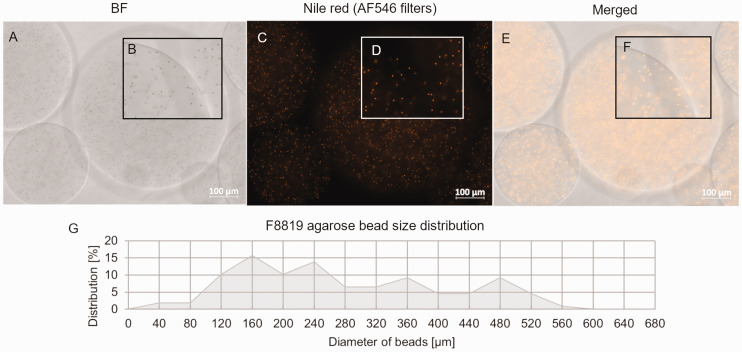
Figure 2.
Size and fluorescence of agarose beads embedded with F8819 latex particles. (a) and (b) Brightfield and (c) and (d) fluorescence microscopy of agarose beads in which F8819 latex particles are embedded, and (e) and (f) merged images. Insets B, D, and F are magnified sections that show latex particles are seen in both brightfield and fluorescence modes. (g) The size distributions of the agarose beads after filtering through a 500-µm mesh.
Table 1.
Data on mice in current and retrospective studies.
| Compound | Conduction of experiment | Number of mice (females) | Age (weeks) | Number of experiments | Procedure and instillation site | ||
|---|---|---|---|---|---|---|---|
| Average | Min | Max | |||||
| F8819 bead-laden agarose beads | Current | 2 (2) | 17.4 | 15.6 | 19.1 | 1 | Bronchoscope, right main bronchus |
| Free F8819 beads | Current | 2 (1) | 21.8 | 12.9 | 30.6 | 2 | Bronchoscope, right main bronchus |
| 25,000 CFU PAM | Retrospective | 17 (8) | 14.2 | 10.1 | 23.1 | 5 | Bronchoscope, trachea |
| 25,000 CFU PAM | Retrospective | 17 (3) | 16.4 | 11.3 | 20.1 | 4 | Transtracheal surgery, trachea |
| 50,000 CFU PAM | Retrospective | 15 (10) | 14.4 | 11.3 | 20.1 | 4 | Bronchoscope, trachea |
| 50,000 CFU PAM | Retrospective | 31 (8) | 11.5 | 6.6 | 27.4 | 10 | Transtracheal surgery, trachea |
| 100,000 CFU PAM | Retrospective | 17 (9) | 11.2 | 8.3 | 14.9 | 4 | Bronchoscope, trachea |
| 100,000 CFU PAM | Retrospective | 15 (10) | 10.8 | 6.9 | 12.9 | 3 | Transtracheal surgery, trachea |
Mortality data from retrospective studies of Pseudomonas aeruginosa and agarose ‘bead’ inocula
Pseudomonas aeruginosa strain M57-15 (PAM57-15, referred to here as PAM), originating as a mucoid clinical isolate, 7 was transformed to carry a transgene expressing the red fluorescent protein mCherry, maintained by gentamicin resistance. 21 PAM-laden agarose beads were prepared and analyzed as described previously. 21 PAM-embedded agarose beads, instilled into the lungs through a transtracheal surgery, ranged in sizes from 30 to 527 µm, with an average size of 206 µm and a median size of 190 µm, and the beads were not filtered. Agarose beads for bronchoscopic instillation ranged from 18 to 692 µm, with an average size of 142 µm and a median size of 123 µm. Mortality and weight data on days 0, 1, 2, and 3 post infection were collected in retrospect from observations (no animals were excluded for the mortality study) from 24 individual infection experiments performed by our laboratory group where different inoculums were used, and the two different instillation methods were compared (see Table 1 and Figure 5(c) for study groups). Weight change of wild-type mice infected with 25,000CFU/50 µL using the transtracheal surgery procedure has been published previously as the control group in Rosenjack et al. 21 (Figure 1). If mice were considered too ill (gross appearance, weight loss >20%), they were discontinued for the infection study. No mice were excluded from the retrospective studies. From the daily weight documentation, mice were noted in retrospect to be either still alive (1) or dead (0) and presented in the Kaplan–Meier plot (Figure 5(a)).
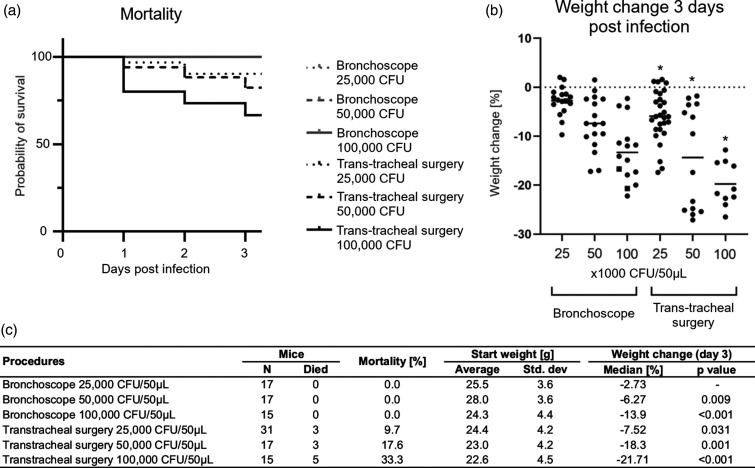
Figure 5.
Effect of procedure on survival and weight change. Three different inoculums of PAM bacteria embedded in agarose beads (25,000, 50,000, or 100,000 CFU) were instilled in the airways of mice via the bronchoscope or through transtracheal surgery. Mortality was observed in a dose-dependent manner only in animals that underwent transtracheal surgery (a). The percent weight change on day 3 (compared with the day of instillation of bacteria; day 0) also shows a dose-dependent weight loss using PAM bacteria (b). Mice that survived to day 3 are shown with a dot, and the lines represent the median for each group. *Some mice in the group died prior to day 3 and are not represented in the graph. Table C shows numbers of deaths after the different procedures and the calculated p-value of weight change on day 3 compared with 25,000 CFU PAM instilled using the bronchoscopic procedure. Weight change increases in a dose-dependent manner for both procedures, whereas numbers of mice that died only increased for the transtracheal procedure.
Visualization of beads in tissue
Murine airways instilled with F8819 Nile red-laden agarose beads or free F8819 beads in the right main bronchus using the bronchoscope were euthanized two hours later, and the lungs were removed. The lungs were rinsed with 1 mL PBS and, for histological sectioning, placed in 5 mL 4% formaldehyde for at least 24 hours at 4°C before being transferred to PBS and kept at 4°C until further processing. The lungs were then incubated in 15% sucrose buffer at 4°C for six to seven hours, followed by 30% sucrose overnight at 4°C before being embedded in Tissue-Tek OCT compound (#4583; Sakura Finetek USA, Torrance, CA), frozen, and kept at –80°C until sectioning at –20°C on a Cryotome. Sections were 7-µm-thick sequential sections and were counterstained with DAPI. Tissue sections were analyzed on a Zeiss Axio Observer 7 LED microscope (Carl Zeiss, White Plains, NY).
For whole-lung 3D modeling, lungs were removed and rinsed in PBS. After embedding in Tissue-Tek OCT, the lungs were snap frozen in liquid nitrogen and stored at –80°C until 10-µm sectioning and imaging on a CryoViz™ (BioInvision, Cleveland, OH) 20 by the Imaging Research Core at Case Western Reserve University. Images were analyzed, and 3D models were created using Amira 3D software (Thermo Fisher Scientific).
Statistics
Weight change percent was calculated as the percent difference in weight from day 0 to day 3 of the experiment. The weight change percent distribution for each group was checked for normality with a Shapiro–Wilk test. Not all groups were normally distributed. So, median values are reported for each. Two-sided Wilcoxon rank sum tests were conducted to compare the smallest-dose bronchoscope group with all other groups. A p-value of ≤0.05 was considered statistically significant. Due to the exploratory nature of these data, no corrections were made for multiple comparisons.
For use of this procedure in prospective studies, power calculations are included for weight loss as an outcome and based on the weight change data on day 3. Examples (Table 2) are given for three different levels of power to detect significant changes in weight: 70%, 80%, and 90% power using a two-tailed analysis with p ≤ 0.05. To estimate the effects of mice that did not survive in the surgical procedure groups, simple linear imputation was used to calculate missing values of day 3 weight change using bacterial inoculum, procedure, and baseline weight (Supplemental Table S1). All analyses were conducted using R v3.6.3 (The R Foundation for Statistical Computing, Vienna, Austria).
Table 2.
Power calculations to detect significant changes in weight for studies involving different bacterial inocula administered by bronchoscopic or surgical procedures.
| Power | Comparing CFU doses | Bronchoscope | Surgery | |||
|---|---|---|---|---|---|---|
| Needed sample size for analysis | Needed sample size for analysis | Mortality-corrected sample size | ||||
| Inoculum 1 | Inoculum 2 | Inoculum 1 | Inoculum 2 | |||
| 70% | 25,000 | 50,000 | 15 | 15 | 17 | 19 |
| 50,000 | 100,000 | 16 | 33 | 41 | 50 | |
| 25,000 | 100,000 | 7 | 5 | 6 | 8 | |
| 80% | 25,000 | 50,000 | 19 | 19 | 22 | 24 |
| 50,000 | 100,000 | 20 | 42 | 51 | 63 | |
| 25,000 | 100,000 | 9 | 7 | 8 | 11 | |
| 90% | 25,000 | 50,000 | 25 | 24 | 27 | 30 |
| 50,000 | 100,000 | 27 | 56 | 68 | 84 | |
| 25,000 | 100,000 | 11 | 8 | 9 | 12 | |
Three different levels of power (70%, 80%, and 90%) were estimated. The difference in mean weight change for each procedure was used together with standard deviation (Supplemental Table S1) to calculate the number of mice needed to perform the analysis of the studies at a significance level of 0.05. Because of bacterial dose-dependent mortality observed for mice undergoing the surgical procedure, the number of mice needed has been mortality corrected. Hence, the number of mice needed for inoculation on day 0 is higher than the number needed for the analysis.
Results
The procedure with the endoscopic system, referred to here as a bronchoscope (Figure 1(c)), was compared with the surgical, transtracheal procedure (Figure 1(d) and (e)). This bronchoscopic system provides visualization of the airway via a camera and a fiber optic to provide light for guidance, as shown in Figure 1(a). From the initial entry into the mouth, the system shows its benefits, first revealing the vocal cords so that the catheter can proceed into the trachea (see schematic and photos in Figure 1(b)) when the vocal cords separate during breathing (Figure 1(b.1) and (b.2)). Passing the vocal cords reveals the trachea (Figure 1(b.3) and (b.4)) followed by the carina (Figure 1(b.5)). Moving the catheter further into the airways allows entry into the right bronchus (Figure 1(b.6) and (b.7)), all the way to the branch point into the individual lobes (Figure 1(b.8)). Once the catheter is at the desired position, the fiber optic is withdrawn to allow substances to be administered through the catheter.
The system was tested for substance administration using 50 µL of a 1:50 agarose bead solution in PBS containing agarose beads with F8819 1-µm-diameter latex particles labeled with the fluorescent dye Nile red (Figure 2). The F8819 particles were chosen because of their similarity in size to many bacterial species for which the agar bead strategy is used for infection modeling. Animals were euthanized less than an hour after administration. The lungs were then harvested and imaged using a CryoViz™ system that captures brightfield and fluorescence images of sequential 10-µm sections of cryopreserved organs or whole-mouse carcasses. 20 Figure 3(a)–(i) shows sections of murine lungs, comparing distribution of free F8819 latex beads after instillation in the lungs with F8819-laden agarose beads deposited in the lungs. The 3D reconstruction from the lung sections visualizes the distribution of free F8819 latex beads (Figure 3(j)–(m)) and the F8819-laden agarose beads (Figure 3(n)–(q)) immediately after instillation.
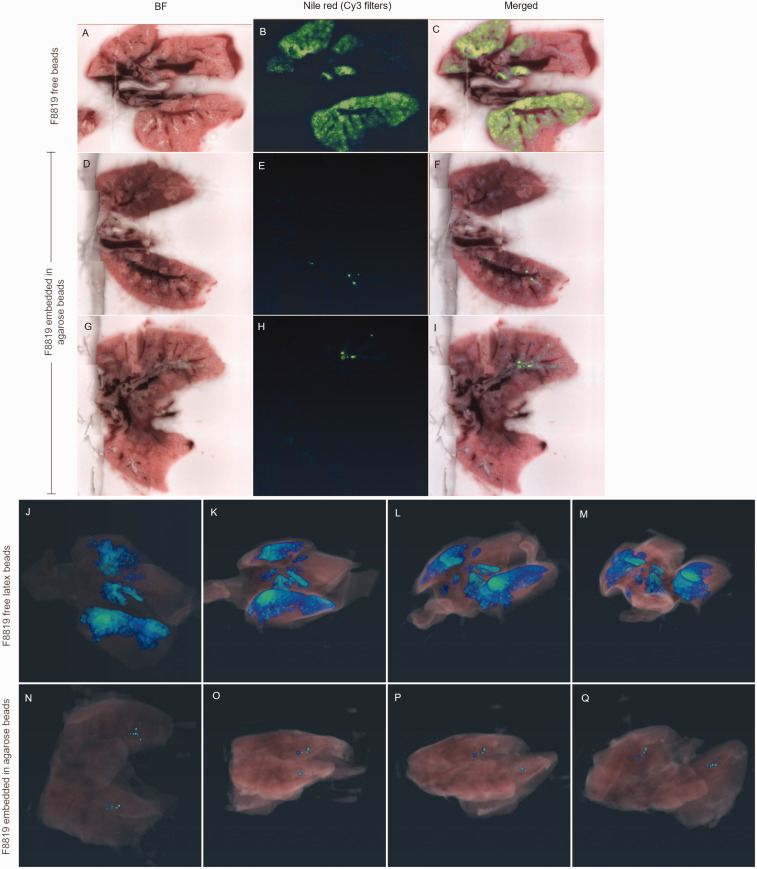
Figure 3.
Imaging whole lungs shows different distribution of fluorescent particles when embedded in agarose compared with free latex beads. Cross-section images from lungs of a mouse administered a suspension of fluorescent F8819 latex particles (a)–(c). Two different cross-sections from lungs of a mouse administered fluorescent F8819 latex particles embedded in agarose beads (d)–(i). (a), (d), and (g) are brightfield; (b), (e), and (h) show Nile red fluorescence, and (c), (f), and (i) are the merged images. Serial images, such as (f) and (i), are compiled to reconstruct a 3D view of the lungs. (j)–(m) show different rotational views of the lungs of a mouse given the F8819 particle suspension, while (n)–(q) show the distribution of F8819 particles embedded in agarose.
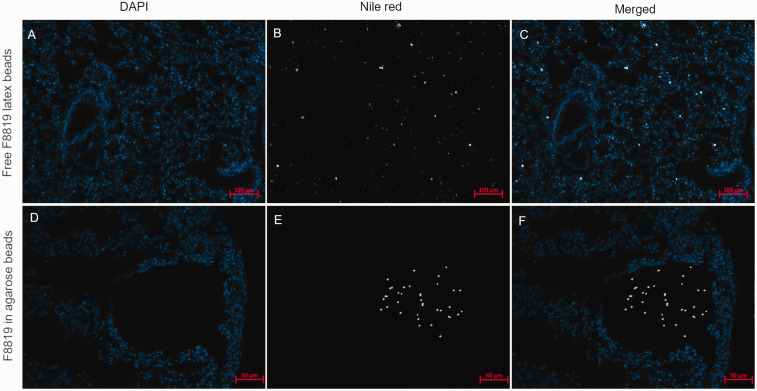
Sections of lungs were also investigated at 20× and 40× magnification for more detailed visualization of the distribution of the latex beads in the tissue (Figure 4). Instillation of free beads in solution shows distribution throughout the lung tissue, whereas instillation of agarose beads shows deposition into the distal parts of the lung but is focal. For a chronic infection model, it is necessary to maintain bacteria within the lungs for extended periods of time, which is accomplished by the agarose bead procedure.
Figure 4.
Comparison of the deposition of F8819-laden agarose beads in the airways and free F8819 latex beads less than two hours after instillation. Nile red signal is shown in white, and nuclei are shown in blue (DAPI stain). (a)–(c) Free F8819 latex beads were found to be distributed all over the lung tissue (bronchi, bronchioles, alveolar tissue). F8819-laden agarose beads were found lodged in the bronchi and in the alveolar tissue (d)–(f) and Nile red signal from F8819 latex beads is visible within agarose beads only (e) and (f).
We then did a retrospective comparison of mortality and weight change from studies where PAM-laden agarose beads were instilled in the lungs using the bronchoscope or our previously used surgical method to create the chronic infection in mice. The method involved a tracheostomy, as illustrated in Figure 1(d) and (e), and is referred to as the transtracheal method. PAM-laden agarose beads (50 μ) were instilled in the trachea in three different inoculums: 25,000, 50,000, and 100,000 CFU. The data revealed more severe effects of surgery than expected. In particular, the mortality rates of the mice were higher in the transtracheal method compared with the bronchoscope, where no deaths were registered (Figure 5(a) and (c)). We observed a dose-dependent relationship between bacterial load and mortality when the surgical method was used (9.7%, 17.6%, and 33.3%), but no deaths when the bronchoscope was used (0%), regardless of inoculum titer. Not only did the surgical technique impact mortality, but it also resulted in greater experimental variation with regard to weight loss, as described below and listed in Table 2. Thus, the surgical procedure requires a greater number of animals due to mortality and outcome variance.
Cachexia is a consequence of chronic infection and thus weight loss is a relevant outcome for these types of studies. Mice used in these studies were both males and females between 6 and 27 weeks old, and mice were randomized between the six study groups as described in Table 1. So, age and sex should not interfere with the observed weight changes in the treatment groups. Figure 5(b) shows the relative weight change caused by the two different procedures and different inoculum titers on day 3 post infection with the PAM-laden agarose beads. It is important to notice that these numbers are likely an underestimate, since mice that died before day 3 are not included in the scatter plot. Most likely, these mice had even greater weight loss on day 3 compared with the mice that survived beyond day 3. Mice that underwent surgery all lost more weight than mice that underwent instillation of bacteria through the bronchoscope. The weight loss was observed to be dose dependent, with high titers (100,000 CFU) giving rise to higher weight loss. Comparing the 100,000 CFU doses given via transtracheal surgery or the bronchoscope reveals that the transtracheal surgery causes higher weight loss and thereby worse outcome for the mice, which is also depicted in the Kaplan–Meier mortality plot, where more mice died following the 100,000 CFU transtracheal surgery. Sample size estimates were calculated from these data, 70%, 80%, and 90% power, for the number of mice needed to detect a significant change in weight for each procedure (Table 2) using different inoculums of bacteria. Since mortality was observed in the surgery groups, we also did simple linear imputation of those mice that died before day 3 to incorporate them in the power calculations (Supplemental Table S2). Using the transtracheal surgery procedure to instill bacteria, at 80% power and two-tailed significance level of 0.05, a study would need 19 mice per group on day 3 (for analysis) to detect a difference in weight loss between the 25,000 and 50,000 CFU groups. Corrected for the expected mortality within those two groups (9.7% and 17.6%, respectively), the number of mice needed on day 0 would be 22 and 24 mice, respectively. Compared with the bronchoscopic approach, the number of mice needed would be 19 mice per group for analysis, which is also the number of mice needed at inoculation, since no mortality is expected. An important thing to notice is that the higher mortality (Figure 5c) of the surgical procedure results in increasing the number of mice needed to be infected in order to have enough mice for analysis. Using the bronchoscopic approach is a great benefit, since it reduces the number of mice needed if one is investigating the effects of bacteria in the lung tissue caused by more natural occurring lung infection and not due to a surgical procedure on the trachea.
Discussion
The goal of this study was to apply a nonsurgical and precise method to deliver substances to the airways and the lungs of mice that could improve our pulmonary infection studies. The bronchoscopic method has already been utilized for delivery to the lungs, 19 but not in a way where the larger agarose beads could be instilled. Our studies add to the utility of this approach in several ways. First, the use of isoflurane allows for more rapid recovery of the animals. Second, removing the fiber optic after the catheter has reached its desired placement allows for instillation of additional and larger substances, such as our agarose beads, to create a more chronic infection in the lung by reducing the clearance rate. Third, we added a fluorescent marker in order to detect where substances or bacteria were deposited, and we were able to detect this by whole-mouse/whole-organ imaging and thereafter 3D reconstructions.
The impact of surgery on animal health, stress, and survival underscores the need to match methodology with the question being addressed. CF lung infections, for example, are not a consequence of tracheostomies or other surgical procedures. So, the ability to carry out bacterial inoculations without surgery is expected to model and reflect actual disease conditions better. The low mortality from the bronchoscopic procedure also reduces the number of mice used for these lung infection studies. The procedure also refines the method of instillation, since we are able to see where in the lungs the compounds are instilled compared with other types of less stressful instillations, such as aerosolization or aspiration directly onto the oropharynx or by using a gavage needle. These other procedures often lead to the compounds ending up in the esophagus instead of the lungs, and depending on what one wants to study, this can be a major source of artefacts and experimental variation.
For experiments in which surgery is not needed or desired, the bronchoscopic system is less stressful and reduces mortality. Our interest is in processes by which animals resolve infections, focusing on immune function in the genetic disease CF. CF mice are fragile, like many murine models of human disease, and therefore minimally stressful methods are needed if these animals are to be useful optimally. Accordingly, methods that decrease variability and maximize survival will improve our ability to generate interpretable results and conclusions. The endoscope used here, effectively a murine bronchoscope, provides an improvement, as it is much less harsh on animals and has increased accuracy and precision for delivering substances to the airways. This bronchoscopic system should allow more accurate delivery of any fluid, suspension, or even gas that one wishes to distribute to the lungs and therefore has applications well beyond CF.
Along with improvements in the delivery process by employing a bronchoscope, we have also developed a method for direct detection of the substances being delivered. Here, we used latex beads labeled with a chemical fluorophore so that deposition of different compounds could be monitored. The fluorescence allows one to readily detect a wide range of delivered materials and differentiate them from exogenous tissue. Including optical markers also allows one to include whole-body fluorescence imaging. The cryoimaging technique shown here offers a global view that is not typically reported but which can provide additional insight in cases where whole-animal anatomy or physiology is of interest. The agarose bead model shows deposition in the distal airways of the lung and has been observed to be more efficient for chronic infection studies compared with delivery of free bacteria that get cleared faster.
The system described here provides improved accuracy for delivery of substances to the airways, does so in a minimally stressful way that improves survival, and allows visual monitoring of bacteria or other substances locally, and throughout the body, by fluorescence microscopy and imaging. The endoscope also reduces variation in endpoints—an important consideration for statistical power and reducing animal numbers in a study.
Supplemental Material
Supplemental material, sj-pdf-1-lan-10.1177_00236772231175553 for A minimally invasive bronchoscopic approach for direct delivery to murine airways and application to models of pulmonary infection by Karen Schelde, Julie Rosenjack, Claire Sonneborn, Anjum Jafri, Michael Kavran, Sarah Brumbaugh, Arne Rietsch, Rebecca J Darrah, Craig A Hodges, Christopher A Flask, Thomas J Kelley and Mitchell L Drumm in Laboratory Animals
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This research was supported by the Carlsberg Foundation Internationalisation fellowship grant (CF17-0157 and CF19-0020), the Imaging Research Core at Case Western Reserve University, the Cystic Fibrosis Foundation (DRUMM19XX0 and DRUMM19R0); the Case Comprehensive Cancer Center (P30 CA043703), the Cleveland Clinical and Translational Science Collaborative (UL1 TR002548), the Cleveland Digestive Diseases Research Core Center (P30 DK097948), The Research Institute for Children’s Health, the Doris and Floyd Kimble Foundation and MPB Charitable Foundation.
ORCID iDs: Karen Schelde https://orcid.org/0000-0002-7162-6938
Mitchell L Drumm https://orcid.org/0000-0003-4739-5153
Supplemental material
Supplemental material for this article is available online.
References
- 1.Bakker-Woudenberg IA. Experimental models of pulmonary infection. J Microbiol Methods 2003; 54: 295–313. [DOI] [PubMed] [Google Scholar]
- 2.Clark GC, Essex-Lopresti A, Moore KA, et al. Common host responses in murine aerosol models of infection caused by highly virulent Gram-negative bacteria from the genera Burkholderia, Francisella and Yersinia. Pathogens 2019; 8(4): 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Melenotte C, Lepidi H, Nappez C, et al. Mouse model of Coxiella burnetii aerosolization. Infect Immun 2016; 84: 2116–2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pechous RD. Intranasal inoculation of mice with Yersinia pestis and processing of pulmonary tissue for analysis. Methods Mol Biol 2019; 2010: 17–28. [DOI] [PubMed] [Google Scholar]
- 5.Lawrenz MB, Fodah RA, Gutierrez MG, et al. Intubation-mediated intratracheal (IMIT) instillation: a noninvasive, lung-specific delivery system. J Vis Exp 2014; e52261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Heeckeren AM, Schluchter MD, Drumm ML, et al. Role of Cftr genotype in the response to chronic Pseudomonas aeruginosa lung infection in mice. Am J Physiol Lung Cell Mol Physiol 2004; 287: L944–952. [DOI] [PubMed] [Google Scholar]
- 7.Heeckeren A, Walenga R, Konstan MW, et al. Excessive inflammatory response of cystic fibrosis mice to bronchopulmonary infection with Pseudomonas aeruginosa. J Clin Invest 1997; 100: 2810–2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Darrah R, Bonfield T, LiPuma JJ, et al. Cystic fibrosis mice develop spontaneous chronic Bordetella airway infections. J Infect Pulm Dis 2017; 3(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Starke JR, Edwards MS, Langston C, et al. A mouse model of chronic pulmonary infection with Pseudomonas aeruginosa and Pseudomonas cepacia. Pediatr Res 1987; 22: 698–702. [DOI] [PubMed] [Google Scholar]
- 10.Gautam S, Stahl Y, Young GM, et al. Quantification of bronchoalveolar neutrophil extracellular traps and phagocytosis in murine pneumonia. Am J Physiol Lung Cell Mol Physiol 2020; 319: L661–L669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonfield TL, Hodges CA, Cotton CU, et al. Absence of the cystic fibrosis transmembrane regulator (Cftr) from myeloid-derived cells slows resolution of inflammation and infection. J Leukoc Biol 2012; 92: 1111–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Heeckeren AM, Schluchter MD, Xue W, et al. Response to acute lung infection with mucoid Pseudomonas aeruginosa in cystic fibrosis mice. Am J Respir Crit Care Med 2006; 173: 288–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Heeckeren AM, Schluchter M, Xue L, et al. Nutritional effects on host response to lung infections with mucoid Pseudomonas aeruginosa in mice. Infect Immun 2004; 72: 1479–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Heeckeren AM, Scaria A, Schluchter MD, et al. Delivery of CFTR by adenoviral vector to cystic fibrosis mouse lung in a model of chronic Pseudomonas aeruginosa lung infection. Am J Physiol Lung Cell Mol Physiol 2004; 286: L717–L726. [DOI] [PubMed] [Google Scholar]
- 15.Van Heeckeren AM, Schluchter MD. Murine models of chronic Pseudomonas aeruginosa lung infection. Lab Anim 2002; 36: 291–312. [DOI] [PubMed] [Google Scholar]
- 16.Soltzberg J, Frischmann S, Van Heeckeren C, et al. Quantitative microscopy in murine models of lung inflammation. Anal Quant Cytol Histol 2011; 33: 245–252. [PMC free article] [PubMed] [Google Scholar]
- 17.Chmiel JF, Konstan MW, Knesebeck JE, et al. IL-10 attenuates excessive inflammation in chronic Pseudomonas infection in mice. Am J Respir Crit Care Med 1999; 160: 2040–2047. [DOI] [PubMed] [Google Scholar]
- 18.Van Heeckeren AM, Tscheikuna J, Walenga RW, et al. Effect of Pseudomonas infection on weight loss, lung mechanics, and cytokines in mice. Am J Respir Crit Care Med 2000; 161: 271–279. [DOI] [PubMed] [Google Scholar]
- 19.Dames C, Akyüz L, Reppe K, et al. Miniaturized bronchoscopy enables unilateral investigation, application, and sampling in mice. Am J Respir Cell Mol Biol 2014; 51: 730–737. [DOI] [PubMed] [Google Scholar]
- 20.Roy D, Steyer GJ, Gargesha M, et al. 3D cryo-imaging: a very high-resolution view of the whole mouse. Anat Rec (Hoboken) 2009; 292: 342–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosenjack J, Hodges CA, Darrah RJ, et al. HDAC6 depletion improves cystic fibrosis mouse airway responses to bacterial challenge. Sci Rep 2019; 9: 10282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Close B, Banister K, Baumans V, et al. Recommendations for euthanasia of experimental animals: Part 1. DGXI of the European Commission. Lab Anim 1996; 30: 293–316. [DOI] [PubMed] [Google Scholar]
- 23.Close B, Banister K, Baumans V, et al. Recommendations for euthanasia of experimental animals: Part 2. DGXT of the European Commission. Lab Anim 1997; 31: 1–32. [DOI] [PubMed] [Google Scholar]
- 24.Kilkenny C, Browne WJ, Cuthill IC, et al. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol 2010; 8: e1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-lan-10.1177_00236772231175553 for A minimally invasive bronchoscopic approach for direct delivery to murine airways and application to models of pulmonary infection by Karen Schelde, Julie Rosenjack, Claire Sonneborn, Anjum Jafri, Michael Kavran, Sarah Brumbaugh, Arne Rietsch, Rebecca J Darrah, Craig A Hodges, Christopher A Flask, Thomas J Kelley and Mitchell L Drumm in Laboratory Animals