Abstract
Background
Drug–drug interactions (DDIs) are common and can result in patient harm. Electronic health records warn clinicians about DDIs via alerts, but the clinical decision support they provide is inadequate. Little is known about clinicians’ real-world DDI decision-making process to inform more effective alerts.
Objective
Apply cognitive task analysis techniques to determine informational cues used by clinicians to manage DDIs and identify opportunities to improve alerts.
Design
Clinicians submitted incident forms involving DDIs, which were eligible for inclusion if there was potential for serious patient harm. For selected incidents, we met with the clinician for a 60 min interview. Each interview transcript was analysed to identify decision requirements and delineate clinicians’ decision-making process. We then performed an inductive, qualitative analysis across incidents.
Setting
Inpatient and outpatient care at a major, tertiary Veterans Affairs medical centre.
Participants
Physicians, pharmacists and nurse practitioners.
Outcomes
Themes to identify informational cues that clinicians used to manage DDIs.
Results
We conducted qualitative analyses of 20 incidents. Data informed a descriptive model of clinicians’ decision-making process, consisting of four main steps: (1) detect a potential DDI; (2) DDI problem-solving, sensemaking and planning; (3) prescribing decision and (4) resolving actions. Within steps (1) and (2), we identified 19 information cues that clinicians used to manage DDIs for patients. These cues informed their subsequent decisions in steps (3) and (4). Our findings inform DDI alert recommendations to improve clinicians’ decision-making efficiency, confidence and effectiveness.
Conclusions
Our study provides three key contributions. Our study is the first to present an illustrative model of clinicians’ real-world decision making for managing DDIs. Second, our findings add to scientific knowledge by identifying 19 cognitive cues that clinicians rely on for DDI management in clinical practice. Third, our results provide essential, foundational knowledge to inform more robust DDI clinical decision support in the future.
Keywords: Health & safety, Adverse events, Health informatics
STRENGTHS AND LIMITATIONS OF THIS STUDY.
Our study addresses a scientific gap in the literature, since it is the first cognitive task analysis that examines clinicians’ drug–drug interactions decision-making process during real-world patient care.
Our interdisciplinary study team was robust, consisting of physician researchers and practitioners, two human factors experts, pharmacist researchers and a practicing clinical pharmacist.
The primarily retrospective nature of the study may be subject to recall bias regarding clinicians’ interpretation of events.
Our study sample of pharmacists consisted primarily of outpatient clinical pharmacists. Thus, our findings are more applicable to hospital or outpatient clinical settings, rather than community-based pharmacies.
Background
Drug–drug interactions (DDIs) are a common cause of adverse drug events for patients.1 DDIs are ‘two or more drugs interacting in such a manner that the effectiveness or toxicity of one or more drugs is altered.’2 DDIs lead to around 245 000 patient hospitalisations in the USA each year,3 costing the healthcare system $1.3 billion.3 Consequences of DDIs to patients can be serious, ranging from ineffective medication treatment, to bleeding, kidney injury and death, yet most DDIs are preventable.4
Electronic health records (EHRs) include many tools to help prevent patient harm, including clinical decision support (CDS) via DDI alerts. Alerts typically appear during medication prescribing. An EHR system includes thousands of potential DDI alerts, which can be both a source of decision support and also consternation for clinicians. High rates of false alarms and numerous, clinically irrelevant DDIs, lead to clinician desensitisation and alert fatigue, sometimes resulting in serious DDIs being overlooked. Additionally, DDI alerts often fail to support real-world clinical decision-making. When prescribers encounter an unfamiliar DDI alert, they may consult reference materials and clinical pharmacists, or bypass the alert altogether with the assumption that the alert is inconsequential.5 6 Prescribers can also become overwhelmed if alerts provide too much information.7 8 Recent studies focus on DDI alert usability. Useful recommendations9 10 and alert evaluation tools,11 12 such as I-MedDeSA13 and TEMAS,14 have been published based on human factors engineering design principles. Newer DDI alert designs include optional Internet links, such as to detailed DDI monographs, for decision-making.10 15 Even with these alert enhancements, error rates in decision-making remained high.15 In our scenario-based simulation, clinicians accessed drug monographs frequently from alerts, which incorporated a number of usability principles, but clinicians still made incorrect decisions for over 25% of DDI alerts.15 Thus, in addition to incorporating known usability principles, there is a need for more in-depth understanding of clinicians’ decision-making process surrounding DDI management for patients.
Little literature examines clinicians’ real-world decision-making for DDIs, even though this knowledge is foundational to developing effective, computerised DDI decision support. For example, we previously conducted field observations in clinical practice and identified 9 factors and 44 subfactors that influenced alert usability.16 That study did not examine decision-making or DDI alerts specifically. In 2019, Bagri et al reported findings from focus groups with hospital pharmacists in a study to understand their perceptions and decision-making related to DDIs.17 They reported themes related to pharmacists’ assessment of DDIs, barriers to detecting DDIs, challenges with DDI alerts and proposed solutions to improve DDI alerts. These types of studies that leverage direct observations and focus groups are useful and necessary, but insufficient to elucidate the nuances of decision-making among clinicians. More targeted methods, such as cognitive task analysis (CTA) and the associated critical decision method (CDM), can be used to understand clinical decision-making, but these techniques have not been applied widely in healthcare. The CDM technique was designed to examine the decision-making of subject matter experts (eg, clinicians) who encounter high-stakes situations (e.g., DDI), yet are able to achieve positive outcomes.18 19 The CDM technique provides rich insights on informational cues needed for effective decision-making.19 20 Therefore, our objective was to apply CDM to identify the cognitive strategies that clinicians use to detect and manage DDIs for patients. This research is part of a larger investigation of clinicians’ decision-making during safety incidents20 21 that has a broader scope involving adverse drug reactions and drug-renal concerns.22 23 In this article, we present findings specific to clinicians’ DDI decision-making and identify opportunities to improve DDI alert design.
Methods
Study design
Our study was conducted with clinicians at a tertiary, Midwestern Veterans Affairs (VA) medical center. Our interdisciplinary study team was robust, consisting of physician researchers and practitioners, two human factors experts, pharmacist researchers and a practicing clinical pharmacist. The study was led by a female researcher, and the study team included both male and female researchers. At the time of this study, the EHR was capable of warning prescribers of 4439 different DDIs. Prescribers and pharmacists receive the same DDI alerts, which include two categories of severity, denoted as a ‘critical’ or ‘significant’ DDI.15 The DDI alert display format, however, can vary depending on whether the clinician is utilising medication ordering (eg, physicians and clinical pharmacists) or dispensing systems (eg, staff pharmacists), which display alerts via a graphical user interface or text-based format, respectively. We asked clinicians to report incidents, and then scheduled individual clinicians for CTA19 24 interviews.20 22 Interviewees provided written consent. We published a detailed study protocol20 and briefly describe methods below. The preparation of this article was informed by the Standards for Reporting Qualitative Research.25
Participants
Clinicians included prescribers (physicians and nurse practitioners) and pharmacists working in inpatient or outpatient care. Prescribers were eligible to participate if they had direct patient care roles. We recruited both staff pharmacists and clinical pharmacists. Throughout this article, we use the term ‘prescribing decisions’, since pharmacists at our study site and associated healthcare system have a broader authority compared with traditional pharmacy practice: clinical pharmacists can prescribe medications, and both staff and clinical pharmacists can make prescribing recommendations and take other actions, such as modifying prescriptions and discontinuing medications. For all clinicians, we excluded residents, other trainees and those with only administrative responsibilities. We invited all eligible clinicians to participate. Each clinician could submit an unlimited number of DDI incidents, but only one DDI incident per clinician was selected for an interview. There were no repeat interviews for any given DDI incident.
Patient and public involvement
Neither patients nor the public were involved in the study design or conduct of this research. Published results will be distributed via e-mail to study participants (ie, clinicians) and shared with physician and pharmacy leaders at the study site, for further distribution to clinicians at their discretion.
Data collection
Incidents
Clinicians were asked to complete a paper incident form when they became aware of a potential DDI concern involving two or more medications during routine clinical care and took any type of action to promote safety. Actions might include, for example, consulting a colleague, prescribing an alternative medication for one or both medications, or educating the patient about the DDI risk. We examined incidents where clinicians effectively managed DDI safety risks, regardless of whether an alert occurred, to build an evidence base to inform improved DDI CDS. Our incident form was previously published20 and was comprised of several items, including, ‘I first became aware of the potential problem by…’, ‘I used these resources to assess or respond to the potential conflict’, ‘The following actions were taken to address the conflict’, with several delineated response options for each item, along with an ‘other’ option for manual entry. Incidents were collected from September 2013 to September 2015. Clinicians’ incident reports were independently screened by a physician and a pharmacist on the research team. Our research objective was not focused on DDI alerts per se, but was to elucidate clinicians’ DDI decision-making strategies. We applied the CDM technique to achieve our objective; this method specifically focuses on incidents of effective decision-making under high stakes, difficult situations. Therefore, as outlined in our published study protocol,20 the reviewers intentionally screened each DDI incident against specific criteria in order to select a sample of high-stakes incidents for CDM interviews and further analyses. Each incident was screened according to five dimensions: (1) DDI was appropriately addressed; (2) DDI had the potential to cause serious injury or harm; (3) DDI required great expertise, coordination or consideration; (4) resolution of the DDI concern is likely to be harder for trainees compared with experienced clinicians and (5) DDI was challenging or unique. Reviewers rated each dimension on a 5-point Likert-type scale (1=strongly disagree and 5=strongly agree). Incidents were selected for interviews when the first two dimensions above were rated as a ‘4’ or higher, and other items were rated ‘3’ or higher. For incidents that met these criteria, a follow-up interview was scheduled within 1 month.20 Each clinician could continue to submit DDI incidents of unlimited number until one of their submitted incidents was selected for an interview or until study data collection concluded.
Cognitive task analysis
CTA methods include in-depth interviews and qualitative data analysis. We used a well-established interview technique18 known as the CDM,19 which is used to reconstruct real-life incidents and capture individual’s information gathering and problem-solving strategies. The interviewer asks questions to reconstruct a timeline of events and collects information about the individuals’ goals during the incident. This includes uncovering strategies that the individual used to detect and solve problems and identifying cues that aided their decisions.26 All interviews were led by a human factors scientist with expertise in medication safety and trained in CTA. Clinicians could access the EHR during interviews to answer questions and reconstruct the incident timeline. Below are sample questions from the published interview guide.20
What cues helped you become aware of the potential drug–drug interaction?
What concerns did you have about this drug–drug interaction?
What, if anything, made it difficult to decide what to do with the patient’s medication(s)?
In what ways did computerised alerts help you notice the right things and take action?
In what ways did alerts hinder you from noticing the right things and taking action?
Interviews were scheduled for 1 hour, audio-recorded, transcribed and deidentified for analysis.
Qualitative analysis
Qualitative analysis was conducted iteratively by an interdisciplinary team in two stages. First, two individuals, a human factors researcher with qualitative data analysis expertise and a medication safety pharmacist, independently analysed each incident to generate a decision requirements table.22 For each decision point during the incident, this table included columns to document the clinician’s associated strategies, cues, information they relied on to make decisions and challenges encountered, along with a corresponding column that includes a human factors analysis of potential system solutions. Analysts met to discuss each table entry until reaching consensus.27 Second, an inductive, qualitative analysis was conducted across tables without a predetermined coding scheme to identify emergent themes.27 28 This analysis team consisted of a human factors researcher, psychologist and pharmacist research assistant. After this team identified initial themes, the psychologist and pharmacist then independently coded the incidents, and discussed any discrepancies until reaching consensus.27 Throughout the analysis process, data that were difficult to code or appeared to represent new themes were discussed by the entire team until reaching consensus.27 Codes were entered into NVivo (V.11, QSR International, Burlington, MA) and multiple codes were assigned if the data clearly related to two or more themes.29 To maintain quality, a sample of four (20%) of the incidents were independently analysed by the entire team periodically.16 Data informed a descriptive model of clinicians’ DDI decision-making process.
Results
Participants and DDI incidents
We contacted 315 clinicians, 45 clinicians responded (14% response rate) and each of them agreed to enroll in the study. No recruited clinicians dropped out, but six clinicians (five physicians, one pharmacist) did not submit any safety incidents. Our final DDI interview sample included 20 clinicians (table 1) and 26 DDI pairs, since four clinicians submitted incident reports involving two or more distinct DDI pairs for the patient. Most DDI incidents were obtained from outpatient care.
Table 1.
Summary of participants and their associated demographic information
| Prescribers (n=11)* | Pharmacists (n=9)† | Total clinicians (N=20) | |
| Age (years) | 48 (34–63) | 37 (30–45) | 43 (30–63) |
| Female | 7 (64%) | 6 (67%) | 13 (65%) |
| Outpatient area of service | 11 (100%) | 6 (67%)‡ | 17 (85%) |
| Generalist practitioner | 5 (45%)§ | 7 (78%) | 12 (60%) |
| VA clinical experience (years) | 13 (3.5–26) | 6.5 (2–13.5) | 10 (2–26) |
Results are presented as mean (range) unless otherwise noted.
*Prescribers: 10 physicians and one nurse practitioner
†Two DDIs were analysed from one pharmacist (DDI incident, plus a separate renal-drug incident that also involved a DDI) and this clinician is represented only once in the table.
‡One pharmacist worked in both inpatient and outpatient settings, but is designated only as outpatient care, to align with the incident setting.
§Prescribers’ specialties: infectious disease, endocrinology, neurology, hematology/oncology and substance abuse.
DDI, drug–drug interaction; VA, veterans affairs.
DDI decision-making model
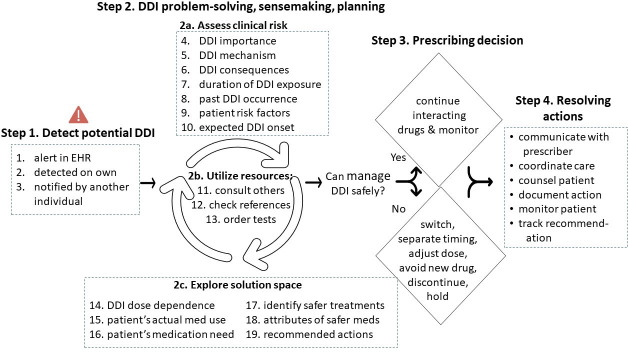
We identified four overarching steps in clinicians’ decision-making process for DDIs, numbered 1–4 in figure 1, which presents the descriptive model of clinicians’ decision-making process for DDIs. No new themes emerged after the first 16 incidents, indicating adequate data saturation. Key aspects of each step are discussed in subsections below. Altogether, across these steps, clinicians relied on 19 information cues to inform their decisions for DDI management, listed in figure 1; step 1 and steps 2a–c. There were no clear differences in decision-making or cues utilised across different clinician types; that is, our findings were supported by both prescribers and pharmacists. Below, we highlight key results for these steps and summarise clinicians’ reported barriers to satisfying these cognitive cues. In the results text below, steps from the model are indicated in bold, with substeps and cognitive cues indicated by italics.
Figure 1.
Descriptive model of clinicians’ DDI decision-making process. This figure shows the coding tree developed during the inductive qualitative analysis, with primary themes shown as ‘steps’ along with associated subthemes listed under each respective step. Step 1 and step 2 list cognitive cues from the inductive qualitative analysis that influenced clinicians’ DDI management. Definitions for each cognitive cue, and examples of quotes, are shown in tables 2–3. DDI, drug–drug interactions; EHR, electronic health records.
Step 1: detect potential DDI
The first essential step for clinicians was to identify any concerning DDIs. Of the DDI incident reports in our sample, 52% of incidents were detected by clinicians via an alert in the EHR during medication ordering or renewal; 43% of incidents clinicians identified DDI based on their own knowledge for example: during EHR chart review, while assessing a new medication, and from a change in the patients’ health status; and the remaining 5% of incidents were brought to the attention of the clinician by another individual. See example quotes in table 2. For almost all incidents (95%) in our sample, clinicians detected the DDI concern before the patient reported any symptoms of a harmful interaction or before dispensing the interacting drug. Reported barriers to DDI detection included EHR alert fatigue and overload, overabundance of text on the alert and lack of a safety alert system due to either paper prescriptions or medications ordered by prescribers from external institutions.
Table 2.
Cognitive cues involved in clinicians’ detection of potentially harmful drug–drug interactions (DDIs), as listed in figure 1 step 1.
| Cognitive cue # | Definition | Quote |
| 1. Alert in EHR | Clinician learns about a potential DDI via a computerised DDI alert in the EHR. | “An alert popped for the linezolid:bupropion interaction.”—physician A |
| 2. Detected on own | Clinician recognises the DDI concern based on their own knowledge about interacting medications, independent of any alert. | “So anytime I see amiodarone or warfarin on the same patient…[I see if] the interaction has already been accounted for….so I do that routinely on any patient that’s on both of those medications.”—pharmacist A |
| 3. Notified by another individual | The clinician becomes aware of a potential DDI because a DDI concern is raised by another individual, such as the patient, caregiver or another clinician. | “…they dispensed the medication, and then the [patient’s] son called back concerned about the drug interaction.”—pharmacist B |
Cues were derived from inductive qualitative analysis of clinicians’ reported incidents. The table above presents a definition for each cue, along with an example of a quote.
DDI, drug–drug interaction; EHR, electronic health record.
Step 2: DDI problem-solving, sensemaking and planning
This second step of the decision-making process was influenced by 16 cognitive cues (figure 1, step 2) that informed clinicians’ DDI management. A definition for each cue, along with an example quote, is shown in table 3A–C. Sometimes, clinicians delayed dispensing of a medication so they would have more time to develop a DDI management plan. Clinicians indicated that their goal for step 2 was to determine whether they could continue both medications as intended while safely managing the potential DDI. Towards this goal, clinicians assessed the clinical risk and explored the solution space in an iterative, parallel manner.
Table 3.
A–C. Cognitive cues involved in clinicians’ process of drug–drug interaction (DDI) problem-solving, sensemaking and planning, as listed in figure 1, step 2.
| Cognitive cue number, and cue | Definition | Quote |
| A. Assess clinical risk | ||
| 4. DDI importance | Drug knowledge information that influenced clinicians’ interpretation of the potential severity of the DDI. | “You always kind of wonder, ‘How serious is this [DDI]?’…does he absolutely need to be on a different medicine [to reduce risks], or should I push, should I optimize what he’s already on?”—physician B, omeprazole: citalopram |
| 5. DDI mechanism | Detailed information about the nature of the DDI, that is, the specific mechanism type of the interaction. | “[I was unaware of this DDI, and the alert] basically said that omeprazole would inhibit the metabolism of the escitalopram, resulting in increased concentrations of escitalopram, which increases your risk of serotonin syndrome.”—pharmacist C, escitalopram: omeprazole |
| 6. DDI consequences | Information about the type or degree of clinical risk to the patient, in terms of side effects or harm. | If they developed low blood pressure [from the DDI], they’re at risk for falls….they could fall and hit their head.”—pharmacist D, amlodipine: clarithromycin |
| 7. Duration of DDI exposure | The patient’s expected duration of exposure to the DDI and associated implications for DDI management. | “I looked at it like this…it’s going to be a short duration for antibiotics….The risk is a little bit less with a short term [exposure to this particular DDI].”—pharmacist E |
| 8. Past DDI occurrence | Past patient or provider experience with a DDI or problematic medication [or similar class] that helped to assess the patient’s susceptibility. Also includes larger patient population experience. | “I’d had a previous experience with a patient who got severely acidotic on linezolid, so [the linezolid:bupropion DDI risk of metabolic acidosis has] been imprinted on my brain.”—physician A, linezolid: bupropion |
| 9. Patient risk factors | Characteristics that make the patient more vulnerable or susceptible to DDI risks. | “So, it’s like all drug alerts are not equal [laughing], even if it’s the same drug-drug interaction, so it [my decision] had to do with patient factors….previously, he had an episode of heart failure that decompensated, so it was not a theoretical risk, it was real, so that’s why I didn’t feel comfortable just stopping the isosorbide [and starting the sildenafil]”—physician C, isosorbide: sildenafil |
| 10. Expected DDI onset | Information on the usual time range of when DDI risks begin to occur, since some DDIs have immediate consequences, while other DDIs only become a problem after weeks, months or more. | “…this one’s a delayed interaction….so with any drug [interaction], you always have this range of possible [times] when the interaction is going to present. So therein lies the challenge.”—pharmacist F, azithromycin: warfarin |
| B. Utilise resources | ||
| 11. Consult others | Contacting another healthcare professional, of similar or different professional degree, for reassurance or to seek guidance on the DDI. | “I called the inpatient clinical pharmacist [for advice]…. I just said to her, ‘He’s on 75 [mg sertraline]… would you think it would be reasonable at least for this amount of time to put him on 50 [mg sertraline during course of Linezolid]?”—pharmacist E, linezolid: sertraline |
| 12. Check references | Examining published reference[s] inform DDI decision-making. | “It’s nice that [I] have more than one reference to go to. That way, you can get different things from different ones… if I can’t get enough information [from] one… I’ll just pull them all up…”—pharmacist D, simvastatin: clarithromycin |
| 13. Order tests | Gathering more information about the patient by running tests or laboratories and interpreting the results. | [Interviewer: What were your thoughts when you were renewing the citalopram and all of sudden this alert came up?] “Oh, sh*t…. we [have] got to do an EKG right now.”—physician D, methadone: citalopram MD |
| C. Explore the solution space | ||
| 14. DDI dose dependence | Information about which DDIs can be appropriately managed via dose adjustments. | “[I was wondering] if there was any dose of the [atorvastatin] we could use [to manage the DDI], or if we really needed to think about using something different.”—pharmacist G, atorvastatin: diltiazem DDI. |
| 15. Patient’s medication use | Performing medication reconciliation activities or gathering information about whether and how often the patient is taking specific medication[s] in order to inform decisions regarding DDI management. | “[The patient] was taking it [omeprazole] for reflux. He hadn’t had any ulcers or anything like that, and so he was actually only taking it intermittently…”—physician E, rilpiverine: omeprazole |
| 16. Patient’s medication need | Information about the indication of an interacting drug, that is, the medical need for the patient to continue with one or more of the drugs involved in the interacting pair | “I asked for medical records from this outside hospital to determine if it was imperative that he’s on this linezolid…”—pharmacist E, linezolid: sertraline |
| 17. Identify safer treatments | Considering safer alternatives to an interacting medication or exploring whether alternative medications introduce another DDI. | “After the alert popped up, I queried Lexi-comp and UptoDate to see if there were less interactions with other SSRIs…”—physician F, omeprazole: escitalopram |
| 18. Attributes of safer medications | Characteristics of alternative medications—beyond DDI safety—such as drug cost, formulary status and treatment effectiveness. | “…if you took the simeprevir out [switched it to avoid the DDI], the treatment duration is longer, the success rate is lower, and the cost is more.”—physician E, efavirenz: simeprivir |
| 19. Recommended actions | Alert terms ‘significant’ and ‘major’ were often ambiguous to clinicians, who instead sought information on the recommended action for managing the DDI, such as ‘stop one medication’, ‘separate the timing of drug administration’ or ‘monitor’ as a means of gauging the DDI’s importance. | “…you can click on this and…it will say, ‘your risk rating [is] D, consider therapy modification’….this is more important [i.e., useful] than what comes up in the alert…”—physician F, omeprazole: escitalopram |
Cues were derived from inductive, qualitative analysis of clinicians’ reported incidents. Numbering of cues is continued from the cognitive cues in figure 1, step 1, and as outlined in table 2. This table presents a definition for each cue, along with an example quote.
DDI, drug–drug interaction; EHR, electronic health record; EKG, electrocardiogram; SSRI medications, selective serotonin reuptake inhibitors.
To further inform their information gathering for either of these aspects, clinicians intermittently utilised resources, as shown in figure 1, step 2b.
In step 2a, when assessing the clinical risk of unfamiliar DDIs, clinicians relied on three of the seven cognitive cues in particular: (1) confirmation that the DDI interaction is clinically important; (2) DDI pharmaceutical or physiological mechanism and (3) potential clinical consequences of the DDI for the patient. For DDI importance, clinicians relied on several sources of drug knowledge, including drug references (eg, Micromedex, Lexicomp and Clinical Pharmacology), drug manufacturers’ black box warnings and DDI alert categorisation (eg, DDI alert displayed as ‘critical’ in the EHR). The length of time during which the patient was, or would be, exposed to the DDI was a factor taken into consideration by almost half (9/20) of participants. Instances where the patient was taking the interacting pair for a long time without problems lessened clinicians’ concern about the DDI, as did situations where the patient would only be taking the drug pair for a short duration (eg, a few days), such as when one interacting medication was an antibiotic. For patient risk factors (table 3A, #9), where clinicians assessed an individual patient’s vulnerability to the DDI, factors varied widely by DDI drug pair: for instance, clinicians considered characteristics such as patient’s age, renal function, mental health, pain level, history of side effects from one of the interacting medications, level of caregiver support and relevant diagnoses (eg, heart condition and dementia). Barriers to step 2a included uncertainty about three cognitive cues in particular: past DDI occurrence, especially whether the patient has taken the interacting combination of medications previously with any ill effects; the expected DDI onset, that is, about how much time it takes for DDI effects to occur, which informs prescribing decisions and DDI safety monitoring and relevant patient risk factors, such as whether the patient or caregiver is capable of monitoring for potential DDI effects.
When clinicians lacked information to satisfy cues in steps 2a or c, they often used resources (step 2b), consulting colleagues or drug references, and checking for congruence across multiple DDI information sources (eg, Micromedex, UpToDate). Clinicians checked references—such as Clinical Pharmacology, Lexicomp, Micromedex and UptoDate—to supplement their own knowledge, assess unfamiliar DDIs, double-check information provided by DDI alerts and associated DDI monographs, or fill in gaps during instances where DDI alerts lacked sufficient information. Clinicians reported two barriers to the use of drug references: vague dosing recommendations and inconsistent information for various DDI details across different references. When two references conflicted, or lacked sufficient detail, clinicians reported consulting a third reference before making a prescribing decision. Another barrier to step 2b included the need to order diagnostic or laboratory tests (eg, EKG, international normalised ratio and cholesterol) prior to making a prescribing decision for DDI management. These tests took time and sometimes delayed the prescribing decision, or in other cases, prescribing decisions needed to be made in the meantime while awaiting test results and then potentially revised later. Additionally, clinicians reported difficulty with reviewing laboratory result trends (eg, international normalised ratio), as related to other aspects of the patients’ care, such as changes to medication dosing, which impeded DDI management. Finally, clinicians reported that, when they wanted to consult others for DDI guidance, finding an appropriate specialist was challenging, which was further exacerbated on night and weekend shifts.
In step 2c, clinicians relied on six cues to identify potential DDI management solutions and inform their prescribing decisions (figure 1). Especially for unfamiliar DDIs, clinicians sought information on whether the DDI was dose dependent vs independent, to determine whether the DDI could be mitigated via dose adjustments, rather than switching to an alternate medication. Clinicians reported several barriers to switching to an alternate medication, including limited therapeutic alternatives to avoid the DDI; variation in medication availability and differences in tablet appearance across medical centres or external healthcare organisations; and added complexity to avoid a DDI by prescribing non-formulary medications or other medications that required special approval. Identifying solutions was also reported as difficult when patients’ medication lists were incomplete and medication reconciliation had not been adequately performed across the patients’ healthcare team prior to detecting the DDI. Furthermore, DDIs involving combination medications, where three or more medications needed to be considered and simultaneously managed, limited options for potential alternate medications and increased the complexity of DDI management.
Step 3: prescribing decision
Clinicians made a variety of prescribing decisions across the DDI pairs (see figure 1 and online supplemental appendix).
bmjopen-2023-075512supp001.pdf (76.7KB, pdf)
Step 4: resolving actions
Clinicians engaged in several activities to carry out prescribing decisions or ensure their decisions were enacted by other clinicians. These are outlined in figure 1, step 4, and are not discussed in depth here, since we previously described these aspects as part of a publication about care coordination from the larger study.21
Discussion
This research is the first to apply CTA to elucidate clinicians’ real-world decision-making for DDIs. Our study provides three key contributions: (1) it is the first to present an illustrative model of clinicians’ real-world decision-making for managing DDIs for patients; (2) our findings add to scientific knowledge by identifying 19 cognitive cues that clinicians routinely use for DDI detection and management in clinical practice and (3) our results provide essential, foundational knowledge to inform more robust CDS for DDIs in the future. Each key contribution is described below.
First, our results yield a valuable, descriptive model (figure 1) of clinicians’ decision-making process for DDIs, from the initial point of potential DDI detection to incident resolution. We did not find any other publication that presents an evidence-based, data-driven model such as this for clinicians’ DDI management. We expect this model can be applied in several ways to improve patient care and safety. For example, healthcare systems can be more thoughtfully designed to support each step of the model, especially step 2 ‘DDI problem-solving, sensemaking and planning’, with clinicians iteratively assessing clinical risk, consulting resources and exploring solutions for DDI management, often simultaneously. Moreover, our results outline barriers to steps within the model, highlighting opportunities for system improvements to enhance clinicians’ efficiency and safety with DDI decision-making. For instance, our results indicate a need for improved EHR data visualisation that allows clinicians to easily view laboratory result trends as related to changes in medication doses and DDI risks. Finally, this model can be incorporated into curricula for healthcare trainees, such as medical and pharmacy students or residents, to help them build evidence-based, mental models for DDI decision-making earlier on in their clinical practice, with the potential to enhance medication safety for patients.
Second, a unique contribution of our study is that it unveils cognitive cues used by clinicians that have not been delineated in prior literature. For example, our study adds significant findings to those of Phansalkar et al, who compared DDI alerts to known human factors principles,30 and found four of the 12 cognitive cues that we identified in figure 1, steps 2a and c for clinicians’ real-world DDI decisions. Romagnoli et al interviewed six drug information experts and conducted a systematic literature review to delineate the information needs of individuals who develop information for publication in DDI references.31 The study comprised a small sample of interview participants, which included clinical pharmacists, drug information compendia editors and academic drug information specialists. Our study adds to the literature by detailing the specific DDI information needs of practicing clinicians. Consequently, our results outline clinicians’ information needs for broader aspects of DDI decision-making, including DDI detection (step 1), resource utilisation (step 2b) and development of potential DDI management solutions (steps 1, 2b and 2c, respectively). Similar to our research, Romagnoli et al identified a set of potential DDI recommendations (see figure 1, step 3, ‘prescribing decisions’); these directly align with our data from practicing clinicians, supporting the validity and comprehensiveness of DDI prescribing decisions of our study sample and associated results.31 Another study team, Bagri et al conducted focus groups with a total of 24 hospital pharmacists, to understand their perceptions of DDIs.17 Their summary of how pharmacists assessed DDIs was analogous to five of the seven cues we identified in step 2a, ‘assess clinical risk’. In other words, their findings accounted for all cues listed under our figure 1, step 2a, except ‘DDI mechanism’ and ‘duration of DDI exposure’. Their study also identified one information need for this step—whether DDI consequences are reversible—that was not identified by our research. The high concordance between our studies for DDI assessment (figure 1 step 2a), however, provides strong evidence of external validity for both of our qualitative studies, at least in terms of this step of the DDI decision-making process. The information needs reported by Bagri et al are largely limited to step 2a ‘DDI assessment’ (figure 1) from our study, with our research providing a more robust delineation of the clinicians’ entire DDI decision-making process.
This study has limitations. We used a specialised data collection form, and clinicians could access the EHR to answer our interview questions,20 but the retrospective nature of our study may be subject to some recall bias regarding clinicians’ interpretation of events. We did not return interview transcripts to clinicians for review nor conduct member checking with participants. There are thousands of potential DDIs, and our study examined a small sample, so there could be other cognitive cues that clinicians use, particularly for rare DDIs, despite evidence that we reached qualitative data saturation for cues. There were no clear differences in decision-making across participant groups. Our sample included a wide variety of naturally occurring DDI incidents, with many types of medication pairs involved and many different patient characteristics, which precludes more nuanced analysis by participant type.21 Future research could apply simulation interviews along with standardised scenarios to systematically compare physicians’ and pharmacists’ approaches to DDI decision-making. Most of our sample (85%, table 1) was obtained from outpatient incidents, so our results are most likely to reflect clinicians’ DDI management in outpatient care. Likewise, our sample of pharmacists consisted primarily of outpatient clinical pharmacists, rather than inpatient clinical pharmacists or staff pharmacists who verify and dispense medications. Thus, our findings are more applicable to the hospital or outpatient clinical setting, rather than community-based pharmacies. For instance, unlike pharmacists in our study, community-based pharmacists typically do not have access to patients’ EHR data to make DDI decisions.
Nonetheless, our results provide evidence to inform more advanced DDI alerts for real-world clinical practice. Historically, EHR DDI alerts were designed in the absence of an in-depth examination of clinicians’ decision-making process during real-world clinical care; our study fills this scientific gap. The problem of alert fatigue for DDI and other alerts is well documented in the literature.9 32 33 For example, a previous VA study1 reported that over 290 000 DDI alerts occurred across six medical centres in a 1 year period. That study only accounted for over-ridden DDI alerts during outpatient medication dispensing; thus, the total alert burden for DDIs alone is estimated to be over 48 000 alerts/year per medical centre.1 15 In this study and our previous research, we found that clinicians’ decision-making was not adequately supported even by clinically relevant EHR DDI alerts.10 16 This was particularly true for step 2 of clinicians’ decision-making process, indicating a need for associated DDI alert enhancements. Table 4 outlines key cognitive cues where alert information from step 2 was particularly lacking, along with proposed alert design recommendations.
Table 4.
Potential recommendations aid clinicians’ DDI decision-making and patient safety.
| Cognitive cue | Alert design recommendation(s) |
| a. Expected DDI onset (step 2 a). Clinicians sought to determine the expected onset of DDI risks, since some DDIs have immediate consequences, while other DDIs only become a problem after weeks or more. This informed clinicians’ DDI management and monitoring. | Indicate in the alert text the expected range of timing for the onset of potential DDI effects (eg, days, weeks, months or years). |
| b. Congruence across DDI reference sources (step 2b). For unfamiliar DDIs, clinicians checked for congruence across DDI information sources (eg, Micromedex and UpToDate). When two sources conflicted, clinicians often consulted a third source before choosing a course of action. (Note: according to an analysis of 14 DDI resources, overlap of DDIs between any two resources is generally<50%.)31 | Provide a means for clinicians to review multiple electronic DDI reference sources directly from the alert, side-by-side. |
| c. DDI dose-dependence (step 2 c). Clinicians expressed a desire to know which DDIs could be safely managed via dose adjustments, vs which DDIs were not dose-dependent. | Explicitly state on the alert whether the DDI is dose-dependent or dose independent. |
| d. Multifaceted, alternative medications (step 2 c). Clinicians sought a safer medication that met each of these criteria: (1) avoids the DDI in question; (2) avoids introducing another DDI with the patient’s other medications listed in the EHR and (3) is available from the organisation’s medication formulary. | Leverage data analytics to populate DDI alerts with ‘smart’ alternative drug options that account for each of these three criteria. |
| e. DDI management recommendation (step 2 c). Alert terms such as ‘significant’ and ‘major’ were often ambiguous to clinicians, who instead sought information on the recommended action for managing the DDI, such as ‘stop one medication’, ‘separate the timing of drug administration’ or ‘monitor’ as a means of gauging the DDI’s importance. | State DDI management recommendation(s) on the alert along with associated patient characteristics (eg, ‘Monitoring possible if the patient indicates a willingness and capability of measuring blood pressure daily.’). |
Above, we highlight key cues used by clinicians during step 2 of the DDI decision-making process (figure 1) that were not well supported by alerts and we provide corresponding DDI alert design recommendations. Cues listed above are depicted in figure 1 model and outlined in table 3.
DDI, drug–drug interaction; EHR, electronic health record.
The organisation where this study was conducted has plans to change EHR vendors, and as researchers we do not have the authority to make implementation decisions for alerts, so the extent to which our recommendations (table 4) will be adopted by alert designers is unknown. Preliminary findings from this research were disseminated at national US scientific meetings. This article will also be shared with national VA administrators, as well as leading EHR and drug alert knowledge venders, to further inform the development of future DDI alerts. For any alert modifications, usability testing and piloting in a clinical setting is warranted to systematically evaluate and strengthen redesigns prior to full-scale implementation.
Conclusions
Our study provides new, foundational evidence regarding clinicians’ DDI decision-making process. To the best of our knowledge, this is the first study to apply cognitive task analysis to delineate clinicians’ decision-making for DDIs. We identified 19 cognitive cues upon which clinicians rely to detect and make decisions regarding DDIs for patients. DDI CDS should be designed to support these information needs. From our results, we propose recommendations to enhance DDI CDS to better support clinicians’ real-world practice, which could subsequently reduce harm to patients. The proposed recommendations from this research should be further evaluated in future usability and clinical studies. Our results suggest that these DDI CDS recommendations could improve clinicians’ decision-making efficiency, confidence and effectiveness by equipping them with the information necessary to manage DDIs in clinical practice.
Supplementary Material
Acknowledgments
The authors thank the study participants for making this work possible. We would like to thank Ms. Rachel Dismore for her assistance with participant recruitment and incident card collection. Donna Burgett at Regenstrief for her assistance with proofreading and formatting. Rachel Dismore, Zamal Franks, Amy Brandt, Linda Collins and Steven Sanchez assisted with IRB procedures.
Footnotes
Contributors: ALR-J wrote the funded grant proposal (VA HSR&D CDA 11-214), with input from PAG and MW. Seven of the authors (ALR-J, KJA, LGM, AJZ, AI, PAG and MW) participated in study planning meetings and contributed to the design of the larger study. LGM trained ALR-J on cognitive task analysis and the critical incident technique. ALR-J conducted all cognitive task analysis interviews. ALR-J and KJA analysed interview transcripts to develop a decision requirements table for each incident. NE and JD analysed decision requirements tables across incidents to identify emergent themes, under the mentorship of ALR-J and KJA. ALR-J drafted the manuscript. All authors (ALR-J, KJA, LGM, JD, NE, AJZ, AI, PAG and MW) reviewed and made important scientific edits to the manuscript and approved it for submission. ALR-J is responsible for the overall content as guarantor. As guarantor, ALR-J accepts full responsibility for the finished work, the conduct of the study, had access to the data, and controlled the decision to publish.
Funding: This work was supported by a VA HSR&D Career Development Award 11-214 (PI: ALR-J) along with the Center for Health Information and Communication, Department of Veterans Affairs, Veterans Health Administration, Health Services Research and Development Service, CIN 13-416 (PI: Weiner). Dr Weiner is Chief of Health Services Research and Development at the Richard L. Roudebush Veterans Affairs Medical Center in Indianapolis, IN. Manuscript writing was supported in part by VA IIR 16-297.
Disclaimer: Views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs or the US government.
Competing interests: LM is co-owner of Applied Decision Science, LLC, a company that studies decision-making in complex environments and utilises the critical decision method. She aided in the design of the cognitive task analysis approach used in this study and trained the interviewer. MW has held stock in Allscripts and Express Scripts Holding Company.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
No data are available.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
This study involves human participants. All study procedures were approved by the Indiana University Institutional Review Board and VA Research and Development Committee. Participants gave informed consent to participate in the study before taking part.
References
- 1.Grizzle AJ, Mahmood MH, Ko Y, et al. Reasons provided by prescribers when overriding drug-drug interaction alerts. Am J Manag Care 2007;13:573–8. [PubMed] [Google Scholar]
- 2.Das S, Behera SK, Xavier AS, et al. Are drug-drug interactions a real clinical concern Perspect Clin Res 2019;10:62–6. 10.4103/picr.PICR_55_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carpenter M, Berry H, Pelletier AL. Clinically relevant drug-drug interactions in primary care. Am Fam Phys 2019;99:558–64. [PubMed] [Google Scholar]
- 4.Baysari MT, Zheng WY, Li L, et al. Optimising computerised decision support to transform medication safety and reduce prescriber burden: study protocol for a mixed-methods evaluation of drug-drug interaction alerts. BMJ Open 2019;9:e026034. 10.1136/bmjopen-2018-026034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Russ AL, Saleem JJ, McManus MS, et al. Computerized medication alerts and prescriber mental models: observing routine patient care. Proc Hum Factors Ergonom Soc Ann Meet 2009;53:655–9. 10.1177/154193120905301105 [DOI] [Google Scholar]
- 6.Russ AL, Zillich AJ, McManus MS, et al. A human factors investigation of medication alerts: barriers to prescriber decision-making and clinical workflow. AMIA Annu Sym Proc 2009:548–52. [PMC free article] [PubMed] [Google Scholar]
- 7.Feldstein A, Simon SR, Schneider J, et al. How to design computerized alerts to safe prescribing practices. Jt Comm J Qual Saf 2004;30:602–13. 10.1016/s1549-3741(04)30071-7 [DOI] [PubMed] [Google Scholar]
- 8.Gallimore JJ, Wong PK. Implementation of electronic systems for prescribing and delivering medication in hospitals: issues in real practice. Proc Hum Factors Ergonom Soc Ann Meet 2007;51:740–4. 10.1177/154193120705101129 [DOI] [Google Scholar]
- 9.Payne TH, Hines LE, Chan RC, et al. Recommendations to improve the usability of drug-drug interaction clinical decision support alerts. J Am Med Inform Assoc 2015;22:1243–50. 10.1093/jamia/ocv011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Russ AL, Zillich AJ, Melton BL, et al. Applying human factors principles to alert design increases efficiency and reduces prescribing errors in a scenario-based simulation. J Am Med Inform Assoc 2014;21:e287–96. 10.1136/amiajnl-2013-002045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marcilly R, Zheng WY, Beuscart R, et al. Comparison of the validity, perceived usefulness and usability of I-Medesa and TEMAS, two tools to evaluate alert system usability: a study protocol. BMJ Open 2021;11:e050448. 10.1136/bmjopen-2021-050448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marcilly R, Zheng W-Y, Quindroit P, et al. Comparison of the validity, perceived usefulness, and usability of I-Medesa and TEMAS, two tools to evaluate alert system usability. Int J Med Inform 2023;175:105091. 10.1016/j.ijmedinf.2023.105091 [DOI] [PubMed] [Google Scholar]
- 13.Zachariah M, Phansalkar S, Seidling HM, et al. Development and preliminary evidence for the validity of an instrument assessing implementation of human-factors principles in medication-related decision-support systems--I-Medesa. J Am Med Inform Assoc 2011;18(Suppl 1):i62–72. 10.1136/amiajnl-2011-000362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zheng WY, Van Dort B, Marcilly R, et al. A tool for evaluating medication alerting systems: development and initial assessment. JMIR Med Inform 2021;9:e24022. 10.2196/24022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Russ AL, Chen S, Melton BL, et al. A novel design for drug-drug interaction alerts improves prescribing efficiency. Jt Comm J Qual Patient Saf 2015;41:396–405. 10.1016/s1553-7250(15)41051-7 [DOI] [PubMed] [Google Scholar]
- 16.Russ AL, Zillich AJ, McManus MS, et al. Prescribers' interactions with medication alerts at the point of prescribing: a multi-method, in situ investigation of the human-computer interaction. Int J Med Inform 2012;81:232–43. 10.1016/j.ijmedinf.2012.01.002 [DOI] [PubMed] [Google Scholar]
- 17.Bagri H, Dahri K, Legal M. Hospital pharmacists' perceptions and decision-making related to drug-drug interactions. Can J Hosp Pharm 2019;72:288–94. [PMC free article] [PubMed] [Google Scholar]
- 18.Hoffman RR, Militello LG. Perspectives on cognitive task analysis: historical origins and modern communities of practice. New York, NY: Taylor and Francis, 2008. 10.4324/9780203809877 [DOI] [Google Scholar]
- 19.Crandall B, Klein GA, Hoffman RR. Working minds: A practitioner’s guide to cognitive task analysis (A Bradford Book). Cambridge, MA: MIT Press, 2006. 10.7551/mitpress/7304.001.0001 [DOI] [Google Scholar]
- 20.Russ AL, Militello LG, Glassman PA, et al. Adapting cognitive task analysis to investigate clinical decision making and medication safety incidents. J Patient Saf 2019;15:191–7. 10.1097/PTS.0000000000000324 [DOI] [PubMed] [Google Scholar]
- 21.Russ-Jara AL, Luckhurst CL, Dismore RA, et al. Care coordination strategies and barriers during medication safety incidents: a qualitative, cognitive task analysis. J Gen Intern Med 2021;36:2212–20. 10.1007/s11606-020-06386-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elkhadragy N, Ifeachor AP, Diiulio JB, et al. Medication decision-making for patients with renal insufficiency in inpatient and outpatient care at a US veterans affairs medical centre: a qualitative, cognitive task analysis. BMJ Open 2019;9:e027439. 10.1136/bmjopen-2018-027439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nguyen KA, Militello LG, Ifeachor A, et al. Strategies prescribers and pharmacists use to identify and mitigate adverse drug reactions in inpatient and outpatient care: a cognitive task analysis at a US veterans affairs medical center. BMJ Open 2022;12:e052401. 10.1136/bmjopen-2021-052401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoffman RR, Crandall B, Shadbolt N. Use of the critical decision method to elicit expert knowledge: a case study in the methodology of cognitive task analysis. Hum Factors 1998;40:254–76. 10.1518/001872098779480442 [DOI] [Google Scholar]
- 25.O’Brien BC, Harris IB, Beckman TJ, et al. Standards for reporting qualitative research: a synthesis of recommendations. Acad Med 2014;89:1245–51. 10.1097/ACM.0000000000000388 [DOI] [PubMed] [Google Scholar]
- 26.Woolley A, Kostopoulou O. Clinical intuition in family medicine: more than first impressions. Ann Fam Med 2013;11:60–6. 10.1370/afm.1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bradley EH, Curry LA, Devers KJ. Qualitative data analysis for health services research: developing taxonomy, themes, and theory. Health Serv Res 2007;42:1758–72. 10.1111/j.1475-6773.2006.00684.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patterson ES, Cook RI, Render ML. Improving patient safety by identifying side effects from introducing bar coding in medication administration. J Am Med Inform Assoc 2002;9:540–53. 10.1197/jamia.m1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Slaughter L, Keselman A, Kushniruk A, et al. A framework for capturing the interactions between Laypersons' understanding of disease, information gathering behaviors, and actions taken during an epidemic. J Biomed Inform 2005;38:298–313. 10.1016/j.jbi.2004.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Phansalkar S, Zachariah M, Seidling HM, et al. Evaluation of medication alerts in electronic health records for compliance with human factors principles. J Am Med Inform Assoc 2014;21:e332–40. 10.1136/amiajnl-2013-002279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Romagnoli KM, Nelson SD, Hines L, et al. Information needs for making clinical recommendations about potential drug-drug interactions: a synthesis of literature review and interviews. BMC Med Inform Decis Mak 2017;17:21. 10.1186/s12911-017-0419-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murad DA, Tsugawa Y, Elashoff DA, et al. Distinct components of alert fatigue in physicians' responses to a noninterruptive clinical decision support alert. J Am Med Inform Assoc 2022;30:64–72. 10.1093/jamia/ocac191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Russ AL, Melton BL, Daggy JK, et al. Pilot evaluation of a method to assess prescribers' information processing of medication alerts. J Biomed Inform 2017;66:11–8. 10.1016/j.jbi.2016.11.011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2023-075512supp001.pdf (76.7KB, pdf)
Data Availability Statement
No data are available.