Abstract
Epidemiological evidence suggests that adverse environmental factors in the nasal cavity may increase the risk for neuropsychiatric diseases. For instance, air pollution and nasal viral infection have been underscored as risk factors for Parkinson’s disease, schizophrenia, and mood disorders. These adverse factors can elicit local inflammation in the nasal cavity, which may in turn influence higher brain function. Nevertheless, evidence that directly supports their causal link is missing. To fill this knowledge gap, we used an inducible mouse model for olfactory inflammation and showed the evidence that this local pathological factor can elicit behavioral abnormalities.
Keywords: Nasal inflammation, Olfactory epithelium, Inducible olfactory inflammation, Schizophrenia, Adolescence, COVID-19, SARS-CoV-2
Olfactory dysfunction has been reproducibly reported across neurodegenerative and neuropsychiatric disorders, such as Alzheimer’s disease (AD), Parkinson’s disease (PD), and schizophrenia (SZ) (Doty, 2017; Moberg et al., 2014). Importantly, olfactory impairments frequently precede neurological and psychiatric manifestations (Brewer et al., 2003; Kamath et al., 2019, 2018; Moberg et al., 2014). Olfactory deficits are capable of identifying patients at high risk for unremitting negative symptoms, particularly anhedonia, and predicting poor outcomes in patients with psychosis (Corcoran et al., 2005; Ishizuka et al., 2010; Moberg et al., 2014).
Odorants are received by olfactory sensory neurons (OSNs) in the olfactory epithelium (OE). OSNs project to glomeruli within the olfactory bulb (OB), and odor information is transferred to higher cerebral cortex, such as the medial prefrontal cortex and orbitofrontal cortex via projection from the primary olfactory cortices (Mori and Sakano, 2021). Therefore, the olfactory deficits can be elicited by deficits in either the peripheral olfactory system (e.g., the OE and OB), or the central olfactory system, or both.
Epidemiological evidence highlights air pollution as an environmental risk factor for multiple neuropsychiatric disorders, such as PD, AD, and SZ (Attademo et al., 2017; Shi et al., 2020). Air pollution can elicit pathological responses in the OE, including inflammatory responses (Ajmani et al., 2016). Viral infection in the nasal cavity directly disturbs the OE as another main stressor (van Riel et al., 2015). The pathological link of viral infection in the nasal cavity and brain disorders has also been suggested in herpes simplex virus and AD (Bathini et al., 2019). During the current outbreak of novel coronavirus disease 2019 (COVID-19), severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and its transmucosal invasion through the nasal cavity are underscored in the context of olfactory dysfunction, possibly followed by deficits of higher brain function associated with psychosis (Steardo et al., 2020). These results imply that disturbance of the peripheral olfactory system may underlie multiple neuropsychiatric disorders. Nevertheless, its causal impact of the peripheral olfactory system and its mechanism for the disease pathophysiology remain to be elucidated.
In rodent models, disturbance of the peripheral olfactory system elicits behavioral changes (Chen et al., 2014; Hendriksen et al., 2015; Moberly et al., 2018). For example, surgical removal of the OB (i.e., olfactory bulbectomy) results in hyperactivity, altered sleep pattern, behavioral despair, and abnormalities in various cognitive behaviors, including fear learning (Hendriksen et al., 2015). Notably, these include odor-independent behaviors, suggesting that olfactory bulbectomy does not simply cause olfactory deficits but also elicits pathophysiological alterations in higher cerebral cortex regulating the odor-independent behaviors. Nevertheless, there remain two major knowledge gaps: (1) although inflammatory response in the OE elicited by air pollution and viral infection may contribute to the etiology of neuropsychiatric conditions, whether olfactory inflammation-induced disturbance in the peripheral olfactory system indeed causes behavioral alterations of neuropsychiatric relevance remains obscure; and (2) the mechanisms by which peripheral olfactory disturbance lead to the pathology of higher cerebral cortex that underlies behavioral abnormalities remain to be investigated.
The goal of the present study is to address the first question described above as the initial step of our long-term efforts to address the overall questions. Accordingly, we used an inducible olfactory inflammation (IOI) mouse model that was generated by crossing the Tet-response element (TRE)-Tumor Necrosis Factor α (TNF-α) transgenic mice with Cyp2 g1-reverse tetracycline trans-activator (rtTA) line and developed in the C57BL/6 background as previously described (Lane et al., 2010). In these previous studies, IOI mice were treated with 0.2 g/kg doxycycline (DOX) food for 6 weeks to induce TNF-α expression in the sustentacular cells of the OE, leading to chronic and local OE inflammation. TNF-α-induced chronic OE inflammation resulted in substantial loss of OSNs, leading to reduced response of the OE to odorants as assessed by electroolfactogram recordings (Lane et al., 2010).
In this study, we examined the behavioral impact of chronic OE inflammation in male and female IOI mice housed on a reversed 12-h light/dark cycle. All tests were conducted during the dark period of the cycle. 12-week (n = 8), 16-week (n = 9), and 20-week (n = 6) old mice were fed a DOX-containing diet (IOI group) or non-DOX-containing diet (control group) for 6 weeks and during behavioral tests including a 1-week habituation. The behavior tests were performed in the following order using the published protocols including ours (Han et al., 2003; Hikida et al., 2007; Johnson et al., 2013; Matsuoka et al., 2008; Saito et al., 2016; Sumitomo et al., 2017; Zhu et al., 2019; Zoubovsky et al., 2011). Olfactory habituation/dis-habituation test: Each mouse was tested during three consecutive 2-min periods for each odor with 2-min intervals and the time that a mouse smelled the swab was recorded. Open field test: Locomotor activity was assessed over a 30-minute period in 40 × 40 cm activity chambers with built-in infrared beams (PAS system, San Diego Instruments Inc., San Diego, CA, USA). Elevated plus maze test: A mouse was placed in the intersection (middle) of the “open” and “closed” arms in the plus maze (San Diego Instruments, Inc.). The number of entries and the time spent in the arms were recorded for 5 min. Three-chamber social approach test: Time spent sniffing of the subject mouse in the 10-min sociability session and 10-min social novelty session were manually measured by the researchers. The heat maps were generated by Ethovision XT 11.0 (Noldus, Leesburg, VA). In the sucrose preference test: During days 1–2 and 3–4, a mouse was habituated with bottle “A” and “B” with water and 1.5 % sucrose, respectively. During days 5–8, bottle A contained 1.5 % sucrose, and bottle B contained drinking water. Sucrose preference on each day was calculated as 100 % × (Vol A/(Vol A + Vol B)). Trace fear conditioning test: This test was conducted on three consequent days in large sound-proof isolation chambers (Med associates Inc.). Mice were subject to associate a pure tone (conditioned stimulus, CS; 3 KHz, 70 dB, 20 s each) with a foot-shock (unconditioned stimulus, US; 2 s, 0.5 mA), with the CS and US being separated by a trace interval (18 s). The CS-US pairing was repeated 4 times at intervals with variable duration (160−200 s). One day after fear acquisition, cued fear retrieval was measured by recording freezing responses to the same pure tones (CS) 4 times in a newly-constructed context, with each tone being delivered at variable intervals (160–200 s). Freezing (%) was scored every 10 s using Video Freeze Software (Med associates Inc.). The interval between different behavioral tests was at least 24 h. Each apparatus was cleaned with 70 % ethanol between individual animals to control for odor cues. All studies were conducted with approved protocols from the Johns Hopkins University Institutional Animal Care and Use Committee and were in accordance with the NIH guidelines for the Care and Use of Laboratory Animals. All data are presented as means ± standard error of the mean (S.E.M.). Statistical comparisons between IOI and control mice were performed using two-tailed unpaired Student t-test. Two-way mixed analyses of variance (ANOVA) were also used for data analysis followed by Bonferroni’s post hoc tests with multiple comparisons in olfactory habituation/dis-habituation test, social interaction test, and trace fear conditioning. A value of p < 0.05 was considered statistically significant.
Given that changes in body weight may nonspecifically affect behavioral phenotypes, we confirmed no difference in body weight between IOI mice and controls after a 6-week DOX treatment (Fig. 1A). We first examined whether chronic OE inflammation impairs olfaction using the olfactory habituation/dis-habituation test. The control mice showed an increased sniffing time at the first presentation of the first odorant and greater response to the second odorant compared with that to the previous odorant, whereas the IOI mice had no response to either odorant, suggesting that olfaction is impaired by chronic OE inflammation in IOI mice (Fig. 1B). Animals were then evaluated in the open field test to measure locomotor activity and anxiety-like behavior. We found no difference in locomotion and rearing between the two groups (Fig. 1C). Since mice with reduced anxiety-like behavior tend to prefer center areas in the open field apparatus, the percentage of time spent in the center of the open field was also measured. There was no signifi-cant difference in the percentage of time spent in the central area between the two groups (Fig. 1C). Consistently, no difference was observed in the percentage of time spent in the open arm between IOI mice and controls in the elevated plus maze test (Fig. 1D).
Fig. 1.
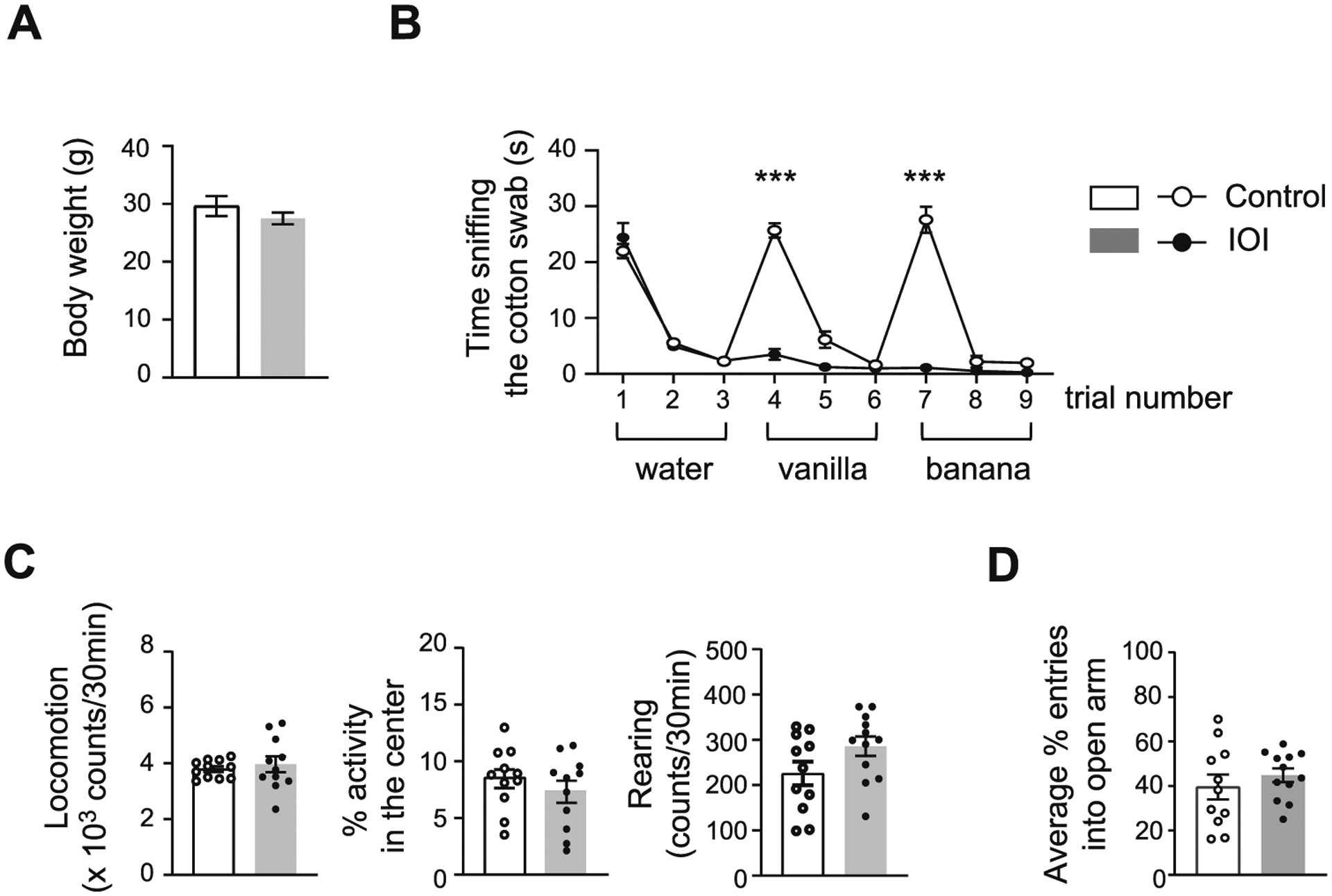
Chronic OE inflammation impairs olfactory discrimination and has no effect on locomotion and anxiety. (A) There was no difference in body weight between IOI mice and control mice. p = 0.2904, t = 1.102, df = 13 determined by Student’s t-test. IOI, n = 8 (7 male, 1 female); Control, n = 7 (6 male, 1 female). Data are presented as the mean ± SEM. (B) The mice were subsequently exposed to water and two different odorants (i.e., vanilla and banana) 3 consecutive times. The control mice showed an increase in time sniffing to water and both odorants for the first time, whereas IOI mice displayed no response to either odorant, suggesting that chronic OE inflammation impairs olfactory discrimination. Dishabituation: odor of water to vanilla; F1,13 = 220.1, ***p < 0.0001 for genotype × odor by two-way ANOVA; Bonferroni’s post hoc tests with multiple comparisons were used to examine the differences between water and vanilla, Control: p < 0.0001; IOI: p = 0.5757. Dishabituation: odor of vanilla to banana; F1,20 = 117.7, ***p < 0.0001 for genotype × odor by two-way ANOVA; Bonferroni’s post hoc tests with multiple comparisons were used to examine the differences between vanilla and banana, Control: p < 0.0001; IOI: p > 0.9999. IOI, n = 8 (7 male, 1 female); Control, n = 7 (6 male, 1 female). Data are presented as the mean ± SEM. (C) IOI mice showed no changes in locomotion (left); p = 0.5720, t = 0.5746, df = 21 determined by Student’s t-test, percentage of time spent in central area (middle); p = 0.3797, t = 0.8984, df = 21 determined by Student’s t-test, and rearing (right); p = 0.0870, t = 1.795, df = 21 determined by Student’s t-test as indicated by the open field test. IOI, n = 12 (7 male, 5 female); Control, n = 11 (6 male, 5 female). Data are presented as the mean ± SEM. (D) No difference was found in the percentage of entries into the open arm between groups as indicated by the elevated plus maze test. p = 0.4068, t = 0.8465, df = 21 determined by Student’s t-test. IOI, n = 12 (7 male, 5 female); Control, n = 11 (6 male, 5 female). Data are presented as the mean ± SEM
In order to evaluate whether OE inflammation affects odor-guided behaviors, we examined social behaviors that highly rely on olfactory cues in the rodents (Ko, 2017), assessed by the three-chamber social interaction test, where the social approach of a mouse toward a stranger mouse trapped in a cage was measured (Zhu et al., 2019). Control mice exhibited preference for exploring a stranger mouse (stranger 1) relative to a cage where an inanimate mouse-like object was located, whereas IOI mice did not show preference for exploring the stranger mouse relative to the inanimate, measured by the total amount of time spent sniffing each cage (Fig. 2A). When another stranger mouse (stranger 2) was placed into the cage, control mice showed preference for exploring stranger 2 relative to stranger 1. In contrast, IOI mice did not exhibit preference for exploring stranger 2 relative to stranger 1 (Fig. 2B). These results suggest that chronic OE inflammation impairs social behaviors in the IOI mouse model.
Fig. 2.
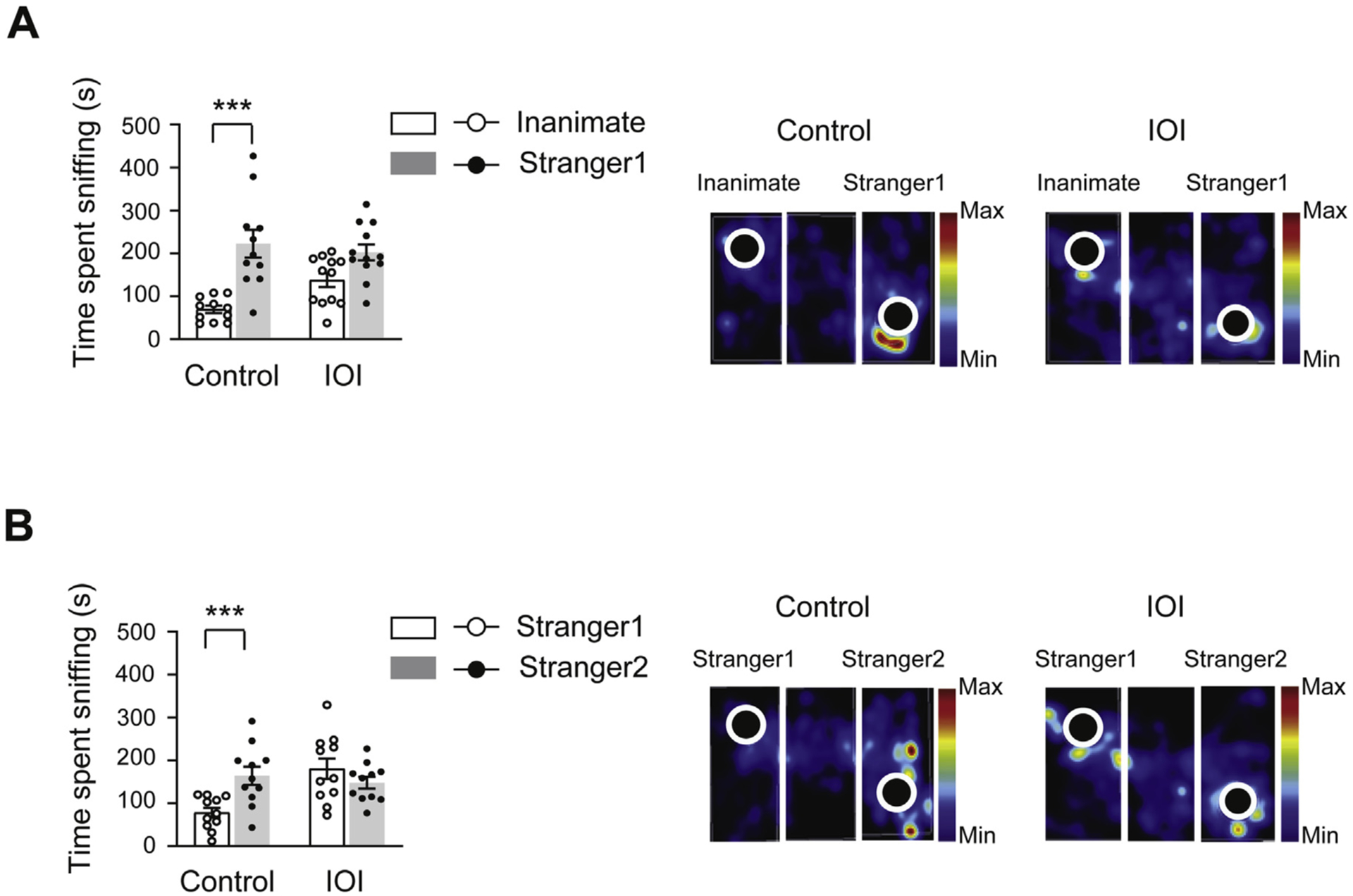
Chronic OE inflammation impairs social behaviors. (A) IOI mice displayed impaired sociability, assessed by time spent sniffing an inanimate and stranger 1 in the three-chambered social interaction test. F1,20= 4.748, p = 0.0415 for genotype × sociability by two-way ANOVA; Bonferroni’s post hoc tests with multiple comparisons were used to examine the differences between the inanimate and stranger mouse 1, Control: ***p < 0.0001; IOI: p = 0.0546. (B) Reduction in preference for social novelty was observed in IOI mice, assessed by measurement of time spent sniffing stranger 1 and stranger 2. F1,40= 10.66, p = 0.0022 for genotype × social novelty by two-way ANOVA; Bonferroni’s post hoc tests with multiple comparisons were used to examine the differences between the stranger mouse 1 and 2, Control: ***p = 0.0036; IOI: p = 0.4221. Representative heat map images (right panels) represent movements of the IOI mice and controls. IOI, n = 12 (7 male, 5 female); Control, n = 11 (6 male, 5 female). Data are presented as the mean ± SEM.
The sucrose preference test is frequently used to measure sensitivity of taste reward and to characterize loss of consummatory pleasure, a component of anhedonia (Scheggi et al., 2018), which may be impaired by disturbance of olfaction. To examine whether consummatory pleasure is impaired by chronic OE inflammation, the IOI mice and controls were subjected to an 8-day sucrose preference test (Zhu et al., 2019). There was no difference in total fluid intake between the IOI mice and controls (Fig. 3A). IOI mice exhibited less preference for the sucrose solution compared with controls (Fig. 3B), suggesting that OE inflammation may dampen consummatory pleasure. Nonetheless, it should be noted that these phenotypes may result from gustatory impairments due to olfac-tory dysfunction.
Fig. 3.
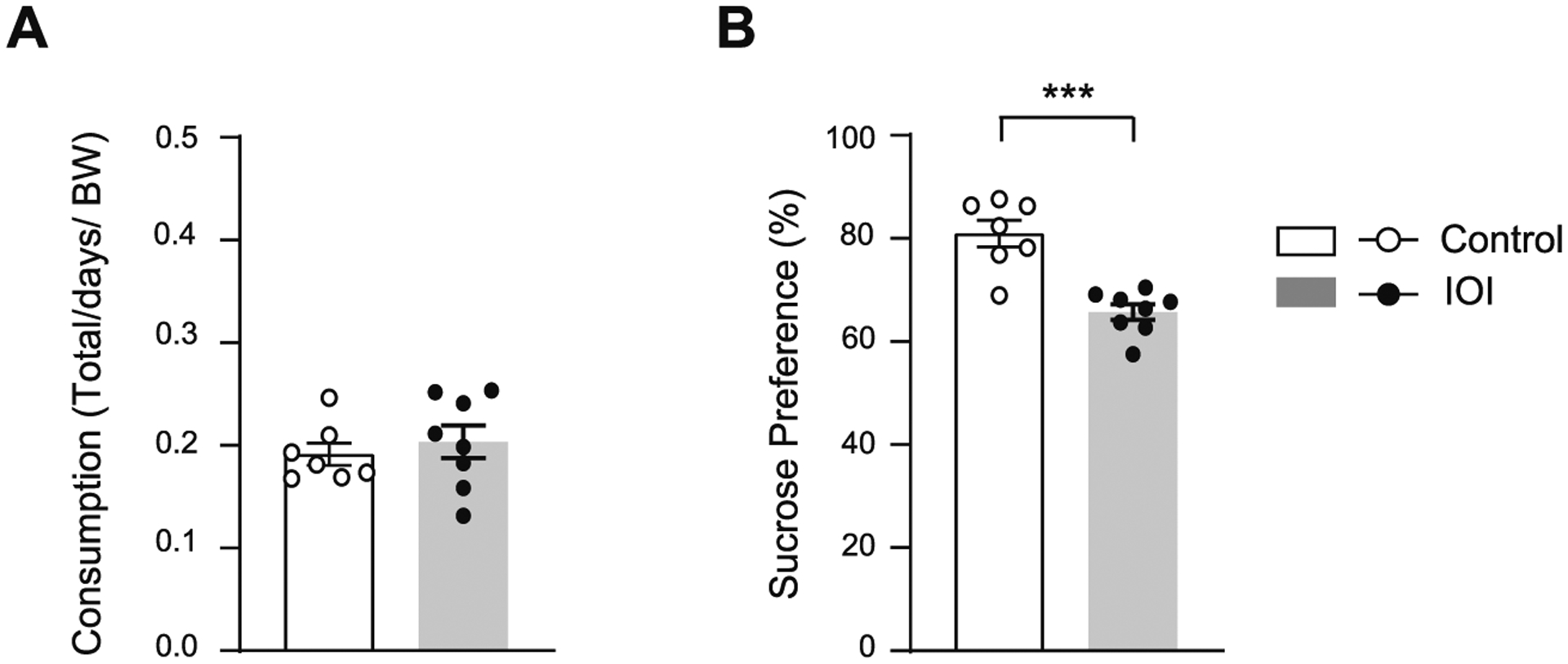
Chronic OE inflammation impairs consummatory behaviors. (A) There was no difference in total fluid intake between the IOI mice and controls. p = 0.5498, t = 0.6141, df = 13 determined by Student’s t-test. (B) IOI mice showed impaired consummatory behaviors reflected by decreased sucrose consumption when given a choice between 1.5 % sucrose and water. ***p = 0.0001, t = 5.326, df = 13 determined by Student’s t-test. IOI, n = 8 (7 male, 1 female); Control, n = 7 (6 male, 1 female). Data are presented as the mean ± SEM.
Chronic nasal inflammation may affect non-odor guided cognitive behaviors. To test this hypothesis, animals were subjected to auditory-cued trace fear conditioning, which is associative learning in which a trace interval between conditioned stimulus (CS) and unconditioned stimulus (US) imposes higher-cognitive processing (Han et al., 2003) (Fig. 4A). Chronic OE inflammation induces exaggerated fear responses to a discrete auditory cue (CS) even before it gets paired with an electric foot-shock (US) during the cued fear acquisition phase, despite no significant effect of the CS on the groups (Fig. 4B). Similar levels of freezing were displayed by IOI mice and controls before tone exposure (Pre tone), and no difference was observed in CS-evoked fear responses between the two groups during cued fear memory retrieval in a different context (Fig. 4C). These results suggest that OE inflammation enhanced auditory sensation, which may affect freezing behaviors during retrieval of conditioned fear. Given that a degree of olfactory inflammation may depend on how much DOX-containing diet each mouse consumes, the variability in acoustic response during the fear acquisition period may derives from varied severity of the olfactory inflammation. We suspect that the observed augmented freezing during the conditioned tone arises from increased sensitivity to auditory stimuli for several reasons. First, as the enhanced freezing was observed even before the first shock, a difference in pain perception may not be a contributing factor. Second, as there was no difference in percent time frozen during the 10-min habituation phase, a between-group difference in potentially anxious responses to the conditioning chamber is not likely to be a contributing factor. Third, multiple lines of evidence support the idea that olfactory deficits can enhance auditory sensation. Specifically, it is known that the loss of a sensory modality can lead to compensatory enhancement of the remaining senses through a process often termed ‘cross-modal plasticity’ (Lee and Whitt, 2015; Meng et al., 2015). Therefore, augmented freezing may be consistently observed even when conditioned with rewarding events (e.g., sucrose/food).
Fig. 4.
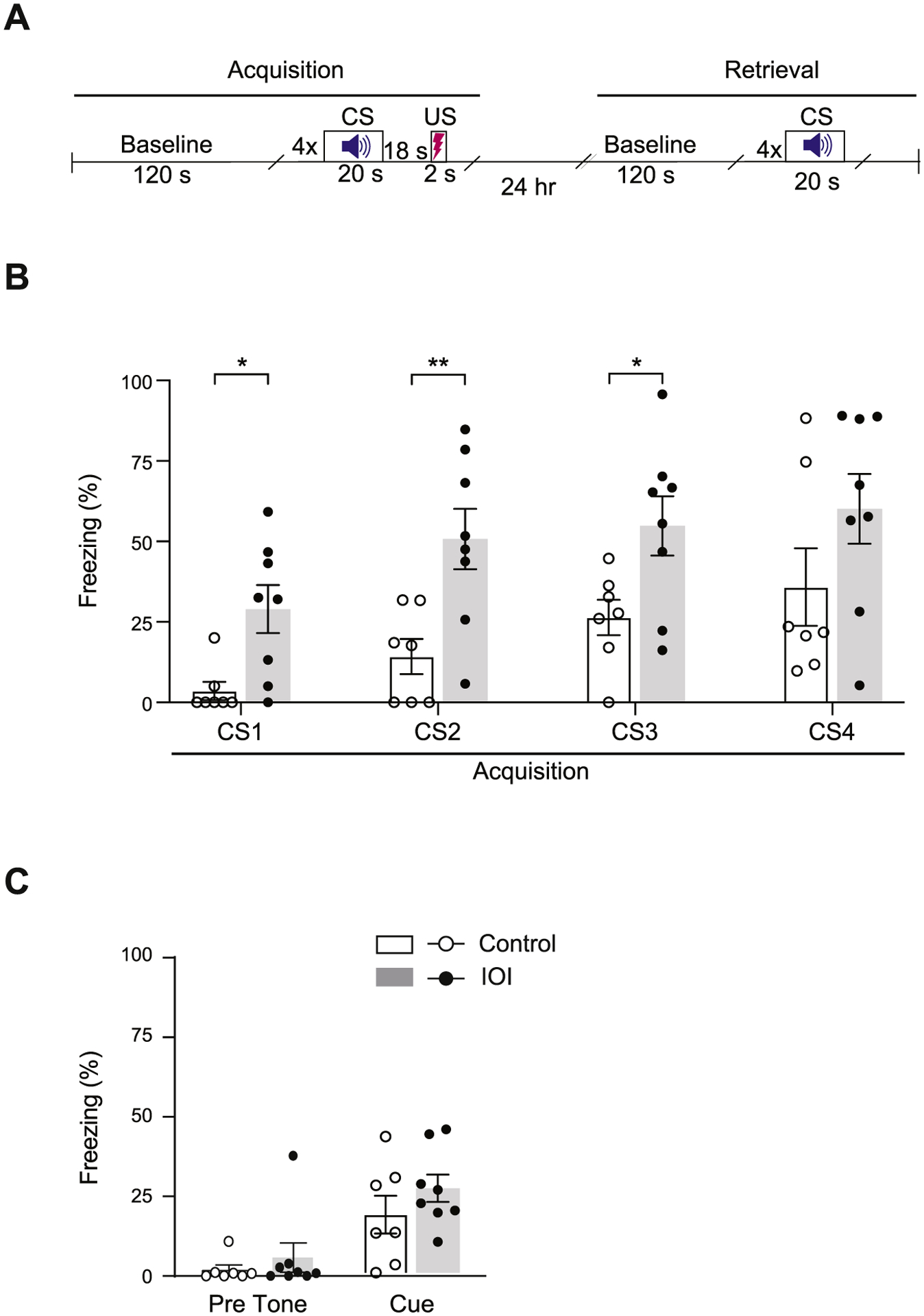
Chronic OE inflammation induces exaggerated fear responses to a discrete auditory cue. (A) Schematic diagram showing the experimental schedule of the trace fear conditioning test. CS: conditioned stimulus, US: unconditioned stimulus. (B) During the fear acquisition period, compared with controls, IOI mice showed a higher percent of time spent freezing as a response to a discrete auditory cue (CS) even before it was paired with a foot-shock (US), despite no significant effect of the CS on the group. Two-way repeated measures ANOVA for group x CS, F3,39 = 0.342, p = 0.795; Bonferroni’s post hoc tests with multiple comparisons were used to examine the differences between Control and IOI mice, CS1: p = 0.01, CS2: p = 0.0065, CS3: p = 0.0240, CS4: p = 0.1556. (C) In the cued fear retrieval phase, similar levels of freezing were displayed by IOI mice and controls before tone exposure (Pre tone), and there was no difference in freezing behavior between the two groups during auditory cue re-exposure in a different context. Two-way ANOVA for group x tone, F1,26 = 0.249, p = 0.622; Bonferroni’s post hoc tests with multiple comparisons were used to examine the differences between Control and IOI mice, Pre tone: p = 0.1968, Cue: p = 0.5412. bar graphs expressed as mean ± SEM; **p < 0.01 and * p < 0.05; IOI, n = 8 (7 male, 1 female); Control, n = 7 (6 male, 1 female).
The main finding of the present study is a set of behavioral phenotypes that are elicited by chronically induced olfactory inflammation in a mouse model. While previous studies demonstrate behavioral changes followed by impairment of the peripheral olfactory system in rodent models (Chen et al., 2014; Hendriksen et al., 2015; Moberly et al., 2018), to the best of our knowledge, this is the first demonstration that local inflammatory insults in the OE can be an initial driver of behavioral abnormalities. Our results support the intriguing hypothesis that chronic OE inflammation may contribute to pathological effect of environmental factors such as air pollutants and viral infection on the central nervous system, leading to neurobehavioral consequences relevant to SZ.
Interestingly, some behavioral phenotypes observed in IOI model are inconsistent with those in other models of olfactory impairment. For instance, olfactory bulbectomy-induced hyperactivity has been repeatedly reported (Hendriksen et al., 2015). In contrast, no change in spontaneous locomotion was observed in IOI model. While the mouse models of genetic inhibition of olfactory function such as the mouse with genetic deletion of cyclic nucleotide gated channel subunit alpha 2 (Cnga2), which play a critical role for regulating odorant signal transduction, and OSN-specific overexpression of M71 odorant receptor produced anxiety-like behaviors (Glinka et al., 2012), IOI mouse showed no abnormalities in the elevated plus maze test. Difference of these results may be explained by different experimental approaches to impair olfactory function. Considering that olfactory deficits are correlated with cognitive abnormalities and negative symptoms of SZ and early onset psychotic patients (Corcoran et al., 2005; de Nijs et al., 2018; Ishizuka et al., 2010; Kamath et al., 2013), relevant behavioral domains in IOI model warrant future investigation.
By taking advantages of the IOI mouse model, in which local OE inflammation can be induced within a specific time window, we will be able to address two key questions in future studies: first, whether there is a “critical period” of olfactory inflammation that may impact higher brain function; second, whether transient olfactory inflammation at the critical period may result in irreversible changes in the brain system, leading to long-term impairments in cognition and emotion. Major psychiatric disorders such as SZ usually occur in specific age ranges, in particular late adolescence and young adulthood, and developmental periods including adolescence are vulnerable to disease-related environmental stressors (Owen et al., 2016). Accordingly, the IOI mouse model will be a novel and useful tool to study the disease trajectory and pathophysiology of major psychiatric disorders such as SZ. Furthermore, by inducing OE inflammation in aged animals, this model may be utilized for studying geriatric disease conditions, including PD and AD.
Acknowledgements
We thank Joi Haskins for critical reading of the manuscript. This work was supported by the National Institutes of Health including MH-094268 Silvio O. Conte center (A.S., A.K.), DA-041208 (A.K.), AG-065168 (A.K.), MH-105660 (A.S.), MH-107730 (A.S.), DC-016106 (A.L.), AI-132590 (A.L.), AT-010984 (X.Z.), as well as foundation grants from Stanley (A.S.), S-R/RUSK (A.S.), and Kanae (Y.H.).
Abbreviations:
- IOI
Inducible olfactory inflammation
- OE
olfactory epithelium
- OB
olfactory bulb
- OSNs
olfactory sensory neurons
- SZ
schizophrenia
- AD
Alzheimer’s disease
- PD
Parkinson’s disease
- DOX
doxycycline
- TNF-α
Tumor necrosis factor α
- CS
conditioned stimulus
- US
unconditioned stimulus
- COVID-19
coronavirus disease 2019
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
Footnotes
Declaration of Competing Interest
The authors declare no competing interests.
References
- Ajmani GS, Suh HH, Pinto JM, 2016. Effects of ambient air pollution exposure on olfaction: a review. Environ. Health Perspect 124, 1683–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attademo L, Bernardini F, Garinella R, Compton MT, 2017. Environmental pollution and risk of psychotic disorders: a review of the science to date. Schizophr. Res 181, 55–59. [DOI] [PubMed] [Google Scholar]
- Bathini P, Brai E, Auber LA, 2019. Olfactory dysfunction in the pathophysiological continuum of dementia. Ageing Res. Rev 55, 100956. [DOI] [PubMed] [Google Scholar]
- Brewer WJ, Wood SJ, McGorry PD, Francey SM, Phillips LJ, Yung AR, Anderson V, Copolov DL, Singh B, Velakoulis D, Pantelis C, 2003. Impairment of olfactory identification ability in individuals at ultra-high risk for psychosis who later develop schizophrenia. Am. J. Psychiatry 160, 1790–1794. [DOI] [PubMed] [Google Scholar]
- Chen Y, Liu X, Jia X, Zong W, Ma Y, Xu F, Wang J, 2014. Anxiety- and depressive-like behaviors in olfactory deficient Cnga2 knockout mice. Behav. Brain Res 275, 219–224. [DOI] [PubMed] [Google Scholar]
- Corcoran C, Whitaker A, Coleman E, Fried J, Feldman J, Goudsmit N, Malaspina D, 2005. Olfactory deficits, cognition and negative symptoms in early onset psychosis. Schizophr. Res 80, 283–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Nijs J, Meijer JH, de Haan L, Meijer CJ, Bruggeman R, van Haren NEM, Kahn RS, Cahn W, 2018. Associations between olfactory identification and (social) cognitive functioning: a cross-sectional study in schizophrenia patients and healthy controls. Psychiatry Res. 266, 147–151. [DOI] [PubMed] [Google Scholar]
- Doty RL, 2017. Olfactory dysfunction in neurodegenerative diseases: is there a common pathological substrate? Lancet Neurol. 16, 478–488. [DOI] [PubMed] [Google Scholar]
- Glinka ME, Samuels BA, Diodato A, Teillon J, Feng Mei D, Shykind BM, Hen R, Fleischmann A, 2012. Olfactory deficits cause anxiety-like behaviors in mice. J. Neurosci 32, 6718–6725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han CJ, O’Tuathaigh CM, van Trigt L, Quinn JJ, Fanselow MS, Mongeau R, Koch C, Anderson DJ, 2003. Trace but not delay fear conditioning requires attention and the anterior cingulate cortex. Proc. Natl. Acad. Sci. U. S. A 100, 13087–13092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendriksen H, Korte SM, Olivier B, Oosting RS, 2015. The olfactory bulbectomy model in mice and rat: one story or two tails? Eur. J. Pharmacol 753, 105–113. [DOI] [PubMed] [Google Scholar]
- Hikida T, Jaaro-Peled H, Seshadri S, Oishi K, Hookway C, Kong S, Wu D, Xue R, Andrade M, Tankou S, Mori S, Gallagher M, Ishizuka K, Pletnikov M, Kida S, Sawa A, 2007. Dominant-negative DISC1 transgenic mice display schizophrenia-associated phenotypes detected by measures translatable to humans. Proc. Natl. Acad. Sci. U. S. A 104, 14501–14506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizuka K, Tajinda K, Colantuoni C, Morita M, Winicki J, Le C, Lin S, Schretlen D, Sawa A, Cascella NG, 2010. Negative symptoms of schizophrenia correlate with impairment on the University of Pennsylvania smell identification test. Neurosci. Res 66, 106–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AW, Jaaro-Peled H, Shahani N, Sedlak TW, Zoubovsky S, Burruss D, Emiliani F, Sawa A, Gallagher M, 2013. Cognitive and motivational deficits together with prefrontal oxidative stress in a mouse model for neuropsychiatric illness. Proc. Natl. Acad. Sci. U. S. A 110, 12462–12467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamath V, Moberg PJ, Kohler CG, Gur RE, Turetsky BI, 2013. Odor hedonic capacity and anhedonia in schizophrenia and unaffected first-degree relatives of schizophrenia patients. Schizophr. Bull 39, 59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamath V, Lasutschinkow P, Ishizuka K, Sawa A, 2018. Olfactory functioning in first-episode psychosis. Schizophr. Bull 44, 672–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamath V, Crawford J, DuBois S, Nucifora FC, Nestadt G, Sawa A, Schretlen D, 2019. Contributions of olfactory and neuropsychological assessment to the diagnosis of first-episode schizophrenia. Neuropsychology 33, 203–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko J, 2017. Neuroanatomical substrates of rodent social behavior: the medial prefrontal cortex and its projection patterns. Front. Neural Circuits 11, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane AP, Turner J, May L, Reed R, 2010. A genetic model of chronic rhinosinusitis-associated olfactory inflammation reveals reversible functional impairment and dramatic neuroepithelial reorganization. J. Neurosci 30, 2324–2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HK, Whitt JL, 2015. Cross-modal synaptic plasticity in adult primary sensory cortices. Curr. Opin. Neurobiol 35, 119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka Y, Jouroukhin Y, Gray AJ, Ma L, Hirata-Fukae C, Li HF, Feng L, Lecanu L, Walker BR, Planel E, Arancio O, Gozes I, Aisen PS, 2008. A neuronal microtubule-interacting agent, NAPVSIPQ, reduces tau pathology and enhances cognitive function in a mouse model of Alzheimer’s disease. J. Pharmacol. Exp. Ther 325, 146–153. [DOI] [PubMed] [Google Scholar]
- Meng X, Kao JP, Lee HK, Kanold PO, 2015. Visual deprivation causes refinement of intracortical circuits in the auditory cortex. Cell Rep. 12, 955–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moberg PJ, Kamath V, Marchetto DM, Calkins ME, Doty RL, Hahn CG, Borgmann-Winter KE, Kohler CG, Gur RE, Turetsky BI, 2014. Meta-analysis of olfactory function in schizophrenia, first-degree family members, and youths at-risk for psychosis. Schizophr. Bull 40, 50–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moberly AH, Schreck M, Bhattarai JP, Zweifel LS, Luo W, Ma M, 2018. Olfac-tory inputs modulate respiration-related rhythmic activity in the prefrontal cortex and freezing behavior. Nat. Commun 9, 1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori K, Sakano H, 2021. Olfactory circuitry and behavioral decisions. Annu. Rev. Physiol 83, 231–256. [DOI] [PubMed] [Google Scholar]
- Owen MJ, Sawa A, Mortensen PB, 2016. Schizophrenia. Lancet 388, 86–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito A, Taniguchi Y, Rannals MD, Merfeld EB, Ballinger MD, Koga M, Ohtani Y, Gurley DA, Sedlak TW, Cross A, Moss SJ, Brandon NJ, Maher BJ, Kamiya A, 2016. Early postnatal GABAA receptor modulation reverses deficits in neuronal maturation in a conditional neurodevelopmental mouse model of DISC1. Mol. Psychiatry 21, 1449–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheggi S, De Montis MG, Gambarana C, 2018. Making sense of rodent models of Anhedonia. Int. J. Neuropsychopharmacol 21, 1049–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Wu X, Danesh Yazdi M, Braun D, Abu Awad Y, Wei Y, Liu P, Di Q, Wang Y, Schwartz J, Dominici F, Kioumourtzoglou MA, Zanobetti A, 2020. Long-term effects of PM2.5 on neurological disorders in the American Medicare population: a longitudinal cohort study. Lancet Planet. Health 4, e557–e565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steardo L Jr., Steardo L, Verkhratsky A, 2020. Psychiatric face of COVID-19. Transl. Psychiatry 10, 261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumitomo A, Ueta K, Mauchi S, Hirai K, Horike K, Hikida T, Sakurai T, Sawa A, Tomoda T, 2017. Ulk1 protects against ethanol-induced neuronal stress and cognition-related behavioral deficits. Neurosci. Res 117, 54–61. [DOI] [PubMed] [Google Scholar]
- van Riel D, Verdijk R, Kuiken T, 2015. The olfactory nerve: a shortcut for influenza and other viral diseases into the central nervous system. J. Pathol 235, 277–287. [DOI] [PubMed] [Google Scholar]
- Zhu X, Nedelcovych MT, Thomas AG, Hasegawa Y, Moreno-Megui A, Coomer W, Vohra V, Saito A, Perez G, Wu Y, Alt J, Prchalova E, Tenora L, Majer P, Rais R, Rojas C, Slusher BS, Kamiya A, 2019. JHU-083 selectively blocks glutaminase activity in brain CD11b(+) cells and prevents depression-associated behaviors induced by chronic social defeat stress. Neuropsychopharmacology 44, 683–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoubovsky SP, Pogorelov VM, Taniguchi Y, Kim SH, Yoon P, Nwulia E, Sawa A, Pletnikov MV, Kamiya A, 2011. Working memory deficits in neuronal nitric oxide synthase knockout mice: potential impairments in prefrontal cortex mediated cognitive function. Biochem. Biophys. Res. Commun 408, 707–712. [DOI] [PMC free article] [PubMed] [Google Scholar]


