Abstract
Over the past decades, there has been an exponential increase in the development of preclinical and clinical nanodelivery systems, and recently, an accelerating demand to deliver RNA and protein-based therapeutics. Organ-specific vasculature provides a promising intermediary for site-specific delivery of nanoparticles and extracellular vesicles to interstitial cells. Endothelial cells express organ-specific surface marker repertoires, that can be used for targeted delivery. This article highlights organ-specific vasculature properties, nanodelivery strategies that exploit vasculature organotropism, and overlooked challenges and opportunities in targeting and simultaneously overcoming the endothelial barrier. Impediments in the clinical translation of vasculature organotropism in drug delivery are also discussed.
Keywords: drug delivery, endothelial barrier, extracellular vesicles, nanodelivery, organ-specific
Graphical Abstract

Introduction
Equally paramount to the molecular mechanisms of a bioactive compound are the macromolecular transport mechanisms, that is, the capacity to reach target sites at sufficient concentrations. The circulatory system serves as the primary distribution mechanism for therapeutic agents administered through systemic routes. In many cases, the vascular endothelium serves as a barrier for access to solid organs. For example, compared to hematological tumors, therapeutic agents are less effective against solid tumors [1]. The ability to interact with and traverse the endothelium is critical for therapeutic efficacy when it comes to extravascular target sites.
Initial studies to identify vasculature targeting ligands employed phage display libraries in mouse models to isolate peptides homing to tumor-associated blood vessels [2]. The coupling of small molecules to identified peptides, such as integrin binding arginine-glycine-aspartate (RGD), led to the development of targeted therapeutic strategies relying on the selective expression of receptors on vasculature, resulting in reduced off-target effects. Such strategies have since been expanded for vasculature homing of synthetic and biological nanoparticles, which is the focus of this article. Previously published literature provides a more in-depth overview of general vasculature targeting strategies [3–5].
The recent advent of RNA therapeutics has brought into focus opportunities and challenges associated with nanoparticle delivery. For example, messenger RNA (mRNA) is a flexible platform through which diverse molecules including antigens [6, 7], antibodies [8], chimeric antigen receptors [9], and protein therapies [10, 11] can be encoded and translated intracellularly. However, the considerable potential of mRNA and other biotherapeutics is limited by the availability of nanocarriers that facilitate delivery to target organs. Notably, mRNA-encoded protein replacement therapies for genetic diseases often rely upon delivery to affected organs. For example, treatment of muscular dystrophies requires efficient expression in skeletal muscle, whilst treatment of cystic fibrosis requires delivery to lung epithelium. Alternatively, mRNA therapeutics can be delivered to hepatocytes, that can serve as general protein production factories [12]. However, delivery to most solid organs and cell types remains unresolved stymying progress in applying mRNA and other emerging biotherapeutics to treat organ-specific diseases. Strategies to target organotropic vasculature are likely to facilitate site-specific delivery of systemically administered drug delivery systems. This article highlights opportunities and challenges in targeting organ-specific vasculature to improve delivery of nanoparticles to meet the accelerating demand to deliver new biologics.
The vascular barrier
The vascular endothelium is comprised of a monolayer of highly specialized endothelial cells on the luminal face of all blood vessels. The endothelial layer plays a paramount role in modulating vascular permeability, adhesiveness, and exchange of materials between the blood and interstitium. Transmembrane adhesion proteins, such as occludin and claudins, maintain junctional integrity between adjacent endothelial cells and consequently, the restrictiveness of the endothelium [13].
Endothelial cells adapt to the distinct niche they are localized in, hence, differing morphologically and physiologically across various organs. Variations in intercellular endothelial cell interactions has led to three distinct classifications of endothelia, namely continuous, fenestrated, and discontinuous (Figure 1). Continuous endothelium makes up the majority of the vasculature, characterized by endothelial cells with regular intercellular tight junctions, whilst being anchored to a continuous basal membrane [14]. Fenestrated endothelium meanwhile, homes transcellular pores, key in facilitating a heightened level of transendothelial material transport. This is particularly relevant to parts of the vasculature present in endocrine glands, the kidneys, and the gastrointestinal tract, which are more functionally involved in filtration and absorption processes [14]. The discontinuous endothelium is distinctive of organs such as the liver and spleen, where substantially large gaps in the endothelium and basement membrane are observed in vascular sinusoids, essential for macromolecular trafficking to maintain immune and metabolic homeostasis [14]. Different endothelial subtypes have also been observed within the same organ. For example, the medium and large blood vessels in the kidneys display a continuous endothelium, while the glomerular and peritubular endothelial cells are highly fenestrated, facilitating filtration and reabsorption of materials, respectively [15, 16]. Heterogeneity in the vascular system is likely to affect mechanisms of nanoparticle transport across the endothelial barrier into the interstitium with fenestrations enabling easier movement of nanoparticles, while a continuous endothelium may necessitate transcytosis. In fact, discontinuous vasculature is a major reason for the accumulation of systemically administered nanoparticles in the liver and spleen [17]. Alternative nanoparticle administration routes, such as intraperitoneal injection or inhalation have been used to reach the pancreas [18] and lungs [19], respectively, circumventing delivery to organs with discontinuous vasculature [18]. However, local injection of nanoparticles, for example, intramuscularly can also result in liver and spleen accumulation due to leakage into the circulation [20].
Figure 1. General representation of the three main types of endothelium.
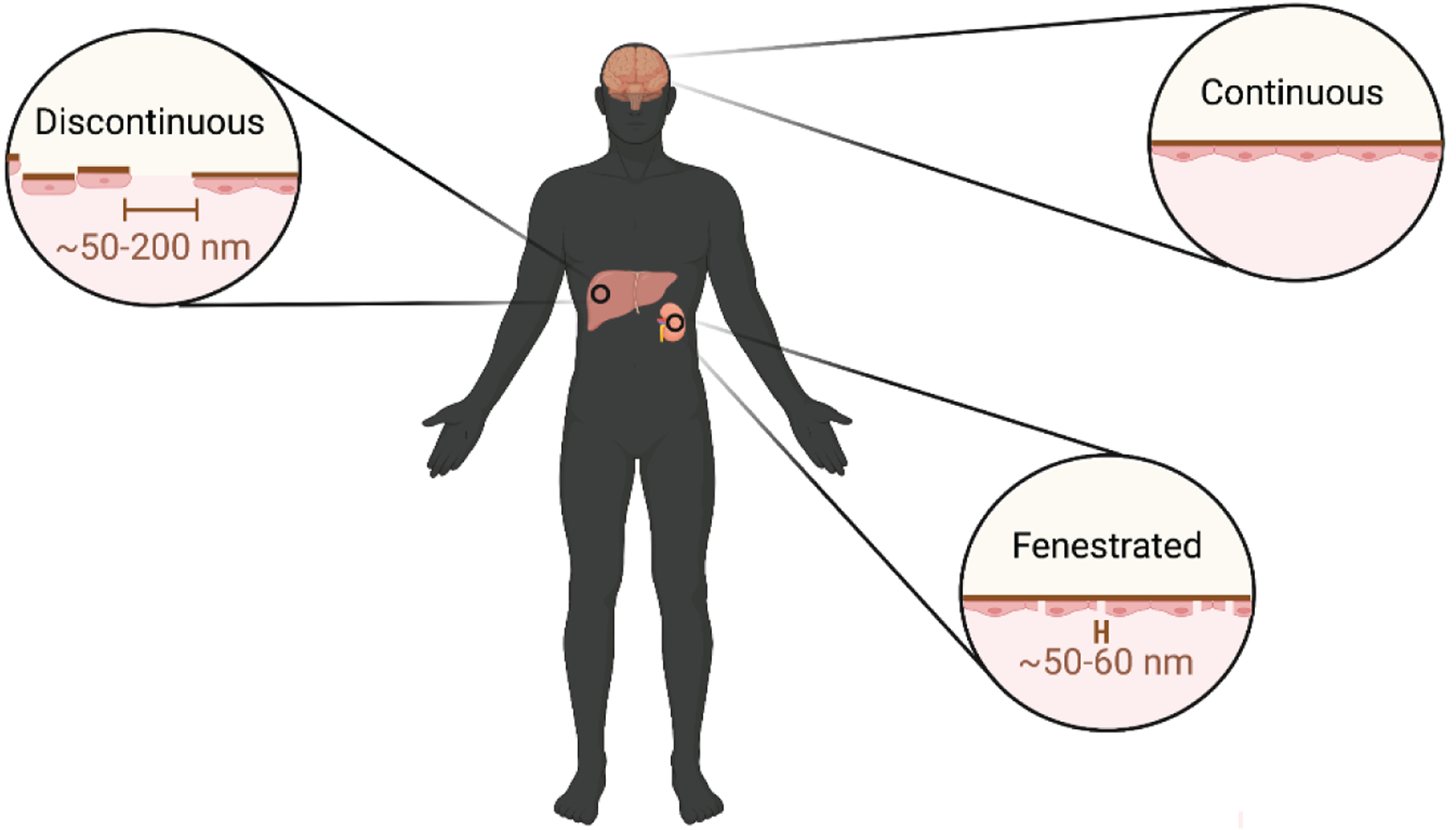
The endothelial structure displays heterogeneity across and within organs, associated with the respective function of the locality. A continuous structure is observed in most organs, such as the brain and lungs, whilst transcellular fenestrations (~50–60 nm) are observed in endocrine and exocrine glands, as well as certain blood vessels within the kidneys, to name a few. Discontinuous endothelium demonstrates paracellular gaps (~50–200 nm), and is primarily observed in organs, such as the liver and spleen, where macromolecular trafficking is significant [14, 24]. Nanoparticles larger than transcellular or paracellular gaps can enter the interstitium via other mechanisms, such as immune cell hitchhiking [25], forced extrusion [26], and endothelial transcytosis [23].
The pervasive continuous endothelium proves to be a formidable barrier for the transport of therapeutic agents between the bloodstream and the interstitium, usually preventing passive crossing of anything larger than 66 kDa, the size of albumin [21]. Systemically administered biological drugs thus encounter substantial difficulties accessing target sites, especially those situated in the interstitium, due to the well-fortified nature of the endothelial barrier.
Conventional drug delivery strategies
In the field of nanomedicine, multiple mechanisms are proposed to enable enhanced drug accumulation at target sites, including the enhanced permeability and retention (EPR) effect and active targeting. The EPR effect relies on the hyperpermeability of vasculature, coined to be “leaky” in inflamed tissues and tumors, coupled with inefficient lymphatic drainage [22]. The former leads to augmented permeability within the endothelial lining, enabling large particles, including macromolecules and nanoparticles, to reach the tumor microenvironment. Concurrently, inefficient lymphatic vessels lead to retention of such formulations within the tumor microenvironment [22]. In addition to the EPR effect, nanoparticles can also engage in trans-endothelial pathways to extravasate across the tumor endothelium to accumulate in solid tumors [23].
Whilst this EPR effects has been well studied and confirmed in small animal models that are ubiquitously used in preclinical studies, it is less apparent in clinical settings (Figure 2). Several reasons attribute to this disparity, including differences in the tumor microenvironment, relative size of solid tumors compared to the host size, and rate of tumor growth [27]. The substantial tumor-to-body weight ratio observed in murine models enables tumors to behave as repositories for the administered drug, reducing off-target effects, and hence, demonstrating promising therapeutic results [28]. However, this is less likely to occur in human pathophysiology, resulting in mismatched clinical observations [29]. Additionally, the immature vasculature that is observed in murine tumor microenvironments is substantially more discontinuous and disorganized than in human ones due to the rapid speed of tumor growth [30, 31]. Accordingly, patient-derived tumor explant models, which are more accurate reflections of clinical tumors, demonstrate much less permeability to nanodelivery systems in comparison to murine xenografts [32]. In addition, elevated interstitial fluid pressure observed intratumorally in humans often appear to work against the EPR effect, impeding the entrance of nanoparticles into the tumor interstitium [33]. In the clinical setting, tumors also display enhanced heterogeneity, including substantial spatiotemporal differences in blood flow, compared to animal models [30, 31]. As a result, patient tumors display unreliably and variable levels of particle extravasation [30]. For example, a gastric cancer patient displayed a fourfold regional difference in nanoparticle accumulation within the same tumor, which was postulated to be due to heterogeneity in terms of vascularization [34].
Figure 2. Differences between the performance of nanodelivery systems in an ideal versus reality setting.
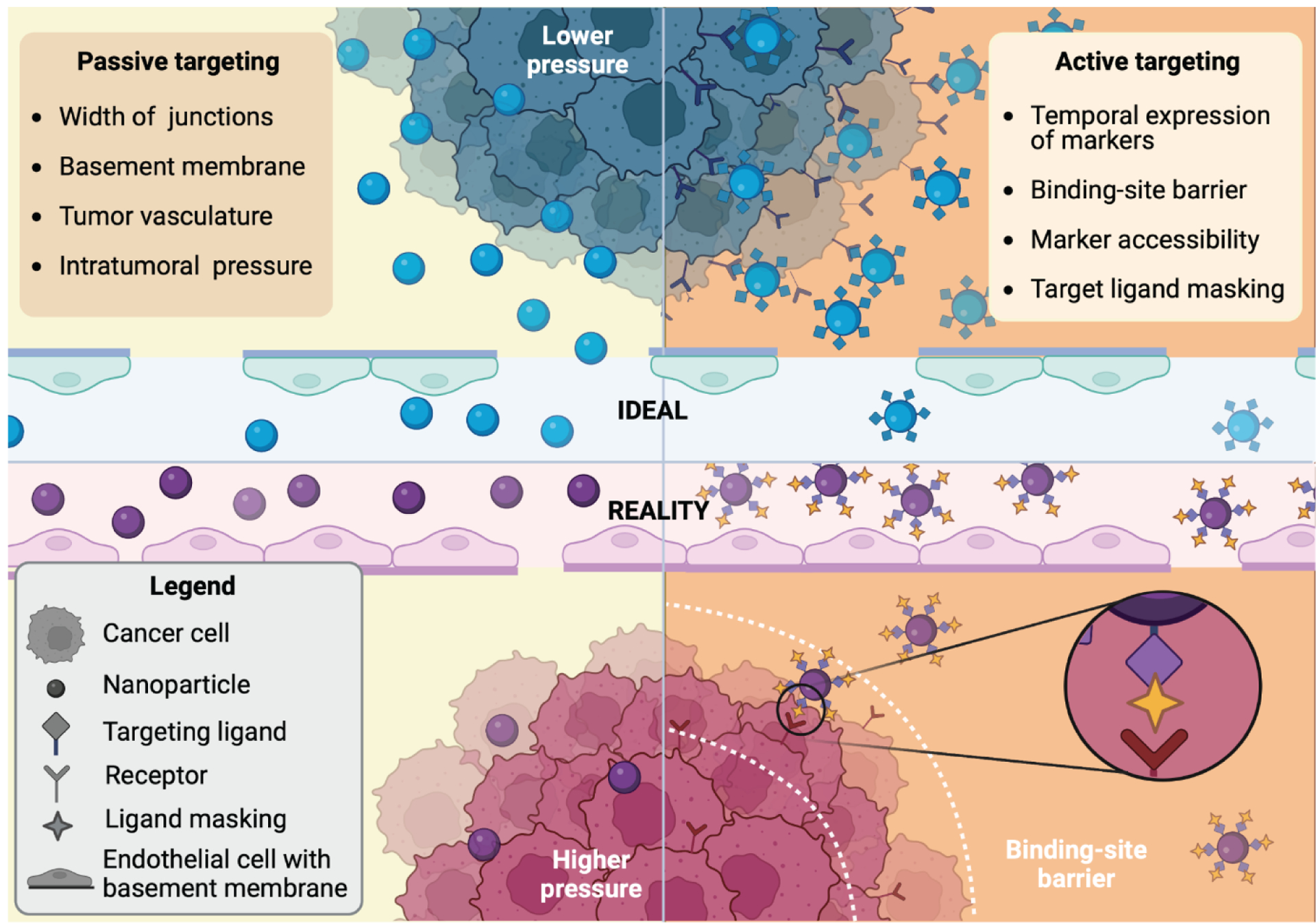
In many cases, the ideal scenarios are observed in the preclinical setting, whereas many more challenges occur in a clinical setting. In terms of passive targeting (left side), small animal models fail to adequately mimic human (patho)physiology. Preclinical models have substantially wider interendothelial junctions and underdeveloped basement membranes. In addition, the tumor vasculature is unable to keep up with the rate of tumor growth, and there is much lower interstitial pressure in comparison to tumors in the clinic. This leads to an overestimation of drug delivery and retention capacity preclinically. With active targeting (right side), nanoparticles, do not have direct access to tumor cells within the interstitium, as they have to cross the vascular barrier. Additionally, tumor cell heterogeneity leads to spatiotemporal variability in the expression of markers. Differences in the species-specific protein corona can lead to unexpected masking of targeting ligands in the clinical setting. The binding site barrier, which arises due to strong binding affinity to receptors closest to the vasculature, leads to poor tumor penetration as the distance from the vessel increases.
The abovementioned contrariety appears to be partially validated by the translational failure encountered by several nanomedicines in clinical settings that demonstrated promising results preclinically. Clinically approved nanoparticles for cancer rely on passive targeting, and have primarily received regulatory approval due to improvements in safety profiles without noteworthy changes in therapeutic potency compared to free drug counterparts [17].
In the preclinical setting, targeting ligands are often employed in conjunction with the passive EPR effect to enhance site-specific delivery of nanoparticles [29]. Examples of this approach is the targeting of ubiquitously upregulated receptors in cancer cells, including transferrin, folate, or integrin, or alternatively, receptors specific to the affected organ, such as human epidermal growth factor receptor 2/neu (HER-2/neu) on breast cancer cells [35]. Active targeting exploits the concept of “magic bullets”, ideally targeting intended parenchymal cells whilst sparing healthy cells, since these receptors are expressed at significantly lower levels in the latter. However, targeting of markers in the tissue interstitium appears to be less straightforward in clinical settings, where multiple clinical failures have occurred [17]. In fact, all nanoparticles to date employing active targeting mechanisms have failed in clinical trials [17]. It is also important to note that antibody-drug conjugates, a class of therapeutics that are not usually classified as nanomedicines, face similar challenges in terms of site-specific delivery, although 14 antibody-drug conjugates have been approved to date, of which seven are prescribed for solid tumors [36]. Challenges with the development of antibody-drug conjugates include inadequate intratissue and intracellular targeting [37].
A likely contributing factor to the failures of active targeting is the use of ligands that recognize molecules that are overexpressed on parenchymal cells in the tissue interstitium. Such targeted nanodelivery systems may lack adequate access to targeted cells due to the endothelial barrier. In the clinical setting, there is also increased spatiotemporal variability in the availability of targeted receptors compared to that observed in animal models [38]. Additionally, the species-specific biomolecular corona [39] could impact ligand masking and interactions with target molecules. In fact, liposomes exposed to mouse plasma were less enriched in opsonins compared those exposed to human plasma [39]. Therefore, the clinical failures of ligand-decorated nanodelivery systems, could be partially due to overlooking the species-specific biological identity of nanoparticles.
Another challenge with active targeting is the binding site barrier, involving the strong binding of ligand-modified therapeutics to the initial receptors that they encounter in the interstitium, hampering further penetration into deeper parts of the tissue (Figure 2) [40]. The vascular endothelium could also present a possible binding site barrier in cases where nanoparticles bind too tightly to endothelial receptors that impede subsequent extravasation into the interstitium.
The vascular endothelium stands as the penultimate barrier for targeted therapeutics to their intended markers on tissues within the interstitium. Hence, decorating therapeutic agents with markers specific to the final site is futile if extravasation across the vascular wall is suboptimal. Exploiting the plethora of surface markers on organ-specific vasculature could prove a promising alternative strategy when it comes to active targeting. Furthermore, studies have shown an upregulation in a myriad of endothelial markers during pathologies, thus potentially serving as disease-specific organotropic targets [41].
Organ-specific features of the vascular endothelium
In addition to the previously mentioned structural variations displayed by the endothelium across various organs, there also exists a large degree of heterogeneity in the endothelial cell phenotypes, including surface markers [42]. Epigenetics [42] and microenvironmental cues, such as substrate topography and hemodynamic fluctuations [43], play a major role in the maturation of endothelial cells into distinct subtypes.
Several endothelial cell markers are constitutively expressed across normal endothelial beds, whereas some display an inducible expression profile correlated to pathological conditions. Constitutive endothelial cell markers include platelet-endothelial cell adhesion molecule-1/cluster of differentiation 31 (PECAM-1/CD31) [44], transferrin/CD71, vascular endothelial cadherin (VE-cadherin/CD144) [45], intercellular adhesion molecule 1 (ICAM-1/CD54) [46, 47], and angiotensin-converting enzyme (ACE/CD143) receptors [48, 49], typically localized on the apical or intercellular interfaces of endothelial cells.
On the other hand, inducible receptors that are expressed only upon activation by certain ligands or in specialized endothelial cells include integrins (for example, αvβ3, αvβ5, and α5β1) [2], and tumor endothelial marker 1 (TEM1/CD248) [50, 51].
Several of the cell surface markers expressed by endothelial cells are organ-specific, be it constitutive or induced, which desirably plays into the notion of organotropic vasculature targeting (Table 1). Vascular adhesion protein-1 (VAP-1) is expressed at elevated levels during inflammation in localities such as the high endothelial venules of the lymph nodes, whilst ACE/CD143 is comparatively more pronounced in the lungs [52–56]. Meanwhile, lymphatic endothelial cells carry markers such as Fms-related tyrosine kinase 4/vascular endothelial growth factor 3 (FLT-4/VEGFR3) and C-X-C chemokine 4 (CXCR4) [57–60]. The endothelium of the blood-brain barrier contains elevated levels of receptors, such as glucose transporter 1 (GLUT-1) and transferrin [61, 62]. With regards to treating solid tumors within the interstitium, it appears that TEM1 is a marker that is only expressed in tumor endothelial cells and not in healthy endothelium [63–65]. Likewise, another set of receptors that could be potentially exploited are integrins, which are enriched within tumor vessels and angiogenic vasculature involved in other pathologies [66, 67].
Table 1.
Endothelial markers and examples of corresponding ligands
| Organ/tissue | Receptor/protein | Status | Changes during pathology | Examples of ligand(s) | Ref |
|---|---|---|---|---|---|
| Pan-endothelial | PECAM-1 (CD31) | Stable | αvβ3 integrin | [71–73] | |
| VE-cadherin (CD144) | Constitutive | Decreased | [14, 74] | ||
| Endoglin (CD105) | Increased | TGF-β1 and β3 in association with TGF-β receptor type II | [75, 76] | ||
| ICAM-1 (CD54) | Increased | LFA-1 integrin | [77] | ||
| TM (CD141) | Constitutive (except brain and liver) | Decreased (Shedding) | [78, 79] | ||
| Lungs | ACE (CD143) | Constitutive | Decreased (Shedding) | [56] | |
| APP | Constitutive | [80, 81] | |||
| Brain (Blood-brain barrier) | GLUT-1 | Constitutive | Increased | Glucose | [61] |
| Transferrin (CD71) | Constitutive | Stable | Transferrin | [62] | |
| LRP-1 | Constitutive | Stable | LDL and PARP-1 | [82, 83] | |
| Lymph Nodes (HEVs) | VAP-1 | Constitutive | Increased | Siglec-9 | [53, 55, 84] |
| Lymph vessels | FLT-4 (VEGFR3) | Constitutive | Increased | VEGFC | [58, 59, 85] |
| PDPN | Constitutive | Increased | [86] | ||
| LYVE-1 | Constitutive | Decreased | [87, 88] | ||
| CXCR4 | Inducible | Increased | CXCL12 | [58] | |
| Adipose Tissue | Prohibitin | Constitutive | Stable | CKGGRAK DC peptide | [89, 90] |
| Bone Marrow | E-selectin (CD62E) | Constitutive | Increased | Glycosphing olipids | [91] |
| Tumors | Integrins αvβ3 and αvβ1 | Induced | Overexpressed (several, including prostate cancer and melanoma) | RGD | [66, 92] |
| CD276 (B7-H3) | Induced | Overexpressed (several, including esophageal cancer and glioma) | TLT-2 receptor | [93, 94] | |
| TEM 8 (ANTXR1) | Induced | Overexpressed (several, including lung and brain cancers) | α3 subunit of collagen VI | [95, 96] | |
| TEM 1 (CD248) | Selectively on tumor endothelium (several, including brain cancer and carcinoma) | Fibronectin, collagen | [64, 65] | ||
| Annexin A1 (ANXA1) | Induced | Cytosolic protein that is selectively externalized on tumor endothelium (several, including lung and breast cancers) | FPR | [80, 97, 98] | |
| E-selectin (CD62E) | Induced | Overexpressed (several, including colon cancer and melanoma) | CLA | [99–101] | |
| Fibronectin extradomain B (EDB) | Induced | Overexpressed (several, including breast cancer and teratocarcinoma) | [102] | ||
| APN (CD 13) | Induced | Overexpressed (several, including breast and bone marrow cancer) | NGR peptide | [103–105] | |
| p32 | Induced | Overexpressed (several, including breast cancer and melanoma) | LyP-1 peptide | [106–108] |
ACE: angiotensin-converting enzyme, ANTXR1: anthrax toxin receptor 1, APN: aminopeptidase N, APP: amyloid precursor protein, B7-H3: B7 homolog 3 protein, CD: cluster of differentiation, CLA: cutaneous lymphocyte associated antigen, CXCL12: C-X-C motif chemokine ligand 12, CXCR4: C-X-C chemokine receptor 4, FLT-4: Fms-related tyrosine kinase 4, FPR: formyl peptide receptor, GLUT-1: glucose transporter 1, GlyCAM-1: glycosylation-dependent cell adhesion molecule-1, ICAM-1: intercellular adhesion molecule 1, LDL: low density lipoprotein, LFA-1: lymphocyte function-associated antigen, LYVE-1: lymphatic vessel endothelial hyaluronan receptor-1, LRP-1: low density lipoprotein receptor-related protein 1, NGR: asparagine-glycine-arginine, PARP-1: poly [ADP-ribose] polymerase 1, PECAM: platelet-endothelial cell adhesion molecule, PSPN: podoplanin, RGD: arginine-glycine-aspartate, Siglec-9: sialic acid-binding Ig-like lectin 9, TEM: tumor endothelial marker, TGF: transforming growth factor beta, TLT-2: triggering receptor expressed on myeloid cell-like transcript 2, TM: thrombomodulin, VAP-1: vascular adhesion protein-1, VE-cadherin: vascular endothelial cadherin, VEGFC: vascular endothelial growth factor C, VEGFR3: vascular endothelial growth factor C.
Being more accessible to systemically administered drugs and delivery systems, tumor-associated endothelial cells are attractive intermediary sites for drug targeting [68–70]. In the oncology setting, direct vasculature targeting for anti-angiogenic purposes may also be advantageous, given that tumor-associated endothelial cells are not characterized by an instable genome, hence being less likely to develop drug resistance than cancer cell counterparts [42].
Organotropic targeting of vascular endothelium with synthetic nanoparticles
Synthetic nanoparticles have been designed to exploit organotropic vasculature. For example, peptides have been used to target drugs towards tumor vasculature, with the integrin-binding RGD peptide being one of the most notable ones. The RGD peptide promotes the accumulation of the drug in angiogenic tissue-associated endothelia overexpressing integrins such as αvβ3 and αvβ5. The relative affinity and specificity of the peptide to target receptors is determined by the amino acids flanking the RGD motif, which influence both the interaction with integrins as well as the conformation of the peptide [109]. Given that the linear version of RGD is highly susceptible to chemical degradation [110], cyclisation of the pentapeptide is typically employed to confer vastly improved physiological stability, as well as selectivity to specific integrin subtypes. Phage display technology has identified several cyclic RGD (cRGD) ligands over the past years, with many demonstrating commendable affinity and specificity for integrin subtypes over native integrin-binding proteins [111, 112]. In addition to binding to vasculature, cRGD ligands conjugated to lipid nanoparticles have been observed to hitchhike on immune cells to attain access to tumor cores [25].
The efficacy of RGD binding to target integrin receptors, however, is heavily influenced by several factors, especially the abundancy of integrin-binding proteins in the blood and other body fluids. Such native proteins contest the interaction between RGD and respective receptors, leading to limited interaction for subsequent effects [113–115]. In order to circumvent this, several motifs and versions of RGD have been explored to identify sequences with improved affinity and avidity to integrin receptors in a physiological setting, with one being internalizing RGD (iRGD) [116], which is also a cyclic form of the original peptide. The iRGD sequence has three distinctive modules, namely a vasculature-targeting RGD motif, a tissue-penetrating C-end rule (CendR) motif, and a protease recognition site [116]. When compared to the original RGD sequence, it demonstrates a substantial improvement in interacting with tumor tissues, and has reported efficacy in a myriad of nanoparticles. This is attributed to the dual targeting characteristic of the peptide, where the RGD motif increases localization to tumor-specific endothelia, whilst the CendR motif markedly improves transcytosis of the therapeutic payload across the vasculature into the tumor interstitium. The latter occurs as a result of protease cleavage of iRGD upon initial integrin binding, resulting in the exposure of the c-terminal of the CendR motif, which can then bind to the b1 domain of neuropilin-1 receptors on the apical end of the endothelia [117]. This interaction subsequently initiates receptor endocytosis, along with the attached drug payload, which eventually is exocytosis at the other end. Another peptide sequence that has been reported in assisting successful delivery of drugs and liposomes is the asparagine–glycine–arginine (NGR) motif peptide, which binds to CD13, an overexpressed marker on tumor endothelial cells [118].
In addition to tumor endothelial cells, synthetic nanoparticles have also been functionalized to target organotropic endothelial cells in other pathologies. The blood-brain barrier is a notoriously formidable impedance to drug delivery into the brain, restricting paracellular flux of solutes [119, 120]. However, several transport pathways have been exploited to facilitate delivery of systemically administered nanoparticles into the brain parenchyma. These pathways include various mechanisms of transcytosis and paracellular transport, the latter enabled by strategies that open tight junctions. Nanoparticles can be designed to hijack endogenous receptor-mediated transcytosis by functionalization with biomolecules that are frequently shuttled across the blood-brain barrier. For example, transferrin receptors provide a plausible route for endocytosis-mediated delivery of transferrin-decorated synthetic nanoparticles to treat central nervous system disorders [121]. Other receptors that are copious on the blood-brain barrier include lipoprotein receptors, which can be exploited by functionalizing nanoparticles with apolipoproteins (Apo) to obtain brain delivery [122]. Lactoferrin receptors have also been explored as another viable option to facilitate transcytotic passage of nanoparticles decorated with lactoferrin in treating Parkinson’s disease [123].
Carrier-mediated transcytosis is an energy-dependent pathway whereby carrier molecules such as glucose and phenylalanine are recognised by corresponding transporter proteins on the luminal face of the endothelium and shuttled across the barrier to the abluminal end. The carrier-mediated route is less likely for the transport of large nanoparticles, due to the small and stereospecific nature of the pores on the cellular surfaces [124]. While some studies have theorized the transport of carbon quantum dots with the aid of transporters such as GLUT1 and alanine, serine, cysteine transporter 2 (ACST2), the relevance of this mechanism to larger nanocarriers has yet to be fully explored [125].
It is worth noting that synthetic nanoparticles designed for active targeting tend to face significant impediments in appropriate binding with target receptors, especially in a clinical setting (Figure 3). This is attributed to the simplistic composition of the nanoparticles, which fails to correspond to the complexity of the biological environment. Natural nanoparticle counterparts such as extracellular vesicles and viruses have demonstrated that efficacious binding with target cells typically arise from multiple ligands binding to corresponding receptors, enhancing overall binding avidity, and subsequently, initiating intracellular signaling to promote endocytosis or membrane fusion [126, 127]. Such targeting moiety combinations are often lacking in synthetic nanoparticles, leading to poor cellular interactions in a heterogonous clinical setting. Additionally, orientation and spatial distribution of ligands on a nanoparticle surface play a central role in facilitating appropriate target receptor interactions [127]. A recent study showed that increasing the density of cRGD on the nanoparticle surface, did not result in increased internalization by target cells [128]. Avoiding unintended immune activation is another critical hurdle for synthetic nanoparticles. Decorating the nanoparticle surface with ligands could lead to enhanced immunological recognition and clearance [17]. Naturally occurring nanoparticles can possess negative regulators that limit immune recognition [129], while such regulators are often overlooked for synthetic nanoparticle design.
Figure 3. Ligand properties of extracellular vesicles (EVs) and synthetic nanoparticles (sNPs).

In comparison to EVs, sNPs often have unfavourable ligand properties that render them with limited targeting capacity in the clinical setting. The lack of negative regulators that limit immune recognition exposes sNPs to increased immune cell uptake and clearance (1). Additionally, functionalization of sNPs with target ligands does not ensure favorable interactions with target cells due to multiple factors. Unideal spatial distribution of target ligands on the sNP surface can lead to inefficient binding to target receptors (2). Incorrect orientation of functionalized ligands on the sNP surface can further exacerbate binding capacity (3). The frequent lack of helper ligands in conjunction to the primary target ligand also results in unfavorable interactions with target cells, resulting in poor intracellular uptake and therapeutic efficacy of the administered sNPs (4).
In addition to surface decorations, molecular substances like bradykinin and leukotrienes or external physical triggers, such as heat, magnetic fields and focused ultrasound can be applied to select regions of the body to increase vasculature permeability and enable nanoparticles to cross the endothelium [130–132]. For example, systemically administered microbubbles with externally applied ultrasound can modify endothelial cells in various ways, including opening tight junctions, upregulating vesicular transport, and causing the formation of cytoplasmic openings, such as channels [130]. Such temporal alteration in regional vascular endothelium can improve the transport of co-administered nanoparticles across vascular barriers through both transcellular and paracellular mechanisms [131, 133, 134]. However, the safety of such mechanisms has to be considered due to the possibility of the concurrent influx of other materials that could result in cerebral toxicity. Examples of mechanisms by which synthetic nanoparticles can overcome biological barriers have been summarized in table 2.
Table 2.
Examples of nanodelivery strategies to overcome vascular barriers
| Description | Mechanism | Refs | |
|---|---|---|---|
| Synthetic nanoparticles | |||
| Multistage | A delivery system with several particles that are active at different stages | The first particle is designed to bind to the vasculature and subsequently releases a second smaller particle that can pass through vasculature gaps | [141, 142, 148] |
| Hijacking receptor-mediated transcytosis | Nanoparticles decorated with biomolecules | Surface functionalized biomolecules, such as apolipoprotein E, transferrin, and lactotransferrin, induce receptormediated transcytosis of nanoparticles across the vasculature | [121–123] |
| Hyperthermia in combination with nanoparticles | Hyperthermia causes disaggregation of the endothelial cytoskeleton, which increases vascular permeability and paracellular nanoparticle transport | [149] | |
| Focused ultrasound in combination with nanoparticles | Focused ultrasound of systemically administered microbubbles increases nanoparticle extravasation through paracellular (and transcellular pathways) | [130, 131, 133] | |
| Co-delivery of nanoparticles with vasoactive small molecules | Vasoactive small molecules increase vascular permeability and promote increased interstitial uptake of nanoparticles through paracellular pathways | [150] | |
| Extracellular vesicles | |||
| Extracellular vesicles containing molecules that | Extracellular vesicle miRNA internalized in brain endothelial cells causes actin disassembly and cytoplasmic relocalisation of tight | [151] | |
| disrupt endothelial junctions | junction proteins, increasing vascular permeability and paracellular transport of noninternalized extracellular vesicles | ||
| Increasing transcytosis levels | Extracellular vesicles containing molecules that increase transcytosis levels in endothelial cells | Extracellular vesicles contain molecules that suppress degradation pathways and enhance recycling pathways following endocytosis, increasing inherently low levels of transcytosis in brain endothelial cells | [152] |
Physiochemical nanoparticle properties such as size, shape, and charge also impact vasculature interactions and drug delivery. For example, the size of nanocarriers affects the extent and rate of clearance, with particles smaller than 10 nm and larger than 200 nm tend to be rapidly cleared by the kidneys and liver, respectively [135, 136]. As a result, particles within the range of 10–100 nm generally display reduced clearance [137, 138]. Another aspect to consider is particle shape, which can impact tissue tropism. One of the first studies assessing the effect of shape, showed that intravenously injected inorganic nanorods displayed fourfold higher transvascular transport in tumors compared to spherical counterparts of the same hydrodynamic size [139]. Subsequent studies demonstrated that rod-shaped nanoparticles with vascular targeting ligands outperform spherical counterparts displaying the same targeting ligands [140]. Such shape-based effects are likely due to differing abilities of the nanoparticle to pass through vascular gaps and differences in the contact area with endothelial cells. Other shape-based design strategies, such as those based on discoidal particles, have exploited disease-specific hemodynamics to enable enhanced binding to the endothelium [141, 142]. Surface charge is also pivotal to nanoparticle-endothelial interactions, with cationic moieties leading to improved passage across the blood-brain barrier, facilitated by adsorptive endocytosis [143, 144]. However, positively charged nanoparticles also have a greater risk of immune cell recognition, clearance, and toxicity [145–147].
Organotropic targeting of vascular endothelium with biological nanoparticles
Extracellular vesicles are cell-released, membrane-bound nanoparticles implicated in intercellular communication, the specificity and purpose of which is dictated by bioactive cargo [153]. Extracellular vesicles can modulate the fate of target cells by transferring cargo to recipient cells or through cell surface signaling. For example, cancer cell-derived extracellular vesicles play a role in promoting metastatic spread, especially by priming the pre-metastatic niche in the target interstitium [154, 155]. The biodistribution of cancer extracellular vesicles during metastasis appears to be determined by a multitude of factors, including surface and internal cargo molecules [156]. Proteomic analyses have discovered distinct integrin patterns expressed by extracellular vesicles with organotropic vasculature features [156]. Additionally, in order to permeabilize the endothelial barrier and subsequently gain access to organ-specific interstitia, cancer extracellular vesicles carry particular microRNAs (miRNAs) that promote vascular remodeling. These miRNAs lead to the downregulation of structural proteins involved in intracellular and intercellular junction integrity, resulting in endothelial remodeling [157]. This has been demonstrated in several tissues, including the bones and brain [151, 158], where extracellular vesicle-derived miRNAs have been observed to disturb the continuity of the endothelium and enhance vascular permeability for eventual tumor cell invasion.
Various strategies have been developed for loading of therapeutic cargo (spanning small molecules to biologics) in extracellular vesicles, including genetic engineering of source cells prior to isolation and modification of extracellular vesicles post-isolation [159, 160]. The benefits of using extracellular vesicles for drug delivery is their complex biomolecular surfaces that are optimized to navigate in a species-specific environment to successfully reach target cells [127, 161]. In addition to the biomolecular corona, complexation with larger structures in circulation could be critical for conferring a biological identity to nanoparticles. Lipoproteins are endogenous delivery system that are highly abundant in circulation and overlap in size with extracellular vesicles and synthetic nanoparticles [162]. Studies have reported that extracellular vesicles form complexes with lipoproteins when mixed together [163, 164] and these complexes were recently found in human plasma [165]. Additionally, a preprint showed that extracellular vesicle-lipoprotein complexes can be transmitted from cell to cell [166]. These findings highlight the need to assess the role of complexation on site-specific targeting of extracellular vesicles and synthetic nanoparticles. In fact, the vascular endothelium is lined with various types of lipoprotein receptors [167], which could potentially contribute to organotropism. Species-specific differences in lipoprotein profiles should also be considered for assessment of lipoprotein-facilitated nanoparticle delivery.
Once extracellular vesicles have crossed the endothelial barrier, navigation through the interstitial space is necessary to reach target cells. A recent study demonstrated that diffusion plays a minor role in determining the spatial distribution of extracellular vesicles in the interstitium [168]. Rather, interstitial flow (convection) and binding to laminin in the extracellular matrix were the main determinants of extracellular vesicle transport in models of the tissue interstitium [168]. Extracellular vesicles from cancer cells with increasing malignant potential were enriched in integrins (α3, α6, and β1), which mediated enhanced accumulation and spatial distribution in a laminin-rich extracellular matrix under flow conditions [168]. It is worth noting that integrins that promote endothelial binding are likely to be distinct from those that promote binding to the extracellular matrix, emphasizing that extracellular vesicles may have complex surface structures that are multifunctional in terms of mediating interactions with different spatiotemporal compartments in the body.
Despite the promise of extracellular vesicles in facilitating site-specific drug delivery, major challenges remain. For example, numerous studies have shown that systemically administered extracellular vesicles exit the circulation within minutes and primarily accumulate in the liver and spleen [127]. On the contrary, endogenously-produced extracellular vesicles display tropism for other organs, as demonstrated in reporter mice [169]. Therefore, a major challenge in the field is replicating the organ-specific targeting of endogenous EVs for drug delivery purposes. There are several potential reasons for the differing biodistribution profiles of internally produced and externally administered extracellular vesicles, including enhanced clearance of the latter due to allogenic/xenogenic effects, infusion rate effects, or damage during isolation, drug loading, storage, and/or labeling [170, 171]. Another challenge is safety concerns associated with drug delivery using cancer cell-derived extracellular vesicles, which are otherwise promising due to presenting organotropic targeting ligands [172]. Cancer cell-derived extracellular vesicles can promote angiogenesis, increase cell proliferation, induce cell migration, and suppress immune responses [173], which could promote progression of existing tumors or cause other unintended side effects. A potential solution is exploiting the surfaces of extracellular vesicles, while discarding internal cargo that may cause unfavorable effects [174]. However, transmembrane extracellular vesicle cargo can also promote tumor progression, as in the case for integrin-α2 subunit (ITAG2), which was shown to mediate migration and invasion of prostate cancer cells [175]. An alternative strategy to obtain extracellular vesicle-based drug delivery systems with organotropic properties is to genetically engineer non-cancerous cells to express targeting ligands, although ligand distribution, orientation, and multi-ligand interactions may fail to mimic ideal conditions. Additionally, obtaining extracellular vesicles from cell culture is challenging from a scalability perspective [176–178]. A promising alternative to cell culture is the use of biofluids as an extracellular vesicle source. In particular, combining isolation methods that have proven clinical scalability for viruses (ultrafiltration/tangential flow filtration [179]) with biofluids as scalable extracellular vesicle sources [180, 181], can address some manufacturing challenges. Extracellular vesicles from biofluids could be further separated to obtain subpopulations that display ideal vasculature targeting properties, although efficient fractionation remains an unresolved hurdle in the field.
Outlook
The clinical success of targeted drug delivery is likely to improve by reassessing the impact of the vasculature barrier. The screening of interactions between nanodelivery systems and organ-specific endothelial cells in vitro seems like a reasonable first step to assess targeting potential. For example, a failure of a nanodelivery system to display preferential interactions with a specific type of endothelium is unlikely to lead to organotropic targeting of the underlying interstitium. However, organ-specific endothelial cell screening assays are rarely implemented in the process of developing drug delivery systems, suggesting a missed opportunity for early-stage selection of promising matches between nanodelivery systems and target organs. The overreliance on leaky vasculature based on preclinical models and/or conceptual flaws in the representation of nanoparticle transport have led to the endothelium being overlooked as a screening tool and major hurdle for successful drug delivery. It has previously been shown that the biomolecular corona that surrounds nanoparticles can dictate organ-specific targeting [182], highlighting the importance of using human circulatory biomolecules (for example, human plasma instead of fetal bovine serum) in endothelial screening assays to more accurately mimic the clinical setting. Ideally, organ-specific hemodynamics should also be considered for in vitro screening assays, as the success of ligand-receptor binding will vary based on the blood flow conditions.
Targeting the vascular endothelium alone is insufficient if the drug delivery system is unable to cross this barrier to gain access to the interstitium. Peptide-based nanotherapeutics, such as those conjugated with iRGD, are designed to facilitate transcytosis of the therapeutic across the vascular barrier for release into the tumor microenvironment [117]. However, these types of approaches have yet to be broadly applied for targeting organ-specific vasculature. Promising strategies for organotropic binding and crossing of the endothelium while circumventing issues with targeting ligands (distribution, orientation, immune recognition, monovalency) include extracellular vesicle-inspired and extracellular vesicle-mediated methods.
Extracellular vesicles have demonstrated multiple intrinsic mechanisms to both target and cross the endothelial barrier. Such mechanisms include engaging in transcytosis by downregulating endocytic degradation pathways, as well as exploiting paracellular transport by modulating actin cytoskeletal dynamics and relocalizing tight junction proteins, as mentioned in table 2 [151, 152]. Uncovering the overlooked extracellular vesicle glycocode represents another opportunity for EV-mediated organotropic vasculature targeting, as changes in extracellular vesicle surface glycans affect biodistribution [183]. Although several studies have characterized individual extracellular vesicle glycans or glycan classes, only a select few have comprehensively identified the extracellular vesicle glycocode [184–188]. Overcoming technical issues with extracellular vesicle isolation and characterization, such as comprehensive glycomics, could aid in an increased understanding of the endothelial-EV interface.
In terms of synthetic nanoparticles, multistage drug delivery systems have been developed, where the first stage involves recognition and binding to inflamed or cancerous vasculature, after which a second component is released, which is optimized for crossing the vasculature wall and interacting with targets in the interstitium [141, 142, 148]. Multistage strategies have the advantage of optimizing ligand-receptor interactions for a specific compartment in the body, for example, blood circulation versus tissue interstitium. Consequently, potential interference of parenchymal targeting ligands with vasculature binding and endothelial ligands with target cell or extracellular matrix recognition in the interstitium can be circumvented by implementing drug delivery systems with sequentially released components. Additionally, the multistage approach overcomes potential issues with the binding site barrier at the endothelium, as the second component does not contain vasculature targeting ligands, and is directly released into the interstitium. The design of separate components optimized for distinct spatiotemporal compartments may be necessary for effective targeting of synthetic delivery system due to an incomplete understanding of how to effectively overcome trade-offs in terms of ligand-receptor interactions at the endothelial versus interstitial interfaces. Extracellular vesicles are likely to display complex surfaces that have multifunctional capacity to bind to target vasculature, extracellular matrix, and parenchymal cells. Alternatively, the discovery that multilayered extracellular vesicles are common among various biofluid and cellular sources [189] brings into question whether potential stimuli-responsive removal of such layers could contribute to the unraveling of surfaces with binding affinities for distinct spatial compartments. An enhanced understanding of extracellular vesicle-mediated transport within the body is likely to substantially contribute to the design of synthetic drug delivery systems for site-specific delivery.
It is evident that there is a pressing need to improve nanotherapeutics, especially those designed to exploit active targeting as their primary mechanism of reaching the intended organs and cells. Systemically administered drugs encounter the vascular endothelium, prior to entering the tissue interstitium, but are often unable to cross this formidable barrier. The vast heterogeneity that endothelial cells display across tissues, having organ-specific surface markers, makes them a plausible intermediary for organotropic targeting. Nanoparticles can thus be equipped with targeting moieties directed at organ-specific endothelial markers together with modalities that further facilitate eventual migration into the tissue interstitium. Taken together, several opportunities and challenges exist regarding the implementation of vasculature organotropism in systemic drug delivery (Table 3). The upcoming decades are likely to provide substantial progress in the preclinical development and clinical translation of synthetic and biological nanoparticles that exploit organ-specific endothelial properties, enabling targeted delivery of new biologics, such as those based on RNA and proteins.
Table 3.
Opportunities and challenges of exploiting vasculature organotropism for systemic drug delivery
| Opportunities |
|---|
|
| Challenges |
|
Acknowledgements
This work was partially funded by The University of Queensland, Australia (JW), the National Institute on Aging, National Institutes of Health, United States under award number R01AG076537 (JW), and The Medical Research Future Fund, Australia under award number MRF2019485 (JW, SWC). The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies. All figures were made in ©BioRender - biorender.com
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Kim SM, Faix PH, Schnitzer JE, Overcoming key biological barriers to cancer drug delivery and efficacy, J Control Release, 267 (2017) 15–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Arap W, Pasqualini R, Ruoslahti E, Cancer Treatment by Targeted Drug Delivery to Tumor Vasculature in a Mouse Model, Science, 279 (1998) 377–380. [DOI] [PubMed] [Google Scholar]
- [3].Teesalu T, Sugahara KN, Ruoslahti E, Mapping of Vascular ZIP Codes by Phage Display, Methods Enzymol, 503 (2012) 35–56. [DOI] [PubMed] [Google Scholar]
- [4].Neri D, Bicknell R, Tumour vascular targeting, Nat Rev Cancer, 5 (2005) 436–446. [DOI] [PubMed] [Google Scholar]
- [5].Smith TL, Sidman RL, Arap W, Pasqualini R, The Vasculome, Elsevier Inc2022, pp. 393–401. [Google Scholar]
- [6].Skowronski DM, De Serres G, Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine, N. Engl. J. Med, 384 (2021) 1576–1577. [DOI] [PubMed] [Google Scholar]
- [7].Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB, McGettigan J, Khetan S, Segall N, Solis J, Brosz A, Fierro C, Schwartz H, Neuzil K, Corey L, Gilbert P, Janes H, Follmann D, Marovich M, Mascola J, Polakowski L, Ledgerwood J, Graham BS, Bennett H, Pajon R, Knightly C, Leav B, Deng W, Zhou H, Han S, Ivarsson M, Miller J, Zaks T, Group CS, Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine, N. Engl. J. Med, 384 (2021) 403–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Stadler CR, Bahr-Mahmud H, Celik L, Hebich B, Roth AS, Roth RP, Kariko K, Tureci O, Sahin U, Elimination of large tumors in mice by mRNA-encoded bispecific antibodies, Nat. Med, 23 (2017) 815–817. [DOI] [PubMed] [Google Scholar]
- [9].Rurik JG, Tombacz I, Yadegari A, Mendez Fernandez PO, Shewale SV, Li L, Kimura T, Soliman OY, Papp TE, Tam YK, Mui BL, Albelda SM, Pure E, June CH, Aghajanian H, Weissman D, Parhiz H, Epstein JA, CAR T cells produced in vivo to treat cardiac injury, Science, 375 (2022) 91–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kormann MS, Hasenpusch G, Aneja MK, Nica G, Flemmer AW, Herber-Jonat S, Huppmann M, Mays LE, Illenyi M, Schams A, Griese M, Bittmann I, Handgretinger R, Hartl D, Rosenecker J, Rudolph C, Expression of therapeutic proteins after delivery of chemically modified mRNA in mice, Nat. Biotechnol, 29 (2011) 154–157. [DOI] [PubMed] [Google Scholar]
- [11].Jirikowski GF, Sanna PP, Maciejewski-Lenoir D, Bloom FE, Reversal of diabetes insipidus in Brattleboro rats: intrahypothalamic injection of vasopressin mRNA, Science, 255 (1992) 996–998. [DOI] [PubMed] [Google Scholar]
- [12].Perez-Garcia CG, Diaz-Trelles R, Vega JB, Bao Y, Sablad M, Limphong P, Chikamatsu S, Yu H, Taylor W, Karmali PP, Tachikawa K, Chivukula P, Development of an mRNA replacement therapy for phenylketonuria, Mol Ther Nucleic Acids, 28 (2022) 87–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Rahimi N, Defenders and challengers of endothelial barrier function, Front Immunol, 8 (2017) 1847–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Aird WC, Phenotypic Heterogeneity of the Endothelium: I. Structure, Function, and Mechanisms, Circ Res, 100 (2007) 158–173. [DOI] [PubMed] [Google Scholar]
- [15].Dumas SJ, Meta E, Borri M, Luo Y, Li X, Rabelink TJ, Carmeliet P, Phenotypic diversity and metabolic specialization of renal endothelial cells, Nature Reviews Nephrology, 17 (2021) 441–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Jourde-Chiche N, Fakhouri F, Dou L, Bellien J, Burtey S, Frimat M, Jarrot PA, Kaplanski G, Le Quintrec M, Pernin V, Rigothier C, Sallée M, Fremeaux-Bacchi V, Guerrot D, Roumenina LT, Endothelium structure and function in kidney health and disease, Nat Rev Nephrol, 15 (2019) 87–108. [DOI] [PubMed] [Google Scholar]
- [17].Wolfram J, Ferrari M, Clinical cancer nanomedicine, Nano Today, 25 (2019) 85–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Melamed JR, Yerneni SS, Arral ML, LoPresti ST, Chaudhary N, Sehrawat A, Muramatsu H, Alameh MG, Pardi N, Weissman D, Gittes GK, Whitehead KA, Ionizable lipid nanoparticles deliver mRNA to pancreatic beta cells via macrophage-mediated gene transfer, Sci Adv, 9 (2023) eade1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Li B, Manan RS, Liang SQ, Gordon A, Jiang A, Varley A, Gao G, Langer R, Xue W, Anderson D, Combinatorial design of nanoparticles for pulmonary mRNA delivery and genome editing, Nat. Biotechnol, (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Di J, Du Z, Wu K, Jin S, Wang X, Li T, Xu Y, Biodistribution and Non-linear Gene Expression of mRNA LNPs Affected by Delivery Route and Particle Size, Pharm. Res, 39 (2022) 105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Komarova Y, Malik AB, Regulation of endothelial permeability via paracellular and transcellular transport pathways, Annu Rev Physiol, 72 (2010) 463–493. [DOI] [PubMed] [Google Scholar]
- [22].Fang J, Nakamura H, Maeda H, The EPR effect: Unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect, Adv Drug Deliv Rev, 63 (2011) 136–151. [DOI] [PubMed] [Google Scholar]
- [23].Sindhwani S, Syed AM, Ngai J, Kingston BR, Maiorino L, Rothschild J, MacMillan P, Zhang Y, Rajesh NU, Hoang T, Wu JLY, Wilhelm S, Zilman A, Gadde S, Sulaiman A, Ouyang B, Lin Z, Wang L, Egeblad M, Chan WCW, The entry of nanoparticles into solid tumours, Nat Mater, 19 (2020) 566–575. [DOI] [PubMed] [Google Scholar]
- [24].Feletou M, The endothelium, part I, Morgan & Claypool Publishers, San Rafael, Calif. (1537 Fourth Street, San Rafael, CA 94901 USA: ), 2011. [Google Scholar]
- [25].Sofias AM, Toner YC, Meerwaldt AE, van Leent MMT, Soultanidis G, Elschot M, Gonai H, Grendstad K, Flobak Å, Neckmann U, Wolowczyk C, Fisher EL, Reiner T, Davies C.d.L., Bjørkøy G, Teunissen AJP, Ochando J, Pérez-Medina C, Mulder WJM, Hak S, Tumor Targeting by αvβ3-Integrin-Specific Lipid Nanoparticles Occurs via Phagocyte Hitchhiking, ACS Nano, 14 (2020) 7832–7846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Romero EL, Morilla M-J, Regts J, Koning GA, Scherphof GL, On the mechanism of hepatic transendothelial passage of large liposomes, FEBS Lett, 448 (1999) 193–196. [DOI] [PubMed] [Google Scholar]
- [27].Harrington KJ, Mohammadtaghi S, Uster PS, Glass D, Peters AM, Vile RG, Stewart JSW, Effective Targeting of Solid Tumors in Patients With Locally Advanced Cancers by Radiolabeled Pegylated Liposomes, Clin Cancer Res, 7 (2001) 243–254. [PubMed] [Google Scholar]
- [28].Nichols JW, Bae YH, Odyssey of a cancer nanoparticle: From injection site to site of action, Nano Today, 7 (2012) 606–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Lammers T, Kiessling F, Hennink WE, Storm G, Drug targeting to tumors: Principles, pitfalls and (pre-) clinical progress, J Control Release, 161 (2012) 175–187. [DOI] [PubMed] [Google Scholar]
- [30].Jain RK, Stylianopoulos T, Delivering nanomedicine to solid tumors, Nat Rev Clin Oncol, 7 (2010) 653–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Michiels C, Tellier C, Feron O, Cycling hypoxia: A key feature of the tumor microenvironment, Biochimica et biophysica acta. Reviews on cancer, 1866 (2016) 76–86. [DOI] [PubMed] [Google Scholar]
- [32].Hare JI, Lammers T, Ashford MB, Puri S, Storm G, Barry ST, Challenges and strategies in anti-cancer nanomedicine development: An industry perspective, Adv Drug Deliv Rev, 108 (2017) 25–38. [DOI] [PubMed] [Google Scholar]
- [33].Heldin C-H, Rubin K, Pietras K, Östman A, High interstitial fluid pressure - an obstacle in cancer therapy, Nat Rev Cancer, 4 (2004) 806–813. [DOI] [PubMed] [Google Scholar]
- [34].Boulikas T, Stathopoulos GP, Volakakis N, Vougiouka M, Systemic Lipoplatin infusion results in preferential tumor uptake in human studies, Anticancer Res, 25 (2005) 3031–3039. [PubMed] [Google Scholar]
- [35].Danhier F, Feron O, Préat V, To exploit the tumor microenvironment: Passive and active tumor targeting of nanocarriers for anti-cancer drug delivery, J Control Release, 148 (2010) 135–146. [DOI] [PubMed] [Google Scholar]
- [36].Fu Z, Li S, Han S, Shi C, Zhang Y, Antibody drug conjugate: the “biological missile” for targeted cancer therapy, Signal Transduct Target Ther, 7 (2022) 93–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Beck A, Goetsch L, Dumontet C, Corvaïa N, Strategies and challenges for the next generation of antibody-drug conjugates, Nat Rev Drug Discov, 16 (2017) 315–337. [DOI] [PubMed] [Google Scholar]
- [38].Denison TA, Bae YH, Tumor heterogeneity and its implication for drug delivery, J Control Release, 164 (2012) 187–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Caracciolo G, Pozzi D, Capriotti AL, Cavaliere C, Piovesana S, La Barbera G, Amici A, Lagana A, The liposome-protein corona in mice and humans and its implications for in vivo delivery, J Mater Chem B, 2 (2014) 7419–7428. [DOI] [PubMed] [Google Scholar]
- [40].Juweid M, Neumann R, Paik C, Perez-Bacete MJ, Sato J, Van Osdol W, Weinstein JN, Micropharmacology of monoclonal antibodies in solid tumors: Direct experimental evidence for a binding site barrier, Cancer Res, 52 (1992) 5144–5153. [PubMed] [Google Scholar]
- [41].Ruoslahti E, Engvall E, Integrins and vascular extracellular matrix assembly, J Clin Invest, 99 (1997) 1149–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Aird WC, Endothelial cell heterogeneity, Cold Spring Harb Perspect Med, 2 (2012) a006429–a006429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Aird WC, Mechanisms of Endothelial Cell Heterogeneity in Health and Disease, Circ Res, 98 (2006) 159–162. [DOI] [PubMed] [Google Scholar]
- [44].Danilov SM, Gavrilyuk VD, Franke FE, Pauls K, Harshaw DW, McDonald TD, Miletich DJ, Muzykantov VR, Lung uptake of antibodies to endothelial antigens : key determinants of vascular immunotargeting, American journal of physiology. Lung cellular and molecular physiology, 24 (2001) L1335–L1347. [DOI] [PubMed] [Google Scholar]
- [45].Gavard J, Endothelial permeability and VE-cadherin: A wacky comradeship, Cell Adh Migr, 7 (2013) 465–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Muro S, Muzykantov V, Targeting of antioxidant and anti-thrombotic drugs to endothelial cell adhesion molecules, Current pharmaceutical design, 11 (2005) 2383–2401. [DOI] [PubMed] [Google Scholar]
- [47].Koning GA, Schiffelers RM, Storm G, Endothelial cells at inflammatory sites as target for therapeutic intervention, Endothelium, 9 (2002) 161–171. [DOI] [PubMed] [Google Scholar]
- [48].Balyasnikova IV, Metzger R, Visintine DJ, Dimasius V, Sun Z-L, Berestetskaya YV, McDonald TD, Curiel DT, Minshall RD, Danilov SM, Selective rat lung endothelial targeting with a new set of monoclonal antibodies to angiotensin I-converting enzyme, Pulm Pharmacol Ther, 18 (2005) 251–267. [DOI] [PubMed] [Google Scholar]
- [49].Balyasnikova IV, Sun ZL, Metzger R, Taylor PR, Vicini E, Muciaccia B, Visintine DJ, Berestetskaya YV, McDonald TD, Danilov SM, Monoclonal antibodies to native mouse angiotensin-converting enzyme (CD143): ACE expression quantification, lung endothelial cell targeting and gene delivery, Tissue Antigens, 67 (2006) 10–29. [DOI] [PubMed] [Google Scholar]
- [50].Spragg DD, Alford DR, Greferath R, Larsen CE, Lee K-D, Gurtner GC, Cybulsky MI, Tosi PF, Nicolau C, Gimbrone MA, Immunotargeting of Liposomes to Activated Vascular Endothelial Cells: A Strategy for Site-Selective Delivery in the Cardiovascular System, Proc Natl Acad Sci U S A, 94 (1997) 8795–8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Christian S, Ahorn H, Koehler A, Eisenhaber F, Rodi H-P, Garin-Chesa P, Park JE, Rettig WJ, Lenter MC, Molecular Cloning and Characterization of Endosialin, a C-type Lectin-like Cell Surface Receptor of Tumor Endothelium, J Biol Chem, 276 (2001) 7408–7414. [DOI] [PubMed] [Google Scholar]
- [52].Michaelis K, Hoffmann MM, Dreis S, Herbert E, Alyautdin RN, Michaelis M, Kreuter J, Langer K, Covalent Linkage of Apolipoprotein E to Albumin Nanoparticles Strongly Enhances Drug Transport into the Brain, J Pharmacol Exp Ther, 317 (2006) 1246–1253. [DOI] [PubMed] [Google Scholar]
- [53].Smith DJ, Salmi M, Bono P, Hellman J, Leu T, Jalkanen S, Cloning of vascular adhesion protein 1 reveals a novel multifunctional adhesion molecule, J Exp Med, 188 (1998) 17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Girard J-P, Springer TA, High endothelial venules (HEVs): specialized endothelium for lymphocyte migration, Immunol Today, 16 (1995) 449–457. [DOI] [PubMed] [Google Scholar]
- [55].Madej A, Reich A, Orda A, Szepietowski JC, Expression of Vascular Adhesion Protein-1 in Atopic Eczema, Int Arch Allergy Immunol, 139 (2006) 114–121. [DOI] [PubMed] [Google Scholar]
- [56].Metzger R, Franke FE, Bohle RM, Alhenc-Gelas F, Danilov SM, Heterogeneous distribution of angiotensin I-converting enzyme (CD143) in the human and rat vascular systems: Vessel, organ and species specificity, Microvasc Res, 81 (2011) 206–215. [DOI] [PubMed] [Google Scholar]
- [57].Mäkinen T, Veikkola T, Mustjoki S, Karpanen T, Catimel B, Nice EC, Wise L, Mercer A, Kowalski H, Kerjaschki D, Stacker SA, Achen MG, Alitalo K, Isolated lymphatic endothelial cells transduce growth, survival and migratory signals via the VEGF-C/D receptor VEGFR-3, EMBO J, 20 (2001) 4762–4773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Zhuo W, Jia L, Song N, Lu X-A, Ding Y, Wang X, Song X, Fu Y, Luo Y, The CXCL12 -CXCR4 chemokine pathway: A novel axis regulates lymphangiogenesis, Clin Cancer Res, 18 (2012) 5387–5398. [DOI] [PubMed] [Google Scholar]
- [59].Veikkola T, Karkkainen M, Claesson-Welsh L, Alitalo K, Regulation of angiogenesis via vascular endothelial growth factor receptors, Cancer Res, 60 (2000) 203–212. [PubMed] [Google Scholar]
- [60].Xu J, Liang J, Meng Y-M, Yan J, Yu X-J, Liu C-Q, Xu L, Zhuang S-M, Zheng L, Vascular CXCR4 expression promotes vessel sprouting and sensitivity to sorafenib treatment in hepatocellular carcinoma, Clin Cancer Res, 23 (2017) 4482–4492. [DOI] [PubMed] [Google Scholar]
- [61].Vannucci SJ, Developmental Expression of GLUT1 and GLUT3 Glucose Transporters in Rat Brain, J Neurochem, 62 (1994) 240–246. [DOI] [PubMed] [Google Scholar]
- [62].Jefferies WA, Brandon MR, Hunt SV, Williams AF, Gatter KC, Mason DY, Transferrin receptor on endothelium of brain capillaries, Nature, 312 (1984) 162–163. [DOI] [PubMed] [Google Scholar]
- [63].Brady J, Neal J, Sadakar N, Gasque P, Human Endosialin (Tumor Endothelial Marker 1) Is Abundantly Expressed in Highly Malignant and Invasive Brain Tumors, J Neuropathol Exp Neurol, 63 (2004) 1274–1283. [DOI] [PubMed] [Google Scholar]
- [64].Rouleau C, Curiel M, Weber W, Smale R, Kurtzberg L, Mascarello J, Berger C, Wallar G, Bagley R, Honma N, Hasegawa K, Ishida I, Kataoka S, Thurberg BL, Mehraein K, Horten B, Miller G, Teicher BA, Endosialin Protein Expression and Therapeutic Target Potential in Human Solid Tumors: Sarcoma versus Carcinoma, Clin Cancer Res, 14 (2008) 7223–7236. [DOI] [PubMed] [Google Scholar]
- [65].Facciponte JG, Ugel S, De Sanctis F, Li C, Wang L, Nair G, Sehgal S, Raj A, Matthaiou E, Coukos G, Facciabene A, Tumor endothelial marker 1-specific DNA vaccination targets tumor vasculature, J Clin Invest, 124 (2014) 1497–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Hatley RJD, Macdonald SJF, Slack RJ, Le J, Ludbrook SB, Lukey PT, An αv-RGD Integrin Inhibitor Toolbox: Drug Discovery Insight, Challenges and Opportunities, Angew Chem Int Ed Engl, 57 (2018) 3298–3321. [DOI] [PubMed] [Google Scholar]
- [67].Sloan EK, Pouliot N, Stanley KL, Chia J, Moseley JM, Hards DK, Anderson RL, Tumor-specific expression of αvβ3 integrin promotes spontaneous metastasis of breast cancer to bone, Breast cancer research : BCR, 8 (2006) R20–R20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Huang RB, Mocherla S, Heslinga MJ, Charoenphol P, Eniola-Adefeso O, Dynamic and cellular interactions of nanoparticles in vascular-targeted drug delivery (review), Mol Membr Biol, 27 (2010) 312–327. [DOI] [PubMed] [Google Scholar]
- [69].Xia W, Low PS, Folate-Targeted Therapies for Cancer, J. Med. Chem, 53 (2010) 6811–6824. [DOI] [PubMed] [Google Scholar]
- [70].Neri D, Bicknell R, Tumour vascular targeting, Nat. Rev. Cancer, 5 (2005) 436–446. [DOI] [PubMed] [Google Scholar]
- [71].Müller AM, Hermanns MI, Skrzynski C, Nesslinger M, Müller K-M, Kirkpatrick CJ, Expression of the Endothelial Markers PECAM-1, vWf, and CD34 in Vivo and in Vitro, Exp Mol Pathol, 72 (2002) 221–229. [DOI] [PubMed] [Google Scholar]
- [72].Hu M, Zhang H, Liu Q, Hao Q, Structural Basis for Human PECAM-1-Mediated Trans-homophilic Cell Adhesion, Sci Rep, 6 (2016) 38655–38655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Piali L, Hammel P, Uherek C, Bachmann F, Gisler RH, Dunon D, Imhof BA, CD31/PECAM-1 Is a Ligand for α vβ 3 Integrin Involved in Adhesion of Leukocytes to Endothelium, J Cell Biol, 130 (1995) 451–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Giannotta M, Trani M, Dejana E, VE-Cadherin and Endothelial Adherens Junctions: Active Guardians of Vascular Integrity, Dev Cell, 26 (2013) 441–454. [DOI] [PubMed] [Google Scholar]
- [75].Gougos A, Letarte M, Primary structure of endoglin, an RGD-containing glycoprotein of human endothelial cells, J Biol Chem, 265 (1990) 8361–8364. [PubMed] [Google Scholar]
- [76].López-Novoa JM, Bernabeu C, The physiological role of endoglin in the cardiovascular system, Am J Physiol Heart Circ Physiol, 299 (2010) H959–H974. [DOI] [PubMed] [Google Scholar]
- [77].Lawson C, Wolf S, ICAM-1 signaling in endothelial cells, Pharmacol Rep, 61 (2009) 22–32. [DOI] [PubMed] [Google Scholar]
- [78].Menschikowski M, Hagelgans A, Eisenhofer G, Tiebel O, Siegert G, Reducing agents induce thrombomodulin shedding in human endothelial cells, Thromb Res, 126 (2010) e88–e93. [DOI] [PubMed] [Google Scholar]
- [79].van Hinsbergh VWM, Endothelium-role in regulation of coagulation and inflammation, Semin Immunopathol, 34 (2012) 93–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Schnitzer JE, Oh P, Li Y, Yu J, Durr E, Krasinska KM, Carver LA, Testa JE, Subtractive proteomic mapping of the endothelial surface in lung and solid tumours for tissue-specific therapy, Nature, 429 (2004) 629–635. [DOI] [PubMed] [Google Scholar]
- [81].Schnitzer JE, Durr E, Yu J, Krasinska KM, Carver LA, Yates JR, Testa JE, Oh P, Direct proteomic mapping of the lung microvascular endothelial cell surface in vivo and in cell culture, Nat Biotechnol, 22 (2004) 985–992. [DOI] [PubMed] [Google Scholar]
- [82].Kim HR, Gil S, Andrieux K, Nicolas V, Appel M, Chacun H, Desmaële D, Taran F, Georgin D, Couvreur P, Low-density lipoprotein receptor-mediated endocytosis of PEGylated nanoparticles in rat brain endothelial cells, Cell Mol Life Sci, 64 (2007) 356–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Demeule M, Currie J-C, Bertrand Y, Ché C, Nguyen T, Régina A, Gabathuler R, Castaigne JP, Béliveau R, Involvement of the low-density lipoprotein receptor-related protein in the transcytosis of the brain delivery vector Angiopep-2, J Neurochem, 106 (2008) 1534–1544. [DOI] [PubMed] [Google Scholar]
- [84].Aalto K, Autio A, Kiss EA, Elima K, Nymalm Y, Veres TZ, Marttila-Ichihara F, Elovaara H, Saanijoki T, Crocker PR, Maksimow M, Bligt E, Salminen TA, Salmi M, Roivainen A, Jalkanen S, Siglec-9 is a novel leukocyte ligand for vascular adhesion protein-1 and can be used in PET imaging of inflammation and cancer, Blood, 118 (2011) 3725–3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Ma L, Li W, Zhang Y, Qi L, Zhao Q, Li N, Lu Y, Zhang L, Zhou F, Wu Y, He Y, Yu H, He Y, Wei B, Wang H, FLT4/VEGFR3 activates AMPK to coordinate glycometabolic reprogramming with autophagy and inflammasome activation for bacterial elimination, Autophagy, 18 (2022) 1385–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Breiteneder-Geleff S, Soleiman A, Kowalski H, Horvat R, Amann G, Kriehuber E, Diem K, Weninger W, Tschachler E, Alitalo K, Kerjaschki D, Angiosarcomas Express Mixed Endothelial Phenotypes of Blood and Lymphatic Capillaries : Podoplanin as a Specific Marker for Lymphatic Endothelium, Am J Pathol, 154 (1999) 385–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Banerji S, Ni J, Wang S-X, Clasper S, Su J, Tammi R, Jones M, Jackson DG, LYVE-1, a New Homologue of the CD44 Glycoprotein, Is a Lymph-Specific Receptor for Hyaluronan, J Cell Biol, 144 (1999) 789–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Johnson LA, Prevo R, Clasper S, Jackson DG, Inflammation-induced Uptake and Degradation of the Lymphatic Endothelial Hyaluronan Receptor LYVE-1, J Biol Chem, 282 (2007) 33671–33680. [DOI] [PubMed] [Google Scholar]
- [89].Pasqualini R, Arap W, Kolonin MG, Saha PK, Chan L, Reversal of obesity by targeted ablation of adipose tissue, Nat Med, 10 (2004) 625–632. [DOI] [PubMed] [Google Scholar]
- [90].Kolonin MG, Saha PK, Chan L, Pasqualini R, Arap W, Reversal of obesity by targeted ablation of adipose tissue, Nat Med, 10 (2004) 625–632. [DOI] [PubMed] [Google Scholar]
- [91].Winkler IG, Barbier V, Nowlan B, Jacobsen RN, Forristal CE, Patton JT, Magnani JL, Lévesque J-P, Vascular niche E-selectin regulates hematopoietic stem cell dormancy, self renewal and chemoresistance, Nat Med, 18 (2012) 1651–1657. [DOI] [PubMed] [Google Scholar]
- [92].Liu Z, Wang F, Chen X, Integrin αvβ3-targeted cancer therapy, Drug Dev. Res, 69 (2008) 329–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Seaman S, Zhu Z, Saha S, Zhang XM, Yang MY, Hilton MB, Morris K, Szot C, Morris H, Swing DA, Tessarollo L, Smith SW, Degrado S, Borkin D, Jain N, Scheiermann J, Feng Y, Wang Y, Li J, Welsch D, DeCrescenzo G, Chaudhary A, Zudaire E, Klarmann KD, Keller JR, Dimitrov DS, St B. Croix, Eradication of Tumors through Simultaneous Ablation of CD276/B7-H3-Positive Tumor Cells and Tumor Vasculature, Cancer Cell, 31 (2017) 501–515.e508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Kontos F, Michelakos T, Kurokawa T, Sadagopan A, Schwab JH, Ferrone CR, Ferrone S, B7-H3: An attractive target for antibody-based immunotherapy, Clin Cancer Res, 27 (2021) 1227–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].St. Croix B, Rago C, Velculescu V, Traverso G, Romans KE, Montgomery E, Lal A, Riggins GJ, Lengauer C, Vogelstein B, Kinzler KW, Genes Expressed in Human Tumor Endothelium, Science, 289 (2000) 1197–1202. [DOI] [PubMed] [Google Scholar]
- [96].Nanda A, Carson-Walter EB, Seaman S, Barber TD, Stampfl J, Singh S, Vogelstein B, Kinzler KW, St Croix B, TEM8 interacts with the cleaved C5 domain of collagen alpha 3(VI), Cancer Res, 64 (2004) 817–820. [DOI] [PubMed] [Google Scholar]
- [97].Hatakeyama S, Sugihara K, Shibata TK, Nakayama J, Akama TO, Tamura N, Wong S-M, Bobkov AA, Takano Y, Ohyama C, Fukuda M, Fukuda MN, Targeted drug delivery to tumor vasculature by a carbohydrate mimetic peptide, Proc Natl Acad Sci U S A, 108 (2011) 19587–19592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Tu Y, Johnstone CN, Stewart AG, Annexin A1 influences in breast cancer: Controversies on contributions to tumour, host and immunoediting processes, Pharmacol Res, 119 (2017) 278–288. [DOI] [PubMed] [Google Scholar]
- [99].Leeuwenberg JFM, Smeets EF, Neefjes JJ, Shaffer MA, Cinek T, Jeunhomme TMAA, Ahern TJ, Buurman WA, E-selectin and intercellular adhesion molecule-1 are released by activated human endothelial cells in vitro, Immunology, 77 (1992) 543–549. [PMC free article] [PubMed] [Google Scholar]
- [100].Kansas GS, Selectins and Their Ligands: Current Concepts and Controversies, Blood, 88 (1996) 3259–3287. [PubMed] [Google Scholar]
- [101].Muz B, Abdelghafer A, Markovic M, Yavner J, Melam A, Salama NN, Azab AK, Targeting E-selectin to Tackle Cancer Using Uproleselan, Cancers (Basel), 13 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Nilsson F, Kosmehl H, Zardi L, Neri D, Targeted delivery of tissue factor to the ED-B domain of fibronectin, a marker of angiogenesis, mediates the infarction of solid tumors in mice, Cancer Res, 61 (2001) 711–716. [PubMed] [Google Scholar]
- [103].Pasqualini R, Koivunen E, Kain R, Lahdenranta J, Sakamoto M, Stryhn A, Ashmun RA, Shapiro LH, Arap W, Ruoslahti E, Aminopeptidase N is a receptor for tumor-homing peptides and a target for inhibiting angiogenesis, Cancer Res, 60 (2000) 722–727. [PMC free article] [PubMed] [Google Scholar]
- [104].Bhagwat SV, Lahdenranta J, Giordano R, Arap W, Pasqualini R, Shapiro LH, CD13/APN is activated by angiogenic signals and is essential for capillary tube formation, Blood, 97 (2001) 652–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Vaidya A, Wang H, Qian V, Gilmore H, Lu ZR, Overexpression of Extradomain-B Fibronectin is Associated with Invasion of Breast Cancer Cells, Cells, 9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Fogal V, Zhang L, Krajewski S, Ruoslahti E, Mitochondrial/cell-surface protein p32/gC1qR as a molecular target in tumor cells and tumor stroma, Cancer Res, 68 (2008) 7210–7218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Roth L, Agemy L, Kotamraju VR, Braun G, Teesalu T, Sugahara KN, Hamzah J, Ruoslahti E, Transtumoral targeting enabled by a novel neuropilin-binding peptide, Oncogene, 31 (2012) 3754–3763. [DOI] [PubMed] [Google Scholar]
- [108].Fogal V, Zhang L, Krajewski S, Ruoslahti E, Mitochondrial/cell-surface protein p32/gC1qR as a molecular target in tumor cells and tumor stroma, Cancer Res, 68 (2008) 7210–7218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Pierschbacher MD, Ruoslahti E, Influence of stereochemistry of the sequence Arg-Gly-Asp-Xaa on binding specificity in cell adhesion, J Biol Chem, 262 (1987) 17294–17298. [PubMed] [Google Scholar]
- [110].BogdanowichKnipp SJ, Chakrabarti S, Siahaan TJ, Williams TD, Dillman RK, Solution stability of linear vs. cyclic RGD peptides, J Pept Res, 53 (1999) 530–541. [DOI] [PubMed] [Google Scholar]
- [111].Koivunen E, Wang B, Ruoslahti E, Phage Libraries Displaying Cyclic Peptides with Different Ring Sizes: Ligand Specificities of the RGD-Directed Integrins, Nature biotechnology, 13 (1995) 265–270. [DOI] [PubMed] [Google Scholar]
- [112].Hölig P, Bach M, Völkel T, Nahde T, Hoffmann S, Müller R, Kontermann RE, Novel RGD lipopeptides for the targeting of liposomes to integrin-expressing endothelial and melanoma cells, Protein Engineering, Design and Selection, 17 (2004) 433–441. [DOI] [PubMed] [Google Scholar]
- [113].Bellis SL, Advantages of RGD peptides for directing cell association with biomaterials, Biomaterials, 32 (2011) 4205–4210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Ruoslahti E, The RGD story: a personal account, Matrix Biol, 22 (2003) 459–465. [DOI] [PubMed] [Google Scholar]
- [115].Plopper GE, McNamee HP, Dike LE, Bojanowski K, Ingber DE, Convergence of integrin and growth factor receptor signaling pathways within the focal adhesion complex, Mol Biol Cell, 6 (1995) 1349–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Teesalu T, Sugahara KN, Kotamraju VR, Ruoslahti E, C-end rule peptides mediate neuropilin-1-dependent cell, vascular, and tissue penetration, Proc Natl Acad Sci U S A, 106 (2009) 16157–16162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Sugahara KN, Teesalu T, Karmali PP, Kotamraju VR, Agemy L, Girard OM, Hanahan D, Mattrey RF, Ruoslahti E, Tissue-Penetrating Delivery of Compounds and Nanoparticles into Tumors, Cancer Cell, 16 (2009) 510–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Pastorino F, Brignole C, Marimpietri D, Cilli M, Gambini C, Ribatti D, Longhi R, Allen TM, Corti A, Ponzoni M, Vascular Damage and Anti-angiogenic Effects of Tumor Vessel-Targeted Liposomal Chemotherapy, Cancer Res, 63 (2003) 7400–7409. [PubMed] [Google Scholar]
- [119].Crone C, Olesen SP, Electrical resistance of brain microvascular endothelium, Brain Res, 241 (1982) 49–55. [DOI] [PubMed] [Google Scholar]
- [120].Roney C, Kulkarni P, Arora V, Antich P, Bonte F, Wu A, Mallikarjuana NN, Manohar S, Liang H-F, Kulkarni AR, Sung H-W, Sairam M, Aminabhavi TM, Targeted nanoparticles for drug delivery through the blood–brain barrier for Alzheimer’s disease, J Control Release, 108 (2005) 193–214. [DOI] [PubMed] [Google Scholar]
- [121].Wiley DT, Webster P, Gale A, Davis ME, Transcytosis and brain uptake of transferrin-containing nanoparticles by tuning avidity to transferrin receptor, Proc. Natl. Acad. Sci. U. S. A, 110 (2013) 8662–8667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Neves AR, Queiroz JF, Lima SAC, Reis S, Apo E-Functionalization of Solid Lipid Nanoparticles Enhances Brain Drug Delivery: Uptake Mechanism and Transport Pathways, Bioconjugate Chem, 28 (2017) 995–1004. [DOI] [PubMed] [Google Scholar]
- [123].Hu K, Shi Y, Jiang W, Han J, Huang S, Jiang X, Lactoferrin conjugated PEG-PLGA nanoparticles for brain delivery: Preparation, characterization and efficacy in Parkinson’s disease, Int J Pharm, 415 (2011) 273–283. [DOI] [PubMed] [Google Scholar]
- [124].Jones AR, Shusta EV, Blood-brain barrier transport of therapeutics via receptor-mediation, Pharm Res, 24 (2007) 1759–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Zheng M, Ruan S, Liu S, Sun T, Qu D, Zhao H, Xie Z, Gao H, Jing X, Sun Z, Self-Targeting Fluorescent Carbon Dots for Diagnosis of Brain Cancer Cells, ACS Nano, 9 (2015) 11455–11461. [DOI] [PubMed] [Google Scholar]
- [126].Yamauchi Y, Helenius A, Virus entry at a glance, J Cell Sci, 126 (2013) 1289–1295. [DOI] [PubMed] [Google Scholar]
- [127].Witwer KW, Wolfram J, Extracellular vesicles versus synthetic nanoparticles for drug delivery, Nature reviews. Materials, 6 (2021) 103–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].De Lorenzi F, Rizzo LY, Daware R, Motta A, Baues M, Bartneck M, Vogt M, van Zandvoort M, Kaps L, Hu Q, Thewissen M, Casettari L, Rijcken CJF, Kiessling F, Sofias AM, Lammers T, Profiling target engagement and cellular uptake of cRGD-decorated clinical-stage core-crosslinked polymeric micelles, Drug Deliv Transl Res, 13 (2023) 1195–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Shimizu A, Sawada K, Kobayashi M, Yamamoto M, Yagi T, Kinose Y, Kodama M, Hashimoto K, Kimura T, Exosomal CD47 Plays an Essential Role in Immune Evasion in Ovarian Cancer, Mol. Cancer Res, 19 (2021) 1583–1595. [DOI] [PubMed] [Google Scholar]
- [130].Sheikov N, McDannold N, Vykhodtseva N, Jolesz F, Hynynen K, Cellular mechanisms of the blood-brain barrier opening induced by ultrasound in presence of microbubbles, Ultrasound Med Biol, 30 (2004) 979–989. [DOI] [PubMed] [Google Scholar]
- [131].Olsman M, Sereti V, Mühlenpfordt M, Johnsen KB, Andresen TL, Urquhart AJ, Davies C.d.L., Focused Ultrasound and Microbubble Treatment Increases Delivery of Transferrin Receptor-Targeting Liposomes to the Brain, Ultrasound Med Biol, 47 (2021) 1343–1355. [DOI] [PubMed] [Google Scholar]
- [132].Li X, Vemireddy V, Cai Q, Xiong H, Kang P, Li X, Giannotta M, Hayenga HN, Pan E, Sirsi SR, Mateo C, Kleinfeld D, Greene C, Campbell M, Dejana E, Bachoo R, Qin Z, Reversibly Modulating the Blood–Brain Barrier by Laser Stimulation of Molecular-Targeted Nanoparticles, Nano Lett, 21 (2021) 9805–9815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Beccaria K, Canney M, Bouchoux G, Puget S, Grill J, Carpentier A, Blood-brain barrier disruption with low-intensity pulsed ultrasound for the treatment of pediatric brain tumors: a review and perspectives, Neurosurg Focus, 48 (2020) E10–E10. [DOI] [PubMed] [Google Scholar]
- [134].Timbie KF, Afzal U, Date A, Zhang C, Song J, Wilson Miller G, Suk JS, Hanes J, Price RJ, MR image-guided delivery of cisplatin-loaded brain-penetrating nanoparticles to invasive glioma with focused ultrasound, J Control Release, 263 (2017) 120–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Kibria G, Hatakeyama H, Ohga N, Hida K, Harashima H, The effect of liposomal size on the targeted delivery of doxorubicin to Integrin αvβ3-expressing tumor endothelial cells, Biomaterials, 34 (2013) 5617–5627. [DOI] [PubMed] [Google Scholar]
- [136].Golombek SK, May JN, Theek B, Appold L, Drude N, Kiessling F, Lammers T, Tumor targeting via EPR: Strategies to enhance patient responses, Adv Drug Deliv Rev, 130 (2018) 17–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Yao Y, Zhou Y, Liu L, Xu Y, Chen Q, Wang Y, Wu S, Deng Y, Zhang J, Shao A, Nanoparticle-Based Drug Delivery in Cancer Therapy and Its Role in Overcoming Drug Resistance, Frontiers in molecular biosciences, 7 (2020) 193–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Fang C, Shi B, Pei Y-Y, Hong M-H, Wu J, Chen H-Z, In vivo tumor targeting of tumor necrosis factor-α-loaded stealth nanoparticles: Effect of MePEG molecular weight and particle size, Eur J Pharm Sci, 27 (2006) 27–36. [DOI] [PubMed] [Google Scholar]
- [139].Chauhan VP, Popović Z, Chen O, Cui J, Fukumura D, Bawendi MG, Jain RK, Fluorescent nanorods and nanospheres for real-time in vivo probing of nanoparticle shape-dependent tumor penetration, Angew Chem Int Ed Engl, 50 (2011) 11417–11420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Kolhar P, Anselmo AC, Gupta V, Pant K, Prabhakarpandian B, Ruoslahti E, Mitragotri S, Using shape effects to target antibody-coated nanoparticles to lung and brain endothelium, Proc. Natl. Acad. Sci. U. S. A, 110 (2013) 10753–10758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Venuta A, Wolfram J, Shen H, Ferrari M, Post-nano strategies for drug delivery: multistage porous silicon microvectors, Journal of Materials Chemistry B, 5 (2017) 207–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Wolfram J, Shen H, Ferrari M, Multistage vector (MSV) therapeutics, J. Control. Release, 219 (2015) 406–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Herve F, Ghinea N, Scherrmann JM, CNS delivery via adsorptive transcytosis, AAPS J, 10 (2008) 455–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Helm F, Fricker G, Liposomal conjugates for drug delivery to the central nervous system, Pharmaceutics, 7 (2015) 27–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Froehlich E, The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles, Int J Nanomedicine, 7 (2012) 5577–5591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Lu W, Wan J, She Z, Jiang X, Brain delivery property and accelerated blood clearance of cationic albumin conjugated pegylated nanoparticle, J Control Release, 118 (2007) 38–53. [DOI] [PubMed] [Google Scholar]
- [147].Oh W-K, Kim S, Choi M, Kim C, Jeong YS, Cho B-R, Hahn J-S, Jang J, Cellular Uptake, Cytotoxicity, and Innate Immune Response of Silica−Titania Hollow Nanoparticles Based on Size and Surface Functionality, ACS Nano, 4 (2010) 5301–5313. [DOI] [PubMed] [Google Scholar]
- [148].Mi Y, Mu C, Wolfram J, Deng Z, Hu TY, Liu X, Blanco E, Shen H, Ferrari M, A Micro/Nano Composite for Combination Treatment of Melanoma Lung Metastasis, Advanced healthcare materials, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Kong G, Braun RD, Dewhirst MW, Characterization of the effect of hyperthermia on nanoparticle extravasation from tumor vasculature, Cancer Res, 61 (2001) 3027–3032. [PubMed] [Google Scholar]
- [150].Kuo Y-C, Lee C-L, Methylmethacrylate–sulfopropylmethacrylate nanoparticles with surface RMP-7 for targeting delivery of antiretroviral drugs across the blood–brain barrier, Colloids Surf B Biointerfaces, 90 (2012) 75–82. [DOI] [PubMed] [Google Scholar]
- [151].Tominaga N, Kosaka N, Ono M, Katsuda T, Yoshioka Y, Tamura K, Lötvall J, Nakagama H, Ochiya T, Brain metastatic cancer cells release microRNA-181c-containing extracellular vesicles capable of destructing blood-brain barrier, Nat Commun, 6 (2015) 6716–6716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Morad G, Carman CV, Hagedorn EJ, Perlin JR, Zon LI, Mustafaoglu N, Park T-E, Ingber DE, Daisy CC, Moses MA, Tumor-Derived Extracellular Vesicles Breach the Intact Blood–Brain Barrier via Transcytosis, ACS Nano, 13 (2019) 13853–13865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].van Niel G, D’Angelo G, Raposo G, Shedding light on the cell biology of extracellular vesicles, Nat. Rev. Mol. Cell Biol, 19 (2018) 213–228. [DOI] [PubMed] [Google Scholar]
- [154].Peinado H, Alečković M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, Hergueta-Redondo M, Williams C, García-Santos G, Ghajar CM, Nitadori-Hoshino A, Hoffman C, Badal K, Garcia BA, Callahan MK, Yuan J, Martins VR, Skog J, Kaplan RN, Brady MS, Wolchok JD, Chapman PB, Kang Y, Bromberg J, Lyden D, Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET, Nat Med, 18 (2012) 883–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Peinado H, Zhang H, Matei IR, Costa-Silva B, Hoshino A, Rodrigues G, Psaila B, Kaplan RN, Bromberg JF, Kang Y, Bissell MJ, Cox TR, Giaccia AJ, Erler JT, Hiratsuka S, Ghajar CM, Lyden D, Pre-metastatic niches: Organ-specific homes for metastases, Nat Rev Cancer, 17 (2017) 302–317. [DOI] [PubMed] [Google Scholar]
- [156].Hoshino A, Costa-Silva B, Shen T-L, Rodrigues G, Hashimoto A, Tesic Mark M, Molina H, Kohsaka S, Di Giannatale A, Ceder S, Singh S, Williams C, Soplop N, Uryu K, Pharmer L, King T, Bojmar L, Davies AE, Ararso Y, Zhang T, Zhang H, Hernandez J, Weiss JM, Dumont-Cole VD, Kramer K, Wexler LH, Narendran A, Schwartz GK, Healey JH, Sandstrom P, Jørgen Labori K, Kure EH, Grandgenett PM, Hollingsworth MA, De Sousa M, Kaur S, Jain M, Mallya K, Batra SK, Jarnagin WR, Brady MS, Fodstad O, Muller V, Pantel K, Minn AJ, Bissell MJ, Garcia BA, Kang Y, Rajasekhar VK, Ghajar CM, Matei I, Peinado H, Bromberg J, Lyden D, Tumour exosome integrins determine organotropic metastasis, Nature, 527 (2015) 329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Fang JH, Zhang ZJ, Shang LR, Luo YW, Lin YF, Yuan Y, Zhuang SM, Hepatoma cell-secreted exosomal microRNA-103 increases vascular permeability and promotes metastasis by targeting junction proteins, Hepatology, 68 (2018) 1459–1475. [DOI] [PubMed] [Google Scholar]
- [158].Zhou W, Fong Miranda Y., Min Y, Somlo G, Liu L, Palomares Melanie R., Yu Y, Chow A, O’Connor Sean Timothy F., Chin Andrew R., Yen Y, Wang Y, Marcusson Eric G., Chu P, Wu J, Wu X, Li Arthur X., Li Z, Gao H, Ren X, Boldin Mark P., Lin Pengnian C., Wang Shizhen E., Cancer-Secreted miR-105 Destroys Vascular Endothelial Barriers to Promote Metastasis, Cancer Cell, 25 (2014) 501–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Busatto S, Iannotta D, Walker SA, Di Marzio L, Wolfram J, A Simple and Quick Method for Loading Proteins in Extracellular Vesicles, Pharmaceuticals (Basel), 14 (2021) 356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Herrmann IK, Wood MJA, Fuhrmann G, Extracellular vesicles as a next-generation drug delivery platform, Nature nanotechnology, 16 (2021) 748–759. [DOI] [PubMed] [Google Scholar]
- [161].Busatto S, Pham A, Suh A, Shapiro S, Wolfram J, Organotropic drug delivery: Synthetic nanoparticles and extracellular vesicles, Biomed. Microdevices, 21 (2019) 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Busatto S, Walker SA, Grayson W, Pham A, Tian M, Nesto N, Barklund J, Wolfram J, Lipoprotein-based drug delivery, Adv Drug Deliv Rev, 159 (2020) 377–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Sodar BW, Kittel A, Paloczi K, Vukman KV, Osteikoetxea X, Szabo-Taylor K, Nemeth A, Sperlagh B, Baranyai T, Giricz Z, Wiener Z, Turiak L, Drahos L, Pallinger E, Vekey K, Ferdinandy P, Falus A, Buzas EI, Low-density lipoprotein mimics blood plasma-derived exosomes and microvesicles during isolation and detection, Sci. Rep, 6 (2016) 24316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Busatto S, Yang Y, Walker SA, Davidovich I, Lin WH, Lewis-Tuffin L, Anastasiadis PZ, Sarkaria J, Talmon Y, Wurtz G, Wolfram J, Brain metastases-derived extracellular vesicles induce binding and aggregation of low-density lipoprotein, J Nanobiotechnology, 18 (2020) 162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Busatto S, Yang Y, Iannotta D, Davidovich I, Talmon Y, Wolfram J, Considerations for extracellular vesicle and lipoprotein interactions in cell culture assays, J Extracell Vesicles, 11 (2022) e12202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Pham M-T, Lee J-Y, Ritter C, Thielemann R, Haselmann U, Funaya C, Laketa V, Rohr K, Bartenschlager R, Endosomal egress and intercellular transmission of hepatic ApoE-containing lipoproteins and its exploitation by the hepatitis C virus, bioRxiv, (2022) 2022.2012.2008.519703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Herz J, Hui DY, Lipoprotein receptors in the vascular wall, Curr. Opin. Lipidol, 15 (2004) 175–181. [DOI] [PubMed] [Google Scholar]
- [168].Sariano PA, Mizenko RR, Shirure VS, Brandt AK, Nguyen BB, Nesiri C, Shergill BS, Brostoff T, Rocke DM, Borowsky AD, Carney RP, George SC, Convection and extracellular matrix binding control interstitial transport of extracellular vesicles, J Extracell Vesicles, 12 (2023) e12323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Luo W, Dai Y, Chen Z, Yue X, Andrade-Powell KC, Chang J, Spatial and temporal tracking of cardiac exosomes in mouse using a nano-luciferase-CD63 fusion protein, Commun Biol, 3 (2020) 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Walker SA, Davidovich I, Yang Y, Lai A, Goncalves JP, Deliwala V, Busatto S, Shapiro S, Koifman N.a., Salomon C, Talmon Y, Wolfram J, Sucrose-based cryoprotective storage of extracellular vesicles, Extracellular Vesicle, 1 (2022) 100016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Walker S, Busatto S, Pham A, Tian M, Suh A, Carson K, Quintero A, Lafrence M, Malik H, Santana MX, Wolfram J, Extracellular vesicle-based drug delivery systems for cancer treatment Theranostics, In press; (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M, Molina H, Kohsaka S, Di Giannatale A, Ceder S, Singh S, Williams C, Soplop N, Uryu K, Pharmer L, King T, Bojmar L, Davies AE, Ararso Y, Zhang T, Zhang H, Hernandez J, Weiss JM, Dumont-Cole VD, Kramer K, Wexler LH, Narendran A, Schwartz GK, Healey JH, Sandstrom P, Labori KJ, Kure EH, Grandgenett PM, Hollingsworth MA, de Sousa M, Kaur S, Jain M, Mallya K, Batra SK, Jarnagin WR, Brady MS, Fodstad O, Muller V, Pantel K, Minn AJ, Bissell MJ, Garcia BA, Kang Y, Rajasekhar VK, Ghajar CM, Matei I, Peinado H, Bromberg J, Lyden D, Tumour exosome integrins determine organotropic metastasis, Nature, 527 (2015) 329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Kalluri R, McAndrews KM, The role of extracellular vesicles in cancer, Cell, 186 (2023) 1610–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Shao M, Lopes D, Lopes J, Yousefiasl S, Macário-Soares A, Peixoto D, Ferreira-Faria I, Veiga F, Conde J, Huang Y, Chen X, Paiva-Santos AC, Makvandi P, Exosome membrane-coated nanosystems: Exploring biomedical applications in cancer diagnosis and therapy, Matter, 6 (2023) 761–799. [Google Scholar]
- [175].Gaballa R, Ali HEA, Mahmoud MO, Rhim JS, Ali HI, Salem HF, Saleem M, Kandeil MA, Ambs S, Abd Elmageed ZY, Exosomes-Mediated Transfer of Itga2 Promotes Migration and Invasion of Prostate Cancer Cells by Inducing Epithelial-Mesenchymal Transition, Cancers (Basel), 12 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Iannotta D, Yang M, Celia C, Di Marzio L, Wolfram J, Extracellular vesicle therapeutics from plasma and adipose tissue, Nano Today, In press, doi: j.nanotod.2021.101159 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Beetler DJ, Di Florio DN, Bruno KA, Ikezu T, March KL, Cooper LT, Wolfram J Jr., Fairweather D, Extracellular vesicles as personalized medicine, Mol. Aspects Med, (2022) 101155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Ghodasara A, Raza A, Wolfram J, Salomon C, Popat A, Clinical Translation of Extracellular Vesicles, Advanced healthcare materials, n/a 2301010. [DOI] [PubMed] [Google Scholar]
- [179].Wolf MW, Reichl U, Downstream processing of cell culture-derived virus particles, Expert Rev Vaccines, 10 (2011) 1451–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Tian M, Ticer T, Wang Q, Walker S, Pham A, Suh A, Busatto S, Davidovich I, Al-Kharboosh R, Lewis-Tuffin L, Ji B, Quinones-Hinojosa A, Talmon Y, Shapiro S, Ruckert F, Wolfram J, Adipose-Derived Biogenic Nanoparticles for Suppression of Inflammation, Small, 16 (2020) e1904064. [DOI] [PubMed] [Google Scholar]
- [181].Wang X, Pham A, Kang L, Walker SA, Davidovich I, Iannotta D, TerKonda SP, Shapiro S, Talmon Y, Pham S, Wolfram J, Effects of Adipose-Derived Biogenic Nanoparticle-Associated microRNA-451a on Toll-like Receptor 4-Induced Cytokines, Pharmaceutics, 14 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Qiu M, Tang Y, Chen J, Muriph R, Ye Z, Huang C, Evans J, Henske EP, Xu Q, Lung-selective mRNA delivery of synthetic lipid nanoparticles for the treatment of pulmonary lymphangioleiomyomatosis, Proc. Natl. Acad. Sci. U. S. A, 119 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Nishida-Aoki N, Tominaga N, Kosaka N, Ochiya T, Altered biodistribution of deglycosylated extracellular vesicles through enhanced cellular uptake, J Extracell Vesicles, 9 (2020) 1713527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Goncalves JP, Deliwala VJ, Kolarich D, Souza-Fonseca-Guimaraes F, Wolfram J, The cancer cell-derived extracellular vesicle glycocode in immunoevasion, Trends Immunol, (2022). [DOI] [PubMed] [Google Scholar]
- [185].Yang M, Walker SA, Aguilar JS Leon Diaz De, Davidovich I, Broad K, Talmon Y, Borges CR, Wolfram J, Extracellular vesicle glucose transporter-1 and glycan features in monocyte-endothelial inflammatory interactions, Nanomedicine, (2022) 102515. [DOI] [PubMed] [Google Scholar]
- [186].Walker SA, Aguilar JS Leon Diaz De, Busatto S, Wurtz GA, Zubair AC, Borges CR, Wolfram J, Glycan Node Analysis of Plasma-Derived Extracellular Vesicles, Cells, 9 (2020) 1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Williams C, Royo F, Aizpurua-Olaizola O, Pazos R, Boons GJ, Reichardt NC, Falcon-Perez JM, Glycosylation of extracellular vesicles: current knowledge, tools and clinical perspectives, J Extracell Vesicles, 7 (2018) 1442985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Pendiuk Goncalves J, Walker SA, Aguilar JS Leon Diaz de, Yang Y, Davidovich I, Busatto S, Sarkaria J, Talmon Y, Borges CR, Wolfram J, Glycan Node Analysis Detects Varying Glycosaminoglycan Levels in Melanoma-Derived Extracellular Vesicles, Int J Mol Sci, 24 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Broad K, Walker SA, Davidovich I, Witwer K, Talmon Y, Wolfram J, Unraveling multilayered extracellular vesicles: Speculation on cause, J Extracell Vesicles, 12 (2023) e12309. [DOI] [PMC free article] [PubMed] [Google Scholar]


