Abstract
The breakthrough of programmed cell death protein 1 (PD‐1) blockade therapy has changed the clinical treatment of non‐small cell lung cancer (NSCLC) in the past few years. The success of PD‐1 blockade therapy has been attributed to high tumor mutation burden and high immunogenicity of lung cancer cells. To further improve the efficacy of NSCLC immunotherapy and overcome the resistance of lung cancer cells to immune checkpoint blockade, new approaches that enhance the active immune response, such as neoantigen vaccines and cellular‐based therapies, are urgently required. Neoantigens are considered ideal targets for cancer immunotherapy because of their high immunogenicity and specificity. In this mini review, we first discuss the current advances in neoantigen vaccines for treating cancers and then review the results of preclinical studies and early‐phase human clinical trials of neoantigen‐based therapies for NSCLC. Finally, we focus on the identification of neoantigens in patients with NSCLC and review the candidate mutations reported by recent studies and our investigations. The review concludes that, in addition to immune checkpoint blockade, approaches targeting neoantigens are promising for improving the efficacy of NSCLC immunotherapy.
Keywords: immunotherapy, lung cancer, neoantigen, vaccine
In our institute, two strategies for identifying individual‐specific new antigens were established and compared in patients with advanced solid tumors, including lung cancer. In the first strategy, second‐generation sequencing of somatic mutations was conducted for each patient, followed by prediction for potential high‐affinity mutant epitopes. In the second strategy, a shared library of neoantigen peptides was constructed. Twenty‐one mutated genes were selected with a mutation frequency of >10% in solid tumors from the COSMIC database and were further analyzed in 2430 solid tumor sequencing samples from the TCGA database. Twenty‐nine ideal hot spot mutations, including KRAS, TP53, CTNNB1, EGFR, BRAF, PIK3CA, and GNAS, were identified. The neoantigen library was constructed to identify neoantigens in a timely and convenient manner.
INTRODUCTION
Lung cancer is one of the leading causes of death worldwide. Many patients with lung cancer are diagnosed at the late stage and show poor prognosis because of ambiguous symptoms. Approximately 1.6 million deaths due to lung cancer are reported every year worldwide. 1 , 2 Lung cancer is classified into two major subtypes: non‐small cell lung cancer (NSCLC) that accounts for 80%–85% of the total cases and small cell lung cancer (SCLC). Over the past few decades, although the survival time of patients with NSCLC has considerably improved because of advances in chemotherapy and molecular‐targeted therapy, some patients may develop drug resistance after the initial response to these therapies. Therefore, new therapeutic approaches are urgently required to target and eliminate invading tumor cells.
In the past few years, programmed cell death protein 1 (PD‐1) blockade therapy has become the standard treatment for NSCLC. However, recent clinical trials have revealed that only approximately 15%–25% of NSCLC patients respond to PD‐1 blockade therapy regardless of the expression level of programmed cell death ligand 1 (PD‐L1). 3 , 4 , 5 Some patients show primary resistance to PD‐1 blockade therapy, while other patients develop acquired drug resistance during the immunotherapy process. According to previous studies, the mechanisms of resistance to PD‐1 blockade therapy probably involve several factors such as abnormal gut microbiome composition, 6 activation of parallel immune inhibitory pathways such as Tim‐3 or Lag‐3, 7 and downregulation of antigen presentation. Specifically, epidermal growth factor receptor (EGFR) mutation‐positive patients, who constitute approximately 10%–17% of Caucasian patients with lung adenocarcinoma and 30%–65% of Asian patients with lung cancer, show limited clinical benefits following PD‐1 or PD‐L1 blockade therapy. 3 , 5 , 8 , 9 Therefore, alternative immunotherapeutic approaches are required to improve the clinical outcome of patients with advanced NSCLC.
To date, several efforts have been made to develop vaccines for NSCLC treatment. Melanoma‐associated antigen (MAGE‐A3), a tumor‐associated antigen (TAA), is primarily expressed in approximately 39.2% of patients with NSCLC. Although a vaccine targeting MAGE‐A3 showed promising results in the phase II clinical trial involving MAGE‐A3‐positive lung cancer patients, no improvement was observed in the progression‐free survival (PFS) or overall survival (OS) of the patients in the subsequent phase III clinical trial. 10 , 11 Most NSCLC patients show a high expression level of EGFR; hence, vaccines targeting EGFR are considered another important immunotherapeutic strategy. CIMAvax‐EGF vaccine, a human recombinant vaccine targeted to treat advanced NSCLC, 12 , 13 showed significant improvement in the OS rate of NSCLC patients (11.7 vs. 5.33 months) in phase III clinical trials. Other reported clinical trials of vaccines for advanced NSCLC include those of the vaccine targeting talactoferrin, TG4010 vaccine targeting the MUC‐1 protein, and belagenpumatucel‐L vaccine. 14 , 15 , 16 , 17 , 18 These vaccines have shown improvements in the PFS or OS of patients with NSCLC. However, these trials were conducted on a relatively small number of patients; hence, large‐scale prospective studies are required to further elucidate the relevance and utility of these personalized vaccines.
The identification of neoantigens is one of the most significant breakthroughs in NSCLC therapy. Neoantigen‐targeted therapy is a promising immunotherapeutic strategy for treating advanced solid cancers. In this article, we first review the recent preclinical and clinical studies on the development of neoantigen‐based therapies for NSCLC and then focus on advances in neoantigen identification in both preclinical and clinical studies.
NEOANTIGEN IN TUMOR IMMUNOTHERAPY
Neoantigens are tumor‐specific antigens (TSAs) derived from somatic gene mutations such as nonsynonymous point mutations, insertion–deletion, gene fusion, and frameshift mutations. Distinct from TAAs that are present in both tumor tissues and normal tissues, neoantigens are expressed only in tumor tissues. Hence, cancer vaccines targeting neoantigens have major advantages, including high specificity, improved safety, and low immune tolerance. 19 A strong correlation between favorable clinical benefits of the immune checkpoint blockade therapy and tumor mutation burden (TMB) was observed in various solid cancers, including melanoma, NSCLC, colorectal cancer, and cholangiocarcinoma. 20 , 21 , 22 , 23 Strategies targeting neoantigens of these cancers have shown promising efficacy in immunotherapy studies, particularly in preclinical and clinical studies conducted in the recent 5–10 years. The clinical application of a neoantigen‐based immunotherapy was first reported by Tran et al. in 2014. A late‐stage cholangiocarcinoma patient was treated with T cells recognizing the ERBB2IP neoantigen, and the tumor was effectively controlled. 24 In a follow‐up study, the same team successfully identified neoantigens recognized by self T cells from 10 digestive tract tumors in nine patients by using similar methods of constructing micro‐sequences (tandem minigene). 25 German researchers also successfully constructed a 5′‐RNA‐linked neoantigen vaccine in three animal tumor models through sequencing and bioinformatics analysis. The results revealed that the novel RNA neoantigen vaccine could effectively control tumor growth and lung metastasis. 26 Based on clinical trials conducted in July 2017, the US and German teams confirmed the remarkable efficacy of personalized neoantigen vaccines targeting tumor mutations in treating malignant melanoma. 27 , 28 Among six patients with melanoma, four patients showed complete response and no tumor recurrence within 32 months of treatment. The tumors of the remaining two patients completely disappeared after they received PD‐1 blockade therapy.
TMB IN LUNG CANCER
Targeted therapy for driver mutations in lung adenocarcinoma cells is used as a standard treatment for cancer; however, this approach has the following limitations 29 , 30 , 31 , 32 : (1) almost half of the patients are not drug‐sensitive because of the lack of sensitive driver mutations; (2) patients may show resistance, thus making it difficult to achieve long‐term clinical effects; and (3) targeting agents in squamous cell carcinoma remain unidentified. Therefore, in patients without sensitive driver mutations of adenocarcinoma and squamous cell carcinoma, versatile therapies, including immunotherapy, play an important role in treating tumors and may overcome tumor resistance. 33
The mutation landscape of different lung tumor types has been reported in an earlier study. The study found that the frequency of somatic mutations varied greatly among different tumor types, ranging from 4 to 938 362 mutations in 7042 patients with lung tumor, that is, approximately 0.001 mutation per MB to more than 400 mutations per MB. Moreover, the mutations were the least in some childhood cancers and showed frequent occurrence in patients with chronic mutagenic factors, such as lung cancer (smoking) and malignant melanoma (ultraviolet radiation). Lung squamous cell carcinoma, lung adenocarcinoma, and SCLC ranked second, third, and fifth, respectively, among these tumors. 34 Missense mutation, frameshift translocation, and mRNA splicing variants that alter posttranslational processes are the most common types of mutations found in NSCLC cells. 35
MUTATION BURDEN AND MUTATION SIGNATURES DETERMINE THE SENSITIVITY OF LUNG CANCER TO IMMUNOTHERAPY
A report published in Science in 2015 indicated that the best responders of anti‐PD‐1 therapy were those cancers that were mainly caused by chronic exposure to mutagens such as ultraviolet light and cigarette smoking, for example, melanomas and NSCLCs. The study showed that the mutation load of patients with clinical benefits of immunotherapy was significantly higher than that of patients with no clinical benefits (302 mutations vs. 148 mutations). The patients were assigned to the mutation high‐load group and low‐load group, with the cutoff value of 209 mutations. The objective response rate was significantly higher in patients with a high mutation load (63% vs. 0); moreover, the PFS time was also longer in patients with a high mutation load (14.5 months vs 3.7 months). A noteworthy finding is that patients with a history of smoking showed significantly higher sensitivity to treatment than nonsmoking patients. 36 Three responders with the highest mutation burden were identified based on deleterious mutations in the genes POLD1, POLE, and MSH2. Of the 14 patients with positive clinical outcome, seven had KRAS mutations, and only one of the 17 patients who did not show clinical benefits had KRAS mutations. 37 Mechanistic studies revealed that neoantigen‐reactive lymphocytes relevant to the mutant antigens were detected among the peripheral blood lymphocytes of patients with a positive clinical outcome. Neoantigen‐specific T cells from responder patients showed an effector phenotype different from that of T cells from nonresponders. 24 , 38 Another study showed that the combination of high TMB with the apolipoprotein B mRNA editing enzyme, the catalytic polypeptide‐like (APOBEC) mutation signature, could predict immunotherapy responders in an NSCLC cohort. 39 Moreover, frameshift mutations caused by insertion or deletion are likely to produce more immunogenic tumor‐specific neoantigens and to induce more infiltration of activated CD4 +T cells, thereby conferring better response to immune checkpoint inhibitors (ICIs) in patients with melanoma, renal cell carcinoma, and lung cancer. 40 These findings laid the theoretical foundation for the continued success of anti‐PD‐1 therapy in special NSCLC patients.
TOLERANCE MECHANISM OF LUNG CANCER IMMUNOTHERAPY
In‐depth research on the tolerance mechanism of immunotherapy can enable to improve its efficacy. A study published in Cancer Discovery in 2016 conducted a genome‐wide sequencing analysis and showed that among 42 lung cancer patients who received treatment with either anti‐PD‐1 antibodies or anti‐CTLA‐4 antibodies alone or in combination, those who developed resistance had a high mutation load. In theory, these patients should be more sensitive to immune checkpoint blockade; however, the results contradicted this theory. This could be attributed to two possible reasons: (1) tumor heterogeneity: tumor cells rich in neoantigens are inhibited, and the remaining tumor cells proliferate significantly, leading to tumor progression; (2) neoantigen deletion: deficiency in the production of neoantigen epitopes with high major histocompatibility complex (MHC) affinity. A comparison of tumor samples before and after tolerance revealed that the mutations that disappeared were the ones that were highly expressed in lung cancer cells, including mutations in KRAS, TP53, ARID1A, RB1, MYC, and SMARCA4. During the emergence of immune tolerance, neoantigen‐specific T cells in the peripheral blood of patients remained inactivated. 41 A neoantigen vaccine functions by increasing the number of neoantigen‐specific T cells in tumors. In future research, we expect to overcome immune tolerance by the replacement of a single neoantigen epitope with multiple neoantigen epitopes; this approach may effectively overcome the resistance caused by tumor heterogeneity.
STUDIES ON NEOANTIGEN VACCINES FOR TREATING LUNG CANCER
A study by Tongji Medical University analyzed 18 175 MHC class II epitopes by analyzing data from 147 patients with lung adenocarcinoma in The Cancer Genome Atlas (TCGA) database. A total of 8804 neopeptides (375 strong binders and 8429 weak binders) following the presentation by a type II human leukocyte antigen (HLA) molecule (HLA DRB1) were predicted from the database by using the NetMHCIIpan 3.1 method. The presentation of mutant peptides comprising 54 strong binders and 896 weak binders was detected for the HLA DRB1*01:01. The study also found that the most mutated genes producing new epitopes were KRAS, TTN, RYR2, MUC16, TP53, USH2A, ZFHX4, KEAP1, STK11, FAT3, NAV3, and EGFR. 42 Other studies from the same research center indicated that, compared to the wild‐type EGFR epitope, the 19 exon deletion mutation of EGFR induced a stronger serum immune response than the EGFR L858R point mutation, and this known deletion mutation could serve as a unique target for immunotherapy in Asian patients with NSCLC. 43 These results support the feasibility of using personalized neoantigen epitopes presented by HLA class II molecules as vaccine candidates for the immunotherapy of NSCLC.
In another study, T cell responses to neoantigen epitopes of lung cancer cells were investigated. The authors screened T cell responses to neoepitopes by using peripheral blood samples of five NSCLC patients. T cell responses were detected by the stimulation of approximately 8.8% of the screened candidate antigens, with an average of 1–7 identified antigens per patient. A majority of responses were not stimulated by the shared antigen but by patient‐specific mutations. In two of these patients, CD4+ T cells that recognized the KRAS G12V epitope and the ERBB2 (Her2) driver mutation epitope were identified. Moreover, T cell receptors specific for KRAS G12V and Her2‐ITD (internal tandem duplication) were isolated and identified by transfection of T cells. These results indicated the possibility of utilizing neoantigen‐specific T cells for the adoptive transfer therapy or neoantigen vaccination strategy for treating NSCLC. 44
In another recent study, the authors tested the feasibility of administering personalized neoantigen vaccines to lung cancer patients with low TMB. The authors predicted, identified, and verified the new candidate antigen peptides through the next‐generation sequencing approach. By designing a personalized neoantigen vaccine (MyVac) and conducting preclinical verification, the authors not only proved the feasibility of effectively identifying tumor‐specific neoantigens but also confirmed that these neoantigens could function as vaccines for targeting tumor cells by enhancing the antigenicity of tumors with low mutation load; this vaccine is currently being tested and is expected to show success in clinical practice. 45
Thus far, more than 10 clinical studies on the global lung cancer neoantigen vaccine have been registered on the US clinical trial website (Table 1). NEO‐PV‐01 is a personalized neoantigen vaccine in the early‐phase clinical trial; it is applied in combination with anti‐PD‐1 antibodies for treating advanced NSCLC and melanoma. The trial demonstrated that the vaccine was safe and effective in eliciting an immune response of CD4+ and CD8+ T cells. 46 RO7198457 is an RNA‐lipoplex vaccine with up to 20 patient‐specific neoantigens; it is termed as individualized neoantigen‐specific immunotherapy. It is a personalized vaccine designed to induce the production and activation of neoantigen‐responsive T cells in each patient. The vaccine was tested in a phase Ia trial targeting patients with locally advanced or metastatic solid tumors, including lung cancer. The results indicated that the vaccine was not only safe but could also induce strong neoantigen‐specific immune responses. 47 Based on this promising result, another study on RO7198457 combined with anti‐PD‐1 antibodies is currently in progress.
TABLE 1.
Ongoing clinical trials of neoantigen vaccine in lung cancer.
| Trial number | Interventions | Phase | Cancer type | Location | Status |
|---|---|---|---|---|---|
| 1. NCT03908671 | Personalized RNA vaccine encoding neoantigens | Not applicable |
Esophageal cancer non‐small cell lung cancer |
The First Affiliated Hospital of Zhengzhou University | Not yet recruiting |
| 2. NCT03871205 | Neoantigen‐primed DC vaccines | 1 |
Non‐small cell/ small cell lung cancer |
Shenzhen People's Hospital | Not yet recruiting |
| 3. NCT04032847 | ATL001 | 1,2 | Advanced non‐small cell lung cancer | University College London Hospital/Freeman Hospital Newcastle | Recruiting |
| 4. NCT02956551 | Neoantigen‐primed DC vaccine | 1 | Non‐small cell lung cancer | China West Hospital | Recruiting |
| 5. NCT03715985 | NPV‐ds001‐CAF09b + anti‐PD‐1 or anti‐PD‐L | 1 | Melanoma, non‐small cell lung cancer, kidney cancer | Herlev Hospital, Center for Cancer Immune Therapy/Herlev Hospital | Active, not recruiting |
|
6. NCT03639714 |
GRT‐C901 GRT‐R902 Nivolumab Ipilimumab |
1,2 |
Non‐small cell lung cancer Colorectal cancer Gastroesophageal adenocarcinoma Urothelial carcinoma |
The University of Chicago Columbia University Medical Center Tennessee Oncology Virginia Cancer Specialists |
Active, not recruiting |
|
7. NCT03953235 |
GRT‐C903 GRT‐R904 Anti‐PD‐(L)1 Anti‐CTLA‐4 |
1,2 |
Non‐small cell lung cancer Colorectal cancer Pancreatic cancer Solid tumor shared neoantigen‐positive tumors |
The University of Chicago Columbia University Medical Center Tennessee Oncology Virginia Cancer Specialists |
Recruiting |
|
8. NCT03412877 |
Individual patient TCR‐transduced PBL |
2 |
Glioblastoma Non‐small cell lung cancer Ovarian cancer Breast cancer Gastrointestinal/ genitourinary cancer |
National Institutes of Health Clinical Center |
Recruiting |
|
9. NCT03380871 |
NEO‐PV‐01 Pembrolizumab chemotherapy |
1 |
Non‐small cell lung cancer Nonsquamous non‐small cell lung cancer |
University of California Massachusetts General Hospital Dana Farber Cancer Institute | Complicated |
| 10. NCT04998474 | FRAME‐001 personalized vaccine in NSCLC | 2 | Non‐small cell lung cancer |
Erasmus Medical Center The Netherlands Cancer Institute University Medical Center Groningen Leiden University Medical Center |
Not yet recuiting |
| 11. NCT04487093 | Clinical study of neoantigen vaccine combined with targeted drugs in the treatment of non‐small cell lung cancer | 1 | Non‐small cell lung cancer | The First Hospital of Shijiazhuang, Shijiazhuang, Hebei, China |
Recruiting |
| 12. NCT04266730 | Trial of a personalized and adaptive neoantigen dose‐adjusted vaccine concurrently with pembrolizumab | 1 | Squamous cell lung cancer; squamous non‐small cell lung cancer; squamous cell carcinoma of head and neck | Lineberger Comprehensive Cancer Center at University of North Carolina—Chapel Hill Chapel Hill, North Carolina, USA | Not yet recuiting |
| 13. NCT04397926 | Phase I study of individualized neoantigen peptides in the treatment of EGFR mutant non‐small cell lung cancer | 1 | Non‐small cell lung cancer | Sun Yat‐sen University Cancer Center Guangzhou, Guangdong, China |
Recruiting |
| 14. NCT04078269 | MIDRIXNEO‐LUNG dendritic cell vaccine in patients with non‐small cell lung cancer | 1 | Non‐small cell lung cancer | Ghent University Hospital Ghent, Belgium | Unknown |
| 15. NCT05269381 | Personalized neoantigen peptide‐based vaccine in combination with pembrolizumab for the treatment of advanced solid tumors, the PNeoVCA study | 1 | Advanced solid tumors | Mayo Clinic in Florida Jacksonville, Florida, USA |
Recruiting |
| 16. NCT04147078 | Personalized dendritic cell vaccine for postoperative cancer | 1 | Hepatocellular carcinoma; non‐small‐cell lung cancer; colon rectal cancer | Chengdu, Sichuan, China | Recruiting |
Abbreviations: DC, dendritic cell; EGFR, epidermal growth factor receptor; NSCLC, non‐small cell lung cancer; PBL, peripheral blood lymphocytes; TCR, T cell receptor.
Approximately 29.61% of lung cancer patients carry KRAS gene mutations. Neoantigen vaccines based on KRAS mutations are thought to induce immune responses in KRAS‐driven lung cancer. However, the efficacy of these vaccines may be limited because of factors such as the presence of immunosuppressive molecules and cytokines in the tumor microenvironment. The combination of the immunosuppressive molecule blockade therapy or adjuvants is expected to avoid negative immune regulation and promote the success of KRAS‐based vaccine treatment. 48 ALK rearrangement is observed in approximately 5%–6% of NSCLC cases. Preclinical studies and clinical trials have also shown that ALK vaccines containing DNA plasmids encoding the ALK cytoplasmic domains are effective against NSCLC with ALK rearrangement, and tumor‐specific T cell response targeting the vaccine can be detected in patients. 49 , 50
EGFR is the most common driver mutation in NSCLC. A previous study reported the results of a phase I clinical trial of a personalized neoantigen peptide vaccine (PPV) targeting patients with advanced NSCLC after the initiation of the standard therapy. Interestingly, all seven patients with clinical response had tumors carrying EGFR mutations. The EGFR new antigen‐specific T cell response was detected in five of these patients receiving the EGFR mutated PPV. In contrast, none of the other eight patients with wild‐type EGFR showed clinical efficacy after PPV immunization. This study indicates that PPV based on new antigens is safe and feasible, particularly for NSCLC patients with EGFR mutations. This strategy is a promising alternative and is expected to provide new treatment options for tumors showing EGFR‐TKI resistance. 51 Regarding EGFR L858R mutation, a case of an Asian patient with lung squamous cell carcinoma demonstrated dramatic regression of multiple lung metastasis after weekly vaccination with neoepitope peptides. By monitoring the immune response of peripheral blood immune cells of patients, it has been shown that targeting the widely shared EGFR L858R mutation can effectively induce antigen‐specific T lymphocyte immune responses, particularly those restricted to HLA‐A3101 mutations. In addition to the response against the EGFR L858R mutation, patients also developed specific T lymphocyte response targeting STK11, NAVC3, and EPHB1 after the treatment. 52 For mutations that probably occur in nearly 20% of lung cancer patients, the neoantigen epitope has broad application prospects; furthermore, the application of multiepitope neoantigen vaccines is expected to overcome immune resistance that may occur through the use of a single epitope.
IDENTIFICATION OF NEOANTIGENS IN LUNG CANCER
Karasaki et al. 33 described two approaches for selecting neoantigens as vaccines for lung cancer: the “off‐the‐shelf” approach and the “personalized pipeline” approach (Figure 1). For the “off‐the‐shelf” approach, a panel of somatic missense mutations shared by at least 1% of patients with lung cancer was established through the online database. The binding affinity of these mutations was then assessed in 15 lung cancer patients according to different HLA restrictions, and potential neoantigen epitopes were chosen for these patients. Twenty‐two missense mutations were identified for adenocarcinoma, including EGFRL858R, KRAS 12C/V/D/A, BRAF V600E, TP53 R237L, EGFR T790M, and PIK3CA H1047R. Eighteen missense mutations were identified for squamous cell carcinoma, including PI3KCA E545K, NFE2L2 R34Q, KRAS G12C/D/V, EGFR L858R, and TP53R248L. Regarding the “personalized pipeline” approach, neoantigens were selected and identified from missense mutations detected by whole‐exome sequencing of each patient. A median of 59 and 164.5 missense mutations were identified in patients with adenocarcinoma and squamous cell carcinoma, respectively. However, only three “off‐the‐shelf” neoantigens were found to be shared among the neoantigens identified through the “personalized pipeline” approach, and no overlap with the “off‐the‐shelf” neoantigens was observed in the remaining 12 patients. Hence, the author considered that the use of the “off‐the‐shelf pipeline” approach was feasible; however, this approach could not meet the requirements of most patients with lung cancer. Therefore, Karasaki et al. recommended the identification of individual‐specific neoantigens by whole‐exome sequencing for each patient to develop personalized neoantigen vaccines or cell‐based immunotherapies for NSCLC patients.
FIGURE 1.
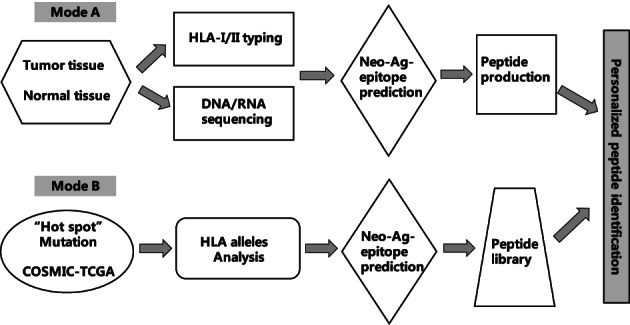
Flow chart of neoantigen identification.
In a study conducted by Chen et al. 53 at our institute, two strategies for identifying individual‐specific new antigens were established and compared in patients with advanced solid tumors, including lung cancer. In the first strategy, second‐generation sequencing of somatic mutations was conducted for each patient, followed by prediction for potential high‐affinity mutant epitopes. In the second strategy, a shared library of neoantigen peptides was constructed. Twenty‐one mutated genes were selected with a mutation frequency of >10% in solid tumors from the COSMIC database and were further analyzed in 2430 solid tumor sequencing samples from the TCGA database. Twenty‐nine ideal hot spot mutations, including KRAS, TP53, CTNNB1, EGFR, BRAF, PIK3CA, and GNAS, were identified. For lung adenocarcinoma, the most frequent mutations were KRAS G12C/V/A/D, EGFR L858R, BRAF V600E, PI3KCA E542K, and TP53 R175H, which constituted 36.96% of the total cases. For squamous cell lung cancer, the most frequent mutations were PIK3CA E545K/E542K and TP53 Y163C/V157F, which constituted 8.38% of the total cases. The neoantigen library was constructed to identify neoantigens in a timely and convenient manner. However, it should be noted that not all mutations result in neoantigen expression. Most somatic mutations detected by sequencing do not lead to effective neoantigen expression. 54 The formation of a neoantigen by a somatic mutation depends on the following factors: (1) the somatic mutation is translated and expressed at the protein level; (2) the mutant protein can be naturally processed into specific epitopes; (3) the epitope has high binding affinity for MHC molecules; and (4) there are adequate neoantigen‐reactive T cells. 19 In our study, the candidate mutant peptides were screened by CD137 staining, tetramer staining assay, or IFN‐γ secreting assay (ELISPOT or cytometric bead array [CBA]) by using autologous peripheral blood mononuclear cells of the patients. An average period of 10 days is required to identify neoepitopes. 53
CONCLUDING REMARKS
Neoantigen vaccine or neoantigen‐reactive T cell strategy has shown remarkable results in both preclinical and clinical settings. However, the cost‐effective identification of candidate antigens limits the clinical application of this strategy. Although neoantigen vaccines have initially shown effective outcomes in the treatment of NSCLC, most of them have been tested in small‐scale phase I or II clinical trials. In future research, it is critical to further validate the safety and effectiveness of neoantigen vaccines by using a large sample size. The combination of neoantigen vaccines with other immunotherapies, including the checkpoint blockade therapy or other strategies, for targeting the immunosuppressive tumor microenvironment should be investigated in future studies.
Su S, Chen F, Xu M, Liu B, Wang L. Recent advances in neoantigen vaccines for treating non‐small cell lung cancer. Thorac Cancer. 2023;14(34):3361–3368. 10.1111/1759-7714.15126
REFERENCES
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics. CA Cancer J Clin. 2017;67(1):7–30. [DOI] [PubMed] [Google Scholar]
- 2. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet‐Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. [DOI] [PubMed] [Google Scholar]
- 3. Borghaei H, Paz‐Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus docetaxel in advanced nonsquamous non‐small‐cell lung cancer. N Engl J Med. 2015;373(17):1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, et al. Pembrolizumab for the treatment of non‐small‐cell lung cancer. N Engl J Med. 2015;372(21):2018–2028. [DOI] [PubMed] [Google Scholar]
- 5. Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, et al. Atezolizumab versus docetaxel in patients with previously treated non‐small‐cell lung cancer (OAK): a phase 3, open‐label, multicentre randomised controlled trial. Lancet. 2017;389(10066):255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Routy B, Le Chatelier E, Derosa L, et al. Gut microbiome influences efficacy of PD‐1‐based immunotherapy against epithelial tumors. Science. 2018;359(6371):91–97. [DOI] [PubMed] [Google Scholar]
- 7. Koyama S, Akbay EA, Li YY, Herter‐Sprie GS, Buczkowski KA, Richards WG, et al. Adaptive resistance to therapeutic PD‐1 blockade is associated with upregulation of alternative immune checkpoints. Nat Commun. 2016;7:10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Durvalumab after Chemoradiotherapy in stage III non‐small‐cell lung cancer. N Engl J Med. 2017;377(20):1919–1929. [DOI] [PubMed] [Google Scholar]
- 9. Herbst RS, Baas P, Kim DW, Felip E, Pérez‐Gracia JL, Han JY, et al. Pembrolizumab versus docetaxel for previously treated, PD‐L1‐positive, advanced non‐small‐cell lung cancer (KEYNOTE‐010): a randomised controlled trial. Lancet. 2016;387(10027):1540–1550. [DOI] [PubMed] [Google Scholar]
- 10. Ruiz R, Hunis B, Raez LE. Immunotherapeutic agents in non‐small‐cell lung cancer finally coming to the front lines. Curr Oncol Rep. 2014;16(9):400. [DOI] [PubMed] [Google Scholar]
- 11. Sienel W, Varwerk C, Linder A, Kaiser D, Teschner M, Delire M, et al. Melanoma associated antigen (MAGE)‐A3 expression in stages I and II non‐small cell lung cancer: results of a multi‐ center study. Eur J Cardiothorac Surg. 2004;25:131–134. [DOI] [PubMed] [Google Scholar]
- 12. Neninger E, Verdecia BG, Crombet T, Viada C, Pereda S, Leonard I, et al. Combining an EGF‐based cancer vaccine with chemotherapy in advanced nonsmall cell lung cancer. J Immunother. 2009;32:92–99. [DOI] [PubMed] [Google Scholar]
- 13. Massarelli E, Papadimitrakopoulou V, Welsh J, Tang C, Tsao AS. Immunotherapy in lung cancer. Transl Lung Cancer Res. 2014;3:53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nemunaitis J, Dillman RO, Schwarzenberger PO, Senzer N, Cunningham C, Cutler J, et al. Phase II study of belagenpumatucel‐L, a transforming growth factor beta‐2 antisense gene‐modified allogeneic tumor cell vaccine in non‐small‐cell lung cancer. J Clin Oncol. 2006;24:4721–4730. [DOI] [PubMed] [Google Scholar]
- 15. Morris JC, Rossi GR, Harold N, Tennant L, Ramsey WJ, Vahanian NN, et al. Potential chemo‐sensitization effect of tergenpumatucel‐L immunotherapy in treated patients with advanced non‐small cell lung cancer (NSCLC). J Clin Oncol. 2013;31:8094. [Google Scholar]
- 16. Ramlau R, Quoix E, Rolski J, et al. A phase II study of Tg4010 (Mva‐Muc1‐Il2) in association with chemotherapy in patients with stage III/IV non‐small cell lung cancer. J Thorac Oncol. 2008;3:735–744. [DOI] [PubMed] [Google Scholar]
- 17. Yang L, Wang L, Zhang Y. Immunotherapy for lung cancer: advances and prospects. Am J Clin Exp Immunol. 2016;5:1–20. [PMC free article] [PubMed] [Google Scholar]
- 18. Quoix E, Ramlau R, Westeel V, Papai Z, Madroszyk A, Riviere A, et al. Therapeutic vaccination with TG4010 and first‐line chemotherapy in advanced non‐small‐ cell lung cancer: a controlled phase 2B trial. Lancet Oncol. 2011;12:1125–1133. [DOI] [PubMed] [Google Scholar]
- 19. Li L, Goedegebuure SP, Gillanders WE. Preclinical and clinical development of neoantigen vaccines. Annals of Oncology. 2017;28(Supplement 12):xii11–xii17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, et al. Genetic basis for clinical response to CTLA‐4 blockade in melanoma. N Engl J Med. 2014;371(23):2189–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Van Allen EM, Miao D, Schilling B, et al. Genomic correlates of response to CTLA‐4 blockade in metastatic melanoma. Science. 2015;350(6257):207–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, et al. Cancer immunology. Mutational landscape determines sensitivity to PD‐1 blockade in non‐ small cell lung cancer. Science. 2015;348(6230):124–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Le DT, Uram JN, Wang H, et al. PD‐1 blockade in tumors with mismatch‐repair deficiency. N Engl J Med. 2015;372(26):2509–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tran E, Turcotte S, Gros A, Robbins PF, Lu YC, Dudley ME, et al. Cancer immunotherapy based on mutation‐specific CD4+ T cells in a patient with epithelial cancer. Science. 2014;344:641–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tran E, Ahmadzadeh M, Lu YC, Gros A, Turcotte S, Robbins PF, et al. Immunogenicity of somatic mutations in human gastrointestinal cancers. Science. 2015;350:1387–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kreiter S, Vormehr M, van de Roemer N, Diken M, Löwer M, Diekmann J, et al. Mutant MHC class II epitopes drive therapeutic immune responses to cancer. Nature. 2015;520:692–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ott PA, Hu Z, Keskin DB, Shukla SA, Sun J, Bozym DJ, et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature. 2017;547(7662):217–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sahin U, Derhovanessian E, Miller M, Kloke BP, Simon P, Löwer M, et al. Personalized RNA mutanome vaccines mobilize poly‐specific therapeutic immunity against cancer. Nature. 2017;547(7662):222–226. [DOI] [PubMed] [Google Scholar]
- 29. Ellis PM, Coakley N, Feld R, Kuruvilla S, Ung YC. Use of the epidermal growth factor receptor inhibitors gefitinib, erlotinib, afatinib, dacomitinib, and icotinib in the treatment of non‐small‐cell lung cancer: a systematic review. Curr Oncol. 2015;22:e183–e215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dempke WC, Suto T, Reck M. Targeted therapies for non‐ small cell lung cancer. Lung Cancer. 2010;67:257–274. [DOI] [PubMed] [Google Scholar]
- 31. Gridelli C, Peters S, Sgambato A, Casaluce F, Adjei AA, Ciardiello F. ALK inhibitors in the treatment of advanced NSCLC. Cancer Treat Rev. 2014;40:300–306. [DOI] [PubMed] [Google Scholar]
- 32. Kohno T, Ichikawa H, Totoki Y, Yasuda K, Hiramoto M, Nammo T, et al. KIF5B‐RET fusions in lung adenocarcinoma. Nat Med. 2012;18:375–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Karasaki T, Nagayama K, Kawashima M, Hiyama N, Murayama T, Kuwano H, et al. Identification of individual cancer‐specific somatic mutations for Neoantigen‐based immunotherapy of lung cancer. J Thorac Oncol. 2016;11(3):324–333. [DOI] [PubMed] [Google Scholar]
- 34. Alexandrov LB, Nik‐Zainal S, et al. Signatures of mutational processes in human cancer. Nature. 2013;500:415–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cho JH. Immunotherapy for non‐small‐cell lung cancer: current status and future obstacles. Immune Netw. 2017;17(6):378–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, et al. Mutational landscape determines sensitivity to PD‐1 blockade in non–small cell lung cancer. Science. 2015;48(6230):124–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dogan S, Shen R, Ang DC, Johnson ML, D'Angelo SP, Paik PK, et al. Molecular epidemiology of EGFR and KRAS mutations in 3,026 lung adenocarcinomas: higher susceptibility of women to smoking‐related KRAS‐ mutant cancers. Clin Cancer Res. 2012;18:6169–6177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Michael F, Suchit J, Marcin K, et al. Late‐differentiated effector neoantigen‐ specific CD8+ T cells are enriched in peripheral blood of non‐small cell lung carcinoma patients responding to atezolizumab treatment. J Immunother Cancer. 2019;7:249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hao C, Wei C, Changcai T, et al. The immune response‐related mutational signatures and driver genes in non‐small‐cell lung cancer. Cancer Sci. 2019;00:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Young KC, Pedro V, Gilberto L, et al. Clinical and immunological implications of frameshift mutations in lung cancer. J Thorac Oncol. 2019;14(10):1807–1817. [DOI] [PubMed] [Google Scholar]
- 41. Anagnostou V, Smith KN, Forde PM, Niknafs N, Bhattacharya R, White J, et al. Evolution of Neoantigen landscape during immune checkpoint blockade in non‐small cell lung cancer. Cancer Discov. 2017;7(3):264–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Weijing C, Dapeng Z, Weibo W, et al. MHC class II restricted neoantigen peptides predicted by clonal mutation analysis in lung adenocarcinoma patients: implications on prognostic immunological biomarker and vaccine design. BMC Genomics. 2018;19:582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Deng P, Dapeng Z, Weijing C, et al. Immunogenicity of Del19 EGFR mutations in Chinese patients affected by lung adenocarcinoma. BMC Immunol. 2019;20:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Veatch JR, Jesernig BL, Kargl J, Fitzgibbon M, Lee SM, Baik C, et al. Endogenous CD4+ T cells recognize neoantigens in lung cancer patients, including recurrent oncogenic KRAS and ERBB2 (Her2) driver mutations. Cancer Immunol Res. 2019;7(6):910–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. McCann K, von Witzleben A, Thomas J, Wang C, Wood O, Singh D, et al. Targeting the tumor mutanome for personalized vaccination in a TMB low non‐small cell lung cancer. J Immunother Cancer. 2022;10(3):e003821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ott PA, Hu‐Lieskovan S, Chmielowski B, Govindan R, Naing A, Bhardwaj N, et al. A phase Ib trial of personalized Neoantigen therapy plus anti‐PD‐1 in patients with advanced melanoma, non‐small cell lung cancer, or bladder cancer. Cell. 2020;183(2):347–362. [DOI] [PubMed] [Google Scholar]
- 47. Braiteh F, LoRusso P, Balmanoukian A, Klempner S, Camidge DR, Hellmann M, et al. Abstract CT169: a phase Ia study to evaluate RO7198457, an individualized Neoantigen specific immunoTherapy (iNeST), in patients with locally advanced or metastatic solid tumors. Cancer Res. 2020;80:CT169. [Google Scholar]
- 48. Zhang Y, Ma JA, Zhang HX, Jiang YN, Luo WH. Cancer vaccines: targeting KRAS‐driven cancers. Expert Rev Vaccines. 2020;19:163–173. [DOI] [PubMed] [Google Scholar]
- 49. First Vaccine for Treating ALK‐Positive Lung Cancer . LUNGevity Found. Available online: https://www.lungevity.org/blogs/ developing‐first‐vaccine‐for‐treating‐alk‐positive‐lung‐cancer (accessed on 17 March 2022).
- 50. Sankar K, Nagrath S, Ramnath N. Immunotherapy for ALK‐rearranged non‐small cell lung cancer: challenges inform promising approaches. Cancer. 2021;13:1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Li F, Deng L, Jackson KR, Talukder AH, Katailiha AS, Bradley SD, et al. Neoantigen vaccination induces clinical and immunologic responses in non‐small cell lung cancer patients harboring EGFR mutations. J Immunother Cancer. 2021;9(7):e002531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Li F, Chen C, Ju T, Gao J, Yan J, Wang P, et al. Rapid tumor regression in an Asian lung cancer patient following personalized neo‐epitope peptide vaccination. Oncoimmulology. 2016;5(12):e1238539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chen F, Zou Z, Du J, et al. Neoantigen identification strategies enable personalized immunotherapy in refractory solid tumors. J Clin Invest. 2019;129(5):2056–2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. 2015;348(6230):69–74. [DOI] [PubMed] [Google Scholar]


