Abstract
Rationale
Dopamine D1 receptor agonists have been shown to improve working memory, but often have a non-monotonic (inverted-U) dose–response curve. One hypothesis is that this may reflect dose-dependent differential engagement of D1 signaling pathways, a mechanism termed functional selectivity or signaling bias.
Objectives and methods
To test this hypothesis, we compared two D1 ligands with different signaling biases in a rodent T-maze alternation task. Both tested ligands (2-methyldihydrexidine and CY208243) have high intrinsic activity at cAMP signaling, but the former also has markedly higher intrinsic activity at D1-mediated recruitment of β-arrestin. The spatial working memory was assessed via the alternation behavior in the T-maze where the alternate choice rate quantified the quality of the memory and the duration prior to making a choice represented the decision latency.
Results
Both D1 drugs changed the alternate rate and the choice latency in a dose-dependent manner, albeit with important differences. 2-Methyldihydrexidine was somewhat less potent but caused a more homogeneous improvement than CY208243 in spatial working memory. The maximum changes in the alternate rate and the choice latency tended to occur at different doses for both drugs.
Conclusions
These data suggest that D1 signaling bias in these two pathways (cAMP vs β-arrestin) has complex effects on cognitive processes as assessed by T-maze alternation. Understanding these mechanisms should allow the identification or discovery of D1 agonists that can provide superior cognitive enhancement.
Keywords: Dopamine D1 agonist, Functional selectivity/signaling bias, Spatial working memory, Rodent T-maze alternation
Introduction
Working memory (WM) deficits are observed in many neurologic and psychiatric disorders, as well as in normal aging. One common preclinical method to identify novel treatments for spatial WM challenges is the rodent T-maze. In this simple behavioral paradigm, in consecutive trials, rats intrinsically tend to choose to visit each of two end arms alternately. This tendency to alternate relies on the implementation of WM that maintains the information of which arm had been entered most recently (i.e., “online”). The rate of alternation (commonly termed as the correct rate) thus can be used to quantify the quality of WM performance (Dudchenko 2001, 2004; Paul et al. 2009; Morellini 2013). Another behavioral measurement in T-maze alternation that is relevant for assessing working memory capacity is choice latency. In early T-maze studies it was termed latency, running time, or choice time (Van der Poel 1973, 1974; Barrett and Livesey 1985). Choice latency is the duration of a rat staying at the intersection of the T-maze while making a choice. Our previous study showed that rats spent the majority of time at the intersection rather than at the runways (Yang and Mailman 2018), suggesting that important cognitive processes (e.g., goal-directed memory retrieval) are occurring during this time period. The choice latency, along with the correct choice rate, is important for working memory evaluation in the T-maze alternation task, but it has not been well studied. The first aim of this study, thus, was to comprehensively examine the latency and its relationship with the correct rate.
Dopamine D1 receptor (D1R) agonists can cause marked cognitive improvement in laboratory animals (Arnsten et al. 1994, 2017; Murphy et al. 1996; Cai and Arnsten 1997; Zahrt et al. 1997; Vijayraghavan et al. 2007; Wang et al. 2019; Yang et al. 2021) and in humans (Mu et al. 2007; Rosell et al. 2015; Huang 2020; Lewis et al. 2022). Although D1 agonists can improve WM-related behavioral performance (e.g., increase the correct rate in T-maze alternation), continued escalation of dose eliminates the improved performance, thus yielding an “inverted-U” response curve (Zahrt et al. 1997; Vijayraghavan et al. 2007; Yang et al. 2021). Several D1R intracellular signaling pathways may be involved in the regulation of this inverted-U response, including cAMP synthesis and signaling initiated by β-arrestin recruitment (Arnsten et al. 2017; Yang 2021; Yang et al. 2021, 2022a, b). The second aim of this study, therefore, was to investigate if differential engagement of D1 signaling pathways (i.e., cAMP vs β-arrestin) might influence the cognitive processes of rats performing T-maze alternation.
We reported previously that two D1 selective agonists, 2-methyldihydrexidine (2MDHX) and CY208243 (CY208), induced similar effects on the correct rate in the T-maze alternation (Yang et al. 2021). The preliminary investigation on the overall choice latency, however, suggested that 2MDHX and CY208 might differ significantly. Interestingly, these two drugs have contrasting D1 signaling profiles. 2MDHX has a modest bias towards D1-mediated β-arrestin-related signaling (full agonist at adenylate cyclase and “super-agonist” at β-arrestin recruitment), whereas CY208 is slightly biased toward D1-mediated cAMP signaling with relatively high intrinsic activity at adenylate cyclase and only partial agonism at β-arrestin recruitment. The different pattern of signal transduction mediated by a drug acting at a single type of receptor (i.e., commonly called functional selectivity or biased signaling) is recognized to be of heuristic importance for developing novel therapies, as well as providing tools for the study of specific signaling (Urban et al. 2007; Kenakin 2012). Using 2MDHX and CY208 as paired probe ligands, we therefore did an in-depth, study on how these compounds affected T-maze alternation. The correct rate and the latency for the respective correct or incorrect choice were analyzed. The results indicate that D1 signaling bias may affect the regulation of WM and other important cognitive processes, suggesting functional selectivity can be a promising strategy for the discovery of novel D1 ligands that may have an improved therapeutic index for cognition.
Methods
Subjects
All animal care and surgical procedures were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and Penn State Hershey Animal Resources Program and were reviewed and approved by IACUC of Penn State College of Medicine. A total of 12 male Sprague–Dawley rats (Charles River Laboratories, Wilmington, MA) were used. They were 226–350 g when received, housed individually, and maintained on a 12-h light–dark cycle with water available ad libitum. They were fed a limited diet of Bio-Serv rat chow to maintain their body weight at 90–95% of free-feeding body weight to permit food to be used for motivation. Highly palatable rewards (chocolate flavor sucrose, Bio-Serv Flemington, NJ) were used during the test. At the end of study, they were about 12 months old.
Apparatus
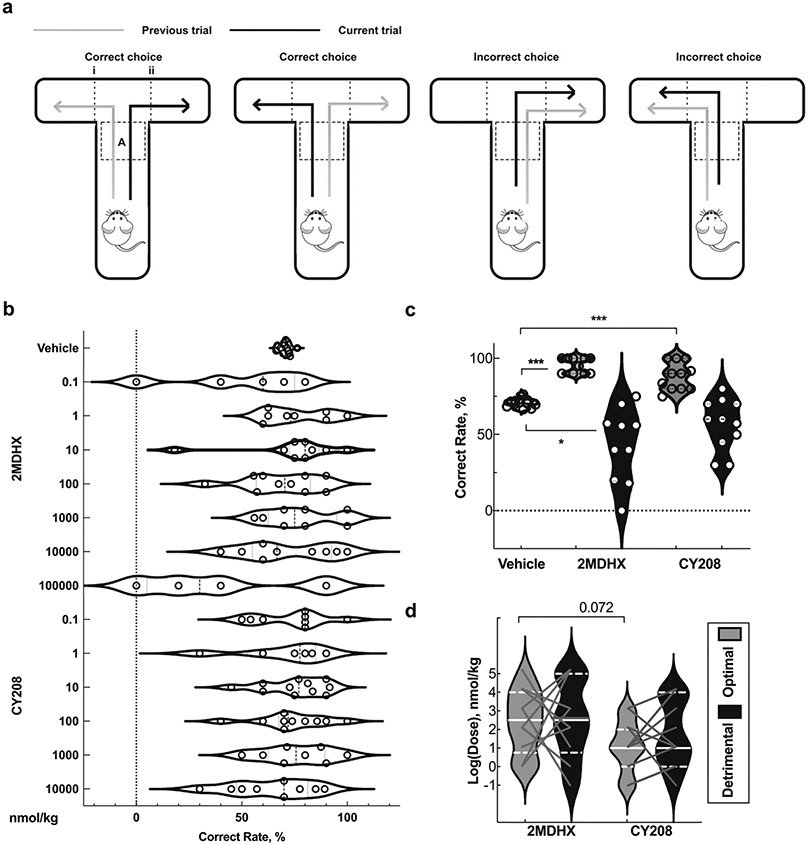
The apparatus was a standard T-maze (Fig. 1a) with one start runway (56 cm long, 10 cm wide, 18 cm high) and two finish arms (41 cm long, 10 cm wide, 18 cm high). The maze was constructed of acrylic polycarbonate with a black floor and clear sides. A CCD camera (30 frames/second, STC-TB33USB-AS, SenTech, Carrollton, TX) was suspended over the top of the maze, and data was captured with a Limelight video recording system (Actimetrics, Coulbourn Instruments, Whitehall, PA) that monitored the free movement in the maze in real time. Pre-defined zone and grids (Fig. 1a) were used to assess the behavior of a subject as staying in zone A to decide and passing grids i or ii to make choices. The Limelight software calculated the duration of stay in the zone and recorded the time point of an animal passing the grids.
Fig. 1.
Change in the correct rate after 2MDHX and CY208 were administered a: T-maze alternation task diagram. b: Change in the correct rate after 2MDHX or CY208 was administered at different nmol/kg doses. c: Change in the correct rate after 2MDHX or CY208 was administered at their respective optimal (grey shade) or detrimental (black shade) doses. d: Summary of the optimal and detrimental doses for each rat. Violin bars show mean and distribution; dots in b and c and lines in d represent an individual rat. Note the significant changes in the correct rate and the animal-to-animal variation induced by 2MDHX and CY208 respectively. *, **, and *** indicate p < 0.05, 0.01, or 0.001 respectively
Drug preparation and administration
2-Methyldihydrexidine (2MDHX), an analog of dihydrexidine, was synthesized by modifications of published procedures (Knoerzer et al. 1995; Yang et al. 2021). CY208,243 (CY208) was purchased from Tocris (Minneapolis, MN). Stock solutions of 100 mM 2MDHX and CY208 were made in DMSO and stored at − 80 °C in the dark. For use, they were diluted in 0.1% ascorbic acid vehicle using a dose range (0.1, 1, 10, 100 nmol/kg, and 1, 10, 100 μmol/kg) suggested by prior experiments (Yang et al. 2021, 2022a). Working solutions of all drugs were prepared freshly prior to subcutaneous injection. The order of drug condition was randomly assigned, and the interval between two drug conditions was at least 5 days of washout for an animal to be reused.
T-maze alternation
Rats were individually trained and tested. They were habituated to all procedures and tested by a single investigator who was blinded to drug treatment conditions. The testing session began when the tester picked up the rat and placed it in the start box of the T-maze. The start box is the lower section of the start runway of the maze that is cordoned off by a solid gate. After the tester raised the gate, the rat needed to run to the intersection of the T where it had to make a choice between the left or right arm of the maze. This first trial was always rewarded with a hand-fed food reward at the end of the finished arm. Then, the rat was gently picked up and placed back in the start box for a predetermined delay. After the delay time had elapsed, the gate was raised, the second trial started and the rat had to make another choice. This continued for a total of 11 trials after the testing session finished. For each trial, rats needed to run to the intersection, make a choice, and reach the end of the arm in less than 2 min; otherwise, the trial was aborted, and the rat was gently picked up and placed back in the start box to restart. The rat intrinsically tends to visit two arms alternately during consecutive trials. This alternation was reinforced by the reward, whereas the wrong/incorrect choice (i.e., the rat continuously visited the same arm) led to no reward. During the entire procedure, there was no visual, odor, or audio cue for the choice. The duration of rats staying at the intersection (zone A in Fig. 1a) was recorded as the choice latency. The rate of alternate choices (i.e., the correct rate) was calculated as follows:
The delay when rats were confined in the start box was constant within a test session but increased as required to maintain the performance of each individual rat (i.e., stable baseline of 60–80% correct). This permitted detection of either improved or impaired performance following drug administration. The delay ranged from 5 to 15 s, consistent with the general temporal scales of WM tasks (Atkinson and Shiffrin 1971).
Experimental design and data analysis
Rats were tested once daily in the T-maze alternation task and rested over each weekend. They were acclimated to all procedures through mock sessions where sham procedures were performed (i.e., needle stick without drug). Once they were performing at a stable, appropriate baseline (60–80% correct), the test session was implemented the next day. First was the vehicle session where an equal volume of 0.1% ascorbate was used. The performance during the vehicle session was consistent with its preceding day’s session (Supplementary Fig. 1 and Table 1) and therefore was used as the baseline. The drug session was performed on the next day of the vehicle session where one drug (2MDHX or CY208) at one dose (0.1, 1, 10, 100 nmol/kg, or 1, 10, 100 μmol/kg) was randomly assigned to be administered. All of the drug and vehicle administration was done 20 min before the task. After a drug test session was completed, there were at least five days for “washout” prior to retesting that animal. During this washout period, rats continued their daily behavioral evaluation in the T-maze alternation without any drug or vehicle administration. Each rat underwent multiple (11–39) test sessions until they either would not perform or performed too well (> 90% correct). If a drug condition was examined in multiple test sessions, its data were pooled together for analysis, since the performance among these multiple sessions was largely consistent (Supplementary Fig. 2). Mean ± SDs are reported unless otherwise specified. Depending on the analysis, MATLAB (MathWorks, Natick MA), SPSS (IBM, Armonk NY), and/or Prism (GraphPad, San Diego, CA) was used.
Table 1.
Change in the correct rate after 2MDHX or CY208 was administered at different doses
| Correct Rate (%) | Vehicle | n | 12 | ||||||
| Mean ± SD | 71 ± 3 | ||||||||
| CV (%) | 4 | ||||||||
| Dose (nmol/kg) | 0.1 | 1 | 10 | 100 | 1000 | 10,000 | 100,000 | ||
| 2MDHX | n | 5 | 8 | 9 | 10 | 8 | 9 | 4 | |
| Mean ± SD | 50 ± 32 | 76 ± 15 | 75 ± 23 | 69 ± 18 | 77 ± 17 | 72 ± 21 | 38 ± 39 | ||
| CV (%) | 63 | 20 | 31 | 26 | 21 | 30 | 103 | ||
| p | 0.218 | 0.319 | 0.619 | 0.731 | 0.315 | 0.888 | 0.185 | ||
| CY208 | n | 8 | 6 | 11 | 10 | 8 | 9 | 0 | |
| Mean ± SD | 73 ± 17 | 70 ± 22 | 75 ± 15 | 74 ± 17 | 76 ± 17 | 64 ± 20 | x | ||
| CV (%) | 23 | 31 | 20 | 23 | 23 | 31 | x | ||
| p | 0.702 | 0.923 | 0.346 | 0.548 | 0.382 | 0.328 | x |
CV indicates animal-to-animal variation. p values of pairwise comparisons are reported on the rows under the mean ± SD and CV. Least significant difference was used for multiple comparisons adjustment. Note no significant changes were detected, but SD and CV both suggest the high animal-to-animal variation as a response to 2MDHX and CY208. x, not tested or not applicable
The effect of the test compound on the correct rate was examined as follows. First, a linear mixed model for repeated measures analysis was used to evaluate whether the correct rate was significantly changed after 2MDHX or CY208 was administered at a specific dose (0.1, 1, 10, 100 nmol/ kg, or 1, 10, 100 μmol/kg). Then, we defined that the dose that led to the highest correct rate was chosen as “optimal” dose for each individual rat, whereas the dose that led to the lowest correct rate was the “detrimental’ dose. The optimal and detrimental doses for each rat were log-transformed and repeated measures ANOVA was used to evaluate whether there was a significant difference between 2MDHX and CY208 regarding optimal and detrimental doses. This included whether the correct rate was changed compared to vehicle after 2MDHX or CY208 was administered at their respective optimal or detrimental dose. We adjusted for multiple pairwise comparisons (see Results). The correct rate animal-to-animal variation was reported as standard deviation (SD) and coefficient of variation (CV) where CV = SD/mean.
The choice latency (i.e., the duration of rats staying at the intersection of the T-maze, zone A in Fig. 1a, to make a choice) also was examined. The average latency to make correct or incorrect choices, respectively, was calculated for each test session. The latency trial-to-trial variation during each test session was measured by its CV. Aborted trials were excluded from these analyses. A linear mixed model for repeated measures analysis was used to evaluate whether the average latency and the trial-to-trial variation were significantly changed after 2MDHX or CY208 was administered at different doses (0.1, 1, 10, 100 nmol/kg, or 1, 10, 100 μmol/kg). The changes in the latency after 2MDHX or CY208 was administered at the respective optimal or detrimental dose (defined above) were analyzed by repeated measures ANOVA. Pearson’s correlation test and linear regression were used to examine if the correct rate was correlated with the choice latency.
Results
Changes in the correct rate after acute 2MDHX or CY208 administration
The correct rate that each rat achieved during vehicle conditions was 71 ± 3%, and the animal-to-animal variation was low (CV = 0.04). Both 2MDHX and CY208 changed the correct rate when administered at different doses, though the statistical analysis did not yield significance (Fig. 1b, Table 1, all p > 0.05), suggesting high animal-to-animal variation responding to 2MDHX and CY208 administration (CV in Table 1, Fig. 1b, Supplementary Fig. 3). We there-after analyzed the correct rate after 2MDHX and CY208 were administered at their optimal or detrimental doses respectively.
Both 2MDHX and CY208 significantly increased the correct rate at their optimal doses (2MDHX = 96 ± 5%, CY208 = 88 ± 9%, both p < 0.05, Table 2, Fig. 1c, Supplementary Table 1), and decreased or had no effect at their detrimental doses (2MDHX = 43 ± 24%, CY208 = 57 ± 17%, Table 2, Fig. 1c, Supplementary Table 1). The optimal and detrimental doses varied among animals, though both 2MDHX and CY208 induced a typical “inverted-U” dose response on most rats, in which a lower (i.e., optimal) dose enhanced the correct rate maximumly, whereas a higher (i.e., detrimental) dose impaired or had no effect (Fig. 1d, Supplementary Fig. 3). Interestingly, CY208 trended to be more potent than 2MDHX for optimal function (log[dose] in nmol/kg: 2MDHX = 2.4 ± 1.7 vs CY208 = 1.1 ± 1.4; p = 0.072, Fig. 1d). The effects on the correct rate were no different between 2MDHX and CY208 (both p > 0.05, Table 2, Fig. 1c). It is, however, noteworthy that only 2MDHX, when administered at its optimal dose, elicited a homogeneous response such that animal-to-animal variation in correct rate was as low as during vehicle condition (CV, 2MDHX = 0.05, p > 0.05, Table 2, Fig. 1c). Conversely, there were significant animal-to-animal variations when 2MDHX was administered at its detrimental dose (2MDHX = 0.56, p < 0.05) and when CY208 was administered at either its detrimental dose or its optimal dose (optimal = 0.10, detrimental = 0.29, both p < 0.05, Table 2, Fig. 1c).
Table 2.
Change in the correct rate after 2MDHX and CY208 were administered at their optimal or detrimental dose, respectively
| Vehicle | 2MDHX | CY208 | |||||
|---|---|---|---|---|---|---|---|
| Optimal | Detrimental | Optimal | Detrimental | ||||
| Correct Rate (%) | Mean ± SD | 71 ± 3 | 96 ± 5 | 43 ± 24 | 88 ± 9 | 57 ± 17 | |
| Vehicle | x | < 0.001 | 0.059 | < 0.001 | 0.209 | ||
| 2MDHX | Optimal | < 0.001 | x | < 0.001 | 0.354 | < 0.001 | |
| Detrimental | 0.059 | 0.001 | x | 0.002 | 1 | ||
| CY208 | Optimal | < 0.001 | 0.354 | 0.002 | x | 0.001 | |
| Detrimental | 0.209 | < 0.001 | 1 | 0.001 | x | ||
| CV (%) | 4 | 5 | 56 | 10 | 29 | ||
| Vehicle | x | 0.475 | 0.014 | 0.025 | 0.023 | ||
| 2MDHX | Optimal | 0.475 | x | 0.019 | 0.210 | 0.042 | |
| Detrimental | 0.014 | 0.019 | x | 0.035 | 0.554 | ||
| CY208 | Optimal | 0.025 | 0.210 | 0.035 | x | 0.176 | |
| Detrimental | 0.023 | 0.042 | 0.554 | 0.176 | x | ||
CV indicates animal-to-animal variation. p values of pairwise comparisons are reported on the rows under the mean ± SD or CV. Bonferroni corrections accounted for multiple comparisons. Note the significant changes in the correct rate and the animal-to-animal variation induced by 2MDHX and CY208.x, not tested or not applicable
Characterization of the choice latency during vehicle sessions
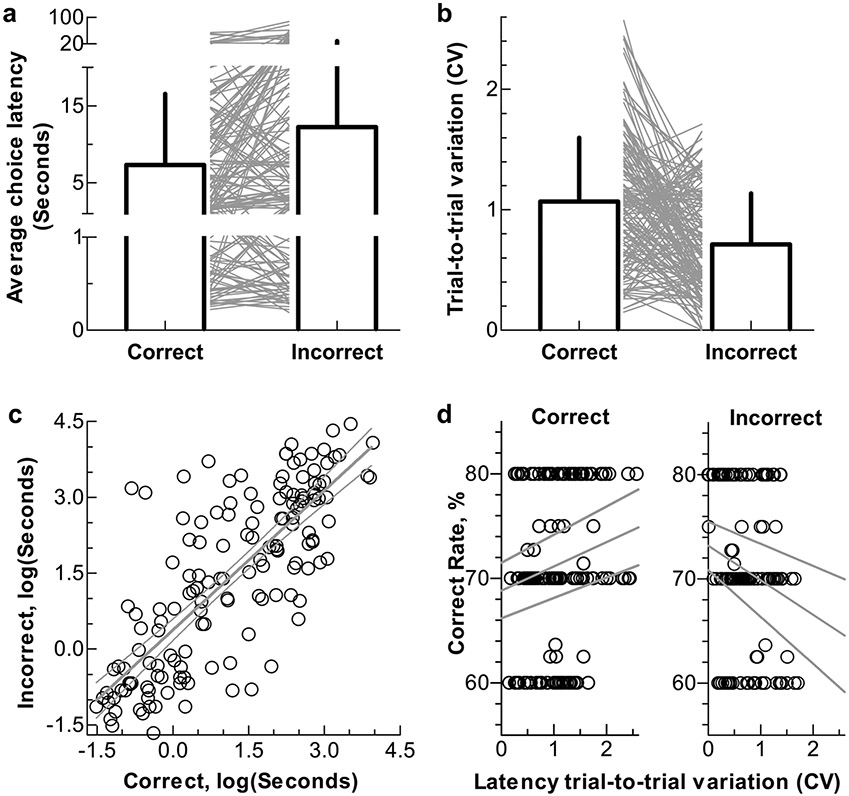
The average time to make choices (latency) that each rat used during vehicle conditions was 10.0 ± 5.5 s. Interestingly, the correct choice latency was significantly shorter than the incorrect choice (in seconds, correct = 7.9 ± 4.5 vs incorrect = 13.3 ± 8.2, p< 0.05, Fig. 2a), but the correct choice latency had more trial-to-trial variation (CV, correct = 1.03 ± 0.28 vs incorrect = 0.71 ± 0.12, p < 0.05, Fig. 2b). There was a correlation between the correct and the incorrect choice latency (linear regression, r2 = 0.43, p < 0.05, Fig. 2c). The hypothesized correlation between the choice latency and the correct rate was, however, not found (all p > 0.05). Interestingly, there were significant correlations between the correct rate and the latency trial-to-trial variation, such that a bigger variation of the correct choice latency and a smaller variation of the incorrect choice latency predicted a higher correct rate (both p < 0.05, Pearson r: correct = 0.17, incorrect = −0.19; Fig. 2d).
Fig. 2.
The choice latency and its relation to the correct rate from the vehicle sessions. a: Summary of the average correct and the incorrect choice latency. b: Summary of the latency trial-to-trial variation. c: Correlation between the correct and the incorrect choice latency. d: Correlation between the latency trial-to-trial variation and the correct rate. Bars show mean and SD; lines in a and b and dots in c and d represent each individual test session. Note the difference between correct and incorrect choices
Changes in the choice latency after acute 2MDHX or CY208 administration
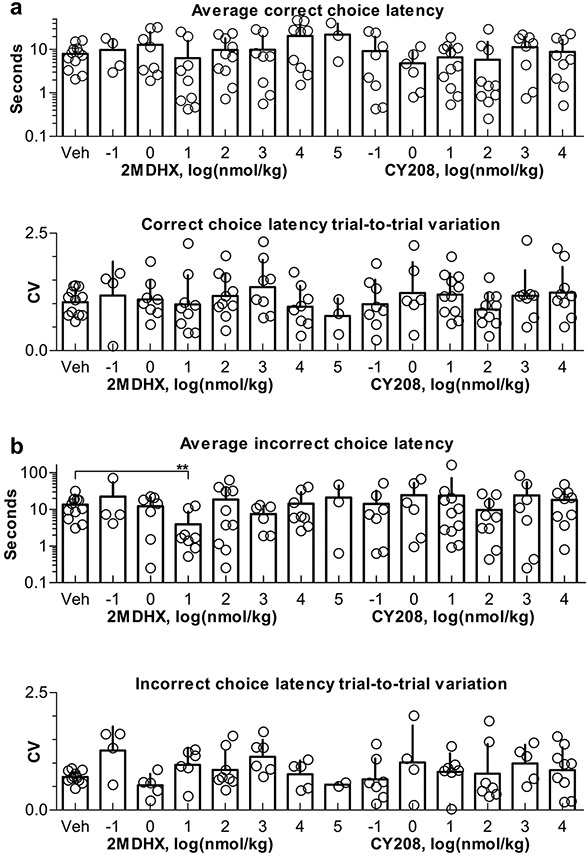
Both 2MDHX and CY208 when administered at different doses caused changes in the choice latency and its trial-to-trial variation although most were not statistically significant (Fig. 3, Tables 3 and 4, all p > 0.05). The exception was that 2MDHX at 10 nmol/kg significantly decreased the incorrect choice latency (p < 0.05, Fig. 3b, Table 4).
Fig. 3.
Change in the choice latency after 2MDHX or CY208 was administered at different doses. Top panel shows the average latency; bottom shows the latency trial-to-trial variation. a: Summary of the correct choices. b: Summary of the incorrect choices. Bars show mean and SD; dots represent each individual rat. Note 10 nmol/kg 2MDHX significantly decreased the incorrect choice latency. ** indicate p < 0.01
Table 3.
Change in the correct choice latency after 2MDHX or CY208 was administered at different doses
| Time to make correct choices (S) | Vehicle | n | 12 | ||||||
| Mean ± SD | 7.9 ± 4.5 | ||||||||
| CV (%) | 103 ± 28 | ||||||||
| Dose (nmol/kg) | 0.1 | 1 | 10 | 100 | 1000 | 10,000 | 100,000 | ||
| 2MDHX | n | 4 | 8 | 9 | 10 | 10 | 8 | 3 | |
| Mean ± SD | 9.7 ± 7.3 | 12.7 ± 12.4 | 6.3 ± 9.3 | 9.7 ± 8.4 | 9.8 ± 10.9 | 20.4 ± 17.7 | 21.9 ± 17.4 | ||
| p | 0.666 | 0.322 | 0.635 | 0.551 | 0.651 | 0.071 | 0.298 | ||
| CV (%) | 117 ± 72 | 109 ± 41 | 99 ± 61 | 116 ± 47 | 135 ± 58 | 93 ± 44 | 74 ± 37 | ||
| p | 0.729 | 0.729 | 0.839 | 0.450 | 0.177 | 0.584 | 0.301 | ||
| CY208 | n | 8 | 6 | 11 | 10 | 8 | 9 | 0 | |
| Mean ± SD | 9.1 ± 10.4 | 4.8 ± 4.2 | 6.6 ± 5.8 | 5.8 ± 9.1 | 11.2 ± 8.9 | 8.8 ± 8.1 | x | ||
| p | 0.761 | 0.171 | 0.539 | 0.506 | 0.330 | 0.794 | x | ||
| CV (%) | 99 ± 52 | 123 ± 66 | 119 ± 45 | 87 ± 36 | 117 ± 54 | 124 ± 55 | x | ||
| p | 0.840 | 0.513 | 0.322 | 0.271 | 0.529 | 0.327 | x |
CV indicates trial-to-trial variation. p values of pairwise comparisons are reported on the rows under the mean ± SD or CV. Least significant difference was used for multiple comparisons adjustment. No significant changes were observed. x, not tested or not applicable
Table 4.
Change in the incorrect choice latency after 2MDHX or CY208 was administered at different doses
| Time to make incorrect choices (S) | Vehicle | n | 12 | ||||||
| Mean ± SD | 13.3 ± 8.2 | ||||||||
| CV (%) | 71 ± 12 | ||||||||
| Dose (nmol/kg) | 0.1 | 1 | 10 | 100 | 1,000 | 10,000 | 100,000 | ||
| 2MDHX | n | 4 | 8 | 9 | 10 | 10 | 8 | 3 | |
| Mean ± SD | 22.2 ± 32.1 | 12.2 ± 8.9 | 3.9 ± 4.7 | 18.3 ± 21.6 | 7.4 ± 5 | 14.2 ± 14.8 | 20.9 ± 23.3 | ||
| p | 0.622 | 0.784 | 0.004 | 0.501 | 0.078 | 0.883 | 0.630 | ||
| CV (%) | 127 ± 51 | 53 ± 24 | 96 ± 36 | 85 ± 40 | 114 ± 36 | 76 ± 30 | 54 ± 6 | ||
| p | 0.110 | 0.189 | 0.140 | 0.332 | 0.030 | 0.691 | 0.053 | ||
| CY208 | n | 8 | 6 | 11 | 10 | 8 | 9 | 0 | |
| Mean ± SD | 14 ± 18 | 24.1 ± 27.7 | 23.5 ± 46.8 | 9.5 ± 10.1 | 23.7 ± 30.9 | 18 ± 15.2 | x | ||
| p | 0.923 | 0.389 | 0.491 | 0.371 | 0.413 | 0.421 | x | ||
| CV (%) | 65 ± 45 | 101 ± 78 | 81 ± 40 | 77 ± 63 | 99 ± 39 | 85 ± 49 | x | ||
| p | 0.782 | 0.484 | 0.497 | 0.775 | 0.173 | 0.395 | x |
CV indicates trial-to-trial variation. p values of pairwise comparisons are reported on the rows under the mean ± SD or CV. Least significant difference was used for multiple comparisons adjustment. Note only 10 nmol/kg 2MDHX significantly decreased the incorrect choice latency. x = not tested or not applicable
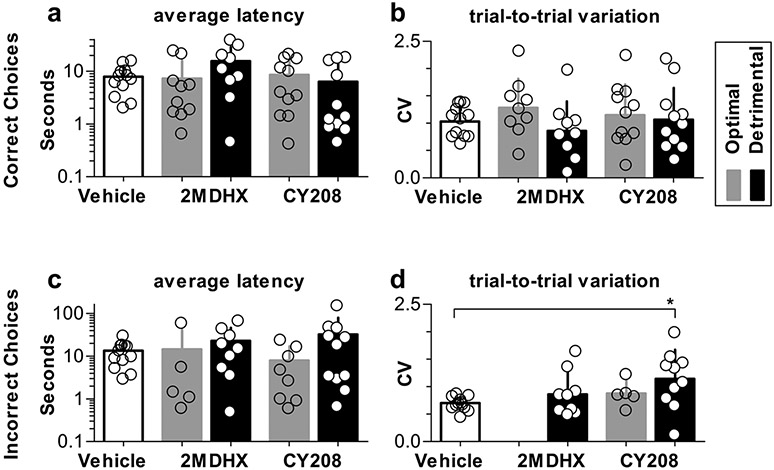
We next examined the changes in the choice latency after 2MDHX or CY208 was administered at their respective “optimal” or “detrimental” doses (see “Methods”, the dose that caused the highest or lowest correct rate). For the correct choices, no significant changes were detected (all p > 0.05, Table 5, Fig. 4a, b). For the incorrect choices, there were no significant changes in the latency (all p > 0.05, Table 6, Fig. 4c), but CY208, when administered at its detrimental dose, significantly increased the latency trial-to-trial variation (CV, CY208 = 1.14 ± 0.53 vs Vehicle = 0.71 ± 0.12, p < 0.05, Table 6, Fig. 4d).
Table 5.
Change in the correct choice latency after 2MDHX and CY208 were administered at their optimal or detrimental dose, respectively
| Vehicle | 2MDHX | CY208 | |||||
|---|---|---|---|---|---|---|---|
| Optimal | Detrimental | Optimal | Detrimental | ||||
| Time to make correct choices (S) | Mean ± SD | 7.9 ± 4.5 | 7.3 ± 8.8 | 15.6 ± 13.3 | 8.5 ± 7.8 | 6.3 ± 7.6 | |
| Vehicle | x | 0.839 | 0.130 | 0.823 | 0.560 | ||
| 2MDHX | Optimal | 0.839 | x | 0.136 | 0.736 | 0.798 | |
| Detrimental | 0.130 | 0.136 | x | 0.185 | 0.089 | ||
| CY208 | Optimal | 0.823 | 0.736 | 0.185 | x | 0.515 | |
| Detrimental | 0.560 | 0.798 | 0.089 | 0.515 | x | ||
| CV (%) | 103 ± 28 | 128 ± 53 | 86 ± 54 | 115 ± 56 | 106 ± 58 | ||
| Vehicle | x | 0.228 | 0.4 | 0.545 | 0.875 | ||
| 2MDHX | Optimal | 0.228 | x | 0.115 | 0.596 | 0.395 | |
| Detrimental | 0.4 | 0.115 | x | 0.258 | 0.43 | ||
| CY208 | Optimal | 0.545 | 0.596 | 0.258 | x | 0.731 | |
| Detrimental | 0.875 | 0.395 | 0.43 | 0.731 | x | ||
CV indicates trial-to-trial variation. p values of pairwise comparisons are reported on the rows under the mean ± SD or CV. Least significant difference was used for multiple comparisons adjustment. Note 2MDHX caused slightly longer correct choice latency compared to CY208 when they were administered at their detrimental doses respectively. x, not tested or not applicable
Fig. 4.
Change in the choice latency after 2MDHX or CY208 was administered at their respective optimal (gray) or detrimental (black) doses. a, b: Summary of the correct choices. c-d: Summary of the incorrect choices. a, c: the average latency; b & d: the latency trial-to-trial variation. Bars show mean and SD; dots represent each individual rat. In panel d, for each rat, there was only one or no incorrect choice after 2MDHX was administered at its optimal dose, therefore not applicable to calculate CV for latency trial-to-trial variation. Note the incorrect choice latency had significantly increased trial-to-trial variation after CY208 was administered at its detrimental dose. * indicate p < 0.05
Table 6.
Change in the incorrect choice latency after 2MDHX and CY208 were administered at their optimal or detrimental doses, respectively
| Vehicle | 2MDHX | CY208 | |||||
|---|---|---|---|---|---|---|---|
| Optimal | Detrimental | Optimal | Detrimental | ||||
| Time to make incorrect choices (S) | Mean ± SD | 13.3 ± 8.2 | 14.3 ± 27 | 22.8 ± 23 | 7.9 ± 9 | 32 ± 46.8 | |
| Vehicle | x | 0.939 | 0.267 | 0.191 | 0.219 | ||
| 2MDHX | Optimal | 0.939 | x | 0.572 | 0.630 | 0.358 | |
| Detrimental | 0.267 | 0.572 | x | 0.101 | 0.574 | ||
| CY208 | Optimal | 0.191 | 0.630 | 0.101 | x | 0.123 | |
| Detrimental | 0.219 | 0.358 | 0.574 | 0.123 | x | ||
| CV (%) | 71 ± 12 | N/A | 88 ± 40 | 88 ± 24 | 114 ± 53 | ||
| Vehicle | x | x | 0.228 | 0.171 | 0.027 | ||
| 2MDHX | Optimal | x | x | x | x | X | |
| Detrimental | 0.228 | x | x | 0.994 | 0.235 | ||
| CY208 | Optimal | 0.171 | x | 0.994 | x | 0.203 | |
| Detrimental | 0.027 | x | 0.235 | 0.203 | x | ||
CV indicates trial-to-trial variation. p values of pairwise comparisons are reported on the rows under the mean ± SD or CV. Least significant difference was used for multiple comparisons adjustment. N/A is because there was only one or no incorrect choice after 2MDHX was administered at its optimal dose, therefore not applicable to calculate CV for trial-to-trial variation. Note CY208 significantly increased trial-to-trial variation of the incorrect choice latency after being administered at its detrimental dose. x, not tested or not applicable
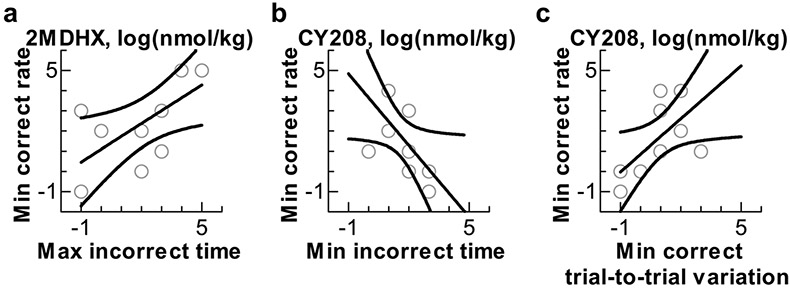
We then examined if there were correlations between the doses that caused the highest or lowest correct rate (i.e., the optimal and detrimental doses) and the doses that caused the longest or shortest latency and largest and least latency trial-to-trial variation. Three pairs yielded significance. The detrimental dose of 2MDHX was positively correlated with the dose to cause the longest incorrect choice latency (linear regression, r2 = 0.46, p < 0.05, Fig. 5a); the detrimental dose of CY208 was negatively correlated with the dose that caused the shortest incorrect choices latency (linear regression, r2 = 0.37, p < 0.05, Fig. 5b), and positively correlated with the dose that caused the least trial-to-trial variation of correct choice latency (linear regression, r2 = 0.38, p < 0.05, Fig. 5c).
Fig. 5.
Correlations between the doses that caused changes in the choice latency and the doses that caused changes in the correct rate. a: The positive correlation between the detrimental dose of 2MDHX (i.e., min correct rate) and the dose to cause the longest incorrect choice latency (i.e., max incorrect latency). b: The negative correlation between the detrimental dose of CY208 (i.e., min correct rate) and the dose to cause the shortest incorrect choice latency (i.e., min incorrect latency). c: The positive correlation between the detrimental dose of CY208 (i.e., min correct rate) and the dose to cause the least trial-to-trial variation of the correct choice latency (i.e., min variation of correct latency). Dots represent each individual rat
Discussion
We previously reported the effects of two D1 agonists 2MDHX and CY208 on rats performing the T-maze alternation (Yang et al. 2021). At that time, only a portion of the experiments recorded the time to make choices, making it difficult to examine comprehensively the choice latency and its relation to the correct rate in the T-maze alternation. Purposefully, in the current study, all the test sessions were video recorded to assess choice latency. There were three major findings in the following categories: (1) relevance of the changes in the correct rate by different D1 agonists and doses; (2) the latency regarding correct and incorrect choices respectively; and (3) the relationship between the correct rate and the choice latency. These observations shed light on how different D1 agonists, potentially through their biased signaling, operate in the context of spatial working memory.
Drug properties influence the correct rate
We first sought to replicate our previous finding re. correct rate, namely that both 2MDHX and CY208 yielded “inverted-U” dose–response curves in which a lower dose enhanced the correct rate and > 10-folder higher doses impaired it or had no effect (Yang et al. 2021). It was surprising at first to not find significant changes in the correct rate for either drug at any tested doses. Further inspection, however, revealed that this was due to high animal-to-animal variation in sensitivity to drug challenge, i.e., dose responses were markedly different among the rats used in the current study.
Because we had used a within-subject, dose–response design, we were able to determine the optimal and detrimental dose for each rat (Supplementary Fig. 3), the analysis of which showed the same expected pattern of dose–response (i.e., “inverted U”), but markedly different values for the optimal dose. Thus, the optimal dose for some rats actually was detrimental to others (Supplementary Fig. 4). This finding of very heterogeneous rat-specific response to D1 agonists was reported previously for other dopaminergic drugs (Yang et al. 2022c) and also found clinically (Peck 2016). The exact mechanisms remain to be elucidated, but if this murine experiment translates to the clinic, it highlights the careful thought that will need to go into D1 drug development trials for cognition-related endpoints. Indeed, our results showed that the correct rate was significantly and overwhelmingly increased by both 2MDHX and CY208 on all rats when they were administered at their respective optimal doses (i.e., individual effective dose), consistent with the literature that D1 agonists can cause a large effective size on cognitive enhancement (Arnsten et al. 1994, 2017; Schneider et al. 1994; Murphy et al. 1996; Cai and Arnsten 1997; Zahrt et al. 1997; Mu et al. 2007; Vijayraghavan et al. 2007; Rosell et al. 2015; Wang et al. 2019; Huang 2020; Yang et al. 2021). Our data highlight the importance of a personalized approach to maximize an individual’s benefit (and to be able to detect a significant difference).
Another finding of note is the difference between 2MDHX and CY208 for the correct rate. Although 2MDHX was slightly less potent than CY208, it caused remarkably less variance at its optimal dose. Despite the inter-subject differences in the optimal dose, this consistency in the optimal dose could be an important property if it translates to humans. Complex factors can contribute to these differences in behavioral effects induced by 2MDHX and CY208, including but not limited to the higher β-arrestin functional selectivity of 2MDHX, and their differences in D1 affinity (see below).
A comprehensive examination of the choice latency
One goal of this study was to have a better understanding of the choice latency in the T-maze alternation. Our previous work only examined the overall latency, regardless of the correct or incorrect choices, whereas the current study examined them separately. The results showed that the correct choice latency was significantly shorter than for the incorrect choice. This contrasts with the expected speed-accuracy trade-off in which shorter latency usually indicates a “rushed” decision that likely will lead to an incorrect choice (Mulder et al. 2010; Karalunas et al. 2012; Tamm et al. 2012; Vallesi et al. 2013). Instead, the “slow-motion” or “prolonged” inaction (longer latency) prior to the incorrect choices suggests that inattention may be the major contributor to the “wrong” behavior in the T-maze alternation (Mulder et al. 2010; Karalunas et al. 2012; Tamm et al. 2012; Vallesi et al. 2013). Based on this assumption, we hypothesized that shorter latency (i.e., less inaction or inattention) may predict more correct choices. The results, however, did not support this hypothesis; no correlations were observed between the latency and the correct rate. Another hypothesis is that a metacognitive process may happen during the T-maze task, whereby the rat knows that it does or does not know the correct answer (Miyamoto et al. 2017; Rutishauser 2021). Interestingly, further inspection showed a significantly larger trial-to-trial variation of the correct choice latency compared to the incorrect choice. This marked variation of the correct choice latency may be an indicator of cognitive processes that were constantly adjusting for T-maze alternation. Based on this assumption, we hypothesized that higher variation of the correct choice latency (i.e., more cognitive adjustment) may predict a higher correct rate; this was supported by correlational analysis. The correlation analyses also showed that higher variation of the incorrect choice latency predicted more incorrect choices (in other words, lower correct rate), probably because of the extremely prolonged inattention. These findings on the choice latency are crucial for the interpretation of the D1 drug effects on the T-maze alternation reported previously (Yang et al. 2021) and below.
Our previous work found that 2MDHX, but not CY208, significantly shortened the choice latency (Yang et al. 2021). The current study replicated this finding and further revealed that the shortened latency was only for incorrect, not correct choices. If the long latency of the incorrect choices is an indication of inaction/inattention (as discussed above), the results with 2MDHX (shortened incorrect choice latency, i.e., lessened inattention) suggest that 2MDHX might be superior in rescuing inattention-related cognitive deficits (Mulder et al. 2010; Karalunas et al. 2012; Tamm et al. 2012; Vallesi et al. 2013), a potentially important implication in attention-deficit/hyperactivity disorder (ADHD). This is consistent with findings from our recent work in which 2MDHX was superior to the ADHD medication methylphenidate for enhancing cognitive processes in an ADHD rat model (Yang et al. 2022c).
Implications and limitations for clinical pharmacology
We tested two D1 agonists at a broad dose range (six-log orders: 0.1 nmol/kg to 100 μmol/kg). The effective doses of both 2MDHX and CY208 for behavioral changes in the T-maze alternation occur at very low D1R fractional occupancies, consistent with the monkey and human studies that evaluated low doses of D1 agonists on cognition (Andersen and Jansen 1990; Murray and Waddington 1990; Abbott et al. 1991; Knoerzer et al. 1995). The selectivity of most D1 experimental ligands in the literature, plus the reversal by selective D1 antagonists (Yang et al. 2021, 2022c), suggest that the biphasic curves and the other effects of 2MDHX and CY208, as summarized above, are a consequence largely of D1 activation. Conversely, at the high range of doses we used, off-target receptor effects may contribute to effects. Thus, our view is that differences between low doses of 2MDHX and CY208 are a consequence of differential engagement of multiple D1-mediated intracellular signaling pathways that play critical roles in the regulation of cognitive effects (Arnsten et al. 2017; Wang et al. 2019; Yang 2021; Yang et al. 2021, 2022a, b), not off-target effects.
Both 2MDHX and CY208 are D1 agonists with high receptor selectivity (Andersen and Jansen 1990; Murray and Waddington 1990; Abbott et al. 1991; Knoerzer et al. 1995; Yang et al. 2021), but their signaling biases are subtly different. 2MDHX is a full agonist at adenylate cyclase and a “super-agonist” at β-arrestin recruitment. Conversely, CY208 has high, but not full, intrinsic activity at adenylate cyclase, but less intrinsic activity at β-arrestin (Markstein et al. 1992; Knoerzer et al. 1995; Yang et al. 2021). It has been suggested that differences between D1-mediated cAMP and β-arrestin signaling may have important implications for D1 drugs as cognitive enhancers, and many believe that β-arrestin activation is a crucial factor. This may be a cause, in part, of the more homogenous effects of 2MDHX in causing performance improvement. This is an interesting area for future studies, especially with the availability of new D1 agonists that are highly biased with essentially no β-arrestin recruitment activity.
Our data associated D1 signaling bias with complex modulation of spatial WM, but this does not prove causation. Determining the exact brain mechanisms of these two D1 agonists is well beyond the scope of this study. We balanced a mechanistically informative design with one that maximized the pharmacological translation of the results, understanding that this left many questions to be answered. Future studies should utilize site-specific drug administration recognizing that different brain regions may have heterogenous response to D1 signaling bias (Goldman-Rakic 1995; Leonard et al. 2003; Klyubin et al. 2008; Beaulieu and Gainetdinov 2011; Huang et al. 2012; Jiang et al. 2013; Thathiah et al. 2013; Yang et al. 2021), and can lead to divergent behavioral outcomes than those seen after systematic administered. Additionally, clinical use will require understanding the effects of chronic administration that also was beyond the scope of this study.
Our findings, regarding the relationship between the choice latency and the correct rate, showed that for both 2MDHX and CY208, their detrimental doses aligned with the doses that caused the longest incorrect choice latency (i.e., the most inaction or inattention), whereas their optimal doses did not align with the doses that caused most beneficial changes in the choice latency. This suggests that the useful effects of D1 agonists may diverge significantly among subdomains of cognitive process (e.g., choice latency vs correct rate, potentially representing attention vs memory). More studies using other behavioral paradigms are needed to further investigate this hypothesis. Thus, the current study furthered our understanding of how D1 signaling bias might influence spatial WM and underscored the importance of both discovery of novel D1 ligands and further exploration of the involved mechanisms.
Supplementary Material
Acknowledgements
The authors thank Dr. Xuemei Huang for her insight into these results and supportive comments during the study, and Susan Kocher and Natalia Loktionova for their invaluable technique support.
This work was supported by the Brain & Behavior Research Foundation Young Investigator Award (19469), Children’s Miracle Network Research Grant (2022–2023) and Trainee Grant (2023-2024), the National Institutes of Health (R01 NS105471, RF1 AG071675), and the Penn State Translational Brain Research Center.
Portions of this work were presented at the International Behavioral Neuroscience Society Annual Meeting in June 2023 (Niagara Falls, Ontario, Canada)
Footnotes
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s00213-023-06440-5.
Conflict of interest RBM is a consultant for Cerevel Therapeutics and also is an inventor of D1-related technology. His conflicts of interest have been disclosed and are managed by the Pennsylvania State University. JW has a research contract with Supernus Pharmaceuticals and is a consultant for Ironshore Pharmaceuticals and Adlon Pharmaceuticals.
Data Availability
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
References
- Abbott B, Starr BS, Starr MS (1991) CY 208–243 behaves as a typical D-1 agonist in the reserpine-treated mouse. Pharmacol Biochem Behav 38:259–263 [DOI] [PubMed] [Google Scholar]
- Andersen PH, Jansen JA (1990) Dopamine receptor agonists: selectivity and dopamine D1 receptor efficacy. Eur J Pharmacol 188:335–347 [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Cai JX, Murphy BL, Goldman-Rakic PS (1994) Dopamine D1 receptor mechanisms in the cognitive performance of young adult and aged monkeys. Psychopharmacology 116:143–151 [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Girgis RR, Gray DL, Mailman RB (2017) Novel dopamine therapeutics for cognitive deficits in schizophrenia. Biol Psychiat 81:67–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson RC, Shiffrin RM (1971) The control of short-term memory. Sci Am 225:82–90 [DOI] [PubMed] [Google Scholar]
- Barrett J, Livesey PJ (1985) Low level lead effects on activity under varying stress conditions in the developing rat. Pharmacol Biochem Behav 22:107–118 [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Gainetdinov RR (2011) The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev 63:182–217 [DOI] [PubMed] [Google Scholar]
- Cai JX, Arnsten AF (1997) Dose-dependent effects of the dopamine D1 receptor agonists A77636 or SKF81297 on spatial working memory in aged monkeys. J Pharmacol Exp Ther 283:183–189 [PubMed] [Google Scholar]
- Dudchenko PA (2001) How do animals actually solve the T maze? Behav Neurosci 115:850–860 [PubMed] [Google Scholar]
- Dudchenko PA (2004) An overview of the tasks used to test working memory in rodents. Neurosci Biobehav Rev 28:699–709 [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS (1995) Cellular basis of working memory. Neuron 14:477–485 [DOI] [PubMed] [Google Scholar]
- Huang C, Wu J, Liao R, Zhang W (2012) SKF83959, an agonist of phosphatidylinositol-linked D(1)-like receptors, promotes ERK1/2 activation and cell migration in cultured rat astrocytes. PLoS ONE 7:e49954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Lewis MM, Van Scoy LJ, De Jesus S, Eslinger PJ, Arnold AC, Miller AJ, Fernandez-Mendoza J, Snyder B, Harrington W, Kong L, Wang X, Sun D, Delnomdedieu M, Duvvuri S, Mahoney SE, Gray DL, Mailman RB (2020) The D1/D5 dopamine partial agonist PF-06412562 in advanced-stage parkinson’s disease: a feasibility study. J Parkinsons Dis 10:1515–1527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang T, Yu JT, Tan MS, Zhu XC, Tan L (2013) beta-Arrestins as potential therapeutic targets for Alzheimer’s disease. Mol Neurobiol 48:812–818 [DOI] [PubMed] [Google Scholar]
- Karalunas SL, Huang-Pollock CL, Nigg JT (2012) Decomposing attention-deficit/hyperactivity disorder (ADHD)-related effects in response speed and variability. Neuropsychology 26:684–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenakin T (2012) The potential for selective pharmacological therapies through biased receptor signaling. Bmc Pharmacol Toxico 13:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klyubin I, Betts V, Welzel AT, Blennow K, Zetterberg H, Wallin A, Lemere CA, Cullen WK, Peng Y, Wisniewski T, Selkoe DJ, Anwyl R, Walsh DM, Rowan MJ (2008) Amyloid beta protein dimer-containing human CSF disrupts synaptic plasticity: prevention by systemic passive immunization. J Neurosci 28:4231–4237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoerzer TA, Watts VJ, Nichols DE, Mailman RB (1995) Synthesis and biological evaluation of a series of substituted benzo[a]phen-anthridines as agonists at D1 and D2 dopamine receptors. J Med Chem 38:3062–3070 [DOI] [PubMed] [Google Scholar]
- Leonard SK, Anderson CM, Lachowicz JE, Schulz DW, Kilts CD, Mailman RB (2003) Amygdaloid D1 receptors are not linked to stimulation of adenylate cyclase. Synapse (New York, NY) 50:320–333 [DOI] [PubMed] [Google Scholar]
- Lewis MM, Van Scoy LJ, Mailman RB, De Jesus S, Hakun J, Kong L, Yang Y, Snyder B, Duvvuri S, Gray DL, Huang X (2022) Dopamine D1 agonist effects in late-stage Parkinson’s disease. medRxiv: 2022.04.30.22270885 [Google Scholar]
- Markstein R, Seiler MP, Jaton A, Briner U (1992) Structure activity relationship and therapeutic uses of dopaminergic ergots. Neurochem Int 20(Suppl):211S–214S [DOI] [PubMed] [Google Scholar]
- Miyamoto K, Osada T, Setsuie R, Takeda M, Tamura K, Adachi Y, Miyashita Y (2017) Causal neural network of metamemory for retrospection in primates. Science 355:188–193 [DOI] [PubMed] [Google Scholar]
- Morellini F (2013) Spatial memory tasks in rodents: what do they model? Cell Tissue Res 354:273–286 [DOI] [PubMed] [Google Scholar]
- Mu Q, Johnson K, Morgan PS, Grenesko EL, Molnar CE, Anderson B, Nahas Z, Kozel FA, Kose S, Knable M, Fernandes P, Nichols DE, Mailman RB, George MS (2007) A single 20 mg dose of the full D1 dopamine agonist dihydrexidine (DAR-0100) increases prefrontal perfusion in schizophrenia. Schizophr Res 94:332–341 [DOI] [PubMed] [Google Scholar]
- Mulder MJ, Bos D, Weusten JMH, van Belle J, van Dijk SC, Simen P, van Engeland H, Durston S (2010) Basic impairments in regulating the speed-accuracy tradeoff predict symptoms of attention-deficit/hyperactivity disorder. Biol Psychiat 68:1114–1119 [DOI] [PubMed] [Google Scholar]
- Murphy BL, Arnsten AF, Jentsch JD, Roth RH (1996) Dopamine and spatial working memory in rats and monkeys: pharmacological reversal of stress-induced impairment. J Neurosci 16:7768–7775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray AM, Waddington JL (1990) New putative selective agonists at the D-1 dopamine receptor: behavioural and neurochemical comparison of CY 208–243 with SK&F 101384 and SK&F 103243. Pharmacol Biochem Behav 35:105–110 [DOI] [PubMed] [Google Scholar]
- Paul CM, Magda G, Abel S (2009) Spatial memory: theoretical basis and comparative review on experimental methods in rodents. Behav Brain Res 203:151–164 [DOI] [PubMed] [Google Scholar]
- Peck RW (2016) The right dose for every patient: a key step for precision medicine. Nat Rev Drug Discov 15:145–146 [DOI] [PubMed] [Google Scholar]
- Rosell DR, Zaluda LC, McClure MM, Perez-Rodriguez MM, Strike KS, Barch DM, Harvey PD, Girgis RR, Hazlett EA, Mailman RB, Abi-Dargham A, Lieberman JA, Siever LJ (2015) Effects of the D1 dopamine receptor agonist dihydrexidine (DAR-0100A) on working memory in schizotypal personality disorder. Neuropsychopharmacology 40:446–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutishauser U (2021) Metamemory: Rats know the strength of their memory. Curr Biol: CB 31:R1432–r1434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JS, Sun ZQ, Roeltgen DP (1994) Effects of dihydrexidine, a full dopamine D-1 receptor agonist, on delayed response performance in chronic low dose MPTP-treated monkeys. Brain Res 663:140–144 [DOI] [PubMed] [Google Scholar]
- Tamm L, Narad ME, Antonini TN, O’Brien KM, Hawk LW Jr, Epstein JN (2012) Reaction time variability in ADHD: a review. Neurotherapeutics 9:500–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thathiah A, Horre K, Snellinx A, Vandewyer E, Huang Y, Ciesielska M, De Kloe G, Munck S, De Strooper B (2013) beta-arrestin 2 regulates Abeta generation and gamma-secretase activity in Alzheimer’s disease. Nat Med 19:43–49 [DOI] [PubMed] [Google Scholar]
- Urban JD, Clarke WP, von Zastrow M, Nichols DE, Kobilka B, Weinstein H, Javitch JA, Roth BL, Christopoulos A, Sexton PM, Miller KJ, Spedding M, Mailman RB (2007) Functional selectivity and classical concepts of quantitative pharmacology. J Pharmacol Exp Ther 320:1–13 [DOI] [PubMed] [Google Scholar]
- Vallesi A, D’Agati E, Pasini A, Pitzianti M, Curatolo P (2013) Impairment in flexible regulation of speed and accuracy in children with ADHD. J Int Neuropsychol Soc: JINS 19:1016–1020 [DOI] [PubMed] [Google Scholar]
- Van der Poel AM (1973) Registration of choice direction in a T-maze in rats under the influence of N-methyl-4-piperidyl cyclopentyl methylethynyl glycolate (PCMG), a centrally acting cholinolytic. Psychopharmacologia 31:271–290 [DOI] [PubMed] [Google Scholar]
- Van der Poel AM (1974) The effect of some cholinolytic drugs on a number of behavioural parameters measured in the T-maze alternation test: dose-response relationships. Psychopharmacologia 37:45–58 [DOI] [PubMed] [Google Scholar]
- Vijayraghavan S, Wang M, Birnbaum SG, Williams GV, Arnsten AF (2007) Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat Neurosci 10:376–384 [DOI] [PubMed] [Google Scholar]
- Wang M, Datta D, Enwright J, Galvin V, Yang ST, Paspalas C, Kozak R, Gray DL, Lewis DA, Arnsten AFT (2019) A novel dopamine D1 receptor agonist excites delay-dependent working memory-related neuronal firing in primate dorsolateral prefrontal cortex. Neuropharmacology 150:46–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y (2021) Functional selectivity of dopamine D1 receptor signaling: retrospect and prospect. Int J Mol Sci 22:11914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Mailman RB (2018) Strategic neuronal encoding in medial prefrontal cortex of spatial working memory in the T-maze. Behav Brain Res 343:50–60 [DOI] [PubMed] [Google Scholar]
- Yang Y, Lee SM, Imamura F, Gowda K, Amin S, Mailman RB (2021) D1 dopamine receptors intrinsic activity and functional selectivity affect working memory in prefrontal cortex. Mol Psychiatry 26:645–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Kocher SD, Lewis MM, Mailman RB (2022a) Dose-dependent regulation on prefrontal neuronal working memory by dopamine D1 agonists: evidence of receptor functional selectivity-related mechanisms. Front Neurosci 16:898051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Lewis MM, Huang X, Dokholyan NV, Mailman RB (2022b) Dopamine D(1) receptor-mediated beta-arrestin signaling: Insight from pharmacology, biology, behavior, and neurophysiology. Int J Biochem Cell Biol 148:106235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Lewis MM, Kong L, Mailman RB (2022c) A dopamine D(1) agonist versus methylphenidate in modulating prefrontal cortical working memory. J Pharmacol Exp Ther 382:88–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahrt J, Taylor JR, Mathew RG, Arnsten AF (1997) Supranormal stimulation of D1 dopamine receptors in the rodent prefrontal cortex impairs spatial working memory performance. J Neurosci 17:8528–8535 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.