Abstract
Telomeres play a key role in chromosomal maintenance and stability. To date, few studies have investigated the association of leukocyte telomere length with risk of cancer incidence and all-cause mortality in a large prospective cohort, particularly of the Asian population. Relative telomere lengths in genomic DNA from peripheral blood samples were quantified using a validated quantitative real-time PCR among 26 540 middle-aged or older Chinese adults. Hazard ratios (HRs) and 95% confidence intervals (CIs) of cancer and deaths by quintiles of telomere length were calculated using the Cox proportional hazards regression method with adjustment for age, sex and other potential confounders. After baseline blood collection, 4353 persons developed cancer and 7609 died. Participants with the longest decile of telomeres had a 26% (95% CI: 11%-44%) higher risk of total cancer incidence compared to the shortest decile after controlling for age, sex and other potential founders (Ptrend < .0001). In contrast, longer telomeres were associated with lower risk of all-cause mortality (HR = 0.93; 95% CI: 0.84–1.03), noncancer death (HR = 0.81; 95% CI: 0.71–0.92), specifically, death from chronic obstructive pulmonary disease and pneumonia (HR = 0.79, 95% CI: 0.70–0.89) and digestive diseases (HR = 0.60, 95% CI: 0.42–0.88). Our findings demonstrated that longer telomeres are associated with increased risk of cancer development overall and several common cancer types including breast, rectal, prostate, pancreatic cancer and lung adenocarcinoma. Our study also confirmed that longer telomeres are associated with a reduced risk of non-cancer related death.
Keywords: all-cause mortality, biomarkers, cancer incidence, prospective cohort study, telomere length
1 |. INTRODUCTION
With an estimated 9.6 million deaths worldwide and 609 640 deaths, accounting for more than 20% of total death, in the United States in 2018, cancer is considered as one of the major leading causes of death and a significant public health burden.1,2 Several lines of evidence support the notion that telomere length is implicated in the etiology of cancer and risk of mortality. Telomeres are specialized structures comprised of multiple repeats of the TTAGGG sequence located at the termini of chromosomes that maintain genome integrity and stability by preventing end-to-end fusion.3,4 Telomeres shorten with each cycle of cell division and are generally considered as an indicator of aging at a cellular level of organisms. Therefore, telomeres have been proposed and extensively studied as a biomarker of aging and age-related diseases such as cardiovascular disease, cancer and diabetes.5,6
Telomere length is largely heritable.7 Single nucleotide polymorphisms have been identified in the genes encoding enzymes involved with telomere biology such as telomerase reverse transcriptase (TERT), telomerase RNA component (TERC), nuclear assembly factor 1 (NAF1), oligonucleotide/oligosaccharide-binding fold-containing protein 1 (OBFC1), regulator of telomere length 1 (RTEL1) and phox domain containing serine/threonine kinase-like (PXK).8,9 Gender and ethnicity have a significant impact on telomere length too.10,11 Environmental factors also impact telomere length including body mass index (BMI),12 cigarette smoking,13 exercise14 and dietary factors.15 Biomarkers that can predict risk of cancer may offer an opportunity for the development and implementation of preventive strategies to lower cancer incidence and cancer and overall mortality. Telomere length has been explored as a potential biomarker for cancer risk. Notably, both shorter and longer telomere lengths have been founded to be associated with increased risk of cancer in observational studies. Although the underlying biological mechanisms of action for higher risk of cancer due to longer telomere length are not well understood, it has been speculated that germline mutations in telomere maintenance genes cause upregulation of telomerase, which in turn, prolong survival of precancerous cells and induce further cell divisions with subsequent carcinogenic mutations.16
Previous prospective cohort studies investigating the relation between telomere length with risks of total mortality, cancer-specific mortality, and/or cancer incidence have been predominantly conducted in American and European populations. The results from these studies remain inconclusive; some found an inverse association17–26 whereas others reported null findings27–33 Data on other ethnic populations are sparse. We have previously reported that longer telomere length is associated with increased risk of developing several cancer types including lung adenocarcinoma,34 gastric cancer,35 breast cancer,36 colorectal cancer37 and pancreatic cancer.38 Data on the role of telomere length on the risk of total mortality and total cancer risk have been lacking, especially in Asian populations who have distinct genetic risk factors and environmental and lifestyle factors profile patterns than their white counterparts.
The primary aim of the present study was to examine the associations of leukocyte telomere length with risk of all-cause and cause-specific mortality in a large prospective cohort of Chinese adults living in Singapore. In the same cohort, we also investigated the impact of telomere length on the risk of developing any cancer as well as 17 specific cancer types with sufficient numbers for a robust estimation of risk.
2 |. METHODS
2.1 |. Study design and population
Participants included in our study were part of the Singapore Chinese Health Study (SCHS). The design and methods of this cohort study have been described in detail elsewhere.39 Briefly, the SCHS is a population-based prospective cohort study of 63 257 Chinese men and women aged 45 to 74 years enrolled from April 1993 through December 1998. The subjects were from the two major dialect groups of Chinese in Singapore (Hokkiens or Cantonese) who were residents of government housing estates where the majority of the Singapore population live in. Each subject completed an in-person interview at recruitment where information on demographics, lifestyle factors, medical history, family history of cancer, reproductive history (women only) and dietary intake over the last year via a 165-item food frequency questionnaire were collected by a trained interviewer. All surviving participants were re-contacted after baseline and updated information on active and passive cigarette smoking, alcohol drinking, tea and coffee drinking, medical history, current body weight and height, physical activity, current menopausal status of women, history of respiratory diseases, blood transfusion, use of medication and indoor air pollution from home cooking through follow-up 1 (1999–2004) and follow-up 2 (2006–2010) interviews. Blood and single-void urine specimens were collected from a random 3% of the study cohort subjects 1 year after initiation of the study and was extended to all surviving subjects in 2000. By April 28, 2005, 346 cohort subjects, representing 54% of eligible subjects, provided blood samples. All specimens were separated into their various components and were subsequently stored at −80°C until analyzed. The present investigation included 28 125 participants after excluding 221 participants with missing values due to failed assays for telomere length measurement.
2.2 |. Mortality and cancer incidence ascertainment
All incident cases of cancer among the cohort participants were identified through the record linkage analysis with the Singapore Cancer Registry. Specific cancer types were coded according to the International Classification of Diseases and Related Health Problems 10th revision (ICD-10) as follows: cancers of the head and neck (C00-C14 and C30-C33), stomach (C16), colon (C18), rectum (C19-C21), pancreas (C25), breast (C50), uterine (C53–54), bladder (C67), prostate (C61) and lymphoma (C81–85). For lung cancer, we focus on lung adenocarcinoma C34.x with morphological codes 8140, 8250, 8260, 8310, 8480; lung squamous cell carcinoma C34.x with 8070, 8071 and 856, all of them with malignant behavior code /3.
All deaths among cohort participants were ascertained through linkage analysis with the Singapore Registry of Births and Deaths.40 The underlying causes of deaths were coded according to the International Classification of Diseases and Related Health Problems ninth revision (ICD-9) up to December 31, 2011, or the ICD 10th revision (ICD-10) from 2012 to 2017. Primary cause of death was used for analysis. Separate analyses were conducted for the following cause-specific mortalities: cancer (ICD-9 codes 140–208, ICD-10 codes C00-C97), ischemic heart disease (ICD-9 codes 410–414; ICD-10 codes I20-I25), stroke (ICD-9 codes 430–438, ICD-10 codes I60-I69), respiratory causes (such as pneumonia ICD-9 codes 480–486, ICD-10 codes J12-J18), chronic obstructive pulmonary disease (ICD-9 codes 490–496, ICD-10 codes J40-J47) digestive system diseases (ICD-9 codes 520–579, ICD-10 codes K00-K95) and genitourinary diseases (ICD-9 codes 580–629, ICD-10 codes N00-N99).
As of December 31, 2017, there were only 56 participants known to be lost to follow-up due to migration out of Singapore or other reasons, suggesting that identification of incident cancer cases and vital statistics among cohort participants was virtually complete.
2.3 |. Measurement of telomere length
Genomic DNA was extracted from whole blood using QIAamp 96 DNA Blood Kits (Qiagen, Valencia, CA) according to the manufacturer’s protocol. Detailed information on telomere length measurement has been previously described.34 In brief, telomere lengths were quantified using a high throughput validated monochrome multiplex qPCR technique developed by Cawthon,41 which is the desirable method in large epidemiological studies. Relative telomere length was measured by comparing signals from telomere repeat copy number (T) to a single gene copy number (S) for albumin in experimental DNA samples in comparison to a reference DNA. This method allows calculation of a relative T/S ratio, which is proportional to mean telomere length.
Standard reference DNA sample was prepared in four concentrations (4.0, 0.8, 0.16, 0.032 ng/μl) from a pool of 77 samples in the Singapore Chinese Health Study by serial dilution in every 96-well plate. Telomere length measures of these samples were within 10% of the population mean. Resulting data were used to generate the standard DNA curve for relative measurement. All specimens were run in duplicate, and the mean value of two measures was used in the final statistical analysis. The average inter-assay coefficient variation obtained from quality control samples was 3.5% indicating that the method is well reproducible. We have also performed terminal restriction fragment (TRF) assay on 100 DNA samples selected from the SCHS using the Southern blot method42 and found that one unit of relative telomere length was equivalent to 6.19 kilobases of TRF (unpublished).
2.4 |. Statistical analyses
Telomere length was divided into quintiles or deciles according to the distribution of all cohort participants with available telomere length measurement. Analysis of covariance (ANCOVA) was used to examine the effect of demographics, lifestyle factors, and other major risk factors for risk of mortality and/or cancer on telomere length in continuous scale with adjustment for age, sex, and dialect group (Hokkien or Cantonese). Kruskal-Wallis or Chi-square test was used to compare the median or categorical variables of telomere length. For the analysis of total cancer and cancer-specific incidence, 1585 subjects with a history of invasive cancer (except nonmelanoma skin cancer) at the time of telomere length assessment were excluded. Therefore, the final analysis for cancer incidence data in our study included 26 540 subjects.
For each participant, person-years of follow-up for mortality analysis were counted from the date of blood sample collection until the date of death or December 31, 2017, whichever occurred first. For the cancer incidence analysis, person-years of follow-up were calculated from the date of blood sample collection to the date of diagnosis of cancer, death or December 31, 2016, the latest date for cancer incidence data available, whichever came first.
We used Cox proportional hazards regression method to estimate the hazard ratios (HRs) and 95% confidence intervals (CIs) of all-cause and cause-specific mortality, as well as for the risk of total cancer incidence and selected cancer-specific incidence. Similar Cox proportional hazards regression models were used to estimate HRs of these endpoints per SD change of telomere length. The assumptions of proportionality for Cox models were confirmed based on scaled Schoenfeld residuals.43 Tests for linear trend in risk of the endpoints with telomere length were performed by using the quintiles or deciles of telomere length as an ordinal variable in the Cox model.
In multivariate analysis, we controlled for age at blood collection (years), sex (men or women), dialect group (Hokkien or Cantonese), BMI (<18.5, 18.5 to <23, 23 to <27.5, 27.5+ kg/m2), level of education (no formal education, primary school, or secondary school and above), smoking status (never, former, or current smoker), number of cigarettes per day, number of years of smoking, alcohol consumption (nondrinker, <7 or ≥7 drinks per week), weekly vigorous work or strenuous sports (no or yes), number of hours of sleep per night (≤6, 7–8 or ≥9), self-reported history of physician-diagnosed diabetes mellitus (yes or no), hypertension (yes or no), stroke (yes or no), ischemic heart disease (yes or no) and cancer (yes or no). For the risk of female cancers including breast and uterine, we further adjusted for age when the menstrual period became regular, age at first live birth (<10, 21–25, 26–30 or ≥31 years), number of live births (0, 1–2, 3–4 or ≥5), age at menopause (≤49, 50–54 or ≥55 years), use of hormone therapy (never, ever, or current), use of oral contraceptives (no or yes) and family history of breast cancer (no or yes).
Statistical analyses were performed using SAS 9.4 software package (SAS Institute, Cary, North Carolina), with a two-sided test of the alpha level at 0.05 as a significant level.
3 |. RESULTS
The mean (SD) of age among all participants at blood collection was 62.9 (7.7) years and the mean (SD) of relative telomere length was 1.02 (0.23). Table 1 shows the baseline demographics and lifestyle characteristics of the study participants (n = 28 125) by the 1st, 5th and 10th deciles of telomere length. Relative telomere length was significantly longer in younger participants and women compared to older and men participants, respectively (both P values < .0001). Telomere length was also significantly longer among participants who were never-smokers and nonalcoholic drinkers than those who were current/former cigarette smokers and alcohol drinkers (P < .0001 and P = .002, respectively). BMI and physical activity levels were comparable across different deciles of telomere length.
TABLE 1.
Selected baseline demographic and lifestyle characteristics of the 28 125 study participants by deciles of increasing relative telomere length in the Singapore Chinese Health Study
| Characteristics | Total | First decile (shortest) | Fifth decile | 10th decile (longest) | P a |
|---|---|---|---|---|---|
| Relative telomere length, median (ranges) | 28 125 | 0.70 (0.64, 0.73) | 0.97 (0.96, 0.98) | 1.42 (1.35, 1.53) | <.0001b |
| Age at sample collection (years), mean (SD) | 28 125 | 66.8 (7.9) | 63.1 (7.5) | 59.9 (7.0) | <.0001 |
| Body mass indexc (kg/m2), mean (SD) | 28 125 | 23.1 (3.5) | 23.3 (3.6) | 23.4 (3.5) | .34 |
| Sex, n (%) | <.0001 | ||||
| Men | 12 850 | 1608 (57.2) | 1338 (47.6) | 1010 (35.9) | |
| Women | 15 275 | 1204 (42.8) | 1474 (52.4) | 1802 (64.1) | |
| Level of educationc, n (%) | <.0001 | ||||
| No formal education | 5950 | 668 (23.8) | 563 (20.0) | 591 (21.0) | |
| Primary school | 12 736 | 1343 (47.8) | 1268 (45.1) | 1253 (44.6) | |
| Secondary school and above | 9439 | 801 (28.5) | 981 (34.9) | 968 (34.4) | |
| Smoking statusc, n (%) | <.0001 | ||||
| Never | 19 089 | 1637 (58.2) | 1889 (67.2) | 2107 (74.9) | |
| Former | 4496 | 660 (23.5) | 449 (16.0) | 337 (12.0) | |
| Current | 4540 | 515 (18.3) | 474 (16.9) | 368(13.1) | |
| Cigarettes per dayd, mean (SD) | 9036 | 17.6 (13.1) | 17.3 (13.4) | 16.1 (12.2) | .46 |
| Years of smokingd, mean (SD) | 9036 | 37.0 (14.9) | 34.2 (13.7) | 31.3 (13.5) | .60 |
| Alcohol consumptionc, (drinks/week), n (%) | .002 | ||||
| None | 22 948 | 2287 (81.3) | 2286 (81.3) | 2298 (81.7) | |
| <7 | 3924 | 382 (13.6) | 385 (13.7) | 417 (14.8) | |
| ≥7 | 1253 | 143 (5.1) | 141 (5.0) | 97 (3.5) | |
| Weekly physical activity, n (%) | .10 | ||||
| No | 18 126 | 1799 (64.0) | 1805 (64.2) | 1836 (65.3) | |
| Yes | 9999 | 1013 (36.0) | 1007 (35.8) | 976 (34.7) | |
| Sleeping hours per day, mean (SD) | .0002 | ||||
| ≤6 hours | 9352 | 985 (35.0) | 952 (33.9) | 963 (34.3) | |
| 7–8 hours | 16 992 | 1613 (57.4) | 1702 (60.5) | 1682 (59.8) | |
| ≥9 hours | 1781 | 214 (7.6) | 158 (5.6) | 167 (5.9) | |
| History of hypertension, n (%) | .49 | ||||
| No | 17 158 | 1705 (60.6) | 1706 (60.7) | 1670 (59.4) | |
| Yes | 10 967 | 1107 (39.4) | 1106 (39.3) | 1142 (40.6) | |
| History of diabetes mellitus, n (%) | .06 | ||||
| No | 24 103 | 2362 (84.0) | 2428 (86.3) | 2412 (85.8) | |
| Yes | 4022 | 450 (16.0) | 384 (13.7) | 400 (14.2) | |
| History of stroke, n (%) | <.0001 | ||||
| No | 27 060 | 2674 (95.1) | 2707 (96.3) | 2718 (96.7) | |
| Yes | 1065 | 138 (4.9) | 105 (3.7) | 94 (3.3) | |
| History of ischemic heart disease, n (%) | <.0001 | ||||
| No | 26 036 | 2512 (89.3) | 2622 (93.2) | 2666 (94.8) | |
| Yes | 2089 | 300 (10.7) | 190 (6.8) | 146 (5.2) | |
| History of cancer, n (%) | .06 | ||||
| No | 26 540 | 2624 (93.3) | 2673 (95.1) | 2630 (93.5) | |
| Yes | 1585 | 188 (6.7) | 139 (4.9) | 182 (6.5) |
P values were determined by using the ANCOVA test for continuous variables adjusted for age at sample collection, sex (men or women) and dialect (Hokkien or Cantonese) or Pearson's chi-square test for categorical variables. Percentages may not add up to 100% due to rounding.
P value is derived from the Kruskal-Wallis test.
Updated with data from the first follow-up interview.
Among only current and former smokers.
During an average of 13.2 years of follow-up, there were a total of 7609 deaths including 2660 deaths from cancer. Longer telomeres were significantly associated with reduced risk of all-cause mortality and noncancer mortality in a dose-dependent manner before adjustment for other potential risk factors (Figure 1). After adjustment for age, sex, BMI, cigarette smoking, alcohol consumption, physical activity, sleep pattern and other lifestyle and demographic factors, the magnitude for the inverse association between the relative telomere length and risk of total mortality considerably attenuated but remained statistically significant. Compared to the 1st (lowest) decile, the 10th (highest) decile of telomere length was associated with a significant 7% lower risk of overall death (Ptrend = .02) and a significant 19% lower risk of noncancer death (Ptrend < .0001; Figure 2). In contrast, participants with the highest decile of telomere length had a significantly 18% higher risk of death from cancer compared to those with the shortest telomeres (Ptrend = .008) after adjustment for multiple potential confounding factors. We further calculated the odds ratio of cancer death vs noncancer death cases and observed a significant positive association between telomere length and risk of cancer mortality (data not shown). For cause-specific mortality, increasing telomere length was significantly associated with decreased risk of mortality from chronic obstructive pulmonary disease (COPD) and pneumonia (HR per 1-SD increase = 0.79, 95% CI: 0.70–0.89) and digestive diseases (HR per 1-SD increase = 0.60, 95% CI: 0.42–0.88). There was no association for risk of mortality due to ischemic heart disease, stroke, and genitourinary diseases (Figure 3). When data were analyzed by quintiles of telomere length, similar results were observed (Table S1).
FIGURE 1.
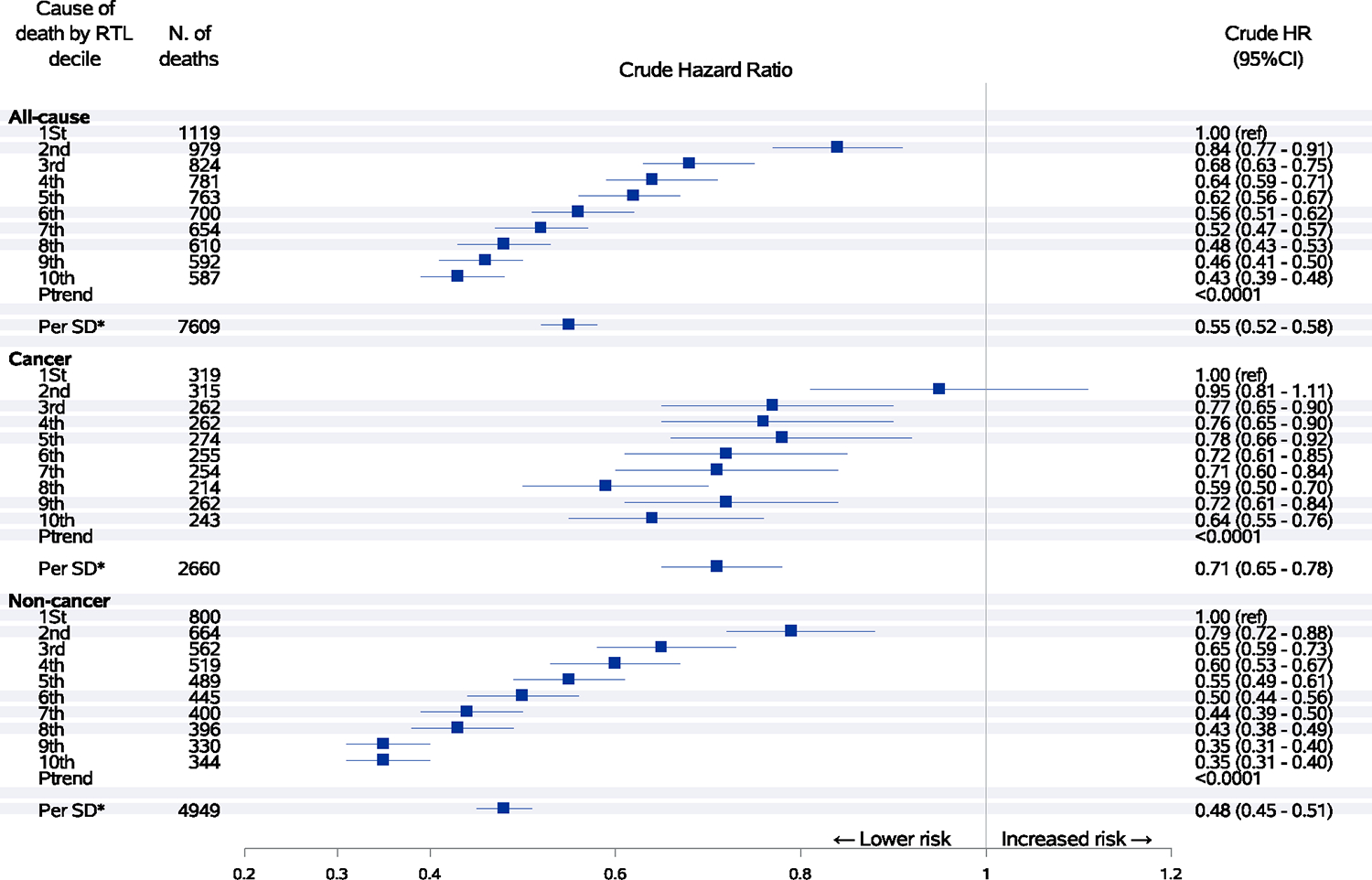
Associations between relative telomere length and risk of all-cause, cancer and noncancer mortality. Hazard ratios with 95% confidence intervals of mortality from all causes, cancer and noncancer causes by decile or one-SD increment of telomere length. Crude hazard ratios
FIGURE 2.
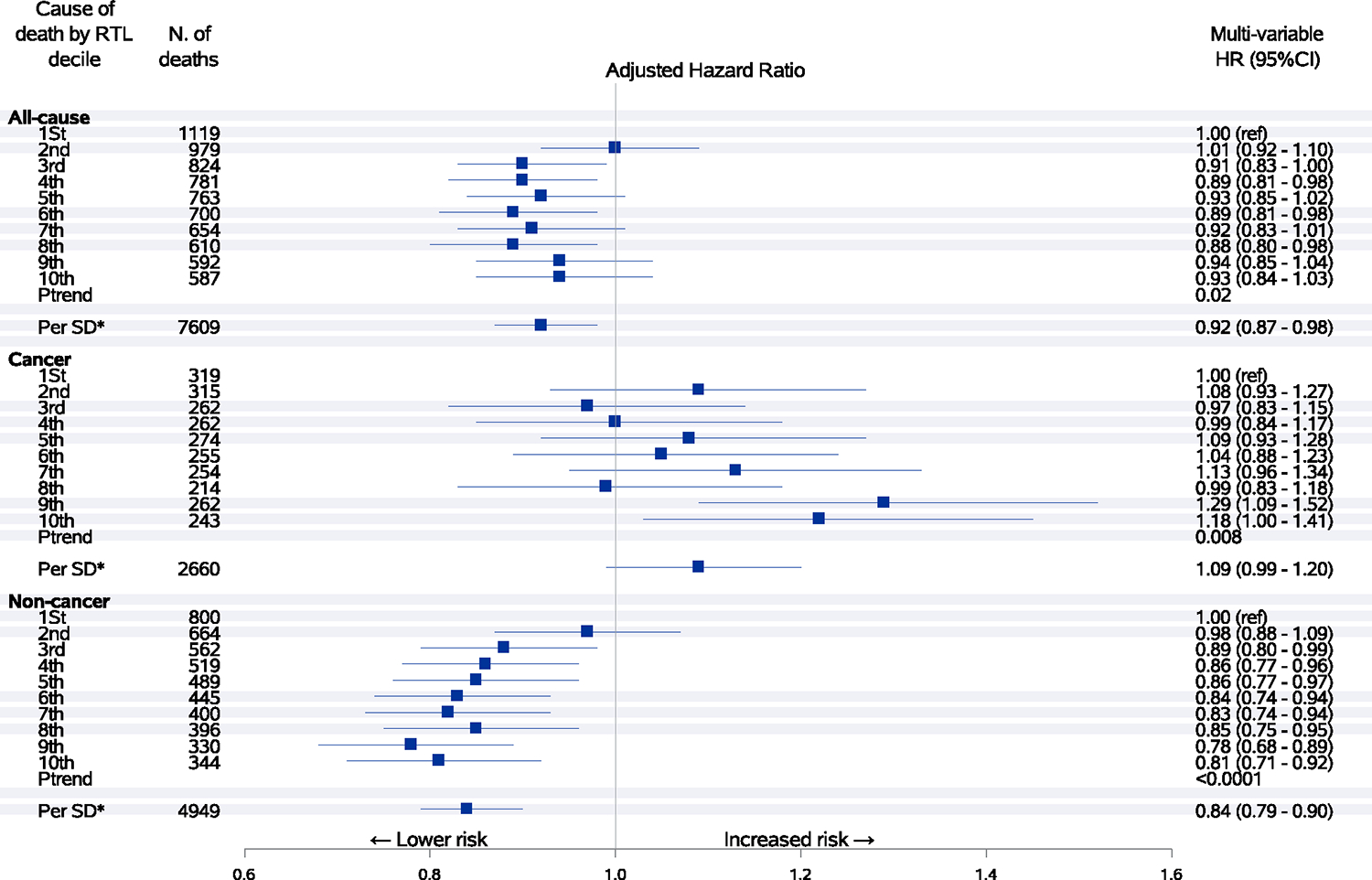
Associations between relative telomere length and risk of all-cause, cancer and noncancer mortality. Hazard ratios with 95% confidence intervals of mortality from all causes, cancer and noncancer causes by decile or one-SD increment of telomere length. Hazard ratios were adjusted for age at blood collection, sex, dialect group, body mass index category, level of education, smoking status, number of cigarettes per day, number of years of smoking, daily alcohol consumption, weekly vigorous work or strenuous sports, number of hours of sleep per night and self-reported histories of physician-diagnosed diabetes mellitus, hypertension, stroke, ischemic heart disease and cancer. Longer telomeres were associated with a lower risk of mortality from all causes and noncancer related causes but associated with a higher risk of mortality from cancer
FIGURE 3.
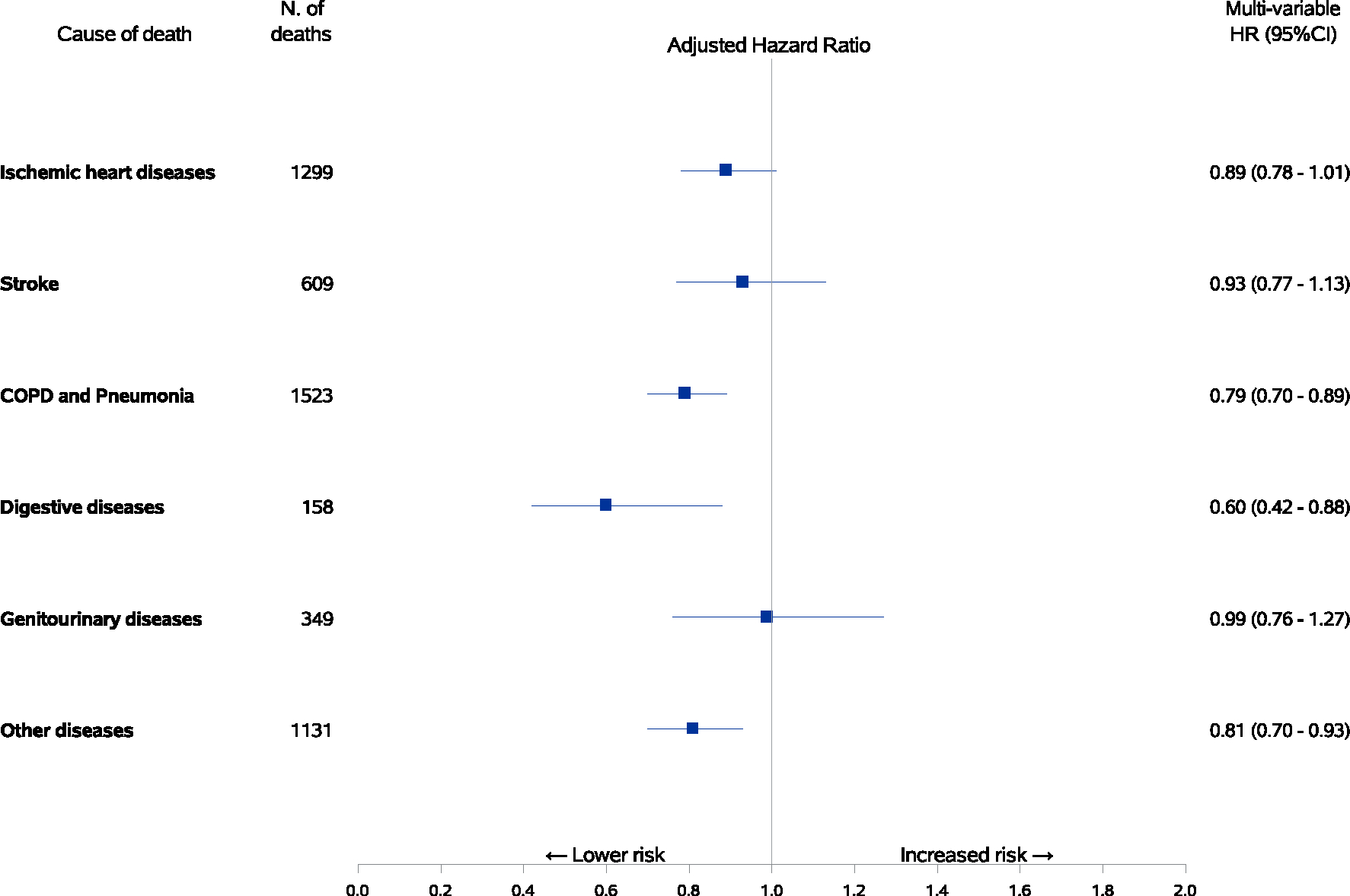
Associations between relative telomere length and risk of cause-specific mortality. Hazard ratios with 95% confidence intervals of mortality from selected causes other than cancer by one-SD increment of telomere length with adjustment for age at blood collection, sex, dialect group, body mass index category, level of education, smoking status, number of cigarettes per day, number of years of smoking, daily alcohol consumption, weekly vigorous work or strenuous sports, number of hours of sleep per night and self-reported histories of physician-diagnosed diabetes mellitus, hypertension, stroke, ischemic heart disease and cancer. Longer telomeres were associated with a lower risk of mortality from chronic obstructive pulmonary disease (COPD) and digestive diseases
Over a total of 332 947 person-years of follow-up (average of 12.5 years per subject), 4353 participants who were free of cancer at the time of blood collection developed cancer. Multivariate-adjusted HRs (95% CI) for total cancer incidence according to deciles of telomere length are presented in Figure 4. Comparing to the first decile, the 10th decile of telomere length was associated with a 26% increased risk of developing any malignancy (95% CI: 11%-44%; Ptrend < .0001).
FIGURE 4.
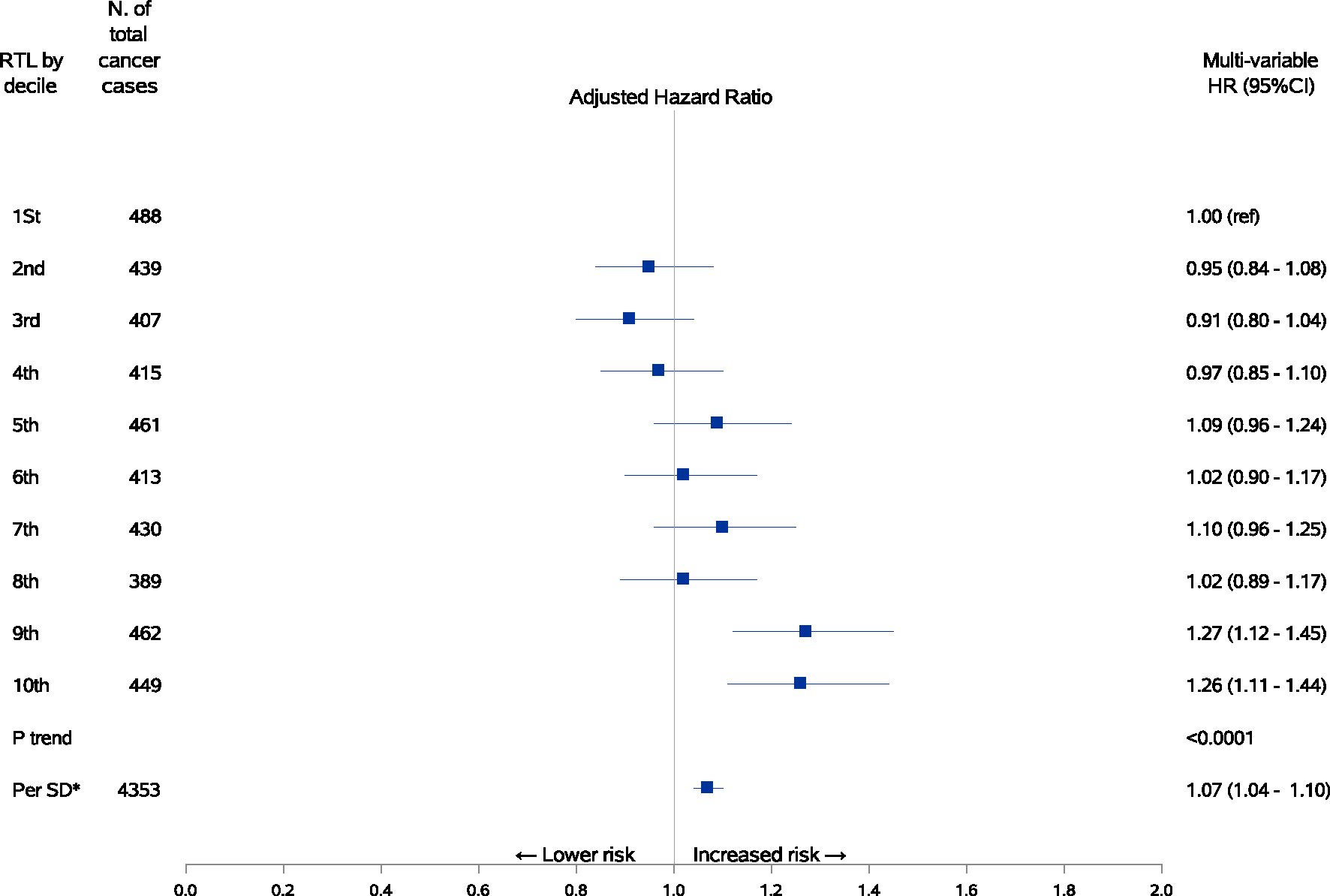
Associations between relative telomere length and risk of total cancer incidence. Hazard ratios with 95% confidence intervals of total cancer incidence by decile or one-SD increment of telomere length with adjustment for age at blood collection, sex, dialect group, body mass index category, level of education, smoking status, number of cigarettes per day, number of years of smoking, daily alcohol consumption, weekly vigorous work or strenuous sports, number of hours of sleep per night and self-reported histories of physician-diagnosed diabetes mellitus, hypertension, stroke, ischemic heart disease and cancer. Longer telomeres were associated with a higher risk of total cancer incidence
For specific cancer types, longer telomeres were associated with higher HRs of lung adenocarcinomas (HR per 1-SD increase = 1.34, 95% CI: 1.19–1.52), pancreatic cancer (HR per 1-SD increase = 1.26, 95% CI: 1.04–1.53), rectal cancer (HR per 1-SD increase = 1.14, 95% CI: 1.01–1.29), and breast cancer (HR per 1-SD increase = 1.11, 95% CI: 1.01–1.23) (Figure 5). Telomere length was associated with a non-statistically significant increased risk of remaining cancer sites. In addition, we examined the relationship between telomere length in quintiles and the risk of total and specific sites of cancer (Table S2).
FIGURE 5.
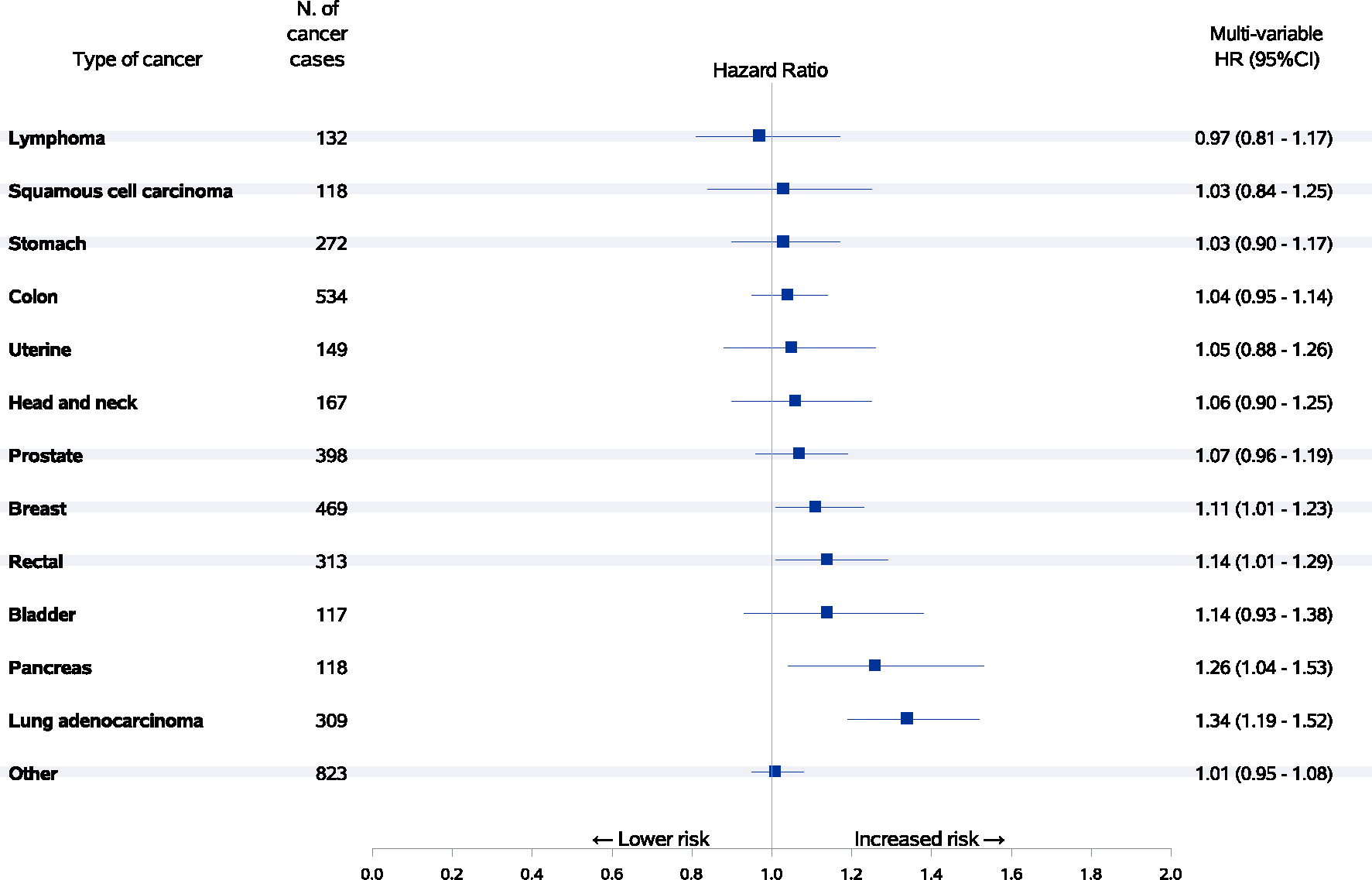
Associations between relative telomere length and risk of cancer-specific incidences. Hazard ratios with 95% confidence intervals of selected specific cancer types with at least 100 cases per one-SD increment of telomere length with adjustment for age at blood collection, sex, dialect group, body mass index category, level of education, smoking status, number of cigarettes per day, number of years of smoking, daily alcohol consumption, weekly vigorous work or strenuous sports, number of hours of sleep per night and self-reported histories of physician-diagnosed diabetes mellitus, hypertension, stroke, ischemic heart disease and cancer. Longer telomeres were associated with a significantly higher risk of breast cancer, rectal cancer, pancreatic cancer and lung adenocarcinoma
In a sensitivity analysis to rule out the potential impact of cancer or other health outcomes on telomere length, we further excluded study participants with the diagnosis of cancer or other health outcomes and the observed person-years that occurred within the first 2 years post the measurement of telomere length. The results of this sensitivity analysis were almost the same as those derived from the entire cohort. For example, the HRs (95% CIs) of total mortality, total cancer incidence and lung cancer adenocarcinoma among participants with the highest quintile of relative telomere length were 0.93 (0.86–1.01; Ptrend = .04), 1.31 (1.19–1.45; Ptrend < .0001) and 2.94 (2.00–4.32; Ptrend < .0001), respectively, as compared to the lowest quintile.
4 |. DISCUSSION
In this large prospective cohort of middle-aged Chinese men and women in Singapore, we found that longer telomere length was associated with significantly reduced risks of all-cause mortality and non-cancer related mortality. On the other hand, longer telomere length was related to an elevated risk of total cancer incidence and cancer mortality, independent of other comorbidities, and established risk factors for mortality. We observed a similar pattern of results for various specific cancer types. This is, to our best knowledge, the first population-based study in an Asian population that evaluates the role of telomere length and all-cause and cause-specific mortality risk.
Findings from previous prospective studies on telomere length and all-cause mortality are inconsistent. Cawthon et al17 conducted the first study on the association between telomere length in blood and mortality in 143 unrelated Utah residents aged 60 to 97 years. After 18 years of follow-up and 101 death cases, they observed that shorter telomere was associated with 1.86-fold higher risk of all-cause mortality (95% CI: 1.22–2.83; P = .004). In a prospective cohort of 64 637 Danish participants with 7607 total deaths,21,24 shorter telomere length was found to be associated with a 40% increase in the risk of all-cause mortality comparing the highest with the lowest decile of telomere length (multi-variable adjusted HR = 1.40; 95% CI: 1.25–1.57). These findings have been confirmed in other studies,18,20,23,25,26 and more recently in a meta-analysis with more than 21 700 deaths.44 Our findings are corroborated by these results and further support an inverse association between telomere length and risk of all-cause mortality.
No study with a prospective design has yet explored the association between leukocyte telomere length and risk of total cancer or cancer-specific mortality in an Asian population. Two cohort studies, one conducted in a Danish population22,24 with 2420 cancer deaths, and the other in an Italian population45 with only 44 cancer deaths, both showed that shorter rather than longer telomeres were significantly associated with a higher risk of cancer mortality. In contrast to these findings, we observed a significant positive association between telomere length and risk of cancer mortality after adjustment for a comprehensive list of cancer mortality risk factors. It is noteworthy that we found a strong inverse association between telomere length and risk of cancer mortality in our crude statistical model without adjustment for potential confounding factors (HR = 0.64; 95% CI: 0.55–0.76) for participants with the highest vs the lowest decile of telomere length. In addition, other studies conducted in Caucasian populations to date reported no significant associations between telomere length and cancer mortality.18,19,28–30 In our study, when the potential confounders were adjusted, the direction of the association was reversed and the magnitude of the association was remarkably changed, suggesting that these factors significantly confounded the associations between telomere length and risk of cancer mortality and incidence. The measurement of and adjustment for the demographic, lifestyle and other risk factors for cancer are critically important to reduce potential residual confounding. This may partly explain the inconsistent results between ours and previous studies. Other characteristics of the previous studies such as a smaller number of cases, shorter length of follow-up, different environmental and genetic risk factors between the study populations may also contribute to the observed inconsistent results.
The observed elevated risk of total cancer incidence in association with longer telomere length conforms with our data on the risk of cancer mortality. In the most recent meta-analysis32 of prospective studies with close to 14 000 cases, longer leukocyte telomere length was marginally associated with increased risk of total cancer incidence (OR = 1.09; 95% CI: 0.95–1.24). However, the majority (ie, more than 92%) of the included study participants were European descents. Also, there was considerable heterogeneity in the characteristics of these study populations such as different geographical locations, male/female ratio, age at the measurement of telomere length, as well as difference in study design, sample size, DNA extraction method and telomere length measurement method among the studies included in that meta-analysis (I2 = 70.4%, P for heterogeneity test < .001). In our previous analyses in the same cohort, we found a positive association for longer telomere length with increased risk of multiple specific cancer types including lung adenocarcinoma,34 breast,36 colorectal37 and pancreatic38 based on the Taiwan Cancer Screening Program Cohort in Asian participants in which the associations cancers. To date, there are only three nested case-control studies conducted within the Shanghai Women’s Health Study and one nested case-control study of telomere length in peripheral blood leucocytes with the incidence of total lung cancer (a positive association),46 breast cancer (an inverse association),47 colorectal cancer (a U-shaped association)48 and hepatocellular carcinoma (no association)49 have been evaluated and produced mixed results. The discrepancy in results between our study and prior studies in Asian or Caucasian populations might be due to difference in the length of follow-up, prevalence rates of established risk factors (ie, smoking, diabetes, physical activity or obesity), study design, and/or the duration of follow-up from the assessment of telomere length (ie, blood collection) and the occurrence of cancer (ie, the date of cancer diagnosis).
Although the underlying mechanism is not yet understood, the observed associations between telomere length and increased risk of cancer mortality and cancer incidence may be explained through the biology of telomeres. Genetic disposition to longer telomeres may influence both cancer incidence and mortality through the telomere maintenance pathway. Longer telomeres may allow damaged cells to survive longer and continue to divide and acquire additional mutations, leading to the malignant transformation50 In addition, certain genetic mutations are associated with enhanced telomerase activity, resulting in telomere lengthening and immortalization of cells, a hallmark of cancer cells31,51–53 It has also been suggested that individuals with a suppressive immune system have a slower pace of telomere shortening because of a lower rate of cell division.54 Immune suppression is found to be associated with an increased risk of cancer.55 The biological mechanisms of the underlying observed associations in our study may also be explained partly by the existing complex theories of cellular senescence and evolutionary theory of antagonistic pleiotropy. Cellular senescence, a presumably irreversible growth arrest mechanism, has been hypothesized to be four-faceted in which opposing but biologically connected processes such as tumor suppression, tumor promotion, aging, and tissue repair each play a role.56 For example, senescent cells may promote age-related diseases such as cancer by depleting tissues of stem or progenitor cells,57 and secretion of cytokines, proteases and other proteins58 that disrupt the structure and function of tissues and have adverse inflammatory properties. Increasing senescence cells throughout life may also promote the reactivation of telomerase and longer telomeres. Some animal studies have shown that reactivation of telomerase enhances tumorigenesis thus antitelomerase therapy is currently an active cancer research strategy.59,60
As proposed by Williams61 more than 60 years ago, and known as the evolutionary theory of antagonistic pleiotropy, some biological processes such as telomere function may enhance fitness in early life while having a deleterious impact later in life. Consistent with the hypothesis of antagonistic pleiotropy, it is tempting to propose that the observed positive association between telomere length and risk of developing total cancer is suggestive of a trade-off between proliferative degenerative diseases of aging such as cancer and non-proliferative degenerative diseases of aging including cardiovascular diseases (CVD).
Strength of our study includes a large sample size in a single prospective study where telomere length was measured in more than 28 000 participants, which in turn, provided strong statistical power, resulting in robust risk estimates. Another strength of the present study is our ability to collect a comprehensive set of demographics, lifestyle factors, including smoking, alcohol consumption, dietary factors, BMI, physical activity and history of diseases that may influence leukocyte telomere length. The simultaneous adjustment for these potential confounding factors minimized the potential confounding effect on the association between leukocyte telomere length and the risk of endpoints in our analysis. The prospective study design with a long-term follow-up also minimized the potential impact of disease progression on telomere length, which ruled out the possibility of a reverse causation relationship between telomere length and risk of disease outcomes (ie, cancer).
Our study also has some limitations. First, telomere length was measured only at one point in time, which limited our ability to investigate the telomere length attrition rate and the risk of developing disease outcomes (ie, cancer) over time. Second, telomere length was measured in blood leukocytes, which may not be representative of the target tissue(s) where cancer developed. Third, as in any observational studies, residual confounding due to unmeasured or imprecisely measured factors such as BMI, cigarette smoking and physical inactivity could not be completely ruled out.
In conclusion, we found that longer telomere length was associated with a significantly reduced risk of all-cause mortality and non-cancer related mortality, but was associated with a higher risk of total cancer incidence and cancer mortality, independent of other comorbidities and established risk factors for death. A similar pattern for longer telomere length and elevated risk was also observed for several cancer types including lung adenocarcinoma and cancers of the breast, rectal, genitourinary system, pancreas and so on. Our findings support a potential role of longer telomeres in the pathogenesis of both cancer-specific types and noncancer diseases and thus might have clinical relevance to be used as a part of aging and other biomarkers for an eventual risk prediction model.
Supplementary Material
What’s new?
Telomere length is a promising biomarker for cancer risk, with longer- and shorter-than-expected telomeres variously linked to increased risk of malignancy. Among Asian populations, however, relationships between telomere length, cancer incidence, and cancer mortality remain understudied. Here, investigation of more than 26 500 Chinese adults with greater than 12 years of health follow-up shows that longer telomere length is associated with significantly elevated risk of total and cancer-specific incidence and mortality and with reduced risk of all-cause and non-cancer-related mortality. The data highlight the relevance of longer telomere length in the development and outcome of specific cancer types and non-cancerous diseases.
ACKNOWLEDGEMENTS
We thank Shalane Porter and Dinesha Walek of the University of Minnesota Genetic Center for measuring telomere length using the qPCR method. We also thank Siew-Hong Low of the National University of Singapore for supervising the fieldwork of the Singapore Chinese Health Study; the Singapore Cancer Registry for the identification of cancer cases and the Singapore Chinese Health Study subjects for their participation in our study. Our study was supported by the United States NIH/NCI (R01 CA144034 and UM1 CA182876) grants; NIH/NCI T32CA186873 training grant in cancer epidemiology; Singapore National Medical Research Council grant (NMRC/CSA/0055/2013).
Abbreviations:
- ANCOVA
analysis of covariance
- BMI
body mass index
- CI
confidence interval
- COPD
chronic obstructive pulmonary disease
- CVD
cardiovascular diseases
- HR
hazard ratio
- ICD
International Classification of Diseases
- NAF1
nuclear assembly factor 1
- OBFC1
oligonucleotide/oligosaccharide-binding fold-containing protein 1
- PCR
polymerase chain reaction
- PXK
Phox domain containing serine/threonine kinase-like
- RTEL1
regulator of telomere length 1
- SCHS
Singapore Chinese Health Study
- TERC
telomerase RNA component
- TERT
telomerase reverse transcriptase
Footnotes
CONFLICT OF INTEREST
All authors declared no financial disclosure or conflict of interest.
ETHICS STATEMENT
All study participants provided informed consent before completing the questionnaires. The institutional review boards at the National University of Singapore and the University of Pittsburgh approved our study.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of this article.
DATA AVAILABILITY STATEMENT
The dataset analyzed in the current study is available from the corresponding author on reasonable request.
REFERENCES
- 1.Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144:1941–1953. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. [DOI] [PubMed] [Google Scholar]
- 3.Blackburn EH. Telomeres and telomerase: their mechanisms of action and the effects of altering their functions. FEBS Lett. 2005;579:859–862. [DOI] [PubMed] [Google Scholar]
- 4.Blackburn EH, Greider CW, Szostak JW. Telomeres and telomerase: the path from maize, Tetrahymena and yeast to human cancer and aging. Nat Med. 2006;12:1133–1138. [DOI] [PubMed] [Google Scholar]
- 5.von Zglinicki T, Martin-Ruiz CM. Telomeres as biomarkers for ageing and age-related diseases. Curr Mol Med. 2005;5:197–203. [DOI] [PubMed] [Google Scholar]
- 6.Sanders JL, Newman AB. Telomere length in epidemiology: a biomarker of aging, age-related disease, both, or neither? Epidemiol Rev. 2013;35:112–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Slagboom PE, Droog S, Boomsma DI. Genetic determination of telomere size in humans: a twin study of three age groups. Am J Hum Genet. 1994;55:876–882. [PMC free article] [PubMed] [Google Scholar]
- 8.Codd V, Nelson CP, Albrecht E, et al. Identification of seven loci affecting mean telomere length and their association with disease. Nat Genet. 2013;45:422–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pooley KA, Bojesen SE, Weischer M, et al. A genome-wide association scan (GWAS) for mean telomere length within the COGS project: identified loci show little association with hormone-related cancer risk. Hum Mol Genet. 2013;22:5056–5064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nawrot TS, Staessen JA, Gardner JP, Aviv A. Telomere length and possible link to X chromosome. Lancet (London, England). 2004;363:507–510. [DOI] [PubMed] [Google Scholar]
- 11.Aviv A, Shay J, Christensen K, Wright W. The longevity gender gap: are telomeres the explanation? Sci Aging Knowledge Environ. 2005; 2005:pe16. [DOI] [PubMed] [Google Scholar]
- 12.Gielen M, Hageman GJ, Antoniou EE, et al. Body mass index is negatively associated with telomere length: a collaborative cross-sectional meta-analysis of 87 observational studies. Am J Clin Nutr. 2018;108:453–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Astuti Y, Wardhana A, Watkins J, Wulaningsih W. Cigarette smoking and telomere length: a systematic review of 84 studies and meta-analysis. Environ Res. 2017;158:480–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin X, Zhou J, Dong B. Effect of different levels of exercise on telomere length: a systematic review and meta-analysis. J Rehabil Med. 2019;51:473–478. [DOI] [PubMed] [Google Scholar]
- 15.Rafie N, Golpour Hamedani S, Barak F, Safavi SM, Miraghajani M. Dietary patterns, food groups and telomere length: a systematic review of current studies. Eur J Clin Nutr. 2017;71:151–158. [DOI] [PubMed] [Google Scholar]
- 16.Blackburn EH, Epel ES, Lin J. Human telomere biology: a contributory and interactive factor in aging, disease risks, and protection. Science (New York, NY). 2015;350:1193–1198. [DOI] [PubMed] [Google Scholar]
- 17.Cawthon RM, Smith KR, O’Brien E, Sivatchenko A, Kerber RA. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet (London, England). 2003;361:393–395. [DOI] [PubMed] [Google Scholar]
- 18.Fitzpatrick AL, Kronmal RA, Kimura M, et al. Leukocyte telomere length and mortality in the cardiovascular health study. J Gerontol A Biol Sci Med Sci. 2011;66:421–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mons U, Muezzinler A, Schottker B, et al. Leukocyte telomere length and all-cause, cardiovascular disease, and cancer mortality: results from individual-participant-data meta-analysis of 2 large prospective cohort studies. Am J Epidemiol. 2017;185:1317–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Honig LS, Kang MS, Schupf N, Lee JH, Mayeux R. Association of shorter leukocyte telomere repeat length with dementia and mortality. Arch Neurol. 2012;69:1332–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weischer M, Bojesen SE, Cawthon RM, Freiberg JJ, Tybjaerg-Hansen A, Nordestgaard BG. Short telomere length, myocardial infarction, ischemic heart disease, and early death. Arterioscler Thromb Vasc Biol. 2012;32:822–829. [DOI] [PubMed] [Google Scholar]
- 22.Weischer M, Nordestgaard BG, Cawthon RM, Freiberg JJ, TybjaergHansen A, Bojesen SE. Short telomere length, cancer survival, and cancer risk in 47102 individuals. J Natl Cancer Inst. 2013;105:459–468. [DOI] [PubMed] [Google Scholar]
- 23.Batsis JA, Mackenzie TA, Vasquez E, et al. Association of adiposity, telomere length and mortality: data from the NHANES 1999–2002. Int J Obes. 2018;42:198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rode L, Nordestgaard BG, Bojesen SE. Peripheral blood leukocyte telomere length and mortality among 64,637 individuals from the general population. J Natl Cancer Inst. 2015;107:djv074. [DOI] [PubMed] [Google Scholar]
- 25.Deelen J, Beekman M, Codd V, et al. Leukocyte telomere length associates with prospective mortality independent of immune-related parameters and known genetic markers. Int J Epidemiol. 2014;43:878–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marioni RE, Harris SE, Shah S, et al. The epigenetic clock and telomere length are independently associated with chronological age and mortality. Int J Epidemiol. 2018;47:356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bischoff C, Petersen HC, Graakjaer J, et al. No association between telomere length and survival among the elderly and oldest old. Epidemiology. 2006;17:190–194. [DOI] [PubMed] [Google Scholar]
- 28.Martin-Ruiz CM, Gussekloo J, van Heemst D, von Zglinicki T, Westendorp RG. Telomere length in white blood cells is not associated with morbidity or mortality in the oldest old: a population-based study. Aging Cell. 2005;4:287–290. [DOI] [PubMed] [Google Scholar]
- 29.Njajou OT, Hsueh WC, Blackburn EH, et al. Association between telomere length, specific causes of death, and years of healthy life in health, aging, and body composition, a population-based cohort study. J Gerontol A Biol Sci Med Sci. 2009;64:860–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Svensson J, Karlsson MK, Ljunggren O, Tivesten A, Mellstrom D, Moverare-Skrtic S. Leukocyte telomere length is not associated with mortality in older men. Exp Gerontol. 2014;57:6–12. [DOI] [PubMed] [Google Scholar]
- 31.Rode L, Nordestgaard BG, Bojesen SE. Long telomeres and cancer risk among 95 568 individuals from the general population. Int J Epidemiol. 2016;45:1634–1643. [DOI] [PubMed] [Google Scholar]
- 32.Zhang X, Zhao Q, Zhu W, et al. The Association of Telomere Length in peripheral blood cells with cancer risk: a systematic review and meta-analysis of prospective studies. Cancer Epidemiol Biomarkers Prev. 2017;26:1381–1390. [DOI] [PubMed] [Google Scholar]
- 33.Bendix L, Thinggaard M, Fenger M, et al. Longitudinal changes in leukocyte telomere length and mortality in humans. J Gerontol A Biol Sci Med Sci. 2014;69:231–239. [DOI] [PubMed] [Google Scholar]
- 34.Yuan JM, Beckman KB, Wang R, et al. Leukocyte telomere length in relation to risk of lung adenocarcinoma incidence: findings from the Singapore Chinese Health Study. Int J Cancer. 2018;142:2234–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Z, Koh WP, Jin A, Wang R, Yuan JM. Telomere length and risk of developing gastric adenocarcinoma: the Singapore Chinese Health Study. Gastric Cancer. 2018;21:598–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Samavat H, Xun X, Jin A, Wang R, Koh WP, Yuan JM. Association between prediagnostic leukocyte telomere length and breast cancer risk: the Singapore Chinese Health Study. Breast Cancer Res. 2019;21:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luu HN, Qi M, Wang R, et al. Association between leukocyte telomere length and colorectal cancer risk in the Singapore Chinese Health Study. Clin Transl Gastroenterol. 2019;10:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luu HN, Huang JY, Wang R, et al. Association between leukocyte telomere length and the risk of pancreatic cancer: findings from a prospective study. PLoS One. 2019;14:e0221697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hankin JH, Stram DO, Arakawa K, et al. Singapore Chinese health study: development, validation, and calibration of the quantitative food frequency questionnaire. Nutr Cancer. 2001;39:187–195. [DOI] [PubMed] [Google Scholar]
- 40.Singapore Cancer Registry Interim Report. Trends in cancer incidence in Singapore 2010–2014. Vol 2019. Singapore: Singapore Cancer Registry; 2019. [Google Scholar]
- 41.Cawthon RM. Telomere length measurement by a novel monochrome multiplex quantitative PCR method. Nucleic Acids Res. 2009;37:e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kimura M, Stone RC, Hunt SC, et al. Measurement of telomere length by the southern blot analysis of terminal restriction fragment lengths. Nat Protoc. 2010;5:1596–1607. [DOI] [PubMed] [Google Scholar]
- 43.Harrell FE, Lee KL. Verifying assumptions of the Cox proportional hazards model. Proceedings of the Eleventh Annual SAS User’s Group International Conference. Cary, NC: SAS Institute; 1986. p. 823–828. [Google Scholar]
- 44.Wang Q, Zhan Y, Pedersen NL, Fang F, Hagg S. Telomere length and all-cause mortality: a meta-analysis. Ageing Res Rev. 2018;48:11–20. [DOI] [PubMed] [Google Scholar]
- 45.Willeit P, Willeit J, Mayr A, et al. Telomere length and risk of incident cancer and cancer mortality. JAMA. 2010;304:69–75. [DOI] [PubMed] [Google Scholar]
- 46.Lan Q, Cawthon R, Gao Y, et al. Longer telomere length in peripheral white blood cells is associated with risk of lung cancer and the rs2736100 (CLPTM1L-TERT) polymorphism in a prospective cohort study among women in China. PLoS One. 2013;8:e59230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qu S, Wen W, Shu XO, et al. Association of leukocyte telomere length with breast cancer risk: nested case-control findings from the Shanghai Women’s Health Study. Am J Epidemiol. 2013;177:617–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cui Y, Cai Q, Qu S, et al. Association of leukocyte telomere length with colorectal cancer risk: nested case-control findings from the Shanghai Women’s Health Study. Cancer Epidemiol Biomarkers Prev. 2012;21:1807–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zeng H, Wu HC, Wang Q, et al. Telomere length and risk of hepatocellular carcinoma: a nested case-control study in Taiwan cancer screening program cohort. Anticancer Res. 2017;37:637–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aviv A, Anderson JJ, Shay JW. Mutations, cancer and the telomere length paradox. Trends Cancer. 2017;3:253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Walsh KM, Codd V, Smirnov IV, et al. Variants near TERT and TERC influencing telomere length are associated with high-grade glioma risk. Nat Genet. 2014;46:731–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bojesen SE, Pooley KA, Johnatty SE, et al. Multiple independent variants at the TERT locus are associated with telomere length and risks of breast and ovarian cancer. Nat Genet. 2013;45:371–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. [DOI] [PubMed] [Google Scholar]
- 54.Svenson U, Gronlund E, Soderstrom I, Sitaram RT, Ljungberg B, Roos G. Telomere length in relation to immunological parameters in patients with renal cell carcinoma. PLoS One. 2013;8:e55543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Corthay A. Does the immune system naturally protect against cancer? Front Immunol. 2014;5:197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rodier F, Campisi J. Four faces of cellular senescence. J Cell Biol. 2011;192:547–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Drummond-Barbosa D. Stem cells, their niches and the systemic environment: an aging network. Genetics. 2008;180:1787–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Freund A, Orjalo AV, Desprez PY, Campisi J. Inflammatory networks during cellular senescence: causes and consequences. Trends Mol Med. 2010;16:238–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ding Z, Wu CJ, Jaskelioff M, et al. Telomerase reactivation following telomere dysfunction yields murine prostate tumors with bone metastases. Cell. 2012;148:896–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Buseman CM, Wright WE, Shay JW. Is telomerase a viable target in cancer? Mutat Res. 2012;730:90–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Williams GC. Pleiotropy, natural selection, and the evolution of senescence. Evolution. 1957;11:398–411. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset analyzed in the current study is available from the corresponding author on reasonable request.


