Abstract
Background
Tumor‐induced osteomalacia (TIO) belongs to a rare disease of the paraneoplastic syndrome. Phosphate uric mesenchymal tumor (PMT) is the most common cause of TIO, while the possibility of other tumors cannot be excluded.
Case presentation
We present a case of a 36‐year‐old female patient with systemic skeletal abnormalities. The woman complained of low back pain with mild motor dysfunction for 2 years. Laboratory examination showed abnormalities in markers of bone metabolism, parathyroid hormone (PTH), vitamin D and serum phosphorus. Pooled imaging examination indicated extension abnormalities in the skeletal system and a single lesion in the right femoral head. The lesion of the right femoral was imaging with somatostatin receptor‐positive, which was highly suggestive of a single neuroendocrine tumor. CT guided right femoral tumorectomy and bone grafting were performed when medical treatment failed. Postoperative pathological diagnosis was phosphate urinary mesenchymal tumor secreting fibroblast growth factor 23 (FGF23), which accorded with pre‐operative expectations. The postoperative symptoms were effectively relieved, and indicators returned to normal.
Conclusion
The tumors causing TIO exhibited significant heterogeneity in terms of tissue origin, pathological characteristics and biological behavior, but the unique common characteristic is the secretion of FGF23. With significant progress in diagnosis and treatment, the clinical follow‐up of most TIO patients shows a good prognosis, but the prognosis of those with malignant tumors is relatively poor.
Keywords: Etiology, Literature review, Phosphate uric mesenchymal tumor, Treatment, Tumor induced osteomalacia
We presented and analyzed a case of tumor‐induced osteomalacia (TIO) caused by the single phosphate urinary mesenchymal tumor secreting fibroblast growth factor 23 (FGF23) in the right femoral head. The tumors causing TIO exhibited significant heterogeneity and the prognosis of patients with malignant tumors is relatively poor.

Introduction
Osteomalacia is a disease characterized by insufficient or delayed mineralization of osteoid tissue in mature bone tissue. 1 Its etiology is diverse, including old age, vitamin D deficiency caused by insufficient sunlight, intestinal absorption disorders, parathyroid or renal diseases, drugs and poisoning, and certain genetic diseases (such as X‐linked hypophosphatemic rickets, autosomal dominant hypophosphatemic rickets). 1 Among the many causes, the least common is osteomalacia caused by tumor factors, also known as “tumor induced osteomalacia.” 2
Tumor induced osteomalacia (TIO) belongs to a rare disease of the paraneoplastic syndrome (PNS), whose data on prevalence and incidence rate are still lacking. 2 TIO is mostly caused by phosphate uric mesenchymal tumor (PMT), 3 and a small proportion is caused by other tumors. 2 It was clinically characterized with nonspecific symptoms such as bone pain and progressive weakness lasting for months or years. 2 As well as an insufficient understanding of the disease, it is extremely easy to be misdiagnosed or missed clinically. The marked biochemical characteristics include hypophosphatemia caused by renal phosphate consumption, a near normal or significant decrease in 1,25‐dihydroxyvitamin D, and a near normal or elevated level of fibroblast growth factor 23 (FGF23). 3 , 4 FGF23 is considered as a major regulator of phosphate homeostasis on the proximal renal tubules and reducing the expression of the sodium‐phosphate cotransporter (NaPi‐2a and NaPi‐2c), resulting in decreased phosphate reabsorption in the renal tubules. 5 The excessive secretion of FGF23 from tumor cells causes uncontrolled phosphate loss through kidney and decreased bone mineralization, resulting in TIO. 3 , 5 In addition, FGF23 inhibits the expression of 25‐hydroxyvitamin D‐1alpha‐hydroxylase in human proximal tubules, resulting in insufficient 1,25 (OH) 2D levels, thereby reducing intestinal phosphate and calcium absorption. 5
Case Report
History
This case describes an adult female with systemic skeletal abnormalities in detail. A 36‐year‐old woman complained of low back pain with mild motor dysfunction for 2 years. The moderate pain occurs without obvious inducements and is characterized by persistent distension and pain. The lasting pain does not aggravate or alleviate in an active or resting state, and increases at night without radiation pain in both lower limbs. The intensity of pain has gradually increased over the past 2 years, and subcostal pain emerged a month ago. Due to the poor efficacy of physical therapy in local hospitals, the patient received effective measures of symptomatic support treatment from the Pain Department and Endocrinology Department of Wuhan Union Medical College Hospital. Improved evidence supported by further inspection indicated the skeletal system disease during the hospitalizations.
Preoperative Examination
A systematic physical examination was conducted for her and the important signs were as followed below. Multiple sites of thoracic vertebrae were pressed with tenderness or percussion pain, and the thoracic spine is slightly rightward curved. Lasegue's sign and Bragard's sign were negative. The range of motion of the right hip joint is normal. Both Thomas sign and Patrick sign was negative. One superficial lymph node in the neck was slightly enlarged, with a size of about 10mm × 10mm, without tenderness, and with moderate activity. The thyroid gland was not palpable and swollen, and there is no abnormal vascular murmur. No obvious abnormality was found in other systems.
Although the urine phosphorus remained within normal range, the abnormal markers of bone metabolism and PTH (parathyroid hormone) were found, and the serum level of vitamin D and phosphorus was significantly decreased (Table 1).
TABLE 1.
The outcomes of laboratory tests were presented.
| Laboratory indexes | Before surgery | After surgery | Normal range | |||
|---|---|---|---|---|---|---|
| Before medication | After medication | After a week | After 3 months | After 6 months | ||
| Serum PTH | 121 pg/mL | 185.9 pg/ml | NA | NA | NA | 15.0–65.0 pg/mL |
| Serum CrossLap | 54.50 pg/ml | NA | NA | NA | NA | 30–574 pg/ml |
| Serum total 25‐hydroxyvitamin D | 55.10 nmol/L | 53.80 nmol/L | NA | NA | NA | 75–250 nmol/L |
| Serum total PINP | 154.60 ng/ml | NA | NA | NA | NA | 15.1–58.6 ng/ml |
| Serum alkaline phosphatase | 227 U/L | 201 U/L | NA | NA | NA | 40–150 U/L |
| Serum phosphorus | 0.50 mmol/L | 0.46 mmol/L | 1.21 mmol/L | 1.15 mmol/L | 1.2 mmol/L | 0.96–1.62 mmol/L |
| Serum calcium | 2.43 mmol/L | 2.51 mmol/L | 2.31 mmol/L | 2.1 mmol/L | 2.4 mmol/L | 2.25–2.75 mmol/L |
| Urine phosphorus | 18.14 mmol/24 h | 40.36 mmol/24 h | 29.81 mmol/L | NA | NA | 22–48 mmol/24 h |
| Urine calcium | 2.24 mmol/24 h | 1.09 mmol/24 h | 2.39 mmol/24 h | NA | NA | 2.5–7.5 mmol/24 h |
Note: “NA” represents that the data does not exist.
Pooled imaging examination indicated abnormalities in the skeletal system throughout the body. The upper thoracic spine Th3 was with a slightly right lateral curvature of the central cervical thoracic spine (Figure 1). Multiple thoracic and lumbar vertebrae widely presented a double concave sign, and there were multiple compression fractures in the thoracic and lumbar vertebrae.
Fig. 1.

The MRI image of the spine showed: the upper thoracic spine Th3 was with a slightly right lateral curvature of the central cervical thoracic spine; multiple thoracic and lumbar vertebrae widely presented a double concave sign; thoracolumbar spine was with multiple compressibility fractures.
Besides a single lesion in the right femoral head, extensive regions with abnormally active bone metabolism in the rest of the bone system were found through SPECT Whole Body Bone Scanning (Figure 2).
Fig. 2.

The SPECT Whole Body Bone Scanning indicated: the skeletal system was presented extensive regions with abnormally active bone metabolism; a single lesion in the right femoral head was with mild active bone metabolism.
Multiple pathological fractures were found throughout the body, as well as abnormalities in markers of bone metabolism and PTH, vitamin D and serum phosphorus. The above results were highly suggestive of metabolic diseases. However, there were no signs of primary hyperparathyroidism because multiple imaging regions with low density were not typical methoxyisobutylisonitrile (MIBI) positive lesions with the low risk of malignant tumor in the Emission computed tomography (ECT) of parathyroid gland (Figure 3).
Fig. 3.

ECT of parathyroid gland showed multiple imaging regions with low density, but they were not typical methoxyisobutylisonitrile (MIBI) positive lesions.
PET‐CT was conducted further for a comprehensive examination of the whole system, and single lesion of right femoral head, osteoporosis, multiple fractures (including vertebral bodies, bilateral hips and bilateral ribs) and slightly increased metabolism level of bone marrow was found. The possibility of neuroendocrine tumor in other sites or endocrine abnormality cannot be ignored due to the evidence that the performance of the skeletal system was inconsistent with the functional state of the parathyroid gland. Thus, 68Ga‐DOTA‐TATE PET imaging was performed to specific localization of lesions (Figure 4). The lesion of the right femoral was imaging with somatostatin receptor‐positive, which was highly suggestive of a single neuroendocrine tumor.
Fig. 4.

68Ga‐DOTA‐TATE PET indicated: the single lesion of the right femoral was imaging with somatostatin receptor‐positive.
The lesion was manifested in patches of increased density under the articular surface of the right femoral head through preoperative X‐ray and CT scans (Figure 5).
Fig. 5.
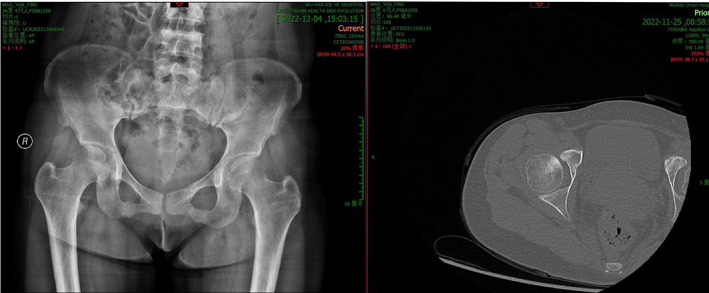
The preoperative X‐ray scan and CT scan showed a patchy increase in density below the articular surface of the right femoral head.
A standardized medication was conducted: oral neutral phosphorus solution, calcium chewable tablets containing vitamin D(D‐Cal) and calcitriol soft capsules (Calcitriol soft capsules) in appropriate doses for a course of 6‐month. Furthermore, desucumab (Prolia) was injected for a course of treatment. However, conservative treatment with drugs has no significant effect.
The preoperative diagnosis was a kind of benign neuroendocrine tumor (with a high likelihood of PMT). A single tumor located under the articular surface of the right femoral head, with a size of about 20mm × 10mm × 10mm, and there were no signs of distant metastasis or lymph node metastasis. Osteomalacia, multiple fractures of the whole body, severe osteoporosis, hyperparathyroidism, hypophosphatemia and Vitamin D deficiency were secondary and caused by the tumor.
Surgery
CT guided right femoral tumorectomy and bone grafting were performed when medical treatment failed, and pathological examination of lesion tissue from surgical resection were conducted simultaneously. After epidural anesthesia, the patient remained in a supine position with CT scans guiding the lesion site. After conventional disinfection, the right anterior curved incision was cut 10 cm layer by layer, and the femoral head was exposed through the anterior approach. A CT scan was performed again to locate the lesion, the surgeon fenestrated anteromedial, and scraped off the lesion tissue. A CT scan was again conducted to confirm complete curettage of the lesion, the surgeon repeatedly rinsed the wound surface, and burned it with an electric knife. Anhydrous alcohol was used to treat the bone surface and repeatedly rinsed the wound surface. Allogeneic bone was implanted and tamped in the bone defect area. After hemostasis and placement of a drainage tube, the operation was successfully completed.
Pathological Examination and Prognosis
Postoperative pathological diagnosis was phosphate urinary mesenchymal tumor which accorded with preoperative expectations, and the tumor cells exhibited staining positive of CD56, SATB2 and FLI‐1(Figure 6).
Fig. 6.

Pathological staining of tumor tissue removed by surgery was accorded with PMT.
The motor function of patient's right hip joint recovered well after surgery, and the physical examination and radiological examination (Figure 7) of the hip joint did not show any significant abnormalities. During the half year follow‐up period, the pain in other sites of the patient was significantly relieved without complications. The general satisfaction of the patient for this treatment was high.
Fig. 7.

Postoperative X‐ray after 3 months did not show any significant abnormalities.
Differential Diagnosis
Currently, due to the lack of unified diagnostic standards, most cases of TIO are exclusive diagnoses. Clinically, It is necessary to distinguish it from the following diseases.
Hereditary Hypophosphatemia
Although the disease often presents with typical symptoms of hypophosphatemia and osteomalacia, it is actually a genetic disease caused by genetic mutations and often has a family history. This case has no family history, and there is a FGF‐23 secretory tumor confirmed by pathological examination.
Primary Hyperparathyroidism
This disease is caused by increased synthesis and secretion of parathyroid hormones caused by primary lesions of the parathyroid gland, such as hyperplasia and tumors, resulting in hypercalcemia and hypophosphatemia. Clinical manifestations include recurrent kidney stones, hematuria, unexplained bone pain, digestive system ulcers, hypertension, and psychiatric neuropathy. The parathyroid gland ECT in this case confirms that there is no abnormality in parathyroid function and it is a secondary change. And most of the clinical symptoms in this case do not match.
Primary Osteoporosis
It is a systemic metabolic bone disease characterized by low bone mass, damage to bone tissue microstructure, increased bone fragility, and susceptibility to fractures. It is divided into postmenopausal osteoporosis, elderly osteoporosis, and idiopathic osteoporosis. The patient is a middle‐aged female with no relevant risk factors or triggers, and normal blood calcium.
Multiple Myeloma
It is a malignant clonal disease of plasma cells, characterized by clonal proliferation of malignant plasma cells in the bone marrow. Clinical manifestations include anemia, osteolytic lesions, widespread osteoporosis, hypercalcemia, bone pain and pathological fractures. This case is characterized by hypophosphatemia, but there are no abnormalities in hemoglobin and serum globulin levels. And most clinical symptoms in this case do not match.
Tarlov Cyst
As a common causes of low back and leg pain, it is the most common in middle‐aged female and often occurs in nerve root cysts within the sacral canal. Clinical symptoms are mostly pain and discomfort in the lumbosacral region and lower limbs, accompanied by mild bowel and bowel dysfunction, perineal numbness, or lower limb claudication. In this case, no cystic lesions were found on the sacrococcygeal MRI, and multiple biochemical examinations were abnormal.
Fanconi Syndrome
It is a clinical syndrome characterized by a group of water electrolyte acid–base metabolic disorders caused by multiple renal tubular dysfunction. Clinically, it is often manifested as renal diabetes, multiple amino acid urine, hypercalciuria, hypophosphatemia, etc. Patients with Fanconi syndrome may exhibit hypophosphatemia, but renal tubular dysfunction is more common. Hereditary Fanconi syndrome often has a positive family history, while acquired Fanconi syndrome has primary diseases or related pathogenic factors. Inquiry of medical history combined with laboratory examination can rule out this disease.
Drug‐induced Hypophosphatemic Osteomalacia
Some anticonvulsants can enhance the reabsorption of phosphate by the kidneys, reducing it by 1α‐ hydroxylase activity, which leads to hypophosphatemia and osteomalacia; Adefovir dipivoxil (ADV) is an important cause of hypophosphatemicosteomalacia in adults. High or long‐term low dose use can increased drug concentration, leading to apoptosis of renal tubular epithelial cells, abnormal renal tubular reabsorption function, and ultimately lead to hypophosphatemic osteomalacia. The patient did not have a history of taking these special medications.
Nutritional Hypophosphatemia
This disease is most commonly caused by vitamin D deficiency in digestive system malabsorption syndrome, such as Crohn's disease, ulcerative colitis, celiac disease, and jejunal ileal bypass obesity, leading to osteomalacia. The patient had no relevant history of these diseases.
Discussion and Literature Review
Case Discussion
In our case, the single phosphate urinary mesenchymal tumor secreting FGF23 in the right femoral head was responsible for osteomalacia which characterized by hypophosphatemia and systemic skeletal changes, resulting in her suffering from the pain over 2 years. The final pathological examinations diagnosed TIO, however, the patient did not show a typical abnormal increase in urine phosphorus before treatment. Two possible explanations may help us understand the contradictory phenomenon. Tumor derived FGF23 secretion may be non‐persistent and exhibit temporal heterogeneity. It is precisely due to the fluctuation of FGF23 levels that there is inconsistency in urinary phosphorus excretion. Due to the lack of detection for FGF23, we cannot verify this point. Furthermore, the negative feedback regulatory mechanism of the body significantly reduces renal phosphate excretion in the presence of long‐term chronic hypophosphatemia.
Patients with TIO typically exhibit non‐specific symptoms such as bone pain, weakness and fractures, causing difficulties in early diagnosis of the disease. The typical biochemical alters with low serum phosphorus content, elevated alkaline phosphatase, low serum 1,25‐dihydroxyvitamin D levels and elevated serum FGF23 levels are almost identical. Once TIO is suspected after other disease factors were excluded, the first step is to determine whether hypophosphatemia exists. In addition, abnormal renal phosphate loss can be determined by calculating the percentage of renal tubular phosphate reabsorption and/or the ratio of renal tubular maximum reabsorption phosphate to glomerular filtration rate. The other laboratory examinations which should be evaluated subsequently included serum calcium, 1,25 (OH) 2D, 25‐OH vitamin D, parathyroid hormone, FGF23, and total or bone specific alkaline phosphatase. In some cases, parathyroid hormone could increase, reflecting secondary hyperparathyroidism caused by low levels of 1,25 (OH) 2D. The serum FGF23 levels should be low in patients with hypophosphatemia, but elevated or close to normal in those with TIO. The sensitive threshold for diagnosing FGF23 induced hypophosphatemia is more than 30 pg/mL of the FGF23 level in patients with hypophosphatemia. 6
Tumor localization is a prominent procedure in diagnosing TIO. It can be quite challenging as tumors are usually small, slow growing, and can occur almost anywhere in the body. 68Ga‐DOTATE PET/CT has the highest sensitivity and specificity for its superiority in higher specificity for the somatostatin receptor SSTR‐2. 7 18FDG PET‐CT may be complementary when scans based on somatostatin imaging do not apply in tumors with a low expression of SSTR‐2. Once suspicious tumor lesions are located through functional imaging, the detections of anatomical imaging should be carried out for more accurate qualitative analysis, including radiography, ultrasound, computed tomography (CT) or magnetic resonance (MR). CT or MR is the preference choice especially for a single lesion, as their high‐resolution imaging helps surgeries determine the subsequent surgical resection plan. Despite the great advancements in diagnostic techniques, some occult tumors cannot be detected even after the use of comprehensive and systematic detection methods. In these cases, patients need to undergo medication treatment under close monitoring as well as the regular imaging examination once a year.
It is integral for all patients to undergo routine medical treatment with phosphate and active vitamin D (calcitriol or alpha calcitriol). In addition, the adjuvant treatment of calcium sensitive receptor agonist Cinacalcet also shows good efficacy, which can significantly reduce the levels of FGF23 and parathyroid hormone and increased reabsorption of phosphate and serum phosphate in renal tubules, resulting in the decreased demand for phosphate and calcitriol supplementation. 8 Complete resection of tumors is currently the standard treatment. However, the alternative treatments have to be provided for patients with tumors that are unsuitable for surgery or unable to be completely removed. Image guided ablation combined with cryoablation or radiofrequency ablation can effectively kill tumor tissue and is the main effective alternative treatment.
Emerging molecular targeted therapies demonstrate great therapeutic potential and can effectively alleviate patients' symptoms. 7 Radiotherapy, chemotherapy, or immunotherapy have been applied as unconventional treatment methods in the treatment of some special malignant tumors that cannot be surgically removed.
Literature Review
A comprehensive literature search was conducted up with the relevant key words via Pubmed and Web of Science from 1959 to 2023. The keywords are: “tumor‐induced osteomalacia” OR “tumor induced osteomalacia.” Over 700 articles were identified through databases searching 63 studies with 123 cases that measured up to the inclusion criteria were included finally. Those included studies were mainly case reports. Additional articles were selected from reference lists of relevant review articles or meta‐analysis articles. Subsequently, etiology and treatment information were summarized with literature review. Although excessive secretion of FGF23 is the common characteristic of these tumors, the histopathological types of them are diverse, which can be divided into two major categories. The first category is the most common phosphate urinary stromal tumors, while the second category is not phosphate urinary stromal tumors which is relatively rare. Most of them are multiple or systemic tumors in soft tissue and bone, a few are limited or solitary tumors occurred in other systems. Therefore, it differs from one another in the specific pathogenesis and treatment.
PMT
PMT, a rare neuroendocrine tumor, is the most common cause of TIO, which is clinically characterized by osteomalacia, phosphonuria, and neoplastic lesions of soft tissue or bone throughout the body. 9 It mainly occurs in adults and rarely occurs in children and its incidence shows no significant difference in gender. 10 Most cases are single and a few cases are multiple. 11 So far, no racial or genetic predisposition has been found. 10 Most PMTs are benign and rarely recur after surgical resection. 11 The incidence of malignant PTMs with distant metastasis is extremely low, and it usually indicated a poor prognosis. 11 The pathological tissue of this tumor is charactered as a highly unusual mix of mildly shaped spindle cells, usually with vascular rich stroma and microcapsular alters. 12 The calcified matrix components with osteoclast‐like giant cells, and metaplastic bone tissue, exhibit characteristics similar to cartilage or bone tissue. 12 The marked biochemical characteristics include hypophosphatemia caused by renal phosphate consumption, a near normal or significant decrease in 1,25‐dihydroxy vitamin D, and a near normal or elevated level of FGF23. 13 , 14
Medical treatment is generally performed with phosphate plus active vitamin D (calcitriol or alpha calcitriol). Most PMTs can be completely cured through tumor resection surgery, and hypophosphatemia and osteomalacia can alleviate subsequently. 9 In cases where the tumor cannot be localized or completely resected, image guided ablation combined with cryoablation or radiofrequency ablation can be used as an alternative treatment. 9 , 13 Small molecule targeted drugs can significantly improve symptoms and inhibit disease progression in unresectable, relatively concealed, or unidentified PMTs, but there is still a lack of evidence regarding their long‐term efficacy and safety. 14 , 15 The anti‐FGF23 monoclonal antibody (burosumab) has been proven to be safe and effective in improving objective indicators and symptoms of TIO. 15 The pan FGFR tyrosine kinase inhibitor BGJ398 has been shown to normalize FGF23 levels and reduce tumor burden in patients with metastatic PMT. 16
Other Tumors
Caution should still be exercised before diagnosing osteomalacia caused by other tumors, unless it is confirmed that they produces FGF23 separately without the coexistence of phosphate mesenchymal tumors. In addition, complete recovery of TIO after surgical resection will contribute to the clinical diagnosis of osteomalacia caused by other types of tumors. Retrospective analysis of literatures found that most of these tumors occurred in vascular, soft tissue and bone, and others originated from the lymphohematopoietic system, nervous system, etc. The causes of TIO include both localized benign lesions and metastatic malignancy. Therefore, these tumors exhibited significant heterogeneity in terms of tissue origin, pathological characteristics and biological behavior, but the unique common characteristic is the secretion of FGF23. Complete resection of the tumors is the most effective treatment. Surgical treatment can effectively cure the disease by cutting off the source. Chemotherapy, radiotherapy, immunotherapy and emerging molecular targeted therapies may be effective for non‐solid tumors that cannot be resected, metastatic malignant tumors that cannot be completely resected as well as tumors that are relatively hidden and temporarily cannot be located. Although these treatments may not completely cure TIO, they can effectively alleviate symptoms or create better conditions for surgical treatment. In addition, the treatment of primary diseases relieved symptoms of patients with TIO, while the long‐term effectiveness was uncertain. For example, patients with hematological tumors complicated with TIO can still benefit from immunotherapy17, 18 Radiotherapy or chemotherapy was applied for the treatment of TIO caused by recurrent or metastatic malignant tumors. 19 , 20 The detail information of the literature review is showed in Table 2.
TABLE 2.
Etiology and treatment information was summarized with literature review.
| Tumor types | Cases | Treatment | Recurrence or metastasis | Reference | ||
|---|---|---|---|---|---|---|
| Vascular tumors | Hemangiopericytoma | 43 | 24 | Surgery | No | 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 |
| Hemangiomas | 8 | Surgery | No | 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 | ||
| Glomangioma | 5 | Surgery | No | 44 , 45 , 46 , 47 , 48 | ||
| Hemangioendothelioma | 2 | Surgery | No | 49 , 50 | ||
| Angiosarcoma | 3 | Surgery; Chemotherapy | Yes | 22 , 51 | ||
| Tumors of bone and soft tissue | Fibro‐osseous lesions | 27 | 1 | Surgery | No | 52 |
| Paget's disease | 1 | Lanreotide treatment | No | 53 | ||
| Nonossifying fibroma | 1 | Surgery | No | 54 | ||
| Ossifying fibroma | 3 | Surgery | No | 55 , 56 , 57 | ||
| Ossifying fibromyxoid tumor | 1 | Surgery; Radiotherapy | Yes | 19 | ||
| Chondroma | 1 | Surgery | No | 58 | ||
| Chondromyxoid fibroma | 1 | Surgery | No | 59 | ||
| Giant cell tumor of tendon sheath | 1 | Surgery | No | 60 | ||
| Giant cell tumor of soft tissue | 2 | Surgery | No | 61 , 62 | ||
| Central giant cell granuloma | 3 | Surgery; Local triamcinolone injection | No | 63 , 64 , 65 | ||
| Giant cell tumor of bone | 4 | Surgery | Partly | 66 , 67 , 68 , 69 | ||
| Fibrous histiocytoma | 1 | Surgery | No | 70 | ||
| Chondrosarcoma | 2 | Surgery | No | 71 , 72 | ||
| Osteosarcoma | 4 | Surgery | No | 66 , 73 , 74 , 75 | ||
| Undifferentiated sarcoma of bone | 1 | Surgery | No | 76 | ||
| Lymphohematopoietic system tumors | Plasmacytoma | 17 | 1 | Surgery; Radiotherapy | Yes | 20 |
| Multiple myeloma | 10 | Radiotherapy; Calcitriol treatment | No | 77 | ||
| B cell non‐Hodgkin's lymphoma | 1 | Immunochemotherapy | No | 17 , 18 | ||
| Natural Killer T‐Cell Lymphoma | 1 | Chemotherapy | No | 78 | ||
| Monoclonal gammopathy of undetermined significance | 2 | Immunochemotherapy;Chemotherapy | No | 18 | ||
| Chronic lymphocytic leukemia | 2 | Immunochemotherapy;Chemotherapy | No | 18 | ||
| Nervous System Tumors | Paraganglioma | 7 | 1 | Surgery | No | 79 |
| Meningiomas | 1 | Burosumab treatment | No | 80 | ||
| Neurofibromatosis | 3 | Surgery | No | 81 , 82 , 83 | ||
| Schwannoma | 2 | Surgery | Partly | 83 | ||
| Lung tumors | Small cell lung cancer | 8 | 8 | Surgery; Chemotherapy | Partly | 84 , 85 , 86 , 87 , 88 , 89 |
| Prostate tumors | Prostate carcinoma | 6 | 6 | Surgery; Chemotherapy; Radiotherapy | Partly | 90 , 91 , 92 , 93 , 94 , 95 |
| Thyroid tumors | Papillary thyroid carcinoma | 2 | 1 | Surgery | No | 96 |
| Anaplastic thyroid carcinoma | 1 | Surgery; Hormone replacement therapy | Yes | 97 | ||
| Kidney tumors | Renal cell carcinoma | 3 | 2 | Surgery | No | 98 , 99 |
| Eosinophilic solid and cystic renal cell carcinoma | 1 | Surgery | No | 100 | ||
| Breast tumors | Breast cancer | 3 | 2 | Surgery; Chemotherapy | Yes | 101 , 102 |
| Fibrocystic nodule of breast | 1 | Surgery | No | 103 | ||
| Ovary tumors | Ovarian teratoma | 2 | 1 | Surgery; Chemotherapy | Yes | 104 |
| Ovarian carcinoma | 1 | Surgery | No | 105 | ||
| Liver tumors | Undifferentiated embryonal sarcoma of liver | 1 | 1 | Surgery; Chemotherapy | No | 106 |
| Colon tumors | Colon adenocarcinoma | 1 | 1 | Surgery | Yes | 107 |
| Bladder tumors | Small cell carcinoma of bladder | 1 | 1 | Surgery | No | 108 |
| Pancreatic tumors | Pancreatic neuroendocrine tumor | 1 | 1 | Somatostatin analogue thereapy | No | 109 |
| Parotid gland tumors | Parotid basal cell adenoma | 1 | 1 | Surgery | No | 110 |
Conclusion
TIO is mostly caused by PMTs, thus caution should be exercised before diagnosing osteomalacia caused by other tumors. The tumors that cause TIO exhibit significant heterogeneity in terms of tissue origin, pathological characteristics and biological behavior, but the unique common characteristic is the secretion of FGF23. Routine phosphate supplementation therapy should be applied to all TIO patients. Complete resection of tumors is currently the standard treatment. Radiotherapy, chemotherapy, or immunotherapy have been applied as unconventional treatment methods in the treatment of some special malignant tumors that cannot be surgically removed. Emerging molecular targeted therapies demonstrate great therapeutic potential and can effectively alleviate patients' symptoms. With significant progress in diagnosis and treatment, the clinical follow‐up of most TIO patients shows a good prognosis, but the prognosis of those with malignant tumors is relatively poor.
Author Contributions
All authors had full access to the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Conceptualization, Zhenhao Zhang and Jiaxin Li; Methodology, Zhenhao Zhang and Jiaxin Li; Investigation, Zhenhao Zhang and Jiaxin Li; Formal Analysis, Zhenhao Zhang; Resources, Zhenhao Zhang and Jiaxin Li; Writing—Original Draft, Zhenhao Zhang and Jiaxin Li; Writing—Review & Editing, Zhenhao Zhang and Jiaxin Li, Zhicai Zhang, ZengwuShao; Visualization, Zhicai Zhang; Supervision, Zengwu Shao.
Conflict of Interest Statement
All the authors declared that they had no conflict of interest in this case report.
Ethical Statement
This study was authorized and approved by the Medical Ethics Committee of Union Hospital Affiliated to Tongji Medical College, Huazhong University of Science and Technology (Application number: [2020] IEC‐J (102)). The patient's medical history information and design comply with the principles of the Helsinki Declaration, fully respecting the informed consent rights of the patient and their families. Written informed consent has been obtained from the patient to publish this paper.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (Grant No. 82072979). We would like to express our gratitude to all those who helped us during writing of this paper. This research received no external funding.
Zhenhao Zhang and Jiaxin Li have contributed equally to this work.
Contributor Information
Zhicai Zhang, Email: zhicaizhang@126.com.
Zengwu Shao, Email: 1985xh0536@hust.edu.cn.
References
- 1. Cianferotti L. Osteomalacia is not a single disease. Int J Mol Sci. 2022;23(23):14896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Annamalai AK, Shinto A, Aram S, Prabhu VA, Santhi R, Gopalakrishnan C, et al. Tumor‐induced osteomalacia. Kidney Int. 2022;102(5):1194. [DOI] [PubMed] [Google Scholar]
- 3. Minisola S, Peacock M, Fukumoto S, Cipriani C, Pepe J, Tella SH, et al. Tumour‐induced osteomalacia. Nat Rev Dis Primers. 2017;3:17044. [DOI] [PubMed] [Google Scholar]
- 4. Folpe AL, Fanburg‐Smith JC, Billings SD, Bisceglia M, Bertoni F, Cho JY, et al. Most osteomalacia‐associated mesenchymal tumors are a single histopathologic entity: an analysis of 32 cases and a comprehensive review of the literature. Am J Surg Pathol. 2004;28(1):1–30. [DOI] [PubMed] [Google Scholar]
- 5. Shimada T, Hasegawa H, Yamazaki Y, Muto T, Hino R, Takeuchi Y, et al. FGF‐23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res. 2004;19(3):429–435. [DOI] [PubMed] [Google Scholar]
- 6. Endo I, Fukumoto S, Ozono K, Namba N, Tanaka H, Inoue D, et al. Clinical usefulness of measurement of fibroblast growth factor 23 (FGF23) in hypophosphatemic patients: proposal of diagnostic criteria using FGF23 measurement. Bone. 2008;42(6):1235–1239. [DOI] [PubMed] [Google Scholar]
- 7. Jan de Beur SM, Miller PD, Weber TJ, Peacock M, Insogna K, Kumar R, et al. Burosumab for the treatment of tumor‐induced Osteomalacia. J Bone Miner Res. 2021;36(4):627–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Geller JL, Khosravi A, Kelly MH, Riminucci M, Adams JS, Collins MT. Cinacalcet in the management of tumor‐induced osteomalacia. J Bone Miner Res. 2007;22(6):931–937. [DOI] [PubMed] [Google Scholar]
- 9. Benson JC, Trejo‐Lopez JA, Nassiri AM, Eschbacher K, Link MJ, Driscoll CL, et al. Phosphaturic mesenchymal tumor. Am J Neuroradiol. 2022;43(6):817–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jiang Y, Xia W‐b, Xing X‐p, et al. Tumor‐induced osteomalacia: an important cause of adult‐onset hypophosphatemicosteomalacia in China: report of 39 cases and review of the literature. J Bone Miner Res. 2012;27(9):1967–1975. [DOI] [PubMed] [Google Scholar]
- 11. Sidell D, Lai C, Bhuta S, Barnes L, Chhetri DK. Malignant phosphaturic mesenchymal tumor of the larynx. Laryngoscope. 2011;121(9):1860–1863. [DOI] [PubMed] [Google Scholar]
- 12. Folpe AL. Phosphaturic mesenchymal tumors: a review and update. Semin Diagn Pathol. 2019;36(4):260–268. [DOI] [PubMed] [Google Scholar]
- 13. Barrera CA, Karp J, Kearns C, Rhodes NG. Phosphaturic mesenchymal tumor. Radiographics. 2023;43(1):e220185. [DOI] [PubMed] [Google Scholar]
- 14. Zhang Z, Jing D, Xuan B, Zhang Z, Wu W, Shao Z. Cellular senescence‐driven transcriptional reprogramming of the MAFB/NOTCH3 axis activates the PI3K/AKT pathway and promotes osteosarcoma progression. Genes Dis. 2023;11(2):952–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jan de Beur SM, Minisola S, Xia W‐B, Abrahamsen B, Body JJ, Brandi ML, et al. Global guidance for the recognition, diagnosis, and management of tumor‐induced osteomalacia. J Intern Med. 2023;293(3):309–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hartley IR, Roszko KL, Li X, Pozo K, Streit J, del Rivero J, et al. Infigratinib reduces fibroblast growth factor 23 (FGF23) and increases blood phosphate in tumor‐induced Osteomalacia. JBMR Plus. 2022;6(8):e10661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Elderman JH, Wabbijn M, de Jongh F. Hypophosphataemia due to FGF‐23 producing B cell non‐Hodgkin's lymphoma. BMJ Case Rep. 2016;2016:bcr2015213954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stewart I, Roddie C, Gill A, Clarkson A, Mirams M, Coyle L, et al. Elevated serum FGF23 concentrations in plasma cell dyscrasias. Bone. 2006;39(2):369–376. [DOI] [PubMed] [Google Scholar]
- 19. Massaccesi M, Miccichè F, Rigante M, Petrone G, Lepre E, Gambacorta MA, et al. Successful treatment of tumor‐induced Osteomalacia by multidisciplinary therapy with radiation to intracranial fibromyxoid tumor. Case Rep Endocrinol. 2021;2021:8841259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chua SC, O'Connor SR, Wong WL, Ganatra RH. Case report: solitary plasmacytoma of bone with oncogenic osteomalacia: recurrence of tumour confirmed by PET/CT. A case report with a review of the radiological literature. Br J Radiol. 2008;81(964):e110–e114. [DOI] [PubMed] [Google Scholar]
- 21. Gitelis S, Ryan WG, Rosenberg AG, Templeton AC. Adult‐onset hypophosphatemicosteomalacia secondary to neoplasm. A case report and review of the pathophysiology. J Bone Jt Surg Am. 1986;68(1):134–138. [PubMed] [Google Scholar]
- 22. Linovitz RJ, Resnick D, Keissling P, Kondon JJ, Sehler B, Nejdl RJ, et al. Tumor‐induced osteomalacia and rickets: a surgically curable syndrome. Report of two cases. J Bone Jt Surg Am. 1976;58(3):419–423. [PubMed] [Google Scholar]
- 23. Miyauchi A, Fukase M, Tsutsumi M, Fujita T. Hemangiopericytoma‐induced osteomalacia: tumor transplantation in nude mice causes hypophosphatemia and tumor extracts inhibit renal 25‐hydroxyvitamin D 1‐hydroxylase activity. J Clin Endocrinol Metab. 1988;67(1):46–53. [DOI] [PubMed] [Google Scholar]
- 24. Seshadri MS, Cornish CJ, Mason RS, Posen S. Parathyroid hormone‐like bioactivity in tumours from patients with oncogenic osteomalacia. Clin Endocrinol. 1985;23(6):689–697. [DOI] [PubMed] [Google Scholar]
- 25. Sweet RA, Males JL, Hamstra AJ, DeLuca HF. Vitamin D metabolite levels in oncogenic osteomalacia. Ann Intern Med. 1980;93(2):279–280. [DOI] [PubMed] [Google Scholar]
- 26. Brociek‐Piłczyńska A, Brodowska‐Kania D, Szczygielski K, et al. A rare combination of tumor‐induced osteomalacia caused by sinonasalglomangiopericytoma and coexisting parathyroid adenoma: case report and literature review. BMC Endocr Disord. 2022;22(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dissanayake AM, Wilson JL, Holdaway IM, Reid IR. Oncogenic osteomalacia: culprit tumour detection whole body magnetic resonance imaging. Intern Med J. 2003;33(12):615–616. [DOI] [PubMed] [Google Scholar]
- 28. Shah R, Lila AR, Jadhav R‐S, Patil V, Mahajan A, Sonawane S, et al. Tumor induced osteomalacia in head and neck region: single center experience and systematic review. Endocr Connect. 2019;8(10):1330–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Catalano PJ, Brandwein M, Shah DK, Urken ML, Lawson W, Biller HF. Sinonasal hemangiopericytomas: a clinicopathologic and immunohistochemical study of seven cases. Head Neck. 1996;18(1):42–53. [DOI] [PubMed] [Google Scholar]
- 30. Sakamoto A, Oda Y, Nagayoshi Y, Iwakiri K, Tamiya S, Iwamoto Y, et al. Glomangiopericytoma causing oncogenic osteomalacia. A case report with immunohistochemical analysis. Arch Orthop Trauma Surg. 2001;121(1–2):104–108. [DOI] [PubMed] [Google Scholar]
- 31. Long Y, Shao F, Lan X. Mediastinal epithelioid hemangioendothelioma revealed on 68Ga‐DOTATATE PET/CT. Clin Nucl Med. 2020;45(5):414–416. [DOI] [PubMed] [Google Scholar]
- 32. Baronofsky SI, Kalbhen CL, Demos TC, Sizemore GW. Oncogenic osteomalacia secondary to a hemangiopericytoma of the hip: case report. Can Assoc Radiol J. 1999;50(1):26–28. [PubMed] [Google Scholar]
- 33. Peters KB, McLendon R, Morse MA, Vredenburgh JJ. Treatment of recurrent intracranial hemangiopericytoma with SRC‐related tyrosine kinase targeted therapy: a case report. Case Rep Oncol. 2010;3(1):93–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Brandwein‐Gensler M, Siegal GP. Striking pathology gold: a singular experience with daily reverberations: sinonasal hemangiopericytoma (glomangiopericytoma) and oncogenic osteomalacia. Head Neck Pathol. 2012;6(1):64–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Auethavekiat P, Roberts JR, Biega TJ, Toney MO, Christensen RS, Belnap CM, et al. Case 3. Oncogenic osteomalacia associated with hemangiopericytoma localized by octreotide scan. J Clin Oncol. 2005;23(15):3626–3628. [DOI] [PubMed] [Google Scholar]
- 36. Agus ZS. Oncogenic hypophosphatemicosteomalacia. Kidney Int. 1983;24(1):113–123. [DOI] [PubMed] [Google Scholar]
- 37. Daniels RA, Weisenfeld I. Tumorous phosphaturicosteomalacia. Report of a case associated with multiple hemangiomas of bone. Am J Med. 1979;67(1):155–159. [DOI] [PubMed] [Google Scholar]
- 38. Salassa RM, Jowsey J, Arnaud CD. Hypophosphatemicosteomalacia associated with “nonendocrine” tumors. N Engl J Med. 1970;283(2):65–70. [DOI] [PubMed] [Google Scholar]
- 39. Turner ML, Dalinka MK. Osteomalacia: uncommon causes. Am J Roentgenol. 1979;133(3):539–540. [DOI] [PubMed] [Google Scholar]
- 40. Yoshikawa S, Kawabata M, Hatsuyama Y, Hosokawa O, Fujita T. A typical vitamin‐D resistant osteomalacia. Report of a case. J Bone Jt Surg Am. 1964;46:46–1007. [PubMed] [Google Scholar]
- 41. Sahnoune I, Tazi‐Mezalek Z, Essaadouni L, Harmouche H, Ismael F, Adnaoui M, et al. Oncogenic osteomalacia in a patient with hemangioma: a clinical diagnosis. Jt Bone Spine. 2006;73(1):115–118. [DOI] [PubMed] [Google Scholar]
- 42. Müssig K, Oksüz MO, Pfannenberg C, et al. Somatostatin receptor expression in an epitheloid hemangioma causing oncogenic osteomalacia. J Clin Endocrinol Metab. 2009;94(11):4123–4124. [DOI] [PubMed] [Google Scholar]
- 43. Vadi SK, Mittal BR, Parihar AS, Kumar R, Singh H, Singh G. 68Ga‐DOTANOC PET/CT in an Atypical Extraskeletal paravertebral hemangioma mimicking as neurogenic tumor in a known case of breast cancer. Clin Nucl Med. 2019;44(5):e364–e366. [DOI] [PubMed] [Google Scholar]
- 44. Muniz CR, Bezerra GAM, da Silva VC, Aguiar PMF, Gerson G, D'Alva CB, et al. Ethmoid glomangioma and oncogenic osteomalacia: a case report. J Med Case Reports. 2021;15(1):348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gökyer A, Sayın S, Küçükarda A, Çelik M, Güldiken S, Çiçin İ. Nasal hemangiopericytoma presenting with oncogenic osteomalasia: a case report and literature review. Curr Probl Cancer. 2021;45(3):100704. [DOI] [PubMed] [Google Scholar]
- 46. Barai R, Tsang T, Cespedes L. Tumour‐induced osteomalacia due to residual benign glomangioma. BMJ Case Rep. 2022;15(11):e250237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gresham MS, Shen S, Zhang YJ, Gallagher K. Anterior Skull Base glomangioma‐induced Osteomalacia. J Neurol Surg Rep. 2017;78(1):e9–e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wang T, Qu S, Ye L. Sinonasal glomus tumour‐induced osteomalacia: a case report. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2014;49(8):691–692. [PubMed] [Google Scholar]
- 49. Nuovo MA, Dorfman HD, Sun CC, Chalew SA. Tumor‐induced osteomalacia and rickets. Am J Surg Pathol. 1989;13(7):588–599. [DOI] [PubMed] [Google Scholar]
- 50. Savage CR, Zimmer LA. Oncogenic osteomalacia from pterygopalatine fossa mass. J Laryngol Otol. 2009;123(9):1052–1054. [DOI] [PubMed] [Google Scholar]
- 51. Sparagana M. Tumor‐induced osteomalacia: long‐term follow‐up of two patients cured by removal of their tumors. J Surg Oncol. 1987;36(3):198–205. [DOI] [PubMed] [Google Scholar]
- 52. Emodi O, Rachmiel A, Tiosano D, Nagler RM. Maxillary tumour‐induced osteomalacia. Int J Oral Maxillofac Surg. 2018;47(10):1295–1298. [DOI] [PubMed] [Google Scholar]
- 53. Vandemergel X, Blocklet D, Decaux G. Positive octreotide scintigraphy and determination of lanreotide activity in Paget's disease of bone associated with phosphate diabetes: a case report. Ann Endocrinol. 2004;65(3):201–204. [DOI] [PubMed] [Google Scholar]
- 54. Asnes RS, Berdon WE, Bassett CA. Hypophosphatemic rickets in an adolescent cured by excision of a nonossifying fibroma. Clin Pediatr. 1981;20(10):646–648. [DOI] [PubMed] [Google Scholar]
- 55. Thi HN, Manh CP, Tuan LT, Le Thi LA, Thanh NN, Vilaiyuk S. Tumor‐induced Osteomalacia associated with a maxillary tumor in children: a case report and review of the literature. J Clin Res Pediatr Endocrinol. 2022;10:4274. [Google Scholar]
- 56. Nomura G, Koshino Y, Morimoto H, Kida H, Nomura S, Tamai K. Vitamin D resistant hypophosphatemicosteomalacia associated with osteosarcoma of the mandible: report of a case. Jpn J Med. 1982;21(1):35–39. [DOI] [PubMed] [Google Scholar]
- 57. Sandoval MAS, Palermo MA, Carrillo R, Bundoc R, Carnate JM, Galsim RJ. Successful treatment of tumour‐induced osteomalacia after resection of an oral peripheral ossifying fibroma. BMJ Case Rep. 2017;2017:bcr2016218637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Shane E, Parisien M, Henderson JE, Dempster DW, Feldman F, Hardy MA, et al. Tumor‐induced osteomalacia: clinical and basic studies. J Bone Miner Res. 1997;12(9):1502–1511. [DOI] [PubMed] [Google Scholar]
- 59. Park JM, Woo YK, Kang MI, Kang CS, Hahn ST. Oncogenic osteomalacia associated with soft tissue chondromyxoid fibroma. Eur J Radiol. 2001;39(2):69–72. [DOI] [PubMed] [Google Scholar]
- 60. Jia C, Shao F, Yang M, Qin C, Lan X. Giant cell tumor of tendon sheath revealed on 68Ga‐DOTA‐TATE PET/CT in a patient with suspicious tumor‐induced Osteomalacia. Clin Nucl Med. 2019;44(6):496–498. [DOI] [PubMed] [Google Scholar]
- 61. Westerberg P‐A, Olauson H, Toss G, et al. Preoperative tumor localization by means of venous sampling for fibroblast growth factor‐23 in a patient with tumor‐induced osteomalacia. Endocr Pract. 2008;14(3):362–367. [DOI] [PubMed] [Google Scholar]
- 62. Battoo AJ, Salih S, Unnikrishnan AG, Jojo A, Bahadur S, Iyer S, et al. Oncogenic osteomalacia from nasal cavity giant cell tumor. Head Neck. 2012;34(3):454–457. [DOI] [PubMed] [Google Scholar]
- 63. Fernández‐Cooke E, Cruz‐Rojo J, Gallego C, Romance AI, Mosqueda‐Peña R, Almaden Y, et al. Tumor‐induced rickets in a child with a central giant cell granuloma: a case report. Pediatrics. 2015;135(6):e1518–e1523. [DOI] [PubMed] [Google Scholar]
- 64. Crossen SS, Zambrano E, Newman B, Bernstein JA, Messner AH, Bachrach LK, et al. Tumor‐induced Osteomalacia in a 3‐year‐old with unresectable central Giant cell lesions. J Pediatr Hematol Oncol. 2017;39(1):e21–e24. [DOI] [PubMed] [Google Scholar]
- 65. Kim YG, Choi YS, Lee SC, Ryu DM. Tumor‐induced osteomalacia associated with lesions in the oral and maxillofacial region: report of two cases. J Oral Maxillofac Surg. 1996;54(11):1352–1357. [DOI] [PubMed] [Google Scholar]
- 66. Gou M, Ma Z. Osteomalacia, renal Fanconi syndrome, and bone tumor. J Int Med Res. 2018;46(8):3487–3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Nitzan DW, Marmary Y, Azaz B. Mandibular tumor‐induced muscular weakness and osteomalacia. Oral Surg Oral Med Oral Pathol. 1981;52(3):253–256. [DOI] [PubMed] [Google Scholar]
- 68. Drezner MK, Feinglos MN. Osteomalacia due to 1α, 25‐dihydroxycholecalciferol deficiency. Association with a giant cell tumor of bone. J Clin Invest. 1977;60(5):1046–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sehli H, Daoud L, Ben Mbarek R, Ghorbel R, Ben Abdelghani K, Charfi H, et al. Osteomalacia and giant cell tumor: a rare case. Tunis Med. 2008;86(9):836–838. [PubMed] [Google Scholar]
- 70. Leow MKS, Dogra S, Ge X, Chuah KL, Liew H, Loke KSH, et al. Paraneoplastic secretion of multiple PhosphatoninsFrom a deep fibrous histiocytoma causing oncogenic Osteomalacia. J Clin Endocrinol Metab. 2021;106(5):e2299–e2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Stone E, Bernier V, Rabinovich S, From GL. Oncogenic osteomalacia associated with a mesenchymal chondrosarcoma. Clin Invest Med. 1984;7(3):179–185. [PubMed] [Google Scholar]
- 72. Zura RD, Minasi JS, Kahler DM. Tumor‐induced osteomalacia and symptomatic looser zones secondary to mesenchymal chondrosarcoma. J Surg Oncol. 1999;71(1):58–62. [DOI] [PubMed] [Google Scholar]
- 73. Boriani S, Campanacci M. Osteoblastoma associated with osteomalacia (presentation of a case and review of the literature). Ital J Orthop Traumatol. 1978;4(3):379–382. [PubMed] [Google Scholar]
- 74. Case records of the Massachusetts General Hospital . Weekly clinicopathological exercises. Case 29–2001. A 14‐year‐old boy with abnormal bones and a sacral mass. N Engl J Med. 2001;345(12):903–908. [DOI] [PubMed] [Google Scholar]
- 75. Hasegawa T, Shimoda T, Yokoyama R, Beppu Y, Hirohashi S, Maeda S. Intracortical osteoblastic osteosarcoma with oncogenic rickets. Skeletal Radiol. 1999;28(1):41–45. [DOI] [PubMed] [Google Scholar]
- 76. Wyman AL, Paradinas FJ, Daly JR. Hypophosphataemicosteomalacia associated with a malignant tumour of the tibia: report of a case. J Clin Pathol. 1977;30(4):328–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Narvaez J, Domingo‐Domenech E, Narvaez JA, Nolla JM, Valverde J. Acquired hypophosphatemicosteomalacia associated with multiple myeloma. Jt Bone Spine. 2005;72(5):424–426. [DOI] [PubMed] [Google Scholar]
- 78. Zheng G, Kanduri SR, Canterbury JP, Nguyen T, Velez JCQ. A case of hypophosphatemia due to oncogenic Osteomalacia in a patient with natural killer T‐cell lymphoma. Kidney Blood Press Res. 2021;46(5):647–651. [DOI] [PubMed] [Google Scholar]
- 79. Wilkins GE, Granleese S, Hegele RG, Holden J, Anderson DW, Bondy GP. Oncogenic osteomalacia: evidence for a humoral phosphaturic factor. J Clin Endocrinol Metab. 1995;80(5):1628–1634. [DOI] [PubMed] [Google Scholar]
- 80. Day AL, Gutiérrez OM, Guthrie BL, Saag KG. Burosumab in tumor‐induced osteomalacia: a case report. Joint Bone Spine. 2020;87(1):81–83. [DOI] [PubMed] [Google Scholar]
- 81. Chadha M, Singh AP, Singh AP. Hypophosphataemicosteomalacia in neurofibromatosis. Acta Orthop Belg. 2009;75(6):847–850. [PubMed] [Google Scholar]
- 82. Haviv YS, Silver J. Late onset oncogenic osteomalacia‐associated with neurofibromatosis type II. Clin Nephrol. 2000;54(5):429–430. [PubMed] [Google Scholar]
- 83. Obo T, Koriyama N, Tokito A, Ogiso K, Nishio Y. Neurofibromatosis type 1 associated with hypophosphatemicosteomalacia due to hypersecretion of fibroblast growth factor 23: a case report. J Med Case Reports. 2020;14(1):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Sauder A, Wiernek S, Dai X, Pereira R, Yudd M, Patel C, et al. FGF23‐associated tumor‐induced Osteomalacia in a patient with small cell carcinoma: a case report and regulatory mechanism study. Int J Surg Pathol. 2016;24(2):116–120. [DOI] [PubMed] [Google Scholar]
- 85. Orr LE. Fanconi syndrome and oat cell carcinoma of the lung. West J Med. 1980;133(3):250–251. [PMC free article] [PubMed] [Google Scholar]
- 86. Laroche M, Arlet P, Ader JL, Durand D, Arlet J, Mazieres B. Phosphate diabetes associated with bone metastases of oat cell lung cancer. J Rheumatol. 1991;18(1):106–109. [PubMed] [Google Scholar]
- 87. Taylor HC, Fallon MD, Velasco ME. Oncogenic osteomalacia and inappropriate antidiuretic hormone secretion due to oat‐cell carcinoma. Ann Intern Med. 1984;101(6):786–788. [DOI] [PubMed] [Google Scholar]
- 88. Robin N, Gill G, van Heyningen C, Fraser W. A small cell bronchogenic carcinoma associated with tumoral hypophosphataemia and inappropriate antidiuresis. Postgrad Med J. 1994;70(828):746–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Tantisattamo E, Ng RCK. Dual paraneoplastic syndromes: small cell lung carcinoma‐related oncogenic osteomalacia, and syndrome of inappropriate antidiuretic hormone secretion: report of a case and review of the literature. Hawaii Med J. 2011;70(7):139–143. [PMC free article] [PubMed] [Google Scholar]
- 90. McMurtry CT, Godschalk M, Malluche HH, Geng Z, Adler RA. Oncogenic osteomalacia associated with metastatic prostate carcinoma: case report and review of the literature. J Am Geriatr Soc. 1993;41(9):983–985. [DOI] [PubMed] [Google Scholar]
- 91. Reese DM, Rosen PJ. Oncogenic osteomalacia associated with prostate cancer. J Urol. 1997;158(31):887. [DOI] [PubMed] [Google Scholar]
- 92. Mak MP, da Costa e Silva VT, Martin RM, et al. Advanced prostate cancer as a cause of oncogenic osteomalacia: an underdiagnosed condition. Support Care Cancer. 2012;20(9):2195–2197. [DOI] [PubMed] [Google Scholar]
- 93. Nakahama H, Nakanishi T, Uno H, Takaoka T, Taji N, Uyama O, et al. Prostate cancer‐induced oncogenic hypophosphatemicosteomalacia. Urol Int. 1995;55(1):38–40. [DOI] [PubMed] [Google Scholar]
- 94. Ramon I, Kleynen P, Valsamis J, Body J‐J, Karmali R. Hypophosphatemia related to paraneoplastic Cushing syndrome in prostate cancer: cure after bilateral adrenalectomy. Calcif Tissue Int. 2011;89(6):442–445. [DOI] [PubMed] [Google Scholar]
- 95. Layman AAK, Joshi S, Shah S. Metastatic prostate cancer presenting as tumour‐induced osteomalacia. BMJ Case Rep. 2019;12(7):e229434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Robertson A, Mansberg R, Mansberg V, Van der Wall H, Hooper M. Tumor‐induced osteomalacia: a case of diagnostic dilemma. Clin Nucl Med. 2007;32(8):631–634. [DOI] [PubMed] [Google Scholar]
- 97. Abate EG, Bernet V, Cortese C, Garner HW. Tumor induced osteomalacia secondary to anaplastic thyroid carcinoma: a case report and review of the literature. Bone Rep. 2016;5:81–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Xie Y, Li H‐z. Oncogenic osteomalacia caused by renal cell carcinoma. J Clin Endocrinol Metab. 2013;98(12):4597–4598. [DOI] [PubMed] [Google Scholar]
- 99. Jin X, Jing H, Li F, Zhuang H. Osteomalacia‐inducing renal clear cell carcinoma uncovered by 99mTc‐Hydrazinonicotinyl‐Tyr3‐octreotide (99mTc‐HYNIC‐TOC) scintigraphy. Clin Nucl Med. 2013;38(11):922–924. [DOI] [PubMed] [Google Scholar]
- 100. Niu Y, Li DM, Liu PP, Zhang HL, Zhong DR. Eosinophilic solid and cystic renal cell carcinoma with tumor‐induced osteomalacia: report of a case. Zhonghua Bing Li Xue Za Zhi. 2021;50(7):829–831. [DOI] [PubMed] [Google Scholar]
- 101. Savva C, Adhikaree J, Madhusudan S, Chokkalingam K. Oncogenic osteomalacia and metastatic breast cancer: a case report and review of the literature. J Diabetes Metab Disord. 2019;18(1):267–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Bhasin B, Velez JCQ. Persistent urinary phosphate wasting in a patient with metastatic breast cancer: What's your diagnosis? Clin Nephrol. 2021;95(2):99–103. [DOI] [PubMed] [Google Scholar]
- 103. Gascón A, Cobeta‐Garcia JC, Iglesias E, Lázaro JM, Muniesa JA. Oncogenic osteomalacia in a patient with a fibrocystic nodule of the breast. Nephrol Dial Transplant. 1999;14(6):1561–1563. [DOI] [PubMed] [Google Scholar]
- 104. Lin H‐A, Shih S‐R, Tseng Y‐T, Chen CH, Chiu WY, Hsu CY, et al. Ovarian cancer‐related Hypophosphatemic Osteomalacia—a case report. J Clin Endocrinol Metab. 2014;99(12):4403–4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Paul J, Jose A, Kingsbury B, Manipadam MT, Kapoor N, Paul TV, et al. Ovarian teratoma causing oncogenic osteomalacia: an instance of serendipity. J Obstet Gynaecol India. 2022;72(4):353–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Olivas‐Mazón R, Martín‐Cazaña M, Pérez‐Mohand P, Garzón‐Lorenzo L, Espino‐Hernández M, Baro‐Fernández M, et al. Tumor‐induced osteomalacia in an adolescent with an undifferentiated embryonal sarcoma of the liver. Pediatr Blood Cancer. 2020;67(7):e28386. [DOI] [PubMed] [Google Scholar]
- 107. Leaf DE, Pereira RC, Bazari H, Jüppner H. Oncogenic osteomalacia due to FGF23‐expressing colon adenocarcinoma. J Clin Endocrinol Metab. 2013;98(3):887–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Cramer SF, Aikawa M, Cebelin M. Neurosecretory granules in small cell invasive carcinoma of the urinary bladder. Cancer. 1981;47(4):724–730. [DOI] [PubMed] [Google Scholar]
- 109. Pickering ME, Bouvier D, Puravet A, Soubrier M, Sapin V, Oris C. Hypophosphatemia related to a neuro‐endocrine tumor of the pancreas: a case report. Clin Biochem. 2022;104:62–65. [DOI] [PubMed] [Google Scholar]
- 110. He Q, Xu Z, Zhang B, Hu W, Zhang X. Tumor‐induced osteomalacia caused by a parotid basal cell adenoma detected by 68Ga‐DOTANOC PET/CT. Clin Nucl Med. 2018;43(6):e198–e199. [DOI] [PubMed] [Google Scholar]


