Abstract
Introduction
Coronary artery disease (CAD) often leads to myocardial ischemia and impaired cardiac function, significantly impacting the well-being and quality of life (QOL) of individuals. The use of drug-coated balloon (DCB) treatment has become a widespread approach in CAD management. However, currently, there is limited evidence available for the meta-analysis of DCB treatment in CAD.
Materials and methods
A systematic search was conducted across databases including PubMed, Cochrane Library, China National Knowledge Infrastructure (CNKI), Wanfang Database, and VIP Database, covering data from the inception of each database up to April 2023. Randomized controlled trials (RCTs) regarding DCB treatment were meticulously chosen based on independent assessment of eligibility and scope by three researchers. Literature screening and data extraction were independently performed by two researchers, while methodological quality of the enrolled studies was assessed using the risk of bias (ROB) tool developed by the Cochrane Collaboration. Meta-analysis was conducted using RevMan 5.3.
Results
Following the screening process, seven studies were included. Four studies demonstrated an odds ratio (OR) of 0.66 for target lesion revascularization (TLR), five reported an OR of 0.41 for postoperative myocardial infarction (MI), four indicated a mean difference (MD) of 6.03 in the degree of stenosis (DOS), five exhibited an MD of 0.13 for late lumen loss (LLL), five reported an OR of 0.33 for cardiac death, and two presented an OR of 1.01 for binary restenosis (BR).
Conclusion
DCB demonstrated a comparable efficacy to drug-eluting stents (DES) in treating CAD, with relatively lower associated risks.
Keywords: Drug-eluting balloon, Drug-eluting stents, Coronary artery disease, Meta-analysis
1. Introduction
With the acceleration of modern lifestyle and the increase in social pressure, people commonly face risk factors for the high prevalence of cardiovascular diseases, such as sedentary behavior, lack of physical activity, excessive intake of highly processed foods, and imbalanced nutrition. These unhealthy habits and dietary behaviors are also contributing factors to the occurrence of contemporary coronary artery disease (CAD), which has become one of the leading causes of death worldwide. Percutaneous coronary intervention (PCI) currently is the primary and effective clinical approach for managing CAD. Through catheter-based methods aimed at alleviating coronary artery stenosis and restore blood flow in occluded vessels, PCI can rapidly improve myocardial perfusion [1]. With the continuous advancement of medical technology, percutaneous coronary intervention techniques have rapidly evolved. Previously, common balloon angioplasty (CBA) faced limitations such as acute vessel closure, vessel elastic recoil, and coronary dissection, thus yielding unfavorable outcomes [2]. The introduction of stents has partially overcome the limitations of CBA. However, bare metal stents (BMS) were associated with excessive neointimal hyperplasia and high rates of restenosis, which stimulated the development of drug-eluting stents (DES) [3]. Nonetheless, DES still poses potential complications such as delayed endothelial healing, stent thrombosis, and late or very late stent malapposition, in which contribute to restenosis [4]. Furthermore, DES may not be highly effective in treating complex coronary lesions, indicating that it may not be the optimal choice for certain types of coronary artery disease, such as small vessel disease (reference vessel diameter ≤2.75 mm). Although DES reduces the rate of in-stent restenosis (ISR) compared to BMS, it remains susceptible to restenosis-related complications, thereby posing potential risks for postoperative patients.
The introduction of drug-coated balloon angioplasty (DCBA) has led to a decreased risk of thrombosis formation due to its shorter inflammatory response time and absence of residual metal stents [5,6]. Furthermore, DCBA has the ability to improve primary patency in target lesions [7]. Notably, DCBA avoids the permanent implantation of foreign materials into the blood vessels, thus preventing potential risks associated with delayed endothelial healing, stent thrombosis, and late or very late stent malapposition seen with DES. DCBA involves coating a conventional balloon with a thin layer of highly concentrated pharmacologically active anti-proliferative drug. During balloon inflation, this drug is transferred to the intimal layer of the vessel wall, facilitating rapid delivery of lipophilic medication and inhibiting smooth muscle cell proliferation [8,9]. The treatment duration for DCBA is relatively short, typically ranging from 30 to 60 s during coronary intervention [10]. This non-stent local drug delivery method preserves the anti-proliferative properties of DES while avoiding challenges like subacute thrombosis, stent fracture, and stent malapposition [11]. In line with the guidelines established by the European Society of Cardiology, clinical investigations exploring the application of DCBA in the treatment of ISR have shown promising outcomes, leading to the categorization of DCBA as a Class Ia indication for ISR treatment. However, the application of DCBA in small vessel disease (SVD) remains a subject of debate.
Currently, a notable deficiency persists in terms of large-scale randomized trials evaluating the efficacy of DCB compared to DES. Individual studies have their limitations. However, the principle of meta-analysis is to systematically collect and summarize literature that meets the same inclusion criteria and has similar baseline characteristics. By pooling data from various sources, a quantitative systematic analysis is conducted to objectively assess the efficacy of DCB compared to DES in CAD.
2. Materials and Methods
2.1. How to retrieve the literatures
The literature screening section was conducted by Tao Yu and Jiang Jun for retrieval and compilation of data. A computer search was conducted in the following databases: China National Knowledge Infrastructure (CNKI), Wanfang Database, VIP Database, PubMed, Web of Science, EM base, and Cochrane Library. The Chinese search terms included “DCB”, “drug-coated balloon”, “coronary artery disease”, “coronary microvascular resistance index”, and “coronary flow reserve”. The English search terms included “coronary artery disease”, “left main coronary artery disease”, “left main disease”, “coronary arteriosclerosis”, “drug-coated balloon”, “DCB”, “drug-eluting stents”, “DES”, and “drug coated stents”. The search period for all databases extended from their inception to May 2023. In cases of disagreement, two researchers discussed and decided whether to include the literature, or a third researcher made the final decision.
2.2. How to enroll and exclude relevant literatures
According to the pre-defined eligibility criteria, the design framework of Participants, Interventions, Comparators, Outcomes, and Study (PICOS) was employed to select the included RCTs. The specific criteria were detailed in Table 1.
Table 1.
Inclusion and exclusion criteria.
| No. | Criteria for enrolling relevant literatures |
|---|---|
| 1 | Study type: RCTs |
| 2 | The subjects were patients undergoing first PCI for target lesions |
| 3 | The Exp group used DCB as the intervention, and the Ctrl group used BMS or DES as the intervention |
| 4 | Lesion reference lumen diameter (RVD) ≥ 2.5 mm |
| 5 | Studies with complete follow-up data |
| No. |
Criteria for excluding relevant literatures |
| 1 |
Patients with coronary bifurcation disease, stent restenosis disease, small vessel disease |
| 2 | Study of routine BMS or DES implantation after DCB |
| 3 | Studies where information was missing or data extraction was not possible |
| 4 | Baseline data were incomplete or statistically different |
2.3. How to extract relevant data
The literature screening section was independently conducted by Tao Yu and Jiang Jun using standardized Microsoft Excel spreadsheets for literature screening and data extraction. They rigorously adhered to the criteria for enrolling and excluding relevant literatures and cross-checked the final results. Any discrepancies were resolved through discussion. The extracted data included I. literature's basic information: title, first author, publication date, source, etc.; II. basic characteristics of the participants: gender distribution, age, sample size, adverse events, etc.; III. key elements of the risk of bias (ROB) assessment: randomization methods, implementation of blinding, allocation concealment, etc.; and IV. outcome measures of interest and corresponding data, such as target lesion revascularization (TLR), characteristics of the target vessel, myocardial infarction (MI), odds ratio (OR), mean difference (MD), etc.
2.4. How to assess the relevant literatures
Using the RevMan 5.3 and following the guidance provided in the Cochrane Handbook for Systematic Reviews 5.3, the enrolled literatures were assessed based on the recommended ROB assessment approach for RCT. This included assessments of random methods, allocation concealment, double-blinding, selective reporting, completeness of experimental data, and other relevant aspects. The assessment categorized the likelihood of bias occurrence as low, unclear, high, or unknown risk. Any discrepancies in the assessment were resolved through discussion between the two reviewers, or a third reviewer was consulted if needed.
2.5. Methods for statistics
The statistical analysis was performed using Review Manager 5.3. For continuous variables, the statistical analysis was conducted using the MD and 95 % confidence interval (CI). For dichotomous data, the analysis was performed using the RR and 95 % CI. The heterogeneity among relevant literatures was assessed using the Cochrane's I2 test. P > 0.1 and I2 < 50 % suggested no heterogeneity among the enrolled literatures, and a fixed-effects model (FEM) was employed. P < 0.1 and I2 > 50 % suggested that the resulting heterogeneity was obvious, and a random-effects model (REM) was applicable. P < 0.05 was considered statistically significant for differences.
3. Results
3.1. Retrieval results and their introduction
A total of 7613 articles were initially retrieved from the databases. After an initial screening, 713 pertinent articles were identified, while 342 duplicate publications were subsequently excluded. Furthermore, 217 were deemed ineligible for various reasons, and an additional 39 articles were excluded from consideration. This culminated in a final selection of 115 articles for further analysis. After reviewing the abstracts and titles, 79 articles were excluded, leaving 36. All 36 articles were then thoroughly read in full text, and 29 articles were excluded due to incorrect study design or incomplete/unobtainable treatment outcomes. Finally, 7 articles were included for the meta-analysis [5,6,[12], [13], [14], [15], [16]]. The visual representation of the selection process was given in Fig. 1, and the baseline characteristics of the enrolled studies were presented in Table 2.
Fig. 1.
Procedures for literature search.
Table 2.
Basic data of the enrolled literature.
| Author | Intervention | Sample size | Male (n (%)) | Age (years old) | Number of patients with hypertension (n (%)) | Number of patients with diabetes (n (%)) | Number of patients with smoking (n (%)) |
|---|---|---|---|---|---|---|---|
| Cortese [5] | DES | 114 | 87 (76.9) | 66 ± 16 | 76 (67.2) | 40 (35.4) | 19 (16.7) |
| DCB | 118 | 83 (70.3) | 64 ± 16 | 77 (65.2) | 45 (38) | 23 (19.5) | |
| Fahrni [6] | DES | 64 | 45 (70.3) | 66.6 ± 11.3 | 57 (89.1) | 23 (36.0) | 30 (46.8) |
| DCB | 47 | 38 (80.9) | 68.1 ± 9.4 | 39 (83.0) | 16 (34.0) | 33 (70.2) | |
| Schellor [12] | DES | 106 | 72 (67.9) | 67.0 ± 13.1 | 93 (87.7) | 38 (35.8) | 43 (40.6) |
| DCB | 104 | 6 9(66.3) | 66.0 ± 11.4 | 82 (78.7) | 28 (26.9) | 35 (33.7) | |
| Tang [13] | DES | 114 | 88 (77.2) | 60.5 ± 10.8 | 86 (75.4 0 | 48 (42.1) | 36 (31.6) |
| DCB | 116 | 77 (66.4) | 60.1 ± 10.5 | 78 (67.2) | 46 (39.7) | 34 (29.4) | |
| Katsumi [14] | DES | 98 | 45 (65.2) | 71 ± 9 | 53 (76.8) | 39 (56.5) | 20 (29.0) |
| DCB | 68 | 38 (70.4) | 71 ± 9 | 38 (70.4) | 33 (61.1) | 10 (18.5) | |
| Yu [16] | DES | 79 | 56 | 64 ± 10.5 | 54 (68.4) | 23 (29.1) | 42 (53.2) |
| DCB | 84 | 62 | 62.6 ± 8.8 | 50 (59.5) | 16 (19.0) | 46 (54.8) | |
| VOS [15] | DES | 60 | 52 (87) | 57.3 ± 8.3 | 19 (32) | 4 (7) | 24 (40) |
| DCB | 60 | 52 (87) | 57.4 ± 9.2 | 18 (30) | 8 (13) | 28 (47) |
Note: values were mean ± SD, n (%), or median (interquartile range).
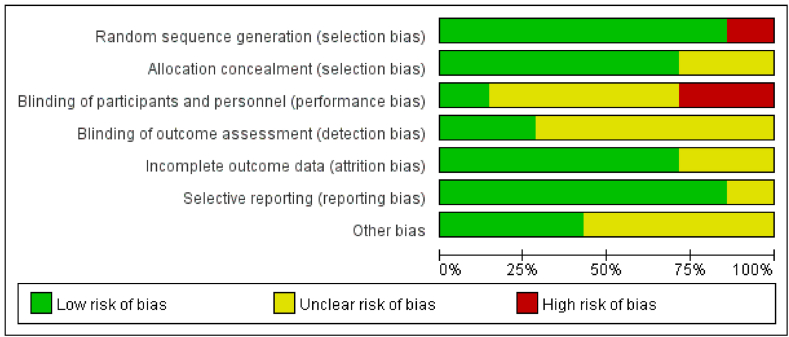
3.2. ROB results
The quality assessment of the enrolled 7 studies revealed that 2 articles (28.5 %) were assigned an A grade, 4 (57.1 %) were assigned a B grade, and 1 (12.3 %) was assigned a C grade. The ROB assessment graph and the summary of the ROB for the enrolled references were presented in Fig. 2, Fig. 3, respectively, both generated using RevMan 5.3. These studies encompassed different types of CAD patients, including stable angina and acute coronary syndrome patients. Due to unexpected situations that arose in some studies, there were instances of patients being transitioned from the drug-eluting stent group to the drug-eluting stent withdrawal group. The criteria for such cases were as follows: (1) in cases of severe conditions, consideration might be given to using drug-eluting stents for treatment; (2) if patients exhibited significant symptoms or impaired cardiac function, consideration might be given to using drug-eluting stents for treatment; and (3) if strongly requested by the patient.
Fig. 2.
ROB of enrolled literatures.
Fig. 3.
Summary graph of ROB. Note: “+”, “-”, and “?” represented low, high, and unclear risk, respectively.
4. Meta-analysis results
4.1. BCE-required drugs
As shown in Table 3, the included studies used the following drugs for BCE treatment. Among them, in addition to 2 studies that did not mention the drug, the remaining 5 studies primarily utilized paclitaxel-eluting scaffolds for BCE filling.
Table 3.
BCE-required drugs.
4.2. TLR
Four studies reported the data on TLR events. The OR was employed as a clinical outcome measure, as demonstrated in Fig. 4. The meta-analysis showed an OR of 0.66 (95 % CI: 0.32–1.37) for posttreatment TLR, with an I2 of 36 % and a P-value of 0.27. These results indicated a low heterogeneity regarding TLR after treatment, and thus a FEM was utilized. The lowest and highest OR values were 0.08 and 1.70, respectively, with a 95 % CI of (0.00, 1.36) and (0.40, 7.28), respectively.
Fig. 4.
Forest plots (FP) of the DCB and DES groups regarding the reconstruction of the TLR.
To further assess the effectiveness of the treatment, a comprehensive analysis was conducted. Fig. 5 presented a FP for posttreatment TLR, indicating a low ROB. Based on these findings, it can be concluded that compared to the DES, treatment with DCB improved the TLR and enhanced blood flow.
Fig. 5.
FP of the DCB and DES groups regarding the TLR.
4.3. MI
Five literatures reported data on postoperative MI events. Using OR as the clinical outcome measure, as demonstrated in Fig. 6, the OR for postoperative MI was 0.41 with a 95 % CI of 0.16–1.04. The heterogeneity for the risk of postoperative MI was low, as indicated by a low I2 value of 17 % and a p-value of 0.06. The lowest and highest OR values were 0.08 and 5.09, respectively, with a 95 % CI of 0.00–1.36 and 0.24–107.18, respectively.
Fig. 6.
FP of DCB group and DES group on MI.
To further observe the treatment effect, a comprehensive analysis was conducted on the risk of postoperative MI. Fig. 7 presented the FPof MI risk in patients after treatment, showing that the studies have low ROB. The above results suggested that DCB treatment is associated with a reduced risk of postoperative MI.
Fig. 7.
FP of DCB group and DES group on MI.
4.4. DOS
Four literatures reported the data on post-treatment DOS, which referred to the % of lumen diameter, within the stented vessel. The MD was deemed as the clinical outcome measure. Fig. 8 demonstrated that the MD for DOS was 6.03, with a 95 % CI of 5.46–6.59. The highest and minimal MD values were 10.10 and 4.40, respectively, with a 95 % CI of 4.69–15.51 and −1.03 - 9.83, respectively.
Fig. 8.
FP of the DCB and DES groups regarding the DOS.
To further evaluate the treatment effect, a comprehensive analysis of the DOS was conducted. Fig. 9 presented a FP of DOS, which assessed heterogeneity among the enrolled literatures and potential publication bias (PB). The small heterogeneity observed among the literatures suggests high accuracy and low ROB. Based on these results, DCB treatment can improve the DOS after the procedure. However, further research is needed to validate these findings.
Fig. 9.
FP of the DCB and DES groups regarding the DOS.
4.5. LLL
5 enrolled literatures reported data on LLL. The MD was undertaken as the clinical outcome measure and revealed a value of −0.13, with a 95 % CI of −0.26 to −0.01 (Fig. 10). The maximal and minimal MD valued were 0.04 and −0.43, respectively, with a 95 % CI of (−0.05, 0.13) and (−0.63, −0.23), respectively.
Fig. 10.
FP of DCB and DES groups on LLL.
Additionally, the LLL was further examined. Fig. 11 showed the FP of LLL, assessing the heterogeneity among the studies and identifying potential outliers. It was observed that four enrolled literatures deviated from the expected distribution, indicating a substantial heterogeneity. These results suggested that DCB treatment yields better outcomes in terms of LLL compared to the DES treatment. However, further research is still needed to validate these findings.
Fig. 11.
FP of DCB group and DES group on LLL.
4.6. Cardiac death
Five enrolled articles reported data on cardiac death events. Using OR as the clinical outcome measure, the OR for cardiac death after treatment was 0.33, with a 95 % CI of 0.10–1.04, as illustrated in Fig. 12. There was no observed heterogeneity among all the enrolled studies, with an I2 value of 0 %, and the p-value was 0.06. These results suggested no visible heterogeneity in the risk of cardiac death among the different groups. The maximal and minimal OR values were 0.17 and 0.50, respectively, with a 95 % CI of 0.02–1.40 and 0.12 to 2.03, respectively.
Fig. 12.
FP of the DCB and DES groups regarding cardiac death.
4.7. BR
Two studies, involving 390 patients, reported data on BR events within the segments. As given in Fig. 13, the results indicated no marked heterogeneity in the risk of BR among the different groups, with an OR of 1.01 and a 95 % CI of 0.50–2.20. The p-value was (P = 0.73). The lowest and highest OR values were 0.91 and 1.17, respectively, with a 95 % CI of 0.37–2.24 and 0.39 to 2.02, respectively.
Fig. 13.
FP of DCB group and DES group on BR.
4.8. Sensitivity analysis
Sensitivity analysis was conducted by changing the analysis models, and the meta-analysis results disclosed no remarkable changes when using different analysis models. This indicates that the enrolled literature demonstrated good stability. Furthermore, additional models such as asymmetry linear regression analysis using the FP can also demonstrate good consistency of the findings.
5. Discussion
This meta-analysis included a compilation of studies comparing the efficacy of DCB versus DES in CAD. The DCB effectiveness and risks were evaluated based on both angiographic outcomes and clinical endpoints. 7 RCTs, comprising 1238 patients undergoing PCI for CAD lesions, were enrolled and analyzed in this meta-analysis. The primary objective of this work was to compare the efficacy and risks of DCB versus DES in CAD. The results revealed that compared to the DES group, the DCB group demonstrated superior outcomes in terms of DOS (MD = 6.03, I2 = 0 %, P < 0.00001). While the difference in LLL (MD = −0.13, I2 = 83 %, P < 0.05) was statistically different, but notable heterogeneity was observed, warranting further analysis to explore the reasons behind it. Although not statistically significant, the DCB group showed favorable trends compared to the DES group in terms of TLR (OR = 0.66, I2 = 36 %, P > 0.05), postoperative MI (OR = 0.41, I2 = 17 %, P > 0.05), and cardiac death (OR = 0.33, I2 = 0 %, P > 0.05). These results suggest that the efficacy and safety of DCB in CAD are not inferior to DES. Compared to DES, DCB exhibits the potential to reduce the occurrence of TLR, decrease LLL, and lower the risks of cardiac death and postoperative MI events. The observed outcomes may be attributed to various pathological and physiological mechanisms related to post-PCI restenosis. Overall, the comprehensive results lend robust support to the utility of DCB as a viable and effective strategy in the management of CAD.
Since its inaugural successful application, the PCI technique has been in a state of continual evolution [17]. Currently, the emergence of the most recent generation DES has reduced the incidence of ISR [18]. However, even with the use of the latest generation DES, the occurrence rate of major adverse cardiac events remains as high as 6.1 % [19]. Despite these advancements, DES faces significant challenges related to long-term ISR and thrombosis, often attributed to vascular vasomotor injury around the stent [20]. On the other hand, despite significant advancements in CAD treatment with DES, complex coronary lesions often carry a higher risk of BR [21]. Additionally, many CAD patients have comorbid diabetes, and these patients exhibit extensive and diffuse vascular lesions with a larger involvement area [22]. Addressing the context of ISR, DES introduces an additional layer of metallic struts, which further reduces the luminal area, potentially complicating the ISR scenario [23].
To mitigate these potential long-term risks, the medical community has delved into various concepts and approaches to avoid permanent implants. Among these approaches, the concept of DCB has emerged as an interventional treatment strategy that eliminates the need for stent implantation. Instead, a DCB delivers medication to the vessel wall, inhibiting neointimal proliferation upon contact. This novel modality introduces the concept of “implant-free intervention” [24], providing a new option for treating small vessel disease. Although the effectiveness and safety of DCB in ISR, bifurcation lesions, and small vessel disease have been confirmed in some RCTs, the results have been inconsistent [[25], [26], [27], [28], [29]]. A network meta-analysis that assessed the most suitable PCI approach for coronary small vessel disease ranked DCB as the second-best treatment based on late lumen loss, the risk of binary restenosis, and follow-up target lesion revascularization. Another meta-analysis based on RCTs compared the safety and efficacy of DCB versus new-generation DES in treating ISR, and the results revealed an effective reduction in LLL and TLR in the short-term follow-up with the new-generation DES. However, both treatment groups exhibited similar rates of major adverse cardiac events, MI, and cardiac death. Additionally, another meta-analysis including 13 studies comparing DCB with second-generation DES for ISR treatment demonstrated similar safety and efficacy between the two approaches based on RCT evidence. Nevertheless, real-world non-randomized evidence suggested a higher risk of all-cause mortality with DCB. Therefore, current research evidence supports the use of either DCB or DES for ISR lesions. Notably, DCB offers the advantage of avoiding supplementary stent implantation, making it potentially more advantageous in managing ISR lesions.
In conclusion, while DCB has undeniable advantages in treating ISR, its role in coronary small vessel disease remains controversial. However, based on current clinical data, DCB emerges as an attractive alternative in cases of primary small vessel disease with high restenosis rates, results comparable to DES in long-term follow-up.
6. Conclusion
The efficacy of DCB in CAD was non-inferior to DES, and it was associated with lower risks. Compared to BMS, DCB could reduce the occurrence of TLR and decrease late lumen loss. Overall, this work indicated the feasibility and effectiveness of DCB treatment in CAD.
Ethical statement
This study does not require review and/or approval from an ethics committee because it is a meta-analysis article that uses publicly available published research data, rather than directly involving human or animal experiments or observations.
This study does not require informed consent as meta-analysis involves the comprehensive analysis and summary of already published research, with the research subjects being publicly available literature rather than specific individual participants.
Data availability statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Additional information
No additional information is available for this paper.
CRediT authorship contribution statement
Xinghua Bai: Data curation, Formal analysis, Investigation, Methodology, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. Chaofeng Shen: Data curation, Resources, Software, Visualization, Writing – original draft. Weizong Zhang: Data curation, Formal analysis, Investigation, Visualization, Writing – original draft. Tao Yu: Software, Validation, Visualization, Writing – original draft. Jun Jiang: Conceptualization, Funding acquisition, Project administration, Writing – original draft, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Bhatt D.L., Steg P.G., Mehta S.R., Leiter L.A., Simon T., Fox K., Held C., Andersson M., Himmelmann A., Ridderstråle W. Ticagrelor in patients with diabetes and stable coronary artery disease with a history of previous percutaneous coronary intervention (THEMIS-PCI): a phase 3, placebo-controlled, randomised trial. Lancet. 2019;394(10204):1169–1180. doi: 10.1016/S0140-6736(19)31887-2. [DOI] [PubMed] [Google Scholar]
- 2.Cheng Y., Sun T., Yin C., Wang S., Li Z., Tao Y., Li Z., Zhang H. Correlations of high-frequency ultrasound evaluation of brachial artery endothelial dilatation and carotid atherosclerosis with glucose and lipid metabolism, inflammatory cytokines, severity of coronary artery disease and vascular endothelial function in elderly patients. Cell. Mol. Biol. 2022;68(5):207–212. doi: 10.14715/CMB/2022.68.5.28. [DOI] [PubMed] [Google Scholar]
- 3.Bates E.R., Tamis-Holland J.E., Bittl J.A., O'Gara P.T., Levine G.N. PCI strategies in patients with ST-segment elevation myocardial infarction and multivessel coronary artery disease. J. Am. Coll. Cardiol. 2016;68(10):1066–1081. doi: 10.1016/j.jacc.2016.05.086. [DOI] [PubMed] [Google Scholar]
- 4.J Bennett Q., Hemptinne De, McCutcheon K. Magmaris resorbable magnesium scaffold for the treatment of coronary heart disease: overview of its safety and efficacy. Expet Rev. Med. Dev. 2019;16(9):757–769. doi: 10.1080/17434440.2019.1649133. [DOI] [PubMed] [Google Scholar]
- 5.Cortese B., Palma G Di, Guimaraes M.G., Piraino D., Orrego P.S., Buccheri D., Rivero F., Perotto A., Zambelli G., Alfonso F. Drug-coated balloon versus drug-eluting stent for small coronary vessel disease: PICCOLETO II randomized clinical trial. JACC Cardiovasc. Interv. 2020;13(24):2840–2849. doi: 10.1016/J.JCIN.2020.08.035. [DOI] [PubMed] [Google Scholar]
- 6.Fahrni G., Scheller B., Coslovsky M., Gilgen N., Farah A., Ohlow M.A., Mangner N., Weilenmann D., Wohrle J., Cuculi F., Leibundgut G., Mobius-Winkler S., Zweiker R., Twerenbold R., Kaiser C., Jeger R., Investigators B.-S. Drug-coated balloon versus drug-eluting stent in small coronary artery lesions: angiographic analysis from the BASKET-SMALL 2 trial. Clin. Res. Cardiol. 2020;109(9):1114–1124. doi: 10.1007/s00392-020-01603-2. [DOI] [PubMed] [Google Scholar]
- 7.Ielasi A., Miyazaki T., Geraci S., Testa L., Abdel-Wahab M., Kawamoto H., Ruparelia N., Sato T., Caramanno G., Bedogni F. Hybrid strategy with a bioresorbable scaffold and a drug-coated balloon for diffuse coronary artery disease: the" no more metallic cages" multicentre pilot experience. Eurointervention: Journal of Europcr in Collaboration with the Working Group on Interventional Cardiology of the European Society of Cardiology. 2016;11(14):e1589–e1595. doi: 10.4244/EIJV11I14A309. [DOI] [PubMed] [Google Scholar]
- 8.Abdallah M.S., Wang K., Magnuson E.A., Spertus J.A., Farkouh M.E., Fuster V., Cohen D.J., Investigators F.T. Quality of life after PCI vs CABG among patients with diabetes and multivessel coronary artery disease: a randomized clinical trial. JAMA. 2013;310(15):1581–1590. doi: 10.1001/jama.2013.279208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aikawa T., Yamaji K., Nagai T., Kohsaka S., Kamiya K., Omote K., Inohara T., Numasawa Y., Tsujita K., Amano T. Procedural volume and outcomes after percutaneous coronary intervention for unprotected left main coronary artery disease—report from the National Clinical Data (J‐PCI Registry) J. Am. Heart Assoc. 2020;9(9) doi: 10.1161/JAHA.119.015404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ni L., Chen H., Luo Z., Yu Y. Bioresorbable vascular stents and drug-eluting stents in treatment of coronary heart disease: a meta-analysis. J. Cardiothorac. Surg. 2020;15:1–7. doi: 10.1186/s13019-020-1041-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matsumura-Nakano Y., Shiomi H., Morimoto T., Yamaji K., Ehara N., Sakamoto H., Takeji Y., Yoshikawa Y., Yamamoto K., Imada K. Comparison of outcomes of percutaneous coronary intervention versus coronary artery bypass grafting among patients with Three-Vessel coronary artery disease in the new-generation drug-eluting stents era (from CREDO-Kyoto PCI/CABG registry Cohort-3) Am. J. Cardiol. 2021;145:25–36. doi: 10.1016/J.AMJCARD.2020.12.076. [DOI] [PubMed] [Google Scholar]
- 12.Scheller B., Ohlow M.A., Ewen S., Kische S., Rudolph T.K., Clever Y.P., Wagner A., Richter S., El-Garhy M., M Bohm R Degenhardt, Mahfoud F., Lauer B. Bare metal or drug-eluting stent versus drug-coated balloon in non-ST-elevation myocardial infarction: the randomised PEPCAD NSTEMI trial. EuroIntervention. 2020;15(17):1527–1533. doi: 10.4244/EIJ-D-19-00723. [DOI] [PubMed] [Google Scholar]
- 13.Tang Y., Qiao S., Su X., Chen Y., Jin Z., Chen H., Xu B., Kong X., Pang W., Liu Y., Yu Z., Li X., Li H., Zhao Y., Wang Y., Li W., Tian J., Guan C., Xu B., Gao R., Investigators R.S.C. Drug-coated balloon versus drug-eluting stent for small-vessel disease: the RESTORE SVD China randomized trial. JACC Cardiovasc. Interv. 2018;11(23):2381–2392. doi: 10.1016/j.jcin.2018.09.009. [DOI] [PubMed] [Google Scholar]
- 14.Katsumi U., Morita N., Kojima Y., Takahashi H., Kawasaki M., Ito R., Kondo H., Minatoguchi S., Yoshida T., Hashimoto Y., Tatsumi T., Kitamura T. Safety and long-term efficacy of drug-coated balloon angioplasty following rotational atherectomy for severely calcified coronary lesions compared with new generation drug-eluting stents. J. Intervent. Cardiol. 2019;2019 doi: 10.1155/2019/9094178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vos N.S., Fagel N.D., Amoroso G., Herrman J.R., Patterson M.S., Piers L.H., van der Schaaf R.J., Slagboom T., Vink M.A. Paclitaxel-coated balloon angioplasty versus drug-eluting stent in acute myocardial infarction: the REVELATION randomized trial. JACC Cardiovasc. Interv. 2019;12(17):1691–1699. doi: 10.1016/j.jcin.2019.04.016. [DOI] [PubMed] [Google Scholar]
- 16.Yu X., Wang X., Ji F., Zhang W., Yang C., Xu F., Wang F. A non-inferiority, randomized clinical trial comparing paclitaxel-coated balloon versus new-generation drug-eluting stents on angiographic outcomes for coronary de novo lesions. Cardiovasc. Drugs Ther. 2022;36(4):655–664. doi: 10.1007/S10557-021-07172-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spadaccio C., Benedetto U. Coronary artery bypass grafting (CABG) vs. percutaneous coronary intervention (PCI) in the treatment of multivessel coronary disease: quo vadis?—a review of the evidences on coronary artery disease. Ann. Cardiothorac. Surg. 2018;7(4):506. doi: 10.21037/acs.2018.05.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stone G.W., Kappetein A.P., Sabik J.F., Pocock S.J., Morice M.-C., Puskas J., Kandzari D.E., Karmpaliotis D., Brown W.M., III, Lembo N.J. Five-year outcomes after PCI or CABG for left main coronary disease. N. Engl. J. Med. 2019;381(19):1820–1830. doi: 10.1056/NEJMoa1909406. [DOI] [PubMed] [Google Scholar]
- 19.Reis A.H. From assessing risk factors to understanding, preventing, and treating cardiovascular diseases: an urgent journey. Discov. Med. 2022;34(173):199–204. [PubMed] [Google Scholar]
- 20.Witberg G., Zusman O., Codner P., Assali A., Kornowski R. Impact of coronary artery revascularization completeness on outcomes of patients with coronary artery disease undergoing transcatheter aortic valve replacement: a meta-analysis of studies using the residual SYNTAX score (Synergy between PCI with Taxus and Cardiac Surgery) Circulation: Cardiovascular Interventions. 2018;11(3) doi: 10.1161/CIRCINTERVENTIONS.117.006000. [DOI] [PubMed] [Google Scholar]
- 21.Yerasi C., Case B.C., Forrestal B.J., Torguson R., Weintraub W.S., Garcia-Garcia H.M., Waksman R. Drug-coated balloon for de novo coronary artery disease: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2020;75(9):1061–1073. doi: 10.1016/j.jacc.2019.12.046. [DOI] [PubMed] [Google Scholar]
- 22.Zhou X., Peng W., Pan S., Lin Z., Pan R., Wang Q. High glucose suppresses osteogenic differentiation and induces mitochondrial dysfunction in osteoblasts via SIRT1/RECQL4 Axis; A laboratory study using mouse cells. J. Biol. Regul. Homeost. Agents. 2022;36(4):889–899. [Google Scholar]
- 23.He Y., Wang L., Song B. Correlation between degenerative aortic valve disease and severity of coronary artery damage in coronary heart disease. Acta Med. Mediterr. 2022;38(3):1649–1653. [Google Scholar]
- 24.Cortese B., Sanchez-Jimenez E. Back to the future: DCB use instead of DES for the treatment of complex, native coronary artery disease. Eur. Heart J. Suppl. 2021;23(Supplement_E):E63–E67. doi: 10.1093/EURHEARTJ/SUAB091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jeger R.V., Eccleshall S., Wan Ahmad W.A., Ge J., Poerner T.C., Shin E.-S., Alfonso F., Latib A., Ong P.J., Rissanen T.T. Drug-coated balloons for coronary artery disease: third report of the international DCB consensus group. Cardiovascular Interventions. 2020;13(12):1391–1402. doi: 10.1016/j.jcin.2020.02.043. [DOI] [PubMed] [Google Scholar]
- 26.Jeger R.V., Farah A., Ohlow M.-A., Mangner N., Möbius-Winkler S., Leibundgut G., Weilenmann D., Wöhrle J., Richter S., Schreiber M. Drug-coated balloons for small coronary artery disease (BASKET-SMALL 2): an open-label randomised non-inferiority trial. Lancet. 2018;392(10150):849–856. doi: 10.1016/S0140-6736(18)31719-7. [DOI] [PubMed] [Google Scholar]
- 27.Loh J.P., Waksman R. Paclitaxel drug-coated balloons: a review of current status and emerging applications in native coronary artery de novo lesions. JACC Cardiovasc. Interv. 2012;5(10):1001–1012. doi: 10.1016/j.jcin.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 28.Mahfoud F., Farah A., Ohlow M.-A., Mangner N., Wöhrle J., Moebius-Winkler S., Weilenmann D., Leibundgut G., Cuculi F., Gilgen N. Drug-coated balloons for small coronary artery disease in patients with chronic kidney disease: a pre-specified analysis of the BASKET-SMALL 2 trial. Clin. Res. Cardiol. 2022;111(7):806–815. doi: 10.1007/S00392-022-01995-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Merinopoulos I., Gunawardena T., Corballis N., Bhalraam U., Gilbert T., Maart C., P Richardson A Ryding, Sarev T., Sawh C. Paclitaxel drug-coated balloon-only angioplasty for de novo coronary artery disease in elective clinical practice. Clin. Res. Cardiol. 2022:1–8. doi: 10.1007/S00392-022-02106-Y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.