Abstract
In Escherichia coli, expression of the carAB operon is subject to cumulative repression, which occurs by ArgR-mediated repression at a downstream promoter, P2, and by pyrimidine-mediated regulation at an upstream promoter, P1. In this study, we show that pyrimidine-mediated regulation occurs in part through a mechanism involving UTP-sensitive reiterative transcription (i.e., repetitive addition of U residues to the 3′ end of a nascent transcript due to transcript-template slippage). In this case, reiterative transcription occurs at the end of a run of three T · A base pairs in the initially transcribed region of the carAB P1 promoter. The sequence of this region is 5′-GTTTGC (nontemplate strand). In the proposed regulatory mechanism, increased intracellular levels of UTP promote reiterative transcription, which results in the synthesis of transcripts with the sequence GUUUUn (where n = 1 to >30). These transcripts are not extended downstream to include structural gene sequences. In contrast, lower levels of UTP enhance normal template-directed addition of a G residue at position 5 of the nascent transcript. This addition precludes reiterative transcription and permits normal transcript elongation capable of producing translatable carAB transcripts. Thus, carAB expression, which is necessary for pyrimidine nucleotide (and arginine) biosynthesis, increases in proportion to the cellular need for UTP. The proposed mechanism appears to function independently of a second pyrimidine-mediated control mechanism that involves the regulatory proteins CarP and integration host factor.
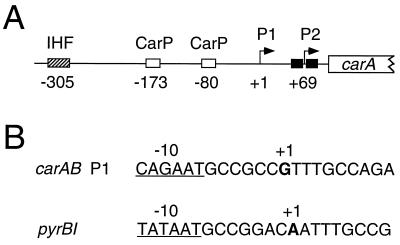
The carAB operon of Escherichia coli encodes the two subunits of carbamoyl phosphate synthetase. This enzyme catalyzes the formation of carbamoyl phosphate, which is an intermediate in the pyrimidine nucleotide and arginine biosynthetic pathways. Expression of the carAB operon is subject to cumulative repression by the end products of each pathway (10). Transcription of the operon is initiated at two tandem promoters designated P1 and P2 (Fig. 1A). Initiation at P1, the more upstream promoter, is negatively regulated by the availability of pyrimidine nucleotides (4, 24). This regulation is poorly understood but requires, at least to some extent, the trans-acting factors CarP and integration host factor (IHF) (9). These proteins bind upstream of promoter P1 and may induce structural modifications and/or the assembly of a nucleoprotein complex required for regulation (7, 8). Initiation at promoter P2 is regulated by arginine-dependent binding of the arginine repressor, ArgR, to two operator sequences that flank the transcriptional start site(s) (4, 24). ArgR binding at promoter P2 does not inhibit transcription initiated at promoter P1.
FIG. 1.
Organization of the carAB regulatory region and sequences of relevant promoter regions. (A) Locations of carAB promoters P1 and P2 and the binding sites for IHF (hatched box), CarP (open boxes), and ArgR (filled boxes). (B) Sequences of the carAB P1 and pyrBI promoter regions. The −10 regions (underlined) and the start sites (boldface) are indicated.
The initially transcribed region of the pyrimidine-sensitive carAB promoter P1 contains the sequence GTTTGC (nontemplate strand), with initiation occurring at the first G residue (Fig. 1B) (4, 24). This region contains a run of three T residues at which reiterative transcription is reported to occur (16). Reiterative transcription is the repetitive addition of a nucleotide (UMP in the case of carAB) caused by slippage between a homopolymeric stretch of nascent transcript and a stretch of ≥3 complementary nucleotides in the DNA template (15). Recently, it has been shown that reiterative transcription involving the repetitive addition of U residues plays a central role in pyrimidine-mediated regulation of pyrBI, codBA, and upp expression in E. coli, although the control mechanisms may differ significantly (19, 27, 31).
In the case of the pyrBI operon, which encodes the two subunits of the pyrimidine nucleotide biosynthetic enzyme aspartate transcarbamylase, a high intracellular level of UTP induces reiterative transcription after synthesis of the nascent transcript AAUUU (Fig. 1B). This reaction produces transcripts with the sequence AAUUUUn (where n = 1 to >30), which cannot be extended downstream to include additional leader and structural gene sequences and which are eventually released from the initiation complex. Consequently, the synthesis of AAUUUUn transcripts precludes the synthesis of short transcripts with the sequence AAUUUG, which are made by normal elongation and can be extended to produce full-length pyrBI transcripts. In this way, pyrBI expression is regulated over a sevenfold range by pyrimidine availability and intracellular UTP levels (19).
In this study, we examined the role of reiterative transcription in pyrimidine-mediated regulation of carAB expression. Our results show that regulation does occur in part through a mechanism involving UTP-sensitive reiterative transcription much like that described for the pyrBI operon. This regulation appears to be independent of regulation mediated by CarP and IHF.
MATERIALS AND METHODS
Bacterial strains and plasmids.
E. coli K-12 strain MC4100 [F− araD139 Δ(argF-lac)U169 rpsL150 thiA1 relA1 deoC1 ptsF25 flbB5301 rbsR] (6) served as a source of chromosomal DNA. Strain CLT42 [MC4100 araD+ car-94] (29) was used as the parent in the construction of λ lysogens. Plasmid pDLC126 (33) was used to construct carAp1::lacZ operon fusions. This plasmid contains a galK::lacZ gene fusion without a promoter. The galK::lacZ gene fusion includes the galK ribosome binding site, the first 54 codons of galK, two spacer codons, and all of lacZ except the first eight codons. Plasmid pDLC126 also contains a unique BamHI site just upstream of the galK::lacZ gene fusion. Operon fusions were made by digesting plasmid pDLC126 with BamHI and ligating the linear plasmid to a BamHI restriction fragment containing either the wild-type or a mutant carAB P1 promoter region. Fusion constructions were screened by restriction mapping and confirmed by DNA sequence analysis with a Sequenase kit (U.S. Biochemicals).
Restriction digests, ligations, and transformations.
Conditions for restriction digests, ligations, and transformations were as previously described (29).
DNA preparations and site-directed mutagenesis.
Plasmid DNA was isolated by use of Qiagen plasmid kits. Chromosomal DNA was prepared as previously described (22). Two types of carAB P1 promoter region fragments were prepared: a short version that does not contain the binding sites for CarP and IHF and a long version that contains these sites. The short fragment, which contains nucleotides −58 to +40 of the promoter region (counting from the transcriptional start site) and flanking DNA containing BamHI restriction sites, was amplified by PCR. The template in the PCR was chromosomal DNA from strain MC4100. The forward and reverse DNA primers were 5′-CGCGGATCCAGCTGATATAAAAAATCCCGCC and 5′-CGCGGATCCAGCTGAATCAATGCAAATCTGC, respectively, with nonpromoter sequences including restriction sites shown in italics. PCR conditions were as follows: 94°C for 4 min; then 94°C for 1 min, 37°C for 1 min, and 72°C for 30 s for 30 cycles; and finally 72°C for 5 min. The long carAB P1 promoter region fragment contains nucleotides −355 to +40 of the promoter region and flanking DNA containing BamHI restriction sites. This fragment was amplified by PCR as described above except the forward primer was 5′-CGCGGATCCAGCTGCCACAAAATATTTGTTATGGTGCA. The resulting PCR products (both short and long versions) were digested with BamHI to trim the fragment ends. The fragments were then inserted separately into plasmid pDLC126 to create carAp1::lacZ operon fusions and also into plasmid pALTER-1 (Promega) for use in site-directed mutagenesis.
Site-directed mutagenesis was used to introduce T-to-G and T-to-C changes at position +3 (Fig. 1) in both the short and the long carAB P1 promoter fragments. Mutagenesis was performed with the Altered Sites II in vitro mutagenesis system (Promega) or by a PCR-based procedure that was essentially the same as that described by Barettino et al. (3). In the latter procedure, DNA was synthesized with Pfu DNA polymerase (Stratagene).
In vitro transcription.
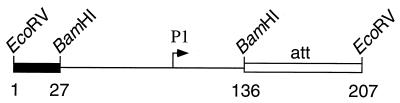
Purified RNA polymerase holoenzyme containing ς70 was prepared as previously described (5, 11). The DNA templates were 207-bp EcoRV restriction fragments containing the short carAB P1 promoter region (with either a wild-type or a mutant promoter) and a downstream segment that included an efficient (∼98%) intrinsic transcriptional terminator, the pyrBI attenuator (32). The presence of the pyrBI attenuator facilitates RNA polymerase release from the DNA template in our multiple-round transcription assay, thereby increasing the synthesis of long (referred to as full-length) transcripts initiated at the carAB P1 promoter and terminated at the attenuator. A general diagram of the DNA templates is shown in Fig. 2. As a control in one experiment, we also used a previously described template containing the pyrBI promoter-leader region (19). All templates were prepared and their concentrations and purity were determined as previously described (26).
FIG. 2.
DNA template for in vitro transcription. bp 1 to 27 are from the multiple cloning site of plasmid pBluescript II SK(−) (Stratagene), bp 28 to 135 are from the BamHI fragment containing the short carAB P1 promoter region (see “DNA preparations and site-directed mutagenesis” in Materials and Methods), and bp 136 to 207 are from a PCR-generated DNA that contains the pyrBI attenuator (att). A transcript initiated at promoter P1 and terminated at the end of the pyrBI attenuator contains 96 nucleotides.
Transcription reaction mixtures (10 μl) contained 10 nM DNA template, 100 nM RNA polymerase, 20 mM Tris-acetate (pH 7.9), 10 mM magnesium acetate, 100 mM potassium glutamate, 0.2 mM Na2EDTA, 0.1 mM dithiothreitol, 200 μM (each) ATP, CTP, and GTP, and 20 to 1,000 μM UTP. In the reaction mixture, one of the nucleoside triphosphates was 32P labeled (2 Ci/mmol; Amersham). Reactions were initiated by addition of RNA polymerase, and the reaction mixtures were incubated at 37°C for 15 min. Heparin (1 μl of a 1-mg/ml solution) was then added to the mixture, and incubation was continued for an additional 10 min. Reactions were terminated by adding 10 μl of stop solution (7 M urea, 2 mM Na2EDTA, 0.25% [wt/vol] each bromophenol blue and xylene cyanol) and placing the samples on ice. The samples were heated at 100°C for 3 min, and an equal volume of each sample was removed and run on a 25% polyacrylamide (29:1 acrylamide:bisacrylamide)–50 mM Tris-borate (pH 8.3)–1 mM Na2EDTA sequencing gel containing 7 M urea (26). Transcripts were visualized by autoradiography and quantitated by scanning the gels with a Molecular Dynamics PhosphorImager.
Transfer of carAp1::lacZ operon fusions from plasmids to the E. coli chromosome.
Wild-type and mutant carAp1::lacZ operon fusions carried on derivatives of plasmid pDLC126 were individually transferred to the chromosome of strain CLT42 by using phage λRZ5 (28). The presence of a single prophage at the λ attachment site was confirmed by PCR analysis (25). In this procedure, the concentration of each primer was 500 nM.
Media and culture methods.
Cells used for enzyme assays and RNA isolations were grown at 37°C with shaking in N−C− medium (1) supplemented with 10 mM NH4Cl, 0.4% (wt/vol) glucose, 0.015 mM thiamine hydrochloride, 1 mM arginine, and either 1 mM uracil or 0.25 mM UMP. Cell growth was monitored and culture samples were harvested during the exponential phase of growth at the same cell density as previously described (18).
Enzyme assays.
Cell extracts were prepared by sonic oscillation (28). β-Galactosidase activity (22) and protein concentrations (21) were determined as previously described.
Isolation of cellular RNA and primer extension mapping.
Cellular RNA was isolated quantitatively as described by Wilson et al. (33). Primer extension mapping of the 5′ ends of carAp1::lacZ transcripts was performed as previously described (20) except that 45 μg of RNA from uracil-grown cells and 30 μg of RNA from UMP-grown cells were used for analysis. The different amounts of RNA, which were isolated from the same mass of cells, reflect the different levels of stable RNA in cells growing at different rates. The primer used in these experiments was 5′-CGCGGATCCAGCTGAATCAATGCAAATCTGC (the reverse primer for PCR synthesis described above), which was labeled with 32P at the 5′ end. The 29 nucleotides at the 3′ end of this primer hybridize to nucleotides 24 to 52 in the carAp1::lacZ transcript. Although this primer would also be expected to hybridize to carAB transcripts, these transcripts are eliminated by mutation in the strains used here (see below).
RESULTS
Demonstration of UTP-sensitive reiterative transcription at the carAB P1 promoter.
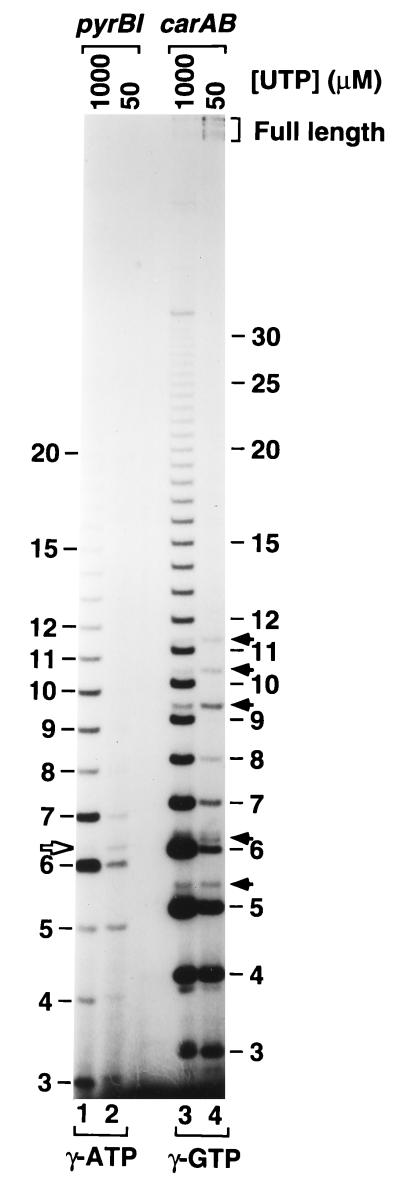
To confirm that reiterative transcription occurs at the carAB P1 promoter and determine whether this reaction is sensitive to the concentration of UTP, we transcribed a DNA template containing this promoter in a reaction mixture containing 200 μM (each) ATP, CTP, and [γ-32P]GTP and either 1,000 or 50 μM UTP. The UTP concentrations selected are similar to those found in E. coli grown under conditions of pyrimidine excess or severe pyrimidine limitation, respectively (2, 23). As a control, we also transcribed a template containing the pyrBI promoter under the same conditions as described above, except that [γ-32P]ATP was used instead of [γ-32P]GTP to 5′ end label the transcripts. Transcripts synthesized in the reactions were separated by gel electrophoresis and visualized by autoradiography.
In the presence of 1,000 μM UTP, the major transcripts produced with the carAB template formed a long ladder of regularly spaced bands in the gel (Fig. 3, lane 3). The spacing between these bands was as expected for transcripts that differ in length by a single nucleotide, and, in general, transcript abundance decreased with increasing transcript length. The longest transcript in the ladder contained more than 30 nucleotides. This pattern, which was also observed with the pyrBI transcripts synthesized at 1,000 μM UTP (Fig. 3, lane 1), is the hallmark of reiterative transcription and a clear indication that this reaction occurred extensively at the carAB P1 promoter. That the ladder transcripts produced by reiterative transcription were indeed initiated at the carAB P1 promoter, and have the expected sequence, GUUUUn, is indicated by their migration pattern in the gel. These transcripts migrated more slowly than pyrBI transcripts containing the same number of nucleotides (i.e., AAUUUn) by an amount indicative of the putative sequence (27). The order for slowing transcript mobility by a particular nucleotide is G > A ≥ U > C (27).
FIG. 3.
Analysis of transcripts initiated at the carAB P1 promoter. DNA templates containing either the pyrBI (control) or the carAB P1 promoter were transcribed in vitro in reaction mixtures containing either 1,000 or 50 μM UTP and analyzed as described in the text. An autoradiograph of the 25% polyacrylamide sequencing gel used to separate the transcripts is shown. The radiolabel included in the reactions is indicated below the gel. The lengths (in nucleotides) of AAUn transcripts initiated at the pyrBI promoter and the pyrBI aborted transcript with the sequence AAUUUG (open arrow) are shown on the left. The lengths of GUn transcripts initiated at the carAB P1 promoter and carAB aborted transcripts containing five or more residues (i.e., GUUUG, GUUUGC, GUUUGCCAG, GUUUGCCAGA, and GUUUGCCAGAA) (filled arrows) are shown on the right. Note that the identities of the very minor bands below GUU and GUUU and above GUUUGC are unknown, although the latter band may be GUUUUG (see below). Full-length transcripts are also indicated (bracket). Heterogeneity in the lengths of these transcripts is due to termination at multiple sites within the intrinsic terminator (data not shown).
Also detectable with 1,000 μM UTP was a ladder of minor transcripts with the longest member containing 11 nucleotides (Fig. 3, lane 3). These transcripts resulted in doublet bands at several positions in the gel, e.g., for 5-, 6-, 9-, 10-, and 11-mers. On the basis of their sequence-dependent mobility in the gel, these bands could be tentatively identified as products of simple abortive initiation (i.e., no repetitive nucleotide addition) at the carAB P1 promoter. Abortive initiation products containing seven and eight nucleotides may also be produced in this reaction, but they are not seen as doublets because they comigrate with more-abundant transcripts generated by reiterative transcription. In addition, major 3- and 4-mer bands corresponding to transcripts with the sequences GUU and GUUU were detected. These transcripts presumably were produced by simple abortive initiation because reiterative transcription cannot occur until at least three U residues have been added to the nascent transcript (14, 34).
In the presence of 50 μM UTP, reiterative transcription at the carAB P1 promoter was nearly eliminated (Fig. 3, lane 4). GUUUUn transcripts containing nine or more residues were no longer detectable (in the exposure shown), and GUUUUn transcripts containing five to eight residues were present at greatly reduced levels, at best. In contrast, synthesis of simple abortive initiation products was either little affected (e.g., 3-, 4-, and 5-mers) or stimulated slightly (e.g., 9-, 10-, and 11-mers). Similar responses were observed with the pyrBI promoter (Fig. 3, lane 2). In addition, the synthesis of full-length carAB transcripts increased more than twofold. The latter transcripts were identified according to their length and dependence on the carAB P1 promoter (data not shown). Although not shown here, synthesis of full-length pyrBI transcripts increases similarly with 50 and 1,000 μM UTP (19). Thus, reiterative transcription at the carAB P1 promoter, like that at the pyrBI promoter, is induced by a high UTP concentration. On the other hand, abortive initiation and full-length transcript synthesis initiated at the two promoters are stimulated by lowering the concentration of UTP.
Additional evidence for the sequence assignments of short carAB transcripts.
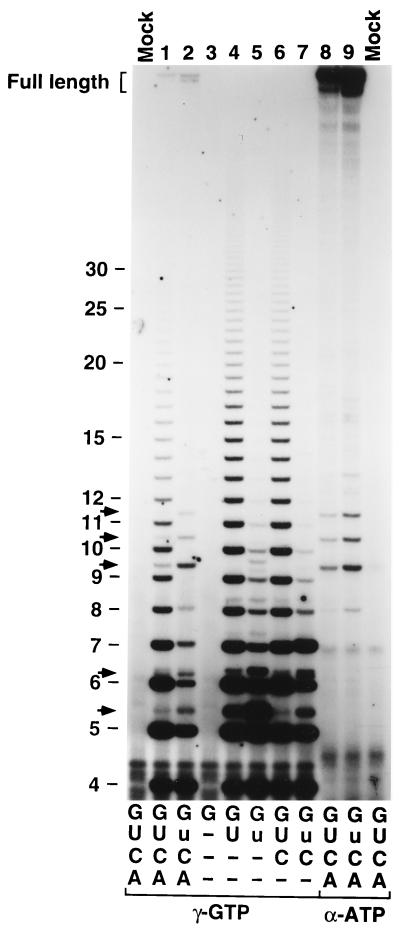
To provide additional evidence in support of our sequence assignments for the carAB transcripts produced by reiterative transcription and simple abortive initiation, we transcribed the carAB P1 promoter template in reaction mixtures containing selected nucleoside triphosphates and different radiolabels. With a reaction mixture containing only 200 μM [γ-32P]GTP, no transcripts were synthesized (Fig. 4, lane 3). With a reaction mixture containing 200 μM [γ-32P]GTP plus 1,000 μM UTP, large amounts of transcripts that appeared identical to the putative GUn and GUUUG transcripts generated with all four nucleoside triphosphates including 1,000 μM UTP were produced (Fig. 4, compare lanes 1 and 4). These results indicate that the putative GUn and GUUUG transcripts indeed contain only G and U residues, consistent with their assigned sequence. For the latter reaction, we did not detect transcripts that migrated with the 9-, 10-, and 11-mer transcripts presumably produced by simple abortive initiation. This result indicates that these transcripts require CTP and/or ATP for synthesis, consistent with their assigned sequences of GUUUGCCAG, GUUUGCCAGA, and GUUUGCCAGAA, respectively.
FIG. 4.
Nucleotide requirements for the synthesis of short transcripts initiated at the carAB P1 promoter. The nucleotides included in each reaction mixture and their concentrations are shown below the gel, with the following abbreviations: A, 200 μM ATP; C, 200 μM CTP; G, 200 μM GTP; U, 1,000 μM UTP; and u, 50 μM UTP (–, no nucleotide). The 32P-labeled nucleotide (i.e., γ-GTP or α-ATP) included in the reaction mixture is also indicated. Mock reactions without RNA polymerase were also analyzed to show bands that were introduced by contaminants in the radiolabel preparations used in this experiment. An autoradiograph of the 25% polyacrylamide gel used to separate the transcripts is shown. The lengths (in nucleotides) of GUn transcripts, aborted transcripts containing five or more residues (i.e., GUUUG, GUUUGC, GUUUGCCAG, GUUUGCCAGA, and GUUUGCCAGAA) (arrows), and full-length transcripts (bracket) are indicated on the left.
When the UTP concentration in the latter reaction mixture was reduced to 50 μM (i.e., the reaction mixture contained 200 μM [γ-32P]GTP and 50 μM UTP), the level of GUUUG transcript synthesis was greatly stimulated, apparently at the expense of GUUUUn transcript synthesis (Fig. 4, compare lanes 4 and 5). This result indicates that either a G or a U residue can be added to the nascent GUUU transcript, as predicted by our sequence assignments, and that the nucleotide selected is determined by the relative concentrations of GTP and UTP. This selection process appears to be a critical step in gene regulation, as will be discussed in detail below. When 200 μM CTP was added to the reaction mixture along with 200 μM [γ-32P]GTP and 1,000 or 50 μM UTP, the level of GUUUG transcript production was greatly reduced. This reduction was accompanied by the accumulation of 7-mer transcript, which was more evident in the reactions with 50 μM UTP (Fig. 4, compare lanes 4 and 5 with lanes 6 and 7). Presumably, the vast majority of 7-mer transcripts synthesized at 50 μM UTP were the result of simple abortive initiation. These results indicate that CTP is required to extend the GUUUG transcript and that the aborted 7-mer is not extended in the absence of ATP. Therefore, we can infer that the aborted 6-mer, 7-mer, and 8-mer contain the sequences GUUUGC, GUUUGCB (where B = C, G, or U), and GUUUGCBA, respectively, again consistent with our sequence assignments.
Another interesting effect of reducing the UTP concentration in the experiments described above (Fig. 4, lanes 4 to 7) was the production of increased amounts of an unidentified 6-mer, which ran above GUUUUU and comigrated with a very minor band produced with all four nucleoside triphosphates (Fig. 4, lanes 1 and 2). The migration pattern and nucleotide content of this unidentified 6-mer indicate that it has the sequence GUUUUG. This transcript could be produced by one cycle of reiterative transcription followed by a switch to nonrepetitive nucleotide addition.
Finally, with a reaction mixture containing 200 μM (each) [α-32P]ATP, CTP, and GTP, and either 1,000 or 50 μM UTP, we detected only transcripts resembling the 8-, 9-, 10-, and 11-mers produced by simple abortive initiation (Fig. 4, compare lanes 8 and 9 with lanes 1 and 2). This result indicates that only these transcripts, and not the putative GUn and shorter aborted transcripts (e.g., GUUUG, GUUUGC, and GUUUGCC), contain one or more A residues. Also, comparison of the levels of [α-32P]ATP and [γ-32P]GTP incorporated into the 8-, 9-, 10-, and 11-mer bands in lanes 1 and 8 (or in lanes 2 and 9) indicates that the 8-mer and 9-mer contain one A residue, the 10-mer contains two A residues, and the 11-mer contains three A residues, as predicted. Thus, this experiment and those described above provide convincing evidence that we have correctly identified the short carAB transcripts.
Effects of UTP concentration on transcription from the carAB P1 promoter.
To examine in more detail the effects of UTP concentration on transcription from the carAB P1 promoter, we transcribed the promoter region in reaction mixtures containing 200 μM (each) ATP, CTP, and [γ-32P]GTP and various concentrations of UTP ranging from 20 to 1,000 μM. The results show that the level of each GUUUUn transcript increased linearly over a wide range when the UTP concentration was increased from 20 to 500 μM (Fig. 5A). This increase was 10-fold in the case of GUUUU and approximately 60-fold for longer GUUUUn transcripts. Above 500 μM, transcript levels were constant or increased slightly, indicating that the UTP concentration was at or near the saturating level for U addition to the transcript. The pattern of increase for one of these transcripts, GU9, is shown in Fig. 5B. Thus, reiterative transcription at the carAB P1 promoter appears to be induced by increasing UTP concentrations similarly to the situation described for the pyrBI promoter (19).
FIG. 5.
Effects of UTP concentration on transcription from the carAB P1 promoter. Standard in vitro transcription reactions in which the UTP concentration was varied as indicated were performed. End-labeled transcripts were separated on a 25% polyacrylamide sequencing gel, visualized by autoradiography, and quantitated. (A) Autoradiograph of the gel. Full-length transcripts (bracket) and nucleotide lengths of transcripts produced by simple abortive initiation are indicated on the left. Nucleotide lengths of GUUUUn transcripts produced by reiterative transcription are shown on the right. (B) The levels of the full-length, GU9, and GU3GC2AG transcripts plotted as a function of UTP concentration. The units for transcript levels are arbitrary.
The effects of UTP concentration on the synthesis of the other carAB transcripts were much different than those described above (Fig. 5A). In the case of transcripts produced by simple abortive initiation, the levels of GUUUG transcripts remained essentially constant while the levels of longer transcripts (at least those that could be measured unambiguously) decreased linearly over a two- to threefold range as the UTP concentration was increased from 20 to 1,000 μM. The decrease in the levels of the aborted 9-mer, GU3GC2AG, is shown in Fig. 5B. Similarly, the levels of full-length transcripts decreased linearly over a 2.5-fold range from 20 to 1,000 μM UTP (Fig. 5B). These results establish an inverse correlation between the synthesis of simple aborted and full-length transcripts and the production of transcripts by reiterative transcription.
Construction and in vitro characterization of carAB P1 promoter mutations that eliminate reiterative transcription.
As the first step in assessing the role of reiterative transcription in pyrimidine-mediated regulation of carAB expression, we constructed two site-directed mutations that introduce either a T-to-G or a T-to-C change at position 3 of the initially transcribed region of the carAB P1 promoter (Fig. 1B). These mutations interrupt the run of three T residues in the initially transcribed region, which is expected to abolish reiterative transcription. To show that the mutations had the anticipated effect, wild-type and mutant DNA templates were transcribed in reaction mixtures containing 200 μM (each) ATP, CTP, and [γ-32P]GTP and 1,000 μM UTP. Analysis of the transcript products indicated that reiterative transcription was in fact eliminated by both mutations, as judged by the absence of a long, regularly spaced ladder of transcripts (Fig. 6, compare lane 1 with lanes 3 and 5).
FIG. 6.
Effects on transcription of the T-to-G and T-to-C mutations at position +3 of the carAB P1 promoter region. Standard in vitro transcription reactions were performed at high (1,000 μM) and low (50 μM) UTP concentrations using either the wild-type (wt) or a mutant DNA template. End-labeled transcripts were separated on a 25% polyacrylamide sequencing gel and visualized by autoradiography. Full-length transcripts (bracket) and nucleotide lengths of transcripts produced with the T-to-C mutant template are indicated on the right. Note that minor (unmarked) bands in the lower part of the autoradiograph are either contaminants in the [γ-32P]GTP preparation or bands which have arisen from an unknown source.
The transcripts produced in the reactions with mutant templates were full-length transcripts and a set of short transcripts up to 12 (T-to-C mutant) or 13 (T-to-G mutant) nucleotides in length. The short transcripts appear to be the products of simple abortive initiation. For the T-to-C mutant, the spacing between transcript bands was exactly as predicted for simple aborted transcripts (Fig. 6, lane 5). For the T-to-G mutant, the gel migration pattern of the short transcripts was more difficult to decipher (Fig. 6, lane 3). There clearly were major bands (e.g., a 4-mer and 6-mer) that migrated as expected for predicted aborted transcripts. However, the identification of minor transcripts was complicated by the presence of doublet bands. These bands most likely arise from initiation at the normal start site and at a second, minor G start site that is created by the T-to-G mutation (see below). Thus, the complex pattern of short transcripts is apparently generated by two overlapping sets of [γ-32P]GTP-labeled aborted transcripts.
We also examined the effects of lowering the UTP concentration to 50 μM on mutant-template transcription. Unlike results with the wild-type template (Fig. 6, lanes 1 and 2), lowering the UTP concentration had no effect on the synthesis of aborted transcripts and had only a minor effect on full-length transcript synthesis (i.e., it slightly altered the selection of termination sites) (Fig. 6, compare lane 3 with lane 4 and lane 5 with lane 6). Interestingly, the level of full-length transcript synthesis with mutant templates was similar to that with the wild-type template at 50 μM UTP but two- to threefold higher than that with the wild-type template at 1,000 μM UTP. Thus, prevention of reiterative transcription either by lowering of the UTP concentration or by mutation apparently allowed increased synthesis of full-length transcripts.
Regulatory effects of carAB P1 promoter mutations that eliminate reiterative transcription.
To directly measure the contribution of reiterative transcription to regulation, we constructed a set of carAp1::lacZ operon fusion strains that contain either the short or the long version of the wild-type or a mutant promoter region. (Note that only the long promoter fragment contains the binding sites for the regulatory proteins CarP and IHF.) The lacZ operon fusions were created by individually inserting a promoter region into plasmid pDLC126 and then transferring the fusion onto phage λRZ5 by recombination. The recombinant phages were used to infect strain CLT42 (car-94 ΔlacZYA), and lysogens carrying a single prophage at the chromosomal λ attachment site were isolated. These strains were designated CLT5174 (short wild-type fusion), CLT5175 (short T-to-G mutant fusion), CLT5177 (short T-to-C mutant fusion), CLT5192 (long wild-type fusion), CLT5202 (long T-to-G mutant fusion), and CLT5203 (long T-to-C mutant fusion). These strains are pyrimidine (and arginine) auxotrophs because the car-94 mutation inactivates carbamoyl phosphate synthetase. The six strains were grown in glucose-minimal salts (plus arginine) medium containing either uracil or UMP as the pyrimidine source, which provides a condition of pyrimidine excess or limitation, respectively. The levels of β-galactosidase in these strains were assayed as an indicator of expression from the carAB P1 promoter (Table 1).
TABLE 1.
Effects of position +3 promoter mutations on expression and regulation of short and long carAp1::lacZ operon fusions
| Strain (carAp1 mutation) | Promoter region length | β-Galactosidase activity (nmol/min/mg)a
|
Fold regulation | |
|---|---|---|---|---|
| Uracil | UMP | |||
| CLT5174 (none [wtb]) | Shortc | 992 | 2,820 | 2.8 |
| CLT5175 (T to G) | Short | 3,610 | 4,910 | 1.4 |
| CLT5177 (T to C) | Short | 2,040 | 2,430 | 1.2 |
| CLT5192 (none [wt]) | Longd | 132 | 2,340 | 18 |
| CLT5202 (T to G) | Long | 563 | 3,480 | 6.2 |
| CLT5203 (T to C) | Long | 305 | 2,710 | 8.9 |
Values are the means of at least three independent determinations with a variation of <8%. Doubling times were 46 ± 2 min for cells grown on uracil and 68 ± 2 min for cells grown on UMP.
wt, wild type.
The short carAB P1 promoter region contained bp −58 to +40.
The long carAB P1 promoter region contained bp −355 to +40.
With the short fusions, the results show that expression directed by the wild-type promoter was regulated over an approximately threefold range by pyrimidine availability. In contrast, only marginal regulation (i.e., 1.2- and 1.4-fold) was observed with the mutant promoters. The loss of regulation was due primarily to increased expression of the mutant fusions under conditions of pyrimidine excess. These results indicate that UTP-dependent reiterative transcription accounts for nearly all of the pyrimidine-mediated negative regulation of the short wild-type operon fusion. Also, it appears that the T-to-G mutation has a general stimulatory effect on fusion expression. With the long fusions, expression from the wild-type promoter was regulated over an 18-fold range by pyrimidine availability. This wider range of regulation was due to a much lower level of operon expression under conditions of pyrimidine excess than that observed with the short wild-type fusion. The effect of the promoter mutations was a reduction in regulation by a factor of 2 (T to C) or 3 (T to G), similar to that observed with the short fusions. The reductions in regulation were again caused by increased expression of the mutant fusions under conditions of pyrimidine excess. The latter results indicate that only part of the pyrimidine-mediated negative regulation of the long operon fusion (and presumably the carAB operon) is due to UTP-sensitive reiterative transcription. Presumably, the additional regulation is provided by an independent mechanism involving CarP and IHF.
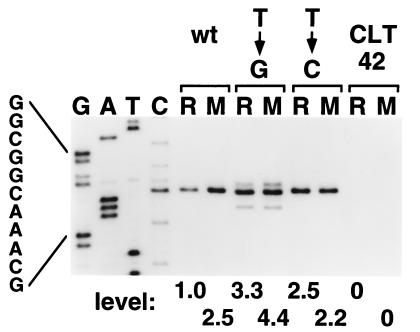
Quantitative primer extension mapping of carAp1::lacZ transcripts initiated at the wild-type and mutant promoters of short operon fusions.
Quantitative primer extension mapping was used to confirm the start sites and measure the levels of carAp1::lacZ transcripts synthesized in the three short operon fusion strains grown on uracil or UMP. With respect to start site(s), the results show that essentially all wild-type and T-to-C mutant transcripts were initiated at the G residue located 7 bases downstream from the promoter −10 region (i.e., the previously identified wild-type start site) (Fig. 7). For the T-to-G mutant, the wild-type start site was used predominantly, but two minor start sites were also employed. The minor start sites are a C residue and the mutant G residue located 6 and 9 bases downstream from the −10 region, respectively (Fig. 1B). Presumably, these minor start sites do not affect regulation, and levels of T-to-G mutant transcripts described below are the sum of the predominant and two minor transcript bands. With respect to transcript levels, the data showed that the wild-type transcript level was 2.5-fold higher in UMP-grown cells than in cells grown on uracil (Fig. 7). In contrast, the pyrimidine source had much smaller effects on the level of transcript produced with the mutant fusions. Overall, the carAp1::lacZ transcript levels roughly paralleled the β-galactosidase activities shown in Table 1. Note that transcripts from the resident carAB operon of the parent strain CLT42 were not detected by this primer extension assay (Fig. 7). Apparently, these transcripts are effectively eliminated by the car-94 mutation.
FIG. 7.
Levels of carAp1::lacZ transcripts synthesized in the short operon fusion strains CLT5174 (wild type [wt]), CLT5175 (T-to-G mutant), and CLT5177 (T-to-C mutant). Cellular RNA was quantitatively isolated from cells grown on either uracil (R) or UMP (M), and transcript levels were measured by primer extension mapping as described in Materials and Methods. An autoradiograph of the 10% polyacrylamide sequencing gel used to analyze the primer extension products is shown. The dideoxy sequencing ladder (G, A, T, C) of the wild-type carAB P1 promoter region, which was used to identify transcripts, was generated with the same primer that was used for primer extension. Note that the sequence is of the template strand. Identical results were obtained by using a sequencing ladder produced with either mutant promoter region. Primer extension product levels, which correspond to transcript levels, were measured with a PhosphorImager. Relative levels are indicated below the appropriate lanes of the autoradiograph. Essentially the same results were obtained in a second, completely independent experiment. As a control, the autoradiograph also shows that transcripts initiated at the carAB P1 promoter were not detected in parent strain CLT42 grown on either uracil or UMP.
DISCUSSION
The in vitro studies described here demonstrate that reiterative transcription involving the repetitive addition of U residues occurs during initiation at the carAB P1 promoter. This reaction produces transcripts with the sequence GUUUUn (where n = 1 to >30), which are apparently released from the transcription initiation complex. The extent of reiterative transcription is directly proportional to the UTP concentration up to approximately 500 μM. Above this concentration, reiterative transcription is stimulated to a much lesser extent. These experiments also indicate an inverse relationship between the levels of reiterative transcription and the synthesis of simple aborted and full-length (i.e., normally elongated) transcripts initiated at the carAB P1 promoter. Furthermore, we showed in vitro that substitution mutations within the run of three T residues in the initially transcribed region of the carAB P1 promoter completely eliminate reiterative transcription.
Our in vivo studies with carAp1::lacZ operon fusion strains indicate that pyrimidine-mediated regulation of operon expression occurs over an 18-fold range, when measured with the long operon fusion that includes the binding sites for CarP and IHF. When the binding sites for these regulatory proteins are deleted, as in the short operon fusion, the range of regulation is reduced to between two- and threefold. Introduction of a promoter mutation that abolishes reiterative transcription into carAp1::lacZ operon fusions showed that nearly all regulation of the short operon fusion was eliminated and that the range of regulation with the long operon fusion was reduced by a factor of 2 to 3. Thus, UTP-sensitive reiterative transcription appears to account for about two- to threefold of the total pyrimidine-mediated regulation, while the remainder of this regulation (i.e., six- to ninefold) presumably occurs by an independent mechanism involving CarP and IHF. The estimated range of regulation by reiterative transcription is consistent with the observed steady-state levels of carAp1::lacZ transcripts produced with short operon fusions containing wild-type and mutant promoters. Primer extension mapping of full-length wild-type transcripts reveals an essentially unique 5′ end without extra U residues, indicating that only transcripts that avoid reiterative transcription can be extended to include downstream sequence.
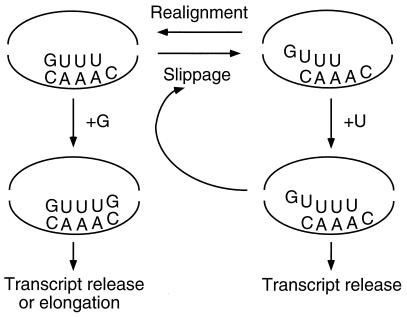
Taken together, our results indicate that regulation of carAp1::lacZ (and carAB) operon expression by UTP-sensitive reiterative transcription occurs by a mechanism analogous to that described for the pyrBI operon and suggest the following regulatory model. Transcription is initiated at the G start site in a UTP concentration-independent manner. After the nascent transcript is extended normally to include four bases and has the sequence GUUU, weak base pairing between the sequences UUU in the transcript and AAA in the DNA template facilitates rapid and reversible slippage between the two strands (Fig. 8). This slippage presumably occurs as a 1-base, upstream shift of the transcript (or at least of the 3′ end of the transcript) (12). When the UTP level is high and the nascent transcript is shifted upstream, the last A of the template AAA sequence directs the efficient addition of the first “extra” U residue to the 3′ end of the transcript. This transcript can be released from the initiation complex, or another round of slippage and U addition can occur. Repetition of this cycle generates transcripts with long runs of U residues at their 3′ ends. Transcripts containing one or more extra U residues are effectively excluded from the normal mode of transcript elongation (i.e., nonrepetitive nucleotide addition). When the UTP level is low, slippage followed by correct repositioning of the GUUU transcript without the addition of an extra U residue are the most likely reactions and permit normal template-directed addition of a G residue to the 3′ end of the transcript. This addition results in more-stable base pairing between the transcript and template and the apparent loss of alternative alignments for the 3′ end of the transcript, which can preclude further slippage. The GUUUG transcript can then be released as a simple aborted transcript or extended further downstream with a certain probability that this transcript will become a full-length carAB transcript.
FIG. 8.
Model showing the alternative fates of the nascent GUUU transcript initiated at the carAB P1 promoter. The GUUU [or GUUU(G/U)] transcript is shown hybridized to the template strand of the initially transcribed region. The curved lines in the complexes indicate the melted strands of DNA. The precise positioning of the 5′ end of the transcript in “slipped” complexes is unknown.
Although described in terms of high and low UTP concentrations, it is important to note that the model does not propose an on-off switch for carAB expression, except perhaps at extreme UTP concentrations. Instead, the model suggests that there is a gradient of operon expression that balances the level of synthesis of carbamoyl phosphate synthetase with the cell’s need for UTP. The UTP concentration determines the frequency of selecting one of two mutually exclusive transcription pathways. One pathway, which is enhanced by an increase in the UTP concentration, leads to the synthesis of nonproductive transcripts. The other pathway, favored by a lowering of the UTP concentration, permits the synthesis of transcripts capable of being translated. Although not included in the model, it is also possible that GTP levels affect carAB expression by influencing the addition of a U or G residue at position 5 of the nascent transcript (17). Physiological conditions that allow GTP levels to modulate the levels of reiterative and productive transcription at the carAB P1 promoter remain to be established.
The full range of pyrimidine-mediated regulation of the carAB P1 promoter apparently requires two independent control mechanisms. Similar situations have been described for a number of E. coli promoters. One function of multiple control mechanisms is regulation of gene expression in response to a wide range of concentrations of a particular effector molecule, with each control mechanism sensitive to a different range of effector concentrations (19, 35). This type of regulation may exist for the carAB P1 promoter. The experiments described in this paper, using pyrimidine auxotrophs grown under conditions of pyrimidine excess or limitation, indicate that the range of CarP-IHF-mediated regulation is essentially the same as that previously measured for pyrimidine prototrophs grown in minimal media with or without a uracil supplement (7, 9). This result indicates that virtually all CarP-IHF-mediated regulation occurs within a range of UTP concentrations between 900 μM and approximately 1.4 mM (23, 30). On the other hand, our results suggest that the bulk of regulation by reiterative transcription occurs at UTP concentrations found in pyrimidine-limited cells (i.e., from 900 to approximately 50 μM). These low UTP levels may be transiently experienced by pyrimidine prototrophs following a shift from a pyrimidine-rich to a pyrimidine-poor environment. Furthermore, we have observed that little (1.4-fold) pyrimidine-mediated regulation of short carAp1::lacZ fusion expression occurs with pyrimidine prototrophs grown in the presence or absence of uracil (13). Thus, regulation of the carAB P1 promoter appears to occur predominantly via a CarP-IHF-mediated mechanism at high UTP concentrations and via UTP-sensitive reiterative transcription at lower UTP concentrations. Note that the regulatory effector of CarP-IHF-mediated regulation does not have to be UTP, but only a molecule whose concentration reflects changes in the UTP level. The identity of this effector awaits further characterization of CarP-IHF-mediated regulation.
An interesting difference revealed by the present study is that the range of regulation provided by UTP-sensitive reiterative transcription at the carAB P1 promoter is much smaller (by roughly a factor of 3) than that observed with the pyrBI promoter (19). Presumably, this effect is due to differences in promoter sequence. There are two obvious differences that could contribute to or account for the effect. First, the sequences at the 5′ end of the carAB and pyrBI transcripts (preceding the U run) are different: G for carAB and AA for pyrBI. Conceivably, the G residue at the end of the carAB transcript could provide stronger base pairing with the template than that provided by the two A residues at the end of the pyrBI transcript. Stronger base pairing would be expected to suppress reiterative transcription and the range of regulation. Second, the locations of the transcription start sites are different for the two promoters: position 7 (counting from the −10 region) for carAB P1 and position 8 for pyrBI (Fig. 1). We recently discovered, using variants of the carAB P1 promoter, that moving the start site from position 7 to position 8 enhances regulation by reiterative transcription by more than twofold (13). The reason for this enhancement is not known. However, this result suggests for the first time that the architecture of the transcription initiation complex can significantly affect reiterative transcription. On the basis of these observations, it appears that the cell could control the expression of numerous operons by employing a similar UTP-sensitive reiterative transcription reaction and achieve different ranges of regulation through minor variations in promoter sequence and/or organization.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grant GM29466 from the National Institutes of Health.
We thank Sara Duesterhoeft for editorial assistance.
REFERENCES
- 1.Alper M D, Ames B N. Transport of antibiotics and metabolite analogs by systems under cyclic AMP control: positive selection of Salmonella typhimurium cya and crp mutants. J Bacteriol. 1978;133:149–157. doi: 10.1128/jb.133.1.149-157.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersen J T, Jensen K F, Poulsen P. Role of transcription pausing in the control of the pyrE attenuator in Escherichia coli. Mol Microbiol. 1991;5:327–333. doi: 10.1111/j.1365-2958.1991.tb02113.x. [DOI] [PubMed] [Google Scholar]
- 3.Barettino D, Feigenbutz M, Valcárcel R, Stunnenberg H G. Improved method for PCR-mediated site-directed mutagenesis. Nucleic Acids Res. 1993;22:541–542. doi: 10.1093/nar/22.3.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bouvier J, Patte J-C, Stragier P. Multiple regulatory signals in the control region of the Escherichia coli carAB operon. Proc Natl Acad Sci USA. 1984;81:4139–4143. doi: 10.1073/pnas.81.13.4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burgess R R, Jendrisak J J. A procedure for the rapid, large-scale purification of Escherichia coli DNA-dependent RNA polymerase involving polymin P precipitation and DNA-cellulose chromatography. Biochemistry. 1975;14:4634–4638. doi: 10.1021/bi00692a011. [DOI] [PubMed] [Google Scholar]
- 6.Casadaban M J. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and Mu. J Mol Biol. 1976;104:541–555. doi: 10.1016/0022-2836(76)90119-4. [DOI] [PubMed] [Google Scholar]
- 7.Charlier D, Gigot D, Huysveld N, Roovers M, Piérard A, Glansdorff N. Pyrimidine regulation of the Escherichia coli and Salmonella typhimurium carAB operons: CarP and integration host factor (IHF) modulate the methylation status of a GATC site present in the control region. J Mol Biol. 1995;250:383–391. doi: 10.1006/jmbi.1995.0384. [DOI] [PubMed] [Google Scholar]
- 8.Charlier D, Hassanzadeh Gh G, Kholti A, Gigot D, Piérard A, Glansdorff N. carP, involved in pyrimidine regulation of the Escherichia coli carbamoylphosphate synthetase operon encodes a sequence-specific DNA-binding protein identical to XerB and PepA, also required for resolution of ColE1 multimers. J Mol Biol. 1995;250:392–406. doi: 10.1006/jmbi.1995.0385. [DOI] [PubMed] [Google Scholar]
- 9.Charlier D, Roovers M, Gigot D, Huysveld N, Piérard A, Glansdorff N. Integration host factor (IHF) modulates the expression of the pyrimidine-specific promoter of the carAB operons of Escherichia coli K12 and Salmonella typhimurium LT2. Mol Gen Genet. 1993;237:273–286. doi: 10.1007/BF00282809. [DOI] [PubMed] [Google Scholar]
- 10.Cunin R, Glansdorff N, Piérard A, Stalon V. Biosynthesis and metabolism of arginine in bacteria. Microbiol Rev. 1986;50:314–352. doi: 10.1128/mr.50.3.314-352.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gonzalez N, Wiggs J, Chamberlin M J. A simple procedure for resolution of Escherichia coli RNA polymerase holoenzyme from core polymerase. Arch Biochem Biophys. 1977;182:404–408. doi: 10.1016/0003-9861(77)90521-5. [DOI] [PubMed] [Google Scholar]
- 12.Guo H-C, Roberts J W. Heterogeneous initiation due to slippage at the bacteriophage 82 late gene promoter in vitro. Biochemistry. 1990;29:10702–10709. doi: 10.1021/bi00499a019. [DOI] [PubMed] [Google Scholar]
- 13.Han, X., and C. L. Turnbough, Jr. Unpublished data.
- 14.Heath, L. S., and C. L. Turnbough, Jr. Unpublished data.
- 15.Jacques J P, Kolakofsky D. Pseudo-templated transcription in prokaryotic and eukaryotic organisms. Genes Dev. 1991;5:707–713. doi: 10.1101/gad.5.5.707. [DOI] [PubMed] [Google Scholar]
- 16.Jin D J. Slippage synthesis at the galP2 promoter of Escherichia coli and its regulation by UTP concentration and cAMP-cAMP receptor protein. J Biol Chem. 1994;269:17221–17227. [PubMed] [Google Scholar]
- 17.Jin D J. A mutant RNA polymerase reveals a kinetic mechanism for the switch between nonproductive stuttering synthesis and productive initiation during promoter clearance. J Biol Chem. 1996;271:11659–11667. [PubMed] [Google Scholar]
- 18.Liu C, Donahue J P, Heath L S, Turnbough C L., Jr Genetic evidence that promoter P2 is the physiologically significant promoter for the pyrBI operon of Escherichia coli K-12. J Bacteriol. 1993;175:2363–2369. doi: 10.1128/jb.175.8.2363-2369.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu C, Heath L S, Turnbough C L., Jr Regulation of pyrBI operon expression in Escherichia coli by UTP-sensitive reiterative RNA synthesis during transcriptional initiation. Genes Dev. 1994;8:2904–2912. doi: 10.1101/gad.8.23.2904. [DOI] [PubMed] [Google Scholar]
- 20.Liu J, Turnbough C L., Jr Effects of transcriptional start site sequence and position on nucleotide-sensitive selection of alternative start sites at the pyrC promoter in Escherichia coli. J Bacteriol. 1994;176:2938–2945. doi: 10.1128/jb.176.10.2938-2945.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 22.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 23.Neuhard J, Nygaard P. Purines and pyrimidines. In: Neidhardt F C, Ingraham J L, Low K B, Magasanik B, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella typhimurium: cellular and molecular biology. Washington, D.C: American Society for Microbiology; 1987. pp. 445–473. [Google Scholar]
- 24.Piette J, Nyunoya H, Lusty C J, Cunin R, Weyens G, Crabeel M, Charlier D, Glansdorff N, Piérard A. DNA sequence of the carA gene and the control region of carAB: tandem promoters, respectively controlled by arginine and the pyrimidines, regulate the synthesis of carbamoyl-phosphate synthetase in Escherichia coli K-12. Proc Natl Acad Sci USA. 1984;81:4134–4138. doi: 10.1073/pnas.81.13.4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Powell B S, Court D L, Nakamura Y, Rivas M P, Turnbough C L., Jr Rapid confirmation of single copy lambda prophage integration by PCR. Nucleic Acids Res. 1994;22:5765–5766. doi: 10.1093/nar/22.25.5765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qi F, Liu C, Heath L S, Turnbough C L., Jr In vitro assay for reiterative transcription during transcriptional initiation by Escherichia coli RNA polymerase. Methods Enzymol. 1996;273:71–85. doi: 10.1016/s0076-6879(96)73007-0. [DOI] [PubMed] [Google Scholar]
- 27.Qi F, Turnbough C L., Jr Regulation of codBA operon expression in Escherichia coli by UTP-dependent reiterative transcription and UTP-sensitive transcriptional start site switching. J Mol Biol. 1995;254:552–565. doi: 10.1006/jmbi.1995.0638. [DOI] [PubMed] [Google Scholar]
- 28.Roland K L, Liu C, Turnbough C L., Jr Role of the ribosome in suppressing transcriptional termination at the pyrBI attenuator of Escherichia coli K-12. Proc Natl Acad Sci USA. 1988;85:7149–7153. doi: 10.1073/pnas.85.19.7149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roland K L, Powell F E, Turnbough C L., Jr Role of translation and attenuation in the control of pyrBI operon expression in Escherichia coli K-12. J Bacteriol. 1985;163:991–999. doi: 10.1128/jb.163.3.991-999.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Southworth, J. P., and C. L. Turnbough, Jr. Unpublished data.
- 31.Tu A-H T, Turnbough C L., Jr Regulation of upp expression in Escherichia coli by UTP-sensitive selection of transcriptional start sites coupled with UTP-dependent reiterative transcription. J Bacteriol. 1997;179:6665–6673. doi: 10.1128/jb.179.21.6665-6673.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Turnbough C L, Jr, Hicks K L, Donahue J P. Attenuation control of pyrBI operon expression in Escherichia coli K-12. Proc Natl Acad Sci USA. 1983;80:368–372. doi: 10.1073/pnas.80.2.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilson H R, Archer C D, Liu J, Turnbough C L., Jr Translational control of pyrC expression mediated by nucleotide-sensitive selection of transcriptional start sites in Escherichia coli. J Bacteriol. 1992;174:514–524. doi: 10.1128/jb.174.2.514-524.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xiong X F, Reznikoff W S. Transcriptional slippage during the transcription initiation process at a mutant lac promoter in vivo. J Mol Biol. 1993;231:569–580. doi: 10.1006/jmbi.1993.1310. [DOI] [PubMed] [Google Scholar]
- 35.Yanofsky C, Kelley R L, Horn V. Repression is relieved before attenuation in the trp operon of Escherichia coli as tryptophan starvation becomes increasingly severe. J Bacteriol. 1984;158:1018–1024. doi: 10.1128/jb.158.3.1018-1024.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]