Abstract
Tn5385 is a ca. 65-kb element integrated into the chromosomes of clinical Enterococcus faecalis strains CH19 and CH116. It confers resistance to erythromycin, gentamicin, mercuric chloride, streptomycin, tetracycline-minocycline, and penicillin via β-lactamase production. Tn5385 is a composite structure containing regions previously found in staphylococcal and enterococcal plasmids. Several transposons and transposon-like elements within Tn5385 have been identified, including conjugative transposon Tn5381, composite transposon Tn5384, and elements indistinguishable from staphylococcal transposons Tn4001 and Tn552. The divergent regions of Tn5385 are linked by a series of insertion sequence (IS) elements (IS256, IS257, and IS1216) of staphylococcal and enterococcal origin. The ends of Tn5385 consist of directly repeated copies of enterococcal IS1216. Within the chromosomes of strains CH19 and CH116, Tn5385 has interrupted an open reading frame with substantial homology to previously described alkyl hydrogen peroxide reductase genes. Segments of this open reading frame in both CH19 and CH116 have been deleted, but the amount of deleted DNA differs for the two insertions. Transfer of Tn5385 from both donors into E. faecalis recipients occurs at a low frequency. Two types of transconjugants have been identified. In one type, the target alkyl hydrogen peroxide reductase open reading frame has been deleted, and sequences flanking Tn5385 in the respective donors are carried over to the transconjugants. These data suggest that the mechanism of Tn5385 insertion into the recipient chromosome in these transconjugants was recombination across flanking regions in the donors and homologous sequences in the recipients. The second type of transconjugant appears to have resulted from excision of Tn5385 from the CH19 chromosome by recombination across the terminal IS1216 elements and insertion into the recipient chromosome by recombination across Tn5381 (within Tn5385) and a previously transferred Tn5381 copy in the recipient chromosome. These data confirm that Tn5385 is a composite structure with genetic material from diverse genera and suggest that it is a functional transposon. They also suggest that chromosomal recombination is a mechanism of genetic exchange in enterococci.
Large, chromosomally located conjugative elements have been found with some frequency in gram-positive bacteria (3, 6, 7, 16). The most prevalent of these elements are the conjugative transposons, which generally confer tetracycline-minocycline resistance encoded by tetM genes and exhibit broad host ranges (23). Conjugative transposons are most commonly 18 to 20 kb in size, although larger variants conferring additional resistance determinants or genes for nisin production have been described in pneumococci and lactococci, respectively (7, 16). The broad host ranges of conjugative transposons suggest that they are important in the dissemination of resistance determinants to diverse genera.
Classic conjugative transposons may be integrated within larger conjugative elements in streptococci and pneumococci. Tn5251, for example, is a Tn916-like tetM-encoding element inserted within a larger transposon that encodes chloramphenicol resistance (Tn5252). Tn5252 bears no structural similarity to conjugative transposons and exhibits site-specific integration into recipient cell genomes on transfer. Integration of Tn5252 into recipient chromosomes is thought to be mediated by transposon-encoded gene products that exhibit structural similarity to site-specific recombinases (14, 27).
We previously reported the chromosomal locations of multiresistance (β-lactamase production, erythromycin resistance, high-level gentamicin resistance, mercuric chloride resistance, streptomycin resistance, and tetracycline resistance) encoding transferable elements in Enterococcus faecalis CH19 and CH116. The transferable elements within which these resistance genes are incorporated in CH19 and CH116 are structurally indistinguishable, if not identical, and have been given the common designation Tn5385.
In this paper, we present the details of the overall structure of Tn5385. We provide evidence that its ends are formed by directly repeated copies of enterococcal insertion sequence IS1216. In addition, we present evidence that integration of Tn5385 into recipient chromosomes occurs by recombination between flanking sequences in the donor and homologous sequences in recipient genomes. A second mechanism of Tn5385 integration into recipient chromosomes, which involves excision of the element by use of the IS1216 ends and integration into the recipient chromosome by recombination across copies of Tn5381, is also described.
MATERIALS AND METHODS
Strains and plasmids.
Relevant bacterial strains, cloning vectors, and recombinant plasmids are listed in Table 1. CH19 and CH116 are clinical E. faecalis strains that were isolated from different patients in the same hospital in 1987. There was no specific epidemiological relationship between these two patients, and the two strains exhibit different plasmid profiles, but their SmaI restriction profiles are identical (18). E. faecalis JH2-7 is a plasmid-free recipient strain used in mating experiments (13). E. faecalis OGIXRF is an OG1 derivative (12). It was constructed by mutating OG1X (Strr) to resistance to fusidic acid and rifampin by sequential inoculation of fusidic acid (25 μg/ml) and rifampin (100 μg/ml) plates with ca. 108 CFU of an overnight culture. Single colonies were harvested and purified, and their resistance phenotypes were confirmed by replating on selective plates containing both rifampin and fusidic acid at the above-mentioned concentrations. CX19 and CV61 are transconjugants resulting from matings between CH19 and JH2-7. CV123 is a transconjugant resulting from a mating between CH19 and OG1XRF. CV34 is a transconjugant resulting from a mating between CH116 and JH2-7.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Resistance phenotypea | Description (reference or source) |
|---|---|---|
| E. faecalis strains | ||
| CH19 | Bla+ Emr Gmr Merr Smr Tcr | Clinical isolate (18) |
| CH116 | Bla+ Emr Gmr Merr Smr Tcr | Clinical isolate (18) |
| JH2-7 | Fusr Rifr | Recipient strain (13) |
| OG1XRF | Fusr Rifr | Recipient strain (12) |
| UV202 | Fusr Rifr | Recombination-deficient recipient strain (28) |
| CX19 | Bla+ Emr Gmr Merr Smr Tcr Fusr Rifr | Transconjugant resulting from mating between CH19 and JH2-7 (18) |
| CV34 | Bla+ Emr Gmr Merr Smr Tcr Fusr Rifr | Tranconjugant resulting from mating between CH116 and JH2-7 (this study) |
| CV123 | Bla+ Emr Gmr Merr Smr Tcr Fusr Rifr | Tranconjugant resulting from mating between CH19 and OG1XRF (this study) |
| CV61 | Bla+ Emr Gmr Merr Smr Tcr Fusr Rifr | Transconjugant resulting from mating between CH19 and JH2-7 (this study) |
| Plasmids | ||
| pUC18 | Ampr | Cloning vector (Bethesda Research Laboratories) |
| pACYC184 | Cmr Tcr | Cloning vector (22) |
| pCRII | Ampr Kmr | Cloning vector for PCR products (Invitrogen) |
| pDL412 | Smr | aadE gene probe (15) |
| pCWR81 | Ampr | 398-bp internal AluI-ClaI fragment of IS1216 cloned into pUC18 (20) |
| pCWR281 | Ampr | 4.3-kb EcoRI-ClaI fragment of CX19, containing the region between the end of Tn5381 and the internal IS1216 within Tn5385, cloned into pUC18 (this study) |
| pCWR303 | Ampr | 650-bp HindIII-ClaI fragment of CV34, containing the left Tn5385-chromosome junction, cloned into pUC18 (this study) |
| pCWR308 | Ampr Smr | 2.6-kb ClaI fragment from CV34, containing the intact streptomycin resistance determinant, cloned into pUC18 (this study) |
| pCWR318 | Ampr | 1.7-kb ClaI fragment from CV123, containing the left Tn5385-chromosome junction, cloned into pUC18 (this study) |
| pCWR343 | Cmr Tcr | 7-kb ClaI fragment of JH2-7, containing the target for Tn5385 insertion, cloned into pACYC184 (this study) |
| pCWR347 | Cmr Tcr | 3.1-kb HindIII fragment of CV34, containing the right Tn5385-chromosome junction, cloned into pACYC184 (this study) |
| pCWR349 | Ampr | Insert from pCWR347 cloned into pUC18 (this study) |
| pCWR351 | Ampr | 1-kb HindIII subfragment of pCWR343, containing the target for Tn5385 insertion, cloned into pUC18 (this study) |
| pCWR389 | Ampr | 5-kb HindIII fragment of CV61, containing the junction of the ends of Tn5385 across a single copy of IS1216, cloned into pUC18 (this study) |
Bla+, β-lactamase positive.
Conjugation experiments.
We previously reported that the entire complement of CH19 resistance determinants transferred to enterococcal recipients at a very low frequency (ca. 10−9 transconjugant/recipient CFU) (18). Conjugation experiments were carried out by mixing 50 μl each of overnight cultures of donor and recipient strains (grown in nonselective brain heart infusion [BHI] broth) in a sterile test tube and then spreading the mixture across a BHI agar plate. Plates were incubated at 37°C overnight. The following day, the confluent growth on the plate was removed with a platinum loop and suspended in 500 μl of sterile saline. Aliquots (150 μl) of this suspension were then plated onto selective plates containing, in most cases, gentamicin (500 μg/ml), fusidic acid (25 μg/ml), and rifampin (100 μg/ml). Plates were incubated for 5 days at 37°C and examined each morning for the appearance of colonies. Colonies were restreaked onto identical plates and tested for associated antimicrobial resistance by being streaked onto BHI agar plates containing fusidic acid (25 μg/ml), rifampin (100 μg/ml), and either erythromycin (10 μg/ml), streptomycin (2,000 μg/ml), or tetracycline (10 μg/ml). Transconjugants were tested for β-lactamase production by using nitrocefin-impregnated discs (BBL Microbiology Systems).
Hybridization experiments.
Genomic DNA was extracted as described previously (21), with the following modifications. After the lysozyme-RNase-proteinase K step (which was shortened to 2 h), the resulting suspension was mixed with a CTAB (hexadecyltrimethyl ammonium bromide)-NaCl solution and heated to 68°C for 20 min. This mixture was then extracted once with phenol-chloroform-isoamyl alcohol (25:24:1) and once with chloroform-isoamyl alcohol. DNA was precipitated with 100% isopropanol, washed with 70% ethanol, and resuspended in TE (50 mM Tris, 10 mM EDTA, pH 7.0) buffer. Genomic DNA was digested with restriction enzymes for 1 to 2 h at 37°C in accordance with the specifications of the manufacturer (Promega, Madison, Wis.). Digested DNA was separated on 0.7 to 1% agarose gels overnight. Separated DNA was denatured, neutralized, transferred to nylon filters by using a negative-pressure transfer apparatus (Pharmacia LKB, Uppsala, Sweden), and baked at 80°C for 1 to 2 h to fix the DNA to the filter. Filters were prehybridized and hybridized with digoxigenin-labeled probes overnight at 68°C and washed under conditions of high stringency in accordance with the specifications of the manufacturer (Boehringer Mannheim, Indianapolis, Ind.).
In most cases, DNA probes were derived from cloned fragments and were labeled either by a random primer method in accordance with the protocol supplied by the manufacturer (Boehringer Mannheim) or by PCR amplification of cloned inserts, using the forward and reverse pUC18 primers and labeling mix as recommended by the manufacturer (Boehringer Mannheim). Probes for the joint region of circularized forms of conjugative transposon Tn5381 were amplified directly from enterococcal genomic DNA as previously described (21). The PCR products were labeled with digoxigenin by a random primer method (Boehringer Mannheim Biochemicals). Since Tn5381 has a single EcoRI site, this probe will hybridize to two fragments of genomic DNA for every Tn5381 insertion. Tn5381 is devoid of SmaI restriction sites, and so this probe will hybridize to only a single fragment in SmaI digests. Circular forms of the transposon are generally present in quantities too small to confuse hybridization results.
Cloning of genomic DNA fragments.
Once fragments of interest were identified by hybridization, they were removed from agarose gels and purified by using a glass bead preparation (Geneclean, La Jolla, Calif.). These fragments were then ligated to like-digested pUC18 or pBCSK+ and transformed into E. coli DH5α (9) or E. coli DH10B (Bethesda Research Laboratories, Gaithersburg, Md.) by electroporation (Bio-Rad, Hercules, Calif.). Transformed preparations were inoculated onto plates containing antimicrobial agents selective for the cloning vectors, and colonies with the appropriate inserts were identified by colony hybridization techniques as previously described (4). These colonies were purified and subcloned as necessary for further sequencing.
PCR amplification.
Several regions were amplified with primers derived from sequences within or flanking Tn5385. Amplification of genomic DNA was performed on a Perkin-Elmer Cetus 9600 thermal cycler, using Taq DNA polymerase, in accordance with standard protocols as recommended by the manufacturer (Perkin-Elmer Cetus, Roche Molecular Systems, Branchburg, N.J.). Variations were introduced into each individual protocol depending on the expected size of the amplification product and the specifics of the primers used. A 10-μl aliquot of the total 50-μl PCR product was loaded on a 0.7 to 1.2% agarose gel for analysis.
DNA sequence analysis.
In most cases, DNA sequencing was performed from cloned DNA on double-stranded templates with an A.L.F. automated sequencing kit and fluorescein- or Cy5 indodicarbocyanine dye-labeled forward and reverse primers. In some cases, sequencing was performed directly from PCR amplification products, using a nested primer. PCR products were purified with QIA-quick PCR purification columns (Qiagen, Inc., Chatsworth, Calif.). Cycle sequencing of these products was performed with a GeneAmp PCR System 9600 thermal cycler (Perkin-Elmer Cetus), using the Cy5 autocycle sequencing kit (Pharmacia LKB) in accordance with the manufacturer’s specifications. Primers used in these experiments are listed in Table 2. Sequences were determined with the ALFExpress automated sequencer (Pharmacia LKB). Sequences were compared with sequences entered into the GenBank, EMBL, DDBJ, and PDB databases by using blastn and blastx local alignment search tools (1) and then further analyzed by using the MacDNAsis version 2.0 (Hitachi, Ltd.) and DNAStar (DNAStar, Madison, Wis.) sequence analysis programs.
TABLE 2.
Custom primers used in amplification and sequencing experiments
| Primer (Fig. 1 designation) | Sequence (5′→3′) | Description |
|---|---|---|
| 346for-80 (A) | TCTGTCACTTGCTTCTCCTCTTT | Alkyl hydrogen peroxide reductase primer |
| IS1216-698Cy5 (B) | TCTCTTCGGGTTTTCGGTCTG | Nested primer for sequencing from within IS1216 |
| IS1216For (C) | AATTTATTGCGTCTCTTTACTGGA | PCR primer for amplifying IS1216-containing fragments |
| IS1216-110Cy5 (D) | CCCACGGCTACAATAATCACA | Nested primer for sequencing from within IS1216 |
| IS1216Rev (E) | CCGTGGGCTACTATCTTCGTT | PCR primer for amplifying IS1216-containing fragments |
| CH3AT1216 (F) | TGAAGAAACACAAAGGGAATG | Primer downstream of aadE gene; used to read outward from left end of Tn5385 |
| Strep DOWN (G) | GTAAAACCGGCTGCCTGGATAG | Downstream of aadE, toward left end of Tn5385 |
| Strep UP (H) | TGAACCACCCTAAAACAATACCTT | Upstream of aadE ORF, toward Tn5381 |
| JointR (I) | CGTGAAGTATCTTCCTACAGT | Primer reading outward from end of Tn5381 |
| JointL (J) | GGATAAATCGTCGTATCAAAG | Primer reading outward from end of Tn5381 |
| 86-301 (K) | AAATAAAAGAACCGAAATACAGG | Primer downstream of β-lactamase gene; used to read outward from right end of Tn5385 |
| 351 forward (L) | GGACAATTTTAGACCCTGCTGAG | Alkyl hydrogen peroxide reductase primer |
GenBank accession number.
The sequence of the target open reading frame (ORF) within the chromosome of JH2-7 has been entered into the GenBank database. The accession number is AF016233.
RESULTS
Structure of Tn5385.
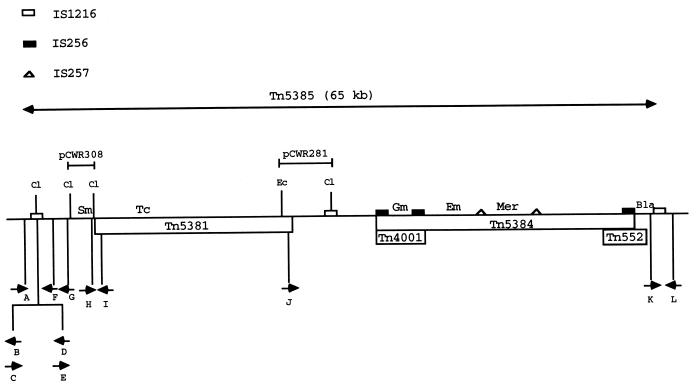
The structure of Tn5385 has been deduced by using a combination of hybridization, cloning, sequencing, and PCR amplification techniques and is shown in Fig. 1. The region between the internal and right-end IS1216 elements has been described in several previous publications (4, 17, 19, 20). The internal structure of Tn5381 has also been described previously (21). Analysis of clones pCWR281 and pCWR308 (Fig. 1) allowed us to determine the relative positions of the internal IS1216 element and the right end of Tn5381 (ca. 3 kb apart) and to show that the ClaI site used to clone the aadE streptomycin resistance gene was located 75 bp from the left terminus of Tn5381. The distance between the aadE gene and the left-end IS1216 element is approximately 4 kb. Partial sequencing of the region immediately internal to the left-end IS1216 revealed 127 bases with 97% sequence identity to an internal region of ORF zeta from Streptococcus pyogenes plasmid pSM19035 (5). This ORF, which is interrupted by IS1216, has no defined function. Sequence analysis of another 400-bp region between the aadE gene and IS1216 revealed no homology with sequences in the database. These data allowed us to construct the detailed map of Tn5385 shown in Fig. 1. Details of primers listed are in Table 2, and their positions are shown in Fig. 1. The total distance between the terminal IS1216 elements of Tn5385 is approximately 65 kb.
FIG. 1.
Schematic portrayal of the structure of Tn5385. Symbols for the different insertion sequences are shown at the top of the figure. Inserts from relevant plasmids are indicated above the figure. Relevant restriction sites are also indicated above the transposon (Cl, ClaI; Ec, EcoRI). The positions of the different resistance genes are noted above the transposon (Bla, β-lactamase gene; Em, ermAM erythromycin resistance gene; Gm, aac6′-aph2" high-level gentamicin resistance gene; Mer, merRAB mercuric chloride resistance genes; Sm, aadE streptomycin resistance gene; and Tc, tetM tetracycline-minocycline resistance gene). Different transposons and transposon-like elements are indicated by boxes below the transposon. Arrows below the transposon represent primers used for amplification and sequencing in these experiments (see Table 2 for details of primers).
Directly repeated copies of IS1216 form the ends of Tn5385.
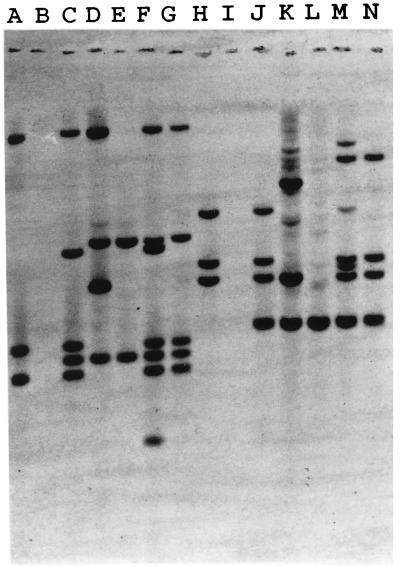
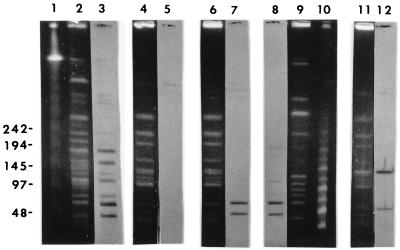
In previously published experiments, we identified IS1216 downstream of the β-lactamase gene within Tn5385 (20). An internal fragment of this IS element was cloned into pUC18 for use as a probe (pCWR91). We detected five copies of IS1216 within the genomes of donor strains CH19 and CH116 (Fig. 2) (the two additional copies observed in CH116 represent a recent [after March 1997] change in the IS1216 profile of this strain). Two transconjugants in which all resistance determinants were transferred were selected for further study. The first, CV34, was the product of a mating between CH116 and JH2-7. The second, CV123, was the product of a mating between CH19 and OG1XRF. JH2-7 has two copies of IS1216 present within its genome (Fig. 2), neither of which is involved in the transfer event. OG1XRF is devoid of IS1216 copies. Hybridization of genomic DNA from CV34 and CV123 after digestion with either HindIII or HincII (neither of which cuts at sites within IS1216) revealed the transfer of three copies of IS1216 in association with the resistance determinants of Tn5385 (Fig. 2). No change in restriction fragment size was observed when the IS1216-hybridizing fragments from donors and transconjugants were compared, suggesting that the three copies of IS1216 were all internal to the transferred region. Pulsed-field gel electrophoresis of SmaI-digested DNA from donors and transconjugants revealed two similarly sized IS1216-hybridizing fragments in CH19, CH116, CV34, and CV123 (Fig. 3). The sizes of these fragments added up to ca. 100 kb, which supported the hypothesis that the transferred region was larger than the 65 kb between the directly repeated IS1216 elements. However, IS1216 hybridization of NotI-digested DNA from OGIXRF and CV123 revealed only one hybridizing fragment (data not shown). The OG1XRF fragment into which insertion occurred increased in size from ca. 280 kb to ca. 350 kb. This size was consistent with the transferred DNA being Tn5385. The assumption that the DNA acquired by the transconjugant consisted of at least 100 kb (based on SmaI digestion of donor and transconjugant DNA) was therefore incorrect. The alternative hypothesis suggested by these findings was that Tn5385 had transferred into a very specific site within the JH2-7 chromosome in which flanking SmaI sites were conserved in the donor and the recipient.
FIG. 2.
Hybridization of genomic DNA from donors, recipients, and transconjugants with an internal fragment of IS1216 (pCWR91). Lanes: A, CV123 (HindIII); B, OG1XRF (HindIII); C, CH19 (HindIII); D, CV61 (HindIII); E, JH2-7 (HindIII); F, CH116 (HindIII); G, CV34 (HindIII); H, CV123 (HincII); I, OG1XRF (HincII); J, CH19 (HincII); K, CV61 (HincII); L, JH2-7 (HincII); M, CH116 (HincII); N, CV34 (HincII).
FIG. 3.
Hybridizations of IS1216 with SmaI-digested genomic DNA from donors and transconjugants. Lanes: 1, megabase size standards (Bio-Rad); 2, CH116; 4, JH2-7; 6, CV34; 9, CV123; 11, CV61. Lanes 3, 5, 7, 8, and 12 represent IS1216 hybridizations of Southern transfers of CH116, JH2-7, CV34, CV123, and CV61, respectively.
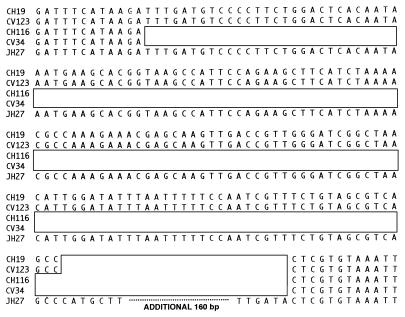
To investigate this possibility, we cloned the left IS1216-chromosome junction from CV123. A subfragment devoid of IS1216 sequences was then used as a probe of genomic digests of OG1XRF, CV123, JH2-7, and CV34. This probe was used to identify the target site for insertion in JH2-7, which was found to be within a 7-kb ClaI fragment. Sequence analysis of the target site revealed an ORF with significant homology to previously described alkyl hydrogen peroxide reductase genes from several different species (2, 10). This target fragment was then used to confirm that the terminal IS1216 elements of Tn5385 represented the ends of the transferred DNA. The junction sequences on both sides of Tn5385 were determined for CH19, CH116, CV34, and CV123, and the target region for OG1XRF was also determined. In all cases, Tn5385 was inserted within the putative alkyl hydrogen peroxide reductase ORF. A comparison of the junction sequences from the different strains is shown in Fig. 4. The right-end Tn5385-chromosome junctions in CH19 and CH116 were identical. The left junctions of the two strains differed. Both left junctions demonstrated deletions of the target ORF. However, the deletion adjacent to Tn5385 (CH116) was 151 bp larger than that adjacent to Tn5385 (CH19). When sequences of junctions from transconjugants CV34 and CV123 were compared with those of the donors, it was noted that the identical deletions present in the donors were carried over to the transconjugants. In addition, base changes characteristic of flanking sequences in the donor (as opposed to the target sequence in the recipient) were also present in the transconjugants. Moreover, the target ORF, intact after PCR amplification in both JH2-7 and OG1XRF, was lost in the transconjugants (Table 3), suggesting that insertion of Tn5385 into the recipient genome occurred by a process that deleted the original target, a finding most consistent with homologous recombination being the mechanism of insertion. In support of this hypothesis, we have never observed transfer of Tn5385 into E. faecalis UV202, a recombination-deficient strain, despite several attempts.
FIG. 4.
Nucleotide sequences of Tn5385-flanking regions in donors and transconjugants and their comparison with the target sequence within JH2-7. Open boxes represent the positions of Tn5385 insertions. To save space, the entire JH2-7 sequence is not shown. The sequence has been entered into the GenBank database under accession no. AF016233.
TABLE 3.
Amplification products of PCR with selected primer pairs
| Strain | Amplification with primer paira:
|
|||
|---|---|---|---|---|
| A-F | K-L | A-L | F-K | |
| CH19 | + | + | − | − |
| CV123 | + | + | − | − |
| CV61 | − | − | + | + |
| CH116 | + | + | − | − |
| CV34 | + | + | − | − |
Evidence for a second insertion mechanism.
We noted a series of transconjugants resulting from a mating between CH19 and JH2-7 for which a different pattern of IS1216 hybridization was observed (Fig. 2 and 3). One such transconjugant (CV61) was selected for further study. CV61 expressed all of the Tn5385-associated resistances. Two of these determinants (β-lactamase production and streptomycin resistance) were encoded by genes at opposite ends of Tn5385 in the donor; this suggested that the entire element had transferred to the recipient cell. In contrast to the transconjugants described above, however, transfer of Tn5385 resulting in CV61 was accompanied by the transfer of two, rather than three, copies of IS1216 (Fig. 2). One of these copies, the copy internal to Tn5385, was identical in size in the donor and the transconjugant. The second copy did not correspond in size to either of the terminal copies of IS1216. IS1216 hybridization of SmaI-digested genomic DNA from CV61 also revealed a hybridization pattern different from those observed with the other class of transconjugants (Fig. 3). β-Lactamase and gentamicin resistance genes hybridized to the larger of the two IS1216-hybridizing SmaI fragments shown in Fig. 3 (data not shown). However, hybridization with the Tn5381 joint probe (21) revealed the presence of these sequences in both of the IS1216-hybridizing bands, suggesting that two copies of Tn5381 were present in the CV61 genome and that both were in relatively close proximity to the transferred IS1216 elements. Hybridization of EcoRI digests of genomic DNA with the Tn5381 joint probe confirmed that a second copy of Tn5381 was present within CV61 (data not shown). We considered the possibility that Tn5385 had circularized by recombination between the two terminal copies of IS1216, was transferred to the recipient cell, and then entered into the chromosome by homologous recombination across Tn5381 within the element and a previously transferred copy of Tn5381 in the recipient chromosome. We cloned the 5-kb IS1216-hybridizing HindIII fragment of CV61 into Escherichia coli, and analysis revealed that sequences internal to the terminal IS1216 elements of Tn5385 flanked the novel IS1216 in this insertion, confirming that Tn5385 had circularized by recombination between the terminal IS1216 elements. The joining of the ends of Tn5385 across IS1216 in the CV61 chromosome was confirmed by amplification of the expected product with primers directing polymerization outward from the ends of the element (Table 3). This product was observable in neither the two donors, nor CV34, nor CV123. PCR amplification of the intact hydrogen peroxide reductase gene in CV61 was also demonstrable, confirming that entry into the recipient chromosome did not occur across this region.
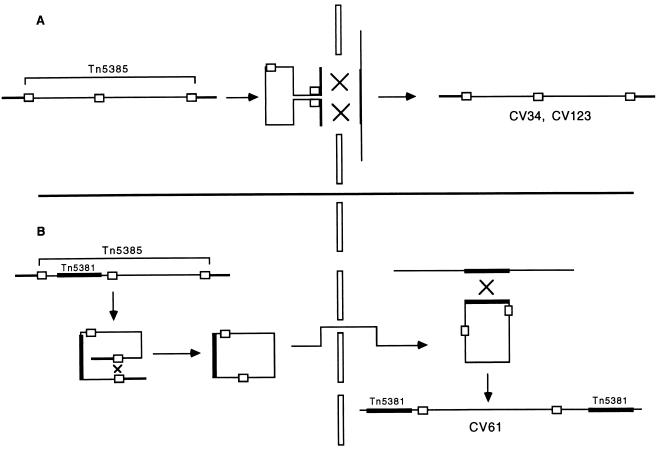
We considered two alternative scenarios for the insertion of Tn5385 into the JH2-7 chromosome resulting in CV61. The first is that insertion represented a cointegration event mediated by Tn5381. We have no experimental data to exclude this possibility. However, the mechanism of transposition of conjugative transposons is well documented to be conservative. Cointegration mediated by replicative transposition of conjugative transposons has never been described for these well-studied elements, so we consider this scenario unlikely. Moreover, the insertion of multiple copies of Tn5381, and other conjugative transposons, into recipient chromosomes during in vitro mating events has been documented (21), lending credibility to the hypothesis involving a previously transferred copy of Tn5381. The second alternative scenario involves insertion into the recipient chromosome by IS element-mediated cointegration. Restriction mapping studies indicate that the maps flanking all of the internal IS elements are unchanged in CV61 in comparison to CH19 (data not shown), arguing against a cointegration event mediated by one of these elements. Once circularized, Tn5385 should theoretically be able to cointegrate with another replicon by using any of the resident IS elements. That we have identified no such insertions to date may simply be due to the fact that we have not searched for them in a systematic and exhaustive fashion. A graphic description of the proposed mechanisms of the two transfer events is illustrated in Fig. 5.
FIG. 5.
Graphic representation of proposed mechanisms for integration of Tn5385 into recipient chromosomes in the two types of transconjugants described in this paper. (A) Proposed mechanism resulting in transconjugants CV34 and CV123. Darkened lines flanking Tn5385 in the donor and recipient chromosomes represent homologous regions across which recombination occurs. (B) Proposed mechanism resulting in transconjugant CV61. The initial step is circularization across terminal IS1216 elements. The circularized form then transfers to the recipient cell and recombines across Tn5381 within the circularized Tn5385 and a previously transferred copy of Tn5381 that has already integrated into the recipient chromosome.
DISCUSSION
We previously reported analyses of regions of Tn5385 that suggested it had evolved as a composite of staphylococcal and enterococcal plasmids and transposons (4, 17). The data reported in this paper further extend these observations. In sum, Tn5385 is a ca. 65-kb composite structure that includes segments characteristic of diverse species and genera, including Staphylococcus aureus (IS257, mercury resistance operon, Tn552-like β-lactamase transposon, and relaxase-mobilization region of small staphylococcal plasmids), broad-host-range enterococci (Tn5381, Tn4001, broad-host-range replication genes, and Tn917), enterococci (IS1216), and S. pyogenes (partial ORF zeta from S. pyogenes plasmid pSM19035). It appears that Tn5385 originated as a plasmid, one which became more complex as it cointegrated with other plasmids. The ability to acquire genetic material from such diverse genera was probably conferred by broad-host-range plasmid conjugation genes that have subsequently been deleted (4). It is intriguing that roughly 38 kb (58%) of Tn5385 is composed of antimicrobial resistance determinants or of structures conferring mobility to such determinants. The concentration of this collection in a small region is reminiscent of integrons in gram-negative bacilli. Although no integron-like mechanism is obvious from the structure of Tn5385, it is tempting to speculate that this collection of determinants represents a gram-positive equivalent of an integron, mediated by the activity of a variety of IS elements. This report represents the first example of such a complex, chromosomally based element in enterococci.
The structure of Tn5385 as described in this paper suggests a mechanism for insertion of the putative composite plasmid into the bacterial chromosome: cointegration mediated by a copy of IS1216 within the presumed plasmid. Supportive of this scenario is the fact that portions of the same ORF are found flanking the terminal, directly repeated IS1216 elements. IS1216 is a member of the large ISS1 family of ISs that characteristically generate 8-bp duplications of the target sequence on insertion; these were not observed in the two Tn5385 insertions detailed in this paper (8). The absence of target duplications flanking Tn5385 is explainable by the occurrence of secondary transposition events resulting in deletions of different segments of the target ORF in CH19 and CH116. In most other respects (e.g., SmaI digest pulsed-field gel electrophoresis patterns and IS6770 hybridization patterns), CH19 and CH116 are indistinguishable. The observed differences in sequence immediately adjacent to the Tn5385 insertion sites in the two strains are consistent with the occurrence of a single insertion event followed by divergence in the quantity of deleted adjacent DNA associated with subsequent rearrangements. Tn5385 appears to require the replication functions of either the chromosome or another plasmid, since the presumed replication origin (for broad-host-range plasmids) has been deleted (4).
Although Tn5385 does not meet the classic definition of a transposon based on currently available information, it exhibits many characteristics of known transposons. First, it is flanked by directly repeated copies of an IS element (the characteristic conformation of composite transposons) known to be insertionally active in enterococci (11). Previous reports have implicated IS1216 in the transposition of vancomycin resistance determinants in E. faecium (11). Second, at least one of the terminal IS1216 copies (the one on the right end) appears to have been involved in the original insertion event, since the insertion sites in both CH19 and CH116 are identical. It is unclear whether the left-end IS1216 elements in CH19 and CH116 are the copies involved in the original insertion, since subsequent rearrangements appear to have occurred. Finally, the chromosomal insertion of Tn5385 in CV61 is consistent with circularization of Tn5385 followed by entry into the recipient chromosome by recombination across copies of conjugative transposons. Evidence of circularization, while not definitive, is highly suggestive that Tn5385 is in fact a transposable element.
There has been debate in the published literature about whether conjugative transposition proceeds by a mechanism resembling a cell fusion event. Torres et al. originally reported the transfer of unlinked chromosomal loci between Bacillus subtilis strains in the presence of conjugative transposon Tn925 in the donor chromosome (26). Showsh and Andrews failed to detect the occurrence of retrotransfer of nonconjugative plasmids in association with conjugative transposition of Tn916 or Tn925, concluding that a cell fusion-like event was unlikely (24). The data presented in this paper are consistent with the occurrence of a cell fusion-like event. The insertion of Tn5385 in recipient chromosomes, resulting in transconjugants CV34 and CV123, bears all of the marks of recombination across regions of chromosomal homology, which would be consistent with cell fusion. The facts that IS1216-hybridizing SmaI fragments are identical in size in donors and transconjugants and that they add up to ca. 100 kb suggest that the area of crossover is substantial and considerably greater than the limits of Tn5385. The carryover of the specific deletions to the transconjugants argues strongly against site-specific insertion of circularized Tn5385 as the mechanism of entry, although circularization into a much larger element than is defined by the terminal IS1216 elements, one that includes the flanking regions of homology and possesses transfer genes, remains a possibility that would not require invoking cell fusion.
Insertion of Tn5385 into the enterococcal chromosome has allowed us to identify a homolog of alkyl hydrogen peroxide reductase genes found in several other genera (2, 10). Mutant strains of Salmonella typhimurium that lack the alkyl hydrogen peroxide reductase gene have been shown to be extremely sensitive to killing by organic hydroperoxides (25). A similar gene in Staphylococcus aureus has been shown to be expressed at increased levels following osmotic stress (2). Preliminary experiments comparing the abilities of recipient and transconjugant strains to grow in the presence of increasing concentrations of hydrogen peroxide revealed no difference in growth rates (data not shown). The role played by this gene in the survival and growth of enterococci remains unclear.
We do not at the present time know what promotes the conjugation event resulting in Tn5385 transfer. A single plasmid is present in CH19 but does not transfer in association with Tn5385 (data not shown). CH116 has no detectable plasmids. It remains possible that transfer is mediated by Tn5381, the conjugative transposon within Tn5385. Tn5381 transfers alone at a much higher frequency than it does when within Tn5385 (ca. 10−6 and 10−9/recipient CFU, respectively) (21). In addition, conjugative transfer of Tn5381 is increased after preincubation of donor strains with tetracycline, apparently because of an increased rate of excision of the element (21). Preincubation with tetracycline does not lead to an increase in the transfer of Tn5385 from either CH19 or CH116 (data not shown), arguing against the possibility that Tn5381 excision stimulates transfer of Tn5385. If, however, Tn5381 transfers by creating cell fusion events, the random transfer of Tn5385 by chromosomal recombination would be expected to occur at a low frequency. Experiments to investigate the role of Tn5381 in the transfer of Tn5385 are planned.
The data presented in this paper emphasize the important role played by insertion elements in the evolution of antimicrobial resistance in gram-positive genera. A circularized form of Tn5385, if it can exist, would be a highly versatile integration element, since it would possess several active IS elements which could stimulate cointegration with other replicons (IS256, three copies; IS1216, two copies; and IS257, two copies). In addition, each of these frequently repeated elements (including Tn5381) can cointegrate with other replicons by homologous recombination, an event which apparently occurred across copies of Tn5381 to result in the transconjugant CV61. The ability of different mobile elements to cooperate in this fashion is a powerful tool for the dissemination of antimicrobial resistance determinants among gram-positive genera.
ACKNOWLEDGMENT
This work was supported by a Merit Review (to L.B.R.) from the Department of Veterans Affairs.
REFERENCES
- 1.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong-Buisseret L, Cole M B, Stewart G S A B. A homologue to the Escherichia coli alkyl hydrogen peroxide reductase AhpC is induced by osmotic upshock in Staphylococcus aureus. Microbiology. 1995. pp. 1655–1661. [DOI] [PubMed] [Google Scholar]
- 3.Ayoubi P, Kilic A O, Vijayakumar M N. Tn5253, the pneumococcal Ω(cat tet) BM6001 element, is a composite structure of two conjugative transposons, Tn5251 and Tn5252. J Bacteriol. 1991;173:1617–1622. doi: 10.1128/jb.173.5.1617-1622.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonafede M E, Carias L L, Rice L B. Enterococcal transposon Tn5384: evolution of a composite transposon through cointegration of enterococcal and staphylococcal plasmids. Antimicrob Agents Chemother. 1997;41:1854–1858. doi: 10.1128/aac.41.9.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ceglowski P, Boitsov A, Chai S, Alonso J C. Analysis of the stabilization system of the pSM19035-derived plasmid pBT233 in Bacillus subtilis. Gene. 1993;136:1–12. doi: 10.1016/0378-1119(93)90441-5. [DOI] [PubMed] [Google Scholar]
- 6.Clewell D B, Gawron-Burke C. Conjugative transposons and the dissemination of antibiotic resistance in streptococci. Annu Rev Microbiol. 1986;40:635–659. doi: 10.1146/annurev.mi.40.100186.003223. [DOI] [PubMed] [Google Scholar]
- 7.Courvalin P, Carlier C. Tn1545: a conjugative shuttle transposon. Mol Gen Genet. 1987;206:259–264. doi: 10.1007/BF00333582. [DOI] [PubMed] [Google Scholar]
- 8.Galas D J, Chandler M. Bacterial insertion sequences. In: Berg D E, Howe M M, editors. Mobile DNA. Washington, D.C: American Society for Microbiology; 1989. pp. 109–162. [Google Scholar]
- 9.Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983;166:577–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 10.Hartford O M, Dowds B C A. Isolation and characterization of a hydrogen peroxide resistant mutant of Bacillus subtilis. Microbiology. 1994;140:297–304. doi: 10.1099/13500872-140-2-297. [DOI] [PubMed] [Google Scholar]
- 11.Heaton M P, Discotto L F, Pucci M J, Handwerger S. Mobilization of vancomycin resistance by transposon-mediated fusion of a VanA plasmid with an Enterococcus faecium sex pheromone-response plasmid. Gene. 1996;171:9–17. doi: 10.1016/0378-1119(96)00022-4. [DOI] [PubMed] [Google Scholar]
- 12.Ike Y, Craig R A, White B A, Yagi Y, Clewell D B. Modification of Streptococcus faecalis sex pheromones after acquisition of plasmid DNA. Proc Natl Acad Sci USA. 1983;80:5369–5373. doi: 10.1073/pnas.80.17.5369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacob A E, Hobbs S J. Conjugal transfer of plasmid-borne multiple antibiotic resistance in Streptococcus faecalis var. zymogenes. J Bacteriol. 1974;117:360–372. doi: 10.1128/jb.117.2.360-372.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kilic A O, Vijayakumar M N, Al-Khaldi S F. Identification and nucleotide sequence analysis of a transfer-related region in the streptococcal conjugative transposon Tn5252. J Bacteriol. 1994;176:5145–5150. doi: 10.1128/jb.176.16.5145-5150.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.LeBlanc D L, Inamine J M, Lee L N. Broad geographical distribution of homologous erythromycin, kanamycin, and streptomycin resistance among group D streptococci of human and animal origin. Antimicrob Agents Chemother. 1986;29:549–555. doi: 10.1128/aac.29.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rauch P J G, De Vos W M. Characterization of the novel nisin-sucrose conjugative transposon Tn5276 and its insertion in Lactococcus lactis. J Bacteriol. 1992;174:1280–1287. doi: 10.1128/jb.174.4.1280-1287.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rice L B, Carias L L, Marshall S H, Bonafede M C. Sequences found on staphylococcal β-lactamase plasmids integrated into the chromosome of Enterococcus faecalis CH116. Plasmid. 1996;35:81–90. doi: 10.1006/plas.1996.0010. [DOI] [PubMed] [Google Scholar]
- 18.Rice L B, Eliopoulos G M, Wennersten C, Goldmann D, Jacoby G A, Moellering R C., Jr Chromosomally mediated β-lactamase production and gentamicin resistance in Enterococcus faecalis. Antimicrob Agents Chemother. 1991;35:272–276. doi: 10.1128/aac.35.2.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rice L B, Marshall S H. Evidence of incorporation of the chromosomal β-lactamase gene of Enterococcus faecalis CH19 into a transposon derived from staphylococci. Antimicrob Agents Chemother. 1992;36:1843–1846. doi: 10.1128/aac.36.9.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rice L B, Marshall S H. Insertions of IS256-like element flanking the chromosomal β-lactamase gene of Enterococcus faecalis CX19. Antimicrob Agents Chemother. 1994;38:693–701. doi: 10.1128/aac.38.4.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rice L B, Marshall S H, Carias L L. Tn5381, a conjugative transposon identifiable as a circular form in Enterococcus faecalis. J Bacteriol. 1992;174:7308–7315. doi: 10.1128/jb.174.22.7308-7315.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rose R E. The nucleotide sequence of pACYC184. Nucleic Acids Res. 1988;16:355. doi: 10.1093/nar/16.1.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scott J R, Churchward G G. Conjugative transposition. Annu Rev Microbiol. 1995;49:367–397. doi: 10.1146/annurev.mi.49.100195.002055. [DOI] [PubMed] [Google Scholar]
- 24.Showsh S A, Andrews R E., Jr Functional comparison of conjugative transposons Tn916 and Tn925. Plasmid. 1996;35:164–173. doi: 10.1006/plas.1996.0019. [DOI] [PubMed] [Google Scholar]
- 25.Storz G, Jacobson F S, Tartaglia L A, Morgan R W, Silveira L A, Ames B N. An alkyl hydroperoxide reductase induced by oxidative stress in Salmonella typhimurium and Escherichia coli: genetic characterization and cloning of ahp. J Bacteriol. 1989;171:2049–2055. doi: 10.1128/jb.171.4.2049-2055.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Torres O R, Korman R Z, Zahler S A, Dunny G M. The conjugative transposon Tn925: enhancement of conjugal transfer by tetracycline in Enterococcus faecalis and mobilization of chromosomal genes in both Bacillus subtilis and E. faecalis. Mol Gen Genet. 1991;225:395–400. doi: 10.1007/BF00261679. [DOI] [PubMed] [Google Scholar]
- 27.Vijayakumar M N, Ayalew S. Nucleotide sequence analysis of the termini and chromosomal locus involved in site-specific integration of the streptococcal conjugative transposon Tn5252. J Bacteriol. 1993;175:2713–2719. doi: 10.1128/jb.175.9.2713-2719.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yagi Y, Clewell D B. Recombination-deficient mutants of Streptococcus faecalis. J Bacteriol. 1980;143:966–970. doi: 10.1128/jb.143.2.966-970.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]