Abstract
Acute respiratory tract infections (ARTI) are caused by respiratory pathogens and range from asymptomatic infections to severe respiratory diseases. These diseases can be life threatening with high morbidity and mortality worldwide. Under the pandemic of coronavirus disease 2019 (COVID-19), little has been reported about the pathogen etiologies and epidemiology of patients suffering from ARTI of all age in Xiamen. Region-specific surveillance in individuals with ARTI of all ages was performed in Xiamen from January 2020 to October 2022. Here, we observed the epidemiological characteristics of thirteen pathogens within ARTI patients and further revealed the difference of that between upper respiratory tract infections (URTI) and lower respiratory tract infections (LRTI). In total 56.36 % (2358/4184) of the ARTI patients were positive for at least one respiratory pathogen. Rhinovirus (RVs, 29.22 %), influenza A (FluA, 19.59 %), respiratory syncytial virus (RSV, 18.36 %), metapneumovirus (MPV, 13.91 %), and adenovirus (ADV, 10.31 %) were the five leading respiratory pathogens. Respiratory pathogens displayed age- and season-specific patterns, even between URTI and LRTI. Compared with other groups, a higher proportion of FluA (52.17 % and 68.75 %, respectively) infection was found in the adult group and the elder group, while the lower proportion of RVs (14.11 % and 11.11 %) infection was also observed in them. Although ARTI cases circulated throughout the year, RVs, FluB, and BoV peaked in autumn, and FluA circulated more in summer. Besides, the co-infectious rate was 8.7 % with the most common for RVs. Logistic regression analyses revealed the correlations between respiratory pathogens and disease types. These results are essential for replenishing epidemiological characteristics of common respiratory pathogens that caused ARTI in Xiamen during the epidemic of COVID-19, and a better understanding of it might optimize the local prevention and clinical control.
Keywords: Acute respiratory tract infections, Upper respiratory tract infections, Lower inspiratory tract infections, Age-specific pattern, Season-specific pattern, Co-infectious rate
1. Introduction
Acute respiratory tract infections (ARTI) act as common illness, leading to millions of emergency or hospitalization each year. According to the data published by World Health Organization (WHO) in 2019, the ARTI, especially those of the lower respiratory tract infections (LRTI), were one of the leading causes for morbidity and mortality, which have been one of the public health problems worldwide [1]. Respiratory pathogens could get explosive during a short period of time due to their high contagion and rapid transmission [2,3]. More than two hundred viruses have been determined as the causative agents for ARTI with varying severity [4], such as respiratory syncytial virus (RSV), metapneumovirus (MPV), and rhinovirus (RVs). Therefore, an excellent understanding of pathogens epidemiology is very meaningful to relieve the burden of public healthcare.
More and more studies have reported the epidemiological features of respiratory pathogens at home and abroad, and a great diversity in pathogenic spectrum across countries even regions was observed owing to the variance of geographical location, humidity, and other factors [[5], [6], [7], [8], [9], [10]]. Currently, the majority of epidemiological studies on ARTI in China were conducted before 2019 [7,[11], [12], [13]]. Since the end of December 2019, coronavirus diseases 2019 (COVID-19) has been declared a pandemic which led to millions of respiratory emergencies [14]. Under the pandemic of COVID-19, the government in China adopt a series of strategies to contain the spread of new coronavirus, including wearing masks, hand hygiene disinfection, quarantine of infected individuals and vaccinations. The implementation of these positive measures might have affected the transmission of common respiratory pathogens that responsible for ARTI [15].
Xiamen, a special economic zone and sub-provincial area in China, exposed to a higher risk of respiratory infections owing to the transmission of respiratory pathogens along the large entering populations each year. Previously, several studies have reported the epidemiological features of respiratory pathogens in children under 2 years in Xiamen [12,16]. However, under the background of COVID-19 pandemic, there is little relevant data on epidemiological characteristics of respiratory pathogens that caused ARTI in all ages of the population in Xiamen. In order to develop reasonable prophylaxis against common respiratory pathogens, 4184 specimens between 2020 and 2022 were analyzed to reveal the characteristics of etiological agents. Of note, the study also indicate the effect of COVID-19 pandemic and positive measures on the transmission of common respiratory pathogens in population, leading to facilitate positive clinical management in the future.
2. Materials and methods
2.1. Study participants
Between January 2020 and October 2022, a retrospective study that enrolling ARTI patients of all ages from outpatient and inpatients at the First Affiliated Hospital of Xiamen University was conducted. ARTI patients were selected according to the following criteria: (1) fever accompanied by at least one symptom of cough, a stuffy nose, rhinorrhea, sore throat, sputum, and headache; (2) the experience of pathogenic detection. Patients with missing clinical records and/or repeated detection for pathogens were excluded from this study. The patients were further divided into the upper respiration tract infections (URTI) group and the lower respiration tract infections (LRTI) group according to their discharge diagnosis and clinical characteristics. The URTI is an illness which involves nose, pharynx and larynx, mainly including sinusitis, pharyngitis, tonsillitis, and laryngitis. And LRTI was predominated by pneumonia, bronchitis, bronchiectasis, and lung infections. The remaining patients with indefinite diagnosis were ignored in subsequent analyses.
2.2. Sample collection and pathogenic detection
Pharyngeal swabs were collected from all patients within 24 h after recruitment. According to normalized protocol, samples were collected using swabs through sampling on the tonsils and the posterior wall of the oropharynx, which were recovered into sterile collection tubes for pathogenic detection.
The total nucleic acids were extracted from clinical samples using nucleic acid extraction or purification kit (1060167, Ning Bo Health Genetech Co., China) according to the manufacture's instructions. After that, multiple pathogens detection was performed using ResP@13 respiratory pathogen multiplex kit according to the manufacturer's protocol (1060071, Ning Bo Health Genetech Co., China), which was based on the multiplex PCR-capillary electrophoresis fragment analysis. The following thirteen respiratory pathogens were tested: influenza A (FluA; pandemic AH7N9, AH1N1, AH3N2, AH5N2), influenza A-H1N1 (FluA-H1N1), influenza A-H3N2 (FluA-H3N2), influenza B (FluB), rhinovirus (RVs), respiratory syncytial virus (RSV; type A and B), metapneumovirus (MPV), adenovirus (ADV), parainfluenza virus (PIV; type Ⅰ-Ⅳ), bocavirus (BoV), coronavirus (CoV; pandemic 229E, OC43, NL63, and HKU1), as well as mycoplasma pneumonia (MP) and Chlamydia.
2.3. Data analysis
Categorical variables were represented as n (%), and the significance were calculated using Pearson's Chi-square test or Fisher's test. Logistic regression analysis was used to evaluate the correlations between respiratory pathogens and disease types. The statistical analyses were all performed based on SPSS v.25.0 software and GraphPad Prism v.8.0.1. P < 0.05 was considered to be statistically significant.
3. Results
3.1. Demographic characteristics
A total of 4645 ARTI patients were recruited and had samples tested, from which 461 specimens were excluded owning to missing clinical data and/or repetitive pathogen detection. Eventually, 4184 specimens were available for further analysis (Table 1). Of the 4184 specimens, the male and female represented 59.06 % and 40.94 % of the population, respectively. More than 85 % of patients aged under 14 years, the majority (28.65 %) of them was patients in pre-school child group, then the number of patients were subsequently decreased with aging. For the seasonality, the most frequent distribution of patients was in summer (1436, 34.32 %), followed by spring (1032, 24.67 %), winter (917, 21.92 %), and autumn (799, 19.10 %).
Table 1.
The demographic and clinical characteristics of patients.
| Total | URTI | LRTI | |
|---|---|---|---|
| N.patients | 4184 | 1332 | 2582 |
| Sex | |||
| Male, n (%) | 2471 (59.06) | 818 (61.41) | 1494 (57.86) |
| Female, n (%) | 1713 (40.94) | 514 (38.59) | 1088 (42.14) |
| Age (years) | |||
| Infant (, <1), n (%) | 727 (17.38) | 160 (12.01) | 534 (20.68) |
| Toddler [1, 3), n (%) | 1063 (25.41) | 439 (32.96) | 579 (22.42) |
| Preschool child [3, 6), n (%) | 1195 (28.65) | 354 (26.58) | 789 (30.56) |
| School child [6, 14), n (%) | 604 (14.44) | 292 (21.92) | 256 (9.91) |
| Adults [14, 60), n (%) | 411 (9.82) | 81 (6.08) | 263 (10.19) |
| Elders [60, ), n (%) | 184 (4.40) | 6 (0.45) | 161 (6.24) |
| Season | |||
| Spring (Mar–May), n (%) | 1032 (24.67) | 373 (28) | 623 (24.13) |
| Summer (Jun–Aug), n (%) | 1436 (34.32) | 468 (35.14) | 872 (33.77) |
| Autumn (Sept–Nov), n (%) | 799 (19.10) | 234 (17.57) | 508 (19.67) |
| Winter (Dec–Feb), n (%) | 917 (21.92) | 257 (19.29) | 579 (22.42) |
| Severity of disease | |||
| Mild (SI = 0), n (%) | / | 1196 (90.06) | 1049 (40.69) |
| Moderate (SI = 1), n (%) | / | 127 (9.56) | 1426 (55.31) |
| Slight severe (2 < SI < 3), n (%) | / | / | 12 (0.47) |
| Severe (SI > 3), n (%) | / | 5 (0.38) | 91 (3.53) |
Abbreviations: URTI, upper respiratory tract infections; LRTI, lower respiratory tract infections. Data were represented as numbers (% percentage).
There were 1332 patients with URTI and 2582 patients with LRTI according to clinical diagnosis, whose demographic distributions among gender, age, and seasons were basically consistent with that of overall cohort. The significant difference of disease severity between URTI group and LRTI group was also observed. As shown in Table 1, more than half of patients with LRTI suffered the moderate pain, while majority severity in patients with URTI was mild.
3.2. Overall positive detection
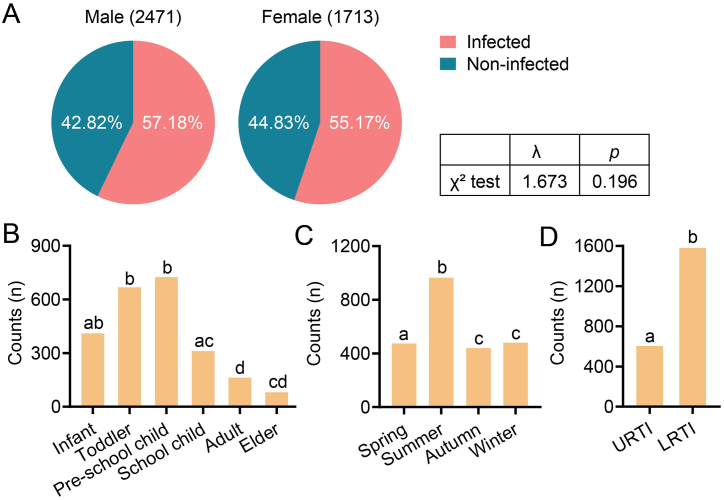
As shown in Table 2, 2358 (56.36 %) patients were positive for at least one respiratory pathogen. Generally, the positive detection of respiratory pathogens was comparable between genders (57.18 % in male and 55.17 % in female) (Fig. 1A). The highest rate of positive detection was observed in pre-school child group (30.75 %), and the rate of positive detection subsequently reduced with aging (Fig. 1B). For the seasonality, the highest positive rate of respiratory pathogens was observed in summer (40.92 %) throughout the year and followed by winter (20.36 %), spring (20.10 %), then autumn (18.62 %) (Fig. 1C). Further analysis revealed a great diversity for positive infections based on diseases types (Fig. 1D, Table 2). Specifically, the patients in toddler group with URTI had a higher positive infections than those with LRTI, while the patients in pre-school child group with LRTI peaked the positive rate.
Table 2.
The positive detection of pathogens across clinical diagnosis among patients.
| Total (2358) | URTI (604) | LRTI (1581) | χ2 test | p | |
|---|---|---|---|---|---|
| Gender, n (%) | 3.17 | 0.075 | |||
| male | 1413 (59.92) | 382 (63.25) | 934 (62.52) | ||
| Female | 945 (40.08) | 222 (36.75) | 647 (59.47) | ||
| Age, n (%) | 96.082 | <0.001 | |||
| Infant (, <1) | 410 (17.39) | 57 (9.44) | 338 (21.38) | ||
| Toddler [1, 3) | 668 (28.33) | 207 (34.27) | 424 (26.82) | ||
| Preschool child [3, 6) | 725 (30.75) | 194 (32.12) | 498 (31.50) | ||
| School child [6, 14) | 311 (13.19) | 116 (19.21) | 154 (9.74) | ||
| Adult [14, 60) | 163 (6.91) | 27 (4.47) | 101 (6.39) | ||
| Elder [60, ) | 81 (3.44) | 3 (0.50) | 66 (4.17) | ||
| Season, n (%) | 20.835 | <0.001 | |||
| Spring (Mar–May) | 474 (20.10) | 129 (21.36) | 328 (20.75) | ||
| Summer (Jun–Aug) | 965 (40.92) | 273 (45.20) | 616 (38.96) | ||
| Autumn (Sep–Nov) | 439 (18.62) | 77 (12.75) | 333 (21.06) | ||
| Winter (Dec–Feb) | 480 (20.36) | 125 (20.70) | 304 (19.23) |
Abbreviations: URTI, upper respiratory tract infections; LRTI, lower respiratory tract infections.
The significance was analyzed by Pearson's Chi-square test. P < 0.05 was considered as statistically significant and shown in corresponding cells.
Fig. 1.
Positive cases of patients based on different dimensions. (A) That of patients based on genders. (B) That of patients based on ages. (C) That of patients based on seasons. (D) That of patients based on disease types. URTI, upper respiratory tract infection. LRTI, lower respiratory tract infection. The p value was calculated by Pearson's Chi-square test. *p < 0.05, **p < 0.01, ***p < 0.001.
3.3. Pathogen spectrum on multiple dimensions
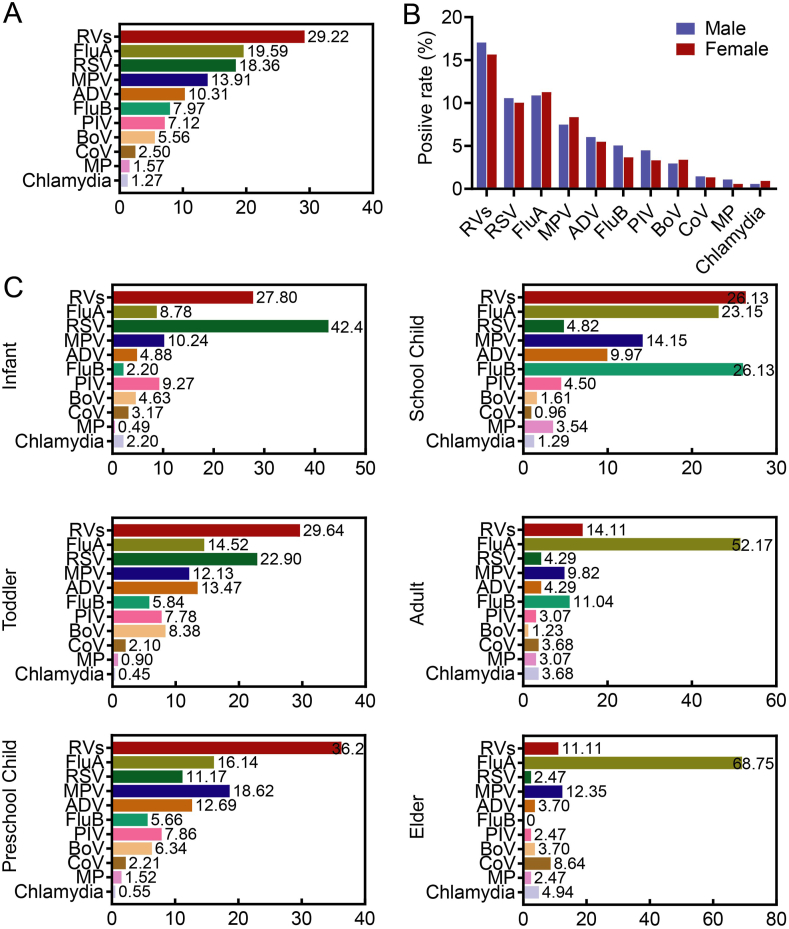
Based on the proportion of positive detection, RVs was the most frequently detected pathogen (account for 29.22 %) in all 2358 positive samples, followed by FluA (19.59 %), RSV (18.36 %), MPV (13.91 %), ADV (10.31 %), FluB (7.97 %), PIV (7.12 %), BoV (5.56 %), CoV (2.5 %), MP (1.57 %), and Chlamydia (1.27 %) (Fig. 2A). Among those who tested positive for FluA, 271 (58.66 %) were typed as FluA-H3N2, and 27 (5.84 %) were FluA-H1N1. In terms of gender, there was no difference in the prevalence of respiratory pathogens (Fig. 2B). Then age-specific characteristic of respiratory pathogens was observed in our study, as reflected by the different ranking of positive pathogens among age groups (Fig. 2C). Children under 14 years were more likely to be infected by RVs. In detail, RVs infection peaked in toddler group (29.64 %), pre-school child group (36.2 %), and school child group (26.13 %), while it drop to 14.11 % in adult group and 11.11 % in elder group. The outbreak of RSV was observed in infant group (42.4 %), and the risk of RSV infection gradually decreased with aging, indicating the age preference of RSV infection. For FluB, it also was the top pathogen in school child group (26.13 %), while a lower proportion was found in other groups. On the contrary, the patients aged more than fourteen years might be more susceptible for FluA, as reflected by the sharply elevated proportion of FluA in adult group (52.17 %) and elder group (68.75 %), while a relatively lower proportion in other groups. What was more, the epidemic of several respiratory pathogens kept stable regardless of age, such as MP and Chlamydia. In short, the age-specific characteristic of respiratory pathogens was determined in our study.
Fig. 2.
Pathogen spectrum of positive cases based on genders and ages. (A) The proportion of positive detection for each pathogen within 2358 positive specimens. (B) The difference of detected rate for each pathogen among genders. (C) The detected rate for each pathogen at per age group. The length of bars and the number behind indicate the proportion of each pathogen for per age group, calculated by its positive cases used as the numerator and the overall positive cases of all pathogens at per age group used as the denominator. The sums of viral infection might be more than 100 % because of the co-infection. RVs, rhinovirus; FluA, influenza A; RSV, respiratory syncytial virus; MPV, metapneumovirus; ADV, adenovirus; FluB, influenza B; PIV, parainfluenza virus; BoV, bocavirus; CoV, coronavirus; MP, mycoplasma pneumonia.
Further analysis revealed that the ranking of pathogens at per age group also varied with disease types. For the patients in infant group, RSV was the most frequent etiology in patients with LRTI, whereas it dropped behind RVs and FluA in patients with URTI. For the patients in school child group, the FluA was characterized as the most active pathogen in patients with URTI, while it down-ranked from first to third in patients with LRTI. These results suggested that all patients at per age group taken together contribute to an altered ranking of pathogens (Supplementary Table 1).
3.4. Seasonality distribution of pathogens
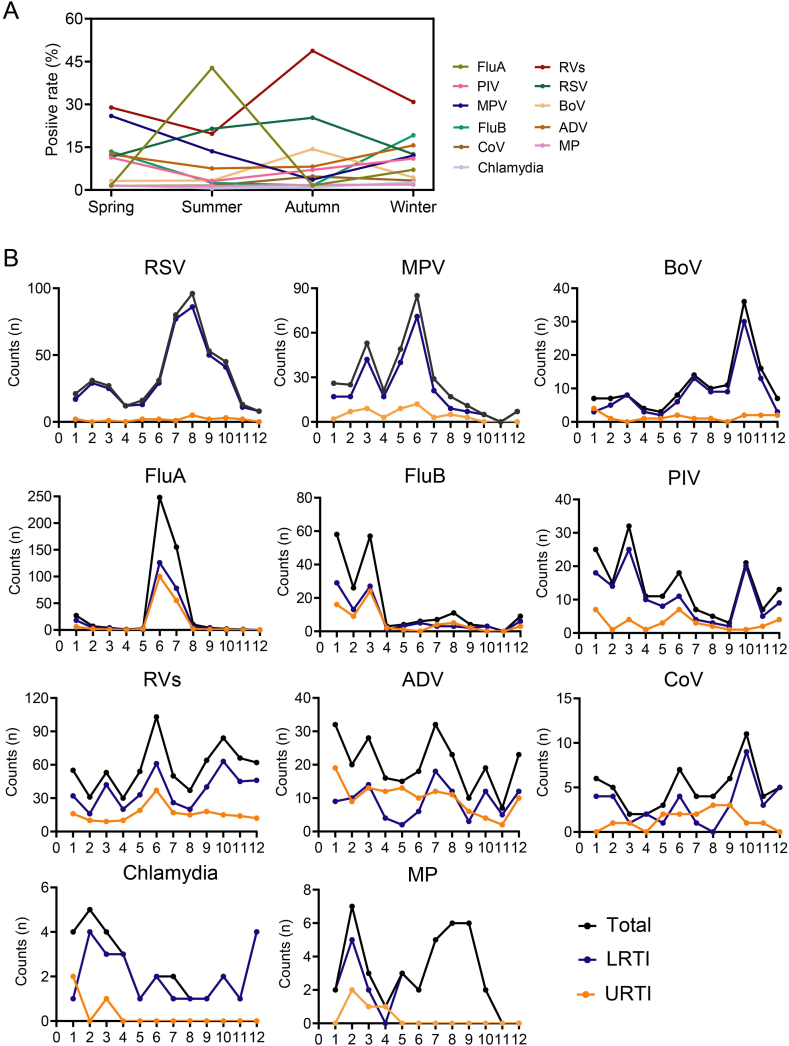
Respiratory pathogens were monthly detected during the study, with the overall positive detection of pathogens ranging from 37.75 % to 71.81 %. And the number and positive detection of ARTI both increased between June and July. Although the highest positive detection of respiratory pathogens in ARTI patients was in summer, the distribution of several viruses exhibited obvious seasonality with drastic changes over the four seasons (Fig. 3A). For example, FluA that mainly predominant by FluA-H3N2 remarkably peaked in summer (42.80 %) and kept silent in other seasons. RVs and RSV generally circulated year round and peaked in autumn (48.75 % and 25.28 %, respectively). ADV and PIV ascended in winter and spring, decreased in summer, slightly rose in autumn. Besides, the epidemic of several pathogens (CoV, MP, and Chlamydia) kept relatively steady that less than 5 % throughout the year. Therefore, the epidemiology of pathogens exhibited season-specific owing to different circulated characteristic for each pathogen (Fig. 3A).
Fig. 3.
Seasonality and time trend of eleven pathogens in patients with ARTI. (A) Seasonal characteristic of eleven pathogen spectrum. (B) The number of positive case for each pathogen over time between URTI and LRTI.
Further analysis found that the epidemic of specific pathogens in patients with URTI was inconsistent with those with LRTI over time. Most RSV, MPV, and BoV were detected in patients with LRTI that peaked in August, June, and October, respectively. While no obvious cyclic occurrence was observed in patients with URTI, as reflected by an extremely low incidence of them. PIV had three peaks throughout the year in patients with LRTI, the first in December to January, the second in March, and the third in October. Only one unimodal peak during December to January was observed in patients with URTI. None of patients with URTI was determined as positive for MP and chlamydia after May of the year, but one unimodal peak would be found in LRTI patients (Fig. 3B). What was more, either FluA or FluB were both common etiologies for URTI and LRTI, whose epidemics were consistent over time. In short, the season-specific distribution of respiratory pathogen should take disease types into account as appropriate.
3.5. Correlation between pathogens and diseases status
The logistic regression analyses were used to assess correlations between positive infection of respiratory pathogens and disease types. We found that patients infected by RSV (13.78, 8.73–21.77), MP (5.54, 1.95–15.71), Chlamydia (5.28, 1.57–17.72), BoV (4.02, 2.37–6.83), MPV (3.2, 2.36–4.36), PIV (2.33, 1.59–3.41), and RVs (1.42, 1.18–1.73) were positively associated with a significantly increased risk for LRTI. In addition, ADV infection was inversely associated with the LRTI in patients (0.45, 0.34–0.6) (Table 3).
Table 3.
Logistic regression of the positive cases for each pathogens and clinical diagnosis of patients.
| Virus | OR (95 % CI) | P |
|---|---|---|
| RSV | 13.78 (8.73, 21.77) | <0.001 |
| MP | 5.54 (1.95, 15.71) | 0.001 |
| Chlamydia | 5.28 (1.57, 17.72) | 0.007 |
| BoV | 4.02 (2.37, 6.83) | <0.001 |
| MPV | 3.20 (2.36, 4.36) | <0.001 |
| PIV | 2.33 (1.59, 3.41) | <0.001 |
| RVs | 1.42 (1.18, 1.73) | <0.001 |
| ADV | 0.45 (0.34, 0.60) | <0.001 |
| FluA | 0.86 (0.70, 1.07) | 0.178 |
3.6. Co-infectious pattern
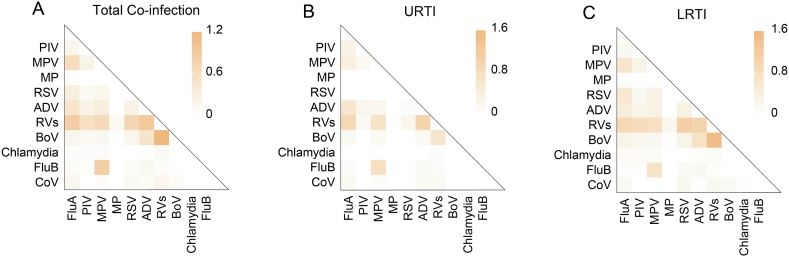
In our study, co-infections that more than one positive pathogens were observed in 364 specimens. A significantly higher detection of co-infections was observed in male (64.29 %) and pre-school group (33.24 %) (p < 0.05). Among the cases of co-infections, the positive kinds of two pathogens were detected in 322 (88.46 %) specimens, three pathogens in 38 (10.44 %) specimens, and four pathogens in 4 (1.10 %) specimens. RVs was the most common pathogen in co-infections, and the most frequent co-detection with RVs was BoV, followed by ADV, RSV, and FluA (Fig. 4A, Supplementary Table 2). However, the co-infections pattern of respiratory pathogens differed between disease types (Fig. 4B and C). The co-infectious pattern within LRTI was basically consistent with overall cohort. For the patients with URTI, not RVs-RSV but RVs-MPV was one of the most common patterns of co-infections. Therefore, it is suggested that the patterns of co-infection varied by clinical diseases.
Fig. 4.
Co-infections pattern of pathogens in patients with ARTI. (A) Co-infections rates were calculated among 4184 patients with ARTI. (B) Co-infections rates were calculated among 1332 patients with URTI. (C) Co-infection rates were calculated among 2582 patients with LRTI. The color of the block indicated the positive rate of co-infections.
4. Discussion
The study that carried out between January 2020 and October 2022 determined the age- and season-specific characteristics of respiratory pathogens. We further explored the difference of epidemiological characteristic in terms of disease types. In the present study, 56.36 % specimens were positive for at least one respiratory pathogen, which was basically consistent with previous surveillance from multiple countries [15,17]. On the contrary, the positive detection with less than 50 % was also reported in several studies that executed in developed and\or developing countries [7,18]. The above contradictions might be affected by the set of pathogen category, assay methods, and study population.
The five leading respiratory pathogens were RVs, FluA, RSV, MPV, and ADV, as have been shown elsewhere [18,19]. As reported previously, influenza was the most frequently detected pathogen in ARTI patients, while the rate of positive detection dropped from 33.8 % to 0.6 % in southern China during the period of COVID-19 epidemic [20]. In this study, the positive rate of FluA and FluB were 15.49 % and 4.49 %, respectively. Meanwhile, the decreased prevalence for FluA, especially the subtype of FluA-H3N2, has been observed previously compared with before COVID-19 pandemic, whose variation could be explained by a series of protective interventions against the COVID-19 outbreak [18,21]. Different from the infection that predominant by influenza, RVs as the most active pathogens in our study. Compared with the prevalence before COVID-19 epidemic, an increased prevalence of RVs was observed, which indicated not all pathogens were restricted by positive prevention [22]. It is suggested that the suitable strategies should be selectively used to treat specific pathogens under the background of COVID-19 pandemic. Different from other studies that merely focus on children, the patients of all age were all analyzed in our study. We found the highest risk for RSV infection was in children under 3 year, further corroborating the age-specific characteristic of RSV infection [5,7,18,23,24], which might be explained by the lower innate immunity response of child than that of adults [25]. In addition, CoV recently has received more attention owing to the discovery and burden of COVID-19 pandemic, CoV was more frequently detected in adult group and elder group in our study, which was consistent with the previous findings [13].
The absence of significant difference in overall positive detection between genders was consistent with previous studies [8,26]. We found that season was an attribute on overall epidemiology with the peak in summer and the trough in autumn. Specially, the prevalence of RVs was persistently high throughout one year. Influenza viruses, especially FluA, is one of the predominant agents for respiratory infections that might lead to severe diseases worldwide [27]. FluA that mainly predominant by the subtype of FluA-H3N2 was detected in summer with the highest rate, in line with previous studies [16,21,28].
According to the logistic regression analysis, the pathogens were more likely to be related to the prevalence of LRTI. Several lines of evidence have indicated that RSV infection was more likely related to LRTI, including pneumonia and asthma attack [29,30]. Similarly, a link between MPV infection and LRTI was observed in our study, which has been reported in previous studies, finding that 97.5 % patients with MPV infection suffered LRTI, including pneumonia and bronchitis [31]. Certain viral infections seem like to be associated with clinical phenotype. ADV was traditionally associated with URTI. Similarly, the correlation between ADV and URTI was observed in our study [32,33]. Although the majority of ARTI remain confined to the top tract [34], they can cause severe manifestations when affecting the lower tract.
Growing evidence showed that the prevalence of respiratory infections might be caused by more than one pathogens [35]. In the present study, co-infections were only detected in 8.7 % of the patients. Several studies have proposed that pathogens could interfere with each other, the detailed interactions between pathogens were still unclear [21,36]. It is possible that interferon and other cytokines that secreted by viruses could induce resistance to possible infections by similar viruses [21]. Consistently, the co-infection between FluA and FluB was rarely detected in our study, which further supported a negative interaction between them [21]. And the co-infection between RVs and other pathogens was frequent except RSV, which might be partly explained by the overlap on the distribution over time [7,19]. In addition, more severe clinical symptoms in LRTI patients with higher co-infections could supported the association between multiple viral infections and higher healthcare burdens [37]. Therefore, knowledge about the specific interactions between pathogens is needed to get epidemic dynamics of respiratory infections, optimizing clinical healthcare and control for patients.
There were some limitations in our study. Firstly, we only take viruses, chlamydia, and MP into analysis, bacterial pathogens that induced ARTI were neglected, which prevented us from acquiring comprehensive information on the epidemiology in this region. Secondly, the patients were enrolled from a single hospital that only represented about 8.23 % patients of Xiamen, so the generality is limited. The findings of the study could serve as a good indication about population-based epidemiology within ARTI in Xiamen. A multiple-center study based on Xiamen population about epidemiology of respiratory pathogens need to be further conducted.
5. Conclusion
In conclusion, our study filled the epidemiology of ARTI patients in all ages during the COVID-19 epidemic. Our findings showed that the epidemiology of respiratory infections in Xiamen vary greatly across age, season, and disease phenotype. RVs exceeded influenza becoming the most frequent cause for ARTI under the background of COVID-19. In addition, respiratory pathogens were more likely to be related to specific clinical phenotype except influenza and CoV. The information may contribute to the authorities for setting up suitable prevention strategies in the primary care setting in the future.
Ethical approval and informed consent
This study has been approved by the Ethics Committee of The Fifth Hospital of Xiamen , with ethics approval reference 2022-XMSDWYY-008. The requirement for informed consent was waived because it was a retrospective study. And clinical data and patients’ identities were protected and anonymous.
Data availability statement
Data associated with the study has not been deposited into a publicly available repository and data will be made available on request.
Funding statement
The study was funded by Fujian Science and Technology Plan Project, China (No.2022D032) and Xiamen Medical and Health Guiding Project, China (No.3502Z20209230).
CRediT authorship contribution statement
Shan Hong: Supervision, Project administration, Investigation, Funding acquisition, Conceptualization. Dan Li: Writing – review & editing, Writing – original draft, Methodology, Investigation, Formal analysis. Yanli Wei: Data curation. Yilin Zheng: Data curation. Jiading Cai: Writing – review & editing. Heping Zheng: Writing – review & editing, Conceptualization. Xuan Zhang: Writing – review & editing. Yulin Deng: Writing – review & editing. Dandan Han: Writing – review & editing. Jia Wang: Writing – review & editing. Linlin Chen: Writing – review & editing. Shujing Li: Writing – review & editing. Weiping Qiu: Writing – review & editing. Min Ren: Supervision, Investigation, Conceptualization. Liangneng Zou: Supervision, Investigation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e22302.
Contributor Information
Min Ren, Email: renmin@xmsdwyy.cn.
Liangneng Zou, Email: zouliangneng@xmsdwyy.cn.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Monto A.S. Epidemiology of viral respiratory infections. Am. J. Med. 2002;112:4s–12s. doi: 10.1016/s0002-9343(01)01058-0. [DOI] [PubMed] [Google Scholar]
- 2.Silver S.A., Beaubien-Souligny W., Shah P.S., Harel S., Blum D., Kishibe T., et al. The prevalence of acute kidney injury in patients hospitalized with COVID-19 infection: a systematic review and meta-analysis. Kidney Medicine. 2021;3(1):83–+. doi: 10.1016/j.xkme.2020.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mutiawati E., Syahrul S., Fahriani M., Fajar J.K., Mamada S.S., Maliga H.A., et al. Global prevalence and pathogenesis of headache in COVID-19: a systematic review and meta-analysis. F1000Research. 2020;9 doi: 10.12688/f1000research.27334.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lauinger I.L., Bible J.M., Halligan E.P., Bangalore H., Tosas O., Aarons E.J., et al. Patient characteristics and severity of human rhinovirus infections in children. J. Clin. Virol. 2013;58(1):216–220. doi: 10.1016/j.jcv.2013.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Conto F., Conversano F., Medici M.C., Ferraglia F., Pinardi F., Arcangeletti M.C., et al. Epidemiology of human respiratory viruses in children with acute respiratory tract infection in a 3-year hospital-based survey in Northern Italy. Diagn. Microbiol. Infect. Dis. 2019;94(3):260–267. doi: 10.1016/j.diagmicrobio.2019.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lei C., Yang L.S., Lou C.T., Yang F., SiTou K.I., Hu H., et al. Viral etiology and epidemiology of pediatric patients hospitalized for acute respiratory tract infections in Macao: a retrospective study from 2014 to 2017. BMC Infect. Dis. 2021;(1):21. doi: 10.1186/s12879-021-05996-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Z.J., Zhang H.Y., Ren L.L., Lu Q.B., Ren X., Zhang C.H., et al. Etiological and epidemiological features of acute respiratory infections in China. Nat. Commun. 2021;12(1) doi: 10.1038/s41467-021-25120-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grunberg M., Sno R., Adhin M.R. Epidemiology of respiratory viruses in patients with severe acute respiratory infections and influenza-like illness in Suriname. Influenza and Other Respiratory Viruses. 2021;15(1):72–80. doi: 10.1111/irv.12791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Falsey A.R., McElhaney J.E., Beran J., van Essen G.A., Duval X., Esen M., et al. Respiratory syncytial virus and other respiratory viral infections in older adults with moderate to severe influenza-like illness. JID (J. Infect. Dis.) 2014;209(12):1873–1881. doi: 10.1093/infdis/jit839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Widmer K., Zhu Y.W., Williams J.V., Griffin M.R., Edwards K.M., Talbot H.K. Rates of hospitalizations for respiratory syncytial virus, human metapneumovirus, and influenza virus in older adults. JID (J. Infect. Dis.) 2012;206(1):56–62. doi: 10.1093/infdis/jis309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sitthikarnkha P., Uppala R., Niamsanit S., Sutra S., Thepsuthammarat K., Techasatian L., et al. Epidemiology of acute lower respiratory tract infection hospitalizations in Thai children: a 5-year national data analysis. Influenza and Other Respiratory Viruses. 2022;16(1):142–150. doi: 10.1111/irv.12911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun Y.P., Zheng X.Y., Zhang H.X., Zhou X.M., Lin X.Z., Zheng Z.Z., et al. Epidemiology of respiratory pathogens among children hospitalized for pneumonia in xiamen: a retrospective study. Infect. Dis. Ther. 2021;10(3):1567–1578. doi: 10.1007/s40121-021-00472-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen A.P.L., Chuang C., Huang Y.C., Wu P.F., Huang S.F., Cheng N.C., et al. The epidemiology and etiologies of respiratory tract infection in Northern Taiwan during the early phase of coronavirus disease 2019 (COVID-19) outbreak. J. Microbiol. Immunol. Infect. 2021;54(5):801–807. doi: 10.1016/j.jmii.2021.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rothan H.A., Byrareddy S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J. Autoimmun. 2020;109 doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Njouom R., Yekwa E.L., Cappy P., Vabret A., Boisier P., Rousset D. Viral etiology of influenza-like illnesses in Cameroon, january-december 2009. JID (J. Infect. Dis.) 2012;206:S29–S35. doi: 10.1093/infdis/jis573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun Y.P., Qiang H.S., Lei S.Y., Zheng X.Y., Zhang H.X., Su Y.Y., et al. Epidemiological features, risk factors, and disease burden of respiratory viruses among hospitalized children with acute respiratory tract infections in xiamen, China. Jpn. J. Infect. Dis. 2022;75(6):537–542. doi: 10.7883/yoken.JJID.2022.097. [DOI] [PubMed] [Google Scholar]
- 17.Bellei N., Carraro E., Perosa A., Watanabe A., Arruda E., Granato C. Acute respiratory infection and influenza-like illness viral etiologies in Brazilian adults. J. Med. Virol. 2008;80(10):1824–1827. doi: 10.1002/jmv.21295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang X.B., Yuan L., Ye C.X., Zhu X., Lin C.J., Zhang D.M., et al. Epidemiological characteristics of respiratory viruses in patients with acute respiratory infections during 2009-2018 in southern China. Int. J. Infect. Dis. 2020;98:21–32. doi: 10.1016/j.ijid.2020.06.051. [DOI] [PubMed] [Google Scholar]
- 19.Ren L., Gonzalez R., Wang Z., Xiang Z., Wang Y., Zhou H., et al. Prevalence of human respiratory viruses in adults with acute respiratory tract infections in Beijing, 2005-2007. Clin. Microbiol. Infection. 2009;15(12):1146–1153. doi: 10.1111/j.1469-0691.2009.02746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feng L.Z., Zhang T., Wang Q., Xie Y.R., Peng Z.B., Zheng J.D., et al. Impact of COVID-19 outbreaks and interventions on influenza in China and the United States. Nat. Commun. 2021;12(1) doi: 10.1038/s41467-021-23440-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng X.Y., Song Z.Y., Li Y.P., Zhang J.J., Wang X.L. Possible interference between seasonal epidemics of influenza and other respiratory viruses in Hong Kong, 2014-2017. BMC Infect. Dis. 2017;17 doi: 10.1186/s12879-017-2888-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geng Y., Hao Y.Y., Xu X.M., Huang R., He F., Ni J., et al. Clinical features and viral etiology of acute respiratory infection in an outpatient fever clinic during COVID-19 pandemic in a tertiary hospital in Nanjing, China. J. Clin. Lab. Anal. 2022;36(12) doi: 10.1002/jcla.24778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reeves R.M., Hardelid P., Gilbert R., Warburton F., Ellis J., Pebody R.G. Estimating the burden of respiratory syncytial virus (RSV) on respiratory hospital admissions in children less than five years of age in England, 2007-2012. Influenza and Other Respiratory Viruses. 2017;11(2):122–129. doi: 10.1111/irv.12443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jain S., Self W.H., Wunderink R.G., Fakhran S., Balk R., Bramley A.M., et al. Community-acquired pneumonia requiring hospitalization among US adults. N. Engl. J. Med. 2015;373(5):415–427. doi: 10.1056/NEJMoa1500245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levy O. Innate immunity of the newborn: basic mechanisms and clinical correlates. Nat. Rev. Immunol. 2007;7(5):379–390. doi: 10.1038/nri2075. [DOI] [PubMed] [Google Scholar]
- 26.Caini S., de Mora D., Olmedo M., Portugal D., Becerra M.A., Mejia M., et al. The epidemiology and severity of respiratory viral infections in a tropical country: Ecuador, 2009-2016. Journal of Infection and Public Health. 2019;12(3):357–363. doi: 10.1016/j.jiph.2018.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eccles R. Understanding the symptoms of the common cold and influenza. Lancet Infect. Dis. 2005;5(11):718–725. doi: 10.1016/S1473-3099(05)70270-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shen J.Y., Niu J.J. Epidemiologic parameters and evaluation of control measure for 2009 novel influenza a (H1N1) in Xiamen, Fujian Province, China. Virol. J. 2012;9 doi: 10.1186/1743-422X-9-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hasan M.I. Respiratory viral infection in early life and development of asthma in childhood: a protocol for systematic review and meta-analysis (vol 98, e15419, 2019) Medicine. 2019;(22):98. doi: 10.1097/MD.0000000000015419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fleming D.M., Pannell R.S., Elliot A.J., Cross K.W. Respiratory illness associated with influenza and respiratory syncytial virus infection. Arch. Dis. Child. 2005;90(7):741–746. doi: 10.1136/adc.2004.063461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen L., Han X.D., Bai L., Zhang J. Clinical characteristics and outcomes in adult patients hospitalized with influenza, respiratory syncytial virus and human metapneumovirus infections. Expert Review of Anti-Infective Therapy. 2021;19(6):787–796. doi: 10.1080/14787210.2021.1846520. [DOI] [PubMed] [Google Scholar]
- 32.Ye S., Wang T.L. Laboratory epidemiology of respiratory viruses in a large children's hospital: a STROBE-compliant article. Medicine. 2018;97(30) doi: 10.1097/MD.0000000000011385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cui B.L., Zhang D.G., Pan H., Zhang F., Farrar J., Law F., et al. Viral aetiology of acute respiratory infections among children and associated meteorological factors in southern China. BMC Infect. Dis. 2015;15 doi: 10.1186/s12879-015-0863-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tregoning J.S., Schwarze J. Respiratory viral infections in infants: causes, clinical symptoms, virology, and immunology. Clin. Microbiol. Rev. 2010;23(1):74. doi: 10.1128/Cmr.00032-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peng D., Zhao D.C., Liu J.T., Wang X., Yang K., Xicheng H., et al. Multipathogen infections in hospitalized children with acute respiratory infections. Virol. J. 2009;6 doi: 10.1186/1743-422X-6-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van den Bergh M.R., Biesbroek G., Rossen J.W.A., Piters W.A.A.D., Bosch A.A.T.M., van Gils E.J.M., et al. Associations between pathogens in the upper respiratory tract of young children: interplay between viruses and bacteria. PLoS One. 2012;7(10) doi: 10.1371/journal.pone.0047711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Semple M.G., Cowell A., Dove W., Greensill J., McNamara P.S., Halfhide C., et al. Dual infection of infants by human metapneumovirus and human respiratory syncytial virus is strongly associated with severe bronchiolitis. JID (J. Infect. Dis.) 2005;191(3):382–386. doi: 10.1086/426457. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data associated with the study has not been deposited into a publicly available repository and data will be made available on request.