Abstract
Prolonged controlled mechanical ventilation (CMV) can cause diaphragm fiber atrophy and inspiratory muscle weakness, resulting in diaphragmatic contractile dysfunction, called ventilator-induced diaphragm dysfunction (VIDD). VIDD is associated with higher rates of in-hospital deaths, nosocomial pneumonia, difficulty weaning from ventilators, and increased costs. Currently, appropriate clinical strategies to prevent and treat VIDD are unavailable, necessitating the importance of exploring the mechanisms of VIDD and suitable treatment options to reduce the healthcare burden. Numerous animal studies have demonstrated that ventilator-induced diaphragm dysfunction is associated with oxidative stress, increased protein hydrolysis, disuse atrophy, and calcium ion disorders. Therefore, this article summarizes the molecular pathogenesis and treatment of ventilator-induced diaphragm dysfunction in recent years so that it can be better served clinically and is essential to reduce the duration of mechanical ventilation use, intensive care unit (ICU) length of stay, and the medical burden.
Keywords: Diaphragm dysfunction, Mechanical ventilation, Reactive oxygen species, Pathogenesis, Prevention and treatment
1. Introduction
Mechanical ventilation (MV) is a life-saving intervention for respiratory support or airway protection, especially in surgical procedures and critical illnesses, and it is used by more than 15 million patients worldwide each year [1]. During controlled mechanical ventilation (CMV), the external physical forces replace active diaphragmatic contractions for pulmonary gas exchange. The diaphragm, which is the primary respiratory muscle, loses its regular autonomous contraction ability and relaxation during CMV [2]. In CMV, the diaphragm is completely unloaded and has no electrical activity. In contrast, under assisted mechanical ventilation (AMV), the diaphragm retained some spontaneous respiration and permitted to intermittently activate the diaphragm during mechanical ventilation. When resuming spontaneous breathing after a prolonged period of CMV, the diaphragm often has a weak contraction ability, making it difficult to wean the patient from the ventilator. This condition occurs due to diaphragm fiber atrophy, inspiratory muscle weakness, and contractile dysfunction during the MV process and is termed ventilator-induced diaphragm dysfunction (VIDD) [[3], [4], [5], [6]].
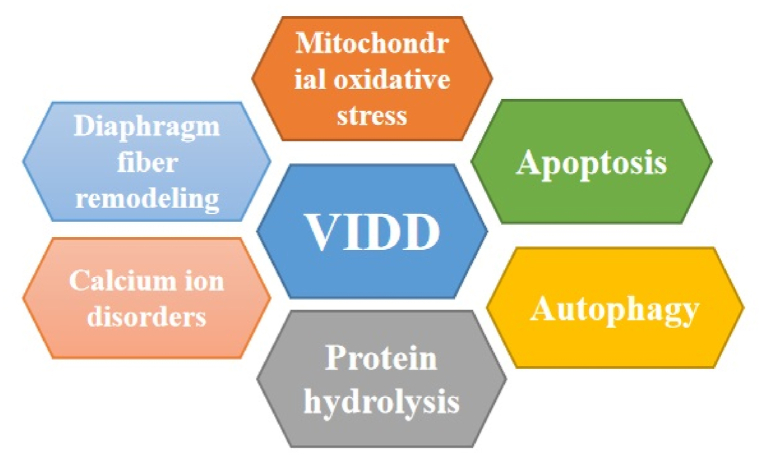
VIDD exerts a remarkable adverse effect on patient survival and prognosis and is a contributing factor of difficulty weaning the patient from MV [7,8]. Critically ill patients are often comorbid with a variety of conditions such as infections, electrolyte disorders and the use of neuromuscular blocking agents. All of these can lead to diaphragmatic weakness. More than 50 % of mechanically ventilated patients rapidly develop VIDD within 24 h of tracheal intubation, and its incidence is associated with prolonged ventilation time, weaning difficulty, and reintubation rates [6,9,10]. Therefore, it is important to gain a detailed understanding of the molecular mechanisms underlying VIDD to develop rational strategies to ameliorate this important clinical problem. Unfortunately, there is a lack of an appropriate clinical measure for VIDD prevention and treatment. Therefore, a detailed understanding of the mechanisms of VIDD and treatment options is essential to reduce the duration of MV use, intensive care unit (ICU) length of stay, and ICU mortality. We herein present a review of the VIDD pathogenesis, particularly on mitochondrial reactive oxygen species (ROS) and oxidative stress, activation of diaphragm protein hydrolase (calpain and caspase-3) activity, reduced protein synthesis, autophagy-lysosome pathway, ubiquitin-proteasome pathway (UPP), disuse atrophy and remodeling of diaphragm fiber, calcium disorders, impaired energy metabolism, and other clinical factors (Fig. 1). The review also discusses preventive and therapeutic approaches for VIDD. There are different effects on the diaphragm during fully controlled and partially assisted ventilation modes, and we focus this review on the controlled ventilation mode.
Fig. 1.
Schematic figure illustrating the signaling pathway implicated in VIDD development. There is the crossover between the various mechanisms. VIDD, ventilator-induced diaphragm dysfunction.
2. Pathogenesis of VIDD
2.1. Mitochondrial ROS and oxidative stress
During prolonged MV, the diaphragm produces excessive amounts of ROS that exceed the body's processing limits, which may lead to oxidative damage to the diaphragm [11,12]. Theoretically, oxidative injury to the diaphragm is caused by the interaction between several major oxidative production pathways. For example, superoxide production either by xanthine oxidase and nicotinamide adenine dinucleotide phosphate (NADPH) oxidase or by mitochondria can cause oxidative damage to the diaphragm during MV. Superoxide production by NADPH oxidase and xanthine oxidase is not the main pathway of MV-induced diaphragmatic ROS production, whereas superoxide production by mitochondria is the main source of diaphragmatic oxidative damage [[13], [14], [15], [16]]. During MV, the muscle loses its normal electrical activity, and calcium ions present in the sarcoplasmic reticulum (SR) leak into the cytoplasm and mitochondria, thereby causing mitochondrial calcium overload, which affects the normal electron transport chain function of the mitochondria, finally leading to increased ROS production [17,18]. Alternatively, the physiological negative thoracic pressure is altered during MV, which reduces diaphragm blood perfusion. This ventilator-induced decline in diaphragmatic oxygenation promotes hypoxia-induced generation of ROS in diaphragm muscle fibers [19]. Finally, during fully supported MV, the diaphragm remains unutilized, and substrate overload and lipid deposition–induced hyperlipidemia and hyperglycemia induces the release of ROS from the diaphragm [20,21]. Hydroxyl radicals (OH−), superoxide anions (O₂−), and hydrogen peroxide (H₂O₂) are common ions of endogenous ROS; although each species has its own characteristics, all species are strong oxidants that can react with proteins and unsaturated fatty acids in the organism, thereby participating in peroxidation reactions and causing damage to the organism [22]. Polyunsaturated fatty acids are the main components of biological membrane phospholipids, and oxygen radicals have a very high affinity to several weak and unsaturated bonds of these fatty acids. On the one hand, free radicals covalently bind to enzymes and receptors on the membrane and alter the activity of membrane components and their structure. On the other hand, sulfhydryl groups on the membrane are oxidized, causing disruption of membrane movement processes and resulting in decreased membrane fluidity and increased membrane fragility. Impaired ion exchange can occur between the cellular interior and exterior regions, degeneration of the cell membrane, and changes in membrane permeability and fluidity, all of which affect the structure and function of the cell membrane [23,24]. The generated oxygen radicals constantly oxidize proteins, causing changes in muscle structure and function and decreasing calcium sensitivity of myofilaments [25,26].
Under normal respiratory conditions, only 1%–3% oxygen is generated in the body during mitochondrial oxidative phosphorylation to produce ROS [22]. In a healthy organism, the production and degradation of ROS should be maintained in the dynamic equilibrium state. Catalase and superoxide dismutase (SOD) are the main ROS-scavenging enzymes in the body [27]. When the extent of ROS production far exceeds the scavenging ability, or when the activity of ROS scavenging enzymes is inhibited, the dynamic equilibrium is disturbed, resulting in oxidative stress injury in the body. Hyatt et al. [28] reported that the expression of 4-hydroxynonenal (4-HNE), a marker of hydrogen peroxide and oxidative damage, was significantly higher in the rat diaphragm than in the control group at 12 h of MV, whereas the fiber cross-sectional area and muscle strength of the diaphragm muscle fibers of rats receiving MV were lower than those in the control group. Powers et al. [29] reported a significant increase in levels of diaphragmatic peroxides after 12 h of MV in rats. However, when mitochondria-targeted antioxidants were used, levels of peroxides were significantly lower in the 12-h mechanically ventilated group than in the group without mitochondria-targeted antioxidants. In MV, ROS production is greatly increased in the organism, and MV for 12 h leads to a substantial increase in mitochondrial ROS production in the rat diaphragm, which also exhibits higher levels of oxidative damage and lower respiratory control rates (RCRs) [28]. Accumulating evidence confirmed elevated levels of ROS in the diaphragm; thus, oxidative stress damage is a prerequisite for diaphragm dysfunction [13,30]. But while most studies on mitochondrial oxidative stress during MV are based on animal experiments, several recent clinical reports have found a different phenomenon. Van den Berg et al. [31]and Breuer et al. [32] found that increased oxidative stress and significant mitochondrial dysfunction were not found in mechanically ventilated clinical patients. We believe that the differences between animal and clinical studies may be due to the following reasons. Firstly, there is a difference in the target population, as animal studies include rats, mice, rabbits, pigs, etc., which are still different from humans; secondly, animal experiments are mostly conducted with healthy animals, whereas in clinical practice, patients requiring mechanical ventilation are often accompanied by a variety of comorbidities (infections, electrolyte disorders, hormone use, multiorgan dysfunction, etc.); and thirdly, there is a difference in the clinical characteristics, as the patients are often accompanied by different diseases and clinical features, which may also affect the outcomes. Although VIDD development involves multiple mechanisms, the development of VIDD is closely related to the release of mitochondrial ROS [33,34]. Mitochondria-derived ROS is a key upstream activator of many pathways in VIDD pathogenesis [35]. Furthermore, increased release of ROS activates enzymes associated with protein hydrolysis, including calpain and caspase, showing marked activation of the lysosomal system and UPP, which can also regulate the activation of apoptotic proteins that together causes protein hydrolysis [14,28,[36], [37], [38]]. This activation process may lead to mitochondrial damage and further drive ROS production, thus creating a vicious cycle. Moreover, the generated ROS activates key autophagy genes, which induce apoptosis and protein hydrolysis, leading to diaphragm atrophy [39]. Mitochondrial oxidative stress plays an important role in inducing abnormal protein catabolism and promoting diaphragm atrophy. In conclusion, these findings suggest that a ventilator-induced increase in mitochondrial ROS release is an upstream signal necessary for prolonged diaphragm atrophy and dysfunction during MV [34,38].
2.2. Protein hydrolase activation
The protein hydrolysis system in VIDD has been extensively studied in recent years. The occurrence of diaphragm atrophy during prolonged MV is inextricably linked to the activation of protein hydrolases [[40], [41], [42]]. Oxidative stress, which is central to the pathogenesis of VIDD, also plays an important role in the activation of catabolic pathways. Protein synthesis in the diaphragm decreases, whereas protein catabolism increases during MV, resulting in diaphragm atrophy. Calpain plays an important role in skeletal muscle homeostasis [43]. But calpain has a function in muscle beyond protein hydrolysis, and its absence can lead to muscular dystrophy [44]. Calpain and caspase are the main protein hydrolases. Calpain is a cysteine protease activated under various conditions and promotes skeletal muscle depletion (e.g., prolonged disuse and sepsis), and calpain has long been known as a calcium-dependent protease that can degrade muscle cytoskeletal proteins [[45], [46], [47]]. Like calpain, caspase-3 is a cysteine protease that can be activated intracellularly through various signaling pathways. Caspase-3, a cysteine protease upregulated by oxidative load, increases calpain activity, upregulates the expression of pro-apoptotic proteins, and causes endogenous apoptosis associated with mitochondrial abnormalities [48]. Calpain and caspase-3 can dissociate the actin-globulin complex, and calpain can degrade large amounts of muscle proteins, promoting myofilament release and facilitating subsequent degradation of myogenic fibers [41,49].
Although several protein hydrolases are activated in the diaphragm during MV, activation of calpain and caspase-3 is essential for MV-induced diaphragm atrophy [28,50]. Hyatt et al. [50] reported that specific inhibition of diaphragmatic calpain activity improved diaphragm protein synthesis and reduced diaphragm atrophy. During MV, increased release of mitochondrial ROS can activate protein hydrolases, and protein hydrolase activation can promote increased ROS production during prolonged MV. Whidden et al. [38] reported that Trolox-mediated protection against MV-induced oxidative stress prevented calpain and caspase-3 activation in the diaphragm. Furthermore, avoidance of MV-induced oxidative stress not only reduced the activation of these proteases but also conferred protection against MV-induced diaphragm muscle fiber atrophy and contractile dysfunction. The mechanism of ROS activation due to proteases, which, some investigators believe may be that oxidants can increase cytoplasmic calcium levels and trigger signaling pathways that activate calpain, has not been conclusively demonstrated. Evidence suggests that MV-induced mitochondrial dysfunction can be successfully avoided by inhibiting calpain activation, preventing MV-induced mitochondrial uncoupling (i.e., a decrease in RCR, and suppressing MV-induced increases in mitochondrial ROS release [28]. The pathophysiological alterations in VIDD are mainly imbalance in protein homeostasis, i.e., decreased protein synthesis and increased protein hydrolysis in the diaphragm, wherein calpain and caspase-3 play an essential role.
2.3. Autophagy
Autophagy is a normal physiological catabolic process that primarily involves the lysosomal degradation of cytoplasmic proteins and organelles. In general, autophagy occurs continuously at low levels in the muscle tissue and involves the lysosomal degradation of cytoplasmic proteins and organelles, which is vital for maintaining the breakdown of dysfunctional cellular components [51]. During prolonged MV, the diaphragm of rodents and humans activates autophagy, as evidenced by increased expression of autophagic proteins and the formation of autophagic vesicles [52]. In VIDD, autophagy is a double-edged sword. Theoretically, depending on the conditions, accelerated autophagy can have either protective or detrimental effects on the skeletal muscle [39]. Particularly, basal levels of autophagy are required for normal muscle function, and deletion of specific autophagy genes can result in significant functional defects. However, autophagy may be significantly upregulated under pathological conditions to accelerate the removal rate of harmful proteins. Increased autophagy induced by MV can, on the one hand, remove damaged organelles and exert a protective effect on the organism; on the other hand, it can promote diaphragm atrophy by eliminating normal structural and cytoplasmic proteins in muscle fibers. Prolonged MV activates autophagy in the diaphragm, but the role of autophagy in MV has been interpreted differently in different studies, probably because of the use of different animal models, duration of ventilation, and interventions [14,52].
Furthermore, evidence suggests that levels of autophagy biomarkers are increased in rodent diaphragms after 8–18 h of full MV support and that MV prolongation increases the expression of essential autophagy proteins (e.g., ATG5, ATG7, and beclin 1) and the number of autophagosomes [39,53]. This was manifested by the upregulated expression of the microtubule-associated protein 1 light chain 3B-II/microtubule-associated protein 1 light chain 3B–I (LC3B-II/I) ratio and a significant increase in autophagosomes [39,54]. MV prolongation activates autophagy in the diaphragm, and accelerated autophagy is a pivotal contributor to VIDD, possibly because of increased ROS production. The current study suggests that MV-induced mitochondrial ROS production is necessary for increases in the expression of crucial autophagic proteins in the diaphragm. Smuder et al. [14] reported that administration of the mitochondria-targeted antioxidant peptide SS-31 prevented both MV-induced expression of autophagy-specific biomarkers and increased autophagic vesicle formation within the diaphragm. Smuder et al. [14] reported that upregulation of autophagy increased cellular ROS production by the action of degrading antioxidant enzymes. Conversely, autophagy inhibition reduced MV-induced ROS production in the diaphragm and prevented the subsequent increase in oxidative stress–stimulated autophagy and suppressing further increases in ROS production and autophagy, thus preventing the positive feedback loop. These results suggest the presence of a regulatory crosstalk between oxidative stress and autophagy.
2.4. Ubiquitin-proteasome pathway
The UPP is involved in most of the life processes in the human body, wherein it regulates protein levels, including growth, development, gene transcription, cell differentiation, and apoptosis. It is a very effective proteolytic pathway in vivo and plays a regulatory role in many cellular metabolisms [55]. The UPP is the most critical and highly selective protein degradation pathway in all eukaryotic organisms, and its specific E3 ligases, namely, atrogin-1 and MuRF1-1, are now widely used as markers of diaphragm fiber atrophy [56,57]. The ATP-dependent UPP is involved in the degradation of myogenic fibronectin during MV [58]. The investigators of one study measured 20S proteasome activity and mRNA and protein levels of two crucial muscle-specific E3 ligases (atrogin-1 and MuRF-1) in the diaphragm during MV [59]. The results showed that MV prolongation significantly elevated the mRNA levels of diaphragm muscle-specific ligases atrogin-1 and MuRF-1 in both groups [60]. Markers of the UPP were significantly upregulated in the diaphragm of mechanically ventilated critically ill patients, as found by Ottenheijm et al. MuRF-1 knockout mice were protected from diaphragmatic contractile weakness during mechanical ventilation [58].
However, animals treated with mitochondria-targeted antioxidants demonstrated significant blunting of the MV-induced elevated mRNA levels of atrogin-1 and MuRF-1 in the diaphragm. A recent report noted increased expression of the E3 ubiquitin ligases MFAbx and MURF-1 at both 12 and 24 h of mechanical ventilation [30]. Smuder et al. [49] [][][]reported that the UPP was active during 12 h of MV and that MV animals treated with proteasome inhibitors had partially attenuated MV-induced decrease in diaphragm contractility, which did not prevent MV-induced diaphragm fiber atrophy. Proteasome inhibitors partially protected the animals against MV-induced diaphragm contractile dysfunction, but UPP activation was not a major contributor to VIDD during 12 h of MV. Overexpression of the mitochondrial antioxidant enzyme SOD2 in the diaphragm partially reduced MV-induced increases in the mRNA expression of the E3 ubiquitin ligase MuRF1 and atrogin-1 [37]. Formation of 4-HNE occurs during the lipid peroxidation cascade, and detection of 4-HNE-modified protein adducts is an excellent biomarker of oxidative muscle damage. MV caused a significant increase in the accumulation of 4-HNE-modified proteins in the diaphragm; however, treatment of animals with proteasome inhibitors failed to inhibit MV-induced increase in oxidative damage in the diaphragm [49]. Therefore, it is speculated that ROS production may promote the activation of the UPP, and conversely, there is no clear evidence of the effect of the UPP on oxidative stress.
2.5. Calcium ion disorders and impaired energy metabolism
Although the exact mechanism remains unclear, changes in intracellular calcium levels also appear to be essential in VIDD. Calcium ions, as one of the intracellular second messengers, are involved in many physiological activities such as muscle contraction, maintenance of neuromuscular excitability, and heartbeat. In the normal innervated skeletal muscle tissue, cytosolic calcium levels are tightly regulated by the electrical activity of the muscle [61]. The skeletal muscle has two calcium channels that regulate intracellular calcium levels. Type I calcium channels (i.e., dihydropyridine channels) are highly expressed in the skeletal muscle and are located in the transverse tubules [62]. These type I channels interact directly with the ryanodine receptor (RyR1) on the SR membrane [17]. Voltage-driven conformational changes in type I channels directly induce RyR1 activation and promote rapid release of calcium ions, thereby triggering muscle contraction. After muscle contraction, the levels of cytoplasmic calcium ions are rapidly restored to the SR through the ATPase calcium pump on the SR, causing muscle relaxation [18]. Thus, in healthy non-contracting muscles, cytoplasmic calcium ions are maintained at low levels.
Sarcoplasmic RYR1 promotes the release of Ca2+ ions required for contraction-coupling, and in the fully supported MV mode, the diaphragm remains in a wasted state, and calcium ion leakage from the SR can be observed [17]. In humans and mice, MV rapidly remodels the RyR1 receptor on the SR membrane through oxidation, s-nitrosylation, and phosphorylation, all of which lead to destabilization of the receptor complex, leakage of calcium ions, and the onset of diaphragm contractile dysfunction [63]. RYR1 of the diaphragm has been remodeled to varying degrees in both human and animal models of MV, while further use of the RYR receptor stabilizers effectively prevents VIDD, suggesting that remodeling of the RYR may be the appropriate mechanism underlying disruption due to intracellular calcium ions [13,17]. Calcium leakage in the SR membrane reduces muscle contractile function and causes loss of muscle mass after prolonged MV in mice [17]. It may also be interesting to investigate whether MV alters the function of the calcium pump in the SR membrane because efficient intracellular calcium transport back to the SR is another critical factor in maintaining muscle calcium homeostasis because, in addition to changes in calcium concentration, impairment of calcium sensitivity occurs by MV. A calcium-sensitizing agent partially restores calcium activation in cells and alleviates VIDD partly [63]. The increase in the cytoplasmic calcium ions may have been due to the atrophy and protein hydrolysis component of VIDD. Calcium can directly activate proteases of the calpain family, and elevated intracellular calcium levels are associated with autophagy, a possible condition underlying diaphragm atrophy in VIDD [64].
Calcium ion leakage causes mitochondrial calcium overload, resulting in mitochondrial dysfunction and insufficient production of adenosine triphosphate (ATP). Because of the inability to form ATP, the progressively higher electrical potential in the mitochondrial membrane generates excessive ROS, and excessive ROS causes damage to mitochondrial DNA and exacerbates the reduction in the number of mitochondria [65]. With 6 h of MV, mitochondrial fission-promoting kinetics-related proteins are activated in the mouse diaphragm muscle, mitochondria undergo rapid fission, and mitochondria within contraction-related muscle fibers preferentially undergo fission, suggesting that disruption of the balance between mitochondrial division and fusion may have also been a potential factor in mitochondrial dysfunction [66]. At the same time, mitochondrial dysfunction that allows a surplus of metabolic substrates such as fatty acids is one main reason for exacerbating mitochondrial dysfunction [20].
2.6. Diaphragm fiber atrophy and remodeling
Diaphragm fiber atrophy occurs due to decreased protein synthesis and increased proteolysis, and the proteolytic pathways of autophagy lysosomes, calpain, caspase-3, and the UPP play essential roles in MV-induced diaphragm fiber atrophy (as described above). A linear relationship exists between human diaphragm fiber atrophy and duration of ventilator use, with atrophy rates averaging 6 % of daily CMV diaphragm thickness [67]. The cross-sectional area of all rat diaphragm fiber types (i.e., type I, type IIa, and type IIx/b) decreases by 10%–15 % within 12 h of CMV control [28,50]. CMV-induced rat diaphragm fiber atrophy increases as time progresses, and the reduction in fiber cross-sectional area approaches 30 % after 18–24 h of MV extension [68]. Shanely et al. [69]. found that the number of type I and type II fibers decreased after 18 h since controlled MV in rats, and the fibers showed more significant atrophy. Although it is difficult to directly compare MV-induced diaphragm fiber atrophy rates between human and animal studies, the temporal pattern of MV-induced diaphragm fiber atrophy is similar between rats and humans, and diaphragm fiber atrophy occurred in both species within 24–48 h of MV initiation [70]. Ottenheijm et al. [58] found that the cross-sectional area of both slow and fast muscle fibres was approximately 25 % smaller and contractility was reduced by more than 50 % in critically ill patients undergoing mechanical ventilation. But in this clinical study, the duration of MV was inconsistent, ranging from as little as a few hours to hundreds of hours. Without adequate prevention and treatment strategies, resting and inactive diaphragms may experience rapid morphological and functional changes after prolonged MV, including accelerated protein degradation, muscle atrophy, and impaired contractility. Many pathogenetic mechanisms underlying the damaging effects of MV on the diaphragm structure and contractility have been demonstrated in animal and human studies of VIDD [[71], [72], [73]]. However, Larsson et al. [74] found no differences in diaphragm fibre structure (myosin heavy chain isoenzyme ratio, cross-sectional area and contractile protein content) between a porcine ICU model and controls on day 5 of CMV. The reason why this result is different from that in the healthy rat model may still be related to the difference in the study population. Notably, both partially supported MV and fully supported MV caused diaphragm fiber atrophy, although diaphragm fiber atrophy induced in partially supported MV occurred at a slower rate than that induced in fully supported ventilation [75].
Myofiber remodeling involves changes in the expression of complex structures and muscle-specific proteins such as myosin heavy chains, myogenic determinants (MYOD), and myogenin [76]. According to the myosin heavy chain isoforms in myosin molecules, myofibers are classified as slow-contracting fibers (type I) and fast-contracting fibers (type II) [59]. During prolonged MV, myosin heavy chain subtypes in the diaphragm appear as so-called hybrid fibers, which is a complex expression of type I and type II fibers in the same muscle fiber, which is transformed from type I fibers. Larsson et al. [74] found a moderate loss of contractile proteins after 9–14 days of CMV treatment given to a porcine ICU model, with the loss of myosin being a uniformly distributed process within the myofibres, and the ratio of myosin to actin being unaffected. In another of his clinical studies, progressive preferential myosin loss was observed in patients with CMV in the ICU for up to 12 days. The myosin:actin ratio decreased from 2.0 at the first biopsy to 0.9 at the final biopsy [77]. Changes in the ratio of myosin to actin have been found to be different in clinical studies and animal experiments. The biggest reason for this may still be the differences in the study population and the existence of different clinical characteristics of the patients. Belcastro et al. [78] showed changes in the expression of myogenic transcription factor regulatory proteins after 24 h of controlled MV in rats, with an increase in the transcript level of diaphragm MYOD and a decrease in the expression of the myogenic regulatory factor myogenin, both of which play an essential role in myofiber remodeling.
2.7. Other clinical factors
Unlike healthy animals undergoing mechanical ventilation, critically ill patients who are mechanically ventilated clinically are often comorbid with many complications. When diaphragmatic weakness is noted in mechanically ventilated patients, other comorbid clinical factors should be considered in addition to the effects caused by mechanical ventilation. Consider first the presence of endocrine and electrolyte disorders such as hypophosphatemia, hypomagnesemia, and hypocalcemia. These can also cause muscle weakness. Chronic hyperglycemia, severe malnutrition, severe untreated renal failure, use of neuromuscular blocking agents, and continued administration of high doses of corticosteroids may lead to decreased muscle strength in mechanically ventilated patients in the ICU. In addition to this another cause of diaphragmatic dysfunction in mechanically ventilated patients is sepsis. Diaphragm function due to sepsis is called sepsis-induced diaphragm dysfunction (SIDD). Many clinical studies have found that diaphragmatic dysfunction is exacerbated in critically ill patients with co-infections. Infection is also one of the main causes of diaphragmatic dysfunction in patients [[79], [80], [81], [82]]. Li et al. [73] [][][]found that both sepsis and MV can cause diaphragm dysfunction, but MV affects diaphragm function more. Demoule et al. [83] also found that prolonged MV exacerbated diaphragm dysfunction in septic rats. Supinski et al. [80] also found in a clinical study that infection was a major contributor to diaphragmatic weakness in critically ill patients. Many critically ill patients in the clinical setting have multiple infections throughout the body, and there are many similarities in the mechanisms of SIDD and VIDD, which work together to cause diaphragmatic dysfunction.
3. Prevention and treatment measures
3.1. Reduction of oxidative stress injury
Oxidative stress is an essential pathophysiological process that causes VIDD, and antioxidants are a potential therapeutic measure. An intravenous infusion of vitamin E analogs with antioxidant activity during MV can prevent VIDD through the reduction of oxidative stress, protein hydrolysis, and contractile muscle dysfunction [33]. Additionally, N-acetylcysteine (NAC), a glutathione precursor, is an alternative clinical agent with antioxidant potential. When controlled ventilated rats received 150 mg/kg of NAC, the NAC effectively prevented oxidation of diaphragm proteins already present at an early stage in the diaphragm by exerting inhibitory effects on 20S proteasome, caspase-3, and calpain activity, thereby eliminating ventilator-related abnormalities in diaphragm function [84]. As for the beneficial effects of NAC, it possesses antioxidant properties of its own, dissolves emboli in the blood vessels, and increases blood flow to the diaphragm. Similarly, the mitochondrial antioxidant SS-31 prevented diaphragm contractile dysfunction and inhibited atrophy. Powers et al. [85] reported a significant increase in the levels of diaphragm peroxides after 12 h of MV in rats. However, when mitochondria-targeted antioxidants were applied, significantly fewer peroxides were measured in the 12-h MV group than in the group without mitochondria-targeted antioxidants. A clinical study also found that enterally administered antioxidants significantly reduced the time to need MV support compared to placebo [86]. In addition to reducing oxidative load, antioxidants can modulate the expression of genes involved in protein hydrolysis; for example, administration of high doses of vitamin E to animals attenuates the expression of several proteases, such as caspase-3 and calpain. Mitochondrial oxidative stress is regulated by the signalling molecules Smad 3, STAT3 and FoxO [87,88]. These drugs may act on molecules upstream of mitochondrial oxidative stress to achieve attenuation of oxidative damage. Therefore, applying antioxidants during MV can help reduce oxidative stress–related damage to the diaphragm, mitigate activation of the diaphragm protein hydrolysis system, and prevent diaphragm dysfunction.
However, the use of antioxidants may reduce the submaximal force [89]. This suggests the importance of low levels of ROS to gain normal muscle strength and restore normal physiological processes. ROS production in the diaphragm during MV is several folds higher than that in the normal diaphragmatic breathing, and excessive production of ROS exceeding the body's limits leads to oxidative stress and damage to the diaphragm [15,84]. Therefore, it is necessary to reduce ROS production and improve VIDD with the use of appropriate antioxidants; conversely, if the ROS production is completely inhibited, then it may reduce diaphragm contractility.
3.2. Inhibition of protein hydrolysis
The protein hydrolysis system activated for various reasons during VIDD is an important therapeutic target. Protein hydrolysis is an essential mechanism of diaphragm fiber atrophy. Disuse diaphragm atrophy is mainly caused by both decreased protein synthesis and increased protein hydrolysis, of which increased protein hydrolysis is the leading cause. Therefore, inhibition of protein hydrolysis during MV can effectively prevent diaphragm atrophy and muscle strength loss. Inhibition of autophagy with an AAV9-mediated dominant negative Atg5 was shown to inhibit MV-induced muscle atrophy dysfunction in rat diaphragm [90]. Maes et al. [91] administered a single intramuscular injection of leupeptinase (for inhibition of calpain or histoproteinase) at MV initiation, and this compound prevented diaphragm fiber atrophy in rats and suppressed impairment of intrinsic contractility. The proteasome inhibitor bortezomib was found to improve the function and atrophy of the quadriceps and diaphragm muscles in rats with heart failure in one study, so we hypothesised that bortezomib might prevent VIDD [92]. However, the epoxymethyl peptide proteasome inhibitor could not replicate the results [49]. This phenomenon may be attributed to the different protein hydrolysis systems. Research on these protease inhibitors is limited to animal studies, and more studies are needed to confirm the findings clinically. Inhibition of protein hydrolysis is essential to prevent diaphragm fiber atrophy; hence, protease inhibitors are also a future direction of treatment.
3.3. Drug treatment
3.3.1. Theophylline
Theophylline or aminophylline can relax airway smooth muscle, dilate bronchi, and enhance histone deacetylase-2, thus reducing the generation of peroxynitrite radicals. Low-dose theophylline drip therapy in patients with VIDD significantly enhanced voluntary diaphragmatic movements, but further investigation is needed because the drug accumulation varies among individuals and may exert adverse effects [93]. In another study, theophylline alleviated neonatal diaphragm fiber atrophy and restored the decrease in transdiaphragmatic pressure caused by resistance-loaded breathing, and it also promoted diaphragmatic perfusion by improving cardiac output and providing diaphragmatic arterial vasodilation [94]. Molecular mechanisms of theophylline are as follows: ① Theophylline can reduce the production of peroxynitrite radicals [95], which can mitigate diaphragmatic oxidative stress damage. ② In MV, the physiological negative thoracic pressure becomes positive, cardiac output decreases, and diaphragmatic blood flow reduces. Theophylline also facilitates diaphragmatic perfusion by improving cardiac output and providing vasodilation in diaphragmatic arterioles [96]. ③ During MV, many inflammatory factors (e.g., interleukin [IL]-6, IL-1β, and tumor necrosis factor-α) are activated. Theophylline can also act as an anti-inflammatory agent by enhancing the action of IL-10 and blocking the translocation of the pro-inflammatory transcription factor NF-κB [93].
3.3.2. Glucocorticoids
Glucocorticoids have antioxidant properties, and they benefit patients with VIDD [97]. Recent studies have investigated the combined effects of high-dose corticosteroid treatment with partially and fully supported MV on diaphragm function in animals [98,99]. Unfortunately, these studies have yielded different results. Animal studies demonstrated that a higher dose of methylprednisolone during MV reduced the degree of type IIx/b fiber atrophy and myogenin depletion in the diaphragm, as evidenced by an upward elevation of the diaphragmatic force-frequency curve. For example, Maes et al. reported that corticosteroids exerted a dose-dependent effect on VIDD in rats during fully supported MV, with high doses of corticosteroids (i.e., 30 mg/kg) providing partial protection against VIDD, whereas low doses of corticosteroids (i.e., 5 mg/kg) exacerbating VIDD [97]. By contrast, Sassoon et al. [100] reported that a high dose of glucocorticoids (i.e., 60 mg/kg) did not confer protection against or exacerbate VIDD in rabbits during fully supported MV, but this dose of glucocorticoids had deleterious effects on the diaphragm during partially supported MV. The differences found in these studies are unclear but may have been due to species differences in response to corticosteroids. How do glucocorticoids work? Glucocorticoids may control diaphragm muscle fiber atrophy during MV by inhibiting calpain activity and lipid peroxidation and controlling diaphragmatic contraction force.
3.3.3. Angiotensin receptor blockers
Passive contraction of the diaphragm induced by MV causes higher mechanical stress in the diaphragm fibers, which results in massive activation of the angiotensin II (Ang II) type I receptor on the diaphragm, leading to G-protein–coupled receptor signaling to initiate oxidative stress–related signaling pathways [101]. Preventing the binding of Ang II to AT1R alone does not cure ventilator-induced diaphragm injury, while the representative angiotensin II receptor blocker olmesartan directly inhibits the activity of AT1Rs to prevent VIDD [102]. Olmesartan can block MV-induced upregulation of STAT3 signaling while attenuating oxidative stress–related damage to diaphragm proteins by reducing mitochondrial uncoupling and inhibiting calpain and caspase-3 activity [103]. The renin-angiotensin system is therefore considered a potential therapeutic target for VIDD.
3.3.4. Calcium sensitizer levosimendan
The calcium sensitizer levosimendan is a positive inotropic agent used in patients with chronic obstructive pulmonary disease who have diaphragmatic dysfunction. It enhances diaphragm contractility by increasing the calcium sensitivity of contractile proteins [104,105]. Muscle contraction requires the binding of troponin and calcium ions before the induction of downstream myofilament contraction. The use of calcium sensitizers suggests that a less amount of calcium is needed to maintain the same magnitude of force production, and the preparation can strongly promote diaphragmatic contractility. However, Roesthuis et al. [106] found that levosimendan did not improve diaphragm contraction efficiency in critically ill patients in a clinical study. Zambelli et al. [107] found that levosimendan did not improve diaphragm contraction efficiency in a rat model of VIDD, even though it preserved the muscle cellular structure. The efficacy of levosimendan in the treatment of VIDD has not been conclusive, and the reasons for this conflicting result are hypothesised as follows. Firstly, there may be differences in the clinical characteristics of the study population; secondly, there are differences in the mode of administration (whether or not pre-administration as well as maintenance doses were given). Although there are no definitive studies of the use of calcium sensitizers in patients with VIDD, levosimendan could be a promising therapeutic agent.
3.3.5. The chaperone co-inducer BGP-15
Heat shock proteins (HSPs) are chaperone proteins whose expression is greatly induced in skeletal muscle in response to environmental or metabolic stress [108]. Particularly, the 72 kDa HSP isoform (HSP72) is upregulated in response to exercise and heat stress. Studies have shown that endurance exercise training prior to the start of MV can lead to a significant increase in diaphragmatic HSP72 levels, sufficient to prevent VIDD [109,110]. The HSP72 co-inducer BGP-15 is a multitarget compound that fluidizes, yet stabilizes, membranes. In a rat model, 10 days of BGP-15 treatment greatly improved diaphragm muscle fiber function (by about 100 %), although it did not reverse diaphragm atrophy [111]. Mnuskina et al. also found that BGP-15 was effective in ameliorating structure-related dysfunction in VIDD [112]. Therefore, BGP-15 may provide an effective intervention strategy to reduce the incidence of VIDD.
3.4. Increase in diaphragm electrical activity
3.4.1. Ventilation mode/pattern selection
Increasing diaphragm muscle electrical activity/contraction during MV can ameliorate the development of VIDD. This has been achieved both by the use of assisted modes of MV and by the induction of activity through electrical stimulation. Fully CMV mode was more likely to induce ROS production in the diaphragm, even after eliminating all voluntary activities of the diaphragm, with a 7.5 % daily reduction in diaphragm thickness, which was more significant at high-pressure support levels than at low-pressure support levels [113]. Partially supported MV can prevent protease activation in the diaphragm, increased expression of UPP components, diaphragm fiber atrophy, and reduced contractile function [114]. Goligher et al. [9]mentioned that if the patient maintains a certain inspiratory effort during mechanical ventilation it might accelerate liberation from ventilation. Maintaining an appropriate level of inspiratory muscle effort and reducing patient-ventilator asynchrony minimizes diaphragm fiber atrophy [115]. In general, with the CMV mode, the diaphragm is completely unloaded and electrically inactive, making it susceptible to VIDD. In contrast, with the AMV mode, the diaphragm retains some spontaneous respiration and allows for intermittent activation of the diaphragm during mechanical ventilation, and thus the incidence of VIDD is greatly reduced.
3.4.2. Phrenic nerve stimulation
In MV, a series of pathophysiological changes caused by a weakened contractile movement of the diaphragm is the main factor in the development of diaphragmatic dysfunction. Phrenic nerve stimulation preserves diaphragm activity and has become a hot research topic in VIDD prevention and treatment. Short-term temporary diaphragmatic pacing has a protective effect on diaphragmatic function in patients on long-term MV. Diaphragmatic pacing can be performed through a transvenous phrenic nerve pacing system placed percutaneously in the left subclavian vein [116]. The transvenous phrenic nerve stimulates inspiratory movements of the diaphragm, thus reducing the support pressure required to reach the ideal tidal volume and facilitating early restoration of the patient's ability to breathe independently [117]. In addition, unilateral phrenic nerve stimulation in patients undergoing cardiothoracic surgery and receiving MV increased the mitochondrial respiratory rate of the diaphragm on the stimulated side [118]. Phrenic nerve stimulation preserves the contractile function of the rat diaphragm after 18 h of MV [119]. The study confirmed that pigs receiving transvenous phrenic nerve pacing synchronized with MV had less diaphragm fiber atrophy than those receiving MV [120]. Therefore, using phrenic nerve stimulation drugs or devices to preserve diaphragmatic contractile activity by stimulating the phrenic nerve may be a new approach in VIDD treatment and prevention, but further studies are needed to demonstrate the safety and feasibility of phrenic nerve stimulation.
3.5. Clinical care
Clinical care includes treating electrolyte imbalances and endocrine disorders including hypoalbuminemia, hypophosphatemia, hypomagnesemia, hyperglycemia, severe untreated renal failure, and hypothyroidism, in addition to avoiding neuromuscular blocking reagent overuse [42,72]. Malnutrition is common in patients receiving MV and associated with poor outcomes, including difficult wound healing, nosocomial infections, and increased mortality [121]. Nutritional support is important; however, no standardized protocol is available regarding the administration route, type of nutrients, and timing of nutrition support. Sepsis is also a major contributor of diaphragmatic dysfunction. Therefore infection control is also an important clinical treatment measure in critically ill patients. Several animal and clinical studies have suggested that adjusting sedation and ventilation mode/pattern to maintain appropriate levels of inspiratory muscle effort and reduce patient-ventilator asynchrony may minimize diaphragm fiber atrophy [9,115].
4. Discussion
This review summarizes the pathogenesis of VIDD occurrence during CMV and the main control measures. Mitochondrial oxidative stress, as the central link in VIDD development, and ROS, a key upstream activator, play a crucial role in the activation of the protein hydrolysis system, disruption of calcium ions, and activation of the cytokine system. Diaphragm fiber atrophy is associated with increased protein hydrolysis, wherein protein hydrolase activation, autophagy, and UPP play a significant role. The pathophysiological mechanisms are intricate, and the factors are intertwined and interact with each other, and numerous studies are still needed to explore the modulating effects between them (Fig. 2). Mitochondrial oxidative stress is central to the pathophysiological mechanisms underlying the development of VIDD. Excessive ROS production causes oxidative damage to cells in a series of reactions. Mitochondrial dysfunction affects ATP production and is the main cause of diaphragm contractile dysfunction. The vicious cycle between oxidative stress and mitochondrial dysfunction exacerbates VIDD development. Activation of the protein hydrolysis system is key to diaphragm fiber atrophy and is one of the main mechanisms leading to diaphragm dysfunction. When oxidative stress is increased, genes associated with protein hydrolysis, including E3 ubiquitin ligase MuRF1 and atrogin-1 and autophagy-related genes, are activated to initiate protein hydrolysis pathways.Calcium ion disorders exacerbate mitochondrial dysfunction and activate calpain, causing an increase in diaphragm protein hydrolysis.
Fig. 2.
This figure summarizes the interconnections between VIDD mechanisms, highlighting the important role of ROS as key upstream activating molecules. ROS, reactive oxygen species; VIDD, ventilator-induced diaphragm dysfunction; UPP, Ubiquitin-proteasome pathway.
However, critically ill patients often have multiple co-morbidities. The causes of diaphragmatic dysfunction also go beyond MV. These studies in healthy animals did not take into account the influence of other risk factors in ICU patients, such as sepsis and multisystem organ failure. Animal studies have demonstrated that VIDD is reversible which is not seen in ICU patients [122,123]. This may be due to the fact that most of the animals in the animal experiments were healthy and only MV was a single factor affecting the diaphragm. In contrast, patients in ICU may have multiple comorbidities such as sepsis and multi-organ dysfunction and are on multiple therapeutic medications, which is the result of a combination of factors. It has been noted that chronic infections may lead to persistent muscle weakness, in which case prolonged diaphragmatic weakness may occur even when off the ventilator [124]. Sepsis in particular is also a major contributor to diaphragmatic dysfunction. Sepsis and MV are common causes of intensive care unit-acquired weakness [125]. In addition, the review focused on complete control of mechanical ventilation, ignoring the effects of patient-ventilator asynchrony and assisted mechanical ventilation. The mechanisms by which they cause damage to the diaphragm and prevent it are questions that need to be addressed. The definitive diagnosis of VIDD in ICU patients is not accessible owing to the presence of multiple confounding factors; in recent years, the use of ultrasound technology has provided great clinical assistance in diagnosing VIDD.
There is no specific clinical treatment for VIDD. Current studies suggest that the use of antioxidants to inhibit oxidative stress, reduce protein hydrolysis, and diaphragm fiber atrophy, as well as the application of some hormonal drugs may be potential treatments. Increasing the activity of the diaphragm by phrenic nerve stimulation, rational selection of ventilator mode parameter modulation, and early weaning from mechanical ventilation are important methods to improve VIDD. Clinical care is also a key aspect. Treating the patient's underlying disease, correcting internal environmental stability, and maintaining nutritional balance are very important steps in VIDD prevention and treatment.
In recent years, most of the publications on VIDD mechanism have focused on animal experiments owing to human ethical constraints. More research in the future should focus on the clinical setting to develop treatments and drugs suitable for application clinically to patients with VIDD. Fortunately, with the development of noninvasive diaphragmatic ultrasound technology, a simpler and noninvasive approach to diaphragm function measurement has become available, making more clinical studies on VIDD possible.
5. Conclusion
MV is commonly used for respiratory support in critically ill patients and diaphragmatic dysfunction is a common and serious clinical problem. VIDD involves many interrelated mechanisms. Diaphragmatic dysfunction is also a result of multiple causative factors. Much of the current research on therapeutic measures for VIDD is still focused on animal studies. More research is needed in the future to find an approach that can be applied to clinically critical patients to reduce the incidence of VIDD.
Ethics statement
All parties (the author, the journal editor, the peer reviewer, the publisher and the society for society-owned or sponsored journals) treating each other with respect and dignity and without discrimination, harassment, bullying or retaliation.
Consent for publication
Not applicable.
Data availability statement
No data was used for the research described in the article.
Funding statement
This study was supported by the Sichuan Science and Technology Program Joint Innovation Key Project Fund (No. 2022YFS0632) and the National Natural Science Foundation of China (No. 81772128).
CRediT authorship contribution statement
Jumei Zhang: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. Jianguo Feng: Data curation, Formal analysis, Investigation, Methodology, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. Jing Jia: Conceptualization, Data curation, Formal analysis, Investigation, Project administration, Resources, Supervision, Validation, Visualization. Xiaobin Wang: Conceptualization, Data curation, Formal analysis, Methodology, Project administration, Supervision, Validation, Writing – review & editing. Jun Zhou: Conceptualization, Data curation, Formal analysis, Methodology, Software, Supervision, Validation, Writing – review & editing. Li Liu: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Abbreviations
- VIDD
Ventilator-induced diaphragm dysfunction
- MV
Mechanical ventilation
- ROS
Reactive oxygen species
- SOD
Superoxide dismutase
- UPP
Ubiquitin-proteasome pathway
- RYR
Ryanodine receptor
- NAC
N-acetylcysteine
- MOS
Mitochondrial oxidative stress
- SR
Sarcoplasmic reticulum
- MYOD
myogenic determinant
- ATP
Adenosine triphosphate
- RCR
respiratory control rate
- ICU
intensive care unit
- NADPH
nicotinamide adenine dinucleotide phosphate
- 4-HNE
4-Hydroxynonenal; IL-6: interleukin 6
References
- 1.Adhikari N., Rubenfeld G.D. Worldwide demand for critical care. Curr. Opin. Crit. Care. 2011;17(6):2620. doi: 10.1097/MCC.0b013e32834cd39c. [DOI] [PubMed] [Google Scholar]
- 2.Heaton C.J., Smith M.A. The diaphragm. Am. Fam. Physician. 1989;39(5):231–236. [PubMed] [Google Scholar]
- 3.Petrof B.J., Jaber S., Matecki S. Ventilator-induced diaphragmatic dysfunction. Curr. Opin. Crit. Care. 2010;16(1):19–25. doi: 10.1097/MCC.0b013e328334b166. [DOI] [PubMed] [Google Scholar]
- 4.Jubran A. Critical illness and mechanical ventilation: effects on the diaphragm. Respir. Care. 2006;51(9):1054–1061. discussion 1062-1054. [PubMed] [Google Scholar]
- 5.Vassilakopoulos T. Ventilator-induced diaphragm dysfunction: the clinical relevance of animal models. Intensive Care Med. 2008;34(1):7–200816. doi: 10.1007/s00134-007-0866-x. [DOI] [PubMed] [Google Scholar]
- 6.Demoule A., Molinari N., Jung B., et al. Patterns of diaphragm function in critically ill patients receiving prolonged mechanical ventilation: a prospective longitudinal study. Ann. Intensive Care. 2016;6(1):2075. doi: 10.1186/s13613-016-0179-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tonnelier A., Tonnelier J.M., Nowak E., et al. Clinical relevance of classification according to weaning difficulty. Respir. Care. 2011;56(5):583–590. doi: 10.4187/respcare.00842. [DOI] [PubMed] [Google Scholar]
- 8.Funk G.C., Anders S., Breyer M.K., et al. Incidence and outcome of weaning from mechanical ventilation according to new categories. Eur. Respir. J. 2010;35(1):88–94. doi: 10.1183/09031936.00056909. [DOI] [PubMed] [Google Scholar]
- 9.Goligher E.C., Dres M., Fan E., et al. Mechanical ventilation-induced diaphragm atrophy strongly impacts clinical outcomes. Am. J. Respir. Crit. Care Med. 2018;197(2):204–213. doi: 10.1164/rccm.201703-0536OC. [DOI] [PubMed] [Google Scholar]
- 10.Jung B., Moury P.H., Mahul M., et al. Diaphragmatic dysfunction in patients with ICU-acquired weakness and its impact on extubation failure. Intensive Care Med. 2016;42(5):853–861. doi: 10.1007/s00134-015-4125-2. [DOI] [PubMed] [Google Scholar]
- 11.Morton A., Smuder A., Wiggs M., et al. Increased SOD2 in the diaphragm contributes to exercise-induced protection against ventilator-induced diaphragm dysfunction. Redox Biol. 2019;20:402–413. doi: 10.1016/j.redox.2018.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Talbert E.E., Smuder A.J., Hudson M.B., et al. A mitochondrial-targeted antioxidant protects against mechanical ventilation-induced diaphragm weakness. Med. Sci. Sports Exerc. 2010;42(Suppl):17–18. [Google Scholar]
- 13.Tang H., Shrager J.B. The signaling network resulting in ventilator-induced diaphragm dysfunction. Am. J. Respir. Cell Mol. Biol. 2018;59(4):417–427. doi: 10.1165/rcmb.2018-0022TR. [DOI] [PubMed] [Google Scholar]
- 14.Smuder A.J., Sollanek K.J., Nelson W.B., et al. Crosstalk between autophagy and oxidative stress regulates proteolysis in the diaphragm during mechanical ventilation. Free Radical Biol. Med. 2017;115:179–190. doi: 10.1016/j.freeradbiomed.2017.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whidden M.A., Smuder A.J., Wu M., et al. Oxidative stress is required for mechanical ventilation-induced protease activation in the diaphragm. J. Appl. Physiol. 2010;108(5):1376–1382. doi: 10.1152/japplphysiol.00098.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McClung J.M., Kavazis A.N., Whidden M.A., et al. Antioxidant administration attenuates mechanical ventilation-induced rat diaphragm muscle atrophy independent of protein kinase B (PKB Akt) signalling. J Physiol. 2007;585(Pt 1):203–215. doi: 10.1113/jphysiol.2007.141119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matecki S., Dridi H., Jung B., et al. Leaky ryanodine receptors contribute to diaphragmatic weakness during mechanical ventilation. Proc Natl Acad Sci U S A. 2016;113(32):9069–9074. doi: 10.1073/pnas.1609707113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matecki S., Jung B., Saint N., et al. Respiratory muscle contractile inactivity induced by mechanical ventilation in piglets leads to leaky ryanodine receptors and diaphragm weakness. J. Muscle Res. Cell Motil. 2017;38(1):17–24. doi: 10.1007/s10974-017-9464-x. [DOI] [PubMed] [Google Scholar]
- 19.Davis R.T., 3rd, Bruells C.S., Stabley J.N., et al. Mechanical ventilation reduces rat diaphragm blood flow and impairs oxygen delivery and uptake. Crit. Care Med. 2012;40(10):2858–2866. doi: 10.1097/CCM.0b013e31825b933a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Picard M., Jung B., Liang F., et al. Mitochondrial dysfunction and lipid accumulation in the human diaphragm during mechanical ventilation. Am. J. Respir. Crit. Care Med. 2012;186(11):1140–1149. doi: 10.1164/rccm.201206-0982OC. [DOI] [PubMed] [Google Scholar]
- 21.Lecuona E., Sassoon C.S., Barreiro E. Lipid overload: trigger or consequence of mitochondrial oxidative stress in ventilator-induced diaphragmatic dysfunction? Am. J. Respir. Crit. Care Med. 2012;186(11):1074–1076. doi: 10.1164/rccm.201209-1735ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sies H. Oxidative stress: a concept in redox biology and medicine. Redox Biol. 2015;4:180–183. doi: 10.1016/j.redox.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zorov D.B., Juhaszova M., Sollott S.J. Mitochondrial ROS-induced ROS release: an update and review. Biochim. Biophys. Acta. 2006;1757(5–6):509–517. doi: 10.1016/j.bbabio.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 24.Chainy G.B.N., Sahoo D.K. Hormones and oxidative stress: an overview. Free Radic. Res. 2020;54(1):1–202026. doi: 10.1080/10715762.2019.1702656. [DOI] [PubMed] [Google Scholar]
- 25.Zhou X.L., Wei X.J., Li S.P., et al. Interactions between cytosolic phospholipase A2 activation and mitochondrial reactive oxygen species production in the development of ventilator-induced diaphragm dysfunction. Oxid. Med. Cell. Longev. 2019 2019:1–12. doi: 10.1155/2019/2561929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Powers S.K., Duarte J., Kavazis A.N., et al. Reactive oxygen species are signalling molecules for skeletal muscle adaptation. Exp. Physiol. 2010;95(1):1–9. doi: 10.1113/expphysiol.2009.050526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nantapong N., Murata R., Trakulnaleamsai S., et al. The effect of reactive oxygen species (ROS) and ROS-scavenging enzymes, superoxide dismutase and catalase, on the thermotolerant ability of Corynebacterium glutamicum. Appl. Microbiol. Biotechnol. 2019;103(13):5355–5366. doi: 10.1007/s00253-019-09848-2. [DOI] [PubMed] [Google Scholar]
- 28.Hyatt H.W., Ozdemir M., Yoshihara T., et al. Calpains play an essential role in mechanical ventilation-induced diaphragmatic weakness and mitochondrial dysfunction. Redox Biol. 2021;38(6) doi: 10.1016/j.redox.2020.101802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Powers S.K., Hudson M.B., Nelson W.B., et al. Mitochondria-targeted antioxidants protect against mechanical ventilation-induced diaphragm weakness. Crit. Care Med. 2011;39(7):1749–1759. doi: 10.1097/CCM.0b013e3182190b62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yong H., Zhou Y., Ye W., et al. PINK1/Parkin-mediated mitophagy in mechanical ventilation-induced diaphragmatic dysfunction. Ther. Adv. Respir. Dis. 2021;15 doi: 10.1177/1753466621998246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van den Berg M., Hooijman P.E., Beishuizen A., et al. Diaphragm atrophy and weakness in the absence of mitochondrial dysfunction in the critically ill. Am. J. Respir. Crit. Care Med. 2017;196(12):1544–1558. doi: 10.1164/rccm.201703-0501OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Breuer T., Bleilevens C., Rossaint R., et al. Dexmedetomidine impairs diaphragm function and increases oxidative stress but does not aggravate diaphragmatic atrophy in mechanically ventilated rats. Anesthesiology. 2018;128(4):784–795. doi: 10.1097/ALN.0000000000002081. [DOI] [PubMed] [Google Scholar]
- 33.Betters J.L., Criswell D.S., Shanely R.A., et al. Trolox attenuates mechanical ventilation-induced diaphragmatic dysfunction and proteolysis. Am. J. Respir. Crit. Care Med. 2004;170(11):1179–1184. doi: 10.1164/rccm.200407-939OC. [DOI] [PubMed] [Google Scholar]
- 34.Falk D.J., Kavazis A.N., Whidden M.A., et al. Mechanical ventilation-induced oxidative stress in the diaphragm: role of heme oxygenase-1. Chest. 2011;139(4):816–824. doi: 10.1378/chest.09-2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moroz N., Maes K., Leduc-Gaudet J.P., et al. Oxidants regulated diaphragm proteolysis during mechanical ventilation in rats. Anesthesiology. 2019;131(3):605–618. doi: 10.1097/ALN.0000000000002837. [DOI] [PubMed] [Google Scholar]
- 36.Kitajima Y., Suzuki N., Nunomiya A., et al. The ubiquitin-proteasome system is indispensable for the maintenance of muscle stem cells. Stem Cell Rep. 2018;11(6):1523–1538. doi: 10.1016/j.stemcr.2018.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morton A.B., Smuder A.J., Wiggs M.P., et al. Increased SOD2 in the diaphragm contributes to exercise-induced protection against ventilator-induced diaphragm dysfunction. Redox Biol. 2019;20:402–413. doi: 10.1016/j.redox.2018.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Whidden M.A., Smuder A.J., Wu M., et al. Oxidative stress is required for mechanical ventilation-induced protease activation in the diaphragm. J. Appl. Physiol. 1985;108(5):1376–1382. doi: 10.1152/japplphysiol.00098.2010. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hussain S.N., Mofarrahi M., Sigala I., et al. Mechanical ventilation-induced diaphragm disuse in humans triggers autophagy. Am. J. Respir. Crit. Care Med. 2010;182(11):1377–1386. doi: 10.1164/rccm.201002-0234OC. [DOI] [PubMed] [Google Scholar]
- 40.van Hees H.W., Schellekens W.J., Andrade Acuña G.L., et al. Titin and diaphragm dysfunction in mechanically ventilated rats. Intensive Care Med. 2012;38(4):702–709. doi: 10.1007/s00134-012-2504-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nelson W.B., Smuder A.J., Hudson M.B., et al. Cross-talk between the calpain and caspase-3 proteolytic systems in the diaphragm during prolonged mechanical ventilation. Crit. Care Med. 2012;40(6):1857–1863. doi: 10.1097/CCM.0b013e318246bb5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dres M., Goligher E.C., Heunks L.M.A., et al. Critical illness-associated diaphragm weakness. Intensive Care Med. 2017;43(10):1441–1452. doi: 10.1007/s00134-017-4928-4. [DOI] [PubMed] [Google Scholar]
- 43.Sorimachi H., Ono Y. Regulation and physiological roles of the calpain system in muscular disorders. Cardiovasc. Res. 2012;96(1):11–22. doi: 10.1093/cvr/cvs157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ojima K., Ono Y., Ottenheijm C., et al. Non-proteolytic functions of calpain-3 in sarcoplasmic reticulum in skeletal muscles. J. Mol. Biol. 2011;407(3):439–449. doi: 10.1016/j.jmb.2011.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smuder A.J., Kavazis A.N., Hudson M.B., et al. Oxidation enhances myofibrillar protein degradation via calpain and caspase-3. Free Radic. Biol. Med. 2010;49(7):1152–1160. doi: 10.1016/j.freeradbiomed.2010.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhu X., van Hees H.W.H., Heunks L., et al. The role of calpains in ventilator-induced diaphragm atrophy. Intensive Care Med Exp. 2017;5(1):14. doi: 10.1186/s40635-017-0127-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ono Y., Sorimachi H. Calpains: an elaborate proteolytic system. Biochim. Biophys. Acta. 2012;1824(1):224–236. doi: 10.1016/j.bbapap.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 48.Smuder A.J., Sollanek K.J., Min K., et al. Inhibition of forkhead boxO-specific transcription prevents mechanical ventilation-induced diaphragm dysfunction. Crit. Care Med. 2015;43(5):e133–e142. doi: 10.1097/CCM.0000000000000928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smuder A.J., Nelson W.B., Hudson M.B., et al. Inhibition of the ubiquitin-proteasome pathway does not protect against ventilator-induced accelerated proteolysis or atrophy in the diaphragm. Anesthesiology. 2014;121(1):115–126. doi: 10.1097/ALN.0000000000000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hyatt H.W., Ozdemir M., Bomkamp M.P., et al. Activation of calpain contributes to mechanical ventilation-induced depression of protein synthesis in diaphragm muscle. Cells. 2022;11(6) doi: 10.3390/cells11061028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liao S.X., Sun P.P., Gu Y.H., et al. Autophagy and pulmonary disease. Ther. Adv. Respir. Dis. 2019;13 doi: 10.1177/1753466619890538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Azuelos I., Jung B., Picard M., et al. Relationship between autophagy and ventilator-induced diaphragmatic dysfunction. Anesthesiology. 2015;122(6):1349–1361. doi: 10.1097/ALN.0000000000000656. [DOI] [PubMed] [Google Scholar]
- 53.Crosstalk between autophagy and oxidative stress regulates proteolysis in the diaphragm during mechanical ventilation. Free Radical Biol. Med. Off. J. Oxygen Soc. 2018;115(1):179–190. doi: 10.1016/j.freeradbiomed.2017.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tang H., Lee M., Khuong A., et al. Diaphragm muscle atrophy in the mouse after long-term mechanical ventilation. Muscle Nerve. 2013;48(2):272–278. doi: 10.1002/mus.23748. [DOI] [PubMed] [Google Scholar]
- 55.Dong H., Nie H., Mei Z., et al. China Medical Herald; 2019. Research Progress of the Application of Ubiquitin Proteasome Pathway. [Google Scholar]
- 56.Files D.C., D'Alessio F.R., Johnston L.F., et al. A critical role for muscle ring finger-1 in acute lung injury-associated skeletal muscle wasting. Am. J. Respir. Crit. Care Med. 2012;185(8):825–834. doi: 10.1164/rccm.201106-1150OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Smuder A.J., Sollanek K.J., Min K., et al. Inhibition of forkhead boxO-specific transcription prevents mechanical ventilation-induced diaphragm dysfunction. Crit. Care Med. 2015;43(5):e133. doi: 10.1097/CCM.0000000000000928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hooijman P.E., Beishuizen A., Witt C.C., et al. Diaphragm muscle fiber weakness and ubiquitin-proteasome activation in critically ill patients. Am. J. Respir. Crit. Care Med. 2015;191(10):1126–1138. doi: 10.1164/rccm.201412-2214OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Levine S., Biswas C., Dierov J., et al. Increased proteolysis, myosin depletion, and atrophic AKT-FOXO signaling in human diaphragm disuse. Am. J. Respir. Crit. Care Med. 2011;183(4):483–490. doi: 10.1164/rccm.200910-1487OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sandri and Marco Protein breakdown in muscle wasting: role of autophagy-lysosome and ubiquitin-proteasome. Int. J. Biochem. Cell Biol. 2013;45(10):2121–2129. doi: 10.1016/j.biocel.2013.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Endo M. Calcium-induced calcium release in skeletal muscle. Physiol. Rev. 2009;89(4):1153–1176. doi: 10.1152/physrev.00040.2008. [DOI] [PubMed] [Google Scholar]
- 62.Catterall W.A. Voltage-gated calcium channels. Cold Spring Harb Perspect Biol. 2011;3(8) doi: 10.1101/cshperspect.a003947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ochala J., Radell P.J., Eriksson L.I., et al. EMD 57033 partially reverses ventilator-induced diaphragm muscle fibre calcium desensitisation. Pflugers Arch. 2010;459(3):475–483. doi: 10.1007/s00424-009-0744-1. [DOI] [PubMed] [Google Scholar]
- 64.Moldoveanu T., Hosfield C.M., Lim D., et al. A Ca(2+) switch aligns the active site of calpain. Cell. 2002;108(5):649–660. doi: 10.1016/s0092-8674(02)00659-1. [DOI] [PubMed] [Google Scholar]
- 65.Yan H., Du J., Chen X., et al. ROS-dependent DNA damage contributes to crizotinib-induced hepatotoxicity via the apoptotic pathway. Toxicol. Appl. Pharmacol. 2019;383 doi: 10.1016/j.taap.2019.114768. [DOI] [PubMed] [Google Scholar]
- 66.Picard M., Azuelos I., Jung B., et al. Mechanical ventilation triggers abnormal mitochondrial dynamics and morphology in the diaphragm. J. Appl. Physiol. 2015;118(9):1161–1171. doi: 10.1152/japplphysiol.00873.2014. 1985. [DOI] [PubMed] [Google Scholar]
- 67.Grosu H.B., Lee Y.I., Lee J., et al. Diaphragm muscle thinning in patients who are mechanically ventilated. Chest. 2012;142(6):1455–1460. doi: 10.1378/chest.11-1638. [DOI] [PubMed] [Google Scholar]
- 68.Wilcox M.E., Rivera M.P., Sassoon C., et al. Clinical year in review II: mechanical ventilation, acute respiratory distress syndrome, critical care, and lung cancer. Proc. Am. Thorac. Soc. 2012;9(4):190–196. doi: 10.1513/pats.201206-033TT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shanely R.A., Van Gammeren D., Deruisseau K.C., et al. Mechanical ventilation depresses protein synthesis in the rat diaphragm. Am. J. Respir. Crit. Care Med. 2004;170(9):994–999. doi: 10.1164/rccm.200304-575OC. [DOI] [PubMed] [Google Scholar]
- 70.Yang L., Luo J., Bourdon J., et al. Controlled mechanical ventilation leads to remodeling of the rat diaphragm. Am. J. Respir. Crit. Care Med. 2002;166(8):1135–1140. doi: 10.1164/rccm.2202020. [DOI] [PubMed] [Google Scholar]
- 71.Dres M., Demoule A. Diaphragm dysfunction during weaning from mechanical ventilation: an underestimated phenomenon with clinical implications. Crit. Care. 2018;22(1) doi: 10.1186/s13054-018-1992-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu Y.Y., Li L.F. Ventilator-induced diaphragm dysfunction in critical illness. Exp Biol Med (Maywood) 2018;243(17–18):1329–1337. doi: 10.1177/1535370218811950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li L.F., Yu C.C., Wu H.P., et al. Reduction in ventilation-induced diaphragmatic mitochondrial injury through hypoxia-inducible factor 1α in a murine endotoxemia model. Int. J. Mol. Sci. 2022;23(3) doi: 10.3390/ijms23031083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ochala J., Renaud G., Llano Diez M., et al. Diaphragm muscle weakness in an experimental porcine intensive care unit model. PLoS One. 2011;6(6) doi: 10.1371/journal.pone.0020558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schepens T., Dres M., Heunks L., et al. Diaphragm-protective mechanical ventilation. Curr. Opin. Crit. Care. 2019;25(1):77–85. doi: 10.1097/MCC.0000000000000578. [DOI] [PubMed] [Google Scholar]
- 76.Shanely R.A., Zergeroglu M.A., Lennon S.L., et al. Mechanical ventilation-induced diaphragmatic atrophy is associated with oxidative injury and increased proteolytic activity. Am. J. Respir. Crit. Care Med. 2002;166(10):1369–1374. doi: 10.1164/rccm.200202-088OC. [DOI] [PubMed] [Google Scholar]
- 77.Cacciani N., Skärlén Å., Wen Y., et al. A prospective clinical study on the mechanisms underlying critical illness myopathy-A time-course approach. J Cachexia Sarcopenia Muscle. 2022;13(6):2669–2682. doi: 10.1002/jcsm.13104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Striano P., Belcastro V. Treatment of myoclonic seizures. Expert Rev. Neurother. 2012;12(12):1411–1417. doi: 10.1586/ern.12.90. ; quiz 1418. [DOI] [PubMed] [Google Scholar]
- 79.Demoule A., Jung B., Prodanovic H., et al. Diaphragm dysfunction on admission to the intensive care unit. Prevalence, risk factors, and prognostic impact-a prospective study. Am. J. Respir. Crit. Care Med. 2013;188(2):213–219. doi: 10.1164/rccm.201209-1668OC. [DOI] [PubMed] [Google Scholar]
- 80.Supinski G.S., Callahan L.A. Diaphragm weakness in mechanically ventilated critically ill patients. Crit. Care. 2013;17(3) doi: 10.1186/cc12792. R120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Petrof B.J. Diaphragmatic dysfunction in the intensive care unit: caught in the cross-fire between sepsis and mechanical ventilation. Crit. Care. 2013;17(4) doi: 10.1186/cc12864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Supinski G.S., Morris P.E., Dhar S., et al. Diaphragm dysfunction in critical illness. Chest. 2018;153(4):1040–1051. doi: 10.1016/j.chest.2017.08.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Le Dinh M., Carreira S., Obert J., et al. Prolonged mechanical ventilation worsens sepsis-induced diaphragmatic dysfunction in the rat. PLoS One. 2018;13(8) doi: 10.1371/journal.pone.0200429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Agten A., Maes K., Smuder A., et al. N-Acetylcysteine protects the rat diaphragm from the decreased contractility associated with controlled mechanical ventilation. Crit. Care Med. 2011;39(4):777–782. doi: 10.1097/CCM.0b013e318206cca9. [DOI] [PubMed] [Google Scholar]
- 85.Powers S., Hudson M., Nelson W., et al. Mitochondria-targeted antioxidants protect against mechanical ventilation-induced diaphragm weakness. Crit. Care Med. 2011;39(7):1749–1759. doi: 10.1097/CCM.0b013e3182190b62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Howe K.P., Clochesy J.M., Goldstein L.S., et al. Mechanical ventilation antioxidant trial. Am. J. Crit. Care. 2015;24(5):440–445. doi: 10.4037/ajcc2015335. [DOI] [PubMed] [Google Scholar]
- 87.Bonetto A., Aydogdu T., Jin X., et al. JAK/STAT3 pathway inhibition blocks skeletal muscle wasting downstream of IL-6 and in experimental cancer cachexia. Am. J. Physiol. Endocrinol. Metab. 2012;303(3):E410–E421. doi: 10.1152/ajpendo.00039.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Meier J.A., Hyun M., Cantwell M., et al. Stress-induced dynamic regulation of mitochondrial STAT3 and its association with cyclophilin D reduce mitochondrial ROS production. Sci. Signal. 2017;10(472) doi: 10.1126/scisignal.aag2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Khawli F.A., Reid M.B. N-acetylcysteine depresses contractile function and inhibits fatigue of diaphragm in vitro. J. Appl. Physiol. 1994;77(1):317–324. doi: 10.1152/jappl.1994.77.1.317. 1985. [DOI] [PubMed] [Google Scholar]
- 90.Smuder A.J., Sollanek K.J., Nelson W.B., et al. Crosstalk between autophagy and oxidative stress regulates proteolysis in the diaphragm during mechanical ventilation. Free Radic. Biol. Med. 2018;115:179–190. doi: 10.1016/j.freeradbiomed.2017.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Maes K., Testelmans D., Powers S., et al. Leupeptin inhibits ventilator-induced diaphragm dysfunction in rats. Am. J. Respir. Crit. Care Med. 2007;175(11):1134–1138. doi: 10.1164/rccm.200609-1342OC. [DOI] [PubMed] [Google Scholar]
- 92.Barreiro E., Puig-Vilanova E., Marin-Corral J., et al. Therapeutic approaches in mitochondrial dysfunction, proteolysis, and structural alterations of diaphragm and gastrocnemius in rats with chronic heart failure. J. Cell. Physiol. 2016;231(7):1495–1513. doi: 10.1002/jcp.25241. [DOI] [PubMed] [Google Scholar]
- 93.Kim W.Y., Park S.H., Kim W.Y., et al. Effect of theophylline on ventilator-induced diaphragmatic dysfunction. J. Crit. Care. 2016;33:145–150. doi: 10.1016/j.jcrc.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 94.De Cunto A., Paviotti G., Bua J., et al. Theophylline increases diaphragmatic contractility in mechanically ventilated newborns. J. Crit. Care. 2017;37:264–265. doi: 10.1016/j.jcrc.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 95.Sugiura H., Kawabata H., Ichikawa T., et al. Inhibitory effects of theophylline on the peroxynitrite-augmented release of matrix metalloproteinases by lung fibroblasts. Am. J. Physiol. Lung Cell Mol. Physiol. 2012;302(8):L764–L774. doi: 10.1152/ajplung.00342.2011. [DOI] [PubMed] [Google Scholar]
- 96.Aydin N.B., Teke T., Toy H., et al. The effect of theophylline on the prevention of mechanical ventilation-induced diaphragm atrophy in rats. Adv. Clin. Exp. Med. 2014;23(1):33–38. doi: 10.17219/acem/37019. [DOI] [PubMed] [Google Scholar]
- 97.Maes K., Testelmans D., Cadot P., et al. Effects of acute administration of corticosteroids during mechanical ventilation on rat diaphragm. Am. J. Respir. Crit. Care Med. 2008;178(12):1219–1226. doi: 10.1164/rccm.200702-296OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Maes K., Testelmans D., Thomas D., et al. High dose methylprednisolone counteracts the negative effects of rocuronium on diaphragm function. Intensive Care Med. 2011;37(11):1865–1872. doi: 10.1007/s00134-011-2337-7. [DOI] [PubMed] [Google Scholar]
- 99.Maes K., Agten A., Smuder A., et al. Corticosteroid effects on ventilator-induced diaphragm dysfunction in anesthetized rats depend on the dose administered. Respir. Res. 2010;11(1):2178. doi: 10.1186/1465-9921-11-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sassoon C.S., Zhu E., Pham H.T., et al. Acute effects of high-dose methylprednisolone on diaphragm muscle function. Muscle Nerve. 2008;38(3):1161–1172. doi: 10.1002/mus.21048. [DOI] [PubMed] [Google Scholar]
- 101.Hall S.E., Ahn B., Smuder A.J., et al. Comparative efficacy of angiotensin II type 1 receptor blockers against ventilator-induced diaphragm dysfunction in rats. Clin Transl Sci. 2021;14(2):481–486. doi: 10.1111/cts.12916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Takezako T., Unal H., Karnik S.S., et al. Current topics in angiotensin II type 1 receptor research: focus on inverse agonism, receptor dimerization and biased agonism. Pharmacol. Res. 2017;123:40–50. doi: 10.1016/j.phrs.2017.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kwon O.S., Smuder A.J., Wiggs M.P., et al. AT1 receptor blocker losartan protects against mechanical ventilation-induced diaphragmatic dysfunction. J. Appl. Physiol. 2015;119(10):1033–1041. doi: 10.1152/japplphysiol.00237.2015. 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.van Hees H.W., Dekhuijzen P.N., Heunks L.M. Levosimendan enhances force generation of diaphragm muscle from patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2009;179(1):41–47. doi: 10.1164/rccm.200805-732OC. [DOI] [PubMed] [Google Scholar]
- 105.Doorduin J., Sinderby C.A., Beck J., et al. The calcium sensitizer levosimendan improves human diaphragm function. Am. J. Respir. Crit. Care Med. 2012;185(1):90–95. doi: 10.1164/rccm.201107-1268OC. [DOI] [PubMed] [Google Scholar]
- 106.Roesthuis L., van der Hoeven H., Sinderby C., et al. Effects of levosimendan on respiratory muscle function in patients weaning from mechanical ventilation. Intensive Care Med. 2019;45(10):1372–1381. doi: 10.1007/s00134-019-05767-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zambelli V., Murphy E.J., Delvecchio P., et al. Treatment with levosimendan in an experimental model of early ventilator-induced diaphragmatic dysfunction. Drug Target Insights. 2023;17:39–44. doi: 10.33393/dti.2023.2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Senf S.M. Skeletal muscle heat shock protein 70: diverse functions and therapeutic potential for wasting disorders. Front. Physiol. 2013 330;4 doi: 10.3389/fphys.2013.00330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Smuder A.J., Min K., Hudson M.B., et al. Endurance exercise attenuates ventilator-induced diaphragm dysfunction. J. Appl. Physiol. 2012;112(3):501–510. doi: 10.1152/japplphysiol.01086.2011. 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Smuder A.J., Morton A.B., Hall S.E., et al. Effects of exercise preconditioning and HSP72 on diaphragm muscle function during mechanical ventilation. J Cachexia Sarcopenia Muscle. 2019;10(4):767–781. doi: 10.1002/jcsm.12427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Salah H., Li M., Cacciani N., et al. The chaperone co-inducer BGP-15 alleviates ventilation-induced diaphragm dysfunction. Sci. Transl. Med. 2016;8(350) doi: 10.1126/scitranslmed.aaf7099. 350ra103. [DOI] [PubMed] [Google Scholar]
- 112.Mnuskina S., Bauer J., Wirth-Hücking A., et al. Single fibre cytoarchitecture in ventilator-induced diaphragm dysfunction (VIDD) assessed by quantitative morphometry second harmonic generation imaging: positive effects of BGP-15 chaperone co-inducer and VBP-15 dissociative corticosteroid treatment. Front. Physiol. 2023;14 doi: 10.3389/fphys.2023.1207802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bissett B., Leditschke I.A., Neeman T., et al. Weaned but weary: one third of adult intensive care patients mechanically ventilated for 7 days or more have impaired inspiratory muscle endurance after successful weaning. Heart Lung. 2015;44(1):15–20. doi: 10.1016/j.hrtlng.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 114.Sassoon C.S., Zhu E., Caiozzo V.J. Assist-control mechanical ventilation attenuates ventilator-induced diaphragmatic dysfunction. Am. J. Respir. Crit. Care Med. 2004;170(6):626–632. doi: 10.1164/rccm.200401-042OC. [DOI] [PubMed] [Google Scholar]
- 115.Hudson M.B., Smuder A.J., Nelson W.B., et al. Partial support ventilation and mitochondrial-targeted antioxidants protect against ventilator-induced decreases in diaphragm muscle protein synthesis. PLoS One. 2015;10(9) doi: 10.1371/journal.pone.0137693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chen Y.H., Hsiao H.F., Li L.F., et al. Effects of electrical muscle stimulation in subjects undergoing prolonged mechanical ventilation. Respir. Care. 2019;64(3):262–271. doi: 10.4187/respcare.05921. [DOI] [PubMed] [Google Scholar]
- 117.Reynolds S.C., Meyyappan R., Thakkar V., et al. Mitigation of ventilator-induced diaphragm atrophy by transvenous phrenic nerve stimulation. Am. J. Respir. Crit. Care Med. 2017;195(3):339–348. doi: 10.1164/rccm.201502-0363OC. [DOI] [PubMed] [Google Scholar]
- 118.Martin A.D., Joseph A.M., Beaver T.M., et al. Effect of intermittent phrenic nerve stimulation during cardiothoracic surgery on mitochondrial respiration in the human diaphragm. Crit. Care Med. 2014;42(2):e152–e156. doi: 10.1097/CCM.0b013e3182a63fdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yang M., Wang H., Han G., et al. Phrenic nerve stimulation protects against mechanical ventilation-induced diaphragm dysfunction in rats. Muscle Nerve. 2013;48(6):958–962. doi: 10.1002/mus.23850. [DOI] [PubMed] [Google Scholar]
- 120.Rohrs E.C., Bassi T.G., Fernandez K.C., et al. Diaphragm neurostimulation during mechanical ventilation reduces atelectasis and transpulmonary plateau pressure, preserving lung homogeneity and [Formula: see text]/[Formula: see text] J. Appl. Physiol. 2021;131(1):290–301. doi: 10.1152/japplphysiol.00119.2021. 1985. [DOI] [PubMed] [Google Scholar]
- 121.Ambrosino N., Vitacca M. The patient needing prolonged mechanical ventilation: a narrative review. Multidiscip Respir Med. 2018 6;13 doi: 10.1186/s40248-018-0118-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Callahan L.A., Supinski G.S. Rapid and complete recovery in ventilator-induced diaphragm weakness--problem solved? J. Appl. Physiol. 2013;115(6):773–774. doi: 10.1152/japplphysiol.00831.2013. 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Thomas D., Maes K., Agten A., et al. Time course of diaphragm function recovery after controlled mechanical ventilation in rats. J. Appl. Physiol. 2013;115(6):775–784. doi: 10.1152/japplphysiol.00302.2012. 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Owen A.M., Patel S.P., Smith J.D., et al. Chronic muscle weakness and mitochondrial dysfunction in the absence of sustained atrophy in a preclinical sepsis model. Elife. 2019;8 doi: 10.7554/eLife.49920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Friedrich O., Reid M.B., Van den Berghe G., et al. The sick and the weak: neuropathies/myopathies in the critically ill. Physiol. Rev. 2015;95(3):1025–1109. doi: 10.1152/physrev.00028.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.