Abstract
The virus interacts with its hosts by developing protein-protein interactions. Most viruses employ protein interactions to imitate the host protein: A viral protein with the same amino acid sequence or structure as the host protein attaches to the host protein's binding partner and interferes with the host protein's pathways. Being opportunistic, viruses have evolved to manipulate host cellular mechanisms by mimicking short linear motifs. In this review, we shed light on the current understanding of mimicry via short linear motifs and focus on viral mimicry by genetically different viral subtypes by providing recent examples of mimicry evidence and how high-throughput methods can be a reliable source to study SLiM-mediated viral mimicry.
Keywords: virus, protein-protein interactions, short linear motifs, mimicry
Introduction
Most viruses use similar strategies to impact cellular circuits by imitating host patterns (Benedict et al., 2002[10]; Chaurushiya et al., 2012[13]; Davey et al., 2011[18]; Finlay and McFadden, 2006[35]). Most of the time, globular domains mediate protein interactions through interactions. It was once believed that only the proteome's globular domains and structured regions could mediate protein-protein interactions (PPIs). However, advances in proteomics have demonstrated that disordered regions with Short Linear Motifs (SLiMs) also significantly contribute to PPIs. Viral cells interact with host cellular proteins via SLiMs that resemble SLiMs in host cells. Several biological activities, such as PPIs, post-translational modifications, regulation, and cell compartment targeting, use SLiMs (Davey et al., 2012[19]; Neduva and Russell, 2005[81]). SLiMs, which are strong and rapidly evolving components found in viruses, cause the rewiring of the virus-host PPIs (vhPPIs) (Chemes et al., 2015[14]). Primarily, a SLiM that is sufficiently visible on a protein surface can govern protein stability; more generally, it can control ligand binding and targeting, which can manage various functions. The human proteome is about 30 % disordered (Garg et al., 2022[38]; Neduva and Russell, 2005[81]; Van Roey et al., 2014[109]). These intrinsically disordered regions (IDRs) and occasionally inaccessible loops inside their folded domains contain SLiMs that are evolutionarily changeable protein regions that can change with a single-point mutation (Neduva and Russell, 2005[81]; Van Roey et al., 2014[109]). IDRs have functional significance but still need extensive characterization (Diella et al., 2008[24]; Hornbeck et al., 2012[52]; Nguyen Ba et al., 2012[83]).
In unrelated proteins, SLiMs may arise de-novo due to convergent evolution, further complicating our understanding of the interactome (Tompa and Csermely, 2004[107]). As per estimation, the human proteome has about 1 million SLiMs (Tompa et al., 2014[108]), explaining the complexity of the regulatory mechanisms of cells. SLiMs in pathogenic proteins are called mimicry motifs because they have similar (if not identical) amino acid composition and function to host SLiMs (Elkhaligy et al., 2021[31]). Many mimicry motifs exist in pathogens, particularly in attachment, penetration, and cytolysis proteins (Goswami et al., 2023[43]). One of the viruses' most well-known mimic motifs is PxxPxR (polyproline motif). This motif has been reported in non-structural 5A proteins (NS5A in hepatitis C virus and Nef protein (Nef in HIV type 1) and establishes interactions with the SH3 domains of host proteins (Shelton and Harris, 2008[102]). The interaction between the spike protein of SARS-CoV-2 receptor binding domain (RBD) and human angiotensin-converting enzyme 2 (ACE2) receptor is well documented (Lan et al., 2020[70]; Ridgway et al., 2023[95]; Zhou et al., 2020[121]). Recently, a study has shown that ORF8 of SARS-CoV-2 mimics IL-17 cytokines, contributing to severe inflammation during COVID-19 (Wu et al., 2022[116]). Domain Motif Interactions (DMI) are the primary mechanism of interaction between viruses and host proteins, where a single target molecule (SLiM) in the virus acts on a single target domain (host domain) (Halehalli and Nagarajaram, 2015[47]). DMI is an important therapeutic target. However, only a few published studies demonstrate the ability of DMIs to be drug targets. DMI targets are challenging due to their transient, heterogeneous, and complex nature. Another issue with DMIs is their physiological and structural properties. (Corbi-Verge and Kim, 2016[16]; Davey et al., 2011[18]). Only a few known DMIs are reported in the human proteome, showing that many are still to be discovered (Tompa et al., 2014[108]). In general, molecular mimicry is an exciting field of research to understand how viruses manipulate host cell routes and how viruses attack host cells (Goswami et al., 2023[43]). Ultimately, these discoveries will develop new antiviral therapeutic drugs (Corbi-Verge and Kim, 2016[16]; Davey et al., 2011[18]; Dyer et al., 2007[29]; Via et al., 2015[110]). Therefore, more advanced techniques (computational or experimental) are needed to study interactions based on SLiM, which is critical to understanding the mechanisms of motif mimicking in viruses (Evans et al., 2009[33]; Segura-Cabrera et al., 2013[101]).
A General Overview of SLiMs
SLiMs are linear recurring peptides composed of 3-10 contiguous residues (Bhowmick et al., 2015[11]; D'haeseleer, 2006[23]; Davey et al., 2012[19]; Weatheritt et al., 2012[111]) often found in the disordered regions of proteins. SLiMs aid in mediating interactions with other partner proteins, although these interactions are transitory and have a low affinity of 1-150 μm (11-14 μm) (Diella et al., 2008[24]). SLiMs usually have only 2-5 defined positions and are challenging to detect by experimental and computational techniques (Neduva and Russell, 2005[81]). SLiMs play a role in various cellular pathways through DMIs, where rapid responses are required for transmission (Gibson, 2009[39]; Pancsa and Fuxreiter, 2012[89]). SLiMs can switch to multiple functionalities using a single-point mutation and are considered molecular switches. This plasticity of SLiMs gives pathogenic proteins an advantage, allowing them to mimic host proteins and aid in the host cell pathway (Dinkel et al., 2014[26]). To date, more than 4,138 new motif instances have been identified. While SLiM discovery is still in its early stages, there has been some progress in recent years. Various new computational tools and techniques have been developed to facilitate SLiM predictions of the protein sequence data. The significant repositories maintaining motif data include the eukaryotic linear motif database (ELM database) (Dinkel et al., 2012[25]; Kumar et al., 2020[68]), PROSITE (Hulo et al., 2006[54]), Linear Motif mediated Protein Interaction Database (LMPID) (Sarkar et al., 2015[99]), Minimotif-Miner (Balla et al., 2006[7]), PepCyber (Gong et al., 2008[42]) and Scansite (Obenauer et al., 2003[84]). The interaction of motif residues with domains suggests that these residues' positions will be conserved over time. Many SLiMs have two or more conserved hygroscopic residues, e.g., the nuclear export sequence (NES) has four (Gibson et al., 2015[40]). SLiMs are highly adaptable and act as molecular switches turned on or off by a single mutation. Such mutations may modify the function of the motifs. This property of SLiMs is being studied to design biological pathways that control different functions (Dueber et al., 2003[27]; Neduva and Russell, 2005[81]). For instance, a single TQG-to-TQT mutation can lead to synaptic transport in the neuron cells (Neduva and Russell, 2005[81]). The small size and polymorphic nature of SLiMs suggest that linear motifs are likely to have independent origins and can be used to find novel motifs that share interaction partners (Bhowmick et al., 2015[11]; Edwards and Palopoli, 2015[30]).
High Throughput PPI Data as the Source for Predicting Motif Mimicry
Proteomic approaches have advanced during the past few years. These approaches play a significant role in identifying protein interactions, especially in understanding host-pathogen interactions. The current advancement in these methods has paved a new way to find novel PPIs. Different studies have discovered several novel interactions. The resulting interactions have been incorporated into several databases to help ease the process of further analysis (Lum and Cristea, 2016[75]). DMIs are used to detect new SLiMs. Unfortunately, most DMI information has been obtained from low-throughput studies (Blikstad and Ivarsson, 2015[12]). However, recently, various high throughput methods, including affinity purification in combination with mass spectrometry, yeast 2-hybrid and coimmunoprecipitations, have been employed to study DMI in multiple organisms (Mihalic et al., 2023[79]). These high throughput methods have enabled the generation of a large PPI dataset that is being used to predict functional DMIs and protein complexes. However, due to the high throughput nature of these high throughput experiments, false positives and false negatives are always present, impacting the chances of successful predictions (Li et al., 2010[71]; Zhang et al., 2015[120]). These high-throughput experiments have been conducted on various domain families, yielding a large amount of PPI data. Today, studies are being undertaken to determine SLiMs and related binding partners in human protein (Rajagopala et al., 2014[93]).
Several methods have been used to study the SLiM-mediated interactions in viruses. Among these methods, two well-known methods are widely used to find vhPPIs. One of these methods is the Yeast two-hybrid (Y2H) method, which has produced a large amount of DMI data. This method splits a transcription factor binding domain and a DNA binding domain to bait or prey proteins. Through the interactions of the prey and bait proteins, the transcription factor leads to the activation of transcription of reporter genes. The advantage of using Y2H is its ability to study one protein or a library of proteins (Rolland et al., 2014[96]). Several successful studies have been carried out in recent years to identify DMIs. For example, SUMO interacting motifs have been discovered that interact with the SUMO1 and SUMO2 proteins (Hecker et al., 2006[51]). Y2H has not only successfully been used for the identification of DMIs but also for characterizing peptide binding motifs through screening peptide libraries. For example, Hu et al. have studied PDZ protein PDZK1 using Y2H screening against a random set of libraries (Hu et al., 2009[53]). Similarly, Guo et al. Investigated PDZ protein LNX using Y2H screening (Guo et al., 2012[46]). Y2H is used to find direct physical interactions between proteins, but most of the Y2H interaction data is transient and includes inter-complex interactions. The chances of false positives and false negatives are high, so Y2H might not be a precise source for binary interaction mapping (Zhang et al., 2015[119]). On the other hand, affinity purification followed by mass spectrometry (AP-MS) and Co-fractionation followed by MS (CoFrac-MS) is more suitable for identifying co-complex interactions, including direct and indirect interactions between proteins (Kim et al., 2010[63]). AP-MS works on purifying baits from cell lysate and detecting copurified proteins (the prey) by MS. The problem with AP-MS data is distinguishing between direct and indirect interactions (Luck et al., 2017[74]; Zhang et al., 2015[119]). CoFrac-MS is based on the fractionation of the protein extracts extensively to separate the protein complexes whose components are then detected by MS (Luck et al., 2017[74]). DMIs are currently underrepresented in available PPI networks and databases (Blikstad and Ivarsson, 2015[12]). During the past few years, these high throughput methods have been essential data sources for SLiM discovery by generating efficient PPI data.
Viral Mimicry by Different Viral Classes
Different computational approaches have been developed in recent years to study vhPPIs across the entire proteome (Dyer et al., 2007[29]; Evans et al., 2009[33]; Segura-Cabrera et al., 2013[101]), but most of these have focused on selected pathogens only (Barnes et al., 2016[9]; Chen et al., 2021[15]; Emamjomeh et al., 2014[32]; Zhang et al., 2017[118]). No study has been conducted to analyze different virus subtypes by their genetic makeup to understand how they disrupt the host cellular machinery responsible for regulatory functions and the infection cycle. So, it is interesting to know how different virus subtypes interact with the host proteins via SLiM. A few examples of experimentally validated mimicry candidates based on viral classes are given in Table 1(Tab. 1) (References in Table 1: Abada et al., 2008[1]; Accardi et al., 2011[2]; Ako-Adjei et al., 2015[3]; Bai and Nicot, 2012[5]; Barbera et al., 2006[8]; de Chassey et al., 2008[21]; Deng et al., 2005[22]; Falson et al., 2015[34]; Ganti et al., 2015[37]; Grzesik et al., 2019[45]; Han et al., 2004[48], 2020[49]; Incrocci et al., 2019[57]; Khan and Geiger, 2021;[62] Kirui et al., 2014[64]; Mechali et al., 2004[77]; Pal and Kundu, 2019[86]; Pan et al., 2018[88]; Ponnusamy et al., 2019[91]; Saridakis et al., 2005[98]; Segura-Cabrera et al., 2013[100]; Tavakolian et al., 2020[106]; Welcker and Clurman, 2005[112]; Zhang et al., 2018[117]).
Table 1. Examples of viral mimicry candidates characterised by viral subtypes.
RNA viruses
RNA viruses are among the most harmful to human health and affect millions worldwide. RNA viruses are single-stranded or double-stranded and reproduce by using RNA-dependent RNA polymerases. A single-stranded virus, such as a retrovirus, infects a host cell with two copies of the single-stranded viral genome. The virus then undergoes reverse transcriptions to create viral DNA entering the host DNA. Viruses, including Ebola virus, HIV-1, Zika virus, Dengue virus, Influenza virus, Yellow fever virus, Adult Human T-cell lymphotropic virus type 1, Poliovirus, SARS virus and Retrovirus, are examples of RNA viruses (Poltronieri et al., 2015[90]). The RNA genome is responsible for generating viral proteins required for viral replication and some additional tasks, such as the template for the replication of genomic sequences, mRNA transcription and the assembly of virions. In some RNA viruses, the viral genome plays a crucial role in multiple processes in the host cell. Different viral and host enzymes interact with the virus genome to perform essential functions that help viruses replicate within host cells. In general, protein and various RNA factors interact with cell pathways to allow viruses to hijack host cell machinery (White et al., 2011[113]). In addition, RNA viruses have a high degree of structural and functional diversity. They can generate new RNA genomes at a rate of 0.4 s (provided the replication machinery is functioning optimally) (Moya et al., 2000[80]). It has been shown that viral hijacking through SLiMs is quite common in RNA viruses (Lieber et al., 2010[72]). Since there is currently no effective vaccine for most RNA viruses, it is essential to understand how the virus infects host cells and replicates by hijacking host cell machinery (Franzosa and Xia, 2011[36]).
Single-stranded RNA viruses
There are two major categories of RNA single-stranded viruses: negative-stranded and positive-stranded. Negative-stranded viruses (NSV) are characterized by their genetic material consisting of a single strand of RNA.
Positive-stranded virus (PSV) viruses are classified as segmented or non-segmented. Segmented viruses comprise families such as orthomyxovirus, Arenavirus, Bunyavirus, etc. Non-segmented viruses consist of families such as paramyxovirus, bornavirus, rhabdovirus, and filovirus. NSVs are characterized by their highly structured genome structures, which can be expressed as nucleocapsid or nucleoprotein complexes where genomic RNA is associated with more than one monomer nucleoprotein (nucleoside) (Green et al., 2014[44]; Ortin and Martin-Benito, 2015[85]). NSVs cause high mortality rates and have been implicated in many disease events, including influenza, measles, and mumps (Ortin and Martin-Benito, 2015[85]). NSVs begin their life cycle by attaching to a host cell, releasing their ssRNA. The released ssRNA is then translated into mRNA within the cell. mRNA is also transcribed to a genomic strand, which acts as a template for the virus genome replication. The transcription is done by a viral polymerase packaged inside the newly formed virion. Once the replicated virion has been assembled, it is released outside the cell (Figure 1B(Fig. 1)) (Stangler et al., 2007[104]).
Figure 1. Replication cycle of ssRNA viruses. A) The replication cycle of PSVs: The virus is attached to a host cell and releases a +sense of its ssRNA. This +sense molecule is then translated into a single polyprotein molecule broken down into a protein. This protein is a template for the replication of the virus. B) The replication cycle of NSVs: The RNA in the NSV is transcribed into an mRNA, which can be further transcribed to a full-length +sense strand. The virus is then packaged and released from the cell, and the virus polymerase plays a role in transcription. Once the virus has been packaged and assembled, it is released outside of the cell (created using BioRender.com).
PSVs, on the other hand, are an essential subclass of RNA viruses in which the RNA genome is plus-stranded. The RNA genome of a PSV serves as the template for the formation of viral proteins. It contains cis-acting RNA segments that regulate viral pathways such as viral replication, transcription, and translation (Liu et al., 2009[73]; Sztuba-Solinska et al., 2011[105]). During the life cycle of PSVs, the virus attaches to the host cell and releases the ssRNA into the cell. The ssRNA is translated into a polyprotein. Polysaccharides are further broken down into various proteins, including viral and RNA-dependent RNA polymerases. A complement of RNA is produced to serve as mRNA and aid in replicating the polysaccharide. Replicated information is assembled into a new virus and released outside the cell (Figure 1A(Fig. 1)) (Stangler et al., 2007[104]).
Generally, PSVs enter the host cell and replicate in its cytoplasm, where the host's immune system creates a hostile environment for viral replication. PSVs solve this problem by concentrating viral proteins in the intracellular space, allowing for continuous viral genome replication. PSVs hijack host factors that are involved in vascular transport and lipid synthesis. These host factors protect the virus replication machinery from the immune system, allowing for safe viral replication and assembly (Harak and Lohmann, 2015[50]). One of the most well-known examples of ssRNA virus motif mimicry is hepatitis C (HCV). HCV hijacks the host cell's machinery by replicating PxxP (known to bind to many SH3 domains in the Src kinases family) (Duro et al., 2015[28]). Another example of a PxxP motif is the hijacking of host cellular machinery in HIV by establishing interactions with the host SH3 domain (Stangler et al., 2007[104]).
Double-stranded RNA viruses
Double-stranded RNA (dsRNA) is widely distributed in various organisms, including animal, plant, microbial, and non-molecular organisms-the first detection of dsRNA in a reovirus was in 1963 by Gomatos and Tamm (Wickner, 1993[114]). Most dsRNA viruses share similar capsid structures, replication strategies, and biochemical and structural characteristics. This is why cognate proteins of similar design and function can be identified from distantly related viruses, providing insight into their shared ancestry. It is well-known that dsRNA viruses replicate within the host cell's cytoplasm. These viruses enter the host cell and convert ssRNA into dsRNA. The genomic dsRNA then undergoes transcription into mRNA, which, upon translation, produces proteins necessary for viral replication (Figure 2(Fig. 2)). dsRNA viruses can replicate/transcribe their RNA within the icosahedral capsid due to the defense mechanisms of eukaryotic systems. These mechanisms detect and inactivate dsRNA through the presence of the inactivation protein PKR or the inactivation protein MDA5 (Mertens, 2004[78]). Furthermore, the viral proteins found in the internal virion-related enzymes and the innermost capsid layer are conserved mainly in most viruses. However, the non-structural proteins found in the outer capsid are diverse in sequence and structural organisation (Mertens, 2004[78]). A well-known example of dsRNA hijacking host cellular machinery is Segment-10 from Bluetongue, which hijacks host cell pathways by mimicking the functions of various host proteins (e.g., NEDD4, TSG101) (Wirblich et al., 2006[115]).
Figure 2. Replication cycle of dsRNA viruses. The virus attaches to the host cell and transcribes its dsRNA into mRNA. This mRNA is then packaged, resulting in the production of an early replicate particle. Subsequently, complementary RNA is produced, resulting in a late replicate particle released as a mature virus (created using BioRender.com).
DNA viruses
The prevalence and diversity of DNA viruses in eukaryotic organisms are significantly lower than that of RNA viruses. However, the emergence of giant viruses (with a genome size more significant than that of bacteria, archaea, and numerous parasitic unicellular organisms) has caused a shift in focus from RNA viruses to DNA viruses (Koonin et al., 2015[65]). DNA viruses are like RNA viruses, using a capsid to bind to and attack host cells (Ng et al., 2012[82]; Rao and Feiss, 2015[94]). Once they enter, they disassemble the virus and release the genome into the cell, transcribing it into mRNA. This mRNA is then translated into proteins, which help them hijack the host cells and make progeny viruses (Krupovic and Forterre, 2015[66]; Ng et al., 2012[82]). These progeny viruses are released outside the host cell, ready to attack other cells. There are two main types of DNA viruses - small (under 10 kb) and large (over 30 kb). Examples of small viruses include HPV and HBV, while giant viruses like Adenoviruses, Poxiviruses, and Herpesviruses are more common (Iyer et al., 2001[58]).
Single-stranded DNA viruses
These simple viruses have a single strand of DNA (ssDNA) as their genome. Most ssDNA viruses contain damaging strand DNA, but some include positive and negative strand DNA (Koonin et al., 2015[65]). ssDNA viruses have various invasion mechanisms depending on the host organisms (eukaryotes, bacteria, archaea, etc.). For example, the bacteriophage family Inoviridae have three different mechanisms of invasion of host cells. Some viruses utilize DDE transposase of IS30 family, IS3 family, IS110 family and IS492 family. Others encode the Serines/Tyrosin superfamily integrases and hijack the host recombinase mechanism. Eukaryotic ssDNA viruses integrate with host cells through the endonuclease function of their rolling ring replication initiation proteins (like bacteria transposon mechanisms) (Krupovic and Forterre, 2015[66]; Rosario et al., 2018[97]). These viruses contain one gene for coding the nucleocapsids in the virus and one for the DNA-encoding DNA replication enzyme. When the virus invades the host cell, it must convert its ssDNA genome to dsDNA, using DNA polymerase in the host cell to produce dsDNA. This dsDNA is then used as a template to transcribe the transcribed RNA into viral proteins. The replicated DNA is then converted to ssDNA, packaged, and reassembled into a new virus. Once packaged, the new virus is released outside the cell to infect new cells (Figure 3(Fig. 3)) (Krupovic and Forterre, 2015[66]).
Figure 3. Replication cycle of ssDNA virus. Replication cycle of ssDNA virus. ssDNA viruses bind and enter their host cells to start their life cycle. As the ssDNA gets inside the vesicle, it releases its ssDNA within the nucleus—next, the ssDNA changes into dsDNA, which is then translated into early cytoplasmic mRNA. The early messenger RNA is then translated into different regulatory proteins that help to replicate the whole genome. Structural proteins are produced by translating the mRNA from the dsDNA transcription. Packaged and released outside the cell are newly duplicated ssDNA and structural proteins (created using BioRender.com).
Double-stranded DNA viruses
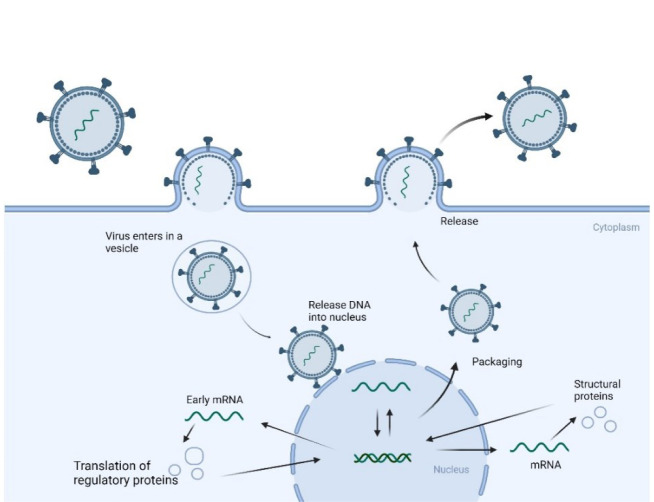
A double-stranded DNA (dsDNA) virus has a single molecule dsDNA as its genome (Wickner, 1993[114]). Many dsDNA virus families infect mammals, such as Hepadnavirus, Papillomirus, Polyomirus, Herpesvirus, Adenovirus, Asfarvirus, and Poxvirus (Koonin et al., 2015[65]). All these viruses, except Asfarvirus, infect humans or animals. dsDNA viruses are often considered the simplest to understand regarding their life cycle. The life cycle of a dsDNA virus starts when a virus attacks the host cell and viral DNA enters the host cell's nucleus, replicating the host genome. Host cell DNA polymerase replicates viral DNA, and then the mRNA is transcribed into various viral proteins. Some of these proteins are capsids in which newly replicated dsDNA is packaged. Once packaged, the packaged virion is released outside the cell to infect other cells (Figure 4(Fig. 4)) (Kazlauskas et al., 2016[60]; Kazlauskas and Venclovas, 2011[61]; Rao and Feiss, 2015[94]). A well-known example of dsRNA motif mimicry is the PxLTXP motif in the HADC serotype 5 E1 protein interacting with the human BS69 protein MYND domain. Hijacking aids viruses in controlling their viral replication at the time of infection (Zhang et al., 2018[117]). Another example of dsDNA virus motif mimicry is where the PDZ-binding motif in the HPDC E6 protein targets host cell PDZ-containing proteins (Accardi et al., 2011[2]; Segura-Cabrera et al., 2013[100]).
Figure 4. Replication cycle of dsDNA virus. The attachment and penetration of the virus into the cell marks the start of the dsDNA virus life cycle. A vesicle carrying the virus enters the cell. Once it enters the nucleus, it releases its dsDNA molecule. The dsDNA gets translated into mRNA once it is within the nucleus. Regulatory proteins are produced through translation of the mRNA in the cytoplasm. Regulatory proteins aid DNA replication and mRNA transcription into structural proteins. After being enclosed in a capsid, the freshly copied DNA and the structural proteins are liberated from the cell (created using BioRender.com).
SLiM-Mediated Prediction of Viral Mimicry
Understanding the functional importance of SLiMs and clarifying their roles in different biological pathways requires computational prediction of these motifs. Various computational SLiM discovery tools have been developed in the post-genomic era to help find putative SLiMs in protein sequences and expand our knowledge of molecular relationships and cellular signalling networks (Idrees et al., 2018[56]). Several SLiM discovery tools have been developed; a few are listed in Table 2(Tab. 2) (References in Table 2: Bailey et al., 2015[6]; de Castro et al., 2006[20]; Jones and Cozzetto, 2015[59]; Krystkowiak and Davey, 2017[67]; Kumar et al., 2022[69]; Palopoli et al., 2015[87]; Prytuliak et al., 2017[92]). Successful prediction of SLiMs using computational tools can also help develop antiviral drugs (Simonetti et al., 2023[103]). The general criteria in such cases are to find SLiMs in viral proteins involved in virus replication, entry, or defence evasion (Davey et al., 2012[17]). Once essential SLiMs have been identified, the next step is to create therapeutic agents (e.g., small molecules, peptides) that imitate or interfere with these SLiMs. This strategy breaks viral protein chains and disrupts viral functions (Mahajan et al., 2021[76]). Most viruses use specific SLiMs to enter host cells; therefore, antiviral methods that block viral entry motifs can help prevent the virus from infecting the cells. Moreover, small peptide-based inhibitors can help interfere with SLiM-mediated interactions, disrupting viral replication and slowing the infection (Bah and Forman-Kay, 2016[4]).
Table 2. SLiM discovery/prediction tools that can help in predicting new mimicry candidates.
In general, computational prediction of SLiM-mediated viral mimicry rapidly progresses, revealing viruses' complex interactions with their hosts. These relationships are necessary for pathogenesis and viral replication. Researchers using advanced computational resources and techniques are finding ways to emulate viral campaigns. This data has the potential to generate novel antiviral therapies in addition to deepening our understanding of vhPPIs. Moreover, developing antiviral strategies using SLiMs as targets is a multi-faceted approach that involves understanding the intricacies of vhPPIs and designing interventions that disrupt these interactions. It's a promising avenue in the battle against viral infections, and continued research in this field may yield innovative antiviral therapies that are effective and less prone to resistance development (Bah and Forman-Kay, 2016[4]).
Conclusion
Viruses enter the host cell and start their replication process to propagate. Due to their restricted genome size, viruses have evolved to utilize host cellular machinery to continue their life cycle and escape the host defense system. Viruses use short sequences of linear motifs to mimic and hijack host cellular proteins. Therefore, it's essential to understand the short linear motif-mediated interactions between viruses and their host proteins. This review covers the current knowledge of viral mimicry and gaps in knowledge that will be important for future studies to investigate.
Declaration
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgments
The authors thank the University of New South Wales, Sydney, and Dr. Richard J. Edwards.
Author contributions
SI wrote the manuscript. KRP, and PMH helped in revision. TS helped in making figures.
Footnotes
This review article contains partial excerpts from Idrees' dissertation (2020[55]).
References
- 1.Abada R, Dreyfuss-Grossman T, Herman-Bachinsky Y, Geva H, Masa SR, Sarid R. SIAH-1 interacts with the Kaposi's sarcoma-associated herpesvirus-encoded ORF45 protein and promotes its ubiquitylation and proteasomal degradation. J Virol. 2008;82:2230–2240. doi: 10.1128/JVI.02285-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Accardi R, Rubino R, Scalise M, Gheit T, Shahzad N, Thomas M, et al. E6 and E7 from human papillomavirus type 16 cooperate to target the PDZ protein Na/H exchange regulatory factor 1. J Virol. 2011;85:8208–8216. doi: 10.1128/JVI.00114-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ako-Adjei D, Fu W, Wallin C, Katz KS, Song G, Darji D, et al. HIV-1, human interaction database: current status and new features. Nucleic Acids Res. 2015;43(Database issue):D566–D570. doi: 10.1093/nar/gku1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bah A, Forman-Kay JD. Modulation of intrinsically disordered protein function by post-translational modifications. J Biol Chem. 2016;291:6696–6705. doi: 10.1074/jbc.R115.695056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bai XT, Nicot C. Overview on HTLV-1 p12, p8, p30, p13: accomplices in persistent infection and viral pathogenesis. Front Microbiol. 2012;3:400. doi: 10.3389/fmicb.2012.00400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bailey TL, Johnson J, Grant CE, Noble WS. The MEME Suite. Nucleic Acids Res. 2015;43(W1):W39–W49. doi: 10.1093/nar/gkv416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balla S, Thapar V, Verma S, Luong T, Faghri T, Huang CH, et al. Minimotif Miner: a tool for investigating protein function. Nat Methods. 2006;3:175–177. doi: 10.1038/nmeth856. [DOI] [PubMed] [Google Scholar]
- 8.Barbera AJ, Chodaparambil JV, Kelley-Clarke B, Joukov V, Walter JC, Luger K, et al. The nucleosomal surface as a docking station for Kaposi's sarcoma herpesvirus LANA. Science. 2006;311(5762):856–861. doi: 10.1126/science.1120541. [DOI] [PubMed] [Google Scholar]
- 9.Barnes B, Karimloo M, Schoenrock A, Burnside D, Cassol E, Wong A, et al. Predicting novel protein-protein interactions between the HIV-1 virus and homo sapiens. 2016 IEEE EMBS International Student Conference (ISC); Ottawa, ON, Canada: 2016. pp. 1–4. [DOI] [Google Scholar]
- 10.Benedict CA, Norris PS, Ware CF. To kill or be killed: viral evasion of apoptosis. Nat Immunol. 2002;3:1013–1018. doi: 10.1038/ni1102-1013. [DOI] [PubMed] [Google Scholar]
- 11.Bhowmick P, Guharoy M, Tompa P. Bioinformatics approaches for predicting disordered protein motifs. Adv Exp Med Biol. 2015;870:291–318. doi: 10.1007/978-3-319-20164-1_9. [DOI] [PubMed] [Google Scholar]
- 12.Blikstad C, Ivarsson Y. High-throughput methods for identification of protein-protein interactions involving short linear motifs. Cell Commun Signal. 2015;13:38. doi: 10.1186/s12964-015-0116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chaurushiya MS, Lilley CE, Aslanian A, Meisenhelder J, Scott DC, Landry S, et al. Viral E3 ubiquitin ligase-mediated degradation of a cellular E3: viral mimicry of a cellular phosphorylation mark targets the RNF8 FHA domain. Mol Cell. 2012;46:79–90. doi: 10.1016/j.molcel.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chemes LB, de Prat-Gay G, Sanchez IE. Convergent evolution and mimicry of protein linear motifs in host-pathogen interactions. Curr Opin Struct Biol. 2015;32:91–101. doi: 10.1016/j.sbi.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 15.Chen R, Ishak CA, De Carvalho DD. Endogenous retroelements and the viral mimicry response in cancer therapy and cellular homeostasis. Cancer Discov. 2021;11:2707–2725. doi: 10.1158/2159-8290.CD-21-0506. [DOI] [PubMed] [Google Scholar]
- 16.Corbi-Verge C, Kim PM. Motif mediated protein-protein interactions as drug targets. Cell Commun Signal. 2016;14:8. doi: 10.1186/s12964-016-0131-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davey NE, Cowan JL, Shields DC, Gibson TJ, Coldwell MJ, Edwards RJ. SLiMPrints: conservation-based discovery of functional motif fingerprints in intrinsically disordered protein regions. Nucleic Acids Res. 2012;40:10628–10641. doi: 10.1093/nar/gks854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davey NE, Trave G, Gibson TJ. How viruses hijack cell regulation. Trends Biochem Sci. 2011;36:159–169. doi: 10.1016/j.tibs.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 19.Davey NE, Van Roey K, Weatheritt RJ, Toedt G, Uyar B, Altenberg B, et al. Attributes of short linear motifs. Mol Biosyst. 2012;8:268–281. doi: 10.1039/c1mb05231d. [DOI] [PubMed] [Google Scholar]
- 20.de Castro E, Sigrist CJ, Gattiker A, Bulliard V, Langendijk-Genevaux PS, Gasteiger E, et al. ScanProsite: detection of PROSITE signature matches and ProRule-associated functional and structural residues in proteins. Nucleic Acids Res. 2006;34(Web Server issue):W362–W365. doi: 10.1093/nar/gkl124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Chassey B, Navratil V, Tafforeau L, Hiet MS, Aublin-Gex A, Agaugue S, et al. Hepatitis C virus infection protein network. Mol Syst Biol. 2008;4:230. doi: 10.1038/msb.2008.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deng Z, Atanasiu C, Zhao K, Marmorstein R, Sbodio JI, Chi NW, et al. Inhibition of Epstein-Barr virus OriP function by tankyrase, a telomere-associated poly-ADP ribose polymerase that binds and modifies EBNA1. J Virol. 2005;79:4640–4650. doi: 10.1128/JVI.79.8.4640-4650.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.D'haeseleer P. What are DNA sequence motifs? Nat Biotechnol. 2006;24:423–425. doi: 10.1038/nbt0406-423. [DOI] [PubMed] [Google Scholar]
- 24.Diella F, Haslam N, Chica C, Budd A, Michael S, Brown NP, et al. Understanding eukaryotic linear motifs and their role in cell signaling and regulation. Front Biosci. 2008;13:6580–6603. doi: 10.2741/3175. [DOI] [PubMed] [Google Scholar]
- 25.Dinkel H, Michael S, Weatheritt RJ, Davey NE, Van Roey K, Altenberg B, et al. ELM - the database of eukaryotic linear motifs. Nucleic Acids Res. 2012;40(Database issue):D242–D251. doi: 10.1093/nar/gkr1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dinkel H, Van Roey K, Michael S, Davey NE, Weatheritt RJ, Born D, et al. The eukaryotic linear motif resource ELM: 10 years and counting. Nucleic Acids Res. 2014;42(Database issue):D259–D266. doi: 10.1093/nar/gkt1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dueber JE, Yeh BJ, Chak K, Lim WA. Reprogramming control of an allosteric signaling switch through modular recombination. Science. 2003;301(5641):1904–1908. doi: 10.1126/science.1085945. [DOI] [PubMed] [Google Scholar]
- 28.Duro N, Miskei M, Fuxreiter M. Fuzziness endows viral motif-mimicry. Mol Biosyst. 2015;11:2821–2829. doi: 10.1039/c5mb00301f. [DOI] [PubMed] [Google Scholar]
- 29.Dyer MD, Murali TM, Sobral BW. Computational prediction of host-pathogen protein-protein interactions. Bioinformatics. 2007;23(13):i159–i166. doi: 10.1093/bioinformatics/btm208. [DOI] [PubMed] [Google Scholar]
- 30.Edwards RJ, Palopoli N. Computational prediction of short linear motifs from protein sequences. Methods Mol Biol. 2015;1268:89–141. doi: 10.1007/978-1-4939-2285-7_6. [DOI] [PubMed] [Google Scholar]
- 31.Elkhaligy H, Balbin CA, Gonzalez JL, Liberatore T, Siltberg-Liberles J. Dynamic, but not necessarily disordered, human-virus interactions mediated through SLiMs in viral proteins. Viruses. 2021;13(12):2369. doi: 10.3390/v13122369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Emamjomeh A, Goliaei B, Zahiri J, Ebrahimpour R. Predicting protein-protein interactions between human and hepatitis C virus via an ensemble learning method. Mol Biosyst. 2014;10:3147–3154. doi: 10.1039/c4mb00410h. [DOI] [PubMed] [Google Scholar]
- 33.Evans P, Dampier W, Ungar L, Tozeren A. Prediction of HIV-1 virus-host protein interactions using virus and host sequence motifs. BMC Med Genom. 2009;2:27. doi: 10.1186/1755-8794-2-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Falson P, Bartosch B, Alsaleh K, Tews BA, Loquet A, Ciczora Y, et al. Hepatitis C virus envelope glycoprotein E1 forms trimers at the surface of the virion. J Virol. 2015;89:10333–10346. doi: 10.1128/JVI.00991-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Finlay BB, McFadden G. Anti-immunology: evasion of the host immune system by bacterial and viral pathogens. Cell. 2006;124:767–782. doi: 10.1016/j.cell.2006.01.034. [DOI] [PubMed] [Google Scholar]
- 36.Franzosa EA, Xia Y. Structural principles within the human-virus protein-protein interaction network. Proc Natl Acad Sci U S A. 2011;108:10538–10543. doi: 10.1073/pnas.1101440108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ganti K, Broniarczyk J, Manoubi W, Massimi P, Mittal S, Pim D, et al. The human papillomavirus E6 PDZ binding motif: from life cycle to malignancy. Viruses. 2015;7:3530–3551. doi: 10.3390/v7072785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garg A, Dabburu GR, Singhal N, Kumar M. Investigating the disordered regions (MoRFs, SLiMs and LCRs) and functions of mimicry proteins/peptides in silico. PLoS One. 2022;17(4):e0265657. doi: 10.1371/journal.pone.0265657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gibson TJ. Cell regulation: determined to signal discrete cooperation. Trends Biochem Sci. 2009;34:471–482. doi: 10.1016/j.tibs.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 40.Gibson TJ, Dinkel H, Van Roey K, Diella F. Experimental detection of short regulatory motifs in eukaryotic proteins: tips for good practice as well as for bad. Cell Commun Signal. 2015;13:42. doi: 10.1186/s12964-015-0121-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gomatos PJ, Tamm I. Animal and plant viruses with double-helical RNA. Proc Natl Acad Sci U S A. 1963;50:878–885. doi: 10.1073/pnas.50.5.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gong W, Zhou D, Ren Y, Wang Y, Zuo Z, Shen Y, et al. PepCyber:P~PEP: a database of human protein protein interactions mediated by phosphoprotein-binding domains. Nucleic Acids Res. 2008;36(Database issue):D679–D683. doi: 10.1093/nar/gkm854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goswami S, Samanta D, Duraivelan K. Molecular mimicry of host short linear motif-mediated interactions utilised by viruses for entry. Mol Biol Rep. 2023;50:4665–4673. doi: 10.1007/s11033-023-08389-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Green TJ, Cox R, Tsao J, Rowse M, Qiu SH, Luo M. Common mechanism for RNA encapsidation by negative-strand RNA viruses. J Virol. 2014;88:3766–3775. doi: 10.1128/Jvi.03483-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grzesik P, Pryce EN, Bhalala A, Vij M, Ahmed R, Etienne L, et al. Functional domains of the Herpes Simplex Virus Type 1 tegument protein pUL37: the amino terminus is dispensable for virus replication in tissue culture. Viruses. 2019;11(9):853. doi: 10.3390/v11090853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guo Z, Song E, Ma S, Wang X, Gao S, Shao C, et al. Proteomics strategy to identify substrates of LNX, a PDZ domain-containing E3 ubiquitin ligase. J Proteome Res. 2012;11:4847–4862. doi: 10.1021/pr300674c. [DOI] [PubMed] [Google Scholar]
- 47.Halehalli RR, Nagarajaram HA. Molecular principles of human virus protein-protein interactions. Bioinformatics. 2015;31:1025–1033. doi: 10.1093/bioinformatics/btu763. [DOI] [PubMed] [Google Scholar]
- 48.Han SI, Kawano MA, Ishizu K, Watanabe H, Hasegawa M, Kanesashi SN, et al. Rep68 protein of adeno-associated virus type 2 interacts with 14-3-3 proteins depending on phosphorylation at serine 535. Virology. 2004;320:144–155. doi: 10.1016/j.virol.2003.11.024. [DOI] [PubMed] [Google Scholar]
- 49.Han Z, Dash S, Sagum CA, Ruthel G, Jaladanki CK, Berry CT, et al. Modular mimicry and engagement of the Hippo pathway by Marburg virus VP40: Implications for filovirus biology and budding. PLoS Pathog. 2020;16(1):e1008231. doi: 10.1371/journal.ppat.1008231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harak C, Lohmann V. Ultrastructure of the replication sites of positive-strand RNA viruses. Virology. 2015;479:418–433. doi: 10.1016/j.virol.2015.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hecker CM, Rabiller M, Haglund K, Bayer P, Dikic I. Specification of SUMO1- and SUMO2-interacting motifs. J Biol Chem. 2006;281:16117–16127. doi: 10.1074/jbc.M512757200. [DOI] [PubMed] [Google Scholar]
- 52.Hornbeck PV, Kornhauser JM, Tkachev S, Zhang B, Skrzypek E, Murray B, et al. PhosphoSitePlus: a comprehensive resource for investigating the structure and function of experimentally determined post-translational modifications in man and mouse. Nucleic Acids Res. 2012;40(Database issue):D261–D270. doi: 10.1093/nar/gkr1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hu S, Song E, Tian R, Ma S, Yang T, Mu Y, et al. Systematic analysis of a simple adaptor protein PDZK1: ligand identification, interaction and functional prediction of complex. Cell Physiol Biochem. 2009;24:231–242. doi: 10.1159/000233258. [DOI] [PubMed] [Google Scholar]
- 54.Hulo N, Bairoch A, Bulliard V, Cerutti L, De Castro E, Langendijk-Genevaux PS, et al. The PROSITE database. Nucleic Acids Res. 2006;34(Database issue):D227–D230. doi: 10.1093/nar/gkj063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Idrees S. Predicting motif mimicry in viruses: University of New South Wales, Thesis. Sydney, Australia: 2020. [Google Scholar]
- 56.Idrees S, Perez-Bercoff A, Edwards RJ. Correction: SLiMEnrich: computational assessment of protein-protein interaction data as a source of domain-motif interactions. PeerJ. 2018;6:e5858. doi: 10.7717/peerj.5858/correction-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Incrocci R, McAloon J, Montesano M, Bardahl J, Vagvala S, Stone A, et al. Epstein-Barr virus LMP2A utilizes Syk and PI3K to activate NF-kappaB in B-cell lymphomas to increase MIP-1alpha production. J Med Virol. 2019;91:845–855. doi: 10.1002/jmv.25381. [DOI] [PubMed] [Google Scholar]
- 58.Iyer LM, Aravind L, Koonin EV. Common origin of four diverse families of large eukaryotic DNA viruses. J Virol. 2001;75:11720–11734. doi: 10.1128/JVI.75.23.11720-11734.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jones DT, Cozzetto D. DISOPRED3: precise disordered region predictions with annotated protein-binding activity. Bioinformatics. 2015;31:857–863. doi: 10.1093/bioinformatics/btu744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kazlauskas D, Krupovic M, Venclovas C. The logic of DNA replication in double-stranded DNA viruses: insights from global analysis of viral genomes. Nucleic Acids Res. 2016;44:4551–4564. doi: 10.1093/nar/gkw322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kazlauskas D, Venclovas C. Computational analysis of DNA replicases in double-stranded DNA viruses: relationship with the genome size. Nucleic Acids Res. 2011;39:8291–8305. doi: 10.1093/nar/gkr564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Khan N, Geiger JD. Role of Viral Protein U (Vpu) in HIV-1 infection and pathogenesis. Viruses. 2021;13(8):1466. doi: 10.3390/v13081466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim ED, Sabharwal A, Vetta AR, Blanchette M. Predicting direct protein interactions from affinity purification mass spectrometry data. Algorithms Mol Biol. 2010;5:34. doi: 10.1186/1748-7188-5-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kirui J, Bucci MD, Poole DS, Mehle A. Conserved features of the PB2 627 domain impact influenza virus polymerase function and replication. J Virol. 2014;88:5977–5986. doi: 10.1128/JVI.00508-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Koonin EV, Krupovic M, Yutin N. Evolution of double-stranded DNA viruses of eukaryotes: from bacteriophages to transposons to giant viruses. DNA Habitats and Their RNA Inhabitants. 2015;1341:10–24. doi: 10.1111/nyas.12728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Krupovic M, Forterre P. Single-stranded DNA viruses employ a variety of mechanisms for integration into host genomes. Ann N Y Acad Sci. 2015;1341:41–53. doi: 10.1111/nyas.12675. [DOI] [PubMed] [Google Scholar]
- 67.Krystkowiak I, Davey NE. SLiMSearch: a framework for proteome-wide discovery and annotation of functional modules in intrinsically disordered regions. Nucleic Acids Res. 2017;45(W1):W464–W469. doi: 10.1093/nar/gkx238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kumar M, Gouw M, Michael S, Samano-Sanchez H, Pancsa R, Glavina J, et al. ELM-the eukaryotic linear motif resource in 2020. Nucleic Acids Res. 2020;48(D1):D296–D306. doi: 10.1093/nar/gkz1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kumar M, Michael S, Alvarado-Valverde J, Meszaros B, Samano-Sanchez H, Zeke A, et al. The eukaryotic linear motif resource: 2022 release. Nucleic Acids Res. 2022;50(D1):D497–D508. doi: 10.1093/nar/gkab975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lan J, Ge J, Yu J, Shan S, Zhou H, Fan S, et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020;581(7807):215–220. doi: 10.1038/s41586-020-2180-5. [DOI] [PubMed] [Google Scholar]
- 71.Li X, Wu M, Kwoh CK, Ng SK. Computational approaches for detecting protein complexes from protein interaction networks: a survey. BMC Genomics. 2010;11(Suppl 1):S3. doi: 10.1186/1471-2164-11-S1-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lieber DS, Elemento O, Tavazoie S. Large-scale discovery and characterization of protein regulatory motifs in eukaryotes. PLoS One. 2010;5(12):e14444. doi: 10.1371/journal.pone.0014444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu Y, Wimmer E, Paul AV. Cis-acting RNA elements in human and animal plus-strand RNA viruses. Biochim Biophys Acta. 2009;1789:495–517. doi: 10.1016/j.bbagrm.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Luck K, Sheynkman GM, Zhang I, Vidal M. Proteome-scale human interactomics. Trends Biochem Sci. 2017;42:342–354. doi: 10.1016/j.tibs.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lum KK, Cristea IM. Proteomic approaches to uncovering virus-host protein interactions during the progression of viral infection. Expert Rev Proteomics. 2016;13:325–340. doi: 10.1586/14789450.2016.1147353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mahajan S, Choudhary S, Kumar P, Tomar S. Antiviral strategies targeting host factors and mechanisms obliging +ssRNA viral pathogens. Bioorg Med Chem. 2021;46:116356. doi: 10.1016/j.bmc.2021.116356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mechali F, Hsu CY, Castro A, Lorca T, Bonne-Andrea C. Bovine papillomavirus replicative helicase E1 is a target of the ubiquitin ligase APC. J Virol. 2004;78:2615–2619. doi: 10.1128/jvi.78.5.2615-2619.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mertens P. The dsRNA viruses. Virus Res. 2004;101(1):3–13. doi: 10.1016/j.virusres.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 79.Mihalic F, Simonetti L, Giudice G, Sander MR, Lindqvist R, Peters MBA, et al. Large-scale phage-based screening reveals extensive pan-viral mimicry of host short linear motifs. Nat Commun. 2023;14(1):2409. doi: 10.1038/s41467-023-38015-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Moya A, Elena SF, Bracho A, Miralles R, Barrio E. The evolution of RNA viruses: A population genetics view. Proc Natl Acad Sci U S A. 2000;97:6967–6973. doi: 10.1073/pnas.97.13.6967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Neduva V, Russell RB. Linear motifs: Evolutionary interaction switches. Febs Letters. 2005;579:3342–3345. doi: 10.1016/j.febslet.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 82.Ng TF, Marine R, Wang C, Simmonds P, Kapusinszky B, Bodhidatta L, et al. High variety of known and new RNA and DNA viruses of diverse origins in untreated sewage. J Virol. 2012;86:12161–12175. doi: 10.1128/JVI.00869-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nguyen Ba AN, Yeh BJ, van Dyk D, Davidson AR, Andrews BJ, Weiss EL, et al. Proteome-wide discovery of evolutionary conserved sequences in disordered regions. Sci Signal. 2012;5(215):rs1. doi: 10.1126/scisignal.2002515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Obenauer JC, Cantley LC, Yaffe MB. Scansite 2.0: Proteome-wide prediction of cell signaling interactions using short sequence motifs. Nucleic Acids Res. 2003;31:3635–3641. doi: 10.1093/nar/gkg584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ortin J, Martin-Benito J. The RNA synthesis machinery of negative-stranded RNA viruses. Virology. 2015;479:532–544. doi: 10.1016/j.virol.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 86.Pal A, Kundu R. Human papillomavirus E6 and E7: the cervical cancer hallmarks and targets for therapy. Front Microbiol. 2019;10:3116. doi: 10.3389/fmicb.2019.03116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Palopoli N, Lythgow KT, Edwards RJ. QSLiMFinder: improved short linear motif prediction using specific query protein data. Bioinformatics. 2015;31:2284–2293. doi: 10.1093/bioinformatics/btv155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pan S, Liu X, Ma Y, Cao Y, He B. Herpes simplex virus 1 gamma(1)34.5 protein inhibits STING activation that restricts viral replication. J Virol. 2018;92(20):e01015–e01018. doi: 10.1128/JVI.01015-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pancsa R, Fuxreiter M. Interactions via intrinsically disordered regions: what kind of motifs? IUBMB Life. 2012;64:513–520. doi: 10.1002/iub.1034. [DOI] [PubMed] [Google Scholar]
- 90.Poltronieri P, Sun B, Mallardo M. RNA viruses: RNA roles in pathogenesis, coreplication and viral load. Curr Genomics. 2015;16:327–335. doi: 10.2174/1389202916666150707160613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ponnusamy R, Khatri R, Correia PB, Wood CD, Mancini EJ, Farrell PJ, et al. Increased association between Epstein-Barr virus EBNA2 from type 2 strains and the transcriptional repressor BS69 restricts EBNA2 activity. PLoS Pathog. 2019;15(7):e1007458. doi: 10.1371/journal.ppat.1007458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Prytuliak R, Volkmer M, Meier M, Habermann BH. HH-MOTiF: de novo detection of short linear motifs in proteins by Hidden Markov Model comparisons. Nucleic Acids Res. 2017;45(W1):W470–W477. doi: 10.1093/nar/gkx341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rajagopala SV, Sikorski P, Kumar A, Mosca R, Vlasblom J, Arnold R, et al. The binary protein-protein interaction landscape of Escherichia coli. Nat Biotechnol. 2014;32:285–290. doi: 10.1038/nbt.2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rao VB, Feiss M. Mechanisms of DNA packaging by large double-stranded DNA viruses. Annu Rev Virol. 2015;2:351–378. doi: 10.1146/annurev-virology-100114-055212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ridgway H, Ntallis C, Chasapis CT, Kelaidonis K, Matsoukas MT, Plotas P, et al. Molecular epidemiology of SARS-CoV-2: The dominant role of arginine in mutations and infectivity. Viruses. 2023;15(2):309. doi: 10.3390/v15020309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rolland T, Tasan M, Charloteaux B, Pevzner SJ, Zhong Q, Sahni N, et al. A proteome-scale map of the human interactome network. Cell. 2014;159:1212–1226. doi: 10.1016/j.cell.2014.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rosario K, Mettel KA, Benner BE, Johnson R, Scott C, Yusseff-Vanegas SZ, et al. Virus discovery in all three major lineages of terrestrial arthropods highlights the diversity of single-stranded DNA viruses associated with invertebrates. PeerJ. 2018;6:e5761. doi: 10.7717/peerj.5761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Saridakis V, Sheng Y, Sarkari F, Holowaty MN, Shire K, Nguyen T, et al. Structure of the p53 binding domain of HAUSP/USP7 bound to Epstein-Barr nuclear antigen 1 implications for EBV-mediated immortalization. Mol Cell. 2005;18:25–36. doi: 10.1016/j.molcel.2005.02.029. [DOI] [PubMed] [Google Scholar]
- 99.Sarkar D, Jana T, Saha S. LMPID: a manually curated database of linear motifs mediating protein-protein interactions. Database (Oxford) 2015;2015:bav014. doi: 10.1093/database/bav014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Segura-Cabrera A, Garcia-Perez CA, Guo X, Rodriguez-Perez MA. A viral-human interactome based on structural motif-domain interactions captures the human infectome. PLoS One. 2013;8(8):e71526. doi: 10.1371/journal.pone.0071526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Segura-Cabrera A, Garcia-Perez CA, Guo XW, Rodriguez-Perez MA. A viral-human interactome based on structural motif-domain interactions captures the human infectome. Plos One. 2013;8(8):e71526. doi: 10.1371/journal.pone.0071526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shelton H, Harris M. Hepatitis C virus NS5A protein binds the SH3 domain of the Fyn tyrosine kinase with high affinity: mutagenic analysis of residues within the SH3 domain that contribute to the interaction. Virol J. 2008;5:24. doi: 10.1186/1743-422X-5-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Simonetti L, Nilsson J, McInerney G, Ivarsson Y, Davey NE. SLiM-binding pockets: an attractive target for broad-spectrum antivirals. Trends Biochem Sci. 2023;48:420–427. doi: 10.1016/j.tibs.2022.12.004. [DOI] [PubMed] [Google Scholar]
- 104.Stangler T, Tran T, Hoffmann S, Schmidt H, Jonas E, Willbold D. Competitive displacement of full-length HIV-1 Nef from the Hck SH3 domain by a high-affinity artificial peptide. Biol Chem. 2007;388:611–615. doi: 10.1515/BC.2007.075. [DOI] [PubMed] [Google Scholar]
- 105.Sztuba-Solinska J, Stollar V, Bujarski JJ. Subgenomic messenger RNAs: Mastering regulation of (+)-strand RNA virus life cycle. Virology. 2011;412:245–255. doi: 10.1016/j.virol.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tavakolian S, Goudarzi H, Faghihloo E. Cyclin-dependent kinases and CDK inhibitors in virus-associated cancers. Infect Agent Cancer. 2020;15:27. doi: 10.1186/s13027-020-00295-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tompa P, Csermely P. The role of structural disorder in the function of RNA and protein chaperones. FASEB J. 2004;18:1169–1175. doi: 10.1096/fj.04-1584rev. [DOI] [PubMed] [Google Scholar]
- 108.Tompa P, Davey NE, Gibson TJ, Babu MM. A million peptide motifs for the molecular biologist. Mol Cell. 2014;55:161–169. doi: 10.1016/j.molcel.2014.05.032. [DOI] [PubMed] [Google Scholar]
- 109.Van Roey K, Uyar B, Weatheritt RJ, Dinkel H, Seiler M, Budd A, et al. Short linear motifs: ubiquitous and functionally diverse protein interaction modules directing cell regulation. Chem Rev. 2014;114:6733–6778. doi: 10.1021/cr400585q. [DOI] [PubMed] [Google Scholar]
- 110.Via A, Uyar B, Brun C, Zanzoni A. How pathogens use linear motifs to perturb host cell networks. Trends Biochem Sci. 2015;40:36–48. doi: 10.1016/j.tibs.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 111.Weatheritt RJ, Davey NE, Gibson TJ. Linear motifs confer functional diversity onto splice variants. Nucleic Acids Res. 2012;40:7123–7131. doi: 10.1093/nar/gks442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Welcker M, Clurman BE. The SV40 large T antigen contains a decoy phosphodegron that mediates its interactions with Fbw7/hCdc4. J Biol Chem. 2005;280:7654–7658. doi: 10.1074/jbc.M413377200. [DOI] [PubMed] [Google Scholar]
- 113.White KA, Enjuanes L, Berkhout B. RNA virus replication, transcription and recombination. RNA Biol. 2011;8(2):182–183. doi: 10.4161/rna.8.2.15663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wickner RB. Double-stranded RNA virus replication and packaging. J Biol Chem. 1993;268:3797–3800. [PubMed] [Google Scholar]
- 115.Wirblich C, Bhattacharya B, Roy P. Nonstructural protein 3 of bluetongue virus assists virus release by recruiting ESCRT-I protein Tsg101. J Virol. 2006;80:460–473. doi: 10.1128/JVI.80.1.460-473.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wu X, Xia T, Shin WJ, Yu KM, Jung W, Herrmann A, et al. Viral mimicry of interleukin-17A by SARS-CoV-2 ORF8. mBio. 2022;13(2):e0040222. doi: 10.1128/mbio.00402-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhang A, Tessier TM, Galpin KJC, King CR, Gameiro SF, Anderson WW, et al. The transcriptional repressor BS69 is a conserved target of the E1A proteins from several human adenovirus species. Viruses. 2018;10(12):662. doi: 10.3390/v10120662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhang AD, He LB, Wang YP. Prediction of GCRV virus-host protein interactome based on structural motif-domain interactions. BMC Bioinformatics. 2017;18:145. doi: 10.1186/s12859-017-1500-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhang XF, Ou-Yang L, Hu X, Dai DQ. Identifying binary protein-protein interactions from affinity purification mass spectrometry data. BMC Genomics. 2015;16:745. doi: 10.1186/s12864-015-1944-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhang Y, Lin H, Yang Z, Wang J. Integrating experimental and literature protein-protein interaction data for protein complex prediction. BMC Genomics. 2015;16(Suppl 2):S4. doi: 10.1186/1471-2164-16-S2-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]