Abstract
The larvae of speckled emperor moths (Gynanisa maja) are important plant defoliators in savanna ecosystems of southern Africa and a valuable food resource for indigenous communities. Population explosions of G. maja larvae can negatively impact an area's primary productivity thereby altering herbivory patterns and associated ecosystem processes. Harvests of the larvae enhance socio‐economic livelihoods of local people by providing a source of protein and improving household incomes. We report on a population outbreak of G. maja larvae that occurred in south‐eastern Zimbabwe between December 2022 and January 2023 and discuss the ecological and social significance of the event. A total biomass weight of 5811 tons of G. maja larvae was estimated over the area of the outbreak and extensive defoliation was recorded in Colophospermum mopane trees. We could not associate the outbreak with any obvious environmental conditions and speculate that it may have been caused by subtle triggers that are not easily identified.
Keywords: caterpillar, defoliation, emperor moth, eruption, food source
This paper reports on the ecological footprint of a Gynanisa maja larvae outbreak that occurred between December 2022 and January 2023 in the south‐eastern lowveld of Zimbabwe.
1. INTRODUCTION
Population explosions of insect folivores are important ecological events in savanna ecosystems (Hartnett et al., 2012). The larval (caterpillar) stage of emperor moths (family, Saturniidae) has both an ecological and socio‐economic significance in southern Africa (Bara et al., 2022; Ditlhogo, 1996; Thomas, 2013) with larvae of two species, the anomalous emperor (Gonimbrasia belina Westwood 1894) and the speckled emperor (Gynanisa maja Klug 1836), being important plant defoliators and a food resource for people in the region. Outbreaks of G. belina are relatively frequent and widespread (Bara et al., 2022; Straeuli, 2022), whereas those of G. maja are characterized by extended periods of relatively low incidence punctuated by population explosions that are less frequent (Singh & Satyanarayana, 2009; Thomas, 2013). Apart from incidental observation, G. maja has been little studied and the drivers of its eruptive dynamics are poorly understood.
Larvae of G. maja feed on the leaves of a wide range of savanna tree species but Colophospermum mopane is their most preferred food source (Chanda et al., 2022; Sileshi et al., 2007; Stone, 1991). The larvae are voracious folivores and outbreaks can defoliate large areas of woodland with implications for primary productivity, herbivory and associated cascading effects on ecosystem processes (Carson et al., 2004; Fajvan & Wood, 1996; Hartnett et al., 2012; Styles, 1994). Here we report on a G. maja larvae outbreak in the south‐eastern lowveld of Zimbabwe that occurred between 18 December 2022 and 6 January 2023. We aimed to (1) determine the spatial extent of the outbreak, (2) estimate the biomass of the caterpillars and (3) quantify the degree of tree and shrub defoliation, and recovery.
2. METHODS
The outbreak occurred in the northern region of Gonarezhou National Park (GNP) and extended a short distance into the southern part of neighbouring Malilangwe Wildlife Reserve (MWR) (Figure 1). Rainfall in the affected area is seasonal with most precipitation occurring between November and March (mean ≈ 499 mm per annum at Chipinda Pools, which is situated within the area of the outbreak) (Gandiwa et al., 2016). The vegetation within the affected area is predominantly mopane (C. mopane) woodland on soils derived from basalt, with Combretum imberbe, Philenoptera violacea, Dalbergia melanoxylon, Pterocarpus brenanii and Acacia nigrescens being less common components of the woody layer (Clegg & O'Connor, 2012; Cunliffe et al., 2012).
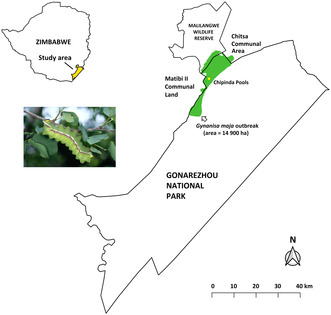
FIGURE 1.
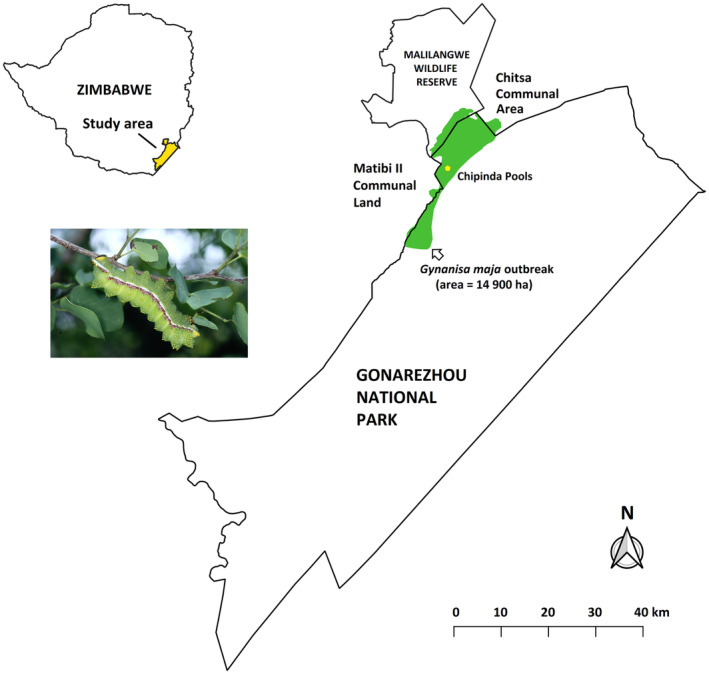
Map showing the location of the Gynanisa maja outbreak. Inset shows G. maja caterpillar on a Colophospermum mopane plant.
The outbreak area was flown using a Savannah‐S fixed‐wing aircraft (ICP, Piedmont, Italy) over a single session and its boundary was digitized using a handheld Global Positioning System device. The expanse of the outbreak was easily discernable on account of the extensive defoliation of the mopane trees. This was then refined by mapping the extent of the defoliation in Quantum GIS v3.26 (QGIS Development Team, 2022) using Sentinel 2 satellite imagery and the Normalized Difference Vegetation Index (NDVI). The NDVI index is sensitive to phenological changes in vegetation cover and is used to measure greenness or the degree of green leaf cover of vegetation (Gandhi et al., 2015; Huang et al., 2021). NDVI values range from −1 to 1 with low values representing low vegetation cover and higher values corresponding to healthy vegetation (Huang et al., 2021).
Three sample plots (each measuring 25 × 5 m) were randomly located within the MWR section of the defoliated area, in areas of medium tree height (3–5 m). Each plot was thoroughly inspected, and the larvae found were collected by hand, and the number per plot was recorded. Most larvae were picked off shrubs and trees, but some were also found on the ground. A sample of 70 caterpillars (5th instar stage) were weighed, and the average mass of a caterpillar was calculated. For each plot, the live biomass of caterpillars per hectare was calculated by multiplying the average weight of a caterpillar by the number counted in the plot and dividing the result by the plot area. The mean density of caterpillar biomass for the outbreak was estimated by averaging the estimates for the three plots. This figure was then multiplied by the area of the outbreak to estimate the total caterpillar biomass.
Following the outbreak event, six sample plots (each measuring 30 × 30 m) were randomly located within the GNP section of the defoliated area for vegetation assessment. Within each plot, the species, height class (shrubs = 0–3 m, trees = >3 m) and canopy dimensions of woody plants were recorded. The degree of defoliation and subsequent foliage regrowth was visually assessed for each woody plant and scores were assigned based on a modified 9‐point scale (0, 1, 2–10, 11–25, 26–50, 51–75, 76–90, 91–95, 96–100%). Spearman's rank correlations (ρ) were used to test the relationship between plant height and the degree of defoliation or recovery (R Core Team, 2023). Informal unstructured interviews were conducted with locals to determine local knowledge of past similar G. maja outbreak events in the area.
3. RESULTS
From satellite image analysis, pixels with NDVI values 0.1 ≤ x ≤ 0.35 marked the defoliated area while values >0.35 represented healthy vegetation and <0.1 corresponded with cleared agricultural fields. The extent of the G. maja outbreak in the study area was estimated at 14,900 ha (Figure 1). A total of 1443 larvae were collected from the three plots sampled at MWR and of these, G. belina larvae constituted only 0.8%, the remainder being G. maja. Where both G. maja and G. belina larvae were recorded, they were found present on the same trees (i.e., no host plant exclusion by either species). The average weight of a caterpillar was 10.2 g, and the mean number of caterpillars per ha was 38,187, which equates to a biomass of 390 kg/ha (Table 1). Given a total outbreak area of 14,900 ha, the total mass of G. maja larvae in the affected area was estimated at 5811 tons.
TABLE 1.
Field data on Gynanisa maja larvae occurrence at Malilangwe Wildlife Reserve and Gonarezhou National Park between December 2022 and January 2023.
| Malilangwe Wildlife Reserve | Gonarezhou National Park | ||||||
|---|---|---|---|---|---|---|---|
| Plot | Number of larvae picked | Number per hectare | Mass per hectare (kg) | Plot | Number of plants sampled | Mean defoliation score | Mean recovery score |
| 1 | 877 | 70,160 | 716 | 1 | 33 | 91–95 | 96–100 |
| 2 | 333 | 26,640 | 272 | 2 | 62 | 11–25 | 96–100 |
| 3 | 222 | 17,760 | 181 | 3 | 63 | 2–10 | 76–90 |
| 4 | 76 | 96–100 | 96–100 | ||||
| 5 | 39 | 91–95 | 96–100 | ||||
| 6 | 44 | 91–95 | 96–100 | ||||
A total of 10 species constituted the 317 woody plants found inside the six plots sampled at GNP with C. mopane making up the majority (84%) (Appendix). Within the six plots, G. maja larvae were only found feeding on C. mopane (Figure 2). The level of defoliation was high (mean rank = 76–90%, mode = 96–100%, n = 267) and similarly, recovery post‐defoliation was also high (mean and mode = 96–100%, n = 267) (Table 1, Figure 3). Plants resprouted within 21 days of defoliation. We found no relationship between plant height and the degree of defoliation (ρ < 0.08, df = 265, p = .189) or recovery (ρ < 0.08, df = 265, p = .173).
FIGURE 2.

Gynanisa maja larvae feeding on a Colophospermum mopane plant (a) and an example of a defoliated stand of mopane trees (b).
FIGURE 3.
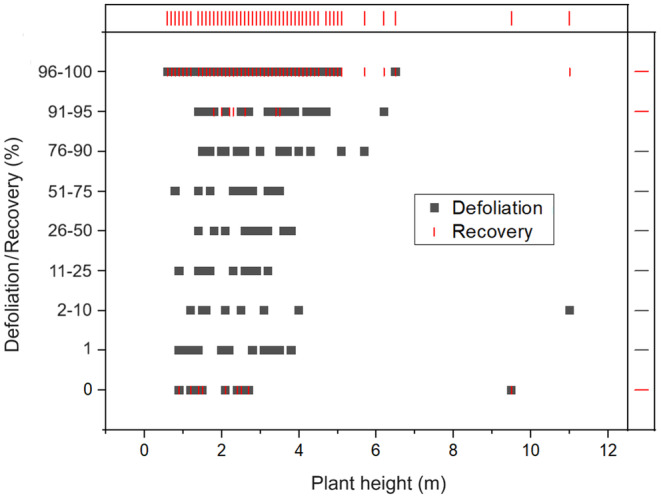
Scatter plot showing the distribution of defoliation and recovery data in relation to plant height.
4. DISCUSSION
This G. maja outbreak was spectacular, and from interviews with locals, it appears that an event of similar magnitude was last witnessed in the 1960s. We note that while G. belina eruptions are common in the area, very few G. belina larvae were recorded during this outbreak (<1%). Either G. maja outcompeted G. belina, or the two species are subject to different eruption triggers. Unfortunately, the long intervals between G. maja outbreaks (≈ 60 years in this case) make it difficult to collect sufficient data to answer these questions. It should also be noted that this outbreak was not an isolated incident but rather one of several outbreaks, albeit smaller, that were reported at the same time in the surrounding communal lands.
The live weight of caterpillars per hectare was equivalent to five times the biomass density of large herbivores at MWR (68 kg/ha), and the total estimated mass of caterpillars (5811 tons) was equivalent to 3369 elephants (using 1725 kg as the average weight of an elephant following Coe et al., 1976). Considering that this represents approximately one third of GNP's estimated population of 10,800 elephants (Dunham, 2022) concentrated within 3% of the park, this represents a substantial biological event. During this outbreak, there were so many caterpillars that the sound of their feeding was clearly audible.
Larvae of G. maja are polyphagous and have been reported to feed on the foliage of several tree species (Sileshi et al., 2007), including Sclerocarya birrea which was present in the sampled plots. Despite there being several species of woody plants in the plots sampled in GNP, larvae were only found on C. mopane. This finding was consistent with the work of Ditlhogo et al. (1996) and Fakazi et al. (2021) on G. belina who showed that larvae are highly selective and almost exclusively feed on C. mopane except in instances where mopane trees are not available. Previous estimates of forage loss from G. maja could not be sourced from the literature but assuming their feeding is comparable to that of G. belina, then following Styles (1994), an estimated 290,550 tons of mopane leaves were consumed during the outbreak.
The effects of defoliation by G. maja on mopane woodlands are potentially substantial. Although G. maja larvae have a relatively short lifespan (6 weeks), their exceptionally large numbers during an eruption give the species an unmatched ability to rapidly defoliate large areas of woodland (Fajvan & Wood, 1996). Though our data indicate that C. mopane showed good regeneration after defoliation, explosion events of this nature can result in 100% loss of leaves over a short period (Hartnett et al., 2012) resulting in a temporary shortage of food for other browsers. Ditlhogo et al. (1996) found that defoliation impacted fruit production in mopane trees with 86% of defoliated plants failing to bear fruit, and shrubs being more severely impacted than trees. In contrast, we found that both shrubs and trees experienced similar levels of defoliation and that there were no differences in foliage recovery.
Defoliation can also disrupt plant community structure through competitive effects of subcanopy species on the canopy layer, which is triggered by increased light penetration to the under‐canopy layer (Carson et al., 2004; Duffy et al., 2018; Fajvan & Wood, 1996). Furthermore, outbreaks of larvae introduce food bursts in the trophic system which can lead to increased insectivore activity and altered energy flow interactions (Carson et al., 2004). Interestingly, bird activity was less than expected during the outbreak, with only hornbills (Bucerotidae) and rollers (Coraciidae) utilising the caterpillars as a source of food. The caterpillars of G. maja have a relatively tough skin and consequently, it may be difficult for other bird species to feed on them. A significant number of dead unconsumed caterpillars were found on the forest floor after the host trees had been defoliated.
Population explosions of edible insects are important events in Sub‐Saharan Africa as they have a socio‐economic benefit to the livelihoods of local communities (Bara et al., 2022; Thomas, 2013). Larvae of G. maja are an important source of protein and harvests can be preserved for future consumption or sold thereby enhancing food security and household incomes (Mbata & Chidumayo, 2003; Nemadodzi et al., 2023). During this outbreak, many people travelled from far and wide to the Chitsa communal area to harvest the caterpillars. Insect eruption events are poorly understood but are generally believed to be triggered by climatic factors, mainly rainfall and temperature (Koricheva et al., 2012; Spear et al., 2021). We could not associate the G. maja outbreak with any obvious environmental conditions and speculate that it may have been caused by subtle triggers that are not easily identified. We, therefore, encourage more research on the species and its relation to climatic and environmental conditions to enable ecological modelling and prediction of future eruption events.
AUTHOR CONTRIBUTIONS
Allan Tarugara: Conceptualization (equal); formal analysis (equal); investigation (lead); methodology (equal); visualization (lead); writing – original draft (lead); writing – review and editing (equal). Bob Mandinyenya: Conceptualization (equal); formal analysis (equal); investigation (equal); methodology (equal); writing – review and editing (equal). Bruce W. Clegg: Conceptualization (equal); formal analysis (equal); investigation (equal); methodology (equal); writing – review and editing (equal).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Supporting information
Appendix S1 and S2.
ACKNOWLEDGEMENTS
We would like to thank Colin and Chrissy Wenham, Callum Duncan, Julius Shimbani and Pardon Hasani for their assistance with fieldwork.
Tarugara, A. , Mandinyenya, B. , & Clegg, B. W. (2023). An outbreak of Gynanisa maja (Lepidoptera: Saturniidae) larvae in the south‐eastern lowveld of Zimbabwe. Ecology and Evolution, 13, e10790. 10.1002/ece3.10790
DATA AVAILABILITY STATEMENT
Data for this study has been submitted in the Dryad repository as ‘Tarugara, Allan; Mandinyenya, Bob; Clegg, Bruce (2023), An outbreak of Gynanisa maja larvae in the south‐eastern lowveld of Zimbabwe’. https://datadryad.org/stash/share/9706jpnqvrGZ45juG_rAywjtL6XItDmKzsT7Jl15AYs.
REFERENCES
- Bara, G. T. , Sithole, R. , & Macheka, L. (2022). The mopane worm (Gonimbrasia belina Westwood): A review of its biology, ecology and utilisation in Zimbabwe. Journal of Insects as Food and Feed, 8(8), 823–836. [Google Scholar]
- Carson, W. P. , Cronin, J. P. , & Long, Z. T. (2004). A general rule for predicting when insects will have strong top‐down effects on plant communities: On the relationship between insect outbreaks and host concentration. In Weisser W. W. & Siemann E. (Eds.), Insects and ecosystem functions (pp. 193–212). Springer‐Verlag. [Google Scholar]
- Chanda, B. , Olweny, C. O. , & Chungu, D. (2022). Indigenous knowledge on host tree preference of the wild edible Gynanisa maja (lepidoptera: Saturniidae) matches with the laboratory test results in western Zambia. African Journal of Agricultural Research, 18(5), 330–339. [Google Scholar]
- Clegg, B. W. , & O'Connor, T. (2012). The vegetation of Malilangwe wildlife reserve, South‐Eastern Zimbabwe. African Journal of Range and Forage Science, 29, 109–131. [Google Scholar]
- Coe, M. J. , Cumming, D. H. , & Phillipson, J. (1976). Biomass and production of large African herbivores in relation to rainfall and primary production. Oecologia, 22, 341–354. [DOI] [PubMed] [Google Scholar]
- Cunliffe, R. , Muller, T. , & Mapaura, A. (2012). Vegetation survey of Gonarezhou National Park. Zimbabwe Parks and Wildlife Management Authority. [Google Scholar]
- Ditlhogo, M. , Allotey, J. , Mpuchane, S. , Teferra, G. , Gashe, B. , & Siame, B. (1996). Interactions between the mopane caterpillar, Imbrasia belina, and its host, Colophospermum mopane in Botswana, Management of Mopane in southern Africa. In Flower C., Wardell‐Johnson G. & Jamieson A. (Eds.), Proceedings of a Conference held at Ogongo Agricultural College (pp. 45–48). University of Namibia. [Google Scholar]
- Ditlhogo, M. K. (1996). The natural history of Imbrasia belina (Westwood) (Lepidoptera: Saturniidae), and some factors affecting its abundance in north‐eastern Botswana. DPhil Thesis. University of Manitoba. [Google Scholar]
- Duffy, K. J. , O'Connor, T. G. , & Collins, O. C. (2018). A lepidopteran (Imbrasia belina) might influence tree‐grass balance of Colophospermum mopane savanna. Theoretical Ecology, 11, 503–513. [Google Scholar]
- Dunham, K. M. (2022). Aerial survey of elephants and other large herbivores in Gonarezhou National Park (Zimbabwe) and some adjacent areas. Gonarezhou Conservation Trust, Gonarezhou National Park. [Google Scholar]
- Fajvan, M. A. , & Wood, J. M. (1996). Stand structure and development after gypsy moth defoliation in the Appalachian plateau. Forest Ecology and Management, 89(1–3), 79–88. [Google Scholar]
- Fakazi, B. H. , Buthelezi, M. N. , Zharare, G. E. , Mlambo, S. , & Fon, F. N. (2021). The occurrence and characteristics of Imbrasia belina (Westwood, 1849) in the subtropical region of KwaZulu‐Natal Province, South Africa. African Entomology, 29(2), 381–391. [Google Scholar]
- Gandhi, G. M. , Parthiban, B. S. , Thummalu, N. , & Christy, A. (2015). Ndvi: Vegetation change detection using remote sensing and GIS–A case study of Vellore District. Procedia Computer Science, 57, 1199–1210. [Google Scholar]
- Gandiwa, E. , Heitkonig, I. M. A. , Eilers, P. H. C. , & Prins, H. H. T. (2016). Rainfall variability and its impact on large mammal populations in a complex of semi‐arid African savanna protected areas. Tropical Ecology, 57(2), 163–180. [Google Scholar]
- Hartnett, D. C. , Ott, J. P. , Sebes, K. , & Ditlhogo, M. K. (2012). Coping with herbivory at the juvenile stage: Responses to defoliation and stem browsing in the African savanna tree Colophospermum mopane . Journal of Tropical Ecology, 28(2), 161–169. [Google Scholar]
- Huang, S. , Tang, L. , Hupy, J. P. , Wang, Y. , & Shao, G. (2021). A commentary review on the use of normalized difference vegetation index (NDVI) in the era of popular remote sensing. Journal of Forestry Research, 32(1), 1–6. [Google Scholar]
- Koricheva, J. , Klapwijk, M. J. , & Björkman, C. (2012). Life history traits and host plant use in defoliators and bark beetles: Implications for population dynamics. Insect Outbreaks Revisited, 20, 175–196. [Google Scholar]
- Mbata, K. J. , & Chidumayo, E. N. (2003). Traditional values of caterpillars (Insecta: Lepidoptera) among the Bisa people of Zambia. International Journal of Tropical Insect Science, 23(4), 341–354. [Google Scholar]
- Nemadodzi, L. E. , Managa, G. M. , & Prinsloo, G. (2023). The use of Gonimbrasia belina (Westwood, 1849) and Cirina forda (Westwood, 1849) caterpillars (lepidoptera: Sarturniidae) as food sources and income generators in Africa. Food, 12(11), 2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- QGIS . (2022). Geographic information system. Open Source Geospatial Foundation. [Google Scholar]
- R Development Core Team . (2023). R Statistical Program version 4.3.1.
- Sileshi, G. , Akinnifesi, F. K. , Ajayi, O. C. , Chakeredza, S. , Kaonga, M. , & Matakala, P. W. (2007). Contributions of agroforestry to ecosystem services in the miombo eco‐region of eastern and southern Africa. African Journal of Environmental Science and Technology, 1(4), 68–80. [Google Scholar]
- Singh, T. V. K. , & Satyanarayana, J. (2009). Insect outbreaks and their management. In Peshin R. & Dhawan A. K. (Eds.), Integrated Pest management: Innovation‐development process (pp. 331–350). Springer. [Google Scholar]
- Spear, M. J. , Walsh, J. R. , Ricciardi, A. , & Zanden, M. J. V. (2021). The invasion ecology of sleeper populations: Prevalence, persistence, and abrupt shifts. Bioscience, 71(4), 357–369. [Google Scholar]
- Stone, S. E. (1991). Foodplants of world Saturniidae. The Lepidopterists' Society. Memoir Number 4, USA, pp. 186.
- Straeuli, R. (2022). Phylogeny of emperor moths and phylogeography of Gonimbrasia belina in southern Africa. MSc Thesis. Stellenbosch University. [Google Scholar]
- Styles, C. (1994). The big value in mopane worms. Farmer's Weekly, 10, 20–22. [Google Scholar]
- Thomas, B. (2013). Sustainable harvesting and trading of mopane worms (Imbrasia belina) in northern Namibia: An experience from the Uukwaluudhi area. International Journal of Environmental Studies, 70, 494–502. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 and S2.
Data Availability Statement
Data for this study has been submitted in the Dryad repository as ‘Tarugara, Allan; Mandinyenya, Bob; Clegg, Bruce (2023), An outbreak of Gynanisa maja larvae in the south‐eastern lowveld of Zimbabwe’. https://datadryad.org/stash/share/9706jpnqvrGZ45juG_rAywjtL6XItDmKzsT7Jl15AYs.


