Abstract
Febrile infection-related epilepsy syndrome (FIRES) is a rare epileptic syndrome characterized by new-onset refractory status epilepticus preceded by a febrile illness. Limited literature exists regarding the relationship between primary immunodeficiencies and immune-mediated epilepsy, and the relationship between new-onset refractory status epilepticus and common variable immunodeficiency (CVID) is not well-understood. We present a case of a 21-year-old female with a history of recurrent sinus infections, asthma, thrombocytopenia, atrioventricular nodal reentrant tachycardia, and neonatal seizures who presented with fever and new-onset status epilepticus. She was ultimately diagnosed with a heterozygous variant in TNFRSF13B c.311G>A (p.Cys104Tyr), which encodes for a tumor necrosis factor receptor implicated in CVID.
Keywords: primary immunodeficiency disease, autoimmune epilepsy, febrile status epilepticus, refractory status epilepticus, common variable immunodeficiency deficiency
Introduction
Febrile infection-related epilepsy syndrome (FIRES) is a rare epileptic syndrome characterized by new-onset refractory status epilepticus (NORSE) preceded by a febrile illness [1]. Studies have shown that elevated levels of pro-inflammatory cytokines, including IL-6, may be involved [1], but limited literature exists regarding the relationship between primary immunodeficiencies and immune-mediated epilepsy. Neurological complications of common variable immunodeficiency (CVID) are rare. The relationship between seizures and CVID is not well understood, particularly with the phenotypic heterogeneity in CVID-associated genes [2]. To our knowledge, none have been reported in association with FIRES/NORSE [3].
This article was previously presented as a meeting abstract at the 2022 AAN Annual Meeting on April 2, 2022.
Case presentation
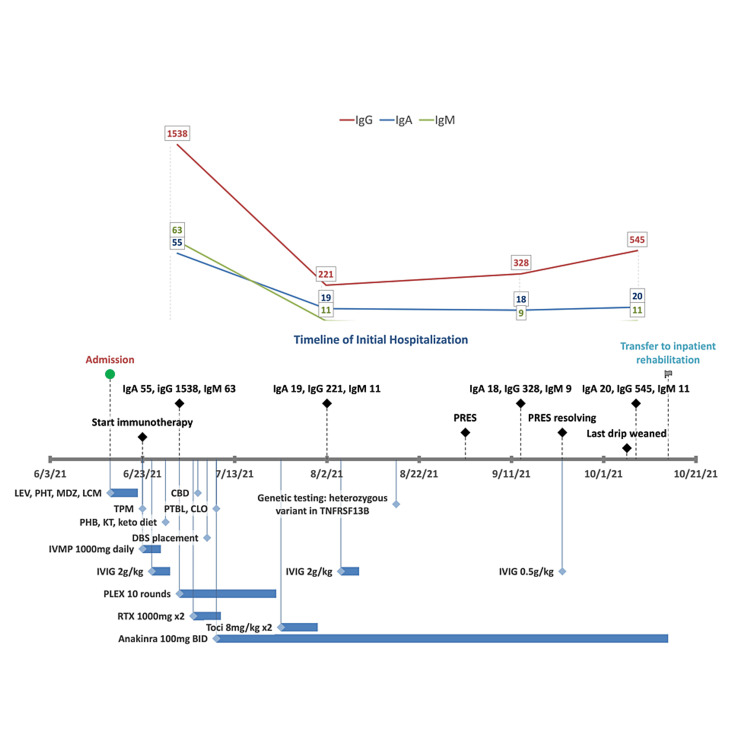
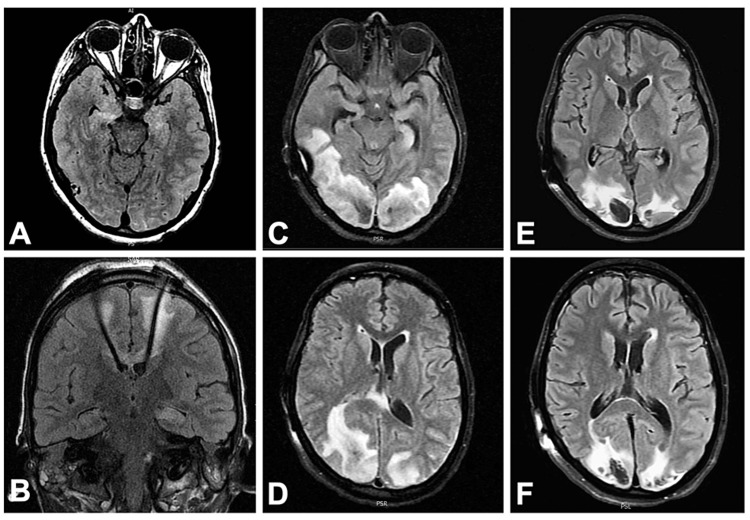
A 21-year-old female presented to the neurocritical care unit with three days of headache, fever, and lethargy followed by new onset status epilepticus consistent with FIRES/NORSE. She had a history of recurrent childhood sinus infections, asthma, thrombocytopenia of unclear etiology, atrioventricular nodal reentry tachycardia, neonatal seizures, and recurrent aseptic meningitis in adolescence. Her initial cerebrospinal fluid (CSF) studies were unremarkable, but they later demonstrated a lymphocytic pleocytosis of 37 cells/uL, an elevated total protein of 124 mg/dL, elevated IL-6 (13.5 pg/ml; normal ≤7.5), and elevated IL-2R (soluble: 64.3 pg/ml; normal ≤26.8). A subsequent CSF analysis demonstrated further IL-6 elevation of 48.8 pg/ml. A brain magnetic resonance imaging (MRI) showed T2 hyperintensities in the mesial temporal lobes concerning limbic encephalitis. However, a comprehensive evaluation for known antibody-mediated autoimmune and paraneoplastic causes of encephalitis was negative except for a low serum glutamic acid decarboxylase (GAD65) antibody titer of 0.25. Diagnostic workup for other inflammatory white matter disorders, rheumatological disease, and infectious causes was negative. During her hospital course, she received multiple antiseizure medications, treatment with the ketogenic diet, bilateral deep brain stimulator (DBS) placement in the anterior thalami, and multiple immunotherapies including intravenous methylprednisolone, intravenous immunoglobulin (IVIG), plasmapheresis, rituximab, tocilizumab, and anakinra (Figure 1). She had a prolonged hospitalization complicated by various nosocomial infections and posterior reversible encephalopathy syndrome (PRES) that improved over the course of several weeks (Figure 2).
Figure 1. Timeline of initial hospitalization and timing of immunoglobulin levels with medication administration and duration.
Abbreviations: LEV = levetiracetam, PHT = phenytoin, MDZ = midazolam, LCM = lacosamide, TPM = topiramate, PHB = phenobarbital, KT = ketamine, keto = ketogenic, PTBL = pentobarbital, CLO = clobazam, CBD = cannabidiol, DBS = deep brain stimulation, IVMP = intravenous methylprednisolone, IVIG = intravenous immunoglobulin, PLEX = plasma exchange, RTX = rituximab; Toci = tocilizumab; immunoglobulins in mg/dL
Figure 2. Evolution of neuroimaging.
A: T2 hyperintensity of bilateral mesial temporal lobes on admission; B: Placement of deep brain stimulator with electrode tips terminating in anterior thalami nuclei; C,D: Confluent posterior predominant, asymmetric subcortical and deep white matter hyperintensity and edema consistent with PRES; E,F: Repeat imaging two weeks later showing resolving prior T2 lesions and development of intraparenchymal hematomas
During her diagnostic workup, she was noted to have a low IgA level of 55 mg/dL (normal 70-400 mg/dL) with a normal IgG level of 1538 mg/dL and IgM of 63 mg/dL, although these levels were obtained one week after she received IVIG 2 g/kg (Figure 1). Unfortunately, a baseline IgG level was not obtained prior to IVIG administration. After receiving two doses of IV rituximab 1000 mg for presumed autoimmune encephalitis, she developed pan-hypogammaglobulinemia with an IgA level of 19 mg/dL, IgG of 221 mg/dL, and IgM of 11 mg/dL with normal IgG subclasses, raising the suspicion for an underlying primary immunodeficiency. Genetic testing revealed a pathogenic heterozygous variant in TNFRSF13B c.311G>A (p.Cys104Tyr). She was started on monthly 0.5 g/kg IVIG, which was held for one month after she developed PRES. She was eventually discharged to an inpatient rehabilitation facility and gradually improved in her motor, fine motor, and speech functions.
Currently, she is 18 months post-discharge, and her seizures remain refractory but improved on several antiseizure medications, DBS use, and anakinra 100 mg subcutaneously three times a day. She has daily short focal onset seizures consisting of gaze deviation and altered awareness. However, her seizures worsened when anakinra was reduced to twice-a-day dosing with the same antiseizure medication dosing and DBS settings. Seizures were reduced again when anakinra was increased back to three times a day. She continued to have undetectable IgM levels <5, low IgA 19, and IgG 474 mg/dL, resulting in frequent infections. Therefore, she is currently on a regimen of 0.5 g/kg IVIG every three weeks. B cell subsets demonstrate continued B cell suppression 15 months after rituximab with 0.4% CD19+ B cells. Thus, further analysis of B subsets was unattainable.
Discussion
The TNFRSF13B gene encodes for transmembrane activator and calcium modulator and cyclophilin ligand interactor (TACI), which is a tumor necrosis factor receptor expressed on marginal zone B cells, plasma cells, and CD27+ memory B cells. TACI promotes T cell-independent antibody responses and plasma cell differentiation and counteracts BAFF-driven B cell activation, affecting isotype switching and immunoglobulin production [4-6]. Studies of TACI deficiency in humans and mouse models demonstrate its important role in toll-like receptor (TLR) pathways [7-10] and the generation of autoreactive B cells [11]. The Cys104Tyr (C104Y) variant found in our patient is a missense variant reported among a cohort of patients with TNFRSF13B variants [12]. The cysteine residue at this amino acid position is highly conserved, with an allele frequency of 0.00016, and no homozygotes, on gnomAD. Functional algorithms with advanced modeling in protein sequence and biophysical properties (SIFT, poly-phen2, Align-GVGD) predict this variant to be probably damaging and deleterious to protein function. This variant is reported in individuals with CVID and IgA deficiency in eight different publications [12-19]. Notably, the Cys104Arg (C104R) variant at the same amino acid position, along with A181E, is one of the most common variants associated with CVID [12,15].
Biallelic/homozygous TNFRSF13B mutations are reported in hypogammaglobulinemia in children, and monoallelic/heterozygous variants are implicated in autoimmune disease in adulthood, although clinical heterogeneity exists even within the same family [11,13]. In a cohort study of 50 patients with TNFRSF13B-associated CVID, patients with heterozygous variants (the most common being C104R) had a higher percentage of autoimmunity compared to control participants without TACI mutations [12]. One study of B cell function from patient-derived biallelic and monoallelic variants in TACI compared to healthy controls demonstrated that TACI plays an important role in B cell tolerance [11]. Only participants with a heterozygous variant in TACI demonstrated impaired immune tolerance and secreted high levels of antinuclear antibodies along with increased circulating B cell lymphoma 6-expressing T follicular helper cells [11]. This suggests that partial TACI protein function in patients with one mutation results in autoimmunity because of impaired removal of autoreactive B cells at a central B cell checkpoint. The most common autoimmune disease in TACI-heterozygous patients was autoimmune thrombocytopenia, a condition that our patient may have developed in childhood. Of the 45 patients with B cell subsets, CD19+ B cell counts were normal in approximately half the patients, but IgD−IgM−CD27+ switched memory B cells were low in 76% of the patients. This decrease in class-switched memory B cells was not reported in patients with biallelic or compound heterozygous mutations. Interestingly, patients with biallelic TACI mutations did not demonstrate autoimmunity, a finding that is consistent with an Italian cohort of 189 CVID patients [12,14]. The Italian study also reported a higher frequency of switched memory B cells compared to heterozygous patients [14].
Clinically, CVID exhibits phenotypic and genetic heterogeneity, including variable severity in the degree of immunodeficiency as well as immune dysregulation and autoimmunity. While many other primary immunodeficiencies exhibit higher rates of monogenic forms, only 2-10% of CVID are monogenic, and a larger subset of CVID likely has complex, rather than monogenic, inheritance [2]. Genes implicated in monogenic CVID include ICOS, TNFRSF13B (TACI), TNFRSF13C (BAFF-R), TNFSF12 (TWEAK), CD19, CD81, CR2 (CD21), MS4A1 (CD20), TNFRSF7 (CD27), IL21, IL21R, LRBA, CTLA4, PRKCD, PLCG2, NFKB1, NFKB2, PIK3CD, PIK3R1, VAV1, RAC2, BLK, IKZF1 (IKAROS), and IRF2BP2 [2].
Neurological complications related to TNFRSF13B mutations are not well understood. However, neurological involvement in CVID includes CNS infections, polyneuropathy, vasculitis, and transverse myelitis [20]. Limited literature exists regarding the relationship between CVID and autoimmune epilepsy. The presence of GAD65 antibodies in our patient’s serum is likely nonspecific as neurological complications from GAD65 antibodies occur at high titers with antibody positivity in the CSF as well as serum [21]. Interestingly, our patient’s seizure control improved after the addition of anakinra, which may be unrelated to the TACI mutation. However, the TACI heterozygous variant may have predisposed her to a cascade of immune dysregulation following a febrile illness.
Given the complex nature of CVID and epilepsy genetics, the TNFRSF13B variant was likely a susceptibility factor for CVID. There were many confounding variables in this case related to treatment including plasma exchange, rituximab, and antiseizure medications. Other factors including gene-gene and gene-environmental interactions likely contributed to the clinical severity of this case. Larger patient cohort studies and functional modeling for this gene are needed to evaluate the potential causal relationship between TACI gene mutations and seizure risks. While there is a broad spectrum in the clinical presentation of TNFRSF13B-associated disease, this diagnosis should still be considered in cases of new-onset refractory status epilepticus with otherwise negative workup for other differential diagnoses. Careful screening prior to initiating immunotherapies such as IVIG and B cell depleting therapy should be performed including quantitative immunoglobulins and lymphocyte subsets.
Conclusions
Mutations in TNFRSF13B are associated with CVID. Biallelic variants in TACI are associated with hypogammaglobulinemia, and heterozygous variants are associated with autoimmune disease. Neurological complications of CVID are rare. Further studies are needed to investigate the causal relationship between CVID and epilepsy given the many potential confounding variables. However, primary immunodeficiencies should be considered in FIRES/NORSE cases, as immunosuppressive medications such as rituximab can unmask an underlying immunodeficiency. Care should be taken in these cases to select the appropriate immunomodulatory therapies.
Acknowledgments
Jennifer Yang is responsible for the study design and concept and initial drafting of the manuscript. Nicholas Scanlon, Wonhee Woo, Jamie Nicole LaBuzetta, Cynthia Gonzalez Gonzalez, Lori Broderick, Taylor A. Doherty, Marc A. Riedl, and Anastasie Dunn-Pirio were responsible for revising the manuscript for content.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained or waived by all participants in this study
References
- 1.New-onset refractory status epilepticus (NORSE) and febrile infection-related epilepsy syndrome (FIRES): state of the art and perspectives. Gaspard N, Hirsch LJ, Sculier C, et al. Epilepsia. 2018;59:745–752. doi: 10.1111/epi.14022. [DOI] [PubMed] [Google Scholar]
- 2.Genes associated with common variable immunodeficiency: one diagnosis to rule them all? Bogaert DJ, Dullaers M, Lambrecht BN, Vermaelen KY, De Baere E, Haerynck F. J Med Genet. 2016;53:575–590. doi: 10.1136/jmedgenet-2015-103690. [DOI] [PubMed] [Google Scholar]
- 3.Structural noninfectious manifestations of the central nervous system in common variable immunodeficiency disorders. van de Ven A, Mader I, Wolff D, et al. J Allergy Clin Immunol Pract. 2020;8:1047–1062. doi: 10.1016/j.jaip.2019.11.039. [DOI] [PubMed] [Google Scholar]
- 4.Transmembrane activator and calcium-modulator and cyclophilin ligand interactor mutations in common variable immunodeficiency. Lee JJ, Ozcan E, Rauter I, Geha RS. Curr Opin Allergy Clin Immunol. 2008;8:520–526. doi: 10.1097/ACI.0b013e3283141200. [DOI] [PubMed] [Google Scholar]
- 5.Transmembrane activator and calcium-modulating cyclophilin ligand interactor mutations in common variable immunodeficiency: clinical and immunologic outcomes in heterozygotes. Zhang L, Radigan L, Salzer U, et al. J Allergy Clin Immunol. 2007;120:1178–1185. doi: 10.1016/j.jaci.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.TACI deficiency - a complex system out of balance. Salzer U, Grimbacher B. Curr Opin Immunol. 2021;71:81–88. doi: 10.1016/j.coi.2021.06.004. [DOI] [PubMed] [Google Scholar]
- 7.Transmembrane activator and calcium modulator and cyclophilin ligand interactor enhances CD40-driven plasma cell differentiation. Castigli E, Wilson SA, Elkhal A, Ozcan E, Garibyan L, Geha RS. J Allergy Clin Immunol. 2007;120:885–891. doi: 10.1016/j.jaci.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The transmembrane activator TACI triggers immunoglobulin class switching by activating B cells through the adaptor MyD88. He B, Santamaria R, Xu W, et al. Nat Immunol. 2010;11:836–845. doi: 10.1038/ni.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.TLR stimulation modifies BLyS receptor expression in follicular and marginal zone B cells. Treml LS, Carlesso G, Hoek KL, et al. J Immunol. 2007;178:7531–7539. doi: 10.4049/jimmunol.178.12.7531. [DOI] [PubMed] [Google Scholar]
- 10.Impaired B cell receptor signaling is responsible for reduced TACI expression and function in X-linked immunodeficient mice. Uslu K, Coleman AS, Allman WR, Katsenelson N, Bram RJ, Alugupalli KR, Akkoyunlu M. J Immunol. 2014;192:3582–3595. doi: 10.4049/jimmunol.1203468. [DOI] [PubMed] [Google Scholar]
- 11.CVID-associated TACI mutations affect autoreactive B cell selection and activation. Romberg N, Chamberlain N, Saadoun D, et al. J Clin Invest. 2013;123:4283–4293. doi: 10.1172/JCI69854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Relevance of biallelic versus monoallelic TNFRSF13B mutations in distinguishing disease-causing from risk-increasing TNFRSF13B variants in antibody deficiency syndromes. Salzer U, Bacchelli C, Buckridge S, et al. Blood. 2009;113:1967–1976. doi: 10.1182/blood-2008-02-141937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The impact of TACI mutations: from hypogammaglobulinemia in infancy to autoimmunity in adulthood. Barroeta Seijas AB, Graziani S, Cancrini C, et al. Int J Immunopathol Pharmacol. 2012;25:407–414. doi: 10.1177/039463201202500210. [DOI] [PubMed] [Google Scholar]
- 14.Clinical associations of biallelic and monoallelic TNFRSF13B variants in Italian primary antibody deficiency syndromes. Pulvirenti F, Zuntini R, Milito C, et al. J Immunol Res. 2016;2016:8390356. doi: 10.1155/2016/8390356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sequence variants of the TNFRSF13B gene in Czech CVID and IgAD patients in the context of other populations. Freiberger T, Ravčuková B, Grodecká L, et al. Hum Immunol. 2012;73:1147–1154. doi: 10.1016/j.humimm.2012.07.342. [DOI] [PubMed] [Google Scholar]
- 16.Clinical variability of family members with the C104R mutation in transmembrane activator and calcium modulator and cyclophilin ligand interactor (TACI) Koopmans W, Woon ST, Brooks AE, Dunbar PR, Browett P, Ameratunga R. J Clin Immunol. 2013;33:68–73. doi: 10.1007/s10875-012-9793-x. [DOI] [PubMed] [Google Scholar]
- 17.TACI mutations and impaired B-cell function in subjects with CVID and healthy heterozygotes. Martinez-Gallo M, Radigan L, Almejún MB, Martínez-Pomar N, Matamoros N, Cunningham-Rundles C. J Allergy Clin Immunol. 2013;131:468–476. doi: 10.1016/j.jaci.2012.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Role of TNFRSF13B variants in patients with common variable immunodeficiency. Martínez-Pomar N, Detková D, Arostegui JI, et al. Blood. 2009;114:2846–2848. doi: 10.1182/blood-2009-05-213025. [DOI] [PubMed] [Google Scholar]
- 19.Mutations in TNFRSF13B encoding TACI are associated with common variable immunodeficiency in humans. Salzer U, Chapel HM, Webster AD, et al. Nat Genet. 2005;37:820–828. doi: 10.1038/ng1600. [DOI] [PubMed] [Google Scholar]
- 20.Neurologic complications of common variable immunodeficiency. Nguyen JT, Green A, Wilson MR, DeRisi JL, Gundling K. J Clin Immunol. 2016;36:793–800. doi: 10.1007/s10875-016-0336-8. [DOI] [PubMed] [Google Scholar]
- 21.Seizures and risk of epilepsy in autoimmune and other inflammatory encephalitis. Spatola M, Dalmau J. Curr Opin Neurol. 2017;30:345–353. doi: 10.1097/WCO.0000000000000449. [DOI] [PMC free article] [PubMed] [Google Scholar]