Abstract
Endometriosis is defined by the presence of extrauterine endometrial-like tissue, which can cause pain and infertility in 10% of reproductive-age women. To date, the pathogenesis is poorly understood resulting in significant diagnostic delays and poor therapeutic outcomes in many women. Small extracellular vesicles (sEVs) (<200 nm) are cell-derived vesicles containing molecules that can influence gene expression and behaviour in target cells. One such cargo are microRNAs (miRNAs), which are short, non-coding RNAs mostly 19–25 nucleotides in length that regulate post-transcriptional gene expression. This mini-review focuses on the role of sEV-miRNAs, which are conceivably better biomarkers for endometriosis than free miRNAs, which reflect the true pathophysiological state in the body, as sEV-encapsulated miRNAs are protected from degradation compared to free miRNA and provide direct cell-to-cell communication via sEV surface proteins. sEV-miRNAs have been implicated in the immunomodulation of macrophages, the proliferation, migration and invasion of endometrial cells, and angiogenesis, all hallmarks of endometriosis. The diagnostic potential of sEV-miRNA was investigated in one study that reported the sensitivity and specificity of two sEV-miRNAs (hsa-miR-22-3p and hsa-miR-320a-3p) in distinguishing endometriosis from non-endometriosis cases. Only three studies have explored the therapeutic potential of sEV-miRNAs in vivo in mice—two looked into the role of sEV-hsa-miR-214-3p in decreasing fibrosis, and one investigated sEV-hsa-miR-30c-5p in suppressing the invasive and migratory potential of endometriotic lesions. While early results are encouraging, studies need to further address the potential influence of factors such as the menstrual cycle as well as the location and extent of endometriotic lesions on miRNA expression in sEVs. Given these findings, and extrapolating from other conditions such as cancer, diabetes, and pre-eclampsia, sEV-miRNAs could present an attractive and urgently needed future diagnostic and therapeutic target for millions of women suffering from endometriosis. However, research in this area is hampered by lack of adherence to the International Society for Extracellular Vesicles 2018 guideline in separating and characterising sEVs, as well as the World Endometriosis Research Foundation Endometriosis Phenome and Biobanking Harmonisation Project protocols.
Keywords: exosomes, small extracellular vesicles, exosomal microRNAs, miRNAs, sEV-miRNAs, endometriosis
Graphical abstract
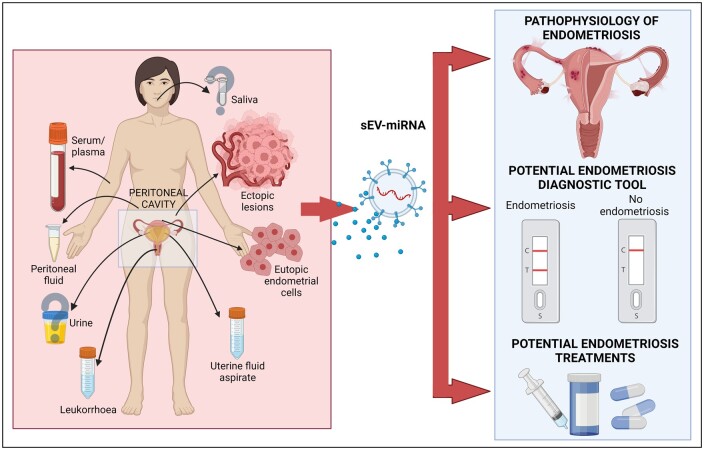
Graphical Abstract.
sEV-miRNAs, which can be isolated from all biological fluids, are essential in the pathophysiology of endometriosis, and potentially in the diagnosis and treatment of endometriosis. This figure was created with BioRender.com. sEV-miRNA, small extracellular vesicle-microRNA.
Introduction
Endometriosis, the presence of extrauterine endometrial-like lesions, affects ∼5–10% of reproductive-aged females (Zondervan et al., 2018) although rare premenarcheal manifestations and postmenopausal endometriosis have been described (Gemmell et al., 2017). Globally, an estimated 190 million reproductive-aged women suffer from endometriosis (Zondervan et al., 2020) although the true prevalence might be higher.
Women with endometriosis often experience severe menstrual and non-menstrual pain involving the lower abdomen, pelvis or lumbosacral region, deep dyspareunia, dyschezia, and dysuria (Uimari et al., 2021) and infertility in 30–50% of cases (Prescott et al., 2016). Patients with endometriosis suffer from chronic fatigue (Ramin-Wright et al., 2018) and are at a higher risk of psychiatric disturbances (56.4%) than those without endometriosis (43.6%) (Pope et al., 2015). Endometriosis is further associated with poorer health-related quality of life mean scores and work productivity as compared to asymptomatic controls (Nnoaham et al., 2011) resulting in an estimated €9.9 billion (equivalent to £8.4 billion) in societal cost in 2012 (Simoens et al., 2012)—a higher economic burden should be assumed today.
Endometriotic lesions most commonly present as superficial peritoneal/serosal lesions of different colours and sizes, as ovarian endometriotic cysts or mostly fibrotic (deep) nodules >5 mm below the peritoneal surface (Cornillie et al., 1990). The extent of pelvic endometriosis is widely described using the revised American Society for Reproductive Medicine (rASRM) classification system into stages I–IV based on direct observation of lesions during surgery (Canis et al., 1997). Infrequently, extrapelvic locations, including abdominal surgical scars (Wang et al., 2016), the umbilicus, diaphragm, thorax, pericardium, and lymph nodes (Ceccaroni et al., 2012), have been described.
To date, the cause of endometriosis is not fully defined. Sampson’s theory of retrograde menstruation (Sampson, 1927), does not explain why only some women develop endometriosis as retrograde menstruation is physiological (Halme et al., 1984). Insufficient public and professional awareness, and the trivilisation of women’s pain (Shah et al., 2010; Samulowitz et al., 2018; De Sanctis et al., 2020), absence of clinically relevant biomarkers (May et al., 2010; Liu et al., 2015; Gupta et al., 2016; Nisenblat et al., 2016a,b,c), and the unspecific nature of endometriosis-associated symptoms (Zondervan et al., 2018) are some of the challenges in diagnosing endometriosis. Previously, the gold standard for diagnosing endometriosis was diagnostic laparoscopy, which may have prevented earlier diagnosis of endometriosis. The latest ESHRE guideline on endometriosis no longer recommends diagnostic laparoscopy as the gold standard diagnostic tool, reserving it rather for women with negative MRI or ultrasound imaging, and/or where empirical treatment has failed (Becker et al., 2022). Current endometriosis treatments are mainly symptomatic and rarely curative while also aiming for fertility preservation if required. All these factors contribute to the 6–8 years (average 6.7 years, measured in 16 clinical centres across 10 countries) waiting time between symptoms onset and endometriosis diagnosis (Nnoaham et al., 2011).
Extracellular vesicles
Extracellular vesicles (EV) are nano-sized membrane-bound vesicles produced by almost all cells in the body. EVs comprise a heterogeneous population and over the years various terms have been used to describe these vesicles, including exosomes, ectosomes, microvesicles (MVs), and microparticles (Fig. 1). The first investigations to describe EVs were two studies (Harding et al., 1984; Pan et al., 1985) that observed 50 nm vesicles released from maturing reticulocytes with transferrin receptors through the process of intraluminal budding of multivesicular endosomes that bind to the lipid cell membrane before their release into the circulation. These vesicles were named ‘exosomes’ by Rose Johnstone, although before the 1980s the term had been used to describe different phenomena such as ‘membrane fragments’ (De Broe et al., 1977), platelet ‘dust’, or cellular debris (Wolf, 1967). Ostensibly, the blood-derived pro-coagulant membrane-derived particles isolated by Chargaff and West (1946) were the platelet ‘dust’ isolated by Wolf (1967), therefore it could be argued that EVs were first observed in 1946.The term was even used to describe isolated 40–1000 nm ‘exosome complexes’ secreted by neoplastic cell lines with a 5′ nucleotidase activity (Trams et al., 1981). Nevertheless, in 1996, these EVs gained renewed research interest with the exosome secretion of Epstein–Barr-virus-transformed B cells (Raposo et al., 1996).
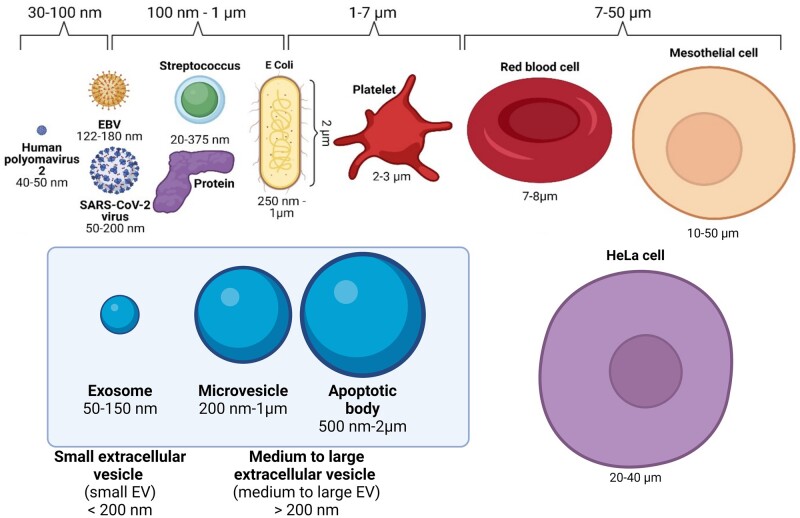
Figure 1.
The diversity of extracellular vesicles produced by cells in the human body. Compared to exosomes, MVs can be defined as EVs that are released directly from the plasma membrane and are on an average larger than exosomes, although their sizes could range from 30 nm to 1 µm (Jeppesen et al., 2019). The terms ‘ectosomes’ and ‘microparticles’ are synonymously used to describe MVs. Like exosomes, MVs are essential for cellular communication and carry cargo such as mRNA, miRNA, lncRNA, and protein. Apoptotic bodies are released as cells undergo apoptotic cell disassembly, where the plasma membrane blebs and the apoptotic membrane protrudes and fragments. Therefore, different EV subtypes can be categorised according to their biogenesis, size, constituent protein, and isolation methods; however, the different criteria for EV subtypes often overlap, and can even contradict each other. This figure was created with BioRender.com. EV, extracellular vesicle; MV, microvesicle; mRNA, messenger RNA; miRNA, microRNA; lncRNA, long non-coding RNA.
Exosomes are small EVs [sEVs, defined as <200 nm (Théry et al., 2018)] of 30–150 nm in size. They are initially formed as an early endosome (EE) through the inward budding of the plasma membrane as it recycles receptors, proteins, and lipids back into the cell. As the EE matures into a late endosome (LE), it traffics and catalogues proteins from the Golgi apparatus, as well as genetic material from the cytoplasm, for degradation or transportation outside of the cell. The LE then forms the multivesicular body (MVB) with the invagination of the MVB membrane to form intraluminal vesicles (ILVs). When ILVs are released into the extracellular space via fusion of the MVB with the cell plasma membrane, they are defined as exosomes. Owing to their biogenesis, exosomes contain proteins involved in the MVB formation (e.g. ALIX, and TSG101), membrane trafficking (e.g. Rab GTPases and annexins), and are enriched in tetraspanins (e.g. CD9, CD63, and CD81) (van Niel et al., 2006; Zöller, 2009; Raposo and Stoorvogel, 2013).
However, there is no consensus on specific markers or specific separation methods for delineating different EV subtypes, beyond their size (Fig. 1). There is no one separation method superior to the other, with one method sacrificing yield versus purity, and vice versa (Théry et al., 2018; Jia et al., 2022). The isolation method of choice will thus depend on the goal of the study, the amount and quality of sample biofluids, and the downstream analyses chosen. The studies included in this mini-review used differential ultracentrifugation (low purity containing protein complexes, medium yield, time consuming, suitable for large sample numbers), size exclusion chromatography (medium-to-high yield, high purity, suitable for low sample numbers), and ready-made isolation kits with precipitation buffers (high yield, low purity as contain polymers, suitable for large sample numbers) or magnetic capture (may be susceptible to charged particles in the sample) (Xu et al., 2016; Jia et al., 2022). The International Society for Extracellular Vesicles (ISEV) 2018 recommendations state that researchers should use the generic term EV unless the precise biogenesis can be captured using live imaging techniques (Théry et al., 2018). ISEV also recommends further describing EV subtypes based on physical and biochemical characteristics and/or conditions/sources (Théry et al., 2018). In this review, we utilise the term sEV, even though many of the studies we include may originally have referred to ‘exosomes’.
sEVs are detected in a variety of bodily fluids (van Niel et al., 2022). They transport cargo that can affect gene expression of target cells in remote parts of the body. Introduction of new receptors, proteins, or genetic material through sEV fusion with the target cells confer new cellular properties that mirror the parent cell’s expression (Zomer et al., 2015). sEVs are implicated in various disease pathologies, including cancer (Hoshino et al., 2015; Zomer et al., 2015), diabetes (Noren Hooten and Evans, 2020), cardiovascular disease (Liu et al., 2021), neurodegenerative disease (Vandendriessche et al., 2020) as well as in benign obstetric and gynaecological conditions such as pre-eclampsia (Murugesan et al., 2022) and endometriosis (Harp et al., 2016; Nazri et al., 2020). A urinary sEV RNA-based diagnostic tool for prostate cancer (Kretschmer et al., 2022), the ExoDx™ Prostate (IntelliScore) (EPI) test, has been used to aid clinical decisions for >50 000 patients so far and is included in the United States National Comprehensive Cancer Network guidelines for early prostate cancer detection.
sEV cargos include the RNA species such as mRNA, piwi-interacting RNA, transfer RNA fragments, microRNA (miRNA), long non-coding RNA (lncRNA), and rRNA (O’Brien et al., 2020). miRNAs are short, non-coding RNAs of ∼19–25 nucleotides in length, which are synthesised via DNA transcription into primary miRNAs, then processed in the cytoplasm into precursor miRNAs, and mature miRNAs (O’Brien et al., 2018). miRNAs regulate post-transcriptional gene expression mainly via targeting mRNAs at the 3′ untranslated region to inhibit mRNA translation into protein or enhance mRNA instability, leading to its degradation (O’Brien et al., 2018). miRNAs can also upregulate gene expression in certain microribonucleoprotein (miRNP) factors or cellular conditions (Vasudevan, 2012) by controlling mRNA transcription rate and translation into proteins as they are shuttled between different subcellular compartments (Makarova et al., 2016).
While the role of miRNA as a diagnostic and therapeutic tool for endometriosis in itself has been an area of burgeoning research interest (Agrawal et al., 2018), sEV-miRNA is better protected from degradation than free miRNA and thus conceivably the better biomarker candidate as it arguably better reflects the true pathophysiological state in the body (Cheng et al., 2014).
In this mini-review, we have included all possible original articles that investigated sEV miRNAs in endometriosis regardless of sEV separation or characterisation methods (Table 1). Literature searches were carried out on PubMed using the terms ‘endometriosis small extracellular vesicles miRna’, ‘endometriosis exosomes miRna’, and ‘endometriosis exosomes miRna’ and studies selected according to quality of hypothesis and methodological rigour in exploring the hypothesis. Review articles were excluded from this mini-review.
Table 1.
Studies investigating the differential expression of sEV-miRNA.
| No. | Sample type | Sample size | sEV isolation methods | sEV concentration and size | sEV protein markers | Methods | Dysregulated sEV miRNA | Study |
|---|---|---|---|---|---|---|---|---|
| 1 | Serum |
|
Differential ultracentrifugation |
|
|
|
↑ hsa-miR-22-3p, hsa-miR-320a-3p | Zhang et al. (2020a) |
|
| ||||||||
| 2 | Serum |
|
|
|
|
|
|
Wu et al. (2022) |
|
| ||||||||
| 3 |
|
|
ExoQuick-TC Exosome Isolation Kit (System Biosciences SBI, USA) |
|
|
|
↓ hsa-miR-214-3p | Zhang et al. (2021) |
| 4 |
|
|
MiRCURY Exosome Isolation Kit (Qiagen) |
|
|
|
|
Khalaj et al. (2019) |
|
| ||||||||
| 5 |
|
|
Total Exosome Isolation kit (Invitrogen) |
|
Not done. |
|
|
Harp et al. (2016) |
|
| ||||||||
| 6 | Eutopic endometrium |
|
ExoQuick-TC Exosome Isolation Kit (System Biosciences SBI, USA) |
|
|
sEV-miRNA sequencing followed by qRT-PCR. |
|
Zhou et al. (2020) |
|
| ||||||||
| 7 |
|
|
ExoQuick-TC Exosome Isolation Kit (System Biosciences SBI, USA) | NTA done and sEVs visualised via TEM according to ‘Materials & Methods’ section but results not reported in the study. | WB done according to ‘Materials & Methods’ section but results not reported in the study. |
|
↓ hsa-miR-15a-5p | Wu et al. (2021) |
| 8 |
|
|
Ultracentrifugation |
|
Not done. |
|
↓ hsa-miR-214-3p | Wu et al. (2018) |
|
| ||||||||
| 9 |
|
|
Differential ultracentrifugation, followed by exosome precipitation using a reagent |
|
|
|
↓ hsa-miR-30c-5p | Zhang et al. (2022) |
| 10 |
|
|
Differential ultracentrifugation |
|
|
|
↑ hsa-miR-301a-3p | Huang et al. (2022) |
|
| ||||||||
| 11 | PF |
|
Differential ultracentrifugation | No information in study. | No information in study. |
|
↓ hsa-miR-130-5p | Chen et al. (2019) |
|
| ||||||||
| 12 |
|
|
Differential ultracentrifugation |
|
|
|
↑ hsa-miR-22-3p | Zhang et al. (2020b) |
| 13 | Uterine aspirate fluid |
|
Size exclusion chromatography exosome isolation kit (Echobiotech, Beijing, China) |
|
|
|
|
Jiang et al. (2022) |
|
| ||||||||
| 14 |
|
|
Differential ultracentrifugation |
|
|
|
↑ hsa-miR-202-3p, hsa-miR-202-5p | Zheng et al. (2023) |
sEV-miRNA, small extracellular vesicle-microRNA; miRNA, microRNA; NTA, nanoparticle tracking analyses; TEM, transmission electron microscopy; WB, Western blot; qRT-PCR, quantitative real time PCR; PF, peritoneal fluid; TSG101, tumour susceptibility gene 101; HSP70, 70 kilodalton heat shock protein; CTGF, connective tissue growth factor—also known as CCN2; α-SMA, α-smooth muscle actin; NC, negative control; HUVEC, human umbilical vein endothelial cells; BCL9, B-cell lymphoma 9; PTEN, phosphatase and tensin homolog; PI3K, phosphatidylinositol 3-kinase; MAPK, mitogen-activated protein kinase.
sEV-miRNA and endometriosis pathophysiology
The roles of sEV-miRNA have been proposed in several key mechanisms of endometriosis pathophysiology (Table 1, Figs 2 and 3), for instance in the immunomodulation of peritoneal macrophages (pMφ), proliferation and migration of ectopic endometriosis lesions (Zhang et al., 2020b, 2022; Huang et al., 2022), and angiogenesis (Harp et al., 2016; Khalaj et al., 2019). Differential expression of sEV-miRNAs isolated from serum/plasma (Khalaj et al., 2019; Zhang et al., 2020a, 2021; Wu et al., 2022), leukorrhoea (Zheng et al., 2023), eutopic endometrium (Khalaj et al., 2019; Zhou et al., 2020; Wu et al., 2021; Zheng et al., 2023), endometrioma (Wu et al., 2021), endometriotic lesion (Harp et al., 2016; Wu et al., 2018; Zhang et al., 2022), peritoneal fluid (PF) (Chen et al., 2019; Khalaj et al., 2019; Zhang et al., 2020b), and uterine aspirate (Jiang et al., 2022) has been demonstrated in sEVs isolated from women with endometriosis compared to controls.
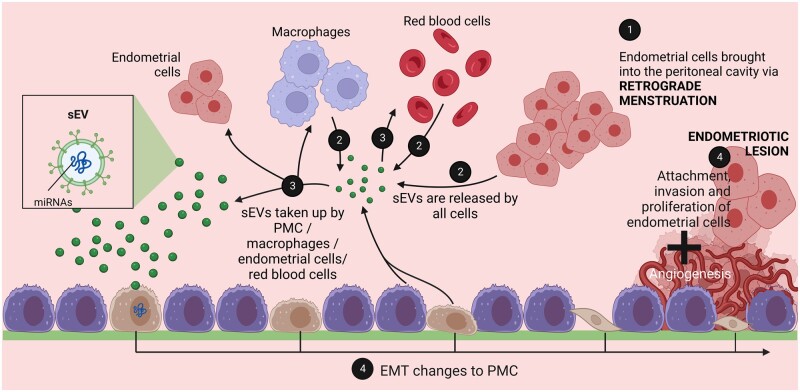
Figure 2.
Retrograde menstruation is only one part of the endometriosis pathophysiology. (1) Retrograde menstruation brings endometrial cells into the peritoneal cavity. (2) sEVs are produced by all cells (endometrial cells, red blood cells, pMφ, andPMCs) in the peritoneal cavity. (3) sEVs containing miRNAs are taken up by endometrial cells, red blood cells, pMφ, and PMC, causing changes to recipient cells. (4) Uptake of sEVs and internalisation of miRNAs promotes proliferation, migration, and invasion of endometrial cells and of existing ectopic lesions, immunomodulation of macrophages, and potentially EMT changes of PMCs, although none of the studies in this review investigated the impact of sEV-miRNA on PMCs. PF-derived sEVs are likely to originate from a variety of cell types including endometrial cells, red blood cells, immune cells, ectopic lesions, and PMCs. This figure was created with BioRender.com. sEV, small extracellular vesicle; miRNA, microRNA; pMφ, peritoneal macrophage; PMC, peritoneal mesothelial cell; EMT, epithelial-to-mesenchymal transition; PF, peritoneal fluid; sEV-miRNA, small extracellular vesicle-microRNA.
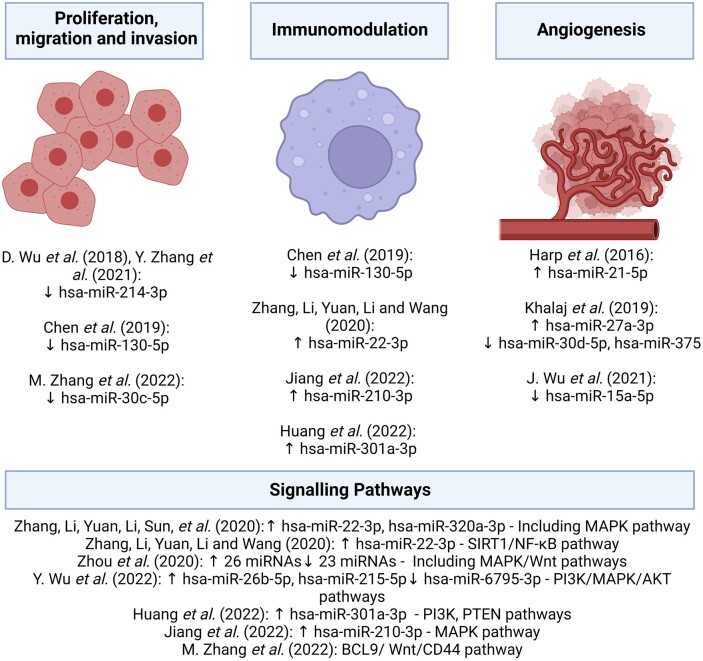
Figure 3.
Proposed sEV-miRNAs involved in the pathophysiology of endometriosis. sEV-miRNAs involved in the pathophysiology of endometriosis through the proliferation, migration, and invasive potential of endometrial cells, immunomodulation, and angiogenesis (formation of blood vessels) to support explanted endometrial cells (ectopic lesions). Studies also investigated endometriosis-specific miRNAs and the signalling pathways involved. This figure was created with BioRender.com. sEV-miRNA, small extracellular vesicle-microRNA.
Immunomodulation, proliferation, and migration
Retrogradely spilt menstrual blood, cells, and tissue can be detected in the abdominal cavity of most women at the time of menstruation (Halme et al., 1984). Therefore, an impaired immune response in combination with the increased proliferative and migratory activity of these cells may contribute to the establishment of endometriosis in some women (Zondervan et al., 2018).
Serum-derived sEVs from women with endometriosis contained higher hsa-miR-22-3p and hsa-miR-320a-3p levels compared to controls, with elevated hsa-miR-22-3p levels in stages III–IV versus stages I–II endometriosis (Zhang et al., 2020a). High hsa-miR-22-3p and hsa-miR-320a-3p levels were also correlated with high serum CA-125 levels in patients with endometriosis (Zhang et al., 2020a). Kyoto Encyclopaedia of Genes and Genomes (KEGG) analysis suggested mitogen-activated protein kinase (MAPK) activity, among others (Zhang et al., 2020a). Increased pMφ-derived sEV-hsa-miR-22-3p in patients compared to controls regulates the sirtuin 1/nuclear factor κB (SIRT1/NF-κB) signalling pathway leading to enhanced ectopic endometrial stromal cells (ESCs) proliferation, migration, and invasion (Zhang et al., 2020b).
Pro-repair phenotype polarisation and reduced phagocytic activity in Mφ exposed to lesion-derived sEVs were mediated by hsa-miR-301a-3p through phosphatidylinositol 3-kinase (PI3K) upregulation and tumour suppressor phosphatase and tensin homologue deleted on chromosome 10 (PTEN) downregulation (Huang et al., 2022). These findings echoed a previous study in an endometriosis mouse model which also revealed decreased total weight and volume of endometriotic lesions following treatment with ESC-derived sEVs (Sun et al., 2019b). Elevated hsa-miR-210-3p in uterine-aspirate-fluid-derived sEVs and eutopic endometrium of patients with endometriosis decreased CD80+ Mφ (a surface marker of pro-inflammatory Mφ) by suppressing c-Jun N-terminal kinase (JNK, protein kinase in MAPK pathway) phosphorylation in Mφ, thus increasing pro-repair Mφ, which could escape immune surveillance. In support of these findings, sEV-treated Mφ increased proliferation, migration, and invasion of endometriosis ESCs compared to control ESCs in a transwell experiment (Jiang et al., 2022). In another study, quantitative RT-PCR-verified endometriosis-specific serum-derived sEV-hsa-miR-26b-5p (downregulated), hsa-miR-215-5p (downregulated), and hsa-miR-6795-3p (upregulated) were found to be involved in the PI3K, MAPK, and protein kinase B (Akt) pathways in a KEGG analysis. Serum-derived sEV-hsa-miR-26b-5p expression was lower in rASRM stages III–IV versus stages I–II endometriosis (Wu et al., 2022).
In a KEGG analysis of 49 differentially expressed sEV-miRNAs from eutopic endometrium of women with endometriosis-related infertility and control endometrium, the Wnt signalling pathway (Zhou et al., 2020) was also implicated, in addition to MAPK, confirming previous findings (Wu et al., 2022; Zhang et al., 2022). Homeobox A10 (HOXA10) and/or leukaemia inhibitory factor (LIF)—targets of 12 miRNAs—were decreased in eutopic endometrium of women with endometriosis-related infertility compared to controls (Zhou et al., 2020).
Higher levels of leukorrhoea-derived sEV-hsa-miR-202-3p and hsa-miR-202-5p in patients with endometriosis compared to controls corresponded to levels in endometriosis eutopic endometrium (Zheng et al., 2023).
Angiogenesis
Neo-angiogenesis, the formation of new blood vessels from existing ones, is a process involved in many physiological (e.g. corpus luteum formation, endometrial proliferation) and pathological processes (e.g. cancer, chronic inflammation) (Folkman, 2001). Retrogradely transplanted endometriotic cells and tissue require a blood supply for proliferation (May and Becker, 2008).
Cultured human umbilical vein endothelial cells (HUVEC) treated with eutopic ESC-derived sEVs from patients with endometriosis exhibited more extensive tube formation compared to those treated with equivalent sEVs from controls (Harp et al., 2016). In this study, ectopic lesion-derived sEVs contained elevated (pro-angiogenic) hsa-miR-21-5p compared to eutopic ESC-derived sEVs from controls. However, it is uncertain what assumptions can be made from this comparison without also comparing eutopic ESC-derived and possibly PF-derived sEVs from patients and controls. Although no specific sEV-miRNAs were investigated, Sun et al. (2019a) observed greater tube formation in endometriosis eutopic ESC-derived sEV-treated HUVECs compared to control eutopic ESC-derived sEVs. Treating HUVECs with plasma-derived sEVs from patients with endometriosis revealed similar findings (Khalaj et al., 2019). Sun et al. (2019a) also observed increased neurite outgrowth induced by endometriosis eutopic ESC-derived sEVs compared to control.
In an RNA sequencing study of control and endometriosis eutopic ESC- and endometrioma-derived sEV-RNA, hsa-miR-15a-5p, involved in vascular endothelial growth factor A (VEGF-A) downregulation, was lower in the cell culture supernatant of patients with endometriosis versus control (Wu et al., 2021). The relation between hsa-circ_0026129, hsa-miR-15a-5p, and ATP6V1A was uncovered in an sEV-competing endogenous RNA (cERNA) network analysis. This showed that increased sEV-hsa-circ_0026129 correlated with decreased hsa-miR-15a-5p and increased ATP6V1A expression in ovarian endometriomas. Dual luciferase reporter assays confirmed that hsa-circ_0026129 competitively binds to hsa-miR-15a-5p, which contributed to low hsa-miR-15a-5p levels thus increasing ATP6V1A expression in ovarian endometriomas compared to controls. ATP6V1A is an oncogene of endometrial cancer, which is linked to the severity of endometriosis and endometrial receptivity (Wu et al., 2021).
PF-derived sEV-miRNAs
PF is rich in immune cells, prostaglandins, interleukin, cytokines, and growth factors that can influence the growth of endometrial lesions (Koninckx, 1998), and owing to its proximity and direct contact with endometriotic lesions, investigating PF for endometriosis-specific sEVs seems a rational approach. Studying the PF could identify the different sEV populations from different cells (e.g. pMφ, red blood cells, endometrial cells, ectopic lesions, peritoneal mesothelial cells), and correlating PF-derived sEV-miRNAs in more accessible fluids (e.g. serum, urine, or saliva) may provide a comprehensive picture of sEV-miRNAs involved in endometriosis and their utility in diagnostics.
In total, four studies investigated PF-derived sEVs in endometriosis. Out of these studies, two groups investigated PF-derived sEV-proteins (Khalaj et al., 2019; Nazri et al., 2020). At present, three studies investigated PF-derived sEV-miRNAs in endometriosis (Chen et al., 2019; Khalaj et al., 2019) including one study reporting on pMφ-derived sEVs (Zhang et al., 2020b).
Increased PF-derived sEV-hsa-miR-130-3p levels, implicated in immune cell function, were found in patients with endometriosis versus controls. In stage III–IV endometriosis, increased PF-derived sEV-hsa-miR-451a and hsa-miR-486-5p were found (Chen et al., 2019). Khalaj et al. (2019) attempted to compare the small RNA and proteome content of endometriotic lesion-derived sEVs versus patients’ matched eutopic endometrium and controls, as well as PF- and plasma-derived sEVs. Accounting for sample types, sEV-miRNAs unique to endometriosis include hsa-miR-30d-5p (upregulated), hsa-miR-27a-3p (downregulated), and hsa-miR-375 (downregulated). Using TargetScan (an online repository for miRNA biological target prediction), Khalaj et al. identified hsa-miR-30d-5p binding sites for thrombospondin-2, alpha-2-antiplasmin, and interferon regulatory factor 4, whereas hsa-miR-375 has binding sites for platelet-derived growth factor subunit A—all of which suggested angiogenesis and pro-inflammatory pathways (Khalaj et al., 2019).
sEV-miRNAs as diagnostic markers for endometriosis
While there is considerable potential for sEV-miRNAs to serve as diagnostic markers for endometriosis, so far only one study has reported the sensitivity and specificity of sEV-miRNAs in diagnosing endometriosis (Zhang et al., 2020a). For serum-derived sEV-hsa-miR-22-3p, the AUC was higher at 85.5% than the AUC of hsa-miR-320a-3p at 82.7%. Combining both miRNAs, the AUC was 88.3% (Zhang et al., 2020a), which is considered to be an encouraging AUC score in discriminating between patients with endometriosis and controls.
sEV-miRNAs for endometriosis treatment
Fourteen studies had identified the differential expression of sEV-miRNAs in endometriosis (Table 1), however, only three studies (Wu et al., 2018; Zhang et al., 2021, 2022) investigated the therapeutic potential of sEV-miRNA in endometriosis in vivo, as described below.
sEV-hsa-miR-214-3p
Endometriosis is a heterogeneous disease with a large range of phenotypes (Zondervan et al., 2020). Although red lesions have been shown to express high levels of VEGF and other growth factors, and are therefore considered by some as the most active, fibrosis is a molecular hallmark and a frequently encountered clinical problem (Burney, 2022). Therefore, non-surgical treatment and/or prevention of fibrosis is an urgently unmet clinical need.
In a study investigating liver fibrosis (Chen et al., 2014), sEV-hsa-miR-214-3p decreased the expression of connective tissue growth factor (CTGF) consequently decreasing the fibrosis of hepatic stellate cells. Thus, hsa-miR-214-3p could be a promising therapeutic target for other fibrotic diseases, including endometriosis, by targeting CTGF. In an endometriosis rodent model (induced by i.p. injection of human endometriosis eutopic ESCs), fibrosis reduction in ectopic lesions was observed when treated with human ectopic ESC-derived sEVs transfected with hsa-miR-214-3p mimics, through CTGF and collagen reduction in ESCs and endometrial epithelial cells (EECs) (n = 3), compared to mice treated with control sEVs (n = 3) and PBS (n = 3) (Wu et al., 2018).
Using a modified, Burns et al. (2012) protocol to induce endometriosis in nude mice (n = 24)—where one experimental group was treated with uterine tissue derived from CTGF-knock-out mice and the other with uterine tissue from wild-type mice—the above findings (Wu et al., 2018) were confirmed (Zhang et al., 2021). The endometriosis-induced mice (n = 24) were divided into four treatment groups (n = 6) of i.p. injections of saline, human serum-derived sEVs, hsa-miR-214-3p mimics, or sEV-hsa-miR-214-3p. This revealed increased fibrosis-related protein expression in endometriotic lesions of sEV-hsa-miR-214-3p-treated mice compared to other treatment groups. Additionally, no differences were seen in mice induced with CTGF-knock-out uterine tissue, confirming the sEV-hsa-miR-214-3p’s CTGF-dependent actions. Finally, hsa-miR-214-3p downregulation and CTGF upregulation in ectopic lesions and eutopic endometrium of patients with endometriosis compared to eutopic endometrium of controls (Wu et al., 2018; Zhang et al., 2021) correlated with lower serum sEV-hsa-miR-214-3p in patients with endometriosis (Zhang et al., 2021).
sEV-hsa-miR-30c-5p
A hypothesis of endometriosis pathophysiology (Nazri et al., 2020) had likened the formation of the endometriotic lesion to the ‘seed and soil’ hypothesis of cancer (Paget, 1889), with sEVs causing increased invasive and migratory potential of retrogradely menstruated endometrial cells, similar to how tumour metastasis is instigated by sEVs (Hoshino et al., 2015). In a study by Zhang et al. (2022), ectopic EECs from endometrioma patients produced sEVs with low hsa-miR-30c-5p, leading to BCL9 (B-cell lymphoma 9) overexpression. BCL9, a co-activator of the Wnt/β-catenin pathway has been implicated in breast cancer metastasis (Wang et al., 2021) and hence may shed light on the invasive and migratory potential of endometriotic lesions.
In contrast, EEC-derived sEVs from control patients transferred hsa-miR-30c-5p, which targets BCL9, thus suppressing the invasive and migratory potential of ectopic EECs (Zhang et al., 2022). Blockage of the BCL9/Wnt/CD44 pathway by sEV-hsa-miR-30c-5p overexpression attenuated ectopic EEC invasive potential in an endometriosis nude mice model injected with sEVs pre-treated with hsa-miR-30c-5p mimic (n = 12, each). Notably, BCL9, Wnt1, β-catenin, c-myc, cyclin D1, CD44, vimentin, and N-cadherin expression was reduced, and E-cadherin increased, corresponding to increased hsa-miR-30c-5p levels. Indeed, attenuating the BCL9/Wnt/CD44 pathway via sEV-hsa-miR-30c-5p overexpression was associated with fewer ectopic nodules in the intestinal walls of nude mice with endometriosis compared to controls (Zhang et al., 2022).
Discussion
Endometriosis is a common heterogeneous disease which lacks clinically reliable non-invasive diagnostic tools as well as disease-specific therapeutic approaches. In this review, we present the currently available literature on sEV-miRNA and its diagnostic and therapeutic potential in the disease. Their roles were studied in various biological fluids and tissues including PF, serum and plasma, leukorrhoea, uterine aspirate fluid, endometrioma as well as eutopic and ectopic endometriotic lesions. However, no studies investigated sEV-miRNAs from peritoneal mesothelial cells (Fig. 2), and since they play a central role in endometriosis (Koks et al., 2000; Nair et al., 2008; Griffith et al., 2010; Knudtson et al., 2020) this is a clear gap in the field and one that warrants further investigation. Additionally, no groups have investigated saliva- or urine-derived sEV-miRNAs.
Of the 14 studies included here, six focused on rASRM stages III–IV endometriosis (Wu et al., 2018, 2021; Khalaj et al., 2019; Zhang et al., 2021, 2022; Jiang et al., 2022). Four studies did not give information about the rASRM stages of endometriosis samples (Harp et al., 2016; Zhang et al., 2020b; Huang et al., 2022; Zheng et al., 2023). One study investigated rASRM stages II–IV (Zhou et al., 2020) and only three studies investigated all rASRM stages (Chen et al., 2019; Zhang et al., 2020a; Wu et al., 2022) although data analyses according to rASRM stage were not included in these studies. Investigating stages I–II endometriosis separately from stages III–IV is essential as increasing evidence shows that these two combined stages of endometriosis could represent different disease entities altogether, with different genetic and biological pathways underpinning the pathophysiology (Painter et al., 2011; Sapkota et al., 2017; Uimari et al., 2017). In addition, testing sEV-miRNAs for their diagnostic potential would arguably be more relevant for superficial disease, which is difficult to visualise even using modern imaging technologies (Nisenblat et al., 2016a).
An additional problem is posed by the control samples used for some studies. Samples from women suffering from other gynaecological abnormalities, such as uterine leiomyomas, ovarian cysts, and ovarian teratomas, may confound results (Table 1). For example, one study reported that sEV-miRNA from human uterine leiomyoma cells was associated with increased proliferation and angiogenesis of uterine leiomyomata (Navarro et al., 2022). Similarly, sEVs from simple ovarian cysts and ovarian cancer have been used to stimulate lymphocytes to understand the differences in gene expression (Li et al., 2017). Three studies had recruited patients with tubal factor infertility as controls (Zhang et al., 2020a, 2022; Wu et al., 2021), although the current implications of this condition on sEV populations are not known. Arguably the most suitable controls in these studies would be those with pelvic pain and negative laparoscopic findings, i.e. symptomatic controls (Tapmeier et al., 2020), although the difficulty of obtaining appropriate controls is widely recognised.
None of the studies investigated sEV-miRNA in all rASRM stages of endometriosis and all phases of the menstrual cycle, including menstruation. As endometriosis is an oestrogen-dependent disease, one could speculate that the changes in sEV cargo and miRNAs may correlate to changes in oestrogen and progesterone levels. Two studies acquired samples in proliferative and secretory phases (Jiang et al., 2022; Wu et al., 2022), four studies only in the proliferative phase (Chen et al., 2019; Wu et al., 2021; Zhang et al., 2021, 2022), and three studies in the secretory phase (Harp et al., 2016; Khalaj et al., 2019; Zhou et al., 2020). Five studies provided no information about the menstrual cycle phases of their samples (Wu et al., 2018; Zhang et al., 2020a,b; Huang et al., 2022; Zheng et al.; 2023). Therefore, regrettably, at the time of writing no research groups have yet analysed and compared sEV-miRNA and -protein in all menstrual cycle phases and all rASRM stages of patients with endometriosis and controls.
Only one study demonstrated the potential for sEV-miRNA as a diagnostic tool for endometriosis although not without the limitations discussed above (Zhang et al., 2020a). Three studies (Wu et al., 2018; Zhang et al., 2021, 2022) evaluated the potential therapeutic properties of sEV-miRNAs in vivo. However, what remains to be investigated is whether treating endometriosis with sEV-has-miR-214-3p (Wu et al., 2018; Zhang et al., 2021) or has-miR-30c-5p (Zhang et al., 2022) would translate to endometriosis symptom relief.
Most importantly, none of these studies could justify using the term ‘sEVs’ or ‘exosomes’ as defined by the ISEV 2018 guidelines (Théry et al., 2018). The recommendations stipulated the use of certain separation methods for specifc downstream analyses taking into account the biological fluid the sEVs are derived from, knowing that there is a trade-off between yield and purity. Compared to ultracentrifugation, differential centrifugation or size-exclusion chromatography, isolating sEVs using precipitation methods (Harp et al., 2016; Khalaj et al., 2019; Zhou et al., 2020; Wu et al., 2021; Zhang et al., 2021) results in a high yield but risks sacrificing EV purity (Gurunathan et al., 2019). To be able to use the term ‘sEVs’, studies must show quantiative analyses of particle yield, with a global concentration of particle yield and particle-to-protein or particle-to-lipid ratios, as well as demonstrating EV proteins from at least three categories (transmembrane, cytosolic, purity control proteins), and visualising the sEVs as single particles. Four studies did not report any quantitative analyses of the particles (Table 1) (Chen et al., 2019; Khalaj et al., 2019; Zhang et al., 2021; Wu et al., 2022). Three studies neglected to perform experiments to confirm sEV proteins (Harp et al., 2016; Wu et al., 2018; Chen et al., 2019). Note that Chen et al. (2019) did not provide any information about its EV characterisation methods and Wu et al. (2021) only mentioned the EV characterisation methods that they employed in their study but did not share the results. Therefore, none of the studies that performed sEV confirmation experiments satisfied the ISEV requirements. With promising results from these studies (Table 1), it is unfortunate that these studies fall short of the minimum requirements of sEV separation methods.
Any study must be independently replicated and validated in a separate cohort, ideally in large multi-national, multi-centre cohort studies. For such studies to be comparable, they should follow ISEV guidance with regards to sEV isolation (Théry et al., 2018); in addition, studies in endometriosis ought to adhere to the WERF EPhect (World Endometriosis Research Foundation Endometriosis Phenome and Biobanking Harmonisation Project) protocols when collecting samples and data (Becker et al., 2014; Fassbender et al., 2014; Rahmioglu et al., 2014; Vitonis et al., 2014).
Conclusion
Endometriosis is a chronic inflammatory disease with a significant impact on women, their families, and society. The lack of clinically usable biomarkers remains one of the obstacles to shortening waiting times between the first symptoms and endometriosis diagnosis. Currently available medical therapies are mainly aimed at hormonal suppression and disregard the heterogeneity of the biological phenotype and clinical need in women suffering from pain and infertility. sEV-miRNAs could be a promising tool to better understand endometriosis pathophysiology or a potential diagnostic and therapeutic tool. Unfortunately, research in this area is hindered by a lack of standardisation in separating and characterising sEVs and adherence to the WERF EPhect protocols.
Contributor Information
Hannah M Nazri, Division of Biomedical Sciences, Warwick Medical School, University of Warwick, Coventry, UK.
Erin Greaves, Division of Biomedical Sciences, Warwick Medical School, University of Warwick, Coventry, UK.
Siobhan Quenby, Division of Biomedical Sciences, Warwick Medical School, University of Warwick, Coventry, UK.
Rebecca Dragovic, Nuffield Department of Women’s & Reproductive Health, Endometriosis CaRe Centre, University of Oxford, Oxford, UK.
Thomas T Tapmeier, Nuffield Department of Women’s & Reproductive Health, Endometriosis CaRe Centre, University of Oxford, Oxford, UK; Department of Obstetrics and Gynaecology, Monash University, Clayton, VIC, Australia; The Ritchie Centre, Hudson Institute of Medical Research, Clayton, VIC, Australia.
Christian M Becker, Nuffield Department of Women’s & Reproductive Health, Endometriosis CaRe Centre, University of Oxford, Oxford, UK.
Data availability
There are no new data associated with this article.
Authors’ roles
H.M.N. conducted the literature review, designed figures, and wrote the manuscript. E.G., S.Q., R.D., T.T.T., and C.M.B. supervised and critically revised the manuscript.
Funding
E.G. received funding from the Medical Research Council (grant number MR/W028255/1). T.T.T. received funding from Endometriosis Australia (grant 20230831) and from the Epworth Medical Foundation and the Julia Argyrou Endometriosis Centre at Epworth HealthCare (Audrey Voss Gynaecology Research Grant). C.M.B. received funding from the Medical Research Council (grant number MR/W028255/1).
Conflict of interest
None declared by all authors.
References
- Agrawal S, Tapmeier T, Rahmioglu N, Kirtley S, Zondervan K, Becker C.. The miRNA mirage: how close are we to finding a non-invasive diagnostic biomarker in endometriosis? A systematic review. Int J Mol Sci 2018;19:599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker C, Bokor A, Heikinheimo O, Horne A, Jansen F, Kiesel L, King K, Kvaskoff M, Nap A, Petersen K. et al. ; ESHRE Endometriosis Guideline Group. ESHRE guideline: endometriosis. Hum Reprod Open 2022;2022:hoac009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burney R. Fibrosis as a molecular hallmark of endometriosis pathophysiology. Fertil Steril 2022;118:203–204. [DOI] [PubMed] [Google Scholar]
- Burns K, Rodriguez K, Hewitt S, Janardhan K, Young S, Korach K.. Role of estrogen receptor signaling required for endometriosis-like lesion establishment in a Mouse Model. Endocrinology 2012;153:3960–3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canis M, Donnez J, Guzick D, Halme J, Rock J, Schenken R, Vernon M.. Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil Steril 1997;67:817–821. [DOI] [PubMed] [Google Scholar]
- Ceccaroni M, Roviglione G, Rosenberg P, Pesci A, Clarizia R, Bruni F, Zardini C, Ruffo G, Placci A, Crippa S. et al. Pericardial, pleural and diaphragmatic endometriosis in association with pelvic peritoneal and bowel endometriosis: a case report and review of the literature. Wideochir Inne Tech Maloinwazyjne 2012;7:122–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chargaff E, West R.. The biological significance of the thromboplastic protein of blood. J Biol Chem 1946;166:189–197. [PubMed] [Google Scholar]
- Chen L, Charrier A, Zhou Y, Chen R, Yu B, Agarwal K, Tsukamoto H, Lee L, Paulaitis M, Brigstock D.. Epigenetic regulation of connective tissue growth factor by MicroRNA-214 delivery in exosomes from mouse or human hepatic stellate cells. Hepatology 2014;59:1118–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Wang K, Xu Y, Guo P, Hong B, Cao Y, Wei Z, Xue R, Wang C, Jiang H.. Alteration of myeloid-derived suppressor cells, chronic inflammatory cytokines, and exosomal miRNA contribute to the peritoneal immune disorder of patients with endometriosis. Reprod Sci 2019;26:1130–1138. [DOI] [PubMed] [Google Scholar]
- Cheng L, Sharples R, Scicluna B, Hill F.. Exosomes provide a protective and enriched source of miRNA for biomarker profiling compared to intracellular and cell-free blood. J Extracell Vesicles 2014;3: 23743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornillie F, Oosterlynck D, Lauweryns J, Koninckx P.. Deeply infiltrating pelvic endometriosis: histology and clinical significance. Fertil Steril 1990;53:978–983. [DOI] [PubMed] [Google Scholar]
- De Broe ME, Wieme RJ, Logghe GN, Roels F.. Spontaneous shedding of plasma membrane fragments by human cells in vivo and in vitro. Clin Chim Acta 1977;81:237–245. [DOI] [PubMed] [Google Scholar]
- De Sanctis V, Soliman A, Daar S, Di Maio S, Elalaily R, Fiscina B, Kattamis C.. Prevalence, attitude and practice of self-medication among adolescents and the paradigm of dysmenorrhea self-care management in different countries. Acta Biomed Atenei Parmensis 2020;91:182–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkman J. Angiogenesis-dependent diseases. Semin Oncol 2001;28:536–542. [DOI] [PubMed] [Google Scholar]
- Gemmell L, Webster K, Kirtley S, Vincent K, Zondervan K, Becker C.. The management of menopause in women with a history of endometriosis: a systematic review. Hum Reprod Update 2017;23: 481–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith J, Liu Y, Tekmal R, Binkley P, Holden A, Schenken R.. Menstrual endometrial cells from women with endometriosis demonstrate increased adherence to peritoneal cells and increased expression of CD44 splice variants. Fertil Steril 2010;93: 1745–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta D, Hull M, Fraser I, Miller L, Bossuyt P, Johnson N, Nisenblat V.. Endometrial biomarkers for the non-invasive diagnosis of endometriosis. Cochrane Database Syst Rev 2016;4:CD012165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurunathan S, Kang M, Jeyaraj M, Qasim M, Kim J-H.. Review of the isolation, characterization, biological function, and multifarious therapeutic approaches of exosomes. Cells 2019;8:307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halme J, Hammond M, Hulka J, Raj S.. Talbert Retrograde menstruation in healthy women and in patients with endometriosis. Obstet Gynecol 1984;64:151–154. [PubMed] [Google Scholar]
- Harding C, Heuser J, Stahl P.. Endocytosis and intracellular processing of transferrin and colloidal gold-transferrin in rat reticulocytes: demonstration of a pathway for receptor shedding. Eur J Cell Biol 1984;35:256–263. [PubMed] [Google Scholar]
- Harp D, Driss A, Mehrabi S, Chowdhury I, Xu W, Liu D, Garcia-Barrio M, Taylor R, Gold B, Jefferson S. et al. Exosomes derived from endometriotic stromal cells have enhanced angiogenic effects in vitro. Cell Tissue Res 2016;365: 187–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino A, Costa-Silva B, Shen T, Rodrigues G, Hashimoto A, Tesic Mark M, Molina H, Kohsaka S, Di Giannatale A, Ceder S. et al. Tumour exosome integrins determine organotropic metastasis. Nature 2015;527:329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Zhu L, Li H, Ye J, Lin N, Chen M, Pan D, Chen Z.. Endometriosis derived exosomal miR-301a-3p mediates macrophage polarization via regulating PTEN-PI3K axis. Biomed Pharmacother 2022;147:112680. [DOI] [PubMed] [Google Scholar]
- Jeppesen D, Fenix A, Franklin J, Higginbotham J, Zhang Q, Zimmerman L, Liebler D, Ping J, Liu Q, Evans R. et al. Reassessment of exosome composition. Cell 2019;177:428–445.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Y, Yu L, Ma T, Xu W, Qian H, Sun Y, Shi H.. Small extracellular vesicles isolation and separation: current techniques, pending questions and clinical applications. Theranostics 2022;12:6548–6575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Chai X, Chen S, Chen Z, Tian H, Liu M, Wu X.. Exosomes from the uterine cavity mediate immune dysregulation via inhibiting the JNK signal pathway in endometriosis. Biomedicines 2022;10:3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalaj K, Miller J, Lingegowda H, Fazleabas A, Young S, Lessey B, Koti M, Tayade C.. Extracellular vesicles from endometriosis patients are characterized by a unique miRNA-lncRNA signature. JCI Insight 2019;4:e128846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudtson J, McLaughlin J, Sultana M, Santos M, Sureshkumar M, Tekmal R, Schenken R.. CD44 variant 6 is involved in the attachment and invasion of endometrial cells to peritoneum’. F S Sci 2020;1:188–194. [DOI] [PubMed] [Google Scholar]
- Koks C, Demir Weusten A, Groothuis P, Dunselman G, de Goeij A, Evers J.. Menstruum induces changes in mesothelial cell morphology. Gynecol Obstet Invest 2000;50:13–18. [DOI] [PubMed] [Google Scholar]
- Koninckx PR, Kennedy SH, Barlow DH.. Endometriotic disease: the role of peritoneal fluid. Hum Reprod Update 1998;4:741–751. [DOI] [PubMed] [Google Scholar]
- Kretschmer A, Tutrone R, Alter J, Berg E, Fischer C, Kumar S, Torkler P, Tadigotla V, Donovan M, Sant G. et al. Pre-diagnosis urine exosomal RNA (ExoDx EPI score) is associated with post-prostatectomy pathology outcome. World J Urol 2022;40: 983–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Yang Y, Xiong A, Wu X, Xie J, Han S, Zhao S.. Comparative gene expression analysis of lymphocytes treated with exosomes derived from ovarian cancer and ovarian cysts. Front Immunol 2017;8:607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Bayado N, He D, Li J, Chen H, Li L, Li J, Long X, Du T, Tang J. et al. Therapeutic applications of extracellular vesicles for myocardial repair. Front Cardiovasc Med 2021;8:758050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu E, Nisenblat V, Farquhar C, Fraser I, Bossuyt P, Johnson N, Hull M.. Urinary biomarkers for the non‐invasive diagnosis of endometriosis. Cochrane Database Syst Rev 2015;2015:CD012019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarova J, Shkurnikov M, Wicklein D, Lange T, Samatov T, Turchinovich A, Tonevitsky A.. Intracellular and extracellular microRNA: an update on localization and biological role. Prog Histochem Cytochem 2016;51:33–49. [DOI] [PubMed] [Google Scholar]
- May K, Becker C.. Endometriosis and angiogenesis. Minerva Ginecol 2008;60: 245–254. [PubMed] [Google Scholar]
- May K, Conduit-Hulbert S, Villar J, Kirtley S, Kennedy S, Becker C.. Peripheral biomarkers of endometriosis: a systematic review. Hum Reprod Update 2010;16:651–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murugesan S, Hussey H, Saravanakumar L, Sinkey R, Sturdivant A, Powell M, Berkowitz D.. Extracellular vesicles from women with severe preeclampsia impair vascular endothelial function. Anesth Analg 2022;134:713–723. [DOI] [PubMed] [Google Scholar]
- Nair A, Nair H, Lucidi R, Kirchner A, Schenken R, Tekmal R, Witz C.. Modeling the early endometriotic lesion: mesothelium-endometrial cell co-culture increases endometrial invasion and alters mesothelial and endometrial gene transcription. Fertil Steril 2008;90:1487–1495. [DOI] [PubMed] [Google Scholar]
- Navarro A, Bariani M, Park H, Zota A, Al-Hendy A.. Report of exosomes isolated from a human uterine leiomyoma cell line and their impact on endometrial vascular endothelial cells’. Pharmaceuticals 2022;15:577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazri H, Imran M, Fischer R, Heilig R, Manek S, Dragovic R, Kessler B, Zondervan K, Tapmeier T, Becker C.. Characterization of exosomes in peritoneal fluid of endometriosis patients’. Fertil Steril 2020;113:364–373.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisenblat V, Bossuyt P, Farquhar C, Johnson N, Hull M.. Imaging modalities for the non-invasive diagnosis of endometriosis. Cochrane Database Syst Rev 2016a;2:CD009591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisenblat V, Bossuyt P, Shaikh R, Farquhar C, Jordan V, Scheffers C, Mol B, Johnson N, Hull M.. Blood biomarkers for the non-invasive diagnosis of endometriosis. Cochrane Database Syst Rev 2016b;2016: CD012179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisenblat V, Prentice L, Bossuyt P, Farquhar C, Hull M, Johnson N.. Combination of the non-invasive tests for the diagnosis of endometriosis’. Cochrane Database Syst Rev 2016c;7:CD012281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nnoaham K, Hummelshoj L, Webster P, D’Hooghe T, de Cicco Nardone F, de Cicco Nardone C, Jenkinson C, Kennedy S, Zondervan K; World Endometriosis Research Foundation Global Study of Women’s Health Consortium. Reprint of: impact of endometriosis on quality of life and work productivity: a multicenter study across ten countries. Fertil Steril 2019;112: 366–373.e8. [DOI] [PubMed] [Google Scholar]
- Noren Hooten N, Evans M.. Extracellular vesicles as signaling mediators in type 2 diabetes mellitus. Am J Physiol Cell Physiol 2020;318:C1189–C1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien J, Hayder H, Zayed Y, Peng C.. Overview of microRNA biogenesis, mechanisms of actions, and circulation. Front Endocrinol (Lausanne) 2018;9:402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien K, Breyne K, Ughetto S, Laurent LC, Breakefield XO.. RNA delivery by extracellular vesicles in mammalian cells and its applications. Nat Rev Mol Cell Biol 2020;21:585–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paget S. The distribution of secondary growths in cancer of the breast. Cancer Metastasis Rev 1889;8:98–101. [PubMed] [Google Scholar]
- Painter JN, Anderson CA, Nyholt DR, Macgregor S, Lin J, Lee SH, Lambert A, Zhao ZZ, Roseman F, Guo Q. et al. Genome-wide association study identifies a locus at 7p15.2 associated with endometriosis. Nat Genet 2011;43:51–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan B, Teng K, Wu C, Adam M, Johnstone R.. Electron microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes. J Cell Biol 1985;101:942–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope C, Sharma V, Sharma S, Mazmanian D.. A Systematic review of the association between psychiatric disturbances and endometriosis. J Obstet Gynaecol Can 2015;37:1006–1015. [DOI] [PubMed] [Google Scholar]
- Prescott J, Farland L, Tobias D, Gaskins A, Spiegelman D, Chavarro J, Rich-Edwards J, Barbieri R, Missmer S.. A prospective cohort study of endometriosis and subsequent risk of infertility. Hum Reprod 2016;31:1475–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramin-Wright A, Kohl Schwartz A, Geraedts K, Rauchfuss M, Wölfler M, Haeberlin F, von Orelli S, Eberhard M, Imthurn B, Imesch P. et al. Fatigue – a symptom in endometriosis’. Hum Reprod 2018;33:1459–1465. [DOI] [PubMed] [Google Scholar]
- Raposo G, Nijman H, Stoorvogel W, Liejendekker R, Harding C, Melief C, Geuze H.. B lymphocytes secrete antigen-presenting vesicles. J Exp Med 1996;183:1161–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raposo G, Stoorvogel W.. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol 2013;200:373–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson J. Peritoneal endometriosis due to the menstrual dissemination of endometrial tissue into the peritoneal cavity. Am J Obstet Gynecol 1927;14:422–469. [Google Scholar]
- Samulowitz A, Gremyr I, Eriksson E, Hensing G.. “Brave Men” and “Emotional Women”: a theory-guided literature review on gender bias in health care and gendered norms towards patients with chronic pain. Pain Res Manag 2018;2018:6358624–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapkota Y, Steinthorsdottir V, Morris A, Fassbender A, Rahmioglu N, De Vivo I, Buring J, Zhang F, Edwards T, Jones S. et al. ; iPSYCH-SSI-Broad Group. Meta-analysis identifies five novel loci associated with endometriosis highlighting key genes involved in hormone metabolism’. Nat Commun 2017;8:15539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah D, Moravek M, Vahratian A, Dalton V, Lebovic D.. Public perceptions of endometriosis: perspectives from both genders. Acta Obstet Gynecol Scand 2010;89:646–650. [DOI] [PubMed] [Google Scholar]
- Simoens S, Dunselman G, Dirksen C, Hummelshoj L, Bokor A, Brandes I, Brodszky V, Canis M, Colombo G, DeLeire T. et al. The burden of endometriosis: costs and quality of life of women with endometriosis and treated in referral centres. Hum Reprod 2012;27: 1292–1299. [DOI] [PubMed] [Google Scholar]
- Sun H, Li D, Yuan M, Li Q, Li N, Wang G.. Eutopic stromal cells of endometriosis promote neuroangiogenesis via exosome pathway. Biol Reprod 2019a;100:649–659. [DOI] [PubMed] [Google Scholar]
- Sun H, Li D, Yuan M, Li Q, Zhen Q, Li N, Wang G.. Macrophages alternatively activated by endometriosis-exosomes contribute to the development of lesions in mice. Mol Hum Reprod 2019b;25:5–16. [DOI] [PubMed] [Google Scholar]
- Tapmeier T, Nazri H, Subramaniam K, Manek S, Garbutt K, Flint E, Cheuk C, Hubbard C, Barrett K, Shepherd E. et al. Protocol for a longitudinal, prospective cohort study investigating the biology of uterine fibroids and endometriosis, and patients’ quality of life: the FENOX study. BMJ Open 2020;10:e032220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Théry C, Witwer K, Aikawa E, Alcaraz M, Anderson J, Andriantsitohaina R, Antoniou A, Arab T, Archer F, Atkin-Smith G. et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles 2018;7:1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trams EG, Lauter CJ, Salem N, Heine U.. Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochim Biophys Acta 1981;645:63–70. [DOI] [PubMed] [Google Scholar]
- Uimari O, Nazri H, Tapmeier T.. Endometriosis and uterine fibroids (leiomyomata): comorbidity, risks and implications. Front Reprod Health 2021;3:750018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uimari O, Rahmioglu N, Nyholt D, Vincent K, Missmer S, Becker C, Morris A, Montgomery G, Zondervan K.. Genome-wide genetic analyses highlight mitogen-activated protein kinase (MAPK) signaling in the pathogenesis of endometriosis. Hum Reprod 2017;32:780–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Niel G, Carter D, Clayton A, Lambert D, Raposo G, Vader P.. Challenges and directions in studying cell–cell communication by extracellular vesicles. Nat Rev Mol Cell Biol 2022;23:369–382. [DOI] [PubMed] [Google Scholar]
- van Niel G, Porto-Carreiro I, Simoes S, Raposo G.. Exosomes: a common pathway for a specialized function. J Biochem 2006;140: 13–21. [DOI] [PubMed] [Google Scholar]
- Vandendriessche C, Bruggeman A, Van Cauwenberghe C, Vandenbroucke R.. Extracellular vesicles in Alzheimer’s and Parkinson’s disease: small entities with large consequences. Cells 2020;9:2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasudevan S. Posttranscriptional upregulation by microRNAs. Wiley Interdiscip Rev RNA 2012;3:311–330. [DOI] [PubMed] [Google Scholar]
- Wang J, Strauss D, Messiou C, Thway K.. Endometriosis of extra-abdominal soft tissues. Int J Surg Pathol 2016;24:497–503. [DOI] [PubMed] [Google Scholar]
- Wolf P. The nature and significance of platelet products in human plasma. Br J Haematol 1967;13:269–288. [DOI] [PubMed] [Google Scholar]
- Wu D, Lu P, Mi X, Miao J.. Exosomal miR-214 from endometrial stromal cells inhibits endometriosis fibrosis. Mol Hum Reprod 2018;24:357–365. [DOI] [PubMed] [Google Scholar]
- Wu J, Fang X, Huang H, Huang W, Wang L, Xia X.. Construction and topological analysis of an endometriosis-related exosomal circRNA-miRNA-mRNA regulatory network. Aging (Albany NY) 2021;13:12607–12630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Yuan W, Ding H, Wu X.. Serum exosomal miRNA from endometriosis patients correlates with disease severity. Arch Gynecol Obstet 2022;305:117–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu R, Greening D, Zhu H, Takahashi N, Simpson R.. Extracellular vesicle isolation and characterization: toward clinical application. J Clin Invest 2016;126:1152–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Li H, Yuan M, Li D, Sun C, Wang G.. Serum exosomal microRNAs as potential circulating biomarkers for endometriosis. Dis Markers 2020a;2020:2456340–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Li H, Yuan M, Li D, Wang G.. Exosomal miR-22-3p derived from peritoneal macrophages enhances proliferation, migration, and invasion of ectopic endometrial stromal cells through regulation of the SIRT1/NF-κB signaling pathway. Eur Rev Med Pharmacol Sci 2020b;24:571–580. [DOI] [PubMed] [Google Scholar]
- Zhang M, Wang X, Xia X, Fang X, Zhang T, Huang F.. Endometrial epithelial cells-derived exosomes deliver microRNA-30c to block the BCL9/Wnt/CD44 signaling and inhibit cell invasion and migration in ovarian endometriosis. Cell Death Discov 2022;8:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Chang X, Wu D, Deng M, Miao J, Jin Z.. Down-regulation of exosomal miR-214-3p targeting CCN2 contributes to endometriosis fibrosis and the role of exosomes in the horizontal transfer of miR-214-3p. Reprod Sci 2021;28:715–727. [DOI] [PubMed] [Google Scholar]
- Zheng L, Sun D, Tong Y.. Exosomal miR-202 derived from leukorrhea as a potential biomarker for endometriosis. J Int Med Res 2023;51:3000605221147183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Lian Y, Jiang J, Wang L, Ren L, Li Y, Yan X, Chen Q.. Differential expression of microRNA in exosomes derived from endometrial stromal cells of women with endometriosis-associated infertility. Reprod Biomed Online 2020;41:170–181. [DOI] [PubMed] [Google Scholar]
- Zöller M. Tetraspanins: push and pull in suppressing and promoting metastasis. Nat Rev Cancer 2009;9:40–55. [DOI] [PubMed] [Google Scholar]
- Zomer A, Maynard C, Verweij FJ, Kamermans A, Schäfer R, Beerling E, Schiffelers RM, de Wit E, Berenguer J, Ellenbroek SIJ. et al. In vivo imaging reveals extracellular vesicle-mediated phenocopying of metastatic behavior. Cell 2015;161:1046–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zondervan K, Becker C, Missmer S.. Endometriosis. N Engl J Med 2020;382: 1244–1256. [DOI] [PubMed] [Google Scholar]
- Zondervan KT, Becker CM, Koga K, Missmer SA, Taylor RN, Viganò P.. Endometriosis. Nat Rev Dis Primers 2018;4:9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
There are no new data associated with this article.