Constitutional protein S (PS) deficiencies are strongly associated with venous thromboembolism (VTE). PS deficiency prevalence is about 2% in patients with VTE compared with 0.03% to 0.1% in general population [1]. Several guidelines for first-line diagnostic assay are currently used. The Groupe Français d’études sur l’Hémostase et la Thrombose recommends to first measure PS activity (PSa) when screening for PS deficiency [2], whereas the International Society on Thrombosis and Haemostasis and the British Society of Haematology guidelines recommend to measure plasma free PS (FPS) then PSa only in case of decreased FPS or discordant symptoms [3,4]. PSa is evaluated through the clotting time of a mixture of platelet poor plasma (PPP), PS-deficient plasma, and activated protein C. Overestimation of PSa is frequent in case of lupus anticoagulant, high-dose heparin, and direct oral anticoagulants. On the contrary, underestimation of PSa is caused by high factor (F) VIII (FVIII) levels and activated protein C resistance (aPCR), mainly due to factor V Leiden (FVL) [2]. Indeed, FVL is responsible for a change in the cleavage site that allows activated protein C (PC) to inactivate the activated factor V (FVa). Although it is hypothesized that aPCR interference can be reduced by adding bovine Va, such as in Staclot Protein S (Diagnostica Stago), previous studies have shown in limited patient cohorts (from 11 to 24 patients) that this interference remained [[5], [6], [7]]. Here, we report a retrospective study done on a larger cohort to determine whether FVL caused interferences for PSa measurements when done in presence of bovine Va.
In this retrospective study, approved by the institutional Ethics Board (CE-2022-81), adult patients included were either outpatients or inpatients at the University Hospital of Strasbourg (Alsace, France) from December 2009 to December 2020. Sex (according to patient’s medical record), age, and laboratory results (PSa, prothrombin time [PT], and FVL status) were retrospectively consolidated. The inclusion criteria were as follows: patients over 18 years old for whom a FVL test and a measurement of PSa have been performed. In addition, only patients for whom a PT was performed on the same sample as PSa were included. Exclusion criteria were incomplete laboratory data, abnormal PT (to limit bias due to anticoagulation, especially vitamin K antagonists) and underaged patients. Patients were then assigned either to FVL+ group or to FVL- group according to their FVL status. Then, to limit bias due to the influence of sex and age on PSa, patients within each FVL group were segregated into 3 subgroups according to their sex and age: adult men aged >18 years, women aged between 18 and 50 years, and women >50 years. Patient blood specimens were drawn into BD Vacutainer glass citrated tubes with 0.129 M of trisodium citrate (Becton Dickinson). PPP were then prepared by double-spin centrifugation at 2,500 g for 10 minutes at 20 °C. PPP aliquots were stored in polypropylene tubes at ≤ −20 °C. On the same sample, PSa was measured with Staclot Protein S containing bovine FVa (Diagnostica Stago) and PT was measured with Neoplastine CI or NeoPTimal reagents (Diagnostica Stago), on a STA-R Evolution or a STA-R Max analyzer (Diagnostica Stago) [8]. The FVL status was used as the substitution control and was determined with real-time PCR FVL kit (Roche) on a Roche Diagnostics LightCycler (Roche). Mean age (years), PSa and PT were compared according to FVL status using a parametric t-test with an alpha risk set at 5%. Association between sex and FVL status was tested by chi-squared test with Yates’s correction test or Fisher’s exact test for smaller group and alpha risk set to 5%. A P value ≤ .05 was considered statistically significant. Analyses were performed with Prism version 6.05 (GraphPad Software).
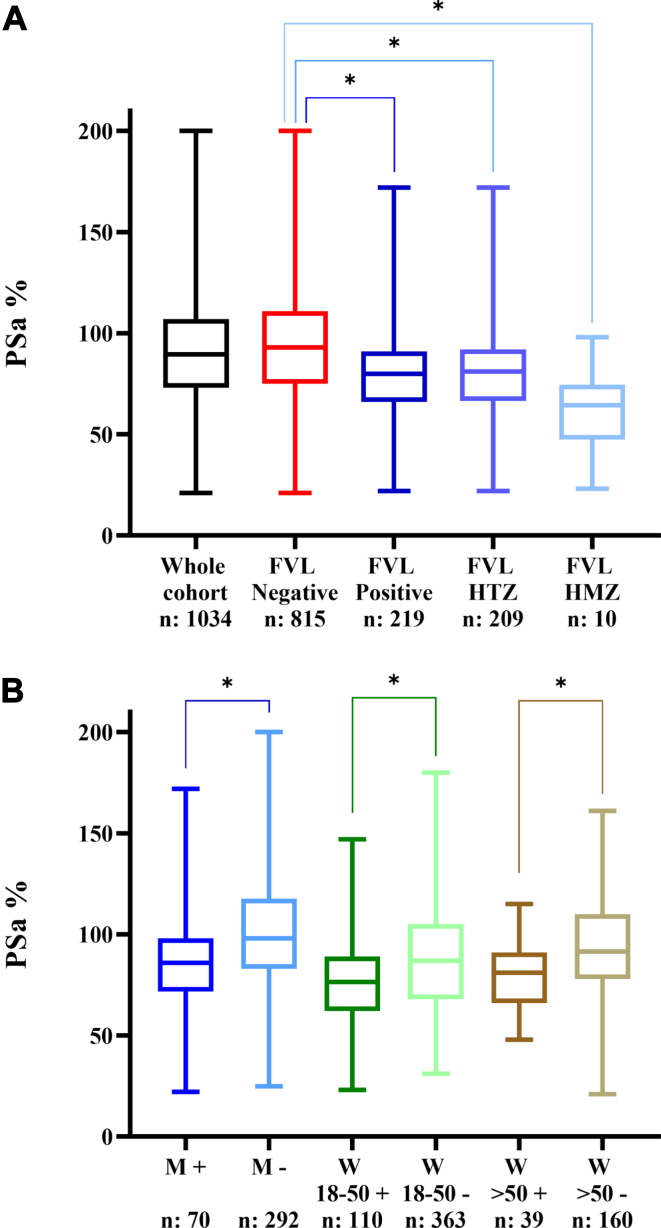
One thousand and thirty-four patients were included in this study, with 362 (35%) men and 672 (65%) women and a mean age of 45.4 years (95% CI: [44.5; 46.4]). The mean PT value was 93.6% (95% CI: [93.1; 94.1]) and the mean PSa was 90.6% (95% CI: [89.1; 92.2]). PSa was significantly lower (P < .001) within the FVL positive group (mean: 79.8%; 95% CI: [77.0; 82.5]) compared to the FVL negative group (mean: 93.6%; 95% CI: [91.8; 95.4]). Age and sex had no significant impact on aPCR status. Mean PSa were significantly different (P < .001) between homozygous FVL patients (61.60%), heterozygous patients (80.64%), and FVL negative patients (93.56%) (Figure A). No significant difference was found when age, sex, and mean PT were considered. Three subgroups were sorted out, consisting in 362 men aged ≥18 years, 473 women aged between 18 and 50 years, and 199 women aged >50 years. Within each subgroup, no significant difference was observed as to mean PT or mean age between FVL+ and FVL− patients. Mean PSa was still significantly different between FVL− and FVL+ patients (P < .001) (Figure B).
Figure.
(A) Variation in protein S activity according to the whole cohort and factor V Leiden mutation. FVL, factor V Leiden; HMZ, homozygous; HTZ, heterozygous. ∗: P value < .001. (B) Protein S activity value according to sex and age. M+ = Male aged >18 years with a positive FVL status, M− = male aged >18 years with a negative FVL status, W 18-50 + = women aged between 18 and 50 years with a positive FVL status, W 18-50 − = women aged between 18 and 50 years with a negative FVL status. W >50 + = women aged >50 years with a positive FVL status, W >50 − = women aged >50 years with a negative FVL status. ∗: P value < .001. N represents the sample size.
PS investigation is essential for optimal management of thrombophilia workups as its deficiency is a risk factor for VTE. First-line laboratory assays are generally either FPS or PSa measurements. The latter are known to be sensitive to aPCR and thus to FVL mutations [7]. Therefore, it was suggested to add bovine FVa to overcome this interference. In this retrospective study on 1,034 patients, we highlighted that FVL interfered on PSa assay, despite bovine Va addition. Indeed, there was a significant difference in PSa measurement regarding the FVL status. This interference had already been described, but only within small patient cohorts (< 25 patients) [9]. Moreover, 2 of these studies determined aPCR status with a functional coagulation assay, which is known to be less specific than molecular biology. Therefore, our study is the first to demonstrate the interference on a statistically adequate cohort including >1000 patients with a highly specific method regarding FVL status. The risk of PS deficiency misdiagnosis should be taken in account since FVL prevalence is about 3% to 5% in Caucasian population and even reaches 9% to 10% in some regions such as Alsace (France), Sweden, or Cyprus [10]. Few alternatives to limit this interference have been suggested. For instance, it is possible to dilute patient plasmas to reduce FVL impact on PSa measurement, this improves assay specificity [9]. FPS measurement is also an alternative since it is not influenced by FVL. However, FPS cannot detect type II PS deficiency. A reliable assay is still, therefore, mandatory. Limitations of this study are its retrospective nature and a possible bias due to anticoagulation (especially direct oral anticoagulants and vitamin K antagonists) since these data were not collected. Nevertheless, this bias seems limited as patients with abnormal PT were excluded, a large number of patients were included, and there was no statistical difference as to sex, age, or PT between the FVL+ and FVL− groups. To finish, due to ethical constraints, the impact of race or ethnicity of participants on interference could not be assessed. In conclusion, this study provided strong argument to prove the persistence of FVL interference during PSa exploration despite bovine Va addition within reagents. Pathologists and clinicians need to be aware of this FVL interference, to avoid PS deficiency misdiagnosis.
Acknowledgments
Funding
Strasbourg University Hospital supported this study. This sponsor had no role on the design, methods, subject recruitment, data collections, analysis, or preparation of this report.
Author contributions
A.H. and B.P. designed the study, collected, and analyzed data. B.P. wrote the manuscript. D.R., J.W., L.M., and L.S. revised intellectual content.
Relationship disclosure
There are no competing interests to disclose.
Footnotes
Handling Editor: Dr Pantep Angchaisuksiri
References
- 1.Aiach M., Alhenc-Gelas M., Borgel D., Emmerich J., Gandrille S., Picard V. Mutations des protéines de la coagulation et thromboses [Coagulation factor mutations and thrombosis] Med Sci (Paris) 2006;22:985–989. doi: 10.1051/medsci/20062211985. [DOI] [PubMed] [Google Scholar]
- 2.Gruel Y., Morange P., Alhenc-Gelas M., Gouin-Thibault I., De Maistre E., De Raucourt E., et al. Thrombophilia testing: proposals of the 2020 GFHT. Rev Francoph Hémost Thromb. 2020;2:93–126. [Google Scholar]
- 3.Marlar R.A., Gausman J.N., Tsuda H., Rollins-Raval M.A., Brinkman H.J.M. Recommendations for clinical laboratory testing for protein S deficiency: communication from the SSC committee plasma coagulation inhibitors of the ISTH. J Thromb Haemost. 2021;19:68–74. doi: 10.1111/jth.15109. [DOI] [PubMed] [Google Scholar]
- 4.Baker P., Platton S., Gibson C., Gray E., Jennings I., Murphy P., et al. Guidelines on the laboratory aspects of assays used in haemostasis and thrombosis. Br J Haematol. 2020;191:347–362. doi: 10.1111/bjh.16776. [DOI] [PubMed] [Google Scholar]
- 5.D’Angelo S.V., Mazzola G., Della Valle P., Testa S., Pattarini E., D’Angelo A. Variable interference of activated protein C resistance in the measurement of protein S activity by commercial assays. Thromb Res. 1995;77:375–378. doi: 10.1016/0049-3848(95)93841-m. [DOI] [PubMed] [Google Scholar]
- 6.Faioni E.M., Boyer-Neumann C., Franchi F., Wolf M., Meyer D., Mannucci P.M. Another protein S functional assay is sensitive to resistance to activated protein C. Thromb Haemost. 1994;72:648. [PubMed] [Google Scholar]
- 7.Jennings I., Kitchen S., Cooper P., Makris M., Preston F.E. Sensitivity of functional protein S assays to protein S deficiency: a comparative study of three commercial kits. J Thromb Haemost. 2003;1:1112–1114. doi: 10.1046/j.1538-7836.2003.00215.x. [DOI] [PubMed] [Google Scholar]
- 8.Valadier J., Touitou E., Guis F., Hamri L., Izmirlian E., Attard S., et al. Evaluating the performance of a new thromboplastin (Stago) with an ISI =1: STA-NeoPTimal (Stago) Spectra Biologie. 2018;237:66–71. [Google Scholar]
- 9.Tripodi A., Asti D., Chantarangkul V., Biguzzi E., Mannucci P.M. Interference of factor V Leiden on protein S activity: evaluation of a new prothrombin time-based assay. Blood Coagul Fibrinolysis. 2007;18:543–546. doi: 10.1097/MBC.0b013e328201ca8a. [DOI] [PubMed] [Google Scholar]
- 10.Stephan D. Mutation du facteur V: Europe, Suède, Alsace. J Médecine Vasc. 2019;44:124. [Google Scholar]