Abstract
In this issue of Cell Reports Methods, Sadi et al. present a nuclear magnetic resonance approach for quantitative assessment of protein interactions with lipid membranes. It is sensitive, applicable to diverse membrane systems, covers a broad range of KDs, and does not require large amounts of material.
In this issue of Cell Reports Methods, Sadi et al. present a nuclear magnetic resonance approach for quantitative assessment of protein interactions with lipid membranes. It is sensitive, applicable to diverse membrane systems, covers a broad range of KDs, and does not require large amounts of material.
Main text
The interactions of proteins with cell membranes have many important physiological consequences for cell function. So-called amphitrophic or peripheral membrane proteins normally exist in solution but are capable of interacting with lipid membranes.1,2 One category of interest pertains to pore-forming proteins that can permeabilize lipid membranes by forming transmembrane pores using a very exquisite mechanism.2,3 This includes binding to a lipid membrane, oligomerization at the membrane plane, and conformational changes to enable pore formation. Quantifying the interactions of such proteins with lipid membranes is important for understanding their molecular mechanisms of action as well as for developing approaches to manipulate their activity, since many of them play a role in disease or can be used in biomedical applications, such as drug delivery or sensing by using nanopores.
Various approaches have been developed to measure molecular interactions that take advantage of different physical phenomena. For example, the fluorescence of a protein based on the aromatic amino acid tryptophan can be used as a probe for monitoring membrane binding. When the environment of these intrinsic probes changes upon binding to the membrane, this becomes visible as a strong change in the emitted light and can be easily measured.4 Extrinsic probes have also been added to a protein, which has the advantage of them being able to be very sensitive to any change in their environment.5 Some other approaches also require protein labeling, for example, microscale thermophoresis.6 Surface plasmon resonance7 or quartz crystal microbalance8 can report binding in real time but require immobilization of one of the binding partners, which can lead to artifacts due to steric hindrance or failure to expose a binding site. Isothermal microcalorimetry, on the other hand, provides a label-free assessment of the thermodynamic parameters of binding.9 However, it requires high concentrations of some binding partners, which is sometimes difficult to achieve. Thus, each of the above and other approaches that are used to quantify protein-membrane interactions has its limitations, and new ways to measure molecular interactions with lipid membranes are desirable.
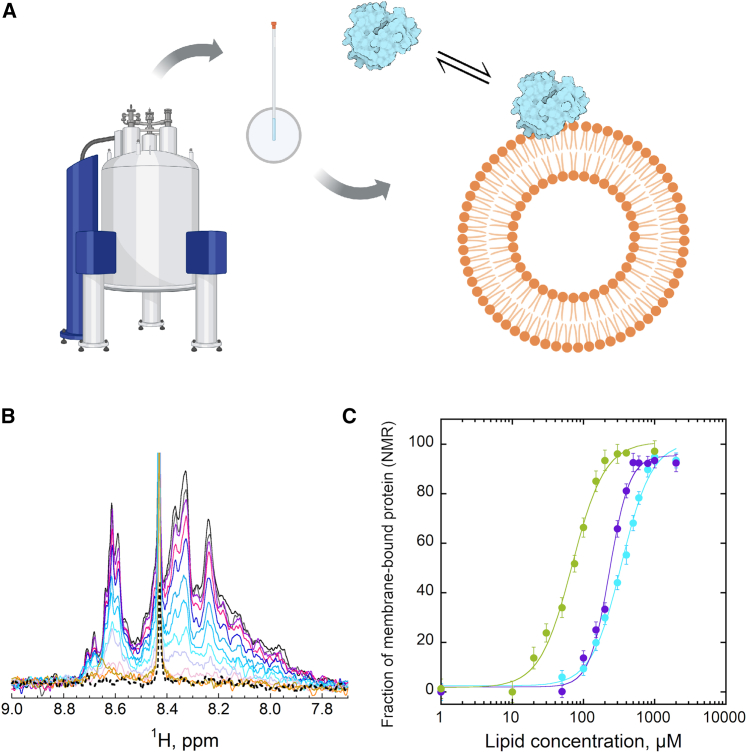
In their manuscript in this issue of Cell Reports Methods, Sadi and colleagues10 developed an alternative method, binding to lipid vesicles (B2LiVe), for determination of partitioning of amphitrophic proteins to lipid membranes. The nuclear magnetic resonance (NMR)-based approach involves selective excitation of amide resonances of proteins, which avoids interference from other components in the experimental system. B2LiVe does not require labeling because it relies only on the amide hydrogen nuclei of protein backbones. The method uses NMR spectrometry to measure the amide proton NMR spectra of proteins in solution and bound to lipid vesicles. By carefully choosing the experimental conditions, the NMR signal of the protein decreases upon membrane partitioning due to the slow tumbling rates of the liposomal model membrane systems (Figure 1). Therefore, the decrease in NMR signal provides direct information about the fraction of protein bound. When the decrease in NMR signal intensity (or fraction of a bound protein) was plotted as a function of lipid concentration, affinity constants could be derived and compared with other approaches.
Figure 1.
B2LiVe approach for quantifying interactions of proteins with lipid membranes
(A) Nuclear magnetic resonance (NMR) spectroscopy is used in B2LiVe to monitor interactions of proteins with model lipid membranes, such as liposomes. Approximately 200 μL of protein solution at micromolar concentrations is placed in an NMR tube, and small volumes of liposomes solution is sequentially added.
(B) The intensity of 1D NMR signal decreases when liposomes are added and protein binds to lipid membranes. Here, data for apo-myoglobin are shown from Sadi et al.10
(C) To determine affinity constants, the fraction of membrane-bound proteins, derived from data in (B), were plotted against lipid concentration. The binding curves are shown for apo-Mb (cyan), translocation domain of diptheria toxin (violet), and pore-forming toxin anthrolysin O (green).10
(A) was created with BioRender (https://biorender.com/).
The authors validate their method using two common approaches for measuring protein binding to lipid membranes, fluorescence, and circular dichroism spectroscopy. They also use several different proteins including apo-myoglobin, the translocation domain of diptheria toxin, and anthrolysin O, a pore-forming toxin from bacterium Bacillus anthracis, in combination with lipid vesicles varying in size and having unilamellar or multilamellar composition. Several control proteins that do not interact with lipid membranes were used to show that the lack of membrane binding did not affect the NMR signal (or tryptophan fluorescence) at similar concentrations. They extend the characterization of the B2LiVe by using peptides derived from the adenylate cyclase toxin of Bordetella pertussis. The peptide that binds to membranes (P233) showed a loss of NMR signal, while the control peptide (P414) did not, providing further evidence that the NMR approach is sensitive enough to monitor membrane partitioning of peptides that are much smaller than some of the proteins used in the study. The membrane partitioning of P233 was also validated by tryptophan fluorescence and far-UV circular dichroism spectroscopy, and both approaches provided similar quantitative parameters for the membrane partitioning process. Finally, by using carefully selected measurement parameters, the authors showed that it was possible to determine the presence of polypeptide segments of the membrane-bound protein that are not associated with the lipid membrane but remain in the aqueous phase.
Some limitations of the approach were identified by authors, and they suggest how the method can be modified to alleviate them. For example, the aggregation of proteins or peptides presents a significant hurdle for many of the commonly used approaches and possibly also for B2LiVe. However, in B2LiVe, as shown in the study, low concentrations of proteins and peptides can be used, which may limit the formation of aggregates.
Overall, the B2LiVe approach provides a label-free alternative to other biophysical methods for characterizing molecular interactions with lipid membranes. The method requires relatively small amounts of proteins (the experiments in this manuscript were performed with micromolar concentrations of peptides and proteins) and is independent of the size of the model membrane vesicles. The liposomal model membrane systems can range from small (tens of nanometers in diameter) to large (several hundred nanometers in diameter) lipid vesicles. It is important to note that the approach is not compromised by the high concentration of lipids in the system. This is a particular problem with optical-based approaches, such as fluorescence spectroscopy, due to the strong light scattering of the vesicles. The current work also convincingly shows that the range of KDs that can be addressed can range from sub-micromolar to millimolar values, depending on a particular system. This is comparable to other approaches and covers the range of many interactions in biological systems.
B2LiVe will most likely prove to be a facile and convenient method for quantifying interactions of peptides and proteins with lipid membranes in integrative structural biology approaches and may be particularly useful for some classes of proteins, including intrinsically disordered proteins, that are difficult to study with other approaches.
Acknowledgments
The work in the author laboratory is supported by the program grant of the Slovenian Research and Innovation Agency P1-0391 (Molecular Interactions).
Declaration of interests
The author declares no competing interests.
References
- 1.Halskau Ø., Muga A., Martínez A. Linking New Paradigms in Protein Chemistry to Reversible Membrane-Protein Interactions. Curr. Protein Pept. Sci. 2009;10:339–359. doi: 10.2174/138920309788922199. [DOI] [PubMed] [Google Scholar]
- 2.Anderluh G., Lakey J.H. Disparate proteins use similar architectures to damage membranes. Trends Biochem. Sci. 2008;33:482–490. doi: 10.1016/j.tibs.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Dal Peraro M., van der Goot F.G. Pore-forming toxins: ancient, but never really out of fashion. Nat. Rev. Microbiol. 2016;14:77–92. doi: 10.1038/nrmicro.2015.3. [DOI] [PubMed] [Google Scholar]
- 4.Hong Q., Gutierrez-Aguirre I., Barlič A., Malovrh P., Kristan K., Podlesek Z., Maček P., Turk D., Gonzalez-Manas J.M., Lakey J.H., Anderluh G. Two-step membrane binding by Equinatoxin II, a pore-forming toxin from the sea anemone, involves an exposed aromatic cluster and a flexible helix. J. Biol. Chem. 2002;277:41916–41924. doi: 10.1074/jbc.M204625200. [DOI] [PubMed] [Google Scholar]
- 5.Malovrh P., Viero G., Serra M.D., Podlesek Z., Lakey J.H., Maček P., Menestrina G., Anderluh G. A novel mechanism of pore formation: membrane penetration by the N-terminal amphipathic region of equinatoxin. J. Biol. Chem. 2003;278:22678–22685. doi: 10.1074/jbc.M300622200. [DOI] [PubMed] [Google Scholar]
- 6.Ameziane-Le Hir S., Paboeuf G., Tascon C., Hubert J.-F., Le Rumeur E., Vié V., Raguénès-Nicol C. Dystrophin Hot-Spot Mutants Leading to Becker Muscular Dystrophy Insert More Deeply into Membrane Models than the Native Protein. Biochemistry. 2016;55:4018–4026. doi: 10.1021/acs.biochem.6b00290. [DOI] [PubMed] [Google Scholar]
- 7.Beseničar M., Maček P., Lakey J.H., Anderluh G. Surface plasmon resonance in protein-membrane interactions. Chem. Phys. Lipids. 2006;141:169–178. doi: 10.1016/j.chemphyslip.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 8.Makino A., Abe M., Ishitsuka R., Murate M., Kishimoto T., Sakai S., Hullin-Matsuda F., Shimada Y., Inaba T., Miyatake H., et al. A novel sphingomyelin/cholesterol domain-specific probe reveals the dynamics of the membrane domains during virus release and in Niemann-Pick type C. FASEB J. 2017;31:1301–1322. doi: 10.1096/fj.201500075R. [DOI] [PubMed] [Google Scholar]
- 9.Alegre-Cebollada J., Cunietti M., Herrero-Galán E., Gavilanes J.G., Martínez-del-Pozo A. Calorimetric scrutiny of lipid binding by sticholysin II toxin mutants. J. Mol. Biol. 2008;382:920–930. doi: 10.1016/j.jmb.2008.07.053. [DOI] [PubMed] [Google Scholar]
- 10.Sadi M., Carvalho N., Léger C., Vitorge B., Ladant D., Guijarro J.I., Chenal A. B2LiVe, a label-free 1D-NMR method to quantify the binding of amphitropic peptides or proteins to membrane vesicles. Cell Reports Methods. 2023;3 doi: 10.1016/j.crmeth.2023.100624. [DOI] [PMC free article] [PubMed] [Google Scholar]