Summary
To better implement mesenchymal stem cell (MSC)-based therapy toward cartilage diseases, a more efficient and less off-target chondrogenesis protocol is needed. Here, we present a protocol to induce human MSC chondrogenesis via Wnt antagonism. We describe steps for pellet formation, Wnt antagonism-based chondrogenic induction, and refreshing the differentiation medium. We detail procedures for characterizing MSC chondrogenesis. By using Wnt antagonism instead of conventional transforming growth factor β-based induction, this protocol avoids the potential for induction of chondrocyte hypertrophy/osteogenesis or other lineages.
For complete details on the use and execution of this protocol, please refer to Hsieh et al. (2023).1
Subject areas: Cell culture, Stem Cells, Cell Differentiation, Biotechnology and bioengineering
Graphical abstract

Highlights
-
•
Protocol for human MSC chondrogenesis based on developmental processes
-
•
Small-molecule-based induction instead of protein factor
-
•
Less non-chondrogenic lineage commitment
-
•
A method applicable for MSCs from various sources
Publisher’s note: Undertaking any experimental protocol requires adherence to local institutional guidelines for laboratory safety and ethics.
To better implement mesenchymal stem cell (MSC)-based therapy toward cartilage diseases, a more efficient and less off-target chondrogenesis protocol is needed. Here, we present a protocol to induce human MSC chondrogenesis via Wnt antagonism. We describe steps for pellet formation, Wnt antagonism-based chondrogenic induction, and refreshing the differentiation medium. We detail procedures for characterizing MSC chondrogenesis. By using Wnt antagonism instead of conventional transforming growth factor β-based induction, this protocol avoids the potential for induction of chondrocyte hypertrophy/osteogenesis or other lineages.
Before you begin
In this protocol, we describe the specific steps for Wnt antagonism-based chondrogenic induction without TGF-β using human iPSC-derived MSCs. We have previously demonstrated the efficacy of this protocol in numerous sources of human MSCs, including from adult bone marrow, fetal placenta, as well as embryonic stem cell-derived MSCs.1 In addition to XAV939, a commonly used Wnt antagonist, described in this protocol, we have also used another Wnt antagonist iCRT-3 to achieve human MSC chondrogenic differentiation. The rapid chondrogenesis induced by Wnt antagonism alone can be observed in both standard 3D pellet culture as well as 2D micromass culture as we have demonstrated in our previous work.1
Institutional permissions
To conduct experiments which require human primary cells, approval must be obtained and relevant regulations must be followed. Researchers must ensure that all experiments are conducted in accordance with applicable guidelines and regulations to ensure the protection and welfare of human subjects.
MSC culture
Timing: 3–5 days
-
1.Culturing and passaging human MSCs.2
-
a.Plate 5 × 105 cells/T75 flask with expansion medium and incubated in humidified incubator with 5% CO2.
-
b.Refresh culture medium every 3 days.
-
c.Passage with trypsin-EDTA when cells reach 80% confluency.
-
a.
CRITICAL: MSCs with passage 3–7 are recommended because of better proliferative capability.3
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, peptides, and recombinant proteins | ||
| L-glutamine (200 mM) | Gibco | Cat#25030081 |
| L-ascorbic acid 2-phosphate | Sigma-Aldrich | Cat#A-8960 |
| ITS liquid media supplement (100×) | Sigma-Aldrich | Cat#I3146 |
| Dexamethasone | Sigma-Aldrich | Cat#D-8893 |
| Penicillin-streptomycin | Gibco | Cat#15140-122 |
| XAV939 | Sigma-Aldrich | Cat#X3004 |
| Alcian blue 8GX | Sigma-Aldrich | Cat#A3157 |
| Experimental models: Cell lines | ||
| Human iPSC-derived MSC | Wang et al.4 | N/A |
| Other | ||
| DMEM, low glucose, pyruvate | Thermo Fisher Scientific | Cat#11885084 |
| Nunc 15 mL conical sterile polypropylene centrifuge tubes | Thermo Fisher Scientific | Cat#339650 |
| Silane-coated slides | Muto Pure Chemicals | Cat#511614 |
Materials and equipment
Complete culture medium
| Reagent | Final concentration | Amount |
|---|---|---|
| Fetal Bovine Serum | 10% | 50 mL |
| L-glutamine | 2 mM | 5 mL |
| DMEM, low glucose, pyruvate | N/A | 445 mL |
| Total | N/A | 500 mL |
Store at 4°C and used within two weeks.
Wnt antagonism-based chondrogenic medium
| Reagent | Final concentration | Amount |
|---|---|---|
| L-glutamine | 2 mM | 5 mL |
| L-AA 2-P | 0.1 mM | 5 mL |
| ITS Liquid Media Supplement (100×) | 1× | 5 mL |
| Dexamethasone | 0.1 μM | 1 mL |
| Penicillin-Streptomycin (10,000 U/mL) | 100 U/mL | 5 mL |
| DMEM, low glucose, pyruvate | N/A | 479 mL |
| XAV939 | 10 μM | 0.5 mL |
| Total | N/A | 500 mL |
Store at 4°C and used within two weeks.
-
•
Alcian blue solution (pH 1.0): Dissolve 1 g of Alcian blue (8GX) in 100 mL of 0.1 M hydrochloric acid solution.
CRITICAL: Strong acid, handle with care.
Step-by-step method details
Pellet formation
Timing: 1 day
Formation of 3D MSC pellet at the bottom of tube by centrifugation and 16 h (overnight) culture.4,5,6
-
1.
Harvest MSCs from culture flasks by trypsinization and centrifuge the cells at 300 × g for 5 min.
-
2.
Resuspend the cell pellet in complete culture medium at a concentration of 2×105 cells/mL.
-
3.
Add 1 mL of cell suspension to 15 mL conical centrifuge tube.
-
4.
Centrifuge the tubes at 450 × g for 10 min.
-
5.
Slightly loosen the cap of centrifuge tube and incubate the tubes at 37°C in humidified incubator with 5% CO2 for 16 h (overnight).
Wnt antagonism-based chondrogenic induction
Timing: 1 day
Induce MSC chondrogenic differentiation by replacing the culture medium with Wnt antagonism-based chondrogenic medium.
-
6.Replace the complete culture medium with Wnt antagonism-based chondrogenic medium.
-
a.Carefully remove the liquid from the centrifuge tube and avoid aspirating the MSC pellet.
-
b.Slowly add the 2 mL of Wnt antagonism-based chondrogenic medium along the tube wall to prevent disturbing the pellet.
-
a.
Maintenance/medium refreshment
Timing: 10–20 days
Refresh the differentiation medium.
-
7.Refresh the Wnt antagonism-based chondrogenic medium.
-
a.Carefully remove the liquid from the centrifuge tube and avoid aspirating the MSC pellet.
-
b.Slowly add 2 mL of Wnt antagonism-based chondrogenic medium along the tube wall to prevent disturbing the pellet.
-
a.
-
8.
Maintained in culture for 10–20 days. Refresh the medium every 2–3 days.
Note: 20-day induction can induce more glycosaminoglycans production, but a 10-day induction is sufficient to induce a significant rapid chondrogenesis compared to traditional TGF-β-based method.
Characterization
Timing: 2 days
Characterization of MSC chondrogenic differentiation by chondrogenic gene expression and Alcian blue staining (stain glycosaminoglycans in extracellular matrix of chondrocytes).
- 9.
| Gene Name | Forward | Reverse |
|---|---|---|
| SOX9 | 5′-TTTCCAAGACACAAACATGA-3′ | 5′-AAAGTCCAGTTTCTCGTTGA-3′ |
| COL2A1 | 5′-GGCAATAGCAGGTTCACGTACA-3′ | 5′-CGATAACAGTCTTGCCCCACTT-3′ |
| ACAN | 5′-TCGAGGACAGCGAGGCC-3′ | 5′-TCGAGGGTGTAGCGTGTAGAGA-3′ |
| GAPDH | 5′-CCACCCATGGCAAATTCCATGGCA-3′ | 5′-TCTAGACGGCAGGTCAGGTCCACC-3′ |
Note: Chondrogenic gene expression can be analyzed at any time point of interest during differentiation process.
-
10.Alcian blue staining.8,9
-
a.Staining of the whole pellet.
-
i.Wash sample pellet with PBS.
-
ii.Fix samples with 0.5 mL 4% paraformaldehyde for 10 min at 25°C.
-
iii.Wash with PBS, rinse in 0.1 N HCl for 5 min.
-
iv.Stain the samples with 0.5 mL Alcian blue solution (pH 1.0) for 1 h.
-
v.Wash with 0.5 mL 0.1 N HCl for 5 min. Repeat this step until the solution is colorless.
-
vi.Rinse with ddH2O.
-
vii.Transfer the pellets to a 24-well plate and conduct microscopic imaging with bright field at 40x magnification.
-
i.
-
b.Quantification of Alcian blue staining (elusion).9,10
-
i.Wash the pellets with PBS for 5 min, transfer to PCR tube then remove supernatant.
-
ii.Digest the pellets with 5 μL collagenase I (3 mg/mL in PBS) at 37°C for 3 h.
-
iii.Carefully aspire the supernatant.
-
iv.Dissolve the pellets with 20 μL of 6 M Guanidine HCl by rigorous pipetting.
-
v.Supernatant was transferred to transparent 96-well plate (for absorbance analysis).
-
vi.Add 30 μL of ddH2O (total 50 μL) each well and measure the absorbance at 650 nm (Dilution could be applied if necessary).
-
i.
-
c.Staining of sliced pellet.
-
i.Wash sample pellet with PBS.
-
ii.Fix samples with 4% paraformaldehyde for 10 min at 25°C.
-
iii.Embed the MSC pellet in OCT compound and freeze at ‒80°C.
 Pause point: The sample can be stored at ‒80°C for at least several months.
Pause point: The sample can be stored at ‒80°C for at least several months. -
iv.Cut the pellet into 10 μm sections using a cryostat and place the sections onto microscope slides.
-
v.Rehydrate the sections by washing them with distilled water three times for 2 min each.
-
vi.Rinse in 0.1 N HCl for 5 min.
-
vii.Stain the sections with Alcian blue solution (pH 1.0) for 30 min.
-
viii.Wash with 0.1 N HCl for 5 min.
-
ix.Rinse with ddH2O.
-
x.Dehydrate the sections by dipping them into graded alcohol solutions (70%, 95%, and 100% ethanol) for 2 min each.
-
xi.Clear the sections with xylene for 2 min.
-
xii.Mount the slides with cover slips using mounting medium.
-
xiii.Microscopic imaging.
-
i.
-
a.
Expected outcomes
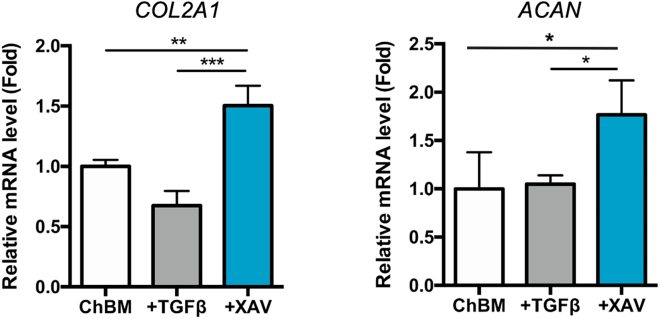
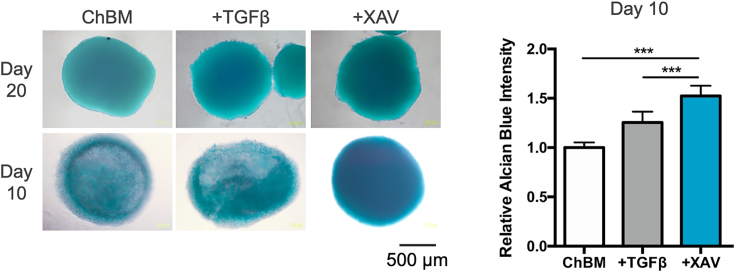
At the pellet formation step, MSCs will form a pellet at bottom of the tube after 16-hour-culture. During chondrogenic induction period, some cells might detach from the pellet, but the remaining pellet will become more condensed. Compared to TGF-β treatment, a significant upregulation of chondrogenic genes COL2A1 and ACAN in the Wnt antagonist-treated group can be detected by qPCR at Day 3 (Figure 1). And at Day 10, significantly stronger Alcian blue staining can be expected in the Wnt antagonist-treated group (Figure 2). A more structured visualization of the pellet can be further examined by Alcian blue staining of histologically sliced pellet (Figure 3). By applying Wnt antagonism instead of TGF-β, a more efficient and rapid MSC chondrogenesis can be achieved.
Figure 1.
Expression levels of chondrogenic gene COL2A1 and ACAN in 3-day pellet-cultured MSCs treated with the indicated modulators for 3 days as quantified by qPCR
Data are represented as mean ± SD. One-way ANOVA: ∗, p < 0.05, ∗∗, p < 0.01, ∗∗∗, p < 0.001.
Figure 2.
Alcian blue staining of pellet-cultured MSCs treated with the indicated modulators (10 ng/mL TGF-β3 or 10 μM XAV) at Day 20 and Day 10 (left panel), with absorbance quantification for Day 10 results (right panel)
Scale bar, 500 μm. Data are represented as mean ± SD. One-way ANOVA: ∗, p < 0.05, ∗∗, p < 0.01, ∗∗∗, p < 0.001.
Figure 3.
Alcian blue staining of the whole pellet (top panel) and sliced pellet (10-μm thickness, bottom panel) of MSCs treated with the indicated modulators (10 ng/mL TGF-β3 or 10 μM XAV) at Day 10
Scale bar, 100 μm.
Limitations
While we have extensively demonstrated the rapid efficiency of this protocol in many sources of human MSCs, we have not assessed longer term impacts and stability of this method (i.e., more than 6 weeks). At the molecular level, although we have found that the Wnt antagonism-based method promotes adherens junction interaction through increased β-catenin and N-cadherin protein expression compared to conventional TGF-β induction, more detailed mechanism of how these interactions are upregulated still require further investigation.
Troubleshooting
Problem 1
The pellet is disintegrated and the cells are dying during pellet culture (related to step 1).
Potential solution
-
•
Movement of the tubes could cause the cap being covered again blocking gas exchange. Check each centrifuge tube again while putting them into the incubator.
Problem 2
Pellet is damaged while changing or refreshing medium (related to steps 2 and 3).
Potential solution
-
•
Use 200 μL pipette instead of larger volume ones to remove the medium. Strong suction pressure could cause damage to pellet structure or sphericality.
Problem 3
Which Wnt inhibitor should be used (related to before you begin).
Potential solution
-
•
Our previously published results show that XAV939 induces stronger GAGs production than iCRT-3 at Day 10 of chondrogenic induction1 (Figure S4). Thus, in the aspect of short-term chondrogenic differentiation, XAV939 is recommended. However, because each modulator and each cell possess their own characteristics and states of the Wnt pathway, testing is still required to determine which is most suitable.11
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, B. Linju Yen (blyen@nhri.edu.tw).
Materials availability
This study did not generate new unique reagents.
Data and code availability
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
Acknowledgments
This work was partially funded by the NHRI (12A1-CSPP06-014 and 12A1-CSGP08-048 to B.L.Y.).
Author contributions
Conceptualization, B.L.Y. and C.-C.H.; methodology, B.L.Y., C.-C.H., and L.C.; investigation, C.-C.H., C.-C.C., and P.-J.H.; writing – original draft, C.-C.H. and B.L.Y.; writing – review and editing, C.-C.H. and B.L.Y.; funding acquisition, B.L.Y.; supervision, B.L.Y.
Declaration of interests
The authors declare no competing interests.
References
- 1.Hsieh C.C., Yen B.L., Chang C.C., Hsu P.J., Lee Y.W., Yen M.L., Yet S.F., Chen L. Wnt antagonism without TGFbeta induces rapid MSC chondrogenesis via increasing AJ interactions and restricting lineage commitment. iScience. 2023;26 doi: 10.1016/j.isci.2022.105713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pittenger M.F., Mackay A.M., Beck S.C., Jaiswal R.K., Douglas R., Mosca J.D., Moorman M.A., Simonetti D.W., Craig S., Marshak D.R. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 3.Yang Y.H.K., Ogando C.R., Wang See C., Chang T.Y., Barabino G.A. Changes in phenotype and differentiation potential of human mesenchymal stem cells aging in vitro. Stem Cell Res. Ther. 2018;9:131. doi: 10.1186/s13287-018-0876-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang L.T., Jiang S.S., Ting C.H., Hsu P.J., Chang C.C., Sytwu H.K., Liu K.J., Yen B.L. Differentiation of Mesenchymal Stem Cells from Human Induced Pluripotent Stem Cells Results in Downregulation of c-Myc and DNA Replication Pathways with Immunomodulation Toward CD4 and CD8 Cells. Stem Cell. 2018;36:903–914. doi: 10.1002/stem.2795. [DOI] [PubMed] [Google Scholar]
- 5.Liu K.J., Wang C.J., Chang C.J., Hu H.I., Hsu P.J., Wu Y.C., Bai C.H., Sytwu H.K., Yen B.L. Surface expression of HLA-G is involved in mediating immunomodulatory effects of placenta-derived multipotent cells (PDMCs) towards natural killer lymphocytes. Cell Transplant. 2011;20:1721–1730. doi: 10.3727/096368911X580590. [DOI] [PubMed] [Google Scholar]
- 6.Yen B.L., Yen M.L., Hsu P.J., Liu K.J., Wang C.J., Bai C.H., Sytwu H.K. Multipotent human mesenchymal stromal cells mediate expansion of myeloid-derived suppressor cells via hepatocyte growth factor/c-met and STAT3. Stem Cell Rep. 2013;1:139–151. doi: 10.1016/j.stemcr.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Craft A.M., Rockel J.S., Nartiss Y., Kandel R.A., Alman B.A., Keller G.M. Generation of articular chondrocytes from human pluripotent stem cells. Nat. Biotechnol. 2015;33:638–645. doi: 10.1038/nbt.3210. [DOI] [PubMed] [Google Scholar]
- 8.Lev R., Spicer S.S. Specific Staining of Sulphate Groups with Alcian Blue at Low Ph. J. Histochem. Cytochem. 1964;12:309. doi: 10.1177/12.4.309. [DOI] [PubMed] [Google Scholar]
- 9.Song J., Baek I.J., Chun C.H., Jin E.J. Dysregulation of the NUDT7-PGAM1 axis is responsible for chondrocyte death during osteoarthritis pathogenesis. Nat. Commun. 2018;9:3427. doi: 10.1038/s41467-018-05787-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frazier S.B., Roodhouse K.A., Hourcade D.E., Zhang L. The Quantification of Glycosaminoglycans: A Comparison of HPLC, Carbazole, and Alcian Blue Methods. Open Glycosci. 2008;1:31–39. doi: 10.2174/1875398100801010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Madan B., Virshup D.M. Targeting Wnts at the Source-New Mechanisms, New Biomarkers, New Drugs. Mol. Cancer Ther. 2015;14:1087–1094. doi: 10.1158/1535-7163.MCT-14-1038. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.