Summary
Simultaneous inhibition of programmed cell death protein-1 (PD-1) and cytotoxic T lymphocyte-associated protein-4 (CTLA-4) with bispecific antibodies may improve efficacy over single-agent treatment while limiting toxicity. Cadonilimab is a humanized, bispecific antibody targeting PD-1 and CTLA-4. This is a phase 1 study of cadonilimab including dose escalation (n = 39) and dose expansion (n = 80). One dose-limiting toxicity event is observed, with the maximum tolerated dose not reached. 6 mg/kg cadonilimab once every 2 weeks is established as the recommended dose for future studies. The most common treatment-related adverse event is infusion-related reaction (18.5%), mostly grade 1/2 in severity. The incidences of any grade and grade ≥3 immune-related adverse events are 44.5% and 6.7%, respectively. The confirmed overall response rate is 13.4%, and the median duration of response is 12.9 months. Cadonilimab is well tolerated and showed promising efficacy in patients with advanced solid tumors. This study is registered with ClinicalTrials.gov: NCT03261011.
Keywords: cadonilimab, PD-1, CTLA-4, immune checkpoint inhibitor, bispecific antibody, advanced solid tumors
Graphical abstract

Highlights
-
•
Cadonilimab is a humanized, bispecific antibody targeting PD-1 and CTLA-4
-
•
Multiple dose levels have been assessed from 0.2 to 25 mg/kg
-
•
Favorable safety profile with a low incidence of immune-related adverse events
-
•
Promising efficacy in solid tumors that are refractory/relapsed to standard therapies
Frentzas et al. evaluate the safety, pharmacokinetics, and efficacy of cadonilimab in patients with advanced solid tumors in this first-in-human study. Cadonilimab shows a favorable safety profile with a low incidence of immune-related adverse events (any grade and grade ≥3) and encourages anti-tumor activity.
Introduction
Immune checkpoint inhibitors (ICIs), such as those targeting cytotoxic T lymphocyte-associated protein-4 (CTLA-4), programmed cell death protein-1 (PD-1), or its ligand, PD-L1, have revolutionized anti-cancer treatment and improved outcomes for patients across many solid tumors.1,2 Despite the proven efficacy of single-agent ICIs, many tumors do not respond to monotherapy, and even if they are responsive, they eventually become resistant to treatment. As a result, various combination therapies have been explored. In particular, combination therapy with a PD-1 or PD-L1 inhibitor and a CTLA-4 inhibitor has yielded improved outcomes in several tumor types, and the US Food and Drug Administration has approved nivolumab plus ipilimumab, targeting PD-1 and CTLA-4, respectively, for the treatment of unresectable/metastatic melanoma, renal cell carcinoma (RCC), metastatic non-small cell lung cancer (NSCLC), hepatocellular carcinoma, unresectable malignant pleural mesothelioma, and high microsatellite instability/mismatch repair-deficient (MSI-H/dMMR) metastatic colorectal cancer.3,4,5,6,7,8,9 The combination of durvalumab (an anti-PD-L1 monoclonal antibody) and tremelimumab (an anti-CTLA-4 monoclonal antibody) is also under investigation for solid tumors with promising results.10,11,12
Although the nivolumab plus ipilimumab combination has demonstrated improved efficacy compared with single-agent ICI, the incidence of grade 3/4 adverse events (AEs) was amplified, particularly with higher doses of ipilimumab.6,13,14 In particular, grade ≥3 immune-related (ir)AEs are more common in patients receiving anti-CTLA-4 monotherapy than in those receiving anti-PD-1 monotherapy, occurring in 15%–24% of patients with ipilimumab compared with 5–10% treated with anti–PD-1 and 1–7% treated with anti–PD-L1 therapies.7,15,16,17,18,19,20,21 The combination of nivolumab plus ipilimumab further increased the incidence of grade ≥3 irAEs to 39.6% and 45%, compared with 18.6% and 15% with ipilimumab alone in the CheckMate067 and CheckMate069 trials, respectively.7,16 These toxicities have been a key factor limiting the broader use of nivolumab plus ipilimumab, despite yielding improved efficacy outcomes. Therefore, new approaches are needed to facilitate enhanced blockade of PD-1 and CTLA-4 with a better toxicity profile.
Cadonilimab (AK104) is a first-in-class, humanized, bispecific antibody targeting PD-1 and CTLA-4 simultaneously. It has been designed as a symmetric tetravalent bispecific antibody that can bind PD-1 and CTLA-4 co-expressed on tumor-infiltrating lymphocytes (TILs) with high affinity, achieving its co-targeting efficacy. PD-1 and CTLA-4 co-expression has been observed in TILs, including CD8+ TILs found in a wide range of solid tumor types, but in not normal peripheral blood lymphocytes.22,23 Pre-clinical data have demonstrated that the preferential retention of cadonilimab in a tumor-like setting may lead to better drug retention in the tumor and contribute to improved safety while achieving antitumor efficacy.23
Here, we report the phase 1a, first-in-human, open-label dose-escalation and the phase 1b dose-expansion study of cadonilimab in solid tumors (ClinicalTrials.gov: NCT03261011). The primary objective was to assess the safety and tolerability of cadonilimab, as well as to determine the maximum tolerated dose (MTD) and the recommended phase 2 dose and schedule of cadonilimab. Secondary and exploratory objectives included describing the pharmacokinetic (PK) parameters of cadonilimab, evaluating potential pharmacodynamic (PD) biomarkers, and exploring its preliminary anti-tumor efficacy.
Results
Patients
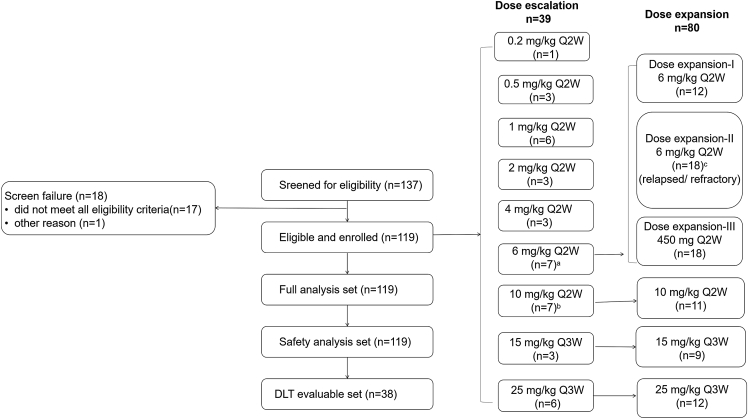
The cutoff date for this analysis was August 27, 2021. A total of 119 patients with advanced solid tumors were enrolled: 39 patients in the phase 1a/dose-escalation phase and 80 patients in the phase 1b/dose-expansion phase. All patients were included in the full analysis set and the safety set; the study flow diagram is shown in Figure 1 (rationale of sample size in each cohort is described in the STAR Methods). Patient demographics and baseline characteristics are detailed in Table 1. Median age of all patients was 61.0 (range 20–85) years, and 43.7% of the patients were female. Patients had received a median of 2 (range 0–8) lines of prior therapy. Mesothelioma was the most common tumor type in the full analysis set (16.8%). Median follow-up was 20.5 months (95% confidence interval [CI] 15.4–22.9).
Figure 1.
Study flow diagram
aOne patient experienced grade 3 infusion-related reaction during the second dose, which resolved within 6 h; this patient was replaced, as she failed to receive >90% of the cadonilimab dose within the first cycle. Although this was not deemed as a dose-limiting toxicity (DLT) event, it was considered of clinical significance and thus included in the DLT-evaluable set per the Dose Escalation Committee agreement.
bIncludes one DLT non-evaluable patient.
cDose-expansion cohort II enrolled patients who had previously received ICI treatment.
For other dose escalation and expansion cohorts, there was no specific requirement regarding prior ICI use. Q2W, once every 2 weeks; Q3W, once every 3 weeks.
Table 1.
Patient demographics and baseline characteristics
| Characteristic | No. of patients | % |
|---|---|---|
| Gender | ||
| Male | 67 | 56.3 |
| Female | 52 | 43.7 |
| Age, years | ||
| Median | 61.0 | – |
| Range | 20–85 | – |
| Race | ||
| White | 104 | 87.4 |
| Asian | 9 | 7.6 |
| Black or African American | 1 | 0.8 |
| Other | 5 | 4.2 |
| Tumor histology | ||
| Mesothelioma | 20 | 16.8 |
| Triple-negative breast cancer | 9 | 7.6 |
| Sarcoma | 8 | 6.7 |
| Neuroendocrine carcinoma | 7 | 5.9 |
| Large cell | 2 | 2.5 |
| Small cell | 3 | 1.7 |
| Unclassified | 2 | 5.9 |
| Colorectal cancer | 7 | 5.9 |
| MSI-H | 4 | 3.4 |
| Unknown | 3 | 2.5 |
| Non-small cell lung cancer | 6 | 5.0 |
| Renal cell carcinoma | 6 | 5.0 |
| Clear cell | 1 | 0.8 |
| Non-clear cell | 5 | 4.2 |
| Esophageal and esophagogastric junction cancer | 5 | 4.2 |
| Uveal melanoma | 5 | 4.2 |
| Endometrial cancer | 4 | 3.4 |
| Head and neck squamous cell carcinoma | 4 | 3.4 |
| Cervical cancer | 3 | 2.5 |
| Cholangiocarcinoma | 3 | 2.5 |
| Gastric cancer | 3 | 2.5 |
| Hepatocellular carcinoma | 3 | 2.5 |
| Ovarian cancer | 3 | 2.5 |
| Pancreatic cancer | 3 | 2.5 |
| Prostate cancer | 3 | 2.5 |
| Small-cell lung cancer | 3 | 2.5 |
| Anal cancer | 2 | 1.7 |
| Penis carcinoma | 2 | 1.7 |
| Urothelial carcinoma | 2 | 1.7 |
| ECOG PS | ||
| 0 | 65 | 54.6 |
| 1 | 54 | 45.4 |
| Prior therapies | ||
| Median | 2.0 | – |
| Range | 0–8 | – |
| Prior ICI treatment | ||
| Yes | 20 | 16.8 |
| Prior PD-1/PD-L1 inhibitors | 20 | 16.8 |
| Prior CTLA-4 inhibitor | 1 | 0.8 |
| No | 99 | 83.2 |
ECOG, Eastern Cooperative Oncology Group; MSI-H, high microsatellite instability; PS, performance status.
Safety
One dose-limiting toxicity (DLT) occurred in one patient receiving 1 mg/kg cadonilimab once every 2 weeks (Q2W). The patient experienced grade 3 aspartate aminotransferase elevation on day 15 of cycle 1 and recovered 7 days later. No other DLTs were observed in the six patients treated at this dose level. Furthermore, no additional DLTs occurred despite escalation to 25 mg/kg once every 3 weeks (Q3W) in six patients for this cohort. The MTD was not reached. The median duration of exposure to cadonilimab was 12.1 (range: 2.0–106.3) weeks. The most common treatment-related AEs (TRAEs) of any grade across all dose levels were infusion-related reaction (18.5%, 22/119), rash (17.6%, 21/119), pruritus (16.8%, 20/119), and fatigue (15.1%,18/119), with the majority being grades 1 and 2 in severity (Table 2). The incidence of grade ≥3 TRAEs was 13.4% (16/119), with infusion-related reaction (5.0%, 6/119) the most frequent, followed by immune-mediated hepatitis (1.7%, 2/119); other events occurred in <1% of patients. Serious TRAEs occurred in 13.4% (16/119) of patients, including seven (5.9%) patients who experienced infusion-related reactions reported as serious TRAEs, mainly due to these patients being admitted for overnight monitoring post-infusion. TRAEs leading to drug discontinuation occurred in nine (7.6%) patients, with infusion-related reaction being the leading cause; five (4.2%) patients who experienced grade ≥3 infusion-related reactions discontinued cadonilimab. There were no TRAEs leading to death.
Table 2.
Most common TRAEs (≥5% of any grade or ≥1% grade 3 or above) at different dose levels
| N (%) | 0.2–2 mg/kg, Q2W, N = 13 |
4 mg/kg, Q2W, N = 3 |
6 mg/kg, Q2W, N = 37 |
10 mg/kg, Q2W, N= 18 |
450mg, Q2W, N = 18 |
15 mg/kg, Q3W, N = 12 |
25 mg/kg, Q3W, N = 18 |
Total, N = 119 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All | Grade ≥3 | All | Grade ≥3 | All | Grade ≥3 | All | Grade ≥3 | All | Grade ≥3 | All | Grade ≥3 | All | Grade ≥3 | All | Grade ≥3 | |
| Infusion-related reaction | 3 (23.1) | 1 (7.7) | 0 (0) | 0 (0) | 8 (21.6) | 1 (2.7) | 2 (11.1) | 0 (0) | 4 (22.2) | 3 (16.7) | 2 (16.7) | 1 (8.3) | 3 (16.7) | 0 (0) | 22 (18.5) | 6 (5.0) |
| Rash | 1 (7.7) | 0 (0) | 1 (33.3) | 0 (0) | 6 (16.2) | 0 (0) | 4 (22.2) | 0 (0) | 4 (22.2) | 0 (0) | 2 (16.7) | 0 (0) | 3 (16.7) | 0 (0) | 21 (17.6) | 0 (0) |
| Pruritus | 1 (7.7) | 0 (0) | 0 (0) | 0 (0) | 6 (16.2) | 0 (0) | 5 (27.8) | 0 (0) | 4 (22.2) | 0 (0) | 3 (25.0) | 0 (0) | 1 (5.6) | 0 (0) | 20 (16.8) | 0 (0) |
| Fatigue | 1 (7.7) | 0 (0) | 0 (0) | 0 (0) | 6 (16.2) | 0 (0) | 2 (11.1) | 0 (0) | 4 (22.2) | 0 (0) | 0 (0) | 0 (0) | 5 (27.8) | 1 (5.6) | 18 (15.1) | 1 (0.8) |
| Nausea | 2 (15.4) | 0 (0) | 0 (0) | 0 (0) | 4 (10.8) | 0 (0) | 2 (11.1) | 0 (0) | 2 (11.1) | 0 (0) | 1 (8.3) | 0 (0) | 3 (16.7) | 0 (0) | 14 (11.8) | 0 (0) |
| Diarrhea | 2 (15.4) | 0 (0) | 0 (0) | 0 (0) | 3 (8.1) | 0 (0) | 1 (5.6) | 0 (0) | 1 (5.6) | 0 (0) | 3 (25.0) | 1 (8.3) | 3 (16.7) | 0 (0) | 13 (10.9) | 1 (0.8) |
| Arthralgia | 1 (7.7) | 0 (0) | 0 (0) | 0 (0) | 1 (2.7) | 0 (0) | 3 (16.7) | 0 (0) | 2 (11.1) | 0 (0) | 0 (0) | 0 (0) | 4 (22.2) | 0 (0) | 11 (9.2) | 0 (0) |
| Hyperthyroidism | 1 (7.7) | 0 (0) | 1 (33.3) | 0 (0) | 1 (2.7) | 0 (0) | 2 (11.1) | 1 (5.6) | 0 (0) | 0 (0) | 4 (33.3) | 0 (0) | 3 (16.7) | 0 (0) | 12 (10.1) | 1 (0.8) |
| Hypothyroidism | 1 (7.7) | 0 (0) | 0 (0) | 0 (0) | 1 (2.7) | 0 (0) | 2 (11.1) | 0 (0) | 0 (0) | 0 (0) | 2 (16.7) | 0 (0) | 2 (11.1) | 0 (0) | 8 (6.7) | 0 (0) |
| Pyrexia | 0 (0) | 0 (0) | 1 (33.3) | 0 (0) | 1 (2.7) | 0 (0) | 3 (16.7) | 1 (5.6) | 1 (5.6) | 0 (0) | 1 (8.3) | 0 (0) | 0 (0) | 0 (0) | 7 (5.9) | 1 (0.8) |
| Rash maculo-papular | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (2.7) | 0 (0) | 2 (11.1) | 0 (0) | 0 (0) | 0 (0) | 1 (8.3) | 0 (0) | 5 (27.8) | 0 (0) | 9 (7.6) | 0 (0) |
| Decreased appetite | 1 (7.7) | 0 (0) | 0 (0) | 0 (0) | 2 (5.4) | 0 (0) | 2 (11.1) | 0 (0) | 0 (0) | 0 (0) | 1 (8.3) | 0 (0) | 0 (0) | 0 (0) | 6 (5.0) | 0 (0) |
| Immune-mediated hepatitis | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (11.1) | 2 (11.1) | 2 (1.7) | 2(1.7) |
AE, adverse event; irAE, immune-related adverse event; Q2W, once every 2 weeks; Q3W, once every 3 weeks; TRAE, treatment-related adverse event.
Of the 22 patients who experienced an infusion-related reaction, the initial episode commonly occurred on the second or third infusion (77.3%, 17/22); a late-emergent or delayed event was observed in a few patients on the fifth infusion (n = 2), on the 22th infusion (n = 1), and on day 2 post-first dose (n = 1). The majority of patients with grade 1 or 2 infusion-related reactions experienced fever and chills. These patients were managed with corticosteroids and symptomatic care, and they were able to continue treatment with pre-medication. Seventeen patients were re-challenged post-initial episode; seven patients had recurrent episodes, while only one patient discontinued due to this reason.
Any grade irAEs occurred in 44.5% of patients, with grade ≥3 irAEs occurring in 6.7% of patients (Table 3). Immune-mediated diarrhea or colitis of any grade was reported in five (4.2%) patients, and only one was grade 3 (immune-related enterocolitis). No myocarditis or pneumonitis was reported. In further exploring the time course of the most common (>5%) and most serious (grade ≥3) TRAEs, these almost always emerged early (i.e., first 2–4 cycles) and more frequently at higher dose levels (see Table S4).
Table 3.
Summary of irAEs at different dose levels of cadonilimab
| 0.2–2 mg/kg, Q2W, N = 13 | 4 mg/kg, Q2W, N = 3 | 6 mg/kg, Q2W, N = 37 | 10 mg/kg, Q2W, N = 18 | 450mg, Q2W, N = 18 | 15 mg/kg, Q3W, N = 12 | 25 mg/kg, Q3W, n = 18 | Total, N = 119 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N (%) | All | Grade ≥3 | All | Grade ≥3 | All | Grade ≥3 | All | Grade ≥3 | All | Grade ≥3 | All | Grade ≥3 | All | Grade ≥3 | All | Grade ≥3 |
| irAEsa | 2 (15.4) | 1 (7.7) | 3 (100.0) | 1 (33.3) | 12 (32.4) | 0 (0) | 11 (61.1) | 3 (16.7) | 7 (38.9) | 0 (0) | 6 (50.0) | 1 (8.3) | 12 (66.7) | 2 (11.1) | 53 (44.5) | 8 (6.7) |
| Dermatologic toxicity | ||||||||||||||||
| Rashb | 0 (0) | 0 (0) | 2 (66.7) | 0 (0) | 6 (16.2) | 0 (0) | 6 (33.3) | 0 (0) | 3 (16.7) | 0 (0) | 5 (41.7) | 0 (0) | 8 (44.4) | 0 (0) | 30 (25.2) | 0 (0) |
| Pruritus | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 5 (13.5) | 0 (0) | 4 (22.2) | 0 (0) | 2 (11.1) | 0 (0) | 1 (8.3) | 0 (0) | 1 (5.6) | 0 (0) | 13 (10.9) | 0 (0) |
| Endocrine toxicity | ||||||||||||||||
| Hyperthyroidism | 1 (7.7) | 0 (0) | 1 (33.3) | 0 (0) | 1 (2.7) | 0 (0) | 2 (11.1) | 1 (5.6) | 0 (0) | 0 (0) | 4 (33.3) | 0 (0) | 3 (16.7) | 0 (0) | 12 (10.1) | 1 (0.8) |
| Hypothyroidism | 1 (7.7) | 0 (0) | 0 (0) | 0 (0) | 1 (2.7) | 0 (0) | 3 (16.7) | 0 (0) | 0 (0) | 0 (0) | 2 (16.7) | 0 (0) | 1 (5.6) | 0 (0) | 8 (6.7) | 0 (0) |
| Type 1 diabetes mellitus | 0 (0) | 0 (0) | 1 (33.3) | 1 (33.3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (5.6) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (1.7) | 1 (0.8) |
| Adrenal insufficiency | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (5.6) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (0.8) | 0 (0) |
| Lymphocytic hypophysitis | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (5.6) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (0.8) | 0 (0) |
| Musculoskeletal toxicity | ||||||||||||||||
| Arthralgia | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (2.7) | 0 (0) | 1 (5.6) | 0 (0) | 1 (5.6) | 0 (0) | 0 (0) | 0 (0) | 4 (22.2) | 0 (0) | 7 (5.9) | 0 (0) |
| Arthritisc | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (5.4) | 0 (0) | 0 (0) | 0 (0) | 1 (5.6) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 3 (2.5) | 0 (0) |
| Dry mouth | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (11.1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (1.7) | 0 (0) |
| Tendonitis | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (2.7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (0.8) | 0 (0) |
| Hepatotoxicity | ||||||||||||||||
| Transaminase increasedd | 2 (15.4) | 1 (7.7) | 0 (0) | 0 (0) | 2 (5.4) | 0 (0) | 1 (5.6) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 5 (4.2) | 1 (0.8) |
| Immune-mediated hepatitis | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (11.1) | 2 (11.1) | 2 (1.7) | 2 (1.7) |
| Gastrointestinal toxicity | ||||||||||||||||
| Diarrhea | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (5.4) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (8.3) | 0 (0) | 1 (5.6) | 0 (0) | 4 (3.4) | 0 (0) |
| Immune-mediated enterocolitis | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (8.3) | 1 (8.3) | 0 (0) | 0 (0) | 1 (0.8) | 1 (0.8) |
| Other | ||||||||||||||||
| Polyneuropathy | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (5.6) | 1 (5.6) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (0.8) | 1 (0.8) |
| Nephritis | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (5.6) | 1 (5.6) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (0.8) | 1 (0.8) |
| Iridocyclitis | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (5.6) | 0 (0) | 1 (0.8) | 0 (0) |
irAE, immune-related adverse event; Q2W, once every 2 weeks; Q3W, once every 3 weeks.
Immune-related events were based on a list of terms specified by sponsor and considered regardless of attribution to treatment or immune relatedness by the investigator.
Includes related terms of rash, rash maculo-papular, rash papular, rash pruritic, rash pustular, and vasculitic rash.
Includes related terms of arthritis and immune-mediated arthritis.
Includes related terms of alanine aminotransferase increased, aspartate aminotransferase increased, and transaminases increased.
PK analysis
PK parameters of cadonilimab during the first intensive sampling (before and after the first dose) and the second intensive sampling (before and after the fourth dose) by dose-level cohorts are shown in Table 4. The concentration-time profiles during the first and second intensive samplings are shown in Figure 2A. The results showed that after intravenous administration, maximum concentrations (Cmax) occurred at the end of infusion in most patients and declined rapidly after that. The time to maximum concentration (Tmax) was approximately 1.07 h, and the mean Cmax was 135 μg/mL. Plasma concentration of cadonilimab decreased to less than 10 μg/mL 15 days after the administration of the first dose at 6 mg/kg, and the concentration-time profile after the fourth dose was similar to that of the first dose (Figure S1A). The observed mean half-life (t1/2) for cadonilimab ranged from 2.7 to 6.9 days, and the mean clearance ranged from 0.89 to 1.72 L/day; the mean volume of distribution for cadonilimab ranged from 5.13 to 7.97 L (Table 4). Cadonilimab exposure increased proportionally in a dose range from 0.5 to 25.0 mg/kg. No obvious drug accumulation was observed at the tested dose levels when cadonilimab was administered Q2W. The PK sampling time points are shown in Tables S1 and S2.
Table 4.
Pharmacokinetic parameters during the first and second intensive samplings after the first and fourth doses of cadonilimab, respectively, presented as mean ± standard deviation unless stated otherwise
| 0.2 mg/kg Q2W | 0.5 mg/kg Q2W | 1.0 mg/kg Q2W | 2.0 mg/kg Q2W | 4.0 mg/kg Q2W | 6.0 mg/kg Q2W | 450 mg Q2W | 10.0 mg/kg Q2W | 15.0 mg/kg Q3Wa | 25.0 mg/kg Q3Wa | |
|---|---|---|---|---|---|---|---|---|---|---|
| First intensive collection (N) | 1 | 3 | 6 | 3 | 3 | 36 | 18 | 18 | 12 | 18 |
| AUC0-t (day∗μg/mL) | 6.67 | 23.1 ± 1.61 | 47.5 ± 12.6 | 99.6 ± 1.51 | 182 ± 25.8 | 315 ± 133 | 306 ± 126 | 633 ± 265 | 987 ± 359 | 2,150 ± 996 |
| Cmax (μg/mL) | 3.24 | 11.6 ± 2.45 | 22.6 ± 6.08 | 38.4 ± 8.78 | 77.8 ± 9.82 | 135 ± 49.4 | 109 ± 24.1 | 261 ± 87.8 | 312 ± 74.1 | 610 ± 202 |
| Tmax (h)b | 1.03 (1.03–1.03) | 4.02 (1.05–4.63) | 1.10 (0.967–4.03) | 1.07 (1.05–1.07) | 1.03 (0.983–1.10) | 1.07 (1.00–4.08) | 1.08 (1.00–23.5) | 1.08 (0.900–3.93) | 1.06 (1.00–4.13) | 1.17 (1.02–70.6) |
| t1/2 (day) | 2.68 | 2.71 ± 0.207 | 2.97 ± 1.64 | 3.73 ± 1.51 | 3.80 ± 0.959 | 3.15 ± 1.18 | 3.93 ± 2.05 | 3.85 ± 1.30 | 4.63 ± 1.81 | 6.92 ± 4.10 |
| Vz (L) | 6.65 | 5.55 ± 0.831 | 5.13 ± 1.50 | 7.41 ± 1.17 | 7.97 ± 0.230 | 6.43 ± 2.69 | 7.50 ± 2.27 | 6.92 ± 2.68 | 7.56 ± 3.44 | 7.80 ± 2.69 |
| CL (L/day) | 1.72 | 1.42 ± 0.104 | 1.43 ± 0.657 | 1.52 ± 0.544 | 1.52 ± 0.413 | 1.47 ± 0.509 | 1.58 ± 0.739 | 1.35 ± 0.570 | 1.21 ± 0.444 | 0.891 ± 0.346 |
| Second intensive collection (N) | – | 1 | 3 | 2 | 2 | 19 | 11 | 12 | – | – |
| AUC0-t,ss (day∗μg/mL) | – | 28.8 | 37.2 ± 28.9 | 33.7 ± 11.0 | 235 ± 32.4 | 389 ± 218 | 426 ± 156 | 722 ± 511 | – | – |
| Cmin,ss (μg/mL) | – | 0.272 | 0.897 ± 0.483 | 0.0527 ± 0.00566 | 4.19 ± 0.205 | 5.50 ± 3.25 | 7.02 ± 4.60 | 8.53 ± 5.49 | – | – |
| Tmax,ss (h)a | – | 1.12 (1.12–1.12) | 1.37 (1.05–1.42) | 2.45 (1.10–3.80) | 1.18 (1.07–1.28) | 1.05 (1.00–4.20) | 1.03 (1.00–4.10) | 1.16 (1.02–5.00) | – | – |
| Cmax,ss (μg/mL) | – | 15.6 | 17.4 ± 4.53 | 29.8 ± 7.28 | 88.0 ± 8.70 | 144 ± 40.3 | 156 ± 79.2 | 282 ± 95.6 | – | – |
| Vz,ss (L) | – | 3.82 | 5.82 ± 0.513 | 6.34 ± 0.595 | 6.56 ± 1.62 | 6.63 ± 3.04 | 7.31 ± 2.14 | 6.97 ± 2.57 | – | – |
| CLss (L/day) | – | 1.25 | 2.66 ± 2.61 | 5.18 ± 0.0588 | 1.05 ± 0.156 | 1.57 ± 1.03 | 1.12 ± 0.387 | 1.47 ± 1.08 | – | – |
| AI | – | 1.01 | 1.07 ± 0.0992 | 1.00 ± 0.0000105 | 1.12 ± 0.0296 | 1.08 ± 0.0692 | 1.15 ± 0.0740 | 1.13 ± 0.148 | – | – |
AI, accumulation index; AUC0-t, area under the serum concentration-time curve from 0 h to the last measurable concentration; AUC0-t,ss, area under the serum concentration-time curve during a dosing interval at steady state; CL, clearance; CLss, clearance at steady state; Cmax, maximum concentration; Cmax,ss, maximum concentration at steady state; Cmin,ss, minimum concentration at steady state; Q2W, once every 2 weeks; Q3W, once every 3 weeks; t1/2, elimination half-life; Tmax, time to maximum concentration; Tmax,ss, time to maximum concentration at steady state; Vz, volume of distribution; Vz,ss, volume of distribution at steady state.
The cohorts of 15 mg/kg Q3W and 25 mg/kg Q3W were evaluated after 10 mg/kg Q2W was deemed safe by the Dose Escalation Committee and according to the protocol. The second intensive sampling for pharmacokinetics was not planned.
Tmax is reported as median (range).
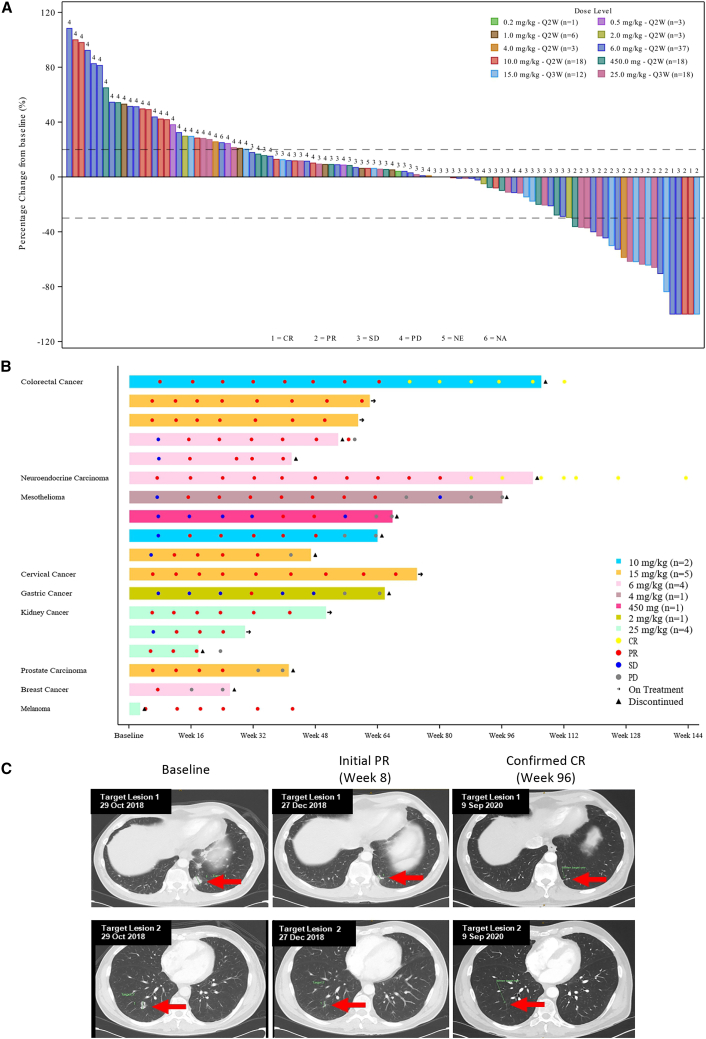
Figure 2.
Tumor response
(A) Waterfall plot of best overall response in all patients.
(B) Tumor responses in responding patients.
(C) Radiological images of target lesion over time in a patient with lung large-cell neuroendocrine carcinoma who achieved CR.
CR, complete response; CRC, colorectal cancer; ICI, immune checkpoint inhibitor; irAE, immune-related adverse event; LCNEC, large-cell neuroendocrine carcinoma; m, month; MSI, microsatellite instability; MSI-H, high microsatellite instability; OS, overall survival; PD, progressive disease; PFS, progression-free survival; PR, partial response; Q2W, once every 2 weeks; Q3W, once every 3 weeks; RCC, renal cell carcinoma; SCC, squamous cell carcinoma; TNBC, triple-negative breast cancer.
PD analysis
The post-dose-to-baseline ratio of CD4+Ki67+ T cells reached a maximum on day 7 after the first dose of cadonilimab among subjects of all cohorts, and the mean post-dose-to-baseline ratio of CD4+Ki67+ T cells was approximately 2- to 3-fold in the 6.0 mg/kg Q2W cohort (Figure 2B). The post-dose-to-baseline ratio of CD4+Ki67+ T cells on day 7 appeared dose dependent in cohorts receiving cadonilimab 0.2 to 10 mg/kg Q2W, as well as in the 15 to 25 mg/kg Q3W cohorts (Figure S1B). Together, this indicated that cadonilimab effectively inhibited CTLA-4 function peripherally.
The receptor occupancy (RO) results showed that the occupancy of cadonilimab on PD-1 and CTLA-4 of peripheral blood CD3+ T cells rapidly reached approximately 80% at day 1 following the first dose at 4.0 mg/kg Q2W or greater dose levels and maintained at a high level following multiple doses at 4.0 mg/kg Q2W or greater doses (Figure 2C). This indicated that cadonilimab has a high affinity to PD-1 and CTLA-4. We also analyzed the correlation between PD biomarker CD4+Ki67+ T cells, RO, and incidence of AEs, as well as anti-tumor efficacy, independently. No correlation was found in this study (data not shown).
Efficacy
Anti-tumor response was observed at dose levels ≥2.0 mg/kg. The confirmed overall response rate (ORR) in all patients was 13.4% (16/119). Two patients had an unconfirmed partial response. Objective response was observed in a substantial proportion of patients with mesothelioma (20%), non-clear cell renal cell carcinoma (RCC) (60%), and microsatellite instability high (MSI-H) colorectal cancer (100%). Among 16 confirmed responses, the median duration of response (DoR) was 12.9 months (range: 2.8–31.3 months). A waterfall plot of best overall response in all 119 patients as well as tumor responses in patients that responded to treatment are shown in Figures 2A and 2B.
Two patients achieved complete response (CR). One was a male, 64-year-old non-smoker who had received no prior systemic treatment for large-cell neuroendocrine carcinoma. The patient received 6 mg/kg Q2W cadonilimab; a partial response (PR) with a 47.5% reduction in lesion size at the first tumor assessment and first CR at week 88 were noted. The patient completed 24 months of cadonilimab treatment with a DoR of 31+ months and a disease-free status of 12+ months. Figure 2C shows the radiological images of the target lesions over time in this patient (tumor responses in 7 patients with neuroendocrine carcinoma are also shown in Figure S2). The second patient was a patient with MSI-H colorectal carcinoma who received 10 mg/kg cadonilimab Q2W. Both patients had ongoing response beyond 24 months of treatment.
Among patients with mesothelioma (n = 20), the majority (85%, 17/20) had epithelioid histology type, all had previously received first-line chemotherapy, 30% received prior second-line therapy, and 20% received >2 lines of prior therapy. Among these patients, the ORR was 20%, and the disease control rate (DCR) was 75.0%. The median DoR was 8.1 months (range: 5.6–12.9 months). The median progression-free survival (PFS) was 5.5 months (95% CI 2.1–12.8), and the median overall survival (OS) was 15.6 months (95% CI 3.5‒not evaluable); the 1-year OS was 60.0% (Figure 3). PD-L1 and mismatch repair (MMR) testing was performed for 9 patients with available archival tissue at baseline. All 9 patients were mismatch repair proficient (pMMR), and only 1 patient with the sarcomatoid subtype tested with a PD-L1 tumor proportion score (TPS) ≥1%.
Figure 3.
Tumor response in patients with mesothelioma
(A) Waterfall plot of best overall response in patients with mesothelioma.
(B) Swimmer plot of treatment duration in patients with mesothelioma.
NA, not available; PD, progressive disease; PR, partial response; Q2W, once every 2 weeks; Q3W, once every 3 weeks; SD, stable disease.
Of the five patients with non-clear cell RCC, PR was achieved in three previously untreated patients (60%) who were treated with 25 mg/kg cadonilimab Q3W. One patient with the chromophobe subtype had an ongoing response for >1 year (Figure S2).
Notably, among 18 patients in the dose-expanded cohort II, 4 patients had primary resistance to previous ICI therapy, and 14 had acquired resistance. Out of 14 patients with prior acquired resistance, 1 achieved PR, 9 achieved stable disease (SD) with a DCR of 55.6% (10/18), 2 achieved PD, and there was no post-baseline tumor evaluation for 2 patients. For the 4 patients with primary resistance, 3 patients experienced PD, and there was no post-baseline tumor evaluation for 1 patient. Seven of the 18 patients (38.9%) had been on treatment for ≥24 weeks. One patient with colorectal cancer (MSI unknown) who had had five lines of prior therapy, including pembrolizumab, achieved a PR with a 44.4% reduction at the fourth tumor assessment. Another patient with cholangiocarcinoma (pMMR status) and treated with 6.0 mg/kg Q2W for 2 years had significant tumor remission post-treatment but was considered not evaluable due to a necrotic portion in the target lesion (Figure S3).
Additionally, PR was observed across different dose levels in sporadic tumor types, such as uveal melanoma, cervical cancer, and gastric cancer. In the 25 mg/kg Q3W cohort, one patient with uveal melanoma achieved a durable response of approximately 42 weeks, despite early discontinuation due to toxicity.
Exploratory analysis
Tumor MMR status was assessed in 54 patients. Among 10 patients with mismatch repair-deficient (dMMR) status, the confirmed ORR was 50%, the median PFS (mPFS) was 15.5 months, and the mOS was 20.5 months. Among 44 patients with pMMR status, three patients (2 mesothelioma and 1 cervical cancer) achieved PR and produced an confirmed ORR of 6.8%; the mPFS was 1.7 months, and the mOS was 8.0 months.
PD-L1 expression was assessed in 59 patients. In patients with PD-L1 TPS ≥1, the ORR and the DCR were 28.6% and 85.7%, respectively, while the mPFS and the mOS were 7.4 and 15.2 months, respectively. In patients with PD-L1 TPS <1, the ORR, DCR, mPFS, and mOS were, respectively, 9.6%, 38.5%, and 1.9 and 8.0 months.
In patients with PD-L1 combined positive score (CPS) ≥1, the ORR, DCR, mPFS, and mOS were the same as those in patients with PD-L1 TPS ≥1, and the results were the same in patients with PD-L1 CPS<1 and PD-L1 TPS<1. Generally, patients with PD-L1-positive tumors achieved a higher response rate and longer mPFS and mOS. Overall distributions of PD-L1 expression and clinical efficacy by different PD-L1 cutoffs are reported in Table S3.
Discussion
This study was the first-in-human investigation of cadonilimab, a bispecific antibody capable of simultaneously binding PD-1 and CTLA-4 with high affinity, with tetravalent and Fc-null design to minimize toxicities. Cadonilimab demonstrated a manageable safety profile with, in particular, a low number of irAEs typically associated with the use of CTLA-4 antibodies. Multiple dose levels were assessed: from 0.2 to 10 mg/kg Q2W, as well as 15 and 25 mg/kg Q3W, as a means of determining the optimal dose and schedule. The MTD was not reached at the maximum administered dose of 25 mg/kg Q3W in this study. Efficacy was seen at doses as low as 2 mg/kg. Based on a combination of safety and PK/PD data, 6 mg/kg cadonilimab Q2W will be taken forward for ongoing and future studies. The safety profile of cadonilimab was favorable across all dose levels and with a low incidence of TRAEs and irAEs.
Cadonilimab-related AEs reported in this study were mostly mild, and the incidence of grade ≥3 TRAEs did not rise with increasing dose levels. Similar to previous observations in therapeutic antibodies,24 infusion-related reactions were frequently noted (22/119, 18.5%), but these were mostly of low grade, and risk of recurrence could be mitigated with pre-medication. At the 6 mg/kg Q2W dose level, only one of the 37 patients experienced a grade 3 infusion-related reaction. Five patients who experienced grade ≥3 infusion-related reactions discontinued treatment, and all recovered with supportive therapies. The toxicity spectrum of cadonilimab was consistent with those of the monotherapy and combination therapies targeting PD-1 and CTLA-4,5,6,7 and no unexpected AE is observed. The incidence of grade ≥3 irAEs (6.7%) was relatively low, and commonly experienced irAEs included rash, hyperthyroidism, and hypothyroidism, which are similar to those reported with anti-PD-(L)1 monotherapy.7 In comparison with MEDI5752 and MGD019, two bispecific anti-PD-1/CTLA-4 antibodies, cadonilimab showed a favorable safety profile with an incidence of grade ≥3 TRAEs of 13.4%. In the first-in-human study of MEDI5752, among 86 patients that received MEDI5752 monotherapy, the incidence of grade 3/4 TRAE was 38.4%.25 In the first-in-human study of MGD019, the incidence of grade ≥3 TRAE rate was 24.2%.26 Of note, colitis (including diarrhea), pneumonitis, and hepatitis were commonly reported as fatal ICI toxic events, and colitis and myocarditis were the most common fatal irAEs to occur in patients treated with anti-PD-(L)1 and CTLA-4 combination therapy.27 In our study, the incidence of colitis was low, with only one patient experiencing grade 3 colitis, and no myocarditis or pneumonitis was observed.
The lower toxicities observed in our study were most likely related to the design features of cadonilimab. Pre-clinical investigation showed that tetravalent cadonilimab possesses higher binding avidity in high-density PD-1 and CTLA-4 settings than in a low-density PD-1 setting, leading to increased retention of cadonilimab in tumors. With an Fc-null design and no binding to Fc receptors, cadonilimab showed minimal antibody-dependent cellular cytotoxicity, antibody-dependent cellular phagocytosis, and interleukin-6/-8 release. These features all likely contribute to the low toxicities of cadonilimab observed in the clinic.23
In addition to the acceptable safety profile, cadonilimab showed a durable response in multiple tumor types. Objective responses were observed in pMMR and PD-L1-negative tumors. Even in patients who had previously received ICI treatment, more than half achieved PR and SD. Of note, response was also seen in tumor types that typically did not respond to anti-PD-1 alone, such as non-clear cell RCC. Currently, there is no satisfactory standard of care for non-clear cell RCC, a heterogeneous subtype of RCC. In KEYNOTE-427, in patients with previously untreated non-clear cell RCC, the ORR was relatively higher in patients with papillary or unclassified RCC (28.8% and 30.8%, respectively) than in those with chromophobe RCC (9.5%) after treatment with pembrolizumab.28 In a separate study, 18 patients with metastatic non-clear cell RCC were treated with nivolumab plus ipilimumab, 33.3% of whom achieved an objective response.29 In the non-clear cell RCC cohort of CheckMate920, of the 46 patients receiving nivolumab plus ipilimumab, two patients achieved CR (papillary, n = 1; unclassified, n = 1), seven achieved PR (papillary, n = 4; unclassified, n = 3), and 17 had SD, with an ORR of 19.6%.30 In our study, although there were only five patients with non-clear cell RCC, three (60%) achieved PR, including one patient with chromophobe RCC who achieved a durable response of approximately 20 months after treatment with 25.0 mg/kg Q3W cadonilimab and remained on treatment at data cutoff. Further studies are needed to explore this profound response to cadonilimab in this tumor type.
In our cohort of patients with mesothelioma, ORRs and DCRs were comparable to that reported with nivolumab plus ipilimumab in the IFCT-1501 MAPS2 study (20% and 75% vs. 28% and 50%, respectively).31 PFS and OS were also similar between our study and IFCT-1501 MAPS2, with mPFSs of 5.5 and 5.6 months and median OSs of 15.6 and 15.9 months, respectively.31 However, in our study, 20% of patients had received ≥2 lines of prior therapy, suggesting that even in heavily pre-treated patients, cadonilimab showed encouraging anti-tumor efficacy, therefore warranting further investigation. Furthermore, 5% of patients who received nivolumab plus ipilimumab in the IFCT-1501 MAPS2 study experienced toxicities leading to death, while no treatment-related deaths occurred in our study.
The expression of Ki67 is a PD biomarker of CTLA-4 and an important index of T cell proliferation by inhibiting CTLA-4.32,33 In our study, the mean post-dose-to-baseline ratio of CD4+Ki67+ T cells at day 7 was approximately 2- to 3-fold with 6 mg/kg Q2W and was maintained at 3-fold up to 25 mg/kg Q3W. These results were consistent with the data from a cynomolgus monkey model of MGD019, another bispecific antibody targeting PD-1 and CTLA-4,34 as well as the data from a phase 1b study of combination durvalumab plus tremelimumab in NSCLC.32 RO is an important marker to evaluate binding capacity of antibody drugs. In our study, the RO on peripheral T cells rapidly reached approximately 80% at doses ≥4 mg/kg, which was consistent with pre-clinical data, demonstrating that cadonilimab possessed high and stable binding activity with both CTLA-4 and PD-1, subsequently leading to T cell activation.23 Previous studies have reported that increased T cell proliferation following ICI administration had no significant impact on prognosis nor on the development of irAEs.35,36
In conclusion, cadonilimab was found to have a favorable toxicity profile and promising efficacy for patients with solid tumors that were refractory/relapsed to standard therapies or for which no effective standard therapy was available. Cadonilimab has been approved by the China National Medical Products Administration for recurrent or metastatic cervical cancer based on the results of a pivotal phase 2 study (ClinicalTrials.gov: NCT03852251). A confirmatory phase 3 study is ongoing for first-line treatment of cervical cancer (ClinicalTrials.gov: NCT04982237), as well as a phase 3 study of cadonilimab in combination with chemotherapy as first-line therapy in gastric cancer (ClinicalTrials.gov: NCT05008783). These data suggest that treatment with cadonilimab is a promising approach to targeting PD-1/CTLA-4, with encouraging efficacy and a markedly more manageable safety profile than previously seen with combination single-agent therapies.
Limitations of the study
The preliminary data in this study indicate that cadonilimab has a favorable safety profile and yields anti-tumor activity in some certain types of tumors; additional study will be needed to substantiate these preliminary findings. The present study is also limited by the small sample size for the exploratory analysis, and it was a post hoc analysis. The efficacy and safety of cadonilimab in patients with different PD-L1 expression will be further explored.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| AK104 | Akeso Biopharma Co., Ltd. | N/A |
| Mouse Anti-Human IgG-AF647 | Southern Biotech | (Clone 4E3)Cat# 9052-31; RRID: AB_2796623 |
| CD45-BV421 | BD Bioscience | (Clone HI30)Cat# 563879; RRID:AB_2744402 |
| CD3-PE | BioLegend | (Clone SK3)Cat# 344806; RRID:AB_10559750 |
| Isotype Mouse IgG1κ-AF647 | BioLegend | (Clone MOPC-21)Cat# 400136; RRID:AB_2832978 |
| BV421 CD3 | BD Bioscience | (SK7) Cat#563798; RRID:AB_2744383 |
| FITC CD4 | BD Bioscience | (SK3 + SK4)Cat#347413; RRID:AB_400297 |
| PE HLA-DR | BD Bioscience | (G46-6) Cat#555812; RRID:AB_396146 |
| PerCP-Cy5.5 CD8 | BD Bioscience | (RPA-T4)Cat#341050; RRID:AB_2811219 |
| AF647 IgG1 | BD Bioscience | (MOPC-21)Cat#557714; RRID:AB_396823 |
| AF647 Ki67 | BD Bioscience | (B56) Cat#558615; RRID:AB_647130 |
| MLH1 (M1) Mouse Monoclonal Primary antibody | VENTANA | 790–5091 |
| MSH2 (G219-1129) Mouse Monoclonal Primary antibody | VENTANA | 790–5093; RRID:AB_2936886 |
| MSH6 (SP93) Rabbit Monoclonal Primary antibody | VENTANA | 790–5092; RRID:AB_2936885 |
| PMS2 (A16-4) Mouse Monoclonal Primary antibody | VENTANA | 790–5094 |
| Biological samples | ||
| Human pre-immunotherapy archived biopsy FFPE tissue samples | NA | NA |
| Chemicals, peptides, and recombinant proteins | ||
| PD1-hFc | Akeso Biopharma Co., Ltd. | N/A |
| CTLA4-mFc-Biotin | Akeso Biopharma Co., Ltd. | N/A |
| Critical commercial assays | ||
| VENTANA PD-L1 (SP263) Assay | VENTANA | 743–7066 |
| OptiView DAB IHC Detection Kit | VENTANA | 760–700 |
| Software and algorithms | ||
| Watson | Thermo Fisher Scientific, Inc. | Laboratory Information Management System v 7.4.2.01 |
| R (Version 4.1.0) | N/A | N/A |
| Other | ||
| Data Acquisition System | Molecular Devices, LLC | Softmax Pro Software v 4.0 installed on a Windows XP |
| BD FACS Canto Cytometer | BD | N/A |
| BD FACS Canto II | BD | N/A |
Resource availability
Lead contact
Further information and requests for resources should be directed to and will be fulfilled by the lead contact, Jayesh Desai (Jayesh.Desai@petermac.org).
Materials availability
This study did not generate new unique reagents.
Experimental model and study participant details
Study participants
This was a multicenter, phase 1 study that was conducted at six centers in Australia. Patients were aged ≥18 years with a histologically or cytologically documented advanced or metastatic solid tumor that had relapsed or was refractory to standard therapies, or for whom no effective standard therapy was available. Patients had adequate organ function and an Eastern Co-operative Group performance status of 0 or 1. Full eligibility criteria are available in Methods S1 in the supplemental information. The protocol was approved to be conducted at all participating hospitals, by the Bellberry and Melbourne Health Human Research Ethics Committees. The trial was conducted in accordance with the Declaration of Helsinki, International Conference on Harmonisation Guidelines for Good Clinical Practice, and applicable laws and regulations. All patients provided written informed consent.
Method details
Study design
For the dose escalation phase, a single patient was enrolled initially at the starting dose of cadonilimab 0.2 mg/kg Q2W after which the dose-escalation phase utilized a 3 + 3+3 dose-escalation procedure, with a minimum of three DLT-evaluable patients at each dose level, and a minimum of six DLT-evaluable patients at the highest dose level. The DLT evaluation period was 28 days. Sequential cohorts each received cadonilimab at a dose level of 0.5, 1.0, 2.0, 4.0 or 6.0 mg/kg Q2W per protocol v1.0. After 6.0 mg/kg Q2W was deemed safe, as the initial highest dose level by the Dose Escalation Committee (DEC), agreement was reached to explore higher dose levels and protocol was amended to include 10 mg/kg Q2W as the highest dose level. In order to explore the feasibility of a Q3W schedule, higher dose levels of 15 mg/kg Q3W and 25 mg/kg Q3W, were evaluated after 10 mg/kg Q2W was deemed safe by the DEC. Dose-expansion cohorts of up to 18 patients per dose levels were enrolled at 6.0 mg/kg Q2W and higher dose levels for selected tumor types, including mesothelioma, RCC and MSI-H/dMMR tumors. Further details for tumor types in the expansion cohorts are listed in Appendix S1. For the 6 mg/kg Q2W regimen, an additional cohort (dose expansion-II) of 18 patients who had previously received ICI treatment were enrolled. A fixed dosing regimen of 450 mg Q2W was also explored. Cadonilimab was administered via intravenous infusion on Days 1 and 15 of each 28-day cycle for Q2W dosing cohorts, and on Day 1 of each 21-day cycle for Q3W cohorts, until disease progression, unacceptable toxicity or a maximum of 24 months.
Safety assessments
AEs were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03. For the dose expansion cohorts, patients were monitored for safety using criteria employed with the dose-escalation cohorts. If ≥ 33% patients experienced DLT, even beyond the DLT evaluation period, enrollment would be paused and re-evaluated before recommencing enrollments. MTD was defined as the highest dose level with an observed incidence of DLT in <33% of the patients enrolled in a cohort level. If all evaluated dose levels demonstrated an observed incidence of DLT in fewer than 33% of patients, the MTD of cadonilimab was not reached. DLT was defined as any grade ≥3 treatment-related toxicity that occurred within the 28-day DLT evaluation period; any grade ≥2 treatment-related toxicity causing a >2-week delay during the DLT evaluation period was also considered a DLT. Further specific DLT definitions and exceptions are listed in Methods S2 in the supplemental information. Tumor response was assessed by investigators according to the Response Evaluation Criteria in Solid Tumors version 1.1. Baseline tumor assessments were performed within the 28 days prior to enrollment. Tumor assessments were performed at baseline, every 8 weeks (±3 days) for patients on the Q2W dosing schedule, or every 6 weeks for the first 24 weeks, then every 9 weeks thereafter for patients on the Q3W dosing schedule until disease progression or study withdrawal.
PK assessments
The endpoints for the PK assessment of cadonilimab included individual subject serum cadonilimab concentrations at different time points after cadonilimab administration. PK parameters were determined by noncompartmental analysis and included, Cmax, area under the concentration–time curve (AUC), clearance, and t1/2.
Serum concentrations of cadonilimab were measured by a validated enzyme-linked immunosorbent assay with a lower limit of quantification of 0.0281 mg/L. Cadonilimab was administered as an intravenous infusion over 60 ± 5 min. Serum samples were collected at Day 1 (prior to infusion, at the end of infusion and 3 h after infusion), Day 2, 4, 8 and Day 15 (prior to the second dose infusion), which was defined as the first intensive collection for the Q2W treatment regimen. The second intensive collection was conducted between Day 43 and Day 57 when the fourth dose was administrated. For patients treated with a Q3W regimen, serum samples were collected at Day 1 (prior to dosing, at the end of dosing, and 3 h after dosing), Day 2, 4, 8 and Day 22 (prior to the second dose infusion), which was defined as the first intensive collection. The details of PK sample collections are shown in Tables S1 and S2.
PD assessments
PD parameters included CTLA-4 suppression (intracellular proliferation marker Ki67), and target engagement biomarker RO on PD-1 and CTLA-4 of peripheral blood T cells, to assess the biological activity of cadonilimab at each escalating dose level cohort in the dose-expansion cohorts.
Circulating quantities of T cells expressing the Ki67 and RO were monitored using qualified flow cytometry-based assays. For Ki67 analyses, 4 mL fresh peripheral blood were obtained in an anti-coagulative tube on Day 1 (pre-dosing and post-dosing), Day 7 and 14. Peripheral blood mononuclear cells were isolated and stained for CD3, CD4, and Ki67 in a certified clinical laboratory. For RO analyses, sample collections were conducted on Day 1 (pre-dosing), Days 2, 8, 15, 29, 57, 113, 169, 225, 281 for Q2W regimens, and Day 1 (pre-dosing), Days 7, 14, 21, 42, 63, 126, 189, and 252 for Q3W treatment regimens.
Proliferation marker Ki67 was assessed as the ratio of CD4+Ki67+ cells in the total CD3+ T cell population following cadonilimab therapy relative to baseline, while target marker was monitored by RO (calculated from mean fluorescence intensity values, mean +standard deviation) of PD-1 and CTLA-4 in the CD3+ T cell population by time point and dose cohort. The correlation between PD marker and AE frequency and efficacy were also analyzed independently.
MMR status and PD-L1 expression assessments
Tumor tissue MMR status were determined by examining the four MMR proteins (MLH1, MSH2, MSH6, PMS2) using VENTANA MMR immunohistochemistry (IHC) panel (VENTANA BenchMark ULTRA platform) on formalin-fixed paraffin-embed tumor tissue. Presence of all four proteins indicates MMR proficiency. Negative expression of one or more protein (<10% nuclear staining in tumor cells) indicates MMR deficiency. PD-L1 expression was assessed on formalin-fixed paraffin-embed tumor tissue using VENTANA PD-L1 SP263 assay (VENTANA BenchMark ULTRA platform). Correlation with efficacy and PD-L1 positivity status was assessed by a cutoff of tumor proportion score (TPS) ≥1% or 5%, and combined positive score (CPS) ≥1. or PD-L1 protein expression was determined using TPS, which was the percentage of viable tumor cells showing partial or complete membrane staining at any intensity. CPS, which was the number of PD-L1–stained cells (tumor cells, lymphocytes, and macrophages) divided by the total number of viable tumor cells and multiplied by 100, was also determined. All interpretations were conducted by pathologists.
Quantification and statistical analysis
Statistical analysis
The enrolled analysis set included all patients who provided consent and subsequently enrolled into the study. The DLT-evaluable set included all patients enrolled in the dose-escalation cohort who had a DLT in the first 4 weeks of the study, or those who did not have a DLT and received at least 90% of the intended cadonilimab dose and completed follow-up through the 28-day DLT evaluation period. The full analysis set included all patients in the enrolled analysis set who received any amount of cadonilimab and had measurable disease at baseline. The safety analysis set included all enrolled patients who received any amount of cadonilimab and had had at least one safety assessment.
Additional resources
This study was registered with ClinicalTrials.gov, number NCT03261011.
Acknowledgments
This study was supported by Akeso Biopharma, Inc., Zhongshan, China. The authors thank all the patients who participated in this study and the investigators and research staff at all study sites. Benchao Chen, Jiawen Tang, and Min Zhang from Akeso Biopharma contributed to PK and PD data analysis, and Yumei Shi and Cunnan Dong from Akeso Biopharma provided professional assistance for the medical review and manuscript preparation. Editorial assistance was provided by Joyce Lee, PhD, CMPP of Nucleus Global Asia Pacific, funded by Akeso Biopharma in accordance with Good Publication Practice (GPP3) guidelines.
Author contributions
S.F., H.K.G., R.C., J.C., B.T., and M.M. were involved in conducting and overseeing the clinical trial as the principal investigators at their study sites. J.D. was involved in conducting and overseeing the clinical trial as the coordinating principal investigator. S.F., H.K.G., R.C., J.C., B.T., M.M., Y.Z., and W.W. were involved in review of medical data. Y.Z. and W.W. were responsible for collection of medical data. S.F., H.K.G., R.C., J.C., B.T., M.M., Y.Z., W.W., D.X., M.W., B.L., M.X., and J.D. were involved in interpretation of the results. S.F., H.K.G., R.C., J.C., B.T., M.M., Y.Z., W.W., D.X., M.W., B.L., M.X., and J.D. were involved in the writing, review, and editing of the manuscript draft. H.K.G., R.C., J.C., B.T., and M.M. were involved in patient recruitment. D.X., M.W., B.L., M.X., and J.D. were involved in the conceptualization of the study. D.X., M.W., B.L., and M.X. were involved in the supervision of the study. All authors read and approved the final manuscript.
Declaration of interests
S.F. has received research funding and honoraria from Amgen; has consulted and advised for Akeso Biopharma and Merck Sharpe and Dohme (MSD); and has received support from Amgen for attending meetings and/or travel and industry trial sponsorship to study site (principal investigator of multiple studies) from Akeso Biopharma, Ambrax, Amgen, AstraZeneca, Aulos, Bristol-Myers Squibb (BMS), BeiGene, Cullinan, Daiichi Sankyo, Edison Oncology, MSD, Pfizer, Takeda, HaiHe Biopharma, Vivace, and WMS. H.K.G. has consulted/advised for BMS, Curis, Merck Serono, and Telix. B.T. has received research funding from Amgen, Astellas, AstraZeneca, Bayer, BMS, Genentech, Ipsen, Janssen, Pfizer, Movember, and MSD; has received honoraria from Amgen, Astellas, AstraZeneca, Bayer, BMS, Ipsen, Janssen, Merck, MSD, Pfizer, Sanofi, and Tolmar; and has served in a consulting/advisory role for Amgen, Astellas, AstraZeneca, Bayer, BMS, Ipsen, IQVIA, Janssen, Merck, MSD, Novartis, Pfizer, Roche, Sanofi, and Tolmar. M.M. has received research funding from BMS and honoraria from BMS, Roche, and the Limbic; has consulted/advised for MSD, the Limbic, Roche, BMS, Takeda, Guardant Health, BeiGene Australia, Amgen, Merck, Lilly, and Novartis; and has received support for attending meetings and/or travel from AstraZeneca. Y.Z., W.W., D.X., M.W., B.L., and M.X. are employees of Akeso Biopharma. J.D. has received institutional research funding from Amgen, AstraZeneca, BeiGene,BMS, GlaxoSmithKline, Roche/Genentech, and Eli Lilly and has consulted/advised for Pierre Fabre, Pfizer, Lilly, Merck KGaA, Roche, Boehringer Ingelheim, Novartis, Antengene, and BeiGene.
Published: October 17, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xcrm.2023.101242.
Supplemental information
Data and code availability
-
•
De-identified data reported in this paper will be shared by the lead contact upon request.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- 1.Brahmer J.R., Lacchetti C., Schneider B.J., Atkins M.B., Brassil K.J., Caterino J.M., Chau I., Ernstoff M.S., Gardner J.M., Ginex P., et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2018;36:1714–1768. doi: 10.1200/JCO.2017.77.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pan C., Liu H., Robins E., Song W., Liu D., Li Z., Zheng L. Next-generation immuno-oncology agents: current momentum shifts in cancer immunotherapy. J. Hematol. Oncol. 2020;13 doi: 10.1186/s13045-020-00862-w. 29-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Myers Squibb B. 2022. OPDIVO U.S. Prescribing Information. [Google Scholar]
- 4.Myers Squibb B. 2022. YERVOY U.S. Prescribing Information. [Google Scholar]
- 5.Baas P., Scherpereel A., Nowak A.K., Fujimoto N., Peters S., Tsao A.S., Mansfield A.S., Popat S., Jahan T., Antonia S., et al. First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): a multicentre, randomised, open-label, phase 3 trial. Lancet. 2021;397:375–386. doi: 10.1016/S0140-6736(20)32714-8. [DOI] [PubMed] [Google Scholar]
- 6.Hellmann M.D., Rizvi N.A., Goldman J.W., Gettinger S.N., Borghaei H., Brahmer J.R., Ready N.E., Gerber D.E., Chow L.Q., Juergens R.A., et al. Nivolumab plus ipilimumab as first-line treatment for advanced non-small-cell lung cancer (CheckMate 012): results of an open-label, phase 1, multicohort study. Lancet Oncol. 2017;18:31–41. doi: 10.1016/S1470-2045(16)30624-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Larkin J., Chiarion-Sileni V., Gonzalez R., Grob J.J., Cowey C.L., Lao C.D., Schadendorf D., Dummer R., Smylie M., Rutkowski P., et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N. Engl. J. Med. 2015;373:23–34。. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lenz H.-J., Van Cutsem E., Luisa Limon M., Wong K.Y.M., Hendlisz A., Aglietta M., García-Alfonso P., Neyns B., Luppi G., Cardin D.B., et al. First-line nivolumab plus low-dose ipilimumab for microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer: the phase II CheckMate 142 study. J. Clin. Oncol. 2022;40:161–170. doi: 10.1200/JCO.21.01015. [DOI] [PubMed] [Google Scholar]
- 9.Motzer R.J., Tannir N.M., McDermott D.F., Arén Frontera O., Melichar B., Choueiri T.K., Plimack E.R., Barthélémy P., Porta C., George S., et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N. Engl. J. Med. 2018;378:1277–1290. doi: 10.1056/NEJMoa1712126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kudo M. Durvalumab plus tremelimumab: A novel combination immunotherapy for unresectable hepatocellular carcinoma. Liver Cancer. 2022;11:87–93. doi: 10.1159/000523702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paz-Ares L., Chen Y., Reinmuth N., Hotta K., Trukhin D., Statsenko G., Hochmair M.J., Özgüroğlu M., Ji J.H., Garassino M.C., et al. Durvalumab, with or without tremelimumab, plus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer: 3-year overall survival update from CASPIAN. ESMO Open. 2022;7 doi: 10.1016/j.esmoop.2022.100408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang B.-C., Li P.-C., Fan J.-Q., Lin G.-H., Liu Q. Durvalumab and tremelimumab combination therapy versus durvalumab or tremelimumab monotherapy for patients with solid tumors: A systematic review and meta-analysis. Medicine. 2020;99 doi: 10.1097/MD.0000000000021273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abdel-Rahman O., Fouad M. Risk of pneumonitis in cancer patients treated with immune checkpoint inhibitors: a meta-analysis. Ther. Adv. Respir. Dis. 2016;10:183–193. doi: 10.1177/1753465816636557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jin C., Zhang X., Zhao K., Xu J., Zhao M., Xu X. The efficacy and safety of nivolumab in the treatment of advanced melanoma: a meta-analysis of clinical trials. OncoTargets Ther. 2016;9:1571–1578. doi: 10.2147/OTT.S96762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hodi F.S., Chesney J., Pavlick A.C., Robert C., Grossmann K.F., McDermott D.F., Linette G.P., Meyer N., Giguere J.K., Agarwala S.S., et al. Combined nivolumab and ipilimumab versus ipilimumab alone in patients with advanced melanoma: 2-year overall survival outcomes in a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol. 2016;17:1558–1568. doi: 10.1016/S1470-2045(16)30366-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hodi F.S., O'Day S.J., McDermott D.F., Weber R.W., Sosman J.A., Haanen J.B., Gonzalez R., Robert C., Schadendorf D., Hassel J.C., et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bellmunt J., De Wit R., Vaughn D.J., Fradet Y., Lee J.-L., Fong L., Vogelzang N.J., Climent M.A., Petrylak D.P., Choueiri T.K., et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N. Engl. J. Med. 2017;376:1015–1026. doi: 10.1056/NEJMoa1613683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reck M., Rodríguez-Abreu D., Robinson A.G., Hui R., Csőszi T., Fülöp A., Gottfried M., Peled N., Tafreshi A., Cuffe S., et al. Pembrolizumab versus chemotherapy for PD-L1–positive non–small-cell lung cancer. N. Engl. J. Med. 2016;375:1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 19.Balar A.V., Galsky M.D., Rosenberg J.E., Powles T., Petrylak D.P., Bellmunt J., Loriot Y., Necchi A., Hoffman-Censits J., Perez-Gracia J.L., et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet. 2017;389:67–76. doi: 10.1016/S0140-6736(16)32455-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaufman H.L., Russell J., Hamid O., Bhatia S., Terheyden P., D'Angelo S.P., Shih K.C., Lebbé C., Linette G.P., Milella M., et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. Lancet Oncol. 2016;17:1374–1385. doi: 10.1016/S1470-2045(16)30364-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patel M.R., Ellerton J., Infante J.R., Agrawal M., Gordon M., Aljumaily R., Britten C.D., Dirix L., Lee K.-W., Taylor M., et al. Avelumab in metastatic urothelial carcinoma after platinum failure (JAVELIN Solid Tumor): pooled results from two expansion cohorts of an open-label, phase 1 trial. Lancet Oncol. 2018;19:51–64. doi: 10.1016/S1470-2045(17)30900-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahmadzadeh M., Johnson L.A., Heemskerk B., Wunderlich J.R., Dudley M.E., White D.E., Rosenberg S.A. Tumor antigen–specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114:1537–1544. doi: 10.1182/blood-2008-12-195792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pang X., Huang Z., Zhong T., Zhang P., Wang Z.M., Xia M., Li B. Cadonilimab, a tetravalent PD-1/CTLA-4 bispecific antibody with trans-binding and enhanced target binding avidity. mAbs. 2023;15:2180794. doi: 10.1080/19420862.2023.2180794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roselló S., Blasco I., García Fabregat L., Cervantes A., Jordan K., ESMO Guidelines Committee Management of infusion reactions to systemic anticancer therapy: ESMO Clinical Practice Guidelines. Ann. Oncol. 2017;28:iv100–iv118. doi: 10.1093/annonc/mdx216. [DOI] [PubMed] [Google Scholar]
- 25.Tran B., Voskoboynik M., Kim S.W., Lemech C., Carcereny E., Rha S.Y., Ahn M.J., Felip E., Lee K.H., Alvarez E.C., et al. Abstract CT016: MEDI5752, a novel PD-1/CTLA-4 bispecific checkpoint inhibitor for advanced solid tumors: First-in-human study[J] Cancer Res. 2022;82(Supplement):CT016. [Google Scholar]
- 26.Berezhnoy A., Sumrow B.J., Stahl K., Shah K., Liu D., Li J., Hao S.S., De Costa A., Kaul S., Bendell J., et al. Development and Preliminary Clinical Activity of PD-1-Guided CTLA-4 Blocking Bispecific DART Molecule. Cell Rep. Med. 2020;1 doi: 10.1016/j.xcrm.2020.100163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang D.Y., Salem J.-E., Cohen J.V., Chandra S., Menzer C., Ye F., Zhao S., Das S., Beckermann K.E., Ha L., et al. Fatal toxic effects associated with immune checkpoint inhibitors: A systematic review and meta-analysis. JAMA Oncol. 2018;4:1721–1728. doi: 10.1001/jamaoncol.2018.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McDermott D.F., Lee J.-L., Ziobro M., Suárez Rodríguez C., Langiewicz P., Matveev V.B., Wiechno P., Gafanov R.A., Tomczak P., Pouliot F., et al. Open-label, single-arm, phase II study of pembrolizumab monotherapy as first-line therapy in patients with advanced non–clear cell renal cell carcinoma. J. Clin. Oncol. 2021;39:1029–1039. doi: 10.1200/JCO.20.02365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gupta R., Ornstein M.C., Li H., Allman K.D., Wood L.S., Gilligan T., Garcia J.A., Merveldt D.V., Hammers H.J., Rini B.I. Clinical activity of ipilimumab plus nivolumab in patients with metastatic non–clear cell renal cell carcinoma. Clin. Genitourin. Cancer. 2020;18:429–435. doi: 10.1016/j.clgc.2019.11.012. [DOI] [PubMed] [Google Scholar]
- 30.Tykodi S.S., Gordan L.N., Alter R.S., Arrowsmith E., Harrison M.R., Percent I., Singal R., Van Veldhuizen P., George D.J., Hutson T., et al. Safety and efficacy of nivolumab plus ipilimumab in patients with advanced non-clear cell renal cell carcinoma: results from the phase 3b/4 CheckMate 920 trial. J. Immunother. Cancer. 2022;10 doi: 10.1136/jitc-2021-003844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scherpereel A., Mazières J., Greillier L., Lantuéjoul S., Dô P., Bylicki O., Monnet I., Corre R., Audigier-Valette C., Locatelli-Sanchez M., et al. Nivolumab or nivolumab plus ipilimumab in patients with relapsed malignant pleural mesothelioma (IFCT-1501 MAPS2): a multicentre, open-label, randomised, non-comparative, phase 2 trial. Lancet Oncol. 2019;20:239–253. doi: 10.1016/S1470-2045(18)30765-4. [DOI] [PubMed] [Google Scholar]
- 32.Antonia S., Goldberg S.B., Balmanoukian A., Chaft J.E., Sanborn R.E., Gupta A., Narwal R., Steele K., Gu Y., Karakunnel J.J., Rizvi N.A. Safety and antitumour activity of durvalumab plus tremelimumab in non-small cell lung cancer: a multicentre, phase 1b study. Lancet Oncol. 2016;17:299–308. doi: 10.1016/S1470-2045(15)00544-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duraiswamy J., Kaluza K.M., Freeman G.J., Coukos G. Dual Blockade of PD-1 and CTLA-4 Combined with Tumor Vaccine Effectively Restores T-Cell Rejection Function in Tumors. Cancer Res. 2013;73:3591–3603. doi: 10.1158/0008-5472.CAN-12-4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berezhnoy A., Sumrow B.J., Stahl K., Shah K., Liu D., Li J., Hao S.-S., De Costa A., Kaul S., Bendell J., et al. Development and preliminary clinical activity of PD-1-guided CTLA-4 blocking bispecific DART molecule. Cell Rep. Med. 2020;1 doi: 10.1016/j.xcrm.2020.100163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Goeje P.L., Poncin M., Bezemer K., Kaijen-Lambers M.E.H., Groen H.J.M., Smit E.F., Dingemans A.-M.C., Kunert A., Hendriks R.W., Aerts J.G.J.V. Induction of peripheral effector CD8 T-cell proliferation by combination of paclitaxel, carboplatin, and bevacizumab in non–small cell lung cancer patients. Clin. Cancer Res. 2019;25:2219–2227. doi: 10.1158/1078-0432.CCR-18-2243. [DOI] [PubMed] [Google Scholar]
- 36.Merchant M.S., Wright M., Baird K., Wexler L.H., Rodriguez-Galindo C., Bernstein D., Delbrook C., Lodish M., Bishop R., Wolchok J.D., et al. Phase I clinical trial of ipilimumab in pediatric patients with advanced solid tumors. Clin. Cancer Res. 2016;22:1364–1370. doi: 10.1158/1078-0432.CCR-15-0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
De-identified data reported in this paper will be shared by the lead contact upon request.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.