Abstract
Background
Clinical trials have shown success in bleed prevention with emicizumab, but real-world data on the effectiveness of emicizumab in preventing serious bleeds in the pediatric population are lacking.
Objectives
To report real-world data on the effectiveness of Emicizumab in pediatric persons with hemophilia A.
Methods
We completed a retrospective chart review of 37 pediatric male patients aged ≤18 years on emicizumab prophylaxis for a median duration of 30.5 months at Children’s Medical Center in Dallas, Texas.
Results
We identified 4 pediatric persons with severe hemophilia A with and without inhibitors who experienced a provoked or unprovoked serious bleed requiring hospitalization.
Conclusion
This study highlights that serious bleeds, both provoked and unprovoked, can occur in pediatric persons with severe hemophilia A. These findings are important for clinicians to provide appropriate counseling/education and recommendation of treatment for pediatric persons with severe hemophilia A through shared decision making. Up-titration of emicizumab or factor VIII replacement needs consideration in persons with hemophilia with suboptimal bleeding control or who participate in activities categorized as moderate- to high-risk activities.
Keywords: bleed, emicizumab, hemophilia A, pediatric, prophylaxis
Essentials
-
•
Real-world data on the effectiveness of emicizumab in preventing serious bleeds are lacking.
-
•
We reviewed charts of 37 pediatric persons with hemophilia A on emicizumab.
-
•
We found 4 pediatric persons with severe hemophilia A on emicizumab with a serious bleed.
-
•
Up-titration of emicizumab or factor VIII replacement needs consideration for serious bleeds.
1. Introduction
Nonfactor substitution therapy was recently introduced into hemophilia care. Emicizumab, the first approved agent in this therapeutic class, is a recombinant, humanized, bispecific monoclonal antibody that bridges factors IXa and X, substituting the role of factor VIIIa to promote hemostasis in persons with hemophilia A [1]. Emicizumab reduces annualized bleeding rates (ABRs) in persons with hemophilia A on emicizumab prophylaxis with and without inhibitors [[2], [3], [4]]. It is injected subcutaneously at 1.5 mg/kg once weekly, 3 mg/kg every 2 weeks, or 6 mg/kg every 4 weeks after a 4-week loading dose phase of 3 mg/kg weekly. The ease of administration via the subcutaneous route and less frequent dosing are appealing to patients [5]. Despite the increase in the utilization of emicizumab, real-world data on the effectiveness of emicizumab in preventing bleeds, particularly with serious bleeds, are scant. We report our experience with serious iliopsoas bleeding in 4 pediatric persons with hemophilia A with and without inhibitors on emicizumab prophylaxis at a single institution.
2. Methods
This was an institutional review board–approved retrospective chart review where we reviewed medical records of persons with hemophilia A who received emicizumab prophylaxis between March 2019 and October 2022 at the Bleeding Disorders and Thrombosis Program at the University of Texas Southwestern, Dallas, Texas. We collected demographics, baseline hemophilia history, details surrounding bleeds, and laboratory information in persons with hemophilia A with and without inhibitors who were on emicizumab prophylaxis.
3. Results and Discussion
Our single-institution review included charts from 37 males aged ≤18 years on emicizumab prophylaxis (median duration of treatment was 33.5 months); among them, 92% had severe hemophilia A (SHA) (n = 34) and 8% had moderate hemophilia A (n = 3); the majority were White (51%; N = 19), followed by Hispanic (30%), Black (11%), and Asian (8%). We identified 4 persons with SHA with and without inhibitors who experienced a serious bleed (provoked and unprovoked) requiring hospitalization.
3.1. Case 1
A 17-year-old Hispanic boy with SHA without an inhibitor on 1.5 mg/kg weekly emicizumab prophylaxis presented with a 2-day history of worsening pain in his lower back, left hip, and thigh and left thigh numbness after wrestling with his friends. The patient had been on emicizumab for 26.4 months with an ABR of 0.45. He reported no missed doses of emicizumab and had previously reported 1 provoked toe bleed while playing soccer. Computed tomography of the abdomen/pelvis revealed an asymmetric enlargement and heterogeneity of the left iliacus and psoas muscles (Figure 1A, B). His hemoglobin (Hgb) level at an outside hospital was 7.4 g/dL, which was decreased from his previous Hgb level of 14.1 g/dL that was measured at his last clinic visit (6 months prior). He received a packed red blood cell transfusion and was transferred to our hospital. His presenting Hgb at our institution was 8.8 g/dL. Partial thromboplastin time (PTT) was not obtained on admission. For this provoked iliopsoas bleed, he was treated with scheduled recombinant factor VIII (rFVIII), was initially placed on strict bed rest, and was allowed to have increased activity with a physical therapist (PT) once his pain and hip extension improved. He was hospitalized for 4 days, with a plan to continue rFVIII for one more day and exercises provided by a PT. Given that he did not have a previous spontaneous bleed while on emicizumab prophylaxis, he continued weekly dosing of emicizumab. He was not evaluated for antidrug antibodies or emicizumab concentration.
Figure 1.
(A, B) Heterogeneous enlargement of the psoas and iliacus muscles (arrows) on computed tomography, consistent with a hematoma in case 1.
3.2. Case 2
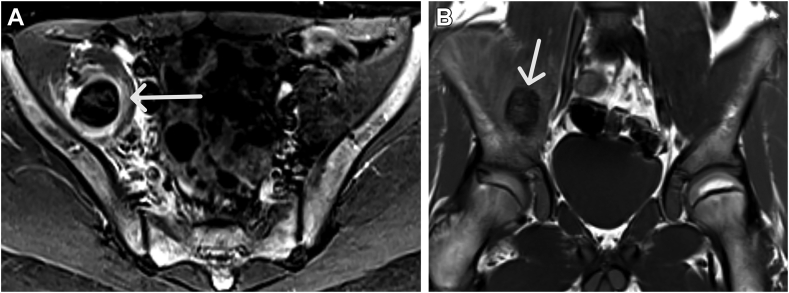
A 14-year-old Black boy with SHA without an inhibitor on 1.5 mg/kg weekly emicizumab prophylaxis presented with a 1.5-week history of right leg pain and swelling and right thigh numbness after sitting and playing video games. The patient had been on emicizumab for 14 months, with an ABR of 0 while on emicizumab. He reported no missed doses of emicizumab. Magnetic resonance imaging of the abdomen/pelvis revealed a hematoma within the right iliacus muscle extending into the right iliopsoas muscle (Figure 2A, B). His Hgb level on arrival was 9.8 g/dL, which had decreased from the previous Hgb level of 12.3 g/dL at his last clinic visit (6 months prior). PTT was not obtained on admission. During his hospitalization, he received scheduled rFVIII, was initially placed on strict bed rest, and was allowed to have increased activity with a PT once his pain and hip extension improved. He was admitted to the hospital for 3 days and discharged with the plan to continue outpatient physical therapy and daily rFVIII until pain resolved. He received 10 days of rFVIII. At a subsequent clinic visit (1.5 years later), his PTT was normal at 24.8 seconds and endogenous thrombin potential (ETP) was 202 nM/min. Given no other spontaneous bleed, he continued weekly dosing of emicizumab and was not evaluated for antidrug antibodies or emicizumab concentration.
Figure 2.
(A, B) Complex hyperintense STIR and hyperintense to muscle T1-W hematoma in the right iliacus muscle (arrows) in case 2. STIR, short-TI inversion recover; T1-W, T1 weighted.
3.3. Case 3
A 16-year-old Hispanic boy with SHA without inhibitor on 3.0 mg/kg biweekly emicizumab presented with a 4-day history of progressive pain/tingling in the left thigh and hip and decreased range of motion while walking for a prolonged period at the local state fair. He had been on emicizumab for 40 months with an ABR of 0 while on emicizumab prophylaxis. He reported no missed doses of emicizumab. Computed tomography of the abdomen/pelvis showed an intramuscular hematoma of the right iliopsoas muscle measuring 2.4 × 2.1 × 7.5 cm (Figure 3A, B). His Hgb level on arrival was 14.8 g/dL, which was previously 15 g/dL at his last clinic visit (3 months prior). His PTT on presentation was normal at 27 seconds. At a previous clinic visit (6 months prior), his ETP was 289 nM/min. During his hospitalization, he received scheduled rFVIII, was initially placed on strict bed rest, and was allowed increased physical activity with a PT once his pain and hip extension improved. He was admitted for 4 days and was discharged with the plan to continue exercise given by a PT and daily rFVIII until pain resolved. He received rFVIII for a total of 7 days. Given no other spontaneous bleed, he continued weekly dosing of emicizumab and was not evaluated for antidrug antibodies or emicizumab concentration.
Figure 3.
(A, B) Diffuse enlargement and mixed high attenuation throughout the left iliopsoas muscle (arrows) in case 3.
3.4. Case 4
A 12-year-old White boy with SHA with an inhibitor on 3 mg/kg biweekly emicizumab prophylaxis with no reported missed doses for 17 months and an ABR of 1.40 (previously provoked knee and thigh bleed) presented with a 4-day history of right groin pain after jumping on a trampoline 1 day prior to onset of pain. Magnetic resonance imaging of the hip revealed intramuscular fluid collection within the left iliopsoas muscle measuring 6.1 × 5.1 × 12.9 cm (Figure 4A, B). His Hgb level on arrival was 12.3 g/dL, and he had a previous Hgb level of 14.2 g/dL at his last clinic visit (3 months prior). PTT was not obtained on admission. He received scheduled recombinant factor VIIa (rFVIIa), was placed on strict bed rest, and was allowed increased physical activity with a PT once his pain and hip extension improved. He was admitted to the hospital for 4 days and discharged with a plan to continue rFVIIa until pain resolved. He received 10 days of rFVIIa due to continued pain. His emicizumab was changed from biweekly to weekly dosing, given the bleed. He was not evaluated for antidrug antibodies or emicizumab concentration given the provoked nature of his bleed.
Figure 4.
(A, B) Hypointense on STIR and T1-W region in the right iliacus muscle (arrows), consistent with a hematoma that contains older blood products in case 4. STIR, short-TI inversion recover; T1-W, T1 weighted.
Herein, we report real-world data of pediatric persons with hemophilia A with and without inhibitors on emicizumab prophylaxis at a single institution. Our experience shows serious bleeding in persons with hemophilia A on emicizumab prophylaxis, requiring hospitalization. These cases emphasize the importance that serious bleeds can occur both spontaneously and provoked in persons with hemophilia A on prophylaxis with emicizumab.
Recent real-world evidence indicates that, in some adults, standard emicizumab regimens cannot control all bleeds [6]. Schmitt et al. [7] reported maintenance dose up-titration in only 3.6% (24/675) due to suboptimal bleeding control. Up-titration was more frequently adopted in adults vs children/adolescents (only 3 were <12 years of age), those previously treated with prophylaxis, and those who received emicizumab 6 mg/kg every 4 weeks [7]. Another real-world report on bleeding patterns of pediatric persons with hemophilia A suggests that bleeding occurred in 43% (22/51) of their patients. Most of these bleeds were minor (29.4% [15/51]) that resolved spontaneously or with antifibrinolytics, and 19.6% (10/51) required additional FVIII treatment. Most of these bleeds were trauma-related and occurred on biweekly regimen of emicizumab prophylaxis [8]. In contrast, one case report by Barg et al. [9] reported a baby with SHA on emicizumab with circumcision bleeding not covered with FVIII replacement. Otherwise, other real-world data on both pediatric and adult patients showed that patients experienced low ABR [10,11], and the most prevalent bleed was traumatic musculoskeletal bleed [12]. In pediatrics alone, real-world data showed to be efficacious (12/13 patients experienced no bleeds) [13] and even eliminated target joints [14].
Steady-state emicizumab is reported to provide approximately 20% of FVIII-like activity [15]. At this level, a mild hemophilia phenotype is assumed with bleeding episodes when provoked. This could explain the bleeds in 2 of our cases participating in the National Hemophilia Foundation categorized moderate- to high-risk activities [16]. However, 2 cases experienced an iliopsoas bleed after being sedentary or walking. A limitation of our study is the lack of ABR data prior to initiating emicizumab prophylaxis. Perhaps these persons with hemophilia A have a more severe bleeding phenotype. Alternatively, these bleeds could be due to compliance issues, inadequate dosing, or the development of drug antibodies. However, all patients report taking their medication appropriately, and in our clinic, we track emicizumab prescriptions, follow patients every 6 months, and adjust dosing when needed. Another limitation of our study is not having PTT data at presentation for all cases. Although it only takes extremely low levels of emicizumab to shorten PTT [17], reassuringly, 2 out of 4 cases had normalized PTT (on presentation and later in clinic). Moreover, ETP (thrombin generation assay) in these same cases, measured prior to or after hospitalization, ranged between 202 and 289 nM/min. At this range, this can have a predicted FVIII activity level of slightly more than 20% [18]. We did not evaluate for drug antibodies, but all patients have not experienced another bleed to date, indirectly ruling out its presence, although antidrug antibodies could have also been transient [19]. Finally, 3 of our 4 cases were Hispanic or Black, which represents the patient population that our program serves. The impact of minority racial composition on clinical outcomes due to socioeconomic factors is plausible but could not be systematically assessed due to a small study at a single center.
While we did not up-titrate emicizumab in our cases, given that they presented with their first spontaneous bleed or bleed was considered provoked, up-titration of emicizumab in cases with suboptimal bleed coverage may need consideration. A dose up-titration to 3 mg/kg weekly of emicizumab has been reported to be well tolerated and resulted in improvement in bleed control for most participants [7]. Alternatively or in addition, factor VIII replacement should be considered, especially in cases where persons with hemophilia A are participating in moderate- to high-risk activities. Very recently available newer, longer-acting FVIII replacement therapies that showed levels >40 IU/dL for most of the week might be the best option for bleed coverage in persons with hemophilia A without inhibitors [20].
In conclusion, we report real-world data of serious bleeding in pediatric patients with SHA with and without inhibitors receiving emicizumab prophylaxis. These cases highlight that emicizumab prophylaxis does not normalize hemostasis and that there is potential for serious bleeds, which is important for clinicians to know to provide appropriate counseling/education and recommendation of treatment for persons with hemophilia A through shared decision making. In addition, repeated education/counseling to families on bleed recognition and early communication with their treatment team is crucial to initiate early and effective treatment to prevent morbidities associated with such bleeds. The World Federation of Hemophilia Treatment Guidelines for an iliopsoas bleed recommend at least 5 days of treatment with FVIII replacement and a longer duration of secondary prophylaxis with a PT [21]. We treated pediatric persons with hemophilia A with at least 5 days of FVIII replacement (longer with pain) and then continued emicizumab as prophylaxis with a PT. Moreover, emicizumab dose up-titration and/or FVIII replacement needs consideration for patients with suboptimal bleeding control, especially in patients who wish to participate in moderate- to high-risk physical activities. Head-to-head comparisons of current novel therapies and studies establishing up-titrating dosing regimens for suboptimal bleed controls are needed to establish individualized treatment plans for preventing bleeds in persons with hemophilia A.
Acknowledgments
Funding
A.Z. is funded by National Heart, Lung, and Blood Institute 1R01HL153963 and American Heart Association 20IPA35320263.
Ethics statement
Not applicable.
Author contributions
J.G. was responsible for conceiving the idea, obtaining and analyzing the data, and writing the manuscript. M.R.H. was responsible for obtaining radiologic images and for revision of the manuscript. A.Z. was responsible for analyzing the data and critical revision of the manuscript. All authors contributed to the literature search and to the approval of final manuscript.
Relationship Disclosure
There are no competing interests to disclose.
Footnotes
Handling Editor: Dr Bethany Samuelson Bannow
References
- 1.Kitazawa T., Igawa T., Sampei Z., Muto A., Kojima T., Soeda T., et al. A bispecific antibody to factors IXa and X restores factor VIII hemostatic activity in a hemophilia A model. Nat Med. 2012;18:1570–1574. doi: 10.1038/nm.2942. [DOI] [PubMed] [Google Scholar]
- 2.Mahlangu J., Oldenburg J., Paz-Priel I., Negrier C., Niggli M., Mancuso M.E., et al. Emicizumab prophylaxis in patients who have hemophilia A without Inhibitors. N Engl J Med. 2018;379:811–822. doi: 10.1056/NEJMoa1803550. [DOI] [PubMed] [Google Scholar]
- 3.Jimenez-Yuste V., Shima M., Fukutake K., Lehle M., Chebon S., Retout S., et al. Emicizumab subcutaneous dosing every 4 weeks for the management of hemophilia A: preliminary data from the pharmacokinetic run-in cohort of a multicenter, open-label, phase 3 study (HAVEN 4) Blood. 2017;130:86. [Google Scholar]
- 4.Pipe S.W., Shima M., Lehle M., Shapiro A., Chebon S., Fukutake K., et al. Efficacy, safety, and pharmacokinetics of emicizumab prophylaxis given every 4 weeks in people with haemophilia A (HAVEN 4): a multicentre, open-label, non-randomised phase 3 study. Lancet Haemotol. 2019;6:e295–e305. doi: 10.1016/S2352-3026(19)30054-7. [DOI] [PubMed] [Google Scholar]
- 5.Kempton C., Trask P., Parnes A., Niggli M., Campinha-Bacote A., Callaghan M.U., et al. Development and testing of the satisfaction questionnaire with intravenous or subcutaneous hemophilia injection and results from the phase 3 HAVEN 3 study of emicizumab prophylaxis in persons with haemophilia A without FVIII inhibitors. Haemophilia. 2021;27:221–228. doi: 10.1111/hae.14222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levy-Mendelovich S., Brutman-Barazani T., Budnik I., Avishai E., Barg A.A., Levy T., et al. Real-world data on bleeding patterns of hemophilia A patients treated with emicizumab. J Clin Med. 2021;10:4303. doi: 10.3390/jcm10194303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmitt C., Mancuso M.E., Chang T., Podolak-Dawidziak M., Petry C., Sidonio R., Jr., et al. Emicizumab dose up-titration in case of suboptimal bleeding control in people with haemophilia A. Haemophilia. 2023;29:90–99. doi: 10.1111/hae.14679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hassan E., Motwani J. Breakthrough bleeding episodes in pediatric severe hemophilia A patients with and without inhibitors receiving emicizumab prophylaxis: a single-center retrospective review. Pediatr Hematol Oncol. 2022;39:418–426. doi: 10.1080/08880018.2021.2004269. [DOI] [PubMed] [Google Scholar]
- 9.Barg A.A., Budnik I., Avishai E., Brutman-Barazani T., Bashari D., Misgav M., et al. Emicizumab prophylaxis: prospective longitudinal real-world follow-up and monitoring. Haemophilia. 2021;27:383–391. doi: 10.1111/hae.14318. [DOI] [PubMed] [Google Scholar]
- 10.McCary I., Guelcher C., Kuhn J., Butler R., Massey G., Guerrera M.F., et al. Real-world use of emicizumab in patients with haemophilia A: bleeding outcomes and surgical procedures. Haemophilia. 2020;26:631–636. doi: 10.1111/hae.14005. [DOI] [PubMed] [Google Scholar]
- 11.Ebbert P.T., Xavier F., Seaman C.D., Ragni M.V. Emicizumab prophylaxis in patients with haemophilia A with and without inhibitors. Haemophilia. 2020;26:41–46. doi: 10.1111/hae.13877. [DOI] [PubMed] [Google Scholar]
- 12.Warren B.B., Chan A., Manco-Johnson M., Branchford B.R., Buckner T.W., Moyer G., et al. Emicizumab initiation and bleeding outcomes in people with hemophilia A with and without inhibitors: a single-center report. Res Pract Thromb Haemost. 2021;5 doi: 10.1002/rth2.12571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Glonnegger H., Andresen F., Kapp F., Malvestiti S., Büchsel M., Zieger B. Emicizumab in children: bleeding episodes and outcome before and after transition to emicizumab. BMC Pediatr. 2022;22:487. doi: 10.1186/s12887-022-03546-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu G., Huang K., Li G., Zhen Y., Li Z., Chen Z., et al. Real-world experience of emicizumab prophylaxis in young children with hemophilia A: retrospective data from China. Front Pediatr. 2022;10 doi: 10.3389/fped.2022.992267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmitt C., Adamkewicz J.I., Xu J., Petry C., Catalani O., Young G., et al. Pharmacokinetics and pharmacodynamics of emicizumab in persons with hemophilia A with factor VIII inhibitors: HAVEN 1 study. Thromb Haemost. 2021;121:351–360. doi: 10.1055/s-0040-1717114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Hemophilia Foundation Playing it safe – bleeding disorders, sports and exercise. 2017. https://vwdconnect.org/wp-content/uploads/2018/02/Playing-It-Safe.pdf
- 17.Müller J., Pekrul I., Pötzsch B., Berning B., Oldenburg J., Spannagl M. Laboratory monitoring in emicizumab-treated persons with hemophilia A. Thromb Haemost. 2019;119:1384–1393. doi: 10.1055/s-0039-1692427. [DOI] [PubMed] [Google Scholar]
- 18.Kizilocak H., Marquez-Casas E., Malvar J., Carmona R., Young G. Determining the approximate factor VIII level of patients with severe haemophilia A on emicizumab using in vivo global haemostasis assays. Haemophilia. 2021;27:730–735. doi: 10.1111/hae.14359. [DOI] [PubMed] [Google Scholar]
- 19.Schmitt C., Emrich T., Chebon S., Fernandez E., Petry C., Yoneyama K., et al. Low immunogenicity of emicizumab in persons with haemophilia A. Haemophilia. 2021;27:984–992. doi: 10.1111/hae.14398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.von Drygalski A., Chowdary P., Kulkarni R., Susen S., Konkle B.A., Oldenburg J., et al. Efanesoctocog alfa prophylaxis for patients with severe hemophilia A. N Engl J Med. 2023;388:310–318. doi: 10.1056/NEJMoa2209226. [DOI] [PubMed] [Google Scholar]
- 21.Srivastava A., Santagostino E., Dougall A., Kitchen S., Sutherland M., Pipe S.W., et al. WFH Guidelines for the management of hemophilia, 3rd edition. Haemophilia. 2020;26:1–158. doi: 10.1111/hae.14046. [DOI] [PubMed] [Google Scholar]