Summary
Chiral 1,2,3,4-tetrahydroquinoxalines are ubiquitous in natural products and bioactive molecules. Herein, we disclose a protocol for stereodivergent asymmetric hydrogenation of disubstituted quinoxalines for the preparation of both cis- and trans-enantioenriched disubstituted tetrahydroquinoxalines (up to >20:1 d.r. and 99% ee). We describe steps for synthesis of ligands and substrate, setup of hydrogenation of disubstituted quinoxalines, and purification of products. Additionally, we provide detailed diagrams of the hydrogenation installation.
For complete details on the use and execution of this protocol, please refer to Liu et al.1
Subject areas: NMR, Mass Spectrometry, Chemistry
Graphical abstract

Highlights
-
•
Asymmetric reaction catalyzed by earth-abundant manganese
-
•
Ligand controlled stereodivergent asymmetric hydrogenation
-
•
Construction of vicinal stereocenters
-
•
Efficient access to 1,2,3,4-tetrahydroquinoxalines
Publisher’s note: Undertaking any experimental protocol requires adherence to local institutional guidelines for laboratory safety and ethics.
Chiral 1,2,3,4-tetrahydroquinoxalines are ubiquitous in natural products and bioactive molecules. Herein, we disclose a protocol for stereodivergent asymmetric hydrogenation of disubstituted quinoxalines for the preparation of both cis- and trans-enantioenriched disubstituted tetrahydroquinoxalines (up to >20:1 d.r. and 99% ee). We describe steps for synthesis of ligands and substrate, setup of hydrogenation of disubstituted quinoxalines, and purification of products. Additionally, we provide detailed diagrams of the hydrogenation installation.
Before you begin
Chiral 1,2,3,4-tetrahydroquinoxalines (THQs) are important heterocyclic structures that are widely present in numerous bioactive molecules.2,3 Asymmetric hydrogenation of readily available quinoxalines provides a straightforward method to access chiral THQs4,5,6). In this regard, asymmetric hydrogenation of mono-substituted quinoxaline substrates has been well developed.7,8,9,10 In contrast, there are few reports on asymmetric hydrogenation of disubstituted quinoxalines, affording mainly cis-selective products.11,12 The trans-selective hydrogenation of ortho di-substituted aromatic compounds is extremely challenging. It is because the face selectivity of the second hydrogenation is strongly induced by the preformed ortho-stereocenter in the first hydrogenation step, resulting in the generation of cis-products with significant superiority.13,14 Therefore, in order to meet the demand for trans-selective products, powerful chiral catalysts with a high level of stereoselectivity control are required to reverse the face selectivity of the second hydrogenation. So far, the only example was reported by Fan and co-workers using a half-sandwich Ru(II) complex in 2011.15 However, the range of substrates was limited to 2,3-dialkyl substituted quinoxaline with small steric hindrance.
Despite these efforts in recent years, the stereodivergent asymmetric hydrogenation of 2,3-disubstituted quinoxalines to access both cis- and trans-chiral THQs remains elusive. The above transformation faces three challenges: (1) the racemization resulting from tautomerism of imine and enamine moieties of semi-hydrogenated intermediates; (2) the induction effect of preformed chiral center for the second hydrogenation step; and (3) the control of reaction sequence of two C-N double bonds in two consecutive hydrogenation steps.
Following our research interest in Mn-catalyzed asymmetric hydrogenation of N-heterocycles,16,17,18,19 we developed an efficient and selective asymmetric hydrogenation of di-substituted quinoxalines via Mn catalysis. The current protocol describes the specific steps for the stereodivergent asymmetric hydrogenation of disubstituted quinoxalines to provide all four stereoisomers of the target THQ products. For the same experiment performed in batch, please refer to Liu et al. (2023).1
Preparation of the reagents and equipment
A complete list of reagents and equipment can be found in the ‘‘key resources table’’ and ‘‘preparation” parts.
Preparation of chiral PNN tridentate ligands
Timing: 30 h
-
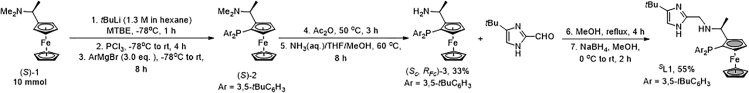
1.Synthesize ligands as the following route (see Figure 1):
-
a.Place (S)-1 (10 mmol, 2.57 g) in a 100 mL flame dried round bottom Schlenk flask equipped with a stir bar.
-
b.Replace the atmosphere of the flask with argon for three times.
-
c.Dissolve (S)-1 (10 mmol, 2.57 g) with anhydrous MTBE (20 mL).
-
d.Add tBuLi ((8.5 mL, 11 mmol, 1.3 M in pentane) dropwise (∼1 mL/min) to the solution with a 10 mL luer lock syringe at ‒78°C (dry ice/acetone bath) and leave the reaction mixture stir for 1 h.
 CRITICAL: Round bottom Schlenk flask, stirring bar and syringe needle (φ0.8 × 200 mm) need to be dried in the oven (80°C–90°C). The moisture content of MTBE is less than 50 ppm. The connection point between the syringe and the needle should be sealed with parafilm to avoid liquid leakage. A double layer argon balloon is needed to protect the tert-butyllithium storage liquid. Please secure the tert-butyl lithium bottle with iron frame and clamp. Before using tert-butyllithium, insert the syringe into the reaction flask that has been previously replaced with argon atmosphere, draw a small amount of argon gas, pull out the needle and purge. Repeat three times to ensure that the syringe is filled with argon. Under the protection of double-layer argon balloons, put the needle of the syringe under the liquid level of tert butyl lithium to extract a certain amount. After leaving the liquid level, put the syringe upward and extract some argon as protection. During the transfer process, keep the syringe always upward.Note: After pulling out the syringe from the reaction flask, the residual tert butyl lithium in the syringe needs to be diluted with 10 mL of anhydrous THF immediately, and then quenched with methanol.
CRITICAL: Round bottom Schlenk flask, stirring bar and syringe needle (φ0.8 × 200 mm) need to be dried in the oven (80°C–90°C). The moisture content of MTBE is less than 50 ppm. The connection point between the syringe and the needle should be sealed with parafilm to avoid liquid leakage. A double layer argon balloon is needed to protect the tert-butyllithium storage liquid. Please secure the tert-butyl lithium bottle with iron frame and clamp. Before using tert-butyllithium, insert the syringe into the reaction flask that has been previously replaced with argon atmosphere, draw a small amount of argon gas, pull out the needle and purge. Repeat three times to ensure that the syringe is filled with argon. Under the protection of double-layer argon balloons, put the needle of the syringe under the liquid level of tert butyl lithium to extract a certain amount. After leaving the liquid level, put the syringe upward and extract some argon as protection. During the transfer process, keep the syringe always upward.Note: After pulling out the syringe from the reaction flask, the residual tert butyl lithium in the syringe needs to be diluted with 10 mL of anhydrous THF immediately, and then quenched with methanol. -
e.Add PCl3 (0.87 mL, 10 mmol, 1.37 g) dropwise to the mixture with a 2.5 mL syringe at ‒78°C (dry ice/acetone bath).Note: The residual PCl3 in the syringe needs to be quenched with ice water in the fume hood.
-
f.Warm up the reaction mixture to 25°C and stir for 4 h.
-
g.Add 3,5-ditertbutylphenyl magnesium bromide (30 mmol in 30 mL of THF) dropwise to this mixture with a 20 mL syringe at ‒78°C.Note: 3,5-ditertbutylphenylmagnesium bromide is commercially available, detailed information can be found in the key resources table. In addition, it can also be prepared in advance, please refer to ref. 20 for detailed information.
-
h.Warm up the reaction mixture to 25°C and stir for 8 h.
-
i.Quench the reaction mixture by slowly adding 20 mL of saturated solution of sodium bicarbonate.
-
j.Extract the mixture with 20 mL of ethyl acetate in a separating funnel for 3 times and dry over anhydrous sodium sulfate.
-
k.Evaporate the solvent to give crude aminophosphine (S)-2 for the next step without purification.
-
l.Transfer (S)-2 to a 100 mL round bottom Schlenk flask and replace the atmosphere of the flask with argon for three times.
-
m.Dissolve (S)-2 with 10 mL of acetic anhydride and stir at 50°C for 3 h.
-
n.Remove the excess acetic anhydride at 25°C under vacuo (∼10 Pa) using an oil pump equipped with a liquid nitrogen protected cold trap.
-
o.Purge the flask three times with argon and protect it with an argon balloon.
-
p.Add a mixed solution of tetrahydrofuran (10 mL), methanol (10 mL) and ammonia water (15 mL, 7 mol/L) to the reaction mixture.
-
q.Heat the mixture to 60°C and stir for 8 h.
-
r.Quench the reaction mixture with 20 mL of ice water.
-
s.Extract with 20 mL of ethyl acetate for 3 times in a separating funnel and dry over anhydrous sodium sulfate.
-
t.Purify by column chromatography (Ø of the column = 4.6 cm) on silica gel (8 cm, 300–400 mesh, petroleum ether (boiling range 60°C–80°C): ethyl acetate = 1:1, approximate 3 L) to afford (Sc, RFc)-3 as a yellow solid (TLC (ethyl acetate), Rf = 0.1).
-
u.Stir a solution of (Sc, RFc)-3 (1 mmol) and 4-tertbutyl imidazole-2-formaldehyde (1.05 mmol) in dry methanol under reflux for 4 h.
-
v.Add NaBH4 (3 mmol, 114 mg) to the mixture at 0°C.
-
w.Stir the reaction mixture at room temperature in air for 2 h.
-
x.Remove the solvent at 45°C under vacuo (approximate 100 mbar) on a rotary evaporator.
-
y.Purify the crude product by column chromatography (Ø of the column = 3.0 cm) on silica gel (8 cm, 300–400 mesh, petroleum ether (boiling range 60°C–80°C): ethyl acetate = 5:1(approximate 500 mL) to 1:1(approximate 1 L)) to yield SL1 (TLC (petroleum ether/EtOAc = 1/1), Rf = 0.1).Note: tert-Butyllithium is extremely flammable. Please strictly follow the Schlenk techniques and prepare a fire blanket in case.Note:SL1 need to be dried thoroughly under high vacuum before transfer and can be stored at ambient surroundings for 1–2 days. Once synthesized, it is best to be kept in the glove box as soon as possible to avoid slow oxidation of phosphine by air.RL1,SL2,RL2 are prepared following similar procedures. For more details, see Liu et al. (2023).1
-
a.
Figure 1.
Ligand preparation reaction process
Preparation of the reagent
Timing: 8 h
-
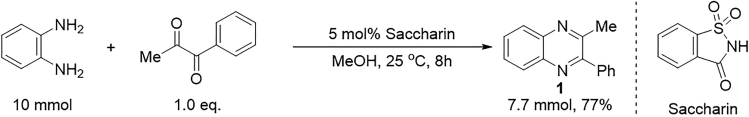
2.Synthesize the reagent 1 as mentioned below:
-
a.Add o-phenylenediamine (10 mmol), 1-phenyl-1,2-propanedione (10 mmol, 1.0 eq.), saccharin (0.5 mmol, 0.05 eq.) and methanol (10 mL) to a 100 mL Schlenk flask.
-
b.Stir the reaction mixture at room temperature for 8 h.
-
c.Monitor the reaction mixture by TLC (petroleum ether/EtOAc = 3/1, o-phenylenediamine: Rf = 0.1, 1-phenyl-1,2-propanedione: Rf = 0.5, quinoxaline 1: Rf = 0.3) with UV-light detection (254 nm).
-
d.Remove the solvent at 45°C under vacuo (approximate 100 mbar) on a rotary evaporator after completion of the reaction (approximate 8 h).
-
e.Purify the crude product by flash column chromatography (Ø of the column = 4.6 cm) on silica gel (8 cm, 300–400 mesh, petroleum ether (boiling range 60°C–80°C): ethyl acetate = 15:1 (approximate 2 L) to give quinoxaline 1 (TLC (petroleum ether/EtOAc = 3/1), Rf = 0.3).
-
a.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, peptides, and recombinant proteins | ||
| (S)-1, 98% | Leyan | CAS: 31886-57-4 |
| tBuLi (1.3 M in hexane) | Energy Chemical | CAS: 594-19-4 |
| tert-Butyl methyl ether, 99.5%, extra dry, with molecular sieves, water ≤50 ppm (byK.F.), Energyseal | J&K Scientific | CAS: 1634-04-4 |
| PCl3, 98% | Energy Chemical | CAS: 7719-12-2 |
| 3,5-ditertbutylphenylmagnesium bromide, 1.0 M in 2-MeTHF, 97% | Energy Chemical | CAS: 204324-71-0 |
| Ac2O (AR) | Energy Chemical | CAS: 108-24-7 |
| Ammonium hydroxide (AR 25%–28%) | Greagent | CAS: 1336-21-6 |
| THF (AR) | Greagent | CAS: 109-99-9 |
| MeOH (AR) | Greagent | CAS: 109-99-9 |
| 4-tertbutyl-1H-imidazole-2-carbaldehyde, 97% | Energy Chemical | CAS: 1339064-78-6 |
| NaBH4, 99% | Sinopharm | CAS: 16940-66-2 |
| o-phenylenediamine, 99.5% | Energy Chemical | CAS: 95-54-5 |
| 1-Phenyl-1,2-propanedione, 98% | Energy Chemical | CAS: 579-07-7 |
| Saccharin, 98% | Energy Chemical | CAS: 81-07-2 |
| Mn(CO)5Br, 99% | Strem Chemicals | CAS: 14516-54-2 |
| tBuOK, 98% | Energy Chemical | CAS: 865-47-4 |
| 15-crown-5, 99% | Energy Chemical | CAS: 33100-27-5 |
| Argon, ≥99.999% | Praxair | GB/T 4842-2017 |
| Hydrogen, ≥99.999% | Praxair | GB/T 3634.1-2006 |
| tAmONa, 98% | Energy Chemical | CAS: 14593-46-5 |
| 1,2-dichlorobenzene, 99.5%, extra dry, with molecular sieves, water ≤50 ppm (byK.F.), Energyseal | J&K Scientific | CAS: 95-50-1 |
| Silica gel for chromatography (300–400 mesh, AR) | Greagent | CAS: 63231-67-4 |
| Petroleum ether (AR) | Greagent | CAS: 8032-32-4 |
| Ethyl acetate (AR) | Greagent | CAS: 141-78-6 |
| Hexane (RG) | Adamas | CAS: 92112-69-1 |
| Deuterated chloroform, 99.8 atom % D | J&K Scientific | CAS: 865-49-6 |
| Other | ||
| Syringe needle (φ0.6 × 25 mm) | Tansoole | Cat# 02026617 |
| Syringe needle (φ0.8 × 200 mm) | Tansoole | Cat# 02044724 |
| Syringe (2 mL) | Tansoole | Cat# 02026617 |
| Chromatography column (Ø 17 mm, 305 mm) | Synthware Glass | Cat# C184173CR |
| Vial (4 mL) | Tansoole | Cat# 02132550 |
| Electronic balance | Mettler Toledo | Cat# BSA124S |
| Nitrile gloves | 3M | Cat# WX300953410 |
| Heating module | Boost | DZ-AHB-PRL |
| Autoclave | Parr | Series 4560 Mini Reactors, 300 mL Specifications |
| Schlenk flask (100 mL) | Synthware | Cat# F909100G |
| Magnetic stirrer | IKA | Cat# RTC basic |
| Thin-layer chromatography (TLC-plates 0.25 mm) | Leyan | Cat# C100053 |
| Rotary evaporator | IKA | Cat# RV 10 basic |
| Vacuum pump | Vacuubrand | Cat# PC 3001 VARIO |
| HPLC analyses | Shimadzu | Cat# LC-20A Prominence |
| CHIRALCEL OD-H column | Daicel | Cat# 250 mm × 4.6 mm × 5 μm |
| 400 MHz NMR spectrometer | Bruker | Cat# Avance III |
| Glove box | Vigor | Cat# SG2400/750TS-F |
Step-by-step method details
Part 1: Procedure for cis-selective asymmetric hydrogenation of 1
Timing: 16 h (for step 1)
Timing: 1 h (for step 2)
In this part, we describe the asymmetric hydrogenation of 1 to (2R,3S)-2 using an in situ generated catalyst from Mn(CO)5Br and SL1. The preparation of 1 and ligands is described above, and the full scope of the transformation is described in Liu et al. (2023).1
-
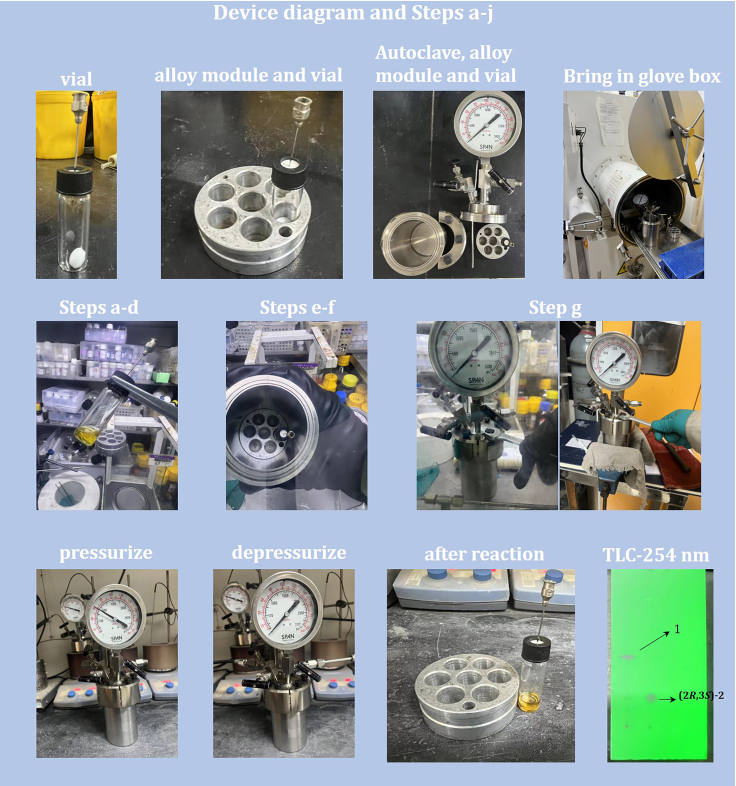
1.Set up the reaction (Schemes 1, 2, and 3; Table 1).
-
a.Add Mn(CO)5Br (1.0 mg, 0.004 mmol, 2 mol %), SL1 (3.4 mg, 0.0044 mmol, 2.2 mol %) and 15-crown-5 (0.5 mL) to a 4 mL vial in the glove box.
-
b.Stir the reaction mixture at 25°C for 10 min.
-
c.Add KOtBu (0.04 mmol, 4.5 mg, 20 mol %) and 1 (0.2 mmol) into the vial.
-
d.Cap the vial with a septum equipped with a syringe needle.
-
e.Place the vial in an alloy module.
-
f.Then place the alloy module to the pre-dried autoclave.
-
g.Seal the autoclave and take it out of the glove box, then tighten the screws with a vice.Note: Steps a-g are completed in the glove box until the autoclave is brought out.
-
h.Purge the autoclave 3 times with hydrogen after sealed.
-
i.Then pressurize to 50 bar and stir at 10°C for 16 h.
-
j.Warm up the autoclave to 25°C and depressurize after reaction in the fume hood (for more details, see Figure 2).
 CRITICAL: Before using the autoclave, please check all parts of the autoclave, including the safety valve, explosion-proof membrane, pressure gauge, etc., and use argon to check the air tightness of the gas line and the autoclave. The autoclave should be handled with care, and the speed of inflation and deflation should be slow. The reactions need to take place in a well-ventilated area. In addition, the hydrogen alarm is necessary. In case of leakage, please immediately close the main valve of the hydrogen cylinder and release the hydrogen in the autoclave and pipeline. Only when the leakage site is checked and repaired can the operation be restarted.
CRITICAL: Before using the autoclave, please check all parts of the autoclave, including the safety valve, explosion-proof membrane, pressure gauge, etc., and use argon to check the air tightness of the gas line and the autoclave. The autoclave should be handled with care, and the speed of inflation and deflation should be slow. The reactions need to take place in a well-ventilated area. In addition, the hydrogen alarm is necessary. In case of leakage, please immediately close the main valve of the hydrogen cylinder and release the hydrogen in the autoclave and pipeline. Only when the leakage site is checked and repaired can the operation be restarted.
-
a.
-
2.Purification of the crude product.
-
a.Take the vial out of the autoclave, and transfer the reaction mixture directly to the flash column chromatography (Ø of the column = 17 mm) on silica gel (10 cm, 300–400 mesh).
-
b.Elute the reaction solvent 15-Crow-5 with 150 mL of petroleum ether (boiling range 60°C–80°C).
-
c.Then purify the mixture with eluent (petroleum ether: ethyl acetate = 4:1, approximate 250 mL) to afford (2R,3S)-2 as brown oil, 42 mg, 95% yield, >20:1 d.r., 93% ee (TLC (petroleum ether/ethyl acetate = 1/2), Rf = 0.2).
-
a.
Scheme 1.
Synthesis of ligands SL1
Scheme 2.
Preparation of quinoxaline 1
Scheme 3.
Asymmetric hydrogenation of 1 to (2R,3S)-2
Table 1.
Quantification of reagents, solvent, and product
| Reagent | Mw (g/mol) | m (mg) | n (mmol) | Equiv. | V (mL) | Conc (M) | Yield (%) |
|---|---|---|---|---|---|---|---|
| 1 | 220 | 44 | 0.2 | 1 | 0.4 | ||
| Mn(CO)5Br | 274 | 1.0 | 0.004 | 0.02 | |||
| SL1 | 773 | 3.4 | 0.0044 | 0.022 | |||
| 15-crown-5 | 0.5 | ||||||
| tBuOK | 112 | 4.5 | 0.04 | 0.2 | |||
| (2R, 3S)-2 | 224 | 42 | 0.19 | 95 |
Figure 2.
Device diagram and steps a–j
The preparation of (2S,3R)-2 is achieved through the same steps using RL1 as the ligand (95% yield, >20:1 d.r., 93% ee).
Part 2: Procedure for trans-selective asymmetric hydrogenation of 1
Timing: 36 h (for step 3)
Timing: 1 h (for step 4)
This section completes the trans-selective asymmetric hydrogenation of 1 to (2S, 3S)-2 (Scheme 4).
-
3.Set up the reaction (Schemes 1 and 2; Table 2).
-
a.Add Mn(CO)5Br (3 mol %, 1.60 mg), SL2 (3.3 mol %, 5.25 mg) and 1,2- dichlorobenzene (0.5 mL) to a 4 mL vial in the glove box.
-
b.Stir the reaction mixture at 25°C for 10 min.
-
c.Add tAmONa (25 mol %, 5.6 mg) and 1 (0.2 mmol) into the vial.
-
d.Cap the vial with a septum equipped with a syringe needle.
-
e.Place the vial in an alloy module.
-
f.Then place the alloy module to the pre-dried autoclave.
-
g.Seal the autoclave and take it out of the glove box, then tighten the screws with a vice.Note: Steps a-g are completed in the glove box until the autoclave is brought out.
-
h.Purge the autoclave 3 times with hydrogen after sealed.
-
i.Then pressurize to 50 bar and stir at 50°C for 36 h.
-
j.Cool down the autoclave to 25°C and depressurize after reaction.
-
a.
-
4.Purification of the crude product.
-
a.Take the vial out of the autoclave, and transfer the reaction mixture directly to the flash column chromatography (Ø of the column = 17 mm) on silica gel (10 cm, 300–400 mesh).Concentrate the resulted residue under vacuo.
-
b.Elute the reaction solvent 1,2-dichlorobenzene with 100 mL of petroleum ether (boiling range 60°C–80°C).
-
c.Then purify the mixture with eluent (petroleum ether : ethyl acetate = 4:1, approximate 150 mL) to afford (2S,3S)-2 as brown oil, 29 mg, 65% yield, 3.2:1 d.r., 99% ee (TLC (petroleum ether/ethyl acetate = 1/2), Rf = 0.3).
-
a.
Scheme 4.
Asymmetric hydrogenation of 1 to (2S,3S)-2
Table 2.
Quantification of reagents, solvent, and product
| Reagent | Mw (g/mol) | m (mg) | n (mmol) | Equiv. | V (mL) | Conc (M) | Yield (%) |
|---|---|---|---|---|---|---|---|
| 1 | 220 | 44 | 0.2 | 1 | 0.4 | ||
| Mn(CO)5Br | 274 | 1.5 | 0.006 | 0.03 | |||
| SL2 | 797 | 5.3 | 0.0066 | 0.033 | |||
| o-DCB | 0.5 | ||||||
| tAmONa | 110 | 5.6 | 0.05 | 0.25 | |||
| (2S, 3S)-2 | 224 | 29 | 0.13 | 65 |
The preparation of (2R,3R)-2 is achieved through the same steps using RL2 as the ligand (65% yield, 2.6:1 d.r., 99% ee).
Expected outcomes
Two cis-enantiomers ((2R,3S)-2 or (2S,3R)-2) were obtained in 95% yields, >20:1 d.r., 93% ee and two trans-enantiomers ((2S,3S)-2 or (2R,3R)-2) were achieved with decreased diastereoselectivities (3.2 :1 d.r., 2.6:1 d.r., respectively) but extremely high ee values (99% ee).
Quantification and statistical analysis
Analytical data

[α]25D = -23.4 (c = 0.7, CHCl3).
1H NMR (400 MHz, CDCl3) δ 7.35–7.24 (m, 5H), 6.69–6.60 (m, 2H), 6.59–6.53 (m, 2H), 4.51 (d, J = 3.2 Hz, 1H), 4.11–3.52 (m, 3H), 0.95 (d, J = 6.5 Hz, 3H).
13C NMR (101 MHz, CDCl3) δ 141.82, 133.19, 132.39, 128.21, 127.58, 127.35, 119.22, 118.36, 114.80, 113.93, 58.55, 49.48, 17.66.
HPLC retention times (OD-H, eluent: Hexanes/i-PrOH = 80/20, 313 nm, flow rate: 1.0 mL/min, T = 25°C): tR = 11.8 min (minor), tR = 13.8 min (major).

[α]25D = = -34.7 (c = 1.1, CHCl3).
1H NMR (400 MHz, CDCl3) δ 7.47–7.30 (m, 5H), 6.74–6.51 (m, 4H), 3.92 (d, J = 7.4 Hz, 1H), 3.87–3.50 (m, 2H), 3.45–3.33 (m, 1H), 1.00 (d, J = 6.2 Hz, 3H).
13C NMR (101 MHz, CDCl3) δ 141.01, 134.12, 133.05, 128.60, 128.09, 128.04, 118.68, 118.57, 114.09, 113.84, 61.76, 52.38, 18.87.
HRMS (ESI) calcd. for [C15H17N2]+([M + H]+): 225.1392, found: 225.1384.
HPLC retention times (OD-H, eluent: Hexanes/i-PrOH = 80/20, 313 nm, flow rate: 1.0 mL/min, T = 25°C): tR = 7.1 min (minor), tR = 8.9 min (major).
Limitations
The protocol is limited to the asymmetric hydrogenation of 2-alkyl-3-aryl quinoxalines. For the reactions of 2,3-dialkyl quinoxalines, please refer to Liu et al. (2023)1 for the optimized conditions.
Troubleshooting
Problem 1
Step 1a & 3a: the reaction system is sensitive to air and moisture.
Potential solution
Solvents used in the reaction were firstly degassed by three freeze-pump-thaw cycles and then distilled under argon protection after dehydration or dried over activated 3Å molecular sieves.
Problem 2
Step 1i & 3i: Magnetic stir bar may fail to stir due to the increased distance to the stirrer.
Potential solution
It is necessary to select magnetic stir bars with good magnetism and verify its functionality in advance.
Problem 3
Step 1d & 3d: The needle may be blocked by debris from the septum, preventing hydrogen from entering the vial.
Potential solution
Replace it with a unobstructed needle.
Problem 4
Step 1h & 3h: Air in the gas line may enter the autoclave and cause the catalyst to be deactivated.
Potential solution
Before pressurizing the autoclave, the line should be purged three times with argon or hydrogen.
Problem 5
Step 1t: The yield may be lower than expected.
Potential solution
In Step 1n, acetic anhydride needs to be removed completely under high vacuum. In Step 1p, ammonia may escape from the reaction mixture due to heat, and ammonia can be added 1–2 times to promote the reaction.
Resource availability
Lead contact
Further information and requests for resources should be directed to and will be fulfilled by the lead contact, Qiang Liu (qiang_liu@mail.tsinghua.edu.cn).
Materials availability
This study did not generate new unique reagents, all compounds have been described in the original article; see Liu et al.1
Data and code availability
-
•
All data reported in this paper will be shared by the lead contact upon request.
-
•
This paper does not report the original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
-
•
The published article includes all [datasets/code] generated or analyzed during this study, see Liu et al.1
Acknowledgments
Financial support from the National Key R&D Program of China (2021YFF0701600) and the National Natural Science Foundation of China (22225103 and 22171159) is greatly appreciated.
Author contributions
M.W. and Q.L. designed and wrote the protocol with inputs from all the authors. C.L. and M.W. performed the experimental data. Q.L. supervised the project. All authors approved the final version of the manuscript for submission.
Declaration of interests
The authors declare no competing interests.
Contributor Information
Mingyang Wang, Email: wmy19@mails.tsinghua.edu.cn.
Qiang Liu, Email: qiang_liu@mail.tsinghua.edu.cn.
References
- 1.Liu C., Liu X., Liu Q. Stereodivergent asymmetric hydrogenation of quinoxalines. Chem. 2023;9:2585–2600. doi: 10.1016/j.chempr.2023.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vitaku E., Smith D.T., Njardarson J.T. Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA Approved Pharmaceuticals. J. Med. Chem. 2014;57:10257–10274. doi: 10.1021/jm501100b. [DOI] [PubMed] [Google Scholar]
- 3.Taylor R.D., MacCoss M., Lawson A.D.G. Rings in Drugs. J. Med. Chem. 2014;57:5845–5859. doi: 10.1021/jm4017625. [DOI] [PubMed] [Google Scholar]
- 4.Kim A.N., Stoltz B.M. Recent Advances in Homogeneous Catalysts for the Asymmetric Hydrogenation of Heteroarenes. ACS Catal. 2020;10:13834–13851. doi: 10.1021/acscatal.0c03958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wiesenfeldt M.P., Nairoukh Z., Dalton T., Glorius F. Selective Arene Hydrogenation for Direct Access to Saturated Carbo- and Heterocycles. Angew. Chem. Int. Ed. 2019;58:10460–10476. doi: 10.1002/anie.201814471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang D.-S., Chen Q.-A., Lu S.-M., Zhou Y.-G. Asymmetric Hydrogenation of Heteroarenes and Arenes. Chem. Rev. 2012;112:2557–2590. doi: 10.1021/cr200328h. [DOI] [PubMed] [Google Scholar]
- 7.Tang W., Xu L., Fan Q.-H., Wang J., Fan B., Zhou Z., Lam K.-h., Chan A.S.C. Asymmetric Hydrogenation of Quinoxalines with Diphosphinite Ligands: A Practical Synthesis of Enantioenriched, Substituted Tetrahydroquinoxalines. Angew. Chem. Int. Ed. 2009;48:9135–9138. doi: 10.1002/anie.200904518. [DOI] [PubMed] [Google Scholar]
- 8.Chen Q.-A., Wang D.-S., Zhou Y.-G., Duan Y., Fan H.-J., Yang Y., Zhang Z. Convergent Asymmetric Disproportionation Reactions: Metal/Brønsted Acid Relay Catalysis for Enantioselective Reduction of Quinoxalines. J. Am. Chem. Soc. 2011;133:6126–6129. doi: 10.1021/ja200723n. [DOI] [PubMed] [Google Scholar]
- 9.Chen Q.-A., Gao K., Duan Y., Ye Z.-S., Shi L., Yang Y., Zhou Y.-G. Dihydrophenanthridine: A New and Easily Regenerable NAD(P)H Model for Biomimetic Asymmetric Hydrogenation. J. Am. Chem. Soc. 2012;134:2442–2448. doi: 10.1021/ja211684v. [DOI] [PubMed] [Google Scholar]
- 10.Fleischer S., Zhou S., Werkmeister S., Junge K., Beller M. Cooperative Iron–Brønsted Acid Catalysis: Enantioselective Hydrogenation of Quinoxalines and 2 H-1,4-Benzoxazines. Chem. Eur J. 2013;19:4997–5003. doi: 10.1002/chem.201204236. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Z., Du H. A Highly cis-Selective and Enantioselective Metal-Free Hydrogenation of 2,3-Disubstituted Quinoxalines. Angew. Chem. Int. Ed. 2015;54:623–626. doi: 10.1002/anie.201409471. [DOI] [PubMed] [Google Scholar]
- 12.Urban S., Ortega N., Glorius F. Ligand-Controlled Highly Regioselective and Asymmetric Hydrogenation of Quinoxalines Catalyzed by Ruthenium N-Heterocyclic Carbene Complexes. Angew. Chem. Int. Ed. 2011;50:3803–3806. doi: 10.1002/anie.201100008. [DOI] [PubMed] [Google Scholar]
- 13.Wang D.-S., Chen Q.-A., Li W., Yu C.-B., Zhou Y.-G., Zhang X. Pd-Catalyzed Asymmetric Hydrogenation of Unprotected Indoles Activated by Brønsted Acids. J. Am. Chem. Soc. 2010;132:8909–8911. doi: 10.1021/ja103668q. [DOI] [PubMed] [Google Scholar]
- 14.Shi L., Ye Z.-S., Cao L.-L., Guo R.-N., Hu Y., Zhou Y.-G. Enantioselective Iridium-Catalyzed Hydrogenation of 3,4-Disubstituted Isoquinolines. Angew. Chem. Int. Ed. 2012;51:8286–8289. doi: 10.1002/anie.201203647. [DOI] [PubMed] [Google Scholar]
- 15.Qin J., Chen F., Ding Z., He Y.-M., Xu L., Fan Q.-H. Asymmetric Hydrogenation of 2- and 2,3-Substituted Quinoxalines with Chiral Cationic Ruthenium Diamine Catalysts. Org. Lett. 2011;13:6568–6571. doi: 10.1021/ol2029096. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y., Zhu L., Shao Z., Li G., Lan Y., Liu Q. Unmasking the Ligand Effect in Manganese-Catalyzed Hydrogenation: Mechanistic Insight and Catalytic Application. J. Am. Chem. Soc. 2019;141:17337–17349. doi: 10.1021/jacs.9b09038. [DOI] [PubMed] [Google Scholar]
- 17.Liu C., Wang M., Liu S., Wang Y., Peng Y., Lan Y., Liu Q. Manganese-Catalyzed Asymmetric Hydrogenation of Quinolines Enabled by π–π Interaction. Angew. Chem. Int. Ed. 2021;60:5108–5113. doi: 10.1002/anie.202013540. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y., Wang M., Li Y., Liu Q. Homogeneous manganese-catalyzed hydrogenation and dehydrogenation reactions. Chem. 2021;7:1180–1223. doi: 10.1016/j.chempr.2020.11.013. [DOI] [Google Scholar]
- 19.Liu C., Wang M., Xu Y., Li Y., Liu Q. Manganese-Catalyzed Asymmetric Hydrogenation of 3H-Indoles. Angew. Chem. Int. Ed. 2022;61 doi: 10.1002/anie.202202814. [DOI] [PubMed] [Google Scholar]
- 20.Ribar P., Valenta L., Šolomek T., Juríček M. Rules of Nucleophilic Additions to Zigzag Nanographene Diones. Angew. Chem. Int. Ed. 2021;60:13521–13528. doi: 10.1002/anie.202016437. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
-
•
All data reported in this paper will be shared by the lead contact upon request.
-
•
This paper does not report the original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
-
•
The published article includes all [datasets/code] generated or analyzed during this study, see Liu et al.1