Summary
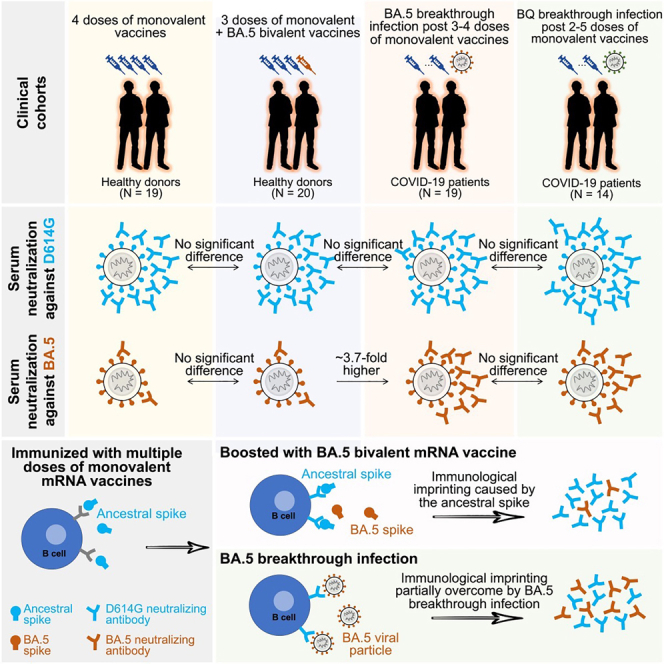
To combat the evolving SARS-CoV-2 Omicron variants, bivalent COVID-19 mRNA vaccines, encoding both ancestral and Omicron BA.5 spikes, have replaced monovalent vaccines in numerous countries. However, fourth doses of either vaccine result in similar neutralizing antibody titers against Omicron subvariants, raising the possibility of immunological imprinting. To address this, we investigate antibody responses in 72 participants given three doses of a monovalent mRNA vaccine, followed by a bivalent or monovalent booster, or those with breakthrough infections with BA.5 or BQ. Bivalent boosters do not show notably higher binding or virus-neutralizing titers against various SARS-CoV-2 variants compared to monovalent ones. However, breakthrough infections lead to significantly better neutralization of Omicron subvariants. Multiple analyses, including antigenic mapping, suggest that the ancestral spike in bivalent vaccines is causing deep immunological imprinting, preventing broadening of antibodies to the BA.5 component, thereby defeating its intended goal. Its removal from future vaccine compositions is therefore strongly recommended.
Keywords: SARS-CoV-2, COVID-19, bivalent mRNA vaccine, immunological imprinting, Omicron BA.5 spike, ancestral spike, serum neutralization, antigenic distance
Graphical abstract
Highlights
-
•
Monovalent and BA.5 bivalent mRNA vaccine boosters induced similar antibody responses
-
•
BA.5 breakthrough infections yielded higher neutralizing activity than vaccine boosters
-
•
The ancestral spike in BA.5 bivalent vaccines caused deep immunological imprinting
-
•
Bivalent boosters did not yield superior antibody responses due to immune imprinting
Wang et al. report that bivalent COVID-19 boosters did not yield superior antibody responses against SARS-CoV-2 Omicron subvariants compared to the original monovalent vaccines. This result is likely due to immunological imprinting caused by the inclusion of the ancestral spike, which should be removed from future vaccines.
Introduction
The FDA recently amended the emergency use authorization for the bivalent (ancestral/BA.5) COVID-19 mRNA vaccines to streamline the vaccination schedule and to allow older and immunocompromised individuals to receive additional booster shots.1 However, several studies have reported that serum-neutralizing antibody titers against SARS-CoV-2 Omicron BA.5 and subsequent subvariants after a bivalent vaccine booster were not discernibly better than after a monovalent (ancestral) booster,2,3,4 though some groups concluded the otherwise.5,6 The component of the ancestral spike in the SARS-CoV-2 bivalent mRNA vaccines may result in the immunological imprinting. However, the extent to which immunological imprinting occurs with the ancestral spike remains unclear.
The concept of immunological imprinting, also known as original antigenic sin, was initially described in the context of influenza.7 It was observed that prior exposure to an influenza strain could influence the immune response to subsequent influenza exposure, whether through vaccination or infection. Notably, antibody titers were found to be highest against influenza strains encountered during childhood.7,8 In our study, we have conducted an in-depth analysis of antibody profiles from various SARS-CoV-2 vaccinee cohorts as well as breakthrough infected patient cohorts to study the issue of immunological imprinting caused by the ancestral spike in the current SARS-CoV-2 bivalent mRNA vaccines. Our findings highlight that the ancestral spike exacerbates immunological imprinting and should be eliminated from future vaccine compositions.
Results and discussion
We collected serum from 72 individuals who had received three doses of vaccines followed by a monovalent or bivalent booster or who had experienced a BA.5 or BQ breakthrough infection. Clinical details for all cases are provided in Table S1 and summarized in Table S2. We performed immunoassays to quantify serum antibodies that bind the spike proteins of D614G, BA.5, and BQ.1.1. Each serum sample was also tested in pseudovirus assays to determine neutralizing antibody titers against the ancestral D614G strain and a panel of Omicron subvariants, including BA.2, BA.5, BQ.1.1, CH.1.1, XBB.1.5, and the current surging XBB.1.16.
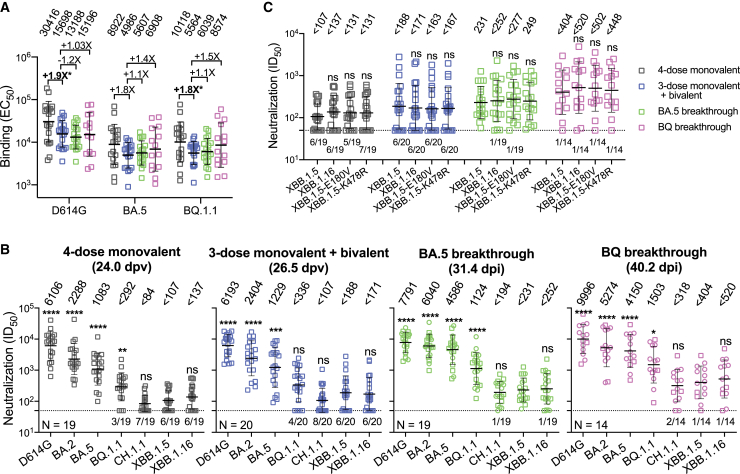
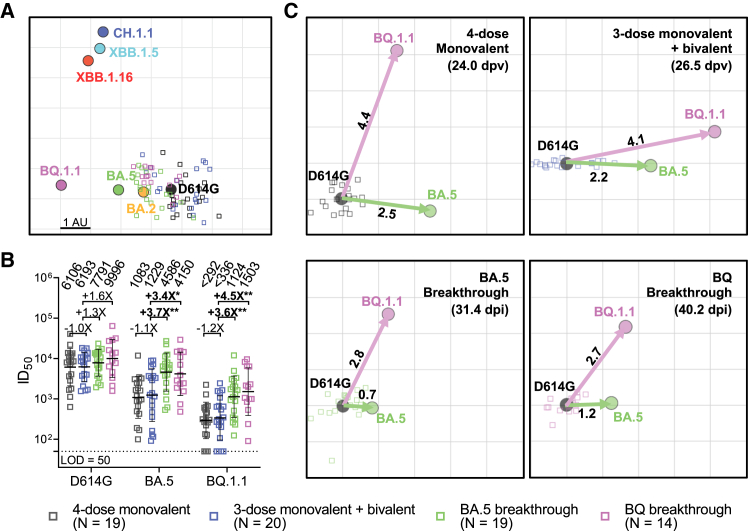
Each cohort exhibited roughly similar (<2-fold difference) serum binding antibody titers to D614G, BA.5, and BQ.1.1 spike proteins (Figure 1A). As for serum SARS-CoV-2-neutralizing antibodies, all cohorts had the highest titers against D614G but substantially lower titers against the Omicron subvariants, particularly the currently dominant XBB.1.5 and the emergent XBB.1.16 (Figure 1B). Notably, the extent of antibody evasion exhibited by XBB.1.16 and its spike point mutants (E180V and K478R) was comparable to that of XBB.1.5 (Figure 1C). The data in Figure 1B were further analyzed in three ways. First, antigenic cartography revealed that sera from the "monovalent" and "bivalent" cohorts were substantially overlapping and centered around the ancestral strain, whereas sera from BA.5 and BQ breakthrough cohorts were similarly shifted toward BA.5 and BQ.1.1 (Figure 2A). Second, the above findings prompted replotting of a subset of the data to specifically examine the issue of immunological imprinting9 (Figure 2B). Serum-neutralizing antibodies against D614G were similar for all cohorts, with small differences that did not reach statistical significance. However, titers against BA.5 or BQ.1.1 were significantly higher for BA.5 (3.4- to 3.7-fold) and BQ.1.1 (3.6- to 4.5-fold) breakthrough cohorts. These findings made clear the role of the ancestral spike in immunological imprinting in that exposure of the immune system to both the ancestral and BA.5 spikes did not elicit discernibly better BA.5-neutralizing antibodies, whereas exposure to only BA.5 spike (through infection) in the absence of the ancestral spike did elicit such antibodies. That BQ.1.1 and BA.5 results were similar should not be surprising since BQ.1.1 is a direct descendant of BA.5. Third, we created antigenic maps based on the neutralization data for each of the clinical cohorts (Figure 2C). The antigenic distances from D614G to BA.5 or to BQ.1.1 were similar for the “monovalent” and “bivalent” vaccine cohorts. In contrast, these antigenic distances were substantially shortened with BA.5 or BQ breakthrough infection, and these differences reached high-level statistical significance (Figure S1). This analysis graphically demonstrates that inclusion of the ancestral spike in the bivalent vaccine precludes the broadening of neutralizing antibodies to BA.5, which was clearly evident in the breakthrough infection cases.
Figure 1.
SARS-CoV-2 antibody responses to D614G and Omicron subvariants following monovalent booster, bivalent booster, or breakthrough infection
(A) EC50 titers of binding antibodies in the serum samples from participants who received four doses of a monovalent mRNA vaccine (4-dose monovalent), three doses of a monovalent mRNA vaccine followed by one dose of a bivalent vaccine (3-dose monovalent + bivalent), and experienced BA.5 (BA.5 breakthrough) or BQ (BQ breakthrough) breakthrough infections after two to four doses of vaccine.
(B) ID50 titers of neutralizing antibodies in the serum samples from “4-dose monovalent,” “3-dose monovalent + bivalent,” “BA.5 breakthrough,” and “BQ breakthrough” cohorts against D614G and Omicron subvariants. Breakthrough serum samples were separated into two sub-groups using two distinct symbols. Square symbols indicate samples from individuals who received three doses of monovalent vaccines followed by a breakthrough infection. Round symbols indicate samples from individuals who received four or five doses of monovalent vaccines and subsequently had a breakthrough infection. dpv, days post last vaccination; dpi, days post infection. The numbers in parentheses represent the mean days post last vaccination (dpv) or the mean days post infection (dpi).
(C) Serum-neutralizing ID50 titers against XBB.1.5, XBB.1.16, and XBB.1.5 carrying the individual spike mutations found in XBB.1.16. Values above symbols indicate the geometric mean EC50 or IC50 titers for each cohort. The neutralization assay limit of detection (LOD) is 50 (dotted line), and the number of samples below the LOD is denoted above the x axis. Comparisons were made against “3-dose monovalent + bivalent” cohort (A) or XBB.1.5 (B and C) by Mann-Whitney tests. ns, not significant; ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; ∗∗∗∗p < 0.0001. Sample sizes (n) are shown in (B). See also Tables S1 and S2.
Figure 2.
Deep immunological imprinting caused by the ancestral spike in bivalent COVID-19 mRNA vaccines impaired serum-neutralizing antibody responses to Omicron subvariants
(A) Antigenic map derived from the neutralization data in Figure 1B. SARS-CoV-2 variants and sera are shown as colored circles and squares, respectively. The x and y axes represent antigenic units (AU), with each grid corresponding to a 2-fold serum dilution of the neutralization titer, as defined by the RACMACS package (https://github.com/acorg/Racmacs/tree/master).
(B) Neutralizing antibody responses induced by a fourth dose of a bivalent mRNA vaccine compared to a fourth dose of the original monovalent booster or breakthrough infections. The values above the symbols indicate the geometric mean ID50 titer for each cohort. The assay limit of detection (LOD = 50) is represented by a dotted line. Comparisons were made against 3-dose monovalent + bivalent cohort by Mann-Whitney test, and the statistical significance is represented as ∗p < 0.05 or ∗∗p < 0.01. The numbers in parentheses represent the mean days post last vaccination (dpv) or the mean days post infection (dpi).
(C) Antigenic maps for individual cohorts against D614G, BA.5, and BQ.1.1. Arrows indicate the antigenic distances from D614G to BA.5 (green) and BQ.1.1 (magenta). See also Figure S1.
Much of the world’s population has been immunologically exposed to the ancestral spike of SARS-CoV-2 through either vaccination or infection or both. The inclusion of this spike in our COVID-19 vaccines will continue to skew our antibody responses toward what we have already seen and away from what we wish to elicit going forward. Therefore, based on observations made herein, we put forth a strong recommendation to remove the ancestral spike from future COVID-19 vaccines to improve variant-specific immunogenicity for the foreseeable future. However, it is worth noting that imprinting with pre-Omicron strains may not necessarily compromise protection against severe disease,10 and complete elimination of imprinting toward the ancestral strain may not be achievable with a variant-specific monovalent vaccine.11
To date, there is no evidence suggesting that the virus is regressing back to the ancestral strain; however, the impact of changing the vaccine composition on viral evolution remains to be seen. Continuous and intensive surveillance of the evolution of SARS-CoV-2 will be essential for making appropriate revisions to vaccine composition and maximizing the benefits of contemporary COVID-19 vaccines.
Limitations of the study
The study has limitations: (1) vesicular stomatitis virus-based pseudoviruses were used instead of live viruses for the neutralization assay. While pseudovirus neutralization assays offer advantages such as high throughput, formal qualification/validation, and biosafety benefits, they differ from live virus assessments. Nevertheless, previous research12,13,14 has demonstrated significant correlations between pseudovirus and live virus neutralization assays when evaluating antibody responses to SARS-CoV-2. (2) Our cohorts were not completely controlled for factors such as age and gender. Nonetheless, it is worth noting that the number of days between the sample collection and fourth vaccine doses was comparable between the monovalent and bivalent vaccine groups, indicating both should be representative of peak antibody responses to the boosters. The cohorts differed in terms of age and the proportion of Moderna vaccines, with the bivalent cohort being younger and having a higher proportion of Moderna vaccines. Given the higher mRNA dose present in Moderna vaccines compared to the Pfizer formulations, we would expect both of these factors to bias titers in favor of the bivalent cohort. Additionally, breakthrough sera samples were collected from individuals who received varying doses of monovalent mRNA vaccines. But, we separated these samples into two sub-groups: those from individuals who received three doses of monovalent mRNA vaccines and those who had received four or five doses. We did not observe a significant difference in neutralizing titers between these sub-groups. (3) We also acknowledge that the ideal control cohort in our study would consist of participants who received three doses of the original monovalent mRNA vaccine, followed by one dose of a BA.5 monovalent vaccine. Since such a cohort is not available in the real world, we instead used serum samples from patients with a BA.5 breakthrough infection, even though these cannot be directly compared to samples from non-replicative mRNA vaccinations. (4) In this study, we tested only the serum neutralization induced by monovalent and bivalent vaccines, as well as by breakthrough infection. However, in addition to humoral responses, CD4+ and CD8+ T cell responses also may contribute to protection, but these were not investigated in this study. (5) Finally, we did not examine cellular or mucosal immunity, in which data on imprinting are still lacking. Cellular immunity is an anticipated contributor to vaccine efficacy, while mucosal immunity is the first line of defense having the potential ability of blocking virus transmission.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Peroxidase AffiniPure goat anti-human IgG (H + L) antibody | Jackson ImmunoResearch | Cat# 109-035-003 RRID: AB_2337577 |
| Bacterial and virus strains | ||
| VSV-G pseudotyped ΔG-luciferase | Kerafast | Cat# EH1020-PM |
| Biological samples | ||
| “4-dose monovalent” sera | This paper | N/A |
| “3-dose monovalent + bivalent” sera | This paper | N/A |
| “BA.5 breakthrough” sera | This paper | N/A |
| “BQ breakthrough” sera | This paper | N/A |
| Chemicals, peptides, and recombinant proteins | ||
| Polyethylenimine (PEI) | Polysciences Inc. | Cat# 23966-100 |
| SARS-CoV-2 D614G S2P | Wang et al.13 | N/A |
| SARS-CoV-2 BA.5 S2P | Wang et al.13 | N/A |
| SARS-CoV-2 BQ.1.1 S2P | Wang et al.15 | N/A |
| Critical commercial assays | ||
| Luciferase Assay System | Promega | Cat# E4550 |
| QuikChange Lightning Site-Directed Mutagenesis Kit | Agilent | Cat# 210518 |
| TMB substrate | Sigma-Aldrich | Cat# T0440 |
| Experimental models: Cell lines | ||
| HEK293T | ATCC | Cat# CRL-3216; RRID: CVCL_0063 |
| Vero-E6 | ATCC | Cat# CRL-1586; RRID: CVCL_0574 |
| Recombinant DNA | ||
| pCMV3-D614G | Wang et al.13 | N/A |
| pCMV3-BA.2 | Wang et al.13 | N/A |
| pCMV3-BA.4/5 | Wang et al.13 | N/A |
| pCMV3-BQ.1.1 | Wang et al.15 | N/A |
| pCMV3-CH.1.1 | Wang et al.16 | N/A |
| pCMV3-XBB.1.5 | This paper | N/A |
| pCMV3-XBB.1.16 | This paper | N/A |
| pCMV3-XBB.1.5-E180V | This paper | N/A |
| pCMV3-XBB.1.5-K478R | This paper | N/A |
| Software and algorithms | ||
| GraphPad Prism 9 | GraphPad Software Inc | https://www.graphpad.com/scientific-software/prism/ |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact Author David D. Ho (dh2994@cumc.columbia.edu).
Materials availability
All reagents generated in this study are available from the lead contact with a completed Materials Transfer Agreement.
Experimental model and study participant details
Patients and vaccinees
The sera analyzed in this study were obtained from four cohorts: the "4-dose monovalent", “3-dose monovalent + bivalent”, “BA.5 breakthrough” and “BQ breakthrough”. The first cohort consisted of individuals who received four doses of the monovalent COVID-19 mRNA vaccine, whereas the second cohort received three doses of the monovalent COVID-19 mRNA vaccine followed by one dose of the Pfizer or Moderna bivalent mRNA vaccine. The last two cohorts comprised of patients who had Omicron BA.5 or BQ breakthrough infection after vaccination. All individuals with breakthrough infections after vaccination with ancestral spike were documented as having a symptomatic disease, either confirmed by sequence or PCR. The participants on immunosuppressant medications were excluded from this study. To determine prior SARS-CoV-2 infection status, serum samples were evaluated using anti-nucleocapsid protein (NP) ELISA.
The study collected sera from two sources. The first source of sera was obtained from the Immunity-Associated with SARS-CoV-2 Study (IASO), an ongoing cohort study that began in 2020 at the University of Michigan.17 The participants in the IASO study provided written informed consent, and the serum samples were collected under a protocol approved by the Institutional Review Board of the University of Michigan Medical School. The second source was Columbia University Irving Medical Center, where a subset of vaccinee and breakthrough sera were collected. All subjects provided written informed consent, and the serum collections were performed under protocols reviewed and approved by the Institutional Review Board of Columbia University. More detailed information for each case can be found in Table S1, while a summary is provided in Table S2.
Cell lines
The Vero-E6 cells (CRL-1586) and HEK293T cells (CRL-3216) were obtained from the American Type Culture Collection (ATCC). The cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin. The cells were maintained in a 5% CO2 atmosphere at 37°C.
Method details
Construction of SARS-CoV-2 spike plasmids
Plasmids that encode the spike proteins of various SARS-CoV-2 variants, including D614G, BA.2, BA.5, BQ.1.1, and XBB.1.5, were previously created.3,13,15,18,19 The spikes XBB.1.16, XBB.1.5-E180V, and XBB.1.5-K478R were generated using the QuikChange II XL site-directed mutagenesis kit according to the manufacturer’s instructions (Agilent). Before use in experiments, the sequences of all constructs were verified by Sanger sequencing.
Pseudovirus production
The pseudotyped SARS-CoV-2 variants were created by replacing the native glycoprotein of the vesicular stomatitis virus (VSV) with the spike protein of the relevant SARS-CoV-2 variant.20 To achieve this, HEK293T cells were transfected with a plasmid that encodes the relevant spike protein using polyethyleneimine (PEI) at a concentration of 1 mg/mL. The transfected cells were then incubated at 37°C in a 5% CO2 atmosphere for 24 h, followed by infection with the VSV-G pseudotyped ΔG-luciferase (G∗ΔG-luciferase, Kerafast). Following a 2-h incubation period at 37°C, the infected HEK293T cells were washed three times and cultured in fresh medium for an additional 24 h. The resulting supernatants were collected, centrifuged to remove any precipitates, and stored at −80°C. To prevent any contamination from VSV-G pseudotyped ΔG-luciferase, the viral stock was pre-incubated with 20% I1 hybridoma (anti-VSV-G) supernatant (ATCC; CRL-2700) for 1 h at 37°C before infecting the target cells.
Pseudovirus neutralization
To ensure consistent viral input, the pseudovirus titers were standardized before conducting the neutralization assay. The heat-inactivated sera were then tested in triplicate using 96-well plates and were serially diluted 4-fold in media, beginning with a 1:50 dilution. After the sera were diluted, they were combined with the pseudoviruses and incubated at 37°C for 1 h. Vero-E6 cells were then added to each well at a density of 3 × 104 cells/well and were incubated at 37°C with 5% CO2 for 10 h. Following the incubation, the cells were lysed, and luciferase activity was measured using the Luciferase Assay System (Promega) and SoftMax Pro v.7.0.2 (Molecular Devices), according to the manufacturer’s instructions.
ELISA
The S2P spike trimer protein of D614G, BA.5, and BQ.1.1 were generated according to previously described methods.15 To determine the binding titers of serum samples to D614G, BA.5, and BQ.1.1 spikes, we immobilized 50 ng/well of S2P trimer onto ELISA plates and incubated them overnight at 4°C. Subsequently, the ELISA plates were blocked with 300 μL blocking buffer (1% BSA and 10% bovine calf serum (BCS) (Sigma)) in PBS at 37°C for 2 h. Afterward, serum samples were serially diluted by 5-fold from 100× using dilution buffer (1% BSA and 20% BCS in PBS) and incubated in the ELISA plates at 37°C for 1 h. After incubation, 10,000-fold diluted Peroxidase AffiniPure goat anti-human IgG (H + L) antibody (Jackson ImmunoResearch) was added and incubated for 1 h at 37°C. The plates were washed between each step with PBST (0.5% Tween 20 in PBS). Finally, the TMB substrate (Sigma) was added and incubated before the reaction was stopped using 1 M sulfuric acid. Absorbance was measured at 450 nm EC50 values was calculated as the dilutions at which the OD450 readings reached half of the maximal using GraphPad Prism v.9.2.
Antigenic cartography
Antigenic distances between SARS-CoV-2 variants were estimated by integrating the neutralization potency of each serum sample using a previously described antigenic cartography method.21 The map was generated with the Racmacs package (https://acorg.github.io/Racmacs/, v.1.1.4) in R, using 2,000 optimization steps and setting the minimum column basis parameter to 'none'. The mapDistances function in the Racmacs package was employed to calculate antigenic distances, and the average distances for all sera to variants were used to represent the final distances. D614G served as the center of sera for each group, the seeds for each antigenic map were manually adjusted to ensure that BA.5 was displayed in the horizontal direction relative to the sera.
Quantification and statistical anaylysis
Serum neutralization ID50 values and antibody and hACE2 neutralization IC50 values were calculated using a five-parameter dose-response curve in GraphPad Prism v.9.2. Evaluations of statistical significance were performed employing two-tailed Wilcoxon matched-pairs signed-rank tests using GraphPad Prism v.9.2 software. Levels of significance are indicated as follows: ns, not significant; ∗, p < 0.05; ∗∗, p < 0.01; ∗∗∗, p < 0.001; and ∗∗∗∗, p < 0.0001. The SPR data was processed and fit to a 1:1 binding model using Biacore Evaluation Software.
Acknowledgments
We thank Sho Iketani for providing BQ.1.1 S2P spike trimer protein. This study was supported financially by the SARS-CoV-2 Assessment of Viral Evolution Program, NIAID, NIH (subcontract no. 0258-A709-4609 under federal contract no. 75N93021C00014) and the Gates Foundation (project no. INV019355) awarded to D.D.H., as well as the NIAID, NIH (subcontract under contract number 75N93019C00051) awarded to A.G. The authors express their gratitude to David Manthei, Carmen Gherasim, Emily Stoneman, Adam Lauring, Victoria Blanc, Pamela Bennett-Baker, Savanna Sneeringer, Theresa Kowalski-Dobson, Alyssa Meyers, Zijin Chu, Hailey Kuiken, Lonnie Barnes, Ashley Eckard, Kathleen Lindsey, Dawson Davis, Aaron Rico, Daniel Raymond, Mayurika Patel, and Nivea Vydiswaran from the IASO study team for their contribution in providing the serum samples.
Author contributions
L.L. and D.D.H. conceived the study. Q.W., A.R.T., and L.L. performed experiments. R.V. and A.G. collected serum samples. Y.G. generated antigenic cartography. Q.W., Y.G., L.L., and D.D.H. analyzed the results and wrote the manuscript. L.L. and D.D.H. directed and supervised the project. All authors reviewed and approved of the manuscript.
Declaration of interests
The authors declare potential conflicts of interest as follows: D.D.H. is a co-founder of TaiMed Biologics and RenBio, as well as a board director for Vicarious Surgical; he also serves as a consultant to WuXi Biologics, Brii Biosciences, and Veru; and he receives research support from Regeneron. A.G. served on a scientific advisory board for Janssen Pharmaceuticals.
Published: October 30, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xcrm.2023.101258.
Contributor Information
Aubree Gordon, Email: gordonal@umich.edu.
Lihong Liu, Email: ll3411@cumc.columbia.edu.
David D. Ho, Email: dh2994@cumc.columbia.edu.
Supplemental information
Data and code availability
Data reported in this paper will be shared by the lead contact upon request.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- 1.FDA . 2023. Coronavirus (COVID-19) Update: FDA Authorizes Changes to Simplify Use of Bivalent mRNA COVID-19 Vaccines.https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-changes-simplify-use-bivalent-mrna-covid-19-vaccines [Google Scholar]
- 2.Wang Q., Bowen A., Valdez R., Gherasim C., Gordon A., Liu L., Ho D.D. Antibody Response to Omicron BA.4-BA.5 Bivalent Booster. N. Engl. J. Med. 2023;388:567–569. doi: 10.1056/NEJMc2213907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Q., Bowen A., Tam A.R., Valdez R., Stoneman E., Mellis I.A., Gordon A., Liu L., Ho D.D. SARS-CoV-2 neutralising antibodies after bivalent versus monovalent booster. Lancet Infect. Dis. 2023;23:527–528. doi: 10.1016/S1473-3099(23)00181-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collier A.R.Y., Miller J., Hachmann N.P., McMahan K., Liu J., Bondzie E.A., Gallup L., Rowe M., Schonberg E., Thai S., et al. Immunogenicity of BA.5 Bivalent mRNA Vaccine Boosters. N. Engl. J. Med. 2023;388:565–567. doi: 10.1056/NEJMc2213948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis-Gardner M.E., Lai L., Wali B., Samaha H., Solis D., Lee M., Porter-Morrison A., Hentenaar I.T., Yamamoto F., Godbole S., et al. Neutralization against BA.2.75.2, BQ.1.1, and XBB from mRNA Bivalent Booster. N. Engl. J. Med. 2023;388:183–185. doi: 10.1056/NEJMc2214293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zou J., Kurhade C., Patel S., Kitchin N., Tompkins K., Cutler M., Cooper D., Yang Q., Cai H., Muik A., et al. Improved Neutralization of Omicron BA.4/5, BA.4.6, BA.2.75.2, BQ.1.1, and XBB.1 with Bivalent BA.4/5 Vaccine. bioRxiv. 2022 doi: 10.1101/2022.11.17.516898. Preprint at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Monto A.S., Malosh R.E., Petrie J.G., Martin E.T. The Doctrine of Original Antigenic Sin: Separating Good From Evil. J. Infect. Dis. 2017;215:1782–1788. doi: 10.1093/infdis/jix173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koutsakos M., Ellebedy A.H. Immunological imprinting: Understanding COVID-19. Immunity. 2023;56:909–913. doi: 10.1016/j.immuni.2023.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wheatley A.K., Fox A., Tan H.X., Juno J.A., Davenport M.P., Subbarao K., Kent S.J. Immune imprinting and SARS-CoV-2 vaccine design. Trends Immunol. 2021;42:956–959. doi: 10.1016/j.it.2021.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chemaitelly H., Ayoub H.H., Tang P., Hasan M.R., Coyle P., Yassine H.M., Al-Khatib H.A., Smatti M.K., Al-Kanaani Z., Al-Kuwari E., et al. Immune Imprinting and Protection against Repeat Reinfection with SARS-CoV-2. N. Engl. J. Med. 2022;387:1716–1718. doi: 10.1056/NEJMc2211055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alsoussi W.B., Malladi S.K., Zhou J.Q., Liu Z., Ying B., Kim W., Schmitz A.J., Lei T., Horvath S.C., Sturtz A.J., et al. SARS-CoV-2 Omicron boosting induces de novo B cell response in humans. Nature. 2023;617:592–598. doi: 10.1038/s41586-023-06025-4. [DOI] [PubMed] [Google Scholar]
- 12.Wang P., Nair M.S., Liu L., Iketani S., Luo Y., Guo Y., Wang M., Yu J., Zhang B., Kwong P.D., et al. Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7. Nature. 2021;593:130–135. doi: 10.1038/s41586-021-03398-2. [DOI] [PubMed] [Google Scholar]
- 13.Wang Q., Guo Y., Iketani S., Nair M.S., Li Z., Mohri H., Wang M., Yu J., Bowen A.D., Chang J.Y., et al. Antibody evasion by SARS-CoV-2 Omicron subvariants BA.2.12.1, BA.4, & BA.5. Nature. 2022 doi: 10.1038/s41586-022-05053-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmidt F., Weisblum Y., Muecksch F., Hoffmann H.H., Michailidis E., Lorenzi J.C.C., Mendoza P., Rutkowska M., Bednarski E., Gaebler C., et al. Measuring SARS-CoV-2 neutralizing antibody activity using pseudotyped and chimeric viruses. J. Exp. Med. 2020;217 doi: 10.1084/jem.20201181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Q., Iketani S., Li Z., Liu L., Guo Y., Huang Y., Bowen A.D., Liu M., Wang M., Yu J., et al. Alarming antibody evasion properties of rising SARS-CoV-2 BQ and XBB subvariants. Cell. 2023;186:279–286.e8. doi: 10.1016/j.cell.2022.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Q., Li Z., Guo Y., Mellis I.A., Iketani S., Liu M., Yu J., Valdez R., Lauring A.S., Sheng Z., et al. Evolving antibody evasion and receptor affinity of the Omicron BA.2.75 sublineage of SARS-CoV-2. bioRxiv. 2023 doi: 10.1101/2023.03.22.533805. Preprint at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simon V., Kota V., Bloomquist R.F., Hanley H.B., Forgacs D., Pahwa S., Pallikkuth S., Miller L.G., Schaenman J., Yeaman M.R., et al. PARIS and SPARTA: Finding the Achilles' Heel of SARS-CoV-2. mSphere. 2022;7 doi: 10.1128/msphere.00179-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu L., Iketani S., Guo Y., Chan J.F.W., Wang M., Liu L., Luo Y., Chu H., Huang Y., Nair M.S., et al. Striking antibody evasion manifested by the Omicron variant of SARS-CoV-2. Nature. 2022;602:676–681. doi: 10.1038/s41586-021-04388-0. [DOI] [PubMed] [Google Scholar]
- 19.Iketani S., Liu L., Guo Y., Liu L., Chan J.F.W., Huang Y., Wang M., Luo Y., Yu J., Chu H., et al. Antibody evasion properties of SARS-CoV-2 Omicron sublineages. Nature. 2022;604:553–556. doi: 10.1038/s41586-022-04594-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu L., Wang P., Nair M.S., Yu J., Rapp M., Wang Q., Luo Y., Chan J.F.W., Sahi V., Figueroa A., et al. Potent neutralizing antibodies against multiple epitopes on SARS-CoV-2 spike. Nature. 2020;584:450–456. doi: 10.1038/s41586-020-2571-7. [DOI] [PubMed] [Google Scholar]
- 21.Smith D.J., Lapedes A.S., de Jong J.C., Bestebroer T.M., Rimmelzwaan G.F., Osterhaus A.D.M.E., Fouchier R.A.M. Mapping the antigenic and genetic evolution of influenza virus. Science. 2004;305:371–376. doi: 10.1126/science.1097211. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data reported in this paper will be shared by the lead contact upon request.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.