Abstract
Background:
The treatment of epilepsy during early life poses unique challenges – first-line therapies leave many individuals with poorly controlled seizures. In response to the pharmaco-resistance of current first-line anti-seizure drugs (ASDs) during early life, new therapies have emerged. One such therapy is cannabidiol (CBD). While well studied in adult models of epilepsy, it is poorly studied in immature animals. Here we assessed the efficacy of CBD in immature rodent models of the epilepsies.
Methods:
Pups were pre-treated with CBD (1, 10, 50, 100, 200 mg/kg) and assessed for anticonvulsant efficacy using two well-established antiseizure screening models: the pentylenetetrazole (PTZ) and maximal electroshock (MES) models. We assessed drug efficacy in postnatal day (P)7 and P21 rats.
Results:
In the PTZ model, CBD delayed seizure onset in adolescent but not neonatal rats. By contrast, higher doses of CBD reduced seizure duration in both neonatal and adolescent rats in the MES model. The effects of CBD in both models were modest but consistent.
Conclusion:
Efficacy of CBD increased in older as compared to younger animals, producing an age-, model-, and dose-dependent suppression of seizures. These data suggest neonatal seizures (modeled by P7 treatment) may be less responsive to CBD. They also suggest preferential efficacy against tonic seizures as compared to partial motor seizures.
Keywords: cannabinoid, epileptogenesis, pentylenetetrazole, electroshock, neonatal
1. Introduction
Treatment of seizures during a specific window of brain development presents a significant challenge for children with epilepsy. While it is well-established that infants respond poorly to first-line anti-seizure drugs (ASDs) [1–2], the underlying mechanisms that give rise to drug-resistant epilepsy are varied. Distinct characteristics, including age-dependent changes in receptor subunit function [3], immature neuronal circuitry [4], and lack of anti-seizure drug (ASD) specificity [5], all contribute to refractory neonatal epilepsy.
In addition to poor efficacy, there is growing concern about the safety of anti-seizure drugs in neonatal populations. For example, in rodent models, many common anti-seizure drugs (ASDs) such as phenobarbital, phenytoin, and valproate trigger pronounced cell death in multiple regions of the developing brain [6–7]. Moreover, these drugs can produce lasting disruptions in synaptic development [8] and long-lasting changes in cognitive function [9–10] following even brief exposure. Together, efficacy and safety concerns underscore the need to investigate the efficacy of newer drugs during critical windows of brain development.
Phytocannabinoids are targets of increasing interest in the epilepsies. Several phytocannabinoids (i.e., Δ9-tetrahydrocannabidiol [Δ9-THC], Δ8-tetrahydrocannabidiol [Δ8-THC], Cannabinol [CBN]) act on receptors (i.e., CB1 and CB2) of the endocannabinoid system to cause intoxicating and psychoactive effects [14]. By contrast, cannabidiol (CBD) and its propyl analog cannabidivarin (CBDV) have minimal action on cannabinoid receptors, and instead are suggested to primarily work through other mechanisms including action at transient potential vanilloid 1 (TRPV1), GPR55, and adenosine reuptake sites [15–17,38]. While drugs that act on CB1 and CB2 receptors have well-established psychoactive properties, CBD displays minimal psychoactive effects [14].
CBD has long been recognized to have anti-seizure activity, with preclinical studies dating back to the 1970s [12–13]. Recently, CBD was approved for the treatment of Lennox-Gastuat Syndrome, Dravet Syndrome and tuberous sclerosis complex (TSC) in the United States [11,37]. Thus, CBD’s clinical efficacy in otherwise refractory early life epilepsies, coupled with its unique receptor pharmacology compared to other common anti-seizure drugs (ASDs), makes it a particularly intriguing target. However, little is known about its efficacy during the first weeks of life in which the developing brain is most vulnerable to seizures (i.e., neonatal period). Recently our lab has shown that CBD’s analog, CBDV, displays anti-seizure activity during adolescence that is absent during the neonatal period [18]. This is consistent with multiple studies that demonstrate that both age- and model-specific factors impact CBDs efficacy profile [18–20]. Given our findings with CBDV, we sought to determine the preclinical efficacy of CBD during two distinct periods of development (postnatal day [P] 7 and postnatal day [P] 21) using multiple seizure models.
2. Methods
2.1. Animals
Pregnant female Sprague-Dawley rats were obtained from Envigo (Indianapolis, ID) on gestational day 15 and housed in Georgetown’s Department of Comparative Medicine. Animals were maintained in a temperature-controlled room (21°C) with a 12 h light cycle (06:00–18:00). All procedures were performed during the light phase. Procedures were conducted in compliance with a protocol (# 2016–1306) approved by the Georgetown University Animal Care and Use Committee, and consistent with the Guide for the Care and Use of Laboratory Animals [21]. The day of parturition was defined as postnatal day (P) 0. On P7 and P21, pups were pre-treated with drug and tested in one of two seizure models 2 h post injection. Treatments were balanced within groups and across litters, and all experiments had approximately equal numbers of male and females.
2.2. Drugs
Cannabidiol (CBD; Brand Name: Epidiolex; Greenwich Biosciences) was obtained as an oral solution (100mg/mL) from the Georgetown University Hospital pharmacy. CBD was diluted in sesame oil (Sigma-Aldrich, cas no: 8008-74-0), which also was the corresponding vehicle solution. Concentrations of 0.1, 1, 5, 10, and 20 mg/mL were administered intraperitoneally (ip) at a volume of 10 mL/kg to deliver a dose of 1, 10, 50, 100, and 200 mg/kg of drug. Drug doses were selected based on CBD’s pharmacokinetic profile as well as the anticonvulsant range in adult rats [20,22].
2.3. Seizure Testing
CBD’s efficacy was measured using the pentylenetetrazole (PTZ) and maximal electroshock (MES) screening models in P7 and P21 rats. Both models are well-established screening methods that provide broad characterization of drug efficacy in rodents [23]. Additionally, P7 and P21 represent two distinct ages in which there are functional differences in brain development [5]. P7 represents the peak of synaptogenesis and corresponds to a period inclusive of early infancy in humans [40,41]. P21 represents an age at which seizure supporting networks are developed, and behavioral seizure responses begin to reliably mirror those seen in adult animals [42]. In both models, we treated animals with CBD 2 h prior to inducing seizures. This timing is based on prior studies, which demonstrated stable brain levels of CBD up to 4 hours after treatment, and peak brain levels at 1–2 h after treatment in P10 rat pups [19].
2.3.1. Pentylenetetrazole (PTZ) Model
Pentylenetetrazole (PTZ) was dissolved in 0.9% NaCl to make a final concentration of 10 mg/mL. P7 and P21 rats were removed from their home cage, weighed, labeled, and treated with CBD 2h prior to PTZ testing. Following pretreatment with CBD, PTZ was administered subcutaneously (0.01ml/g body weight) and observed for 30 minutes for seizure activity. Seizure onset and duration was recorded by a treatment-blind observer. We used a 100 mg/kg dose of PTZ, as this reliably produces seizures in animals of this age, and is the standard dose used as a chemoconvulsant challenge by our group [18,25] and other groups [24,43].
2.3.2. Seizure Scoring
Seizure activity in P7 rat pups was scored using a 5-point behavioral scoring system modified from Kubova and Mares (1993), as previously described by our lab [24, 25]. The following 5-point scale for measuring minimal and maximal behavioral seizures was: 0=no change in behavior, 0.5=wet dog shakes, 1=myoclonic jerks, 2=unilateral clonus, 3=forelimb clonus, 4=loss of posture, 5=tonic-clonic seizure. Seizures were scored by a treatment-blind observer.
P21 rats display different behavioral characteristics from neonatal rats [26] and required a different scoring method to assess seizure activity following drug exposure. We used a 6-point rating scale, as we have previously described [27]. Seizures were scored as: 1=myoclonic jerk, 2=rapid myoclonic jerks, 3=forelimb clonus (FFC), 4= FFC + rearing, 5=loss of posture, 6=tonic-clonic seizure. Seizures were scored by a treatment-blind observer.
2.3.3. Maximal electroshock (MES) Model
MES is a well-established seizure model to measure drug efficacy [23]. Animals were pre-treated with CBD 2h prior to seizure testing, and P7/ P21 animals received 0.5% tetracaine HCl eyedrops prior to transorbital stimulation. Animals received 200Hz sinusoidal train pluses at 50mA for 300ms using a Ugo Basile stimulator (Model 7800). The current selected was chosen to produce seizures (tonic hindlimb extension) in ~90% of control animals. The classic endpoint of the maximal electroshock model is tonic extension of the hindlimbs. While P21 animals display robust tonic extension, this was less reliable in P7 animals. Therefore, we measured the duration of behavioral seizure activity in both P7 and P21 animals, and the incidence of tonic extension only in P21 animals. Seizure duration was recorded by a treatment-blind observer.
2.4. Statistics
Statistical analysis was performed using GraphPad Prism 8 (GraphPad Software; La Jolla, Ca). Behavioral seizure scores were analyzed using the Kruskal-Wallis test followed by Holm-Šídák corrected multiple comparisons test, as these data are inherently non-normal. Latency data failed normality testing (D’Agostino & Pearson test) and thus were also analyzed by Kruskal-Wallis. The proportion of P21 animals displaying tonic extension was analyzed using Fisher’s Exact test. P-values < 0.05 were considered statistically significant.
3. Results
3.1. Anticonvulsant profile of CBD on PTZ-evoked seizures in neonatal and adolescent rats
Both P7 and P21 rats were subjected to PTZ-evoked seizures to determine the efficacy of CBD at each age. In P7 rats treated with vehicle, all subjects displayed Score 5 seizures, corresponding to tonic-clonic movement of the limbs. CBD was without effect on seizure severity (Kruskal-Wallis H=3.92, p=0.56). At each of the doses tested, the median seizure score remained 5 (Fig 1A). Latency to maximal tonic-clonic seizures was also analyzed as a measurement of anticonvulsant efficacy. Vehicle treated rats displayed a mean latency of 378.9s at P7. CBD displayed dose-dependent effects on latency to seizure onset (Kruskal-Wallis, H=11.82, p=0.037). A dose of 200 mg/kg significantly increased the latency of tonic-clonic seizures while lower doses of CBD had no effect on seizure onset (Holm-Šídák corrected Dunn’s test, p= 0.0070, Fig 1B).
Figure 1.
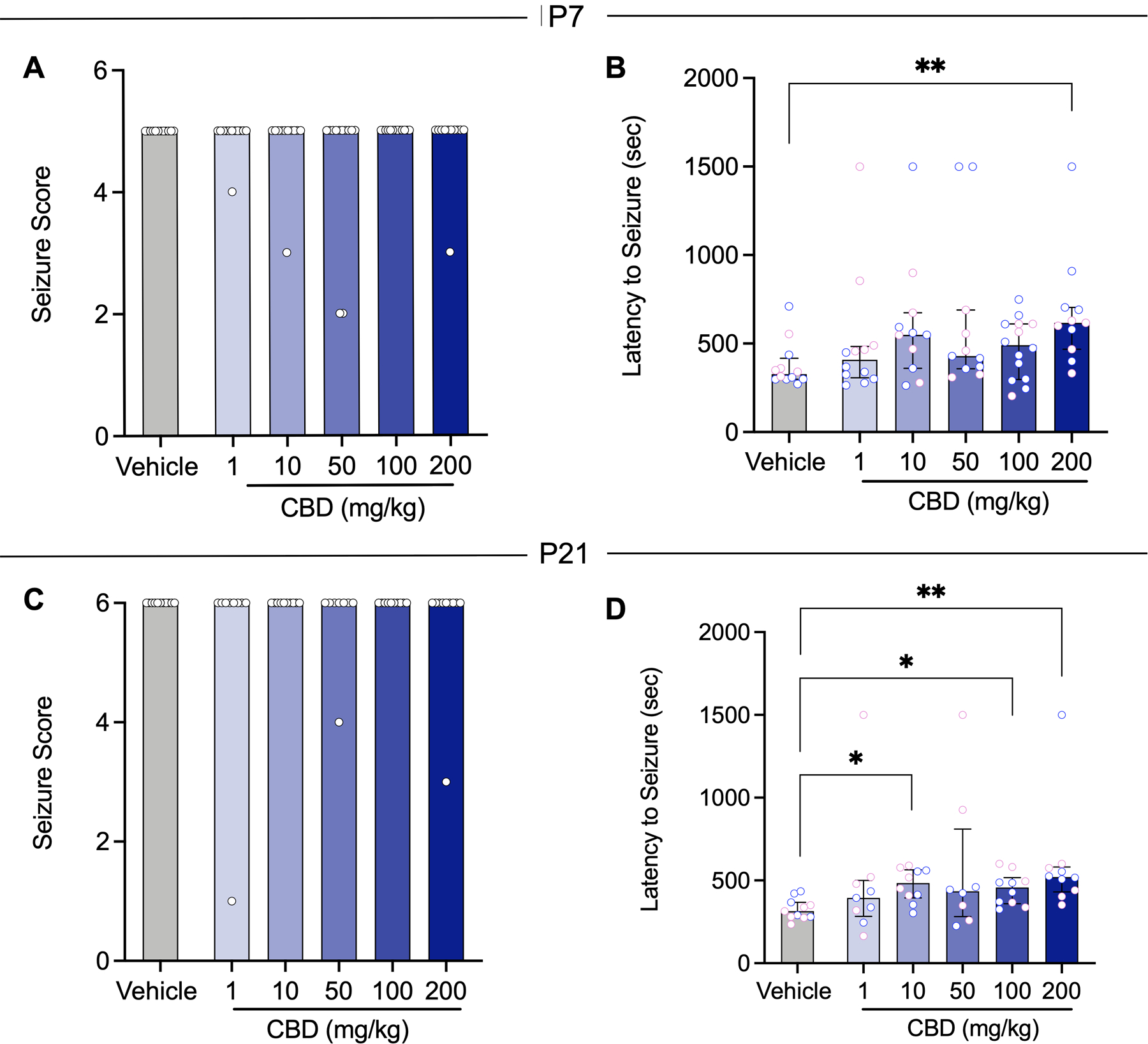
CBD increases latency but does not attenuate the severity of PTZ-induced seizures in immature and adolescent rats. A Mean seizure score as a function of CBD dose in P7 rats (Veh n=12; 1mg/kg n=12; 10mg/kg n=11; 50mg/kg n=12; 100mg/kg n=13; 200mg/kg n=11). B Mean latency to seizure onset as a function of CBD dose in P7 rats (Veh n=12; 1mg/kg n=12; 10mg/kg n=11; 50mg/kg n=11; 100mg/kg n=14; 200mg/kg n=11). C Mean seizure score as a function of CBD dose in P21 rats (Veh n=11; 1mg/kg n=9; 10mg/kg n=10; 50mg/kg n=8; 100mg/kg n=10; 200mg/kg n=10;). D Mean latency to seizure onset as a function of CBD dose in P21 rats (Veh n=11; 1mg/kg n=9; 10mg/kg n=10; 50mg/kg n=8; 100mg/kg n=10; 200mg/kg n=10). Blue data values represent male subjects, and pink data values represent female subjects. Bars show median; error bars (where present) show interquartile range. *p<0.05; ** p<0.01; ***p<0.001; Kruskal Wallis test, followed by Holm-Sidak corrected Dunn’s test.
In P21 vehicle treated rats, all animals displayed Score 6 PTZ-induced seizures corresponding to tonic-clonic movement of the limbs. CBD had no effect on seizure severity at each dose tested, and the median seizure score remained 6 for all groups (Kruskal-Wallis H=3.61, p=0.61, Fig 1C). In P21 rodents, the vehicle treated group displayed a mean latency of 326s for tonic-clonic maximal seizures that was significantly elevated following exposure to 10, 100, and 200 mg/kg of CBD (Kruskal-Wallis, H=15.77, p=0.0075; Holm-Šídák correction, ps= 0.05, 0.001, 0.9492, respectively; Fig 1D).
3.2. Anticonvulsant profile of CBD on MES-evoked seizures in immature and adolescent rats.
Next, we used the MES-evoked seizure model to further assess the sensitivity of CBD in P7 and P21 rodents. We have previously reported that the propyl analog of CBD, CBDV, displays age-dependent efficacy in the MES model [18]. Tonic hindlimb extension was not observed in P7 rats, however, we were able to observe psychomotor seizures and use it as an endpoint to measure total seizure time in the MES-evoked seizure model. Vehicle treated rats displayed a mean seizure duration of 25s at P7, and treatment with 100 and 200 mg/kg of CBD significantly reduced seizure duration (Kruskal-Wallis, H=28.33, p<0.0001; Holm-Šídák correction, ps= 0.0308, 0.0005, respectively; Fig 2A). CBD was without effect at lower doses of 1, 10, and 50 mg/kg of CBD (Kruskal-Wallis, H=28.33, p<0.0001; ps=0.6315, 0.6491, 0.680, respectively).
Figure 2.

CBD alters MES-evoked seizures in immature and adolescent rats. A Mean seizure duration as a function of CBD dose in P7 rats (Veh n=12; 1mg/kg n=12; 10mg/kg n=12; 50mg/kg n=12; 100mg/kg n=11; 200mg/kg n=12). B Mean seizure duration as a function of CBD dose in P21 rats (Veh n=9; 1mg/kg n=10; 10mg/kg n=11; 50mg/kg n=11; 100mg/kg n=11; 200mg/kg n=10). C Percent of P21 animals showing tonic extension following MES-evoked seizures. Blue data values represent male subjects, and pink data values represent female subjects. Bars show mean and standard error of the mean. *p<0.05; ***p<0.001; Kruskal Wallis test, followed by Holm-Sidak corrected Dunn’s test.
In P21 rats, we measured the duration and incidence of tonic hindlimb extension as endpoints of CBDs anticonvulsant efficacy. Vehicle treated rats displayed a mean duration of 10s, and while CBD did not protect against MES-evoked seizures, 100 mg/kg of CBD significantly decreased the duration of tonic seizures (Kruskal-Wallis, H=12.76, p=0.03; Holm-Šídák correction, *p= 0.0374; Fig 2B), an effect not observed at other doses. We also examined the proportion of animals with tonic-hindlimb extension as a function of drug treatment. Fischer’s exact test found no difference in the incidence of tonic-hindlimb extension as a function of CBD treatment (χ2 = 7.45, df = 5, p= 0.1895; Fig 2C).
4. Discussion
Despite cannabidiol (CBD) being approved by the FDA for some rare childhood epilepsies, very few studies have examined CBDs efficacy and safety profile during the neonatal period. Previous reports indicate that changes in receptor subunit function, immature network connectivity, and blood-brain-barrier permeability at various stages of development may all be factors that influence the anticonvulsant efficacy of drugs like CBD [4,5,28]. Historically, the preclinical efficacy of drugs has been assessed in adult rodent models of epilepsy [29] resulting in limited information about the efficacy and safety of CBD during early life. With this scarcity of information, our study focused on the effectiveness of CBD during distinct periods of development.
Our study revealed an age- dependent efficacy profile for CBD. Effects were weak or absent in neonatal rats but were clear and reproducible in adolescent rats. In the PTZ model, CBD dose-dependently delayed seizure onset but did not affect seizure severity in either neonatal or adolescent rats. The latter finding was rather surprising considering previous work that suggests CBD reduces seizure severity in immature [19,30] and adult [20,22,31] rodents. The inability of CBD to reduce seizure severity in our study may more broadly represent CBDs limited pharmacological sensitivity during development [38]. Multiple studies support the idea that there is low expression of receptor targets for CBD that change over the developmental timeline [5,18,28]. For example, previous studies from our lab illustrate changes in mRNA expression levels of TRPV1, TRPV3, and GPR55 from postnatal day 10 to postnatal day 20 in rats [18]. Moreover, while Uttl et al. (2021) reported a reduction in the severity of PTZ-evoked seizures during the early life period, our studies focused on an earlier timepoint (P7) where brain networks and receptor expression levels are less mature [5], potentially explaining the differences in CBD’s impact on seizure severity during development. Uttl et al. (2021) used a different vehicle to dissolve CBD, a shorter interval between CBD administration and testing, a different age (P12), and a different rat strain, all of which may contribute to the differences between our studies. Uttl and colleagues also report stable brain levels for several hours after drug administration using moderate doses (60 mg/kg), suggesting that pharmacokinetics are unlikely to account for the differences between our studies.
While another study has evaluated efficacy of CBD against partial seizures in during the early life period [30], they use the systemic and intrahippocampal kainate models. These models preferentially produce partial seizures, but not generalized tonic-clonic seizures. This suggests that model-specific factors influence CBDs efficacy. Taken together, our findings suggest that a combination of age-related expression levels, seizure model, and dosage of CBD all influence CBDs efficacy in neonatal and adolescent rats. A limitation to the present study is that our chosen models, while well-accepted tools for screening anti-seizure drugs are acute, rather than chronic models of epilepsy. The same holds true for all of the prior studies of CBD efficacy in neonatal rodents. There is a dearth of chronic models that can be used effectively in neonatal rodents. Further translation of these results into either chronic or genetic models of early-life seizures would be of interest. Moreover, future studies examining the impact of CBD on behavior in developing animals would also be of relevance. We anecdotally noted that many animals in the highest dose (200 mg/kg) group displayed sedation after drug treatment.
Preclinical data from our lab suggest that CBDs n-propyl analog, cannabidivarin (CBDV), is effective in multiple seizure models in neonatal rats [18]. However, unlike CBDV, CBD has an atypical effect on seizure severity and duration in all seizure models used in our study. We previously reported that CBDV reduces PTZ-evoked seizure severity but not seizure onset in neonatal rats [18]. This is in comparison to our current study where CBD has no effect on PTZ-evoked seizure severity, but significantly increased the latency to seizure in neonatal rats with a more robust effect in adolescent rats. The same atypical effect of CBD is observed in our MES model. In adolescent rats, we found no effect of CBD to influence tonic hindlimb seizures that was observed in our prior report with CBDV [18]. In addition, we found higher doses of CBD were effective in reducing seizure duration in neonatal rats that was not observed following treatment with CBDV [18].
The paradoxical effect of CBD versus CBDV in neonatal and adolescent rats may represent age-specific, mechanistic, and pharmacological differences of both drugs. In our study and consistent with prior reports, MES-evoked seizures at postnatal day (P) 7 displayed psychomotor seizures compared to the traditional tonic hindlimb extension [32,33]. This is mainly attributed to the immature development of motor pathways yielding an altered phenotypic response during the first week of life [33,34], suggesting that the efficacy of CBD and CBDV may change depending on the maturation of seizure networks during early life. Moreover, information about the pharmacokinetic profile of CBD and CBDV may provide insight into its effectiveness in rodents. Previous work has suggested that CBDV can reach brain concentrations similar to that of CBD [35]. CBD and CBDV display overlapping drug targets (e.g., Trp channels) but the precise targets that mediate anticonvulsant action are only partially understood. In the context of neonatal seizures, we have previously shown reduced efficacy of CBDV against electroshock seizures in TRPV1 knockout mice [18]. Both drugs display agonist responses at TRPV1 channels, and both can desensitize TRPV1. Previous work suggest CBD is a more potent desensitizer of TRPV1 channels than CBDV [36], and displays a narrower index between concentrations that activate and desensitize the receptor. Whether this difference accounts for the differential anticonvulsant profiles of these compounds remains to be determined.
5. Conclusion
In summary, we report that CBD displays weak efficacy against PTZ-evoked seizures in both P7 and P21 rat pups. CBD displayed greater efficacy in the MES model than in the PTZ model. Our findings suggest that developmental stage and seizure type may be critical variables to consider for the use of CBD to treat epilepsy.
Funding.
This research was supported in part by R01HD091994 to PAF.
Abbreviations:
- P
Postnatal day
- CBD
Cannabadiol
- MES
Maximal Electrohock
- PTZ
Pentylenetetrazole
- ANOVA
Analysis of Variance
Footnotes
Conflicts of interest. The authors report no conflicts of interest.
Data Availability Statement:
The datasets generated during and analyzed during the current study are available from the corresponding author on reasonable request
References
- 1.Painter MJ, Scher MS, Stein AD, et al. Phenobarbital compared with phenytoin for the treatment of neonatal seizures. N Engl J Med 1999; 341:485–9. [DOI] [PubMed] [Google Scholar]
- 2.Glass HC, Soul JS, Chu CJ, et al. Response to antiseizure medications in neonates with acute symptomatic seizures. Epilepsia. 2019; 60(3): e20–e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou C, Sun H, Klein PM, Jensen FE. Neonatal seizures alter NMDA glutamate receptor GluN2A and 3A subunit expression and function in hippocampal CA1 neurons. Front Cell Neurosci. 2015; 9:362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holmes GL. The long-term effects of neonatal seizures. Clin Perinatol. 2009;36(4):901–viii. doi: 10.1016/j.clp.2009.07.012 [DOI] [PubMed] [Google Scholar]
- 5.Jensen FE. Neonatal seizures: an update on mechanisms and management. Clin Perinatol. 2009;36(4):881–vii. doi: 10.1016/j.clp.2009.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bittigau P, Sifringer M, Genz K, et al. Antiepileptic drugs and apoptotic neurodegeneration in the developing brain. Natl Acad Sci USA.2002;99(23):15089–15094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forcelli PA, Kim J, Kondratyev A, Gale K. Pattern of antiepileptic drug-induced cell death in limbic regions of the neonatal rat brain. Epilepsia. 2011;52(12):e207–e211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forcelli PA, Janssen MJ, Vicini S, Gale K, 2012a. Neonatal exposure to antiepileptic drugs disrupts striatal synaptic development. Ann. Neurol 72, 363–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gutherz SB, Kulick CV, Soper C, Kondratyev A, Gale K, Forcelli PA. Brief postnatal exposure to phenobarbital impairs passive avoidance learning and sensorimotor gating in rats. Epilepsy Behav. 2014;37:265–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forcelli PA, Kozlowski R, Snyder C, Kondratyev A, Gale K. Effects of neonatal antiepileptic drug exposure on cognitive, emotional, and motor function in adult rats. J Pharmacol Exp Ther. 2012;340(3):558–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.FDA approves first drug comprised of an active ingredient derived from marijuana to treat rare, severe forms of epilepsy. U.S. Food & Drug. FDA Press; AnnouncementsPage.https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm611046.htm [Google Scholar]
- 12.Carlini EA, Leite JR, Tannhauser M, Berardi AC. Letter: Cannabidiol and Cannabis sativa extract protect mice and rats against convulsive agents. J Pharm Pharmacol. 1973;25(8):664–665. [DOI] [PubMed] [Google Scholar]
- 13.Izquierdo I, Orsingher OA, Berardi AC. Effect of cannabidiol and of other cannabis sativa compounds on hippocampal seizure discharges. Psychopharmacologia. 1973;28(1):95–102. [DOI] [PubMed] [Google Scholar]
- 14.Morales P, Hurst DP, Reggio PH. Molecular Targets of the Phytocannabinoids: A Complex Picture. Prog Chem Org Nat Prod. 2017;103:103–131. doi: 10.1007/978-3-319-45541-9_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gray RA, Whalley BJ. The proposed mechanisms of action of CBD in epilepsy. Epileptic Disord. 2020;22(S1):10–15. [DOI] [PubMed] [Google Scholar]
- 16.Morales P, Hurst DP, Reggio PH, 2017. Molecular targets of the phytocannabinoids- A complex picture. Prog. Chem. Org. Nat. Prod 103, 103–131. 10.1007/978-3-319-45541-9_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morano A, Fanella M, Albini M, Cifelli P, Palma E, et al. Cannabinoids in the Treatment of Epilepsy: Current Status and Future Prospects. Neuropsychiatr Dis Treat. 2020;16:381–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huizenga MN, Sepulveda-Rodriguez A, Forcelli PA. Preclinical safety and efficacy of cannabidivarin for early life seizures. Neuropharmacology. 2019;148:189–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uttl L, Hložek T, Mareš P, Páleníček T, Kubová H. Anticonvulsive Effects and Pharmacokinetic Profile of Cannabidiol (CBD) in the Pentylenetetrazol (PTZ) or N-Methyl-D-Aspartate (NMDA) Models of Seizures in Infantile Rats. Int J Mol Sci. 2021;23(1):94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones NA, Glyn SE, Akiyama S, et al. Cannabidiol exerts anti-convulsant effects in animal models of temporal lobe and partial seizures. Seizure. 2012;21(5):344–352. [DOI] [PubMed] [Google Scholar]
- 21.Guide for the Care and Use of Laboratory Animals. Eighth Edition. Institute for Laboratory Animal Research. https://grants.nih.gov/grants/olaw/guide-for-the-care-and-use-of-laboratory-animals.pdf [Google Scholar]
- 22.Jones NA, Hill AJ, Smith I, et al. Cannabidiol displays antiepileptiform and antiseizure properties in vitro and in vivo. J Pharmacol Exp Ther. 2010;332(2):569–577. doi: 10.1124/jpet.109.159145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Löscher W Critical review of current animal models of seizures and epilepsy used in the discovery and development of new antiepileptic drugs. Seizure. 2011;20(5):359–368. [DOI] [PubMed] [Google Scholar]
- 24.Kubová H, Mares P. Anticonvulsant action of oxcarbazepine, hydroxycarbamazepine, and carbamazepine against metrazol-induced motor seizures in developing rats. Epilepsia. 1993;34(1):188–192. [DOI] [PubMed] [Google Scholar]
- 25.Forcelli PA, Soper C, Lakhkar A, Gale K, Kondratyev A. Anticonvulsant effect of retigabine during postnatal development in rats. Epilepsy Res. 2012;101(1–2):135–140. [DOI] [PubMed] [Google Scholar]
- 26.Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroen Clin Neuro. 1972;32:281–294. [DOI] [PubMed] [Google Scholar]
- 27.Forcelli PA, Gale K, Kondratyev A. Early postnatal exposure of rats to lamotrigine, but not phenytoin, reduces seizure threshold in adulthood. Epilepsia. 2011;52:e20–e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rakhade SN, Jensen FE. Epileptogenesis in the immature brain: emerging mechanisms. Nat Rev Neurol. 2009;5(7):380–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chapman KE, Raol YH, Brooks‐Kayal A, 2012. Neonatal seizures: controversies and challenges in translating new therapies from the lab to the isolette. Eur. J. Neurosci 35, 1857–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Friedman LK, Wongvravit JP. Anticonvulsant and Neuroprotective Effects of Cannabidiol During the Juvenile Period. J Neuropathol Exp Neurol. 2018;77(10):904–919. [DOI] [PubMed] [Google Scholar]
- 31.Patra PH, Barker-Haliski M, White HS, et al. Cannabidiol reduces seizures and associated behavioral comorbidities in a range of animal seizure and epilepsy models. Epilepsia. 2019;60(2):303–314. doi: 10.1111/epi.14629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.SCHICKEROVÁ R, MAREŠ P, TROJAN S: Correlation between electrocorticographic and motor phenomena induced by metrazol during ontogenesis in rats. Exp Neurol 84: 153–164, 1984. [DOI] [PubMed] [Google Scholar]
- 33.Mareš P Models of epileptic seizures in immature rats. Physiol Res. 2012;61(Suppl 1):S103–S108. [DOI] [PubMed] [Google Scholar]
- 34.Mares P, Maresová D, Trojan S, Fischer J. Ontogenetic development of rhythmic thalamo-cortical phenomena in the rat. Brain Res Bull. 1982;8(6):765–769. [DOI] [PubMed] [Google Scholar]
- 35.Deiana S, Watanabe A, Yamasaki Y, et al. Plasma and brain pharmacokinetic profile of cannabidiol (CBD), cannabidivarine (CBDV), Δ⁹-tetrahydrocannabivarin (THCV) and cannabigerol (CBG) in rats and mice following oral and intraperitoneal administration and CBD action on obsessive-compulsive behaviour. Psychopharmacology (Berl). 2012;219(3):859–873. [DOI] [PubMed] [Google Scholar]
- 36.De Petrocellis L, Ligresti A, Moriello AS, et al. Effects of cannabinoids and cannabinoid-enriched Cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. Br J Pharmacol. 2011;163(7):1479–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.FDA approves new indication for drug containing active ingredient derived from cannabis to treat seizures in rare genetic disease. U.S. Food & Drug. FDA Press Announcements. Page.https://www.fda.gov/news-events/pressannouncements/fda-approves-new-indication-drug-containing-active-ingredient-derived-cannabis-treat-seizures-rare [Google Scholar]
- 38.Iannotti FA, Hill CL, Leo A, Alhusaini A, Soubrane C, Mazzarella E, Russo E, Whalley BJ, Di Marzo V, Stephens GJ. Nonpsychotropic plant cannabinoids, cannabidivarin (CBDV) and cannabidiol (CBD), activate and desensitize transient receptor potential vanilloid 1 (TRPV1) channels in vitro: potential for the treatment of neuronal hyperexcitability. ACS Chem Neurosci. 2014. Nov 19;5(11):1131–41. [DOI] [PubMed] [Google Scholar]
- 39.Rosenberg EC, Patra PH, Whalley BJ. Therapeutic effects of cannabinoids in animal models of seizures, epilepsy, epileptogenesis, and epilepsy-related neuroprotection. Epilepsy Behav. 2017. May;70(Pt B):319–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dobbing J, Sands J. Comparative aspects of brain growth spurt. Early Human Development. 1979;311:79–83. [DOI] [PubMed] [Google Scholar]
- 41.Semple BD, Blomgren K, Gimlin K, Ferriero DM, Noble-Haeusslein LJ. Brain development in rodents and humans: Identifying benchmarks of maturation and vulnerability to injury across species. Prog Neurobiol. 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kulick C, Gutherz S, Kondratyev A, Forcelli PA. Ontogenic profile of seizures evoked by the beta-carboline DMCM (methyl-6,7-dimethoxy-4-ethyl-β-carboline-3-carboxylate) in rats. Eur J Pharmacol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Velisek L, Kubova H, Pohl M et al. Pentylenetetrazol-induced seizures in rats: an ontogenetic study.Naunyn-Schmiedeberg’s Arch Pharmacol 346, 588–591 (1992). 10.1007/BF00169017 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and analyzed during the current study are available from the corresponding author on reasonable request


