Summary
Nonylphenol (NP), a widely recognized endocrine disruptor, exhibits lipophobic properties that drive its accumulation in adipose tissue, leading to various physiological disruptions. Using Caenorhabditis elegans, this study investigated the effects of NP exposure on lipid homeostasis and physiological indicators. NP exposure increased lipid storage, hindered reproduction and growth, and altered phospholipid composition. Transcriptional analysis revealed NP’s promotion of lipogenesis and inhibition of lipolysis. Metabolites related to lipid metabolism like citrate, amino acids, and neurotransmitters, along with lipids, collectively influenced physiological processes. This work elucidates the complex link between lipid metabolism disturbances and NP-induced physiological disruptions, enhancing our understanding of NP’s multifaceted toxicity.
Subject areas: Biochemistry, Lipid, Toxicology
Graphical abstract

Highlights
-
•
NP induced increased lipid accumulation and disrupted physiology of C. elegans
-
•
NP altered lipid profiles, especially phosphatidylcholines and fatty acids
-
•
NP stimulated lipogenesis and inhibited lipolysis
-
•
Upregulated citramalic acid, amino acids linked to lipid deposition
Biochemistry; Lipid; Toxicology
Introduction
Nonylphenol (NP) is a prominent member of the alkylphenol class and has received significant attention due to its prevalence in various environmental media and toxicological implications.1 Due to its ability to bioconcentrate, extensive research has been conducted on its toxic effects on various species. The toxic effects of NP are multiterminal and complex, including reduced the hatchability of embryos, altered hermaphroditism sex ratio, gamete production, and neurotoxicity, etc.2,3 Most studies on the multi-toxic mechanism of NP have primarily focused on its estrogen-like effects. NP can mimic the natural estrogen 17β-estradiol to regulate endocrine system and thus generate toxic effects on organisms. However, compelling evidence suggests that NP, along with other endocrine disrupting chemicals (EDCs), has been identified as obesogenic chemical that tends to accumulate mainly in lipophilic tissues and negatively impact lipid metabolism of animals,4 consequently leading to a range of adverse impacts on physiological functions.
Lipid storage is characterized by its dynamic nature, undergoing constant modulations in response to exogenous factors, as exemplified by prior studies.5,6,7 The disruption of lipid metabolism or the hormonal regulatory framework by obesogenic chemicals can yield profound ramifications on energy allocation, viability, reproduction, and developmental processes.8 Noteworthy instances include the effect of organofluorine compounds, which accentuate lipid accumulation in Daphnia magna, concurrently leading to reduced lifespan and diminished body size;9 Similarly, obesogenic agents like tributyltin interfere with lipid metabolism, exerting deleterious consequences on the fitness of D. magna;10 Furthermore, bisphenol S perturbs lipid consumption and distribution in the brain, instigating neurotoxicity in zebrafish.11 Given this intricate interplay between lipid dynamics and chemical exposure, it is imperative to accord due attention to lipid-related mechanisms as potential sources of EDCs-induced toxicity, an aspect that may not have yet garnered the gravity it warrants.
The lipophilic properties of NP, recognized for its capacity to accumulate and endure in adipose tissue,12 are confirmed by the identification of NP in all assessed adipose tissue samples.13 NP can reduce glycogen levels in aquatic creatures, prompting greater energy metabolism toward fat storage.14 Furthermore, NP fosters the proliferation and differentiation of adipocytes, contributing to abnormal weight gain.15 The augmentation of storage lipids may additionally expedite the bioaccumulation of NP, creating a positive feedback loop between NP accumulation and fat storage. While the physiological impacts of NP on organisms are broadly understood, the connection between NP-adjusted lipid metabolism and its underlying toxic effects remains undisclosed. The multifaceted repercussions of lipid metabolism disruptions can reverberate throughout the biological system, underscoring the significance of unraveling these pathways in the context of environmental toxicology.
Omics techniques represent a potent tool for unraveling complex biological processes and interrelationships. Among these methodologies, non-targeted metabolomic analysis emerges as a versatile method, capable of furnishing unbiased molecular insights into diverse facets of biological phenomena, encompassing lipid metabolism and life history components such as neurotransmitters, amino acids, and carbohydrates.16,17 The convergence of these multifaceted processes holds the potential to yield enriched insights into the intricate biological pathways susceptible to the influences of toxicants, thereby facilitating the discernment of the underlying mechanisms governing NP multi-toxicity.
Herein, we investigated the effects of NP exposure on lipid storage and physiological indicators at both phenotypic and molecular levels using the Caenorhabditis elegans model as a vehicle. The nematode C. elegans was selected as tested subject due to its suitability as an model organism for investigating lipid metabolism.18,19 Genetic conservation allows ancient features of fat storage pathways to be explored in C. elegans.20 The lipid storage and physiological indicators, including body area and brood size, were simultaneously detected following NP concentrations of 0, 1, 10, 200, and 400 μg L−1. Untargeted metabolome analytical approaches and transcriptome were utilized to understand the molecular mechanism of lipid metabolism and establish global profiles of altered molecular alterations in C. elegans. Finally, the complex effects of lipid-related processes were analyzed from molecular and physiological effects. Through this investigation, we aspire to contribute to a holistic understanding of NP’s multi-toxicity mechanism.
Results
Effects of NP on lipid storage
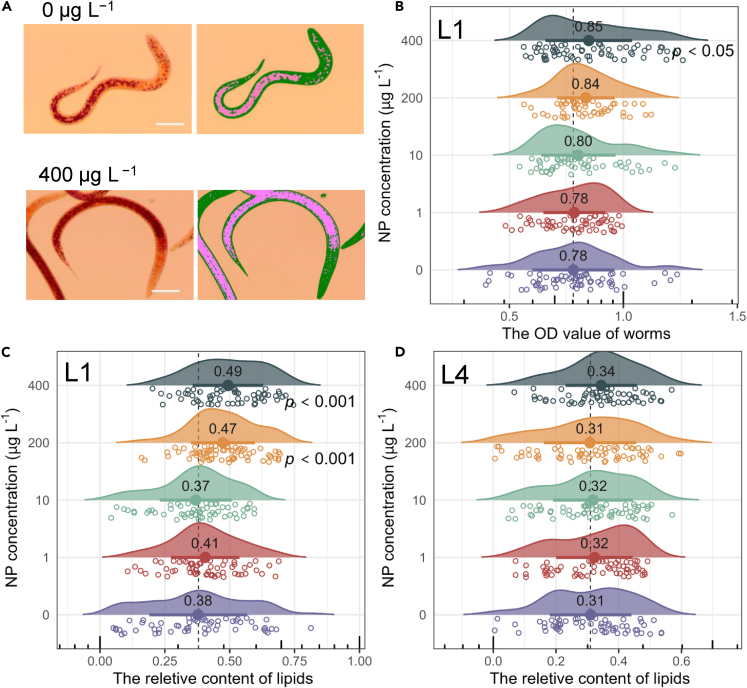
Lipid storage in the worm C. elegans at two life stages was visualized and analyzed in the presence (1–400 μg L−1) and absence of NP (control). For L1 larvae, the results showed that the distribution of lipid droplets was more abundant in the intestines and subcutaneous tissue in the presence of NP (Figure 1A). Optical density (OD) of lipid droplets increased significantly at 400 μg L−1 (Figure 1B), and excessive lipid relative content was observed at 200 and 400 μg L−1 NP concentrations compared to negative control groups (p < 0.05, Figure 1C). Therefore, the fat ratio method was a more sensitive quantitative measurement for assessing fat storage in C. elegans compared to the OD test. In contrast to the L1 larvae, fat storage at the L4 larvae was not affected by the all-NP treatment groups (p > 0.05, Figure 1D).
Figure 1.
Impact of NP exposure on nematode lipid accumulation
(A) Distribution of lipid droplets in nematodes under control and 400 μg L−1 NP exposure. Scale bar, 20 μm.
(B) Lipid density in L1 larval nematodes in response to NP exposure.
(C) Ratio of stored lipids in L1 larval nematodes in response to NP exposure.
(D) Ratio of stored lipids in L4 larval nematodes in response to NP exposure. Data are presented as mean ± standard deviation (SD) (n = 50). ∗p < 0.05 vs. the vehicle control (0.1% DMSO, v/v).
The increased lipid storage in worms exposed to NP was congruent with published data in other species. Hao et al. once elaborated that NP could promote the fat mass of mice,21 and similar condition is also found in the NP-treated fish.22 This demonstrated the consistency of the effects of NP on lipid interference in different organisms. In addition, the life periods that exposure to NP is also a critical factor for adipocytes differentiation.23 On this regard, the onset of stimulation adipogenesis induced by NP exposure was more likely to be early postnatal stage in worms. It is also worth noting that oil red O serves as fixative-based dye that stains lipid species most closely associated with triacylglycerols (TAGs).24 The enhanced lipid deposition by NP exposure indicated that the excessive synthesis of TAGs in C. elegans.
Effects of NP on physiological indicators
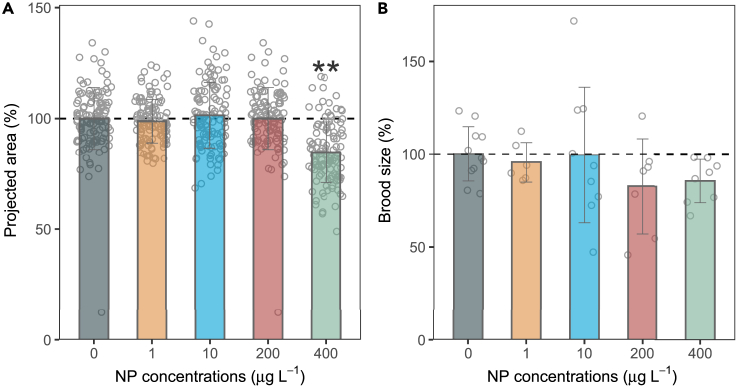
The growth and reproductive processes of the worms were affected to varying degrees by the presence of NP, as depicted in Figure 2. The projected area of each treatment, which was used to characterize growth, exhibited slight fluctuations with the increase of NP concentration (p > 0.05), except for the 400 μg L−1 exposure group which showed a significant decrease (p < 0.05, Figure 2A). Additionally, the brood size of worms in NP treatments of 1 and 10 μg L−1 was comparable to the vehicle control (with a mean of 255–267 posterities per worm). However, in the case of 200 and 400 μg L−1 NP treatments, there was a decrease in the number of offspring per worm (220−228 posterities per worm) (Figure 2B).
Figure 2.
Impact of NP exposure on nematode growth and reproduction
(A) Projected area in response to NP exposure.
(B) Brood size in response to NP exposure. Values represent the mean ± SD. Open circles indicate individual measurements, while the bar represents the mean value. ∗p < 0.05 vs. the vehicle control (0.1% DMSO, v/v).
Low concentration of NP altered storage lipid accumulation, and simultaneously decreased the body area and brood size of C. elegans. Both development and reproduction are energetically costly processes. Evaluated lipid storage probably related to the imbalance of energy allocation to other phycological processes. For example, tributyltin disturbs the homeostasis of neutral lipids by impairing the transfer of TAGs to eggs, and consequently increasing the storage of lipids in adults.10 However, such idea has not been tested in NP yet, and to do so, underlying mechanisms are required to explain the connection between the upregulated lipid storage and affected phycological indicators.
Effects of NP on pharyngeal movements
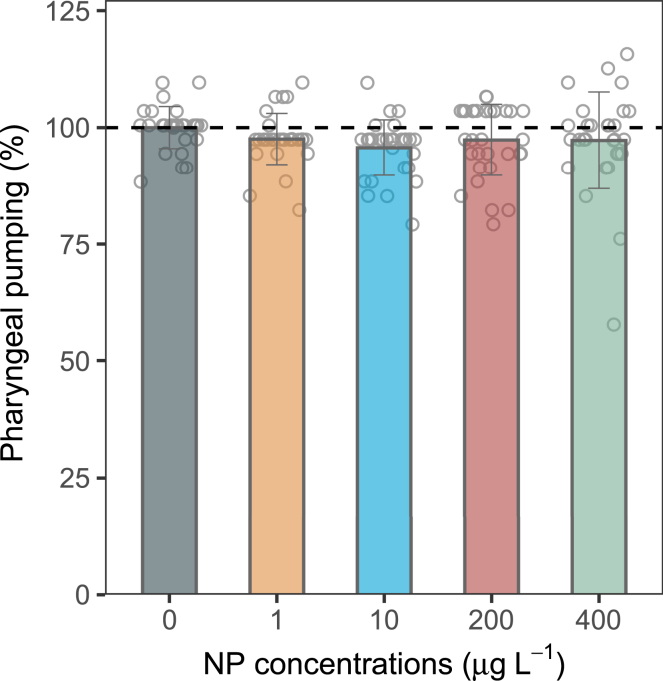
In the context of inducing excessive lipid storage, it has been widely postulated that heightened nutrient intake, assessed by quantifying pharyngeal movements, serves as a key contributory factor among various environmental obesogens.25,26 To gauge the magnitude of food consumption in worms, the frequency of pharyngeal movements was measured.27 Intriguingly, despite various NP concentration treatments, the frequency of pharyngeal movements remained unaltered, maintaining mean values within the range of 31–32 min−1 (p > 0.05) (Figure 3). This observation implies that the altered storage lipids in C. elegans prompted by NP exposure did not correlate with the regulation of appetite. As elucidated earlier, the increase in lipid storage was the result of another specific mechanism of NP exposure.
Figure 3.
Impact of NP exposure on pharyngeal pumping
Open circles indicate individual measurements, while the bar represents the mean value. Values are presented as mean ± SD (n = 30).
Effects of NP on fatty acid composition
Reportedly, the alterations in phenotypes encompassing physiological indicators and fat storage are indicative of an underlying imbalance in fatty acid composition.28 To gain insights into the lipid profiles linked to juvenile worms subsequent to NP exposure, a metabolome analysis was harnessed. To ensure robust analysis, one outlier sample was excluded from both the control and 400 μg L−1 NP groups, guided by principal component analyses (PCA) analysis. Through OPLS-DA analysis, the predictive prowess of the model was discernible, evident from the commendable parameters of positive ion model effective prediction with R2Y at 0.974 and Q2Y at 0.735, and the negative ion model with values of 0.994 and 0.736, respectively (Figure S2). The coherence of the aforementioned findings substantiated the establishment of a robust model for data analyzes. During the identification of metabolites modulated by NP exposure, the criteria of Variable Importance in the Projection (VIP) > 1, fold change (FC) > 1, and a p value <0.05 were collectively fulfilled in the screening of altered metabolites induced by NP exposure.
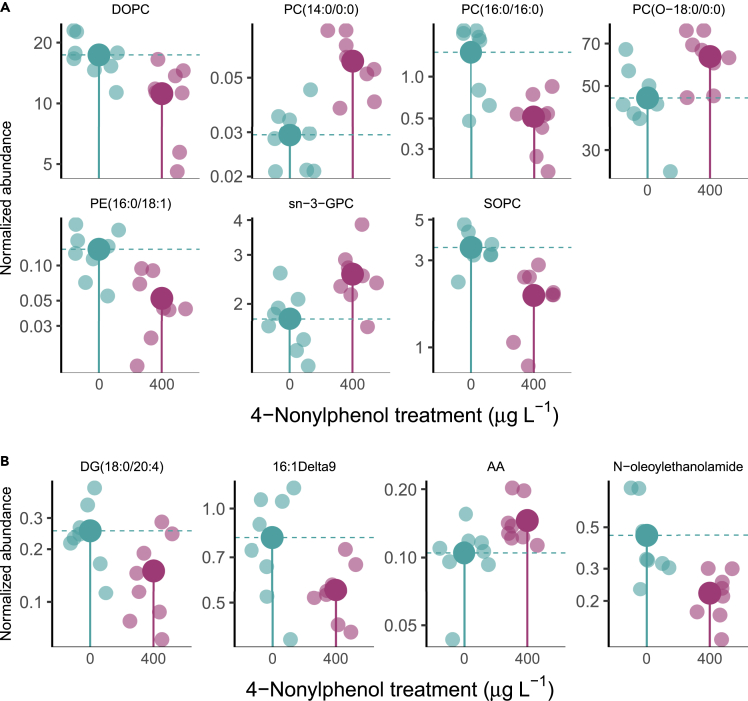
The metabolic fingerprint of the exposed group was significantly discriminated from that of the control group (Figures S1 and S2). NP exposure resulted in 113 metabolites that were up or down-regulated by more than 2-fold compared to the control group, with p value less than 0.05. Out of these metabolites, 11 significantly altered lipid metabolites were identified, mainly concentrated in phosphatidylcholine (PC) and fatty acids (Figure 4). The fold changes of sn-glycero-3-phosphocholine (sn-3-GPC), PC (O-18:0/0:0), and PC (14:0/0:0) were 1.3, 1.9, and 1.9 times higher in the NP treatment group than that of in the control group, respectively. In contrast, phosphatidylglycerol phosphatidylethanolamine (PE, 16:0/18:1), 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC), PC (16:0/16:0), and 1-stearoyl-2-oleoyl-sn-glycero-3-phosphocholine (SOPC) were downregulated by NP exposure versus the control group. In addition to phospholipids, significantly modified monounsaturated and polyunsaturated fatty acids were also present in C. elegans (Figure 3B). Arachidonic acid AA (20:4n-6) exhibited an upregulation trend, while diacylglycerol DG (18:0/20:4), 9-hexadecenoic acid, and N-oleoylethanolamide showed a downregulation trend in the current investigation.
Figure 4.
Lipid alterations in C. elegans induced by NP exposure
(A) Significantly altered phosphatidylcholine.
(B) Significantly altered glycerides and fatty acids. Dark-colored circles represent the mean abundance of altered metabolites, while light-colored circles represent the observed abundance of altered metabolites (n = 9). See also Figures S1–S3.
Phosphatidylcholines account for roughly 32% of the lipid droplets in C. elegans.29 Moreover, most of these identified phosphatidylcholines mediate directly fatty acid and triglyceride metabolism. This could be a significant contributing factor to the alteration of lipid storage resulting from NP exposure. Lipid droplets in C. elegans also contain TAGs, constituting 40–55% of its composition. Nonetheless, this experiment did not identify any significant signals of TAGs in either the vehicle control or 400 μg L−1 NP-treated groups, likely because detecting neutral lipids is challenging due to the lack of charged groups that could be detected by liquid chromatography-tandem mass spectroscopy (LC-MS). Despite this, the results of oil red O staining indicated that the presence of NP enhanced the production of TAGs.
An excessive amount of arachidonic acid indicates a surplus of lipid deposition. For instance, arachidonic acid can both enhance the formation of intracellular lipid droplets and impede cellular lipid metabolic activities using human monocytes as a vehicle.30 Moreover, Arachidonic acid is involved in the metabolic production of phosphatidylcholine,31 which suggests a potential correlation between its levels and the excessive accumulation of lipid droplets observed in this study (Figure 3B). Arachidonic acid also serves as a substrate for various metabolic pathways and directly impacts the concentrations of other metabolites, such as diacylglycerol. The downregulation of diacylglycerol by NP exposure can also be partly explained by the altered levels of some phosphatidylcholine or phosphatidylethanolamine that facilitate diacylglycerol transformation to TAGs via the diacylglycerol acyltransferase pathway.32
Apart from acting as energy storage carriers, lipids play a vital role in various physiological functions. Changes in phosphatidylcholines may signal compromised homeostasis of some neurotransmitters.18 Fatty acids, arachidonic acid and 9-hexadecenoic acid belong to polyunsaturated omega fatty acids, which was both required for growth, reproduction, and neurotransmission.18 This intricate interplay between lipid metabolism and physiological functions underscores the importance of a holistic approach to understanding the multi-toxicity mechanisms of NP.
Alterations of lipid-related metabolites pathways
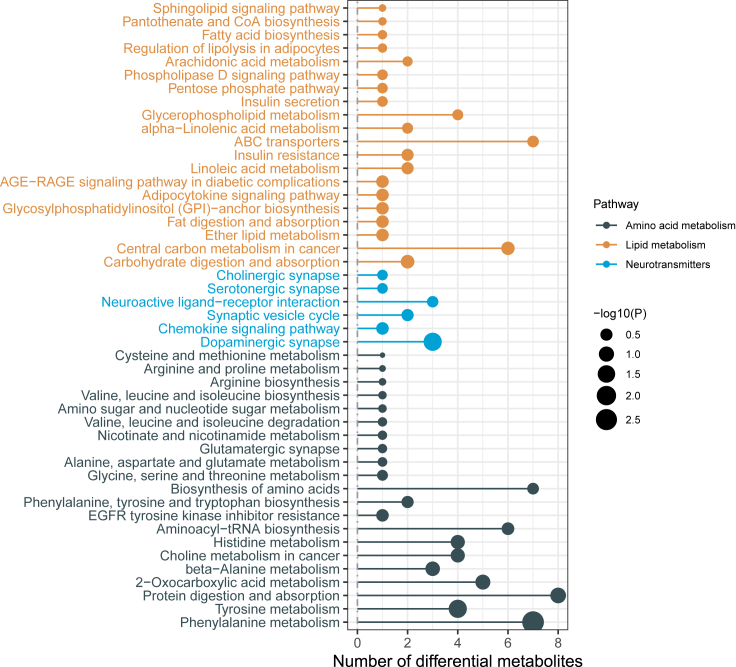
Lipid metabolic pathways exhibit an intricate interplay, intricately intertwined with other molecular processes via specific pathways or small-molecule metabolites. For instance, these pathways interact with pivotal cycles like the glucose metabolism cycle (TCA cycle) and the involvement of acetyl-CoA.33 Employing Kyoto Encyclopedia of Genes and Genomes (KEGG) for a comprehensive analysis of the functionality of all altered metabolites, a total of 47 pathways were delineated, as exemplified in Figure 5. Notably, these pathways perturbed by NP exposure prominently intersect with the functional landscape of carbohydrate, amino acid, and neurotransmitter metabolism, shedding light on their interconnectedness and the ramifications of NP-induced disruptions.
Figure 5.
Identified metabolites in the KEGG pathway of C. elegans exposed to 400 μg L−1 NP
Carbohydrate metabolism
Both carbohydrates and lipids are hydrocarbon-based molecules, and their metabolism is intimately interconnected.18 Citramalic acid, a major intermediate of the tricarboxylic acid cycle (TCA or Krebs cycle), was observed to be highly accumulated after NP exposure (FC = 9.09-fold change compared to control). The high levels of citramalic acid in cells indicate that the energy requirements for other functions have been fulfilled, and the cell will allocate surplus citramalic acid to fatty acids for energy storage. Additionally, ubiquinone (coenzyme-Q) and other terpene quinones regulate ATP levels and energy balance, and showed the strongest correlation with NP in KEGG enrichment. The disruption of energy metabolism resulting from elevated CA and the suppression of mitochondrial ATP production has been noted.34 As uncouplers of oxidative phosphorylation, alkylphenols have been shown to play a crucial role in regulating ATP and energy.35 The environmental hormones Bisphenol A and Bisphenol S, have been linked to interference with cellular mitochondrial function, which is thought to contribute to excessive adipogenesis.36,37 Herein, it is evident that NP-exposed C. elegans exhibited perturbations in the metabolic of glucose cycle and energy activities to variable degrees.
Carboxylic acids and derivatives metabolism
The carboxylic acids and derivatives pathway emerged as the most significant pathway in the KEGG analysis, reflecting its paramount role in the metabolic response to NP exposure. Notably, the discernible alterations in amino acid levels constituted a predominant subset of the annotated differential metabolites associated with NP exposure. This augmentation of amino acids holds the potential to serve as a vital fuel source for intricate metabolic processes, facilitated by their conversion into shared metabolic intermediates within organisms.
The observed elevation in the expression of amino acids could plausibly be linked to the concurrent augmentation in lipid deposition and the heightened levels of citramalic acid. The citramalic acid metabolism cascade generates a multitude of intermediates, including amino acids, which might serve as a substantial contributory factor to the conspicuous upregulation of amino acids, as evidenced by this study. Consequently, the entire spectrum of amino acids can seamlessly integrate into the TCA cycle through acetyl-CoA’s intermediary role.
In particular, the branched-chain amino acids (BCAA), exhibit a transformative capacity by engaging in cyclic metabolic processes via the TCA cycle. This engenders their conversion into both carbohydrates and lipids, besides serving as the elemental building blocks for protein synthesis.38,39 Remarkably, the concentration of BCAA, particularly leucine (FC = 1.9-fold), experienced a substantial surge under the influence of 400 g L−1 NP exposure, markedly contrasting with control conditions in this study. It is imperative to underscore those other amino acids also bear pivotal roles in steering energy metabolism. For instance, it was previously elucidated by Li et al. that leucine operates as an intermediary participant in glycolysis and the electron respiratory chain.40
Neurotransmitters metabolism
The impact of NP exposure extended to the delicate equilibrium of neurotransmitter levels, evidenced by a pronounced enrichment of dopaminergic synaptic functional synapses as identified in the KEGG analysis. Notably, the concentration of dopamine and histamine exhibited a reduction under the influence of 400 μg L−1 NP exposure (FC = 0.77-fold and 0.33-fold), demonstrating a suppressive effect on neurotransmitter biosynthesis due to NP. Consistent with our previous experimental findings, a consistent trend of reduced transcript levels was observed in genes associated with dopamine synthesis in C. elegans exposed to NP, such as cat-1.41 This persistence of NP’s effect on dopamine levels throughout the nematode’s life cycle suggests an enduring impact on neurotransmitter modulation.
Dopamine, derived from tyrosine, exhibited an intriguing counterintuitive trend of elevated levels compared to controls (FC = 1.9-fold). This observation hints at NP’s potential interference with pivotal steps in the conversion of tyrosine to dopamine, perhaps manifesting through compromised activity of tyrosine hydroxylase. Pertinently, the KEGG annotation route pertaining to tyrosine metabolism and tryptophan biosynthesis underwent notable modifications. Notably, tryptophan serves as the exclusive precursor for the production of serotonin (5-HT).42 Interestingly, our prior research delineated a detrimental effect of NP on 5-HT production in C. elegans.41 Nematodes unable to synthesize 5-HT exhibited a propensity for accumulating and synthesizing substantial quantities of lipids.26
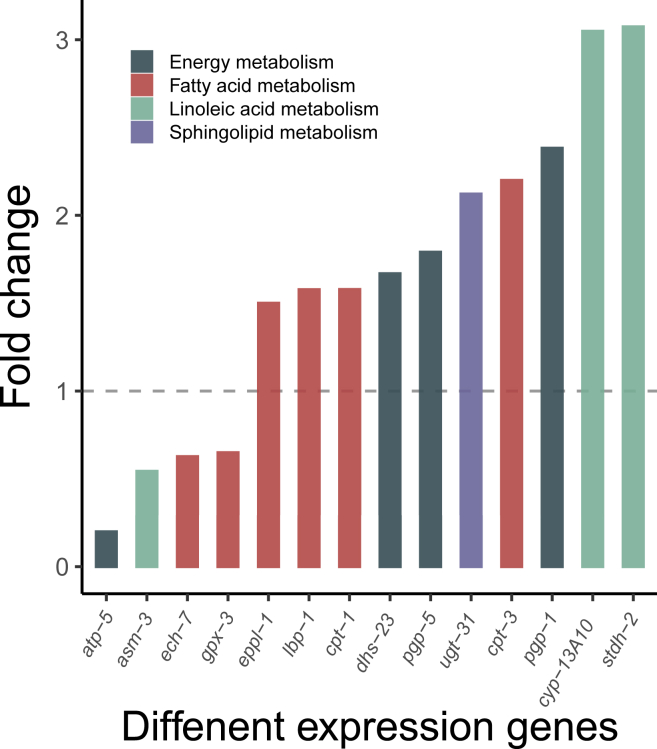
Roles of lipogenic genes in NP-induced lipid alteration
The gene expression profiles were simultaneously depicted with lipid profiles in C. elegans. Methodologically, lipid metabolism-related genes were selected from the profiles of 127 different expression genes (DEGs, FC >2 and p value <0.05), which were manually searched in the total database of lipid metabolites of C. elegans including KEGG and wormbase database et al.43 Specifically, DEGs associated with lipid metabolism were involved in four pathways induced by NP exposure (Figure 6), including linoleic acid metabolism (involved genes: asm-3, cyp13A10, and stdh-2), energy metabolism (involved genes: atp-5, dhs-23, pgp-1, and pgp-5), fatty acid metabolism (involved genes: ech-7, lbp-1, cpt-1, eppl-1, gpx-3, and cpt-3), and sphingolipid metabolism (involved gene: ugt-31). Except for apt-5, asm-3, gpx-3, and ech-7 genes, the transcriptional expression of other genes was upregulated in response to NP exposure.
Figure 6.
Transcriptional profile of lipid-related genes in C. elegans exposed to NP
Data are presented as the fold change compared to the vehicle control (n = 3), with a false discovery rate (FDR) < 0.05.
As anticipated, the genes associated with lipid droplet deconstruction (asm-3) and fatty acid β-oxidation (ech-7) were suppressed. The primary function of the asm-3 gene product is to activate long-chain fatty acids by conjugating them with coenzyme A (CoA) to form Acyl-CoA, and the ech-7 gene encodes enoyl-CoA hydratase enzyme. Downregulation of the genes may herald a decrease in the activity of both enzymes, thus contributing to a decrease in lipid mobilization. The cpt-1 and cpt-3 genes encode carnitine palmitoyl transferase enzyme, and this suggests NP also affects the processes of transporting long-chain fatty acids into the mitochondria for β-oxidation. In addition, the genes related to lipid synthesis processes, i.e., stdh-2 (encodes stearoyl-CoA desaturase enzyme) and cyp-13A10, implicated in fatty acid elongation, was upregulated. Changes in gene expression indicated that NP exposure could stimulate lipogenesis and inhibit lipolysis in worms, partly explaining the increased lipid droplet storage observed in the prior section.
The perturbed transcriptomic and metabolomic profiles resulting from NP exposure were collectively integrated into the KEGG pathway map (Table S1). Notably, the elevated expression of the genes eppl-1 and stdh-2 was correlated with enhancements in the levels of glycerophospholipid species PE (16:0/18:1) and PC (16:0/16:0), alongside the modulation of linoleic acid levels.
In certain animal studies, researchers have investigated transcriptional changes mediated by NP and pathways linked to lipid metabolism. For example, the NP-treatment groups in Daphnia magna and medaka fish observed sphingolipid biogenesis pathway upregulation and Acyl-CoA synthetase family pathway upregulation, respectively.37,44 The gene family expression related to fatty acid synthesis (PPARs and factor-related apoptosis) and degradation (lipoprteinlipase and hormone-sensitive triglyceride lipase) in fish is affected by exposure to NP,22 as observed in the response of C. elegans. In mammals, the transcriptional levels of adipose related target proteins (including factor-related apoptosis, CEBPα, PPARγ) were also upregulated in NP-exposed rats.45 Interaction with other phenolic compounds also contributes to the disruption of lipid-related transcriptional processes. Bisphenol A, NP, and Octylphenol share similar modes of action involving the phenyl hydroxyl group, which has common effects on lipid-related pathways, like de novo lipogenesis processes.46 Consistently, the previous evidence indicates that NP has stimulating effects on lipid synthesis processes in a wide range of species, including both vertebrates and invertebrates.
Discussion
In a holistic perspective, the discernible shift in composition and the highlighted prominence of amino acid in particular, reflect the intricate orchestration of metabolic adaptations orchestrated by NP exposure. This convergence of pathways and substrates underscores the interplay among lipid deposition, carbohydrates and amino acid modulation, and their concerted contributions to the intricate landscape of energy metabolism in the context of NP-induced multi-toxicity.
The interrelation among these processes visually depicted in Figure 7 to elucidate the interrelation among these processes. In a broad sense, the influence of NP was observed to redirect glycolytic and amino acid metabolic pathways toward favoring lipid synthesis, thus culminating in the augmentation of lipid storage within C. elegans. As depicted, while non-targeted metabolomics techniques exhibit the capability to capture a broader spectrum of small molecules, including amino acids, their lack of specificity may inadvertently hinder the detection of numerous lipid molecules.47 However, our findings managed to discern specific fluctuations in the expression of lipid-associated molecules, thus furnishing a nexus between these variations and the physiological toxicity elicited by NP exposure.
Figure 7.
Overview of differentially expressed genes and altered metabolites in C. elegans induced by NP exposure
Upregulated metabolites or genes are indicated in red letters, while downregulated metabolites or genes are indicated in green letters.
Lipids are acknowledged for their involvement in a myriad of physiological functions, thereby underscoring the potential of NP-induced aberrant lipid metabolism to further intercede with adverse outcome pathways. Guided by the amalgamated results from transcriptomics and metabolomics, we postulated plausible connections between lipid-related processes and the deleterious outcomes of NP exposure: (1) Shared regulatory mechanisms governing both physiological markers and lipid metabolism, exemplified by Insulin/IGF-1 signaling and steroid signaling pathways, as identified in this study (Figure 6). These pathways, known to mediate life history and lipid metabolism, are underscored in previous research;48 (2) the perturbed equilibrium between energy storage and expenditure engendered by the surfeit accumulation of lipids. Dysfunctions in genes like atp-5, stdh-2, and the molecule ubiquinone were envisaged to be instrumental in precipitating genital and developmental morphological abnormalities.49 Other EDCs, e.g., tributyltin, pyriproxyfen, and perfluorooctanoic acid, have been documented to concurrently impair the development and reproductive activities of Daphnia magna and fish by perturbing the allocation of energy between life history and lipid storage;4,9,14 (3) hindered conversions of lipids into other molecular entities, such as neurotransmitters and carbohydrates, indirectly impact physiological functions; (4) notably, lipid molecules themselves can regulate physiological indicators, including signaling hormones.
The perturbation of lipid metabolism emerges as a plausible toxic mechanism induced by NP exposure. This phenomenon is substantiated by the observed accumulation of lipid storage within the intestines of C. elegans, as evidenced by specific oil red O staining. Furthermore, the non-targeted metabolomic investigation spotlighted the potential role of altered phospholipid molecules in driving the increase in lipid storage elicited by NP. At the transcriptomic level, the surge in lipid accumulation stems from NP’s directive influence on driving lipid molecules toward the trajectory of lipid synthesis. The dysregulation in lipid metabolism, prompted by NP exposure, emerges as a direct consequence of the excessive abundance of small molecule metabolites, such as citrate and amino acids. The lipid-centric alterations prompted by NP constitute a pivotal mechanistic driver behind the observed physiological shifts in nematodes. This research has advanced our comprehension of NP-induced disruptions in lipid homeostasis within organisms.
Limitation of the study
Lack of lipidomics analysis
Although the non-targeted metabolomic analysis utilized in this study offers a broad perspective on various components such as neurotransmitters, amino acids, and carbohydrates, it may have limited the specific detection of lipid molecules. Consequently, a more targeted lipidomic approach could provide a more detailed and precise understanding of the specific lipid species affected by NP exposure.
Absence of in vivo quantification of nonylphenol
The study did not quantitatively analyze the concentration of NP within the model organism. Understanding the dynamic changes of NP accumulation over time within the organism is crucial for unraveling its potential toxicity and accumulation mechanisms.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Bacterial and virus strains | ||
| E.coli OP50 | CGC | Cat# WBStrain00041969 |
| Chemicals, peptides, and recombinant proteins | ||
| 4-Nonylphenol | Aladdin | Cas# 104-40-5; RRID: AB_2313773 |
| Oil-Red-O | Aladdin | Cas# 1320-06-5; RRID: AB_146295 |
| Glycerine | Aladdin | Cas# 56-81-5; RRID: AB_2313773 |
| Triton X-100 | Aladdin | Cas# 9002-93-1; RRID: AB_286840 |
| LC-MS reagents | CNW Technologies | N/A |
| 2-Chloro-L-phenylalanine | Hengbai Biotechnology | Cas# 103616-89-3; RRID: AB_2313773 |
| Experimental models: Organisms/strains | ||
| N2 Bristol wild-type | CGC | Cat# WBStrain00000001 |
| Software and algorithms | ||
| R | Ross Ihaka and Robert Gentleman | https://www.r-project.org/ |
| Image-Pro Plus | Media Cybernetics | https://mediacy.com/image-pro/ |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Xue Cao (caoxue@szu.edu.cn).
Materials availability
All data generated or analysed in this study are included in this published article.
Data and code availability
-
•
Data reported in this paper will be shared by the lead contact upon request.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
Experimental model and subject details
C. elegans strains and cultivation
The wild-type N2 Bristol strain of C. elegans was acquired from the Caenorhabditis Genetics Centre (GCG) and grown on nutrient-rich nematode growth media (NGM) at a temperature of 20°C. To obtain synchronized worms, eggs were collected from gravid hermaphrodites using the bleach method and transferred to food-rich NGM.50 The eggs of nematodes are covered by a thick cuticle, which makes it difficult for contaminants to enter. Hence, L1 and L4 stage worms, which were 12-h and 24-h post-egg development, were both selected for the assessment of NP toxicity.
Method details
NP exposure tests
To cover the range of NP concentrations found in natural environments, a variety of concentrations were chosen for exposure tests, spanning from 1 to 400 μg L−1, and the control group was established using K solution with 0.1% DMSO. The 4-NP was dissolved in DMSO and then diluted with the K-medium (containing 51 mmol L−1 NaCl and 32 mmol L−1 KCl) to prepare the test solutions (DMSO, 0.1%). During the exposure period, the worms were fed UV-inactivated E. coli OP50 as a food source, and 50% of the NP solution was refreshed daily to keep NP concentration stable. Specifically, 100 worms were placed in 10 mL of the NP-treatment solution for 48 h, with three replicated bakers at each treatment, and relevant endpoints were subsequently evaluated. None of tested concentrations of NP affected survival of worms during the whole exposure periods. All manipulations were performed at a constant temperature of 20 ± 1°C.
Quantification of fat storages
Oil-Red-O is commonly utilized as a molecular probe to label lipid droplets in C. elegans.51 A 1% stock solution of Oil-Red-O was prepared in 60% isopropanol and was heated to 60°C to expedite the dissolution. Exposed worms were collected from NGM agar plates, washed thrice with sterile K liquid, and fixed with 1% paraformaldehyde at 4°C for 30 min before quickly transferred to −80°C for 15 min. Subsequently, the frozen samples were placed in a 43°C water bath and washed thoroughly with PBS (pH = 7). Next, the worms were fixed and stained with a combination of Oil-Red-O and Triton X-100 for 30 min and then placed on slides. The optical microscope (Olympus bx53, Japan) was used to capture the images, which were then analysed with the Image-Pro Plus 6.0 software (Media Cybernetics, Silver Spring, MD). Every treatment concentration was carried out in three biological replicates. To calculate the relative lipid content per worm, the lipid proportion was determined using the following equation: lipid proportion = (total area per worm − area of unstained part per worm)/total area per worm. Additionally, to evaluate the Oil-Red-O positive substance density, the optical density (OD) of each worm value was also measured (n = 60 for each treatment).
Measurements of physiological indicators
The indicators of projected area and brood size were selected to reflect the NP effects on the life history of C. elegans. After being synchronized in the L1 stage, worms were subjected to NP at different dosages for 48 h. For the assay of projected area, the collected worms were incubated at 40°C and further dyed with rose red for 1 h. Following the staining process, the worms were thoroughly rinsed with K buffer three times to ensure complete removal of excess dye. The worms were then prepared for imaging under an optical microscope, and the projected area of the worms were analyzed using Image-Pro Plus software.
For the brood size assay, 10 randomly selected pregnant worms were transferred to fresh NGM plates every 24 h. The original NGM plates were left to incubate at 20°C for an additional 24 h. After this incubation period, each NGM plate was placed under a stereomicroscope for egg counting. To ensure accurate counts, a red-hot platinum needle was used to immobilize and mark each counted worm. This approach allowed for a clearer count of the hatched progeny from the eggs on the NGM plates. The entire process was repeated at 24-h intervals until the worms ceased laying eggs.
To measure pharyngeal movements, 30 worms were randomly selected from each treatment and were placed on NGM medium that was supplemented with E. coli OP50 and kept stable for 1 h. Individuals were then counted under the microscope for the number of pharyngeal pumping events in 1 min.
Untargeted metabolomic and transcriptome analysis
The lipids and lipid-related molecule profile of two groups of C. elegans, where one group was treated with 0 and the other with 400 μg L−1 NP, was investigated using untargeted metabolomic analysis. Each replicate for two groups consisted of approximately 8,000 individuals (n = 10 for each group). The worm samples were freeze-dried and weighted. Extract solution for metabolites was composed of acetonitrile (40% v/v), methanol (40% v/v), and water (20% v/v) (with 2 mg L−1 L-2-Chlorophenylalanine as internal standard). After vortex for 30 s, the samples were homogenized by employing magnetic beads at 35 Hz for 240 s, and then sonicated in an ice-water bath at 4°C for 300 s. The above operation procedure was repeated twice. After that, the samples were allowed to stand at −40°C for 60 min and then centrifuged at 4°C for fifteen min to separate the supernatant. 480 μL of supernatant was pipetted into EP tube and dried under vacuum at 37°C. Add 100 μL of 50% acetonitrile to the dried samples and sonicate in an ice-water bath for 10 min. Then, the samples were centrifuged at 4°C for fifteen min, and 75 μL of supernatants were measured to determine metabolite using LC-MS. The quality control trials were prepared by mixing equal amounts of supernatant from all samples together.
For analysis, samples were taken to ensure the molecules separated by using a 1290 infinity series UHPLC System (Waters, Agilent Technologies) equipped with a UPLC BEH Amide column (2.1-mm length, 100-mm i.d., 1.7-μm thickness). Liquid chromatography phase A was aqueous (pH = 9.75), containing 25 mmol/L ammonium acetate and 25 mmol/L ammonia, and phase B was acetonitrile. The elution gradient analysis as follows: from 0 min to 0.5 min, 95% B; from 0.5 min to 7.0 min, 95%–65% B; from 7.0 min to 8.0 min, 65%–40% B; from 8.0 min to 9.0 min, 40% B; from 9.0 min to 9.1 min, 40%–95% B; from 9.1 min to 12.0 min, 95% B. The temperature of column and auto-sampler was set as 25°C and 4°C, respectively. The injection volume of positive and negative ions was 2 μL, respectively. The Triple TOF 6600 mass spectrometry was used to collect MS/MS spectra data through information-dependent acquisition (IDA) mode. In the mode of IDA, the data acquisition software (Analyst TF 1.7, AB Sciex) based on the MS/MS spectra data and pre-defined criteria, the ions were automatically selected, and their full scan survey MS data were acquired. The 12 most intense ions with intensity greater than 100 were selected for MS/MS for each cycle, with collision-induced dissociation at an energy of 30 eV and a cycle time of 0.56 s. Delustering potential was set as 60 V, and Ion Spray Voltage Floating was modulated as 5000 V (Pos)/-4000 (Neg).
Transcriptome studies of exposure and control groups were carried out in three biological replicates. Briefly, the worms from two treatment groups, 0 and 400 μg L−1 were employed to continue with the transcriptome sequencing study. In addition, the qPCR technique was used to verify the reliability of the data. Detailed approaches are presented in our earlier reports.49
Quantification and statistical analysis
For metabolomic analysis, principal component analyses (PCA) were run to check the homogeneity of each subpopulation and eventually exclude outliers. The partial least squares-discriminant analysis (OPLS-DA) were run to discriminate altered metabolites of worms between the NP-treatment and control group (Figure S1A). To prevent overfitting of simulation results, the reliability of OPLS-DA model was further graded and confirmed.
The data of biological indicators were presented as mean ± standard deviation (SD). The significant difference was calculated using one-way ANOVA, and post-hoc Dunnett's test. The R software (version 4.1.3) and the package "tidyverse" were used to analysis and create all of the figures. Differences between control and exposure groups were considered statistically significant when p-values were less than 0.05 (∗) or 0.01 (∗∗).
Acknowledgments
We thank the Caenorhabditis Genetic Center for providing the Caenorhabditis elegans N2 strain used in this study. This work was supported by the National Natural Science Foundation of China (42206143), the Natural Science Foundation of Shanghai (23ZR1417500), the Fundamental Research Funds for the Central Universities, and Shanghai Sailing Program (23YF1445900).
Author contributions
Conceptualization, F.L. and X.C.; methodology, F.L.; investigation, F.L. and X.C.; writing – original draft, F.L. and X.C.; writing – review & editing, L.Z.; funding acquisition, F.L., L.Z., and X.C.; resources, L.Z.; supervision, X.C. and L.Z.
Declaration of interests
The authors declare no competing interests.
Inclusion and diversity
We support inclusive, diverse, and equitable conduct of research.
Published: November 8, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2023.108417.
Contributor Information
Xue Cao, Email: caoxue@szu.edu.cn.
Lei Zhou, Email: zhoulei@ecust.edu.cn.
Supplemental information
References
- 1.Hong Y., Feng C., Yan Z., Wang Y., Liu D., Liao W., Bai Y. Nonylphenol occurrence, distribution, toxicity and analytical methods in freshwater. Environ. Chem. Lett. 2020;18:2095–2106. [Google Scholar]
- 2.Cailleaud K., Michalec F.-G., Forget-Leray J., Budzinski H., Hwang J.-S., Schmitt F.G., Souissi S. Changes in the swimming behavior of Eurytemora affinis (Copepoda, Calanoida) in response to a sub-lethal exposure to nonylphenols. Aquat. Toxicol. 2011;102:228–231. doi: 10.1016/j.aquatox.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 3.Vidal-Liñán L., Bellas J., Salgueiro-González N., Muniategui S., Beiras R. Bioaccumulation of 4-nonylphenol and effects on biomarkers, acetylcholinesterase, glutathione-S-transferase and glutathione peroxidase, in Mytilus galloprovincialis mussel gills. Environ. Pollut. 2015;200:133–139. doi: 10.1016/j.envpol.2015.02.012. [DOI] [PubMed] [Google Scholar]
- 4.Jordão R., Garreta E., Campos B., Lemos M.F.L., Soares A.M.V.M., Tauler R., Barata C. Compounds altering fat storage in Daphnia magna. Sci. Total Environ. 2016;545–546:127–136. doi: 10.1016/j.scitotenv.2015.12.097. [DOI] [PubMed] [Google Scholar]
- 5.Chen R., Yu Z., Yin D. Multi-generational effects of lindane on nematode lipid metabolism with disturbances on insulin-like signal pathway. Chemosphere. 2018;210:607–614. doi: 10.1016/j.chemosphere.2018.07.066. [DOI] [PubMed] [Google Scholar]
- 6.Wu Q., Zhi L., Qu Y., Wang D. Quantum dots increased fat storage in intestine of Caenorhabditis elegans by influencing molecular basis for fatty acid metabolism. Nanomedicine. 2016;12:1175–1184. doi: 10.1016/j.nano.2016.01.016. [DOI] [PubMed] [Google Scholar]
- 7.Li Z., Yu Z., Cui C., Ai F., Yin D. Multi-generational obesogenic effects of sulfomethoxazole on Caenorhabditis elegans through epigenetic regulation. J. Hazard Mater. 2020;382 doi: 10.1016/j.jhazmat.2019.121061. [DOI] [PubMed] [Google Scholar]
- 8.Lee M.-C., Park J.C., Lee J.-S. Effects of environmental stressors on lipid metabolism in aquatic invertebrates. Aquat. Toxicol. 2018;200:83–92. doi: 10.1016/j.aquatox.2018.04.016. [DOI] [PubMed] [Google Scholar]
- 9.Seyoum A., Pradhan A., Jass J., Olsson P.-E. Perfluorinated alkyl substances impede growth, reproduction, lipid metabolism and lifespan in Daphnia magna. Sci. Total Environ. 2020;737 doi: 10.1016/j.scitotenv.2020.139682. [DOI] [PubMed] [Google Scholar]
- 10.Jordão R., Casas J., Fabrias G., Campos B., Piña B., Lemos M.F.L., Soares A.M.V.M., Tauler R., Barata C. Obesogens beyond Vertebrates: Lipid Perturbation by Tributyltin in the Crustacean Daphnia magna. Environ. Health Perspect. 2015;123:813–819. doi: 10.1289/ehp.1409163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang W., Li Z., Zhang X., Zhang J., Ru S. Bisphenol S Impairs Behaviors through Disturbing Endoplasmic Reticulum Function and Reducing Lipid Levels in the Brain of Zebrafish. Environ. Sci. Technol. 2023;57:582–594. doi: 10.1021/acs.est.2c07828. [DOI] [PubMed] [Google Scholar]
- 12.Heindel J.J., Newbold R., Schug T.T. Endocrine disruptors and obesity. Nat. Rev. Endocrinol. 2015;11:653–661. doi: 10.1038/nrendo.2015.163. [DOI] [PubMed] [Google Scholar]
- 13.Casals-Casas C., Desvergne B. Endocrine disruptors: from endocrine to metabolic disruption. Annu. Rev. Physiol. 2011;73:135–162. doi: 10.1146/annurev-physiol-012110-142200. [DOI] [PubMed] [Google Scholar]
- 14.Lee D.-H., Jo Y.J., Eom H.-J., Yum S., Rhee J.-S. Nonylphenol induces mortality and reduces hatching rate through increase of oxidative stress and dysfunction of antioxidant defense system in marine medaka embryo. Mol. Cell. Toxicol. 2018;14:437–444. [Google Scholar]
- 15.Yu J., Li W., Tang L., Luo Y., Xu J. In vivo and in vitro effects of chronical exposure to nonylphenol on lipid metabolism. Environ. Sci. Eur. 2020;32:87. [Google Scholar]
- 16.Bundy J.G., Davey M.P., Viant M.R. Environmental metabolomics: a critical review and future perspectives. Metabolomics. 2009;5:3–21. [Google Scholar]
- 17.Kim H.M., Lee D.-K., Long N.P., Kwon S.W., Park J.H. Uptake of nanopolystyrene particles induces distinct metabolic profiles and toxic effects in Caenorhabditis elegans. Environ. Pollut. 2019;246:578–586. doi: 10.1016/j.envpol.2018.12.043. [DOI] [PubMed] [Google Scholar]
- 18.Watts J.L., Ristow M. Lipid and carbohydrate metabolism in Caenorhabditis elegans. Genetics. 2017;207:413–446. doi: 10.1534/genetics.117.300106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Z., Yu Z., Yin D. Multi- and trans-generational disturbances of perfluorobutane sulfonate and perfluorohexane sulfonate on lipid metabolism in Caenorhabditis elegans. Chemosphere. 2021;280 doi: 10.1016/j.chemosphere.2021.130666. [DOI] [PubMed] [Google Scholar]
- 20.O'Rourke E.J., Soukas A.A., Carr C.E., Ruvkun G. C. elegans major fats are stored in vesicles distinct from lysosome-related organelles. Cell Metab. 2009;10:430–435. doi: 10.1016/j.cmet.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hao C.J., Cheng X.J., Xia H.F., Ma X. The endocrine disruptor 4-nonylphenol promotes adipocyte differentiation and induces obesity in mice. Cell. Physiol. Biochem. 2012;30:382–394. doi: 10.1159/000339032. [DOI] [PubMed] [Google Scholar]
- 22.Çakmak G., Togan I., Uğuz C., Severcan F. FT-IR spectroscopic analysis of rainbow trout liver exposed to nonylphenol. Appl. Spectrosc. 2003;57:835–841. doi: 10.1366/000370203322102933. [DOI] [PubMed] [Google Scholar]
- 23.Chang L.L., Wun W.S.A., Wang P.S. In utero and neonate exposure to nonylphenol develops hyperadrenalism and metabolic syndrome later in life. I. First generation rats (F(1)) Toxicology. 2012;301:40–49. doi: 10.1016/j.tox.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 24.Ramírez-Zacarías J.L., Castro-Muñozledo F., Kuri-Harcuch W. Quantitation of adipose conversion and triglycerides by staining intracytoplasmic lipids with Oil red O. Histochemistry. 1992;97:493–497. doi: 10.1007/BF00316069. [DOI] [PubMed] [Google Scholar]
- 25.Grün F., Blumberg B. Endocrine disrupters as obesogens. Mol. Cell. Endocrinol. 2009;304:19–29. doi: 10.1016/j.mce.2009.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luo Z., Yu Z., Yin D. Obesogenic effect of erythromycin on Caenorhabditis elegans through over-eating and lipid metabolism disturbances. Environ. Pollut. 2022;294 doi: 10.1016/j.envpol.2021.118615. [DOI] [PubMed] [Google Scholar]
- 27.Avery L., You Y.J. WormBook; 2012. C. elegans Feeding; pp. 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kniazeva M., Sieber M., McCauley S., Zhang K., Watts J.L., Han M. Suppression of the ELO-2 FA elongation activity results in alterations of the fatty acid composition and multiple physiological defects, including abnormal ultradian rhythms, in Caenorhabditis elegans. Genetics. 2003;163:159–169. doi: 10.1093/genetics/163.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Y., Li C., Zhang J., Xu X., Fu L., Xu J., Zhu H., Hu Y., Li C., Wang M., et al. Polyunsaturated fatty acids promote the rapid fusion of lipid droplets in Caenorhabditis elegans. J. Biol. Chem. 2022;298 doi: 10.1016/j.jbc.2022.102179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guijas C., Pérez-Chacón G., Astudillo A.M., Rubio J.M., Gil-de-Gómez L., Balboa M.A., Balsinde J. Simultaneous activation of p38 and JNK by arachidonic acid stimulates the cytosolic phospholipase A2-dependent synthesis of lipid droplets in human monocytes. J. Lipid Res. 2012;53:2343–2354. doi: 10.1194/jlr.M028423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bermúdez M.A., Balboa M.A., Balsinde J. Lipid droplets, phospholipase A(2), arachidonic acid, and atherosclerosis. Biomedicines. 2021;9:2–6. doi: 10.3390/biomedicines9121891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodriguez-Cuenca S., Pellegrinelli V., Campbell M., Oresic M., Vidal-Puig A. Sphingolipids and glycerophospholipids – The “ying and yang” of lipotoxicity in metabolic diseases. Prog. Lipid Res. 2017;66:14–29. doi: 10.1016/j.plipres.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 33.Wang R., Li B., Lam S.M., Shui G. Integration of lipidomics and metabolomics for in-depth understanding of cellular mechanism and disease progression. J. Genet. Genomi. 2020;47:69–83. doi: 10.1016/j.jgg.2019.11.009. [DOI] [PubMed] [Google Scholar]
- 34.De Oliveira Pateis V., Bracht L., dos Santos Castro L., Bueno Franco Salla G., Comar J.F., Valderrama Parizotto A., Peralta R.M., Bracht A. The food additive BHA modifies energy metabolism in the perfused rat liver. Toxicol. Lett. 2018;299:191–200. doi: 10.1016/j.toxlet.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 35.Bragadin M., Perin G., Iero A., Manente S., Rizzoli V., Scutari G. An in vitro study on the toxic effects of nonylphenols (NP) in mitochondria. Chemosphere. 1999;38:1997–2001. doi: 10.1016/s0045-6535(98)00412-3. [DOI] [PubMed] [Google Scholar]
- 36.Liu Q., Shao W., Weng Z., Zhang X., Ding G., Xu C., Xu J., Jiang Z., Gu A. In vitro evaluation of the hepatic lipid accumulation of bisphenol analogs: A high-content screening assay. Toxicol. Vitro. 2020;68 doi: 10.1016/j.tiv.2020.104959. [DOI] [PubMed] [Google Scholar]
- 37.Campos B., Garcia-Reyero N., Rivetti C., Escalon L., Habib T., Tauler R., Tsakovski S., Piña B., Barata C. Identification of metabolic pathways in Daphnia magna explaining hormetic effects of selective serotonin reuptake inhibitors and 4-nonylphenol using transcriptomic and phenotypic responses. Environ. Sci. Technol. 2013;47:9434–9443. doi: 10.1021/es4012299. [DOI] [PubMed] [Google Scholar]
- 38.Wu G. Amino acids: metabolism, functions, and nutrition. Amino Acids. 2009;37:1–17. doi: 10.1007/s00726-009-0269-0. [DOI] [PubMed] [Google Scholar]
- 39.Newgard C.B. Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metab. 2012;15:606–614. doi: 10.1016/j.cmet.2012.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li Y., Man S., Li J., Chai H., Fan W., Liu Z., Gao W. The antitumor effect of formosanin C on HepG2 cell as revealed by 1H-NMR based metabolic profiling. Chem. Biol. Interact. 2014;220:193–199. doi: 10.1016/j.cbi.2014.06.023. [DOI] [PubMed] [Google Scholar]
- 41.Cao X., Wang X., Chen H., Li H., Tariq M., Wang C., Zhou Y., Liu Y. Neurotoxicity of nonylphenol exposure on Caenorhabditis elegans induced by reactive oxidative species and disturbance synthesis of serotonin. Environ. Pollut. 2019;244:947–957. doi: 10.1016/j.envpol.2018.09.140. [DOI] [PubMed] [Google Scholar]
- 42.Hol J.W., Stolker R.J., Klimek M., Stronks D.L., Fekkes D. The tryptophan kynurenine pathway, neopterin and IL-6 during vulvectomy and abdominal hysterectomy. J. Biomed. Sci. 2014;21:102. doi: 10.1186/s12929-014-0102-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang Y., Zou X., Ding Y., Wang H., Wu X., Liang B. Comparative genomics and functional study of lipid metabolic genes in Caenorhabditis elegans. BMC Genom. 2013;14:164. doi: 10.1186/1471-2164-14-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Won H., Woo S., Yum S. Acute 4-nonylphenol toxicity changes the genomic expression profile of marine medaka fish, Oryzias javanicus. Mol. Cell. Toxicol. 2014;10:181–195. [Google Scholar]
- 45.Yu J., Li W., Tang L., Luo Y., Xu J. In vivo and in vitro effects of chronical exposure to nonylphenol on lipid metabolism. Environ. Sci. Eur. 2020;32:87. [Google Scholar]
- 46.Guo J., Mo J., Zhao Q., Han Q., Kanerva M., Iwata H., Li Q. De novo transcriptomic analysis predicts the effects of phenolic compounds in Ba River on the liver of female sharpbelly (Hemiculter lucidus) Environ. Pollut. 2020;264 doi: 10.1016/j.envpol.2020.114642. [DOI] [PubMed] [Google Scholar]
- 47.Gao Y., Chen Y., Yue X., He J., Zhang R., Xu J., Zhou Z., Wang Z., Zhang R., Abliz Z. Development of simultaneous targeted metabolite quantification and untargeted metabolomics strategy using dual-column liquid chromatography coupled with tandem mass spectrometry. Anal. Chim. Acta. 2018;1037:369–379. doi: 10.1016/j.aca.2018.08.042. [DOI] [PubMed] [Google Scholar]
- 48.Hansen M., Flatt T., Aguilaniu H. Reproduction, fat Metabolism, and life span: what is the connection? Cell Metab. 2013;17:10–19. doi: 10.1016/j.cmet.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cao X., Yan C., Wu X., Zhou L., Xiu G. Nonylphenol induced individual and population fluctuation of Caenorhabditis elegans: Disturbances on developmental and reproductive system. Environ. Res. 2020;186 doi: 10.1016/j.envres.2020.109486. [DOI] [PubMed] [Google Scholar]
- 50.Liu F., Luo Q., Zhang Y., Huang K., Cao X., Cui C., Lin K., Zhang M. Trans-generational effect of neurotoxicity and related stress response in Caenorhabditis elegans exposed to tetrabromobisphenol A. Sci. Total Environ. 2020;703 doi: 10.1016/j.scitotenv.2019.134920. [DOI] [PubMed] [Google Scholar]
- 51.Lapierre L.R., Silvestrini M.J., Nuñez L., Ames K., Wong S., Le T.T., Hansen M., Meléndez A. Autophagy genes are required for normal lipid levels in C. elegans. Autophagy. 2013;9:278–286. doi: 10.4161/auto.22930. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
Data reported in this paper will be shared by the lead contact upon request.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.